Abstract
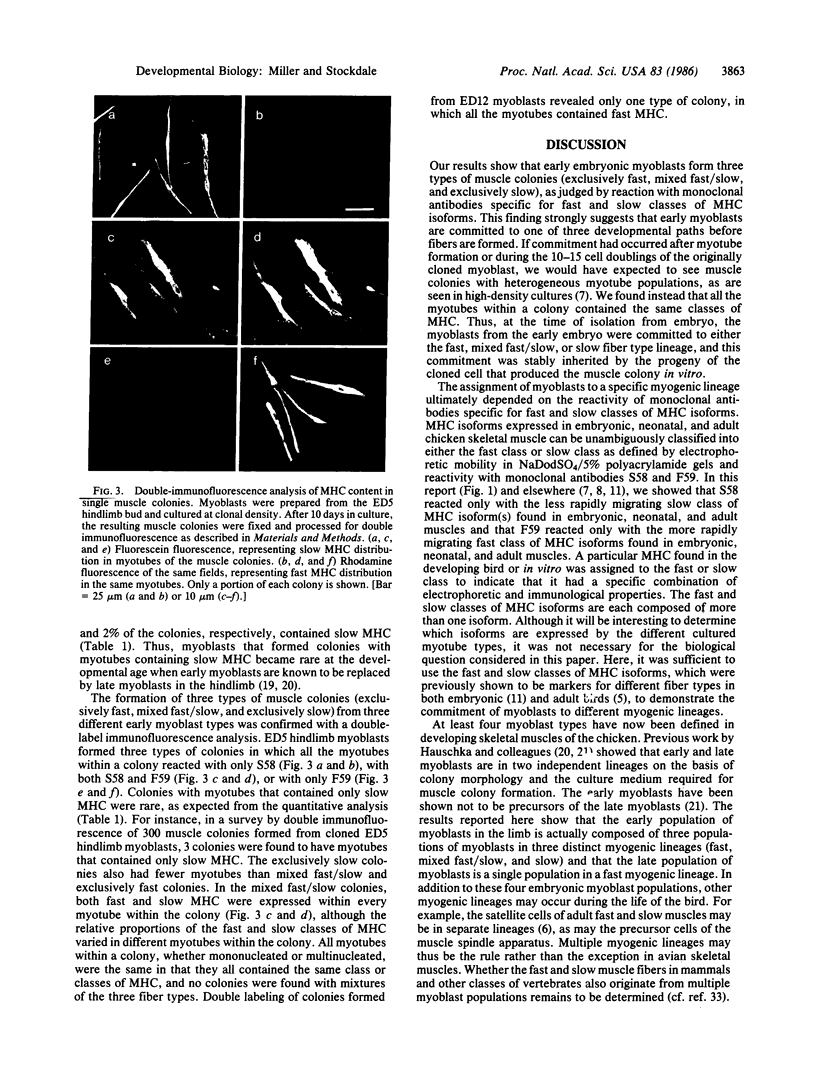
A clonal analysis was used to show that skeletal muscle myoblasts are committed to distinct cell lineages during development. Myoblasts taken from embryonic chicken hindlimb muscles of different ages were cultured at clonal density. The content of fast and slow classes of the myosin heavy chain isoforms in the myotubes of the resulting muscle colonies was determined immunocytochemically with specific monoclonal antibodies that served as markers for the different fiber types. The muscle colonies formed by cloning myoblasts from early hindlimbs (days 4-6 in ovo) were of three types: the most numerous type, in which all myotubes in a colony contained only the fast class of myosin heavy chain; a less numerous type, in which all myotubes in a colony contained both the fast and slow classes of myosin heavy chain isoforms; and a rare type, in which all myotubes in a colony contained only the slow class of myosin heavy chain. The muscle colonies formed by cloning myoblasts from later hindlimbs (days 10-12 in ovo) were, however, all of one type, in which every myotube in a colony contained only fast myosin heavy chain. Thus, myoblasts in the early embryo (days 4-6 in ovo) were a heterogeneous population committed to three myogenic lineages: fast, mixed fast/slow, and slow, whereas myoblasts from the later embryo (days 10-12 in ovo) were only in the fast myogenic lineage. These results suggest that muscle fiber formation is rooted in two developmental phases--an early phase in which diverse fiber types are formed from intrinsically diverse populations of myoblasts and a later phase in which fibers are formed from a single population of myoblasts.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arndt I., Pepe F. A. Antigenic specificity of red and white muscle myosin. J Histochem Cytochem. 1975 Mar;23(3):159–168. doi: 10.1177/23.3.47867. [DOI] [PubMed] [Google Scholar]
- Atsumi S. Development of neuromuscular junctions of fast and slow muscles in the chick embryo: a light and electron microscopic study. J Neurocytol. 1977 Dec;6(6):691–709. doi: 10.1007/BF01176380. [DOI] [PubMed] [Google Scholar]
- Bader D., Masaki T., Fischman D. A. Immunochemical analysis of myosin heavy chain during avian myogenesis in vivo and in vitro. J Cell Biol. 1982 Dec;95(3):763–770. doi: 10.1083/jcb.95.3.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandman E., Matsuda R., Strohman R. C. Developmental appearance of myosin heavy and light chain isoforms in vivo and in vitro in chicken skeletal muscle. Dev Biol. 1982 Oct;93(2):508–518. doi: 10.1016/0012-1606(82)90138-5. [DOI] [PubMed] [Google Scholar]
- Benfield P. A., Lowey S., LeBlanc D. D., Waller G. S. Myosin isozymes in avian skeletal muscles. II. Fractionation of myosin isozymes from adult and embryonic chicken pectoralis muscle by immuno-affinity chromatography. J Muscle Res Cell Motil. 1983 Dec;4(6):717–738. doi: 10.1007/BF00712162. [DOI] [PubMed] [Google Scholar]
