Abstract
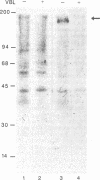
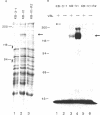
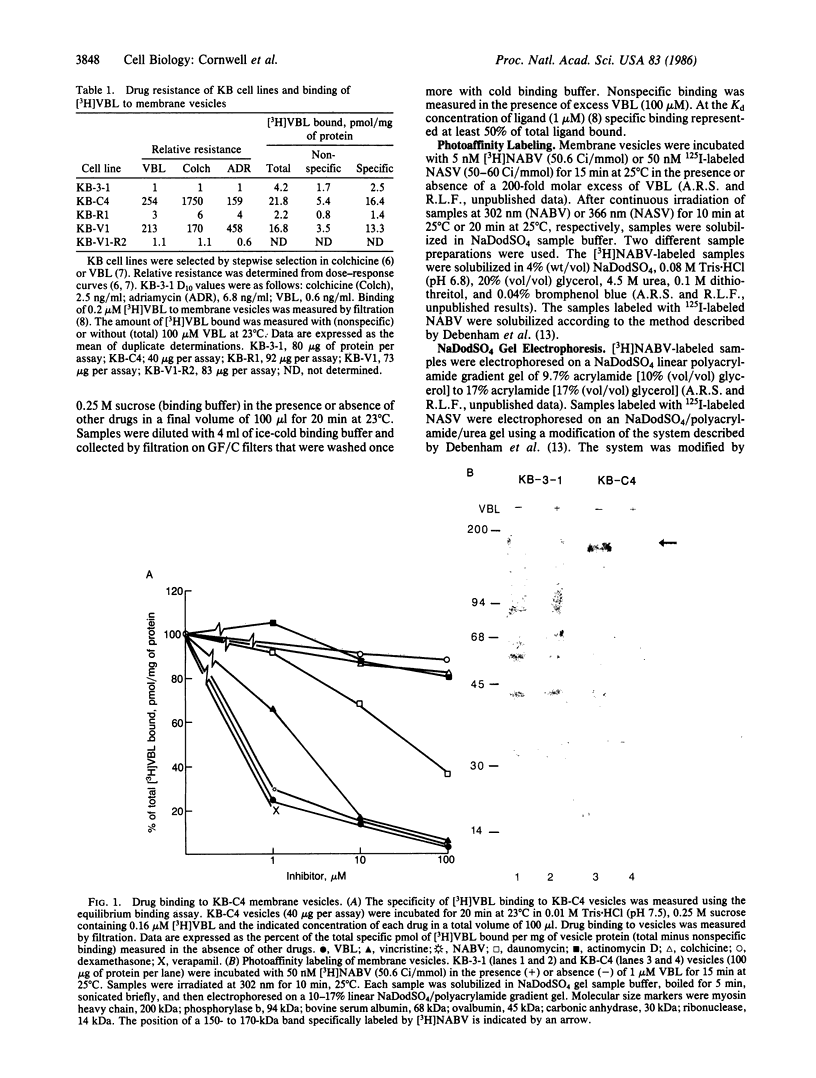
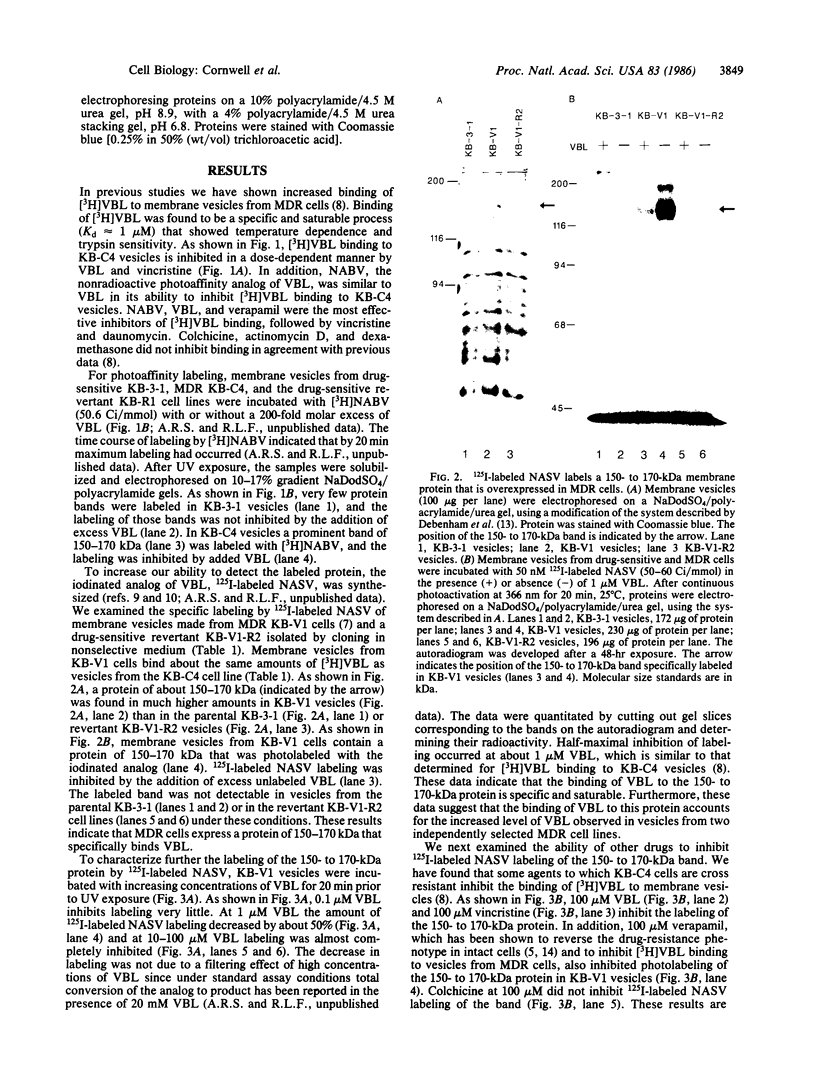
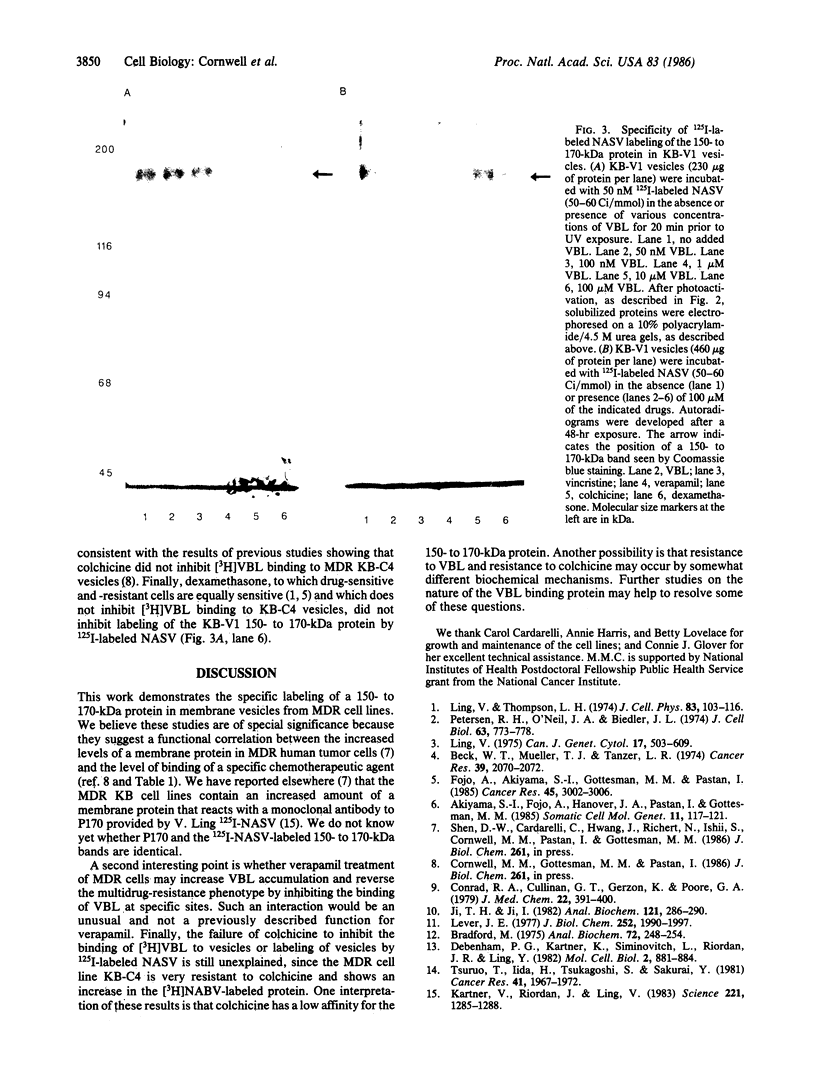
Multiple drug resistance of tumor cells is a common problem in cancer therapy. We have demonstrated that membrane vesicles from highly multidrug-resistant human KB carcinoma cell lines exhibit increased specific and saturable binding of vinblastine. To identify the molecules that bind vinblastine, membrane vesicles from multidrug-resistant cells were exposed to two analogs of vinblastine, N-(p-azido-[3,5-3H]benzoyl)-N'-(beta-aminoethyl)vindesine and N-(p-azido-[3-125I]salicyl)-N'-(beta-aminoethyl)vindesine, that could be photoactivated. Our studies show the specific labeling of a 150- to 170-kDa protein in membrane vesicles from two independently selected multidrug-resistant KB cell lines, which was not seen in drug-sensitive parental or revertant cell lines. The labeling of the high molecular weight protein was inhibited in a dose-dependent manner by vinblastine with half-maximal inhibition at about 1 microM. Photolabeling was also inhibited by 100 microM vincristine or 100 microM verapamil but not by 100 microM colchicine or 100 microM dexamethasone. The data suggest that the 150- to 170-kDa protein may play an important role in the multidrug-resistance phenotype.