Abstract
A dominantly inherited syndrome associated with hypopigmentation, heterochromia irides, colobomatous eyes and bilateral hearing loss has been ascertained in Fleckvieh cattle (German White Fleckvieh syndrome). This syndrome has been mapped to bovine chromosome (BTA) 22 using a genome-wide association study with the bovine high density single nucleotide polymorphism array. An R210I missense mutation has been identified within microphthalmia-associated transcription factor (MITF) as responsible for this syndrome. The mutation is located in the highly conserved basic region of the protein and causes a negative-dominant effect. SOX10 and PAX3 promoter binding site mutations in MITF could be ruled out as causative for the German White Fleckvieh syndrome. Molecular characterization of this newly detected bovine syndrome means a large animal model is now available for the Tietz syndrome in humans.
Introduction
A German Fleckvieh cattle family has been ascertained segregating for a white coat phenotype. Examination of these animals revealed a phenotype similar to incomplete albinism. Incomplete albinism in cattle is characterized by white coat color, pigmentless skin, heterochromia irides and white-yellowish hooves and horns [1], [2]. Incomplete albino cattle have previously been noted in Herefords and Hereford crossbreds. This condition has been considered as an autosomal dominant trait [3]. The German Fleckvieh investigated here distinguishes from Herefords and their crossbreds with incomplete albinism in that the German White Fleckvieh exhibited profound bilateral deafness. The German White Fleckvieh represents the first reported dominant white cattle with heterochromia irides and bilateral hearing loss. In dogs, white coat color and heterochromia irides are often associated with congenital sensorineural deafness [4]. Phenotypes similar to these dominant white cattle are known in humans as Waardenburg Syndrome Type 2A (WS2A) [5] and Tietz syndrome (TS) [6]–[8]. WS2A and TS are auditory-pigmentary syndromes caused by dominantly acting mutations in the MITF gene [9]. Patients with WS2A are experiencing sensorineural hearing loss of varying degrees and show heterochromia irides, early greying and a white forelock and in some cases vestibular dysfunction [5], [9]. Tietz syndrome differs in its phenotype from WS2A in some respects. Deafness is always congenital, profound and bilateral, depigmentation is generalized rather than patchy, but irides are not heterochromatic. Fundus hypopigmentation and vestibular dysfunction are similar in both syndromes, WS2A and TS [6]–[9]. The objectives of this study were to characterize the phenotypes of German White Fleckvieh and to identify the mutation responsible for this newly detected phenotype in cattle using genome-wide association analyses and re-sequencing of MITF, the most likely candidate gene.
Results
Phenotypic characterization of the animals
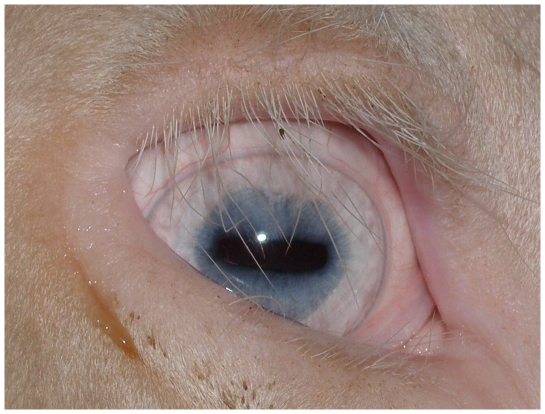
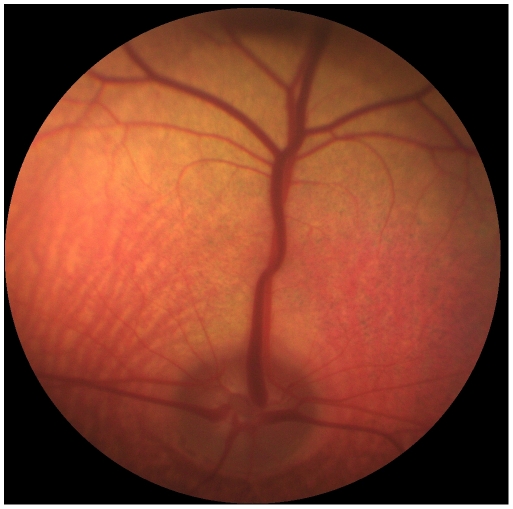
