Abstract
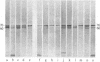
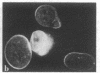
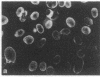
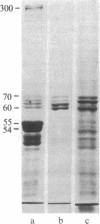
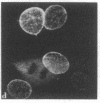
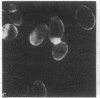
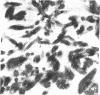
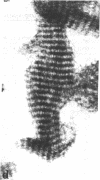
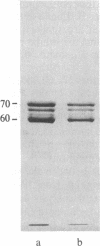
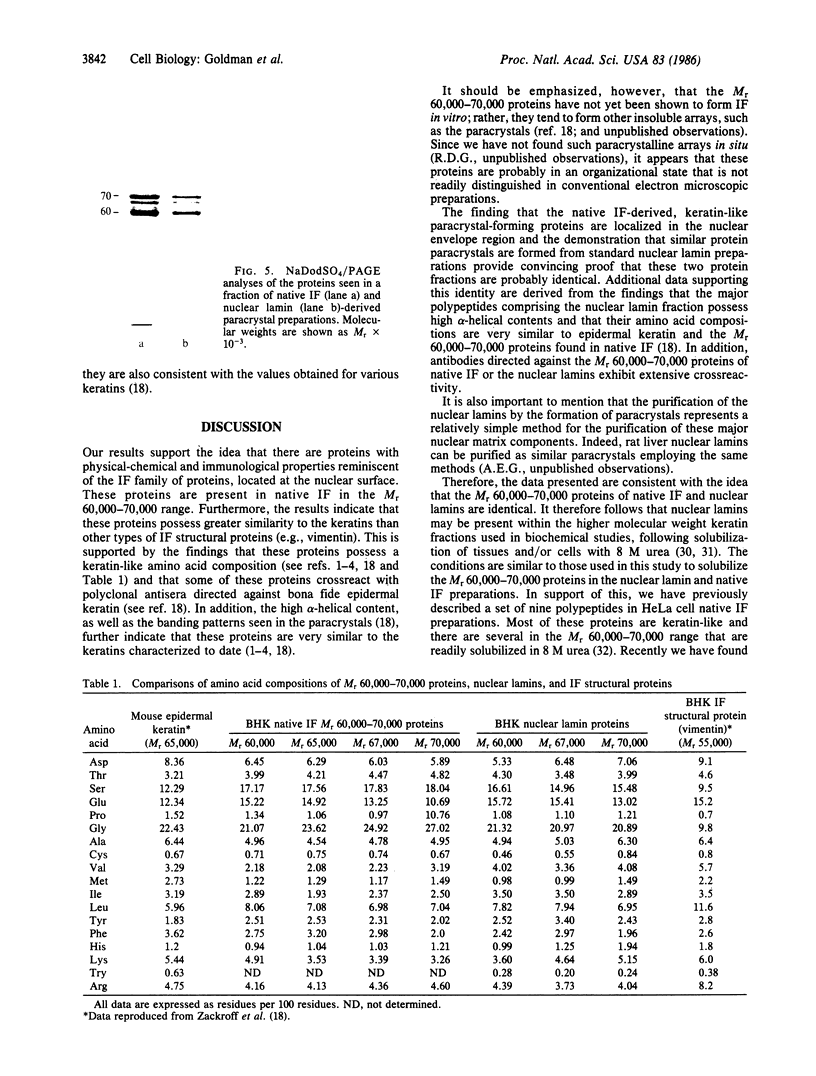
Four proteins of Mr approximately equal to 60,000, 65,000, 67,000, and 70,000 coisolate with the major intermediate filament (IF) structural proteins of BHK-21 cells. These proteins are keratin-like, they form distinctive paracrystals in vitro, and they are concentrated at the nuclear surface. Since these properties indicate similarities with the nuclear lamins, we have prepared conventional fractions of BHK-21 nuclei from which the same type of paracrystal is obtained. Furthermore, biochemical and immunological data demonstrate that the lamins are identical to the Mr 60,000-70,000 proteins found in IF preparations, and both sets of proteins are similar to keratin. These results suggest that an IF-like protein network is present in the nuclear lamina. We speculate that in some unknown way this network connects to the cytoplasmic IF network that courses from the juxtanuclear region to the cell surface. These proposed interconnecting networks may form part of the infrastructure of cytoskeletal-nuclear matrix-connecting links involved in signal transmission between the nuclear and cytoplasmic compartments of eukaryotic cells.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaronson R. P., Blobel G. Isolation of nuclear pore complexes in association with a lamina. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1007–1011. doi: 10.1073/pnas.72.3.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnn J., Staehelin L. A. The structure and function of spot desmosomes. Int J Dermatol. 1981 Jun;20(5):330–339. doi: 10.1111/j.1365-4362.1981.tb00815.x. [DOI] [PubMed] [Google Scholar]
- Aynardi M. W., Steinert P. M., Goldman R. D. Human epithelial cell intermediate filaments: isolation, purification, and characterization. J Cell Biol. 1984 Apr;98(4):1407–1421. doi: 10.1083/jcb.98.4.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissell M. J., Hall H. G., Parry G. How does the extracellular matrix direct gene expression? J Theor Biol. 1982 Nov 7;99(1):31–68. doi: 10.1016/0022-5193(82)90388-5. [DOI] [PubMed] [Google Scholar]
- Blose S. H., Chacko S. Rings of intermediate (100 A) filament bundles in the perinuclear region of vascular endothelial cells. Their mobilization by colcemid and mitosis. J Cell Biol. 1976 Aug;70(2 Pt 1):459–466. doi: 10.1083/jcb.70.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagenais A., Bibor-Hardy V., Simard R. Characterization of lamin proteins in BHK cells. Exp Cell Res. 1984 Dec;155(2):435–447. doi: 10.1016/0014-4827(84)90204-0. [DOI] [PubMed] [Google Scholar]
- Dale B. A. Purification and characterization of a basic protein from the stratum corneum of mammalian epidermis. Biochim Biophys Acta. 1977 Mar 28;491(1):193–204. doi: 10.1016/0005-2795(77)90055-1. [DOI] [PubMed] [Google Scholar]