- Bennett M. R., Davey D. F., Uebel K. E. The growth of segmental nerves from the brachial myotomes into the proximal muscles of the chick forelimb during development. J Comp Neurol. 1980 Jan 15;189(2):335–357. doi: 10.1002/cne.901890209. [DOI] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Butler J., Cosmos E., Brierley J. Differentiation of muscle fiber types in aneurogenic brachial muscles of the chick embryo. J Exp Zool. 1982 Nov 20;224(1):65–80. doi: 10.1002/jez.1402240108. [DOI] [PubMed] [Google Scholar]
- Crow M. T., Olson P. S., Stockdale F. E. Myosin light-chain expression during avian muscle development. J Cell Biol. 1983 Mar;96(3):736–744. doi: 10.1083/jcb.96.3.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crow M. T., Stockdale F. E. Myosin expression and specialization among the earliest muscle fibers of the developing avian limb. Dev Biol. 1986 Jan;113(1):238–254. doi: 10.1016/0012-1606(86)90126-0. [DOI] [PubMed] [Google Scholar]
- Gauthier G. F., Lowey S. Distribution of myosin isoenzymes among skeletal muscle fiber types. J Cell Biol. 1979 Apr;81(1):10–25. doi: 10.1083/jcb.81.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolesz F., Sreter F. A. Development, innervation, and activity-pattern induced changes in skeletal muscle. Annu Rev Physiol. 1981;43:531–552. doi: 10.1146/annurev.ph.43.030181.002531. [DOI] [PubMed] [Google Scholar]
- KONIGSBERG I. R. Clonal analysis of myogenesis. Science. 1963 Jun 21;140(3573):1273–1284. doi: 10.1126/science.140.3573.1273. [DOI] [PubMed] [Google Scholar]
- Kelly A. M., Zacks S. I. The histogenesis of rat intercostal muscle. J Cell Biol. 1969 Jul;42(1):135–153. doi: 10.1083/jcb.42.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laing N. G., Lamb A. H. The distribution of muscle fibre types in chick embryo wings transplanted to the pelvic region is normal. J Embryol Exp Morphol. 1983 Dec;78:67–82. [PubMed] [Google Scholar]
- Lowey S., Risby D. Light chains from fast and slow muscle myosins. Nature. 1971 Nov 12;234(5324):81–85. doi: 10.1038/234081a0. [DOI] [PubMed] [Google Scholar]
- Matsuda R., Spector D. H., Strohman R. C. Regenerating adult chicken skeletal muscle and satellite cell cultures express embryonic patterns of myosin and tropomyosin isoforms. Dev Biol. 1983 Dec;100(2):478–488. doi: 10.1016/0012-1606(83)90240-3. [DOI] [PubMed] [Google Scholar]
- McLennan I. S. Differentiation of muscle fiber types in the chicken hindlimb. Dev Biol. 1983 May;97(1):222–228. doi: 10.1016/0012-1606(83)90079-9. [DOI] [PubMed] [Google Scholar]
- McLennan I. S. Neural dependence and independence of myotube production in chicken hindlimb muscles. Dev Biol. 1983 Aug;98(2):287–294. doi: 10.1016/0012-1606(83)90359-7. [DOI] [PubMed] [Google Scholar]
- McLennan I. S. The development of the pattern of innervation in chicken hindlimb muscles: evidence for specification of nerve-muscle connections. Dev Biol. 1983 May;97(1):229–238. doi: 10.1016/0012-1606(83)90080-5. [DOI] [PubMed] [Google Scholar]