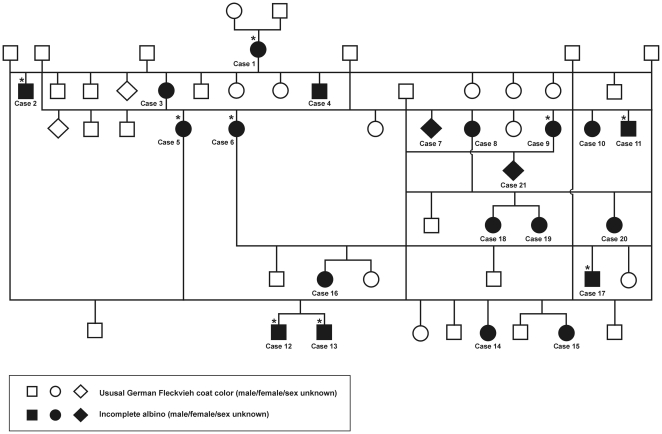
The German White Fleckvieh cattle family exhibited pure white coat color, pink skin without any darker spots or ghost patterns, yellow-white hooves, white horns, pigmentless muzzle, anus, eyelids, eye lashes, cilia, nictitating membranes and conjunctivae (Figure 1). The irides were pale blue in the central part and white towards the periphery (Figure 2). Pupils exhibited typical albinotic light reflection and the ocular fundus was completely or nearly completely albinotic using direct ophthalmoscopy (Figure 3). Optic disks were enlarged and irregular. The white animals were bilaterally deaf. We could not observe behavioural reactions or Preyer's reflex on different sounds. Sensorineural hearing loss could be confirmed using brainstem auditory evoked response (BAER) tests in two young bulls. The mode of inheritance appeared autosomal dominant with full penetrance and all white cattle could be traced to the same white founder dam (Figure 4).
Figure 1. German White Fleckvieh cow exhibiting a colour mutation similar to incomplete albinism with irises being pale blue in the central part and white towards the periphery as well as pigmentless muzzle, eyelashes and eyelids.
Figure 2. Heterochromia irides with the central iris appearing blue and white towards the periphery in the right eye of a German White Fleckvieh bull.
Cilia, eyelashes, eyelids, nictitating membrane and conjunctiva are white.
Figure 3. Ocular fundus of the left eye of a German White Fleckvieh bull showing a subalbinotic fundus with large areas of choriodal hypopigmentation.
Choriodal vessels and white sclera are visible due to the transparent choriodal layer.
Figure 4. Pedigree of the German White Fleckvieh family resembling an incomplete albino phenotype.
The founder animal for all white animals is Case 1. All animals marked with a star were examined on the farm and genotyped for the c.629G>T and g.32387485A>G mutations. Not all spotted progeny are depicted in the pedigree. With exception of Case 13 and Case 17, all white animals marked with a star were genotyped using the bovine high density Illumina beadchip.
Mapping the associated genomic region
A genome-wide association study (GWAS) using the bovine high density Illumina bead chip with 774,660 single nucleotide polymorphisms (SNPs) and TASSEL [10], [11] revealed the genome-wide most significantly (−log10Praw = 254.4; −log10PBonferroni-adjusted = 248.6) associated region on bovine chromosome (BTA) 22 at 33.422, 36.052 and 36.060 Mb (Figure S1). Haplotypes were estimated using these three SNPs and the procedure HAPLOTYPE of SAS/Genetics, version 9.2. The haplotypes of dominant white animals were consistent with inheritance of the mutation from the common founder dam and one joint haplotype for the alleles associated with the dominant white mutation could be confirmed (Table S1). The alleles of the associated haplotype were uncommon in German Fleckvieh and not present in the spotted animals related with German White Fleckvieh.