- Dale B. A., Resing K. A., Lonsdale-Eccles J. D. Filaggrin: a keratin filament associated protein. Ann N Y Acad Sci. 1985;455:330–342. doi: 10.1111/j.1749-6632.1985.tb50420.x. [DOI] [PubMed] [Google Scholar]
- Fey E. G., Capco D. G., Krochmalnic G., Penman S. Epithelial structure revealed by chemical dissection and unembedded electron microscopy. J Cell Biol. 1984 Jul;99(1 Pt 2):203s–208s. doi: 10.1083/jcb.99.1.203s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerace L., Blobel G. Nuclear lamina and the structural organization of the nuclear envelope. Cold Spring Harb Symp Quant Biol. 1982;46(Pt 2):967–978. doi: 10.1101/sqb.1982.046.01.090. [DOI] [PubMed] [Google Scholar]
- Gerace L., Blobel G. The nuclear envelope lamina is reversibly depolymerized during mitosis. Cell. 1980 Jan;19(1):277–287. doi: 10.1016/0092-8674(80)90409-2. [DOI] [PubMed] [Google Scholar]
- Gerace L., Blum A., Blobel G. Immunocytochemical localization of the major polypeptides of the nuclear pore complex-lamina fraction. Interphase and mitotic distribution. J Cell Biol. 1978 Nov;79(2 Pt 1):546–566. doi: 10.1083/jcb.79.2.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerace L., Comeau C., Benson M. Organization and modulation of nuclear lamina structure. J Cell Sci Suppl. 1984;1:137–160. doi: 10.1242/jcs.1984.supplement_1.10. [DOI] [PubMed] [Google Scholar]
- Goldman R. D., Follett E. A. Birefringent filamentous organelle in BHK-21 cells and its possible role in cell spreading and motility. Science. 1970 Jul 17;169(3942):286–288. doi: 10.1126/science.169.3942.286. [DOI] [PubMed] [Google Scholar]
- Goldman R., Goldman A., Green K., Jones J., Lieska N., Yang H. Y. Intermediate filaments: possible functions as cytoskeletal connecting links between the nucleus and the cell surface. Ann N Y Acad Sci. 1985;455:1–17. doi: 10.1111/j.1749-6632.1985.tb50400.x. [DOI] [PubMed] [Google Scholar]
- Jones J. C., Goldman A. E., Steinert P. M., Yuspa S., Goldman R. D. Dynamic aspects of the supramolecular organization of intermediate filament networks in cultured epidermal cells. Cell Motil. 1982;2(3):197–213. doi: 10.1002/cm.970020302. [DOI] [PubMed] [Google Scholar]
- Jones J. C., Goldman A. E., Yang H. Y., Goldman R. D. The organizational fate of intermediate filament networks in two epithelial cell types during mitosis. J Cell Biol. 1985 Jan;100(1):93–102. doi: 10.1083/jcb.100.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lazarides E. Intermediate filaments as mechanical integrators of cellular space. Nature. 1980 Jan 17;283(5744):249–256. doi: 10.1038/283249a0. [DOI] [PubMed] [Google Scholar]
- Lehto V. P., Virtanen I., Kurki P. Intermediate filaments anchor the nuclei in nuclear monolayers of cultured human fibroblasts. Nature. 1978 Mar 9;272(5649):175–177. doi: 10.1038/272175a0. [DOI] [PubMed] [Google Scholar]