- Miller J. B., Crow M. T., Stockdale F. E. Slow and fast myosin heavy chain content defines three types of myotubes in early muscle cell cultures. J Cell Biol. 1985 Nov;101(5 Pt 1):1643–1650. doi: 10.1083/jcb.101.5.1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill M. C., Stockdale F. E. A kinetic analysis of myogenesis in vitro. J Cell Biol. 1972 Jan;52(1):52–65. doi: 10.1083/jcb.52.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips W. D., Bennett M. R. Differentiation of fiber types in wing muscles during embryonic development: effect of neural tube removal. Dev Biol. 1984 Dec;106(2):457–468. doi: 10.1016/0012-1606(84)90245-8. [DOI] [PubMed] [Google Scholar]
- Rubinstein N. A., Kelly A. M. Development of muscle fiber specialization in the rat hindlimb. J Cell Biol. 1981 Jul;90(1):128–144. doi: 10.1083/jcb.90.1.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushbrook J. I., Stracher A. Comparison of adult, embryonic, and dystrophic myosin heavy chains from chicken muscle by sodium dodecyl sulfate/polyacrylamide gel electrophoresis and peptide mapping. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4331–4334. doi: 10.1073/pnas.76.9.4331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutz R., Haney C., Hauschka S. Spatial analysis of limb bud myogenesis: a proximodistal gradient of muscle colony-forming cells in chick embryo leg buds. Dev Biol. 1982 Apr;90(2):399–411. doi: 10.1016/0012-1606(82)90389-x. [DOI] [PubMed] [Google Scholar]
- Rutz R., Hauschka S. Clonal analysis of vertebrate myogenesis. VII. Heritability of muscle colony type through sequential subclonal passages in vitro. Dev Biol. 1982 May;91(1):103–110. doi: 10.1016/0012-1606(82)90013-6. [DOI] [PubMed] [Google Scholar]
- Seed J., Hauschka S. D. Temporal separation of the migration of distinct myogenic precursor populations into the developing chick wing bud. Dev Biol. 1984 Dec;106(2):389–393. doi: 10.1016/0012-1606(84)90237-9. [DOI] [PubMed] [Google Scholar]
- Sréter F. A., Bálint M., Gergely J. Structural and functional changes of myosin during development: comparison with adult fast, slow and cardiac myosin. Dev Biol. 1975 Oct;46(2):317–325. doi: 10.1016/0012-1606(75)90108-6. [DOI] [PubMed] [Google Scholar]
- Umeda P. K., Sinha A. M., Jakovcic S., Merten S., Hsu H. J., Subramanian K. N., Zak R., Rabinowitz M. Molecular cloning of two fast myosin heavy chain cDNAs from chicken embryo skeletal muscle. Proc Natl Acad Sci U S A. 1981 May;78(5):2843–2847. doi: 10.1073/pnas.78.5.2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White N. K., Bonner P. H., Nelson D. R., Hauschka S. D. Clonal analysis of vertebrate myogenesis. IV. Medium-dependent classification of colony-forming cells. Dev Biol. 1975 Jun;44(2):346–361. doi: 10.1016/0012-1606(75)90405-4. [DOI] [PubMed] [Google Scholar]
- White N. K., Hauschka S. D. Muscle development in vitro. A new conditioned medium effect on colony differentiation. Exp Cell Res. 1971 Aug;67(2):479–482. doi: 10.1016/0014-4827(71)90437-x. [DOI] [PubMed] [Google Scholar]
- YAFFE D., FELDMAN M. THE FORMATION OF HYBRID MULTINUCLEATED MUSCLE FIBERS FROM MYOBLASTS OF DIFFERENT GENETIC ORIGIN. Dev Biol. 1965 Apr;11:300–317. doi: 10.1016/0012-1606(65)90062-x. [DOI] [PubMed] [Google Scholar]