Identification of the associated mutation
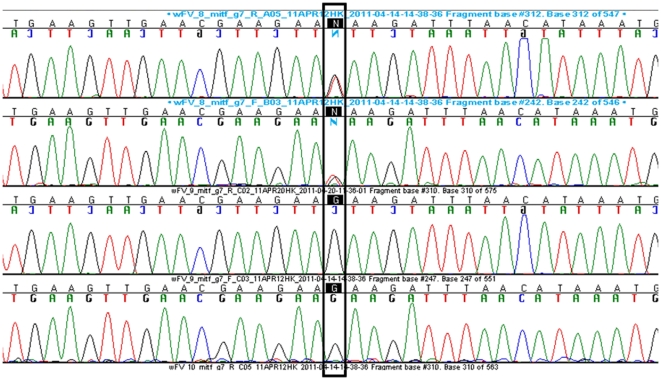
Among 13 genes contained in the associated interval on BTA22 of Btau 4.0/5.2 (Ensemble, http://www.ensembl.org/index.html), we considered the microphthalmia-associated transcription factor (MITF) gene at 32,353,746–32,387,234 bp as the most plausible candidate. MITF is essential for the development and post-natal survival of melanocytes. All MITF mutations reported in humans and mice affect melanocytes to a varying degree, retinal pigment epithelial cells and a few osteoclasts. The resulting phenotypic changes are characterized by pigmentary defects of skin, coat and eyes, deafness and in some instances osteopetrosis or hyperosteosis [9]. We could not see genome-wide significant signals in the GWAS at BTA2, 5, 6, 12, 15, and 29 where possible candidate genes other than MITF are located on the bovine genome assembly. Thus, possible candidates for white coat colour (KIT on BTA6, KITLG on BTA5, PMEL on BTA5, TYR on BTA29) and other Waardenburg syndromes (WS) including PAX3 (WS1, WS3; on BTA2), EDNRB (WS4a; on BTA12), EDN3 (WS4b; on BTA13) and SOX10 (WS2e, WS4c; on BTA5) could be clearly excluded. MITF is a transcription factor of the basic helix-loop-helix zipper (bHLH-Zip) family of proteins controlling differentiating and development of melanocytes, osteoclasts and mast cells [12]. We sequenced all exons of all in silico identified bovine MITF isoforms (Table S2, Figure S2). A missense mutation (c.629G>T, p.210R>I) was identified within exon 7 of the bovine MITF M-Form (NM_001001150) for which all German White Fleckvieh animals were heterozygous (Figure 5). Furthermore, this mutation was not detected in 383 non-completely white controls from ten different breeds (Table S3). In silico analysis using Polyphen predicted a probably damaging effect on the protein with a PSIC score difference of 3.065. The c.629G>T mutation is localized within the region encoding the basic region of the MITF protein involved in DNA binding. The basic domain is highly conserved between all species including vertebrates and invertebrates (Table 1) [9]. In order to evaluate possible effects on MITF expression through mutations in the binding sites of SOX10 and PAX3, we analyzed the MITF M-form promoter region for all previously reported binding sites of SOX10 and PAX3 [9], [13]. Both genes, SOX10 and PAX3 act directly and synergistically to activate the MITF promoter. Mutations in the binding sites of the MITF promoter can abolish transcriptional activation of MITF [13], [14]. We detected eight putative SOX10 and two putative PAX3 binding sites after screening approximately 1 kb of the bovine reference sequence Btau 5.2 upstream of the 5′end of MITF transcription start site. Therefore, we sequenced a 581 bp amplicon containing the putative eight SOX10 and the two putative PAX3 binding sites. Mutation screening revealed a g.32387485A>G SNP at position −212 of the putative SOX10 binding site 7/8. However, this binding site showed only minor effects on transcriptional activity in co-transfection assays with HeLa cells [13]. The mutant G allele appeared generally common in Fleckvieh and even more frequently distributed among German White Fleckvieh. Eight of the nine German White Fleckvieh cattle and two of the spotted German Fleckvieh cattle were homozygous for the G allele (Table 2). At the putative PAX3 binding sites, we could not detect any mutations.
