Abstract
Episodic ataxia type 2 (EA2) is an autosomal dominant disorder arising from CACNA1A mutations, which commonly predict heterozygous expression of Cav2.1 calcium channels with truncated α12.1 pore subunits. We hypothesized that α12.1 truncations in EA2 exert dominant-negative effects on the function of wild-type subunits. Wild-type and truncated α12.1 subunits with fluorescent-protein tags were transiently co-expressed in cells stably expressing Cav auxiliary β subunits, which facilitate α1-subunit functional expression through high-affinity interactions with the alpha interaction domain (AID). Co-expression of wild-type subunits with truncations often resulted in severely reduced whole-cell currents compared to expression of wild-type subunits alone. Cellular image analyses revealed that current suppression was not due to reduced wild-type expression levels. Instead, the current suppression depended on truncations terminating distal to the AID. Moreover, only AID-bearing α12.1 proteins co-immunoprecipitated with Cav β subunits. These results indicate that Cav β subunits may play a prominent role in EA2 disease pathogenesis.
Keywords: CACNA1A, P/Q-type, channelopathy, cerebellum, stress, Cav β subunit
INTRODUCTION
The dominantly-inherited paroxysmal disorder episodic ataxia type 2 (EA2) results from mutations in the CACNA1A gene, which encodes the pore-forming α12.1 subunit of Cav2.1 voltage-gated calcium channels (Ophoff et al., 1996). The Cav2.1 calcium channel subtype regulates neurotransmission throughout the nervous system, but is predominantly expressed within cerebellar Purkinje cells (Usowicz et al., 1992; Stea et al., 1994; Westenbroek et al., 1995). Not surprisingly, cerebellar dysfunction is the primary feature of EA2 attacks, as patients experience bouts of symptoms such as ataxia, migraine and vertigo, in response to emotional, physical or pharmacological stressors (Ptacek, 1998; Jen, 2000; Jen et al., 2004). Although episodic neurological disorders can be characterized by a wide range of symptoms, including epileptic seizures, paroxysmal dyskinesias or periodic paralysis, many also arise from mutations within ion channel genes (Jen, 1999; Ptacek, 1999). Thus, studying the pathophysiological mechanisms of individual diseases such as EA2 may be useful in the development of treatment strategies for episodic channelopathy disorders in general.
Voltage-gated calcium or Cav channels regulate an array of physiological processes including muscle contraction, hormone secretion, neurotransmission, and gene expression. This diversity in function is mainly due to the expression of 10 genetically distinct α1 subunit subtypes that may make up the calcium channel pore (Ertel et al., 2000; Catterall et al., 2005). However, high voltage-activated (HVA) Cav channels are also composed of at least two auxiliary subunits, β and α2δ, which modulate channel kinetics (Singer et al., 1991; Arikkath and Campbell, 2003; Catterall et al., 2005). The auxiliary β subunits also play a crucial role in the functional expression of Cav channels. These non-membrane-spanning subunits promote translocation of α1 subunits from the endoplasmic reticulum (ER) to the plasma membrane through a high affinity association with the alpha interaction domain, AID, which is evolutionarily conserved in all α1 subunit subtypes (Pragnell et al., 1994; De Waard et al., 1996; Bichet et al., 2000).
Functional expression studies involving EA2 mutations have firmly established that non- or hypo-conductive α12.1 subunits cause the disorder (Guida et al., 2001; Jen et al., 2001; Jouvenceau et al., 2001; Wappl et al., 2002; Imbrici et al., 2004; Spacey et al., 2004; Imbrici et al., 2005; Wan et al., 2005b; Jeng et al., 2006), which is largely but not exclusively associated with expression of α12.1 truncation mutants (Ophoff et al., 1996; Yue et al., 1998; Battistini et al., 1999; Denier et al., 1999; Jen et al., 1999; Denier et al., 2001; van den Maagdenberg et al., 2002; Wappl et al., 2002; Subramony et al., 2003; Jen et al., 2004; Mantuano et al., 2004; Eunson et al., 2005; Spacey et al., 2005; Wan et al., 2005a; Wan et al., 2005b; Scoggan et al., 2006). However, the molecular mechanisms by which non-functional α12.1 pores generate disease in EA2 are still debated. Although some studies have suggested that the loss of channel function in EA2 simply induces a haplo-insufficiency of Cav2.1 currents (Wappl et al., 2002; Imbrici et al., 2004; Imbrici et al., 2005), substantial evidence argues that non-conductive α12.1 mutants in EA2 actually suppress the functional contributions of Cav2.1 channels composed of wild-type subunits through a dominant-negative mechanism (Jouvenceau et al., 2001; Raghib et al., 2001; Arikkath et al., 2002; Page et al., 2004; Jeng et al., 2006). Studies have suggested that impaired translation or stability of wild-type α12.1 subunits contributes to EA2 pathogenesis (Raghib et al., 2001; Page et al., 2004), while other evidence implicates the interactions between non-conductive α12.1 mutants and auxiliary β subunits in the dominant-negative suppression of wild-type α12.1 subunit function (Arikkath et al., 2002; Jeng et al., 2006).