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akiyama S., Fojo A., Hanover J. A., Pastan I., Gottesman M. M. Isolation and genetic characterization of human KB cell lines resistant to multiple drugs. Somat Cell Mol Genet. 1985 Mar;11(2):117–126. doi: 10.1007/BF01534700. [DOI] [PubMed] [Google Scholar]
- Beck W. T., Mueller T. J., Tanzer L. R. Altered surface membrane glycoproteins in Vinca alkaloid-resistant human leukemic lymphoblasts. Cancer Res. 1979 Jun;39(6 Pt 1):2070–2076. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Conrad R. A., Cullinan G. J., Gerzon K., Poore G. A. Structure-activity relationships of dimeric Catharanthus alkaloids. 2. Experimental antitumor activities of N-substituted deacetylvinblastine amide (vindesine) sulfates. J Med Chem. 1979 Apr;22(4):391–400. doi: 10.1021/jm00190a008. [DOI] [PubMed] [Google Scholar]
- Debenham P. G., Kartner N., Siminovitch L., Riordan J. R., Ling V. DNA-mediated transfer of multiple drug resistance and plasma membrane glycoprotein expression. Mol Cell Biol. 1982 Aug;2(8):881–889. doi: 10.1128/mcb.2.8.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fojo A., Akiyama S., Gottesman M. M., Pastan I. Reduced drug accumulation in multiply drug-resistant human KB carcinoma cell lines. Cancer Res. 1985 Jul;45(7):3002–3007. [PubMed] [Google Scholar]
- Ji T. H., Ji I. Macromolecular photoaffinity labeling with radioactive photoactivable heterobifunctional reagents. Anal Biochem. 1982 Apr;121(2):286–289. doi: 10.1016/0003-2697(82)90481-x. [DOI] [PubMed] [Google Scholar]