Figure 5. Sequences of exon 7 of the MITF M-form of a dominant German White Fleckvieh and a spotted German Fleckvieh animal.
Base pairs in boxes demonstrate the c.629G>T mutation causing the Tietz-like-syndrome in German White Fleckvieh. The first row shows the forward and the second row the reverse sequence of an affected animal with the heterozygous G/T genotype, the third and fourth row contain the forward and the reverse sequence of a spotted German Fleckvieh animal with the homozygous G/G wildtype.
Table 1. Protein sequence alignment of the MITF basic helix-loop-helix (bHLH) domain in vertebrate species.
| Species/Breed | Protein identifier | Protein sequence |
| German White Fleckvieh | SEARALAKERQKKDNHNLIERRIRFNINDRIKELGTLIPKSNDPDMRWNKGTILKASV | |
| Cow | ENSBTAP00000053693 | SEARALAKERQKKDNHNLIERRRFNINDRIKELGTLIPKSNDPDMRWNKGTILKASV |
| Human | ENSP00000295600 | ********************** R ********************************** |
| Mouse | ENSMUSP00000044938 | ********************** R ********************************** |
| Rat | ENSRNOP00000044350 | ********************** R ********************************** |
| Dog | ENSCAFP00000009730 | ********************** R ********************************** |
| Horse | ENSECAP00000004487 | ********************** R ********************************** |
| Dolphin | ENSTTRP00000006690 | ********************** R ********************************** |
| Elefant | ENSLAFP00000017388 | ********************** R ********************************** |
| Kangaroo rat | ENSDORP00000003119 | ********************** R ********************************** |
| Chicken | ENSGALP00000012434 | ********************** R ********************************** |
| Frog | ENSXETP00000000313 | ********************** R ********************************** |
| Lion's cove | BAD69632 | A*V******************* R ********************************** |
Table 2. Genotype and allele frequencies in German White Fleckvieh and German Fleckvieh genotyped for the g.32387485A>G MITF promoter mutation.
| Breed | n | Coat color | Number of animals | Allele frequency | ||
| G/G | G/A | A/A | G | |||
| German White Fleckvieh | ′ | white | 8 | 1 | - | 0.947 |
| German Fleckvieh related with German White Fleckvieh | 6 | spotted | 2 | 3 | 1 | 0.583 |
Discussion
In German White Fleckvieh, a perfect cosegregation was demonstrated for a missense mutation affecting the DNA binding domain of MITF. Function of protein domains was shown in detail for transcription factor TFEB [15] which shares critically conserved residues with MITF and other bHLH-Zip proteins [16], [17]. Arginine210 seems to be necessary to contact the phosphate backbone of DNA and to build a hydrogen bond to an N-terminal glutamine. In German White Fleckvieh, the p.210R>I substitution probably disrupts both functions as the strong basic amino acid arginine is replaced by the non-polare isoleucine. In humans, the Tietz syndrome is characterized by a missense mutation (N210K) [18] or a 3-bp in-frame deletion (DelR217) [7], [8]. In the semi-dominant oak ridge mouse mutant (Mitfmi-or), the corresponding arginine is replaced by lysine (R216K) which affects the DNA binding domain [19]. In mice, further semi-dominant mutations affecting the DNA binding domain of MITF were seen in the Mitfmi, Mitfmi-wh, Mitfmi-b, Mitfmi-H, Mitfmi-enu5, Mitfmi-bcc2 and Mitfmi-ew mutations [9]. The Mitfmi mutation in the mouse is identical to the human DelR217 mutation. It is worth to mention that the bovine R210I and the human DelR217 and N210K mutations lead to more similar phenotypes than the murine mutations in this critical region. In humans and cattle, the mutations cause a dominant-negative effect with no variable expressivity. Humans and cattle, both are born white and sensorineural hearing loss was always bilateral [6]–[9], [18]. In contrast to humans, cattle show heterochromia irides and this could be caused by the substitution of the non-polare isoleucine amino acid. In mice however, the trait is transmitted in a semi-dominant or recessive fashion and phenotypes differ from human WS2A and TS [9] as well as the German White Fleckvieh reported here. In heterozygous mice, white spotting and/or color dilution is observed and in some cases reduced iris pigment. In the homozygous condition, microphthalmia or even absent eyes, osteopetrosis and in some inner ear defects and reduced fertility have been noted [9]. In humans with WS2A or TS and in addition, in German White Fleckvieh, neither eye defects nor osteopetrosis have been found.