The lack of a clear disease model for EA2 may be due in part to important differences between methods of Cav channel functional expression in the laboratory. Therefore, to further test the hypothesis that α12.1 mutants in EA2 exert dominant-negative effects on Cav2.1 function, we utilized a strategy designed to reliably record whole-cell Cav2.1 currents from channels composed of auxiliary β and α2δ subunits and mixed populations of α12.1 subunits, containing both wild-type isoforms and truncation mutants. We found that non-conductive α12.1 truncations, including those associated with EA2, severely suppressed Cav2.1 currents when co-expressed with wild-type α12.1 subunits. Current suppression was observed despite abundant expression of wild-type α12.1 subunits, demonstrating that these effects were not due to wild-type protein instability. Furthermore, of the several α12.1 truncation mutants tested, only those terminating distal to the AID suppressed Cav2.1 currents. These results are consistent with a dominant-negative model of EA2 disease pathogenesis and further implicate the involvement of Cav β auxiliary subunits in EA2 pathophysiology.
METHODS
Design of full-length and truncated α12.1 subunit cDNAs
Single nucleotide mutations were engineered using the Quickchange mutagenesis PCR method (Stratagene, Cedar Creek, TX) and verified by nucleotide sequence analysis. Wild-type α12.1 subunits N-terminally tagged with cyan or yellow fluorescent protein (CFP-Wt or YFP-Wt) were generated by subcloning an 8 kb Bgl II/BamH I fragment containing a rabbit-human α12.1 subunit chimeric cDNA (Restituito et al., 2000) into the pECFP or pEYFP cloning vectors (Clontech, Paulo Alto, CA). As described previously (Restituito et al., 2000), this chimeric α12.1 cDNA encodes the rabbit brain BI-1 isoform (Genbank accession # X57476) fused to the human α12.1 extended C-terminus, which was cloned from human cerebellum. This full-length α12.1 chimeric cDNA clone translates proteins bearing 93% identity to the full-length human clone AF004884. The EA2 mutation 1443X (Denier et al., 1999) was generated by inserting a point mutation into the YFP-Wt construct using forward primer CGAGTTTCACTAGGACAACGTGCTGTGG and reverse primer CCACAGCACGTTGTCCTAGTGAAACTCG. The 612X mutation, similar to a reported EA2 mutation (van den Maagdenberg et al., 2002) was generated by sublconing a 1.8 kb Bgl II/Sca I digested fragment from the full-length α12.1 cDNA into the pEYFP cloning vector. Point mutations were introduced into this construct to express α12.1 subunits truncated proximal (384X) and distal (402X) to the reported alpha interaction domain, AID (Pragnell et al., 1994), using forward and reverse primers GAAGCTGCGGCGGTAGCAGCAGATTG and CAATCTGCTGCTACCGCCGCAGCTTC, respectively for YFP-384X, and GGTGATCCTCGCATAGGACGAGAGACCGACG and CGTCGGTCTCGTCCTATGCGAGGATCACC for YFP-402X. The 330X mutation (Subramony et al., 2003) was constructed via subcloning a BssH1 (site 1282) BamH1 fragment of the full-length α12.1 cDNA into the pEYFP cloning vector.
Cell culture
β1-HEK cells (Rock et al., 1989; Piedras-Renteria et al., 2001; Barrett et al., 2005; Cao et al., 2005), which stably express human β1c (Genbank accession # U86960; personal communication, Erika Piedras-Renteria, Loyola University, Chicago) and rabbit α2δ subunits (Genbank accession # M21948; Rock et al., 1989) were grown in low-calcium DMEM/F12 media (10% fetal bovine serum, 1% L-glutamine, 0.05% Gentamicin, 1% non-essential amino acids) and transfected with equimolar amounts of wild-type and mutant α12.1 subunit cDNAs using Lipofectamine (Invitrogen, Carlsbad, CA). Cells were plated onto poly-D lysine coated cover slips at low-density approximately 48 hrs post-transfection and recorded or imaged 2–4 hours later.