- Kartner N., Riordan J. R., Ling V. Cell surface P-glycoprotein associated with multidrug resistance in mammalian cell lines. Science. 1983 Sep 23;221(4617):1285–1288. doi: 10.1126/science.6137059. [DOI] [PubMed] [Google Scholar]
- Lever J. E. Active amino acid transport in plasma membrane vesicles from Simian virus 40-transformed mouse fibroblasts. Characteristics of electrochemical Na+ gradient-stimulated uptake. J Biol Chem. 1977 Mar 25;252(6):1990–1997. [PubMed] [Google Scholar]
- Ling V. Drug resistance and membrane alteration in mutants of mammalian cells. Can J Genet Cytol. 1975 Dec;17(4):503–515. doi: 10.1139/g75-064. [DOI] [PubMed] [Google Scholar]
- Ling V., Thompson L. H. Reduced permeability in CHO cells as a mechanism of resistance to colchicine. J Cell Physiol. 1974 Feb;83(1):103–116. doi: 10.1002/jcp.1040830114. [DOI] [PubMed] [Google Scholar]
- Peterson R. H., O'Neil J. A., Biedler J. L. Some biochemical properties of Chinese hamster cells sensitive and resistant to actinomycin D. J Cell Biol. 1974 Dec;63(3):773–779. doi: 10.1083/jcb.63.3.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuruo T., Iida H., Tsukagoshi S., Sakurai Y. Overcoming of vincristine resistance in P388 leukemia in vivo and in vitro through enhanced cytotoxicity of vincristine and vinblastine by verapamil. Cancer Res. 1981 May;41(5):1967–1972. [PubMed] [Google Scholar]