The homozygous genotype of the mutation of the putative SOX10 binding site 7/8 was not specific for the German White Fleckvieh and thus, an effect on the white phenotype can be ruled out. However, this MITF promoter mutation could be associated with the distribution of the white coat color in spotted German Fleckvieh because the bovine reference sequence was from an animal of the Hereford breed.
In conclusion, we have identified the mutation responsible for a Tietz-like-syndrome in cattle. This is the first time that an unambiguous mutation in the MITF gene is identified in non-human and non-rodent mammals. The German White Fleckvieh is a useful large animal model to study human Tietz syndrome and the MITF transcription factor network. This study highlights the strength of trait mapping using high density SNP arrays in domestic animals, particularly with regard to one or a few founder animals for the target trait.
Materials and Methods
Ethics statement
All animal work has been conducted according to the national and international guidelines for animal welfare. The study has been approved by the Lower Saxony state veterinary office Niedersächsisches Landesamt für Verbraucherschutz und Lebensmittelsicherheit, Oldenburg, Germany, and registered under the approval ID 33.42502-05-04A247.
Animals
The genome-wide association study (GWAS) included genomic DNA samples of seven white and 79 spotted German Fleckvieh whereof five samples of the spotted German Fleckvieh were from the same herd as the German White Fleckvieh. From the seven white animals, three animals were males and four females. In controls, females and males were at the same proportions. DNA samples of nine German White Fleckvieh, six German Fleckvieh related with German White Fleckvieh, 89 German Fleckvieh unrelated with German White Fleckvieh and 288 animals from nine other breeds and German Fleckvieh x Red Holstein crossbred animals were used to test the MITF c.629G>T mutation. For cDNA analysis, hair root samples of two white males and one spotted female were used.
On the farm where the German White Fleckvieh had been detected, only the female white animals were used for breeding, whereas all bull calves were used for fattening without siring offspring. Therefore, all white animals had a white mother and a normally spotted German Fleckvieh sire (Figure 4). In total, six female colour variants gave birth to 20 white animals and 24 spotted offspring. Matings among spotted descendants with German Fleckvieh sires did not result in white offspring. The ratio of white to spotted offspring from the six white dams used for breeding was close to the 1∶1 ratio expected for a monogenic autosomal dominant mode of inheritance. All other modes of inheritance are unlikely. The colour variant of the founder cow appears to be caused by a spontaneous mutation as both parents were normally spotted and other white offspring from the parents of the founder dam had not been reported.
Genotyping and genome-wide association analysis
DNA from seven dominant white German Fleckvieh (cases) and 79 spotted German Fleckvieh cattle (controls) randomly sampled from the whole German Fleckvieh cattle population were genotyped using the bovine Illumina high density beadchip with 774,660 single nucleotide polymorphisms (SNPs). Quality control for genotyping was performed using two duplicates. Consistency of genotyping was >0.999. After filtering for a minor allele frequency >0.05 and genotyping rate >0.99, 585,047 SNPs were left for a genome-wide association study (GWAS) among cases and controls. GWAS was performed using Tassel, version 3.0 (URL:http://www.maizegenetics.net/index.php?option=com_content&task=view&id=89&Itemid=119). A general model was employed to control for the data structure in GWAS. The fixed effects in the model included sex and the first eight principal components. Principal components were estimated using TASSEL and a pruned set of SNPs. A sliding window size of 50 SNPs, window shift steps of five SNPs and a pairwise correlation (r2)<0.2 were used for generating this set of 56,535 SNPs. In order to test the consistency of the model, the first four, first six or first ten principal components were employed in additional models. The association results were consistent among the different models and therefore, data from these models are not shown. Genome-wide significance was ascertained through a Bonferroni correction for the number of SNPs tested. Association of haplotypes with the dominant white phenotype was tested using the procedure HAPLOTYPE of SAS/Genetics (Statistical Analysis System, version 9.2, Cary, NC, USA, 2011, http://support.sas.com/documentation /cdl_main/index.html).