Electrophysiology
Whole-cell barium currents were recorded and captured using the patch-clamp technique with an Axopatch 200B amplifier, Digidata 1320A converter, and pCLAMP6 software (Axon instruments, Foster City, CA). Borosilicate micropipettes of 2–4 MΩ resistance were filled with (in mM): 125 CsCl, 5 EDTA, 2 MgCl2, and 5 Glucose (pH 7.2 with CsOH and 275 mOsm with glucose). The recording solution was (in mM): 2.5 BaCl2, 10 HEPES, 110 TEACl, and 30 TEAOH (pH 7.4 with methane sulfonic acid and 300 mOsm with glucose). Cells were viewed at 60x under oil immersion, on an Axiovert S100TV inverted microscope (Zeiss, Jena, Germany), and with CFP and YFP excitation/emission epifluorescence filter sets (Chroma Technology, Rockingham VT). Cells were clamped at −80 mV, and recordings initiated after compensating for capacity transients and series resistance (70%). Linear leak was subtracted using the P/4 method and current records filtered using a 5kHz low-pass Bessel filter. Currents were evoked using 150 ms voltage steps from −60 to 60 mV at intervals of 10 seconds. Peak current density (Peak J, pA pF−1) was measured at 0 mV. Whole-cell current density data for individual cells were fitted with the modified Boltzmann equation: Peak J = G (V−Vrev)[1 + exp (V0.5−V)−k]−1 to compare Cav2.1 channel open probability, where G = relative conductance, V = voltage, Vrev = reversal potential, V0.5 = the voltage of half maximal activation, and k = the slope factor. Rates of activation and inactivation were measured from raw whole-cell current traces at 0, 10, and 20 mV step pulses, with Ipeak = the time to reach the peak current and Ir50 = the % peak current remaining at 50 ms. To control for wild type or mutant isoform expression level bias, dual-fluorescing cells were randomly selected for physiological recordings. To control for potential changes in the β1-HEK cell line following several passages during this study, recordings of control cells transfected with CFP-Wt constructs were obtained throughout for each transfection. Recordings from control cells resulted in robust currents in each case. Only cells surviving through the entire I/V experiment and expressing detectable levels of currents were used for kinetic measurements.
Fluorescence microscopy
Images of randomly-selected β1-HEK cells expressing CFP-tagged wild-type α12.1 subunits and YFP-tagged truncations were captured using an Olympus 1X70 inverted light microscope (Melville, NY) equipped with a Spot RT digital camera (Diagnostic Instruments, Sterling Heights, MI). Cells were treated identically to those used for electrophysiological experiments. Average CFP and YFP pixel intensities of several samples from individual cells imaged during the same sitting were calculated and analyzed following background subtraction using the MetaMorph acquisition and analysis software package (Molecular Devices Corporation, Sunnyvale, CA). Values expressed represent mean relative fluorescence intensities for each group of cells.
Immunoblot and immunoprecipitation
Immunoprecipitation and western blot experiments were performed essentially as previously reported (Restituito et al., 2000). Due to the availability of high quality commercial β3 subtype-specific antisera (Alomone Labs, Israel), co-immunoprecipitation studies were carried out in β3-HEK cells stably expressing rat β3 and α2δ subunits (Kordasiewicz et al., 2006). Briefly, approximately 48 hours post-transfection, HEK cells stably expressing β3 and α2δ subunits (β3-HEK) were lysed with ice-cold buffer (M-PER; Pierce Biotechnology, Rockford, IL) supplemented with 10mM EDTA, 10mM EGTA and protease inhibitors (Complete Mini EDTA-free; Roche, Indianapolis, IN). For western blots, 30 µg of total lysate were subjected to SDS-PAGE on 8% tris-glycine gels (Invitrogen, Carlsbad, CA). Immunoprecipitation was performed by incubating 40 µL of staphylococcus protein A agarose beads (SPA; Invitrogen) with 5 µg of monoclonal anti-GFP (Roche) and 100 µg lysate. To remove unbound proteins, preparations were washed with TBS containing 1% Tween-20 (TBS-t) and then with TBS-t containing 10 mM tris base. Bound proteins were freed from SPA beads by boiling and denaturing followed by SDS-PAGE. For all immunoblots, proteins were transferred to nitrocellulose membranes, which were incubated with monoclonal anti-GFP (western blot) or polyclonal anti-β3 (immunoprecipitation) and species-specific secondary antibodies. Chemiluminescent signals were developed using the ECL western detection kit (Roche).