- Lieska N., Yang H. Y., Goldman R. D. Purification of the 300K intermediate filament-associated protein and its in vitro recombination with intermediate filaments. J Cell Biol. 1985 Sep;101(3):802–813. doi: 10.1083/jcb.101.3.802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maul G. G., Baglia F. A., Newmeyer D. D., Ohlsson-Wilhelm B. M. The major 67 000 molecular weight protein of the clam oocyte nuclear envelope is lamin-like. J Cell Sci. 1984 Apr;67:69–85. doi: 10.1242/jcs.67.1.69. [DOI] [PubMed] [Google Scholar]
- McKeon F. D., Kirschner M. W., Caput D. Homologies in both primary and secondary structure between nuclear envelope and intermediate filament proteins. Nature. 1986 Feb 6;319(6053):463–468. doi: 10.1038/319463a0. [DOI] [PubMed] [Google Scholar]
- Osborn M., Weber K. Intermediate filaments: cell-type-specific markers in differentiation and pathology. Cell. 1982 Dec;31(2 Pt 1):303–306. doi: 10.1016/0092-8674(82)90122-2. [DOI] [PubMed] [Google Scholar]
- Starger J. M., Brown W. E., Goldman A. E., Goldman R. D. Biochemical and immunological analysis of rapidly purified 10-nm filaments from baby hamster kidney (BHK-21) cells. J Cell Biol. 1978 Jul;78(1):93–109. doi: 10.1083/jcb.78.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinert P. M., Cantieri J. S., Teller D. C., Lonsdale-Eccles J. D., Dale B. A. Characterization of a class of cationic proteins that specifically interact with intermediate filaments. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4097–4101. doi: 10.1073/pnas.78.7.4097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinert P. M., Idler W. W., Zimmerman S. B. Self-assembly of bovine epidermal keratin filaments in vitro. J Mol Biol. 1976 Dec 15;108(3):547–567. doi: 10.1016/s0022-2836(76)80136-2. [DOI] [PubMed] [Google Scholar]
- Steinert P. M., Jones J. C., Goldman R. D. Intermediate filaments. J Cell Biol. 1984 Jul;99(1 Pt 2):22s–27s. doi: 10.1083/jcb.99.1.22s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinert P. M., Parry D. A. Intermediate filaments: conformity and diversity of expression and structure. Annu Rev Cell Biol. 1985;1:41–65. doi: 10.1146/annurev.cb.01.110185.000353. [DOI] [PubMed] [Google Scholar]
- Steinert P. M., Steven A. C., Roop D. R. The molecular biology of intermediate filaments. Cell. 1985 Sep;42(2):411–420. doi: 10.1016/0092-8674(85)90098-4. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H. Y., Lieska N., Goldman A. E., Goldman R. D. A 300,000-mol-wt intermediate filament-associated protein in baby hamster kidney (BHK-21) cells. J Cell Biol. 1985 Feb;100(2):620–631. doi: 10.1083/jcb.100.2.620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zackroff R. V., Goldman A. E., Jones J. C., Steinert P. M., Goldman R. D. Isolation and characterization of keratin-like proteins from cultured cells with fibroblastic morphology. J Cell Biol. 1984 Apr;98(4):1231–1237. doi: 10.1083/jcb.98.4.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zackroff R. V., Goldman R. D. In vitro assembly of intermediate filaments from baby hamster kidney (BHK-21) cells. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6226–6230. doi: 10.1073/pnas.76.12.6226. [DOI] [PMC free article] [PubMed] [Google Scholar]