DNA sequence analysis
Genomic DNA was isolated from frozen EDTA stabilized blood samples using an in-house desalting method. For identifying DNA polymorphisms within the bovine MITF gene (Gene ID: 407219), the putative exons of the MITF gene were determined by comparative alignment of the bovine genomic sequence ((NC_007320.4, 157513 bp–618513 bp) versus the known bovine mRNA isoform M (NM_001001150) and the eight human MITF mRNA isoform sequences (NM_000248.3, NM_001184967.1, NM_001184968.1, NM_006722.2, NM_198158.1, NM_00198159.1, NM_00198177.1 and NM_00198178.1) using Spidey (http://www.ncbi.nlm.nih.gov/blast/). For sequence analysis, primer pairs for all identified exons and adjacent intronic regions were designed using PRIMER3 software (http://frodo.wi.mit.edu/cgi-bin/primer3/primer3_www.cgi).
For mutation analysis of the MITF M-form promoter the previously reported murine SOX10 and PAX3 binding sites were identified by searching their core sequences [9] in the bovine reference sequence Btau 5.2 upstream of the 5′end of the putative MITF transcription site. Primer pairs were designed for a 581 bp amplicon that contained all previously reported binding sites for SOX10 (sites S1–S8) and PAX3 (sites P1–P2) [9].
PCR was carried out in 30 µl containing 10 ng genomic DNA according to the standard protocol advised by the manufacturer of the Taq DNA polymerase (Qbiogene Heidelberg, Germany). The subsequent sequencing of the PCR products was performed using the ABI BigDye Terminator v3.1 sequencing kit (Life Technologies, Darmstadt, Germany). The products were analyzed on an automated ABI 3500 capillary sequencer (Life Technologies).
cDNA sequence analysis
RNA was extracted from hair root cells using the RNeasy Universal Tissue kit (Qiagen, Hilden, Germany). The following cDNA synthesis was carried out in a 20 µl reaction volumes using Maxima First strand cDNA synthesis kit (Fermentas, St. Leon-Rot, Germany). The open reading frame of MITF was generated with two amplicons. In addition, specific primer pairs were designed to amplify the predicted different MITF isoforms (Table S2). The RT amplicons were directly sequenced using the PCR primer pairs for sequencing. Sequencing was performed using the BigDye Terminator v3.1 sequencing kit (Life Technologies). The products were analyzed on an ABI 3500 capillary sequencer (Life Technologies).
SNP detection using the ABI SNP detection system
Primer pairs and probes for the MITF c.629G>T SNP were designed using the Custom Taqman SNP genotyping assay service (Table S2). MGB probes were dye-labelled with FAM and VIC, respectively. Genotyping was performed in 12.0 µl reaction volumes containing 6.0 µl SensiMix DNA Kit (Quantace, London, UK), 0.3125 µl 40×Assay (Applied Biosystems, Darmstadt, Germany) and 1.5 µl genomic DNA on an ABI 7300 sequence detection system (Life Technologies). Reaction conditions for a two step PCR were set using ABI instructions. Test samples were run in single reactions, controls for homozygous T/T and G/T genotypes were run in duplex on every analyzed plate. DNA samples of dominant white cattle were used as heterozygous controls while the homozygous T/T genotypes were generated by cloning a heterozygous Mitf_Ex7_M-form amplicon using the Qiagen PCR cloning kit (Qiagen). Clones containing the strand with the mutated base T were confirmed by sequencing.