Statistical analysis
Data expressed as means ± SEM and were statistically analyzed with ANOVA and a post hoc Bonferroni correction using a statistical software package (Statview, SAS Institute).
RESULTS
Truncated α12.1 subunits suppress Cav2.1 currents in a length-dependent fashion
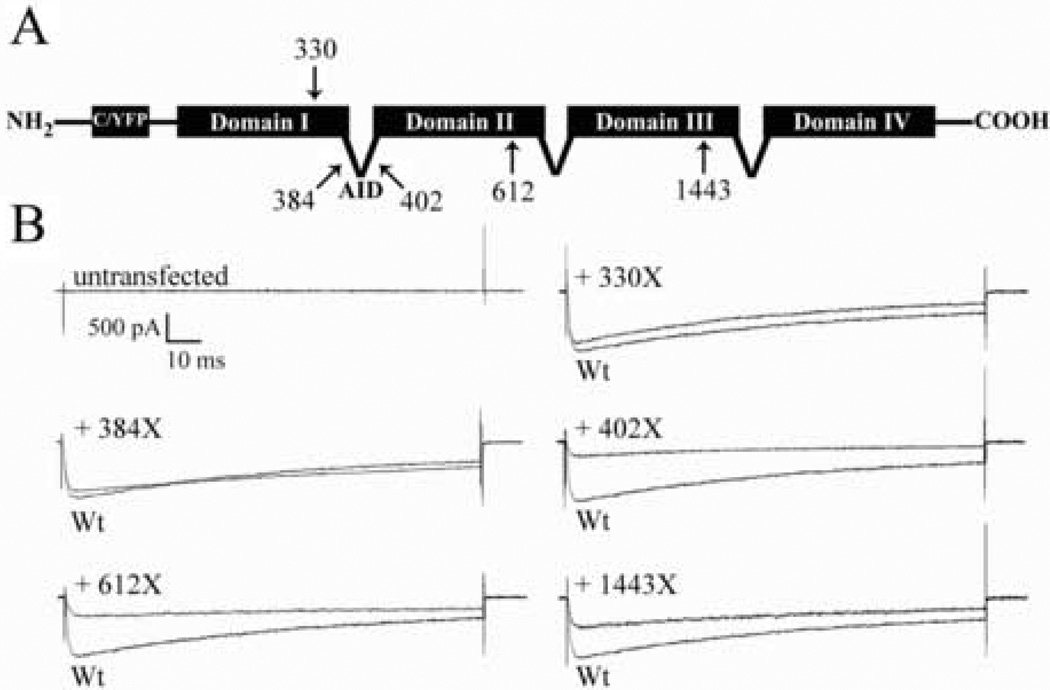
To directly test for a dominant-negative effect of truncated α12.1 subunits on Cav2.1 channel function, we assembled a series of cDNA constructs designed to express wild-type and truncated α12.1 subunits, N-terminally tagged with cyan or yellow fluorescent protein, respectively (CFP and YFP; Figure 1A) and transiently expressed them in human embryonic kidney cells stably expressing Cav β and α2δ auxiliary subunits (β1-HEK cells; Piedras-Renteria et al., 2001). Untransfected β1-HEK cells expressing only the Cav auxiliary subunits failed to exhibit currents at any potential (Figure 1B). In contrast, whole-cell currents from voltage-clamped β1-HEK cells expressing CFP-fluorescing wild-type α12.1 subunits (CFP-Wt) were easily obtainable (Figure 1B, Figure 2), and exhibited a mean peak current density of −75.5 ± 7.9 pA pF−1 at 0 mV (n = 16; Table 1). Consistent with all previous reports, none of the YFP-tagged α12.1 truncations tested here conducted detectable Cav2.1 currents (not shown).
Figure 1. Design and co-expression of CFP-tagged wild-type α12.1 subunits with YFP-tagged truncations.
A. Schematic of CFP-tagged wild-type α12.1 subunits (Wt) and YFP-tagged truncations indicates the amino-acid residue numbers and approximate length of each truncated isoform. The alpha interaction domain, AID, between repeat domains I and II is also marked. B. Current traces depicted were elicited with 0 mV depolarizing pulses from −80 mV holding potentials following transient co-expression of truncations with Wt subunits in β1-HEK cells. Traces from cells co-expressing truncations with Wt subunits are overlaid with those obtained from control cells expressing Wt subunits alone.
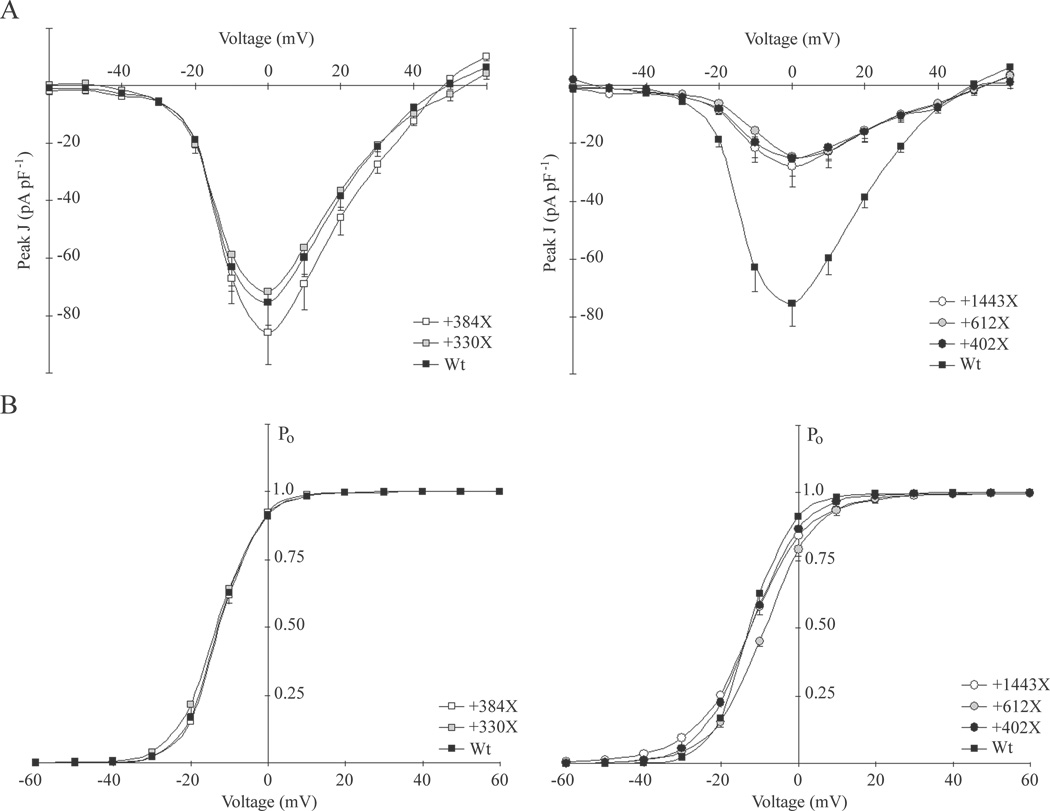
Figure 2. Whole-cell Cav2.1 currents from β1-HEK cells co-expressing α12.1 truncations with wild-type subunits.
Whole-cell currents were elicited with 150 ms step pulses from −60 to +60 mV at intervals of 10 seconds from a hold of −80 mV. A. Mean peak current density responses (Peak J, pA pF−1) plotted against voltage steps (mV) with error bars representing SEM. Current-voltage plots of recordings from cells co-expressing CFP-tagged wild-type α12.1 subunits (Wt) and YFP-tagged truncations terminating proximal to the AID are similar in magnitude to control recordings from control cells expressing Wt subunits alone (Left panel). Current-voltage plots of recordings from cells co-expressing α12.1 truncations terminating distal to the AID reveal severely reduced current densities across all voltages compared to control cells (Right panel). B. Whole-cell current data for individual cells were fitted with the modified Boltzmann equation: Peak J = G (V−Vrev)[1 + exp(V0.5−V)−k]−1 to compare Cav2.1 channel open probability (Po) across all voltage steps. The mean of each group is expressed with error bars representing SEM. Compared to currents from control cells expressing Wt subunits alone, those from cells co-expressing truncations terminating proximal to the AID in most cases exhibited normal Po profile across all voltages (Left panel). Most of the Po profiles of currents from cells co-expressing truncations terminating distal to the AID reveal minor deviations from control, except for currents from cells co-expressing YFP-612X truncations, which appeared to activate at more positive voltages.