All new sequence data and polymorphisms from this study have been submitted to the EMBL/GenBank Data Libraries.
Supporting Information
Manhattan plot of the −log10 P-values for the genome-wide association analysis of the dominant white phenotype in German Fleckvieh from a general model analysis using TASSEL, version 3.0. The highest −log10 p-values (254) were obtained for bovine chromosome 22 at 33 and 36 Mb.
(DOC)
In silico identified bovine MITF isoforms using bovine genomic MITF gene sequences (Gene ID: 407219), bovine mRNA sequences of the MITF isoform M (NM_001001150) and all eight human MITF mRNA isoform sequences (NM_000248.3, NM_001184967.1, NM_001184968.1, NM_006722.2, NM_198158.1, NM_00198159.1, NM_00198177.1 and NM_00198178.1).
(DOC)
Haplotype frequencies and their standard errors (SE) for all animals, German White Fleckvieh (GWF) and controls and haplotype-trait association with χ2- and P-values. The founder dam haplotye conforms to G-A-A.
(DOC)
Primer pairs used for sequencing the bovine MITF gene and mutation detection, amplicon size and annealing temperature (AT) for the amplicons.
(DOC)
Number of animals (n) per breed, their coat color and genotype for the c.629G>T MITF mutation.
(DOC)
Acknowledgments
The authors thank the breeders for sending samples and Heike Klippert-Hasberg and Stefan Neander for their expert technical assistance.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: The authors have no support or funding to report.
References
- 1.Leipold HW, Huston K. Incomplete albinism and heterochromia irides in Herefords. J Hered. 1968;59:3–8. doi: 10.1093/oxfordjournals.jhered.a107635. [DOI] [PubMed] [Google Scholar]
- 2.Leipold HW, Huston K. Histopathology of incomplete albinism and heterochromia irides in the Herefords. Cornell vet. 1969;59:69–75. [PubMed] [Google Scholar]
- 3.Leipold HW, Huston K. Dominant incomplete albinism of cattle. J Hered. 1968;59:223–224. doi: 10.1093/oxfordjournals.jhered.a107698. [DOI] [PubMed] [Google Scholar]
- 4.Rak SG, Distl O. Congenital sensorineural deafness in dogs: a molecular genetic approach toward unravelling the responsible genes. Vet J. 2005;169:188–196. doi: 10.1016/j.tvjl.2004.01.015. [DOI] [PubMed] [Google Scholar]
- 5.Tassabehji M, Newton VE, Read AP. Waardenburg syndrome type 2 caused by mutations in the human microphthalmia (MITF) gene. Nat Genet. 1994;8:251–255. doi: 10.1038/ng1194-251. [DOI] [PubMed] [Google Scholar]
- 6.Tietz W. A syndrome of deaf-mutism associated with albinism showing dominant autosomal inheritance. Am J Hum Genet. 1963;15:259–264. [PMC free article] [PubMed] [Google Scholar]
- 7.Amiel J, Watkon PM, Tassabehji M, Read AP, Winter RM. Mutation of the MITF gene in albinism-deafness syndrome (Tietz syndrome). Clin Dysmorphol. 1998;7:17–20. [PubMed] [Google Scholar]
- 8.Smith SD, Kelley PM, Kenyon JB, Hoover DJ. Tietz syndrome (hypopigmentation/deafness) caused by mutation of MITF. Med Genet. 2000;37:446–448. doi: 10.1136/jmg.37.6.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Steingrímsson E, Copeland NG, Jenkins NA. Melanocytes and the microphthalmia transcription factor network. Annu Rev Genet. 2004;38:365–411. doi: 10.1146/annurev.genet.38.072902.092717. [DOI] [PubMed] [Google Scholar]
- 10.Bradbury PJ, Zhang Z, Kroon DE, Casstevens TM, Ramdoss Y, et al. TASSEL: software for association mapping of complex traits in diverse samples. Bioinformatics. 2007;19:2633–2635. doi: 10.1093/bioinformatics/btm308. [DOI] [PubMed] [Google Scholar]
- 11.Zhang Z, Ersoz E, Lai CQ, Todhunter RJ, Tiwari HK, et al. Mixed linear model approach adapted for genome-wide association studies. Nat Genet. 2010;42:355–360. doi: 10.1038/ng.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moore KJ. Insight into the microphthalmia gene. Trends Genet. 1995;11:442–448. doi: 10.1016/s0168-9525(00)89143-x. [DOI] [PubMed] [Google Scholar]
- 13.Bondurand N, Pingault V, Goerich DE, Lemort N, Sock E, et al. Interaction among SOX10, PAX3 and MITF, three genes altered in Waardenburg syndrome. Hum Mol Genet. 2000;9:1907–1917. doi: 10.1093/hmg/9.13.1907. [DOI] [PubMed] [Google Scholar]
- 14.Potterf SB, Furumura M, Dunn KJ, Arnheiter H, Pavan WJ. Transcription factor hierarchy in Waardenburg syndrome: regulation of MITF expression by SOX10 and PAX3. Hum Genet. 2000;107:1–6. doi: 10.1007/s004390000328. [DOI] [PubMed] [Google Scholar]
- 15.Fisher DE, Parent LA, Sharp PA. High affinity DNA-binding Myc analogs: recognition by an alpha helix. Cell. 1993;72:467–476. doi: 10.1016/0092-8674(93)90122-7. [DOI] [PubMed] [Google Scholar]
- 16.Ferré-D'Amaré AR, Prendergast GC, Ziff EB, Burley SK. Recognition by Max of its cognate DNA through a dimeric b/HLH/Z domain. Nature. 1993;363:38–45. doi: 10.1038/363038a0. [DOI] [PubMed] [Google Scholar]
- 17.Lüscher B, Larsson LG. The basic region/helix-loop-helix/leucine zipper domain of Myc proto-oncoproteins: function and regulation. Oncogene. 1999;18:2955–2966. doi: 10.1038/sj.onc.1202750. [DOI] [PubMed] [Google Scholar]
- 18.Izumi K, Kohta T, Kimura Y, Ishida S, Takahashi T, et al. Tietz syndrome: unique phenotype specific to mutations of MITF nuclear localization signal. Clin Genet. 2008;74:93–95. doi: 10.1111/j.1399-0004.2008.01010.x. [DOI] [PubMed] [Google Scholar]
- 19.Steingrímsson E, Moore KJ, Lamoreux ML, Ferré-D'Amaré AR, Burley SK, et al. Molecular basis of mouse microphthalmia (mi) mutations helps explain their developmental and phenotypic consequences. Nat Genet. 1994;8:256–263. doi: 10.1038/ng1194-256. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Manhattan plot of the −log10 P-values for the genome-wide association analysis of the dominant white phenotype in German Fleckvieh from a general model analysis using TASSEL, version 3.0. The highest −log10 p-values (254) were obtained for bovine chromosome 22 at 33 and 36 Mb.
(DOC)
In silico identified bovine MITF isoforms using bovine genomic MITF gene sequences (Gene ID: 407219), bovine mRNA sequences of the MITF isoform M (NM_001001150) and all eight human MITF mRNA isoform sequences (NM_000248.3, NM_001184967.1, NM_001184968.1, NM_006722.2, NM_198158.1, NM_00198159.1, NM_00198177.1 and NM_00198178.1).
(DOC)
Haplotype frequencies and their standard errors (SE) for all animals, German White Fleckvieh (GWF) and controls and haplotype-trait association with χ2- and P-values. The founder dam haplotye conforms to G-A-A.
(DOC)
Primer pairs used for sequencing the bovine MITF gene and mutation detection, amplicon size and annealing temperature (AT) for the amplicons.
(DOC)
Number of animals (n) per breed, their coat color and genotype for the c.629G>T MITF mutation.
(DOC)