Table 1.
Cav2.1 currents from β1-HEK cells co-expressing α12.1 truncations and wild-type subunits
| Wild type | + 330X | + 384X | + 402X | + 612X | + 1443X | |
|---|---|---|---|---|---|---|
| Jpeak (pA pF−1) | −75.5 ± 7.9 (16) |
−71.8 ± 12.8 (13) |
−85.8 ± 11.2 (7) |
−25.2 ± 5.9b (7) |
−24.5 ± 3.9c (12) |
−27.8 ± 7.1b (9) |
| Cm (pF) | 11.7 ± 0.6 | 9.2 ± 1.0 | 10.2 ± 1.0 | 12.1 ± 1.8 | 11.6 ± 0.8 | 11.5 ± 1.5 |
| G (pA pF−1mV−1) | 2.0 ± 0.2 | 1.8 ± 0.3 | 2.0 ± 0.3 | 0.5 ± 0.1b | 0.7 ± 0.1c | 0.7 ± 0.2b |
| V0.5 (mV) | −12.2 ± 0.8 | −13.0 ± 1.0 | −12.1 ± 0.7 | −11.6 ± 0.6 | −8.6 ± 0.7a | −12.2 ± 1.1 |
| k (mV) | 4.6 ± 0.3 | 5.0 ± 0.4 | 4.7 ± 0.3 | 7.7 ± 1.1 | 6.8 ± 0.6a | 6.3 ± 0.3c |
| Ipeak (ms) | 6.3 ± 0.3 (11) |
5.8 ± 0.4 (7) |
6.7 ± 0.3 (7) |
7.0 ± 0.8 (6) |
7.3 ± 0.5 (11) |
7.3 ± 0.5 (8) |
| Ir50 (%) | 59.4 ± 2.4 | 52.6 ± 7.6 | 70.0 ± 1.9 | 56.1 ± 4.1 | 66.6 ± 2.6 | 53.8 ± 2.9 |
Data are expressed as means ± SEM and tested for significance using ANOVA and post hoc Bonferrroni correction. Jpeak measured at 0 mV step pulse; Ipeak and Ir50 measured at 0, 10 and 20 mV step pulses. (N) = number of cells for current density or kinetic measurements. Kinetic measurements are from recordings of cells that survived throughout the experiment and expressed detectable levels of Cav2.1 currents.
pa < 0.005,
pb < 0.001,
pc < 0.0001.
In several cases, co-expression of wild-type and truncated α12.1 subunits resulted in significantly reduced whole-cell Cav2.1 current densities. Interestingly, the density of currents in co-transfected cells depended on the length of truncated isoform co-expressed. Co-expression of CFP-Wt subunits with either of the YFP-612X or YFP-1443X truncations, similar to mutants predicted from two distinct nonsense mutations associated with EA2 (Denier et al., 1999; van den Maagdenberg et al., 2002), resulted in severe reductions in Cav2.1 currents across all voltages compared to control (Figure 1B, Figure 2A). Cells exhibited mean peak current densities of −24.5 ± 3.9 (n = 12, p < 0.0001) and −27.8 ± 7.1 (n = 9, p < 0.001) pA pF−1 at 0 mV, when co-expressing CFP-Wt subunits with YFP-612X or YFP-1443X truncations, respectively (Table 1). In addition, co-expression of YFP-612X truncations caused a slight but statistically-significant hyperpolarizing shift in channel activation relative to control (Figure 2B, Table 1).
In contrast, currents from cells co-expressing YFP-330X truncations, which corresponds to one possible outcome of a CACNA1A splice-site mutation reported by our group (Subramony et al., 2003), were similar in appearance (Figure 1B) and magnitude (Table 1) to those obtained from control cells expressing CFP-Wt subunits alone, with a mean peak current density of 71.8 ± 12.8 pA pF−1 at 0 mV (n = 13, p > 0.5). There were no significant kinetic changes associated with co-expression of YFP-330X truncations (Table 1, Figure 2). The lack of effects associated with YFP-330X co-expression indicates that the reported CACNA1A splice-site mutation may not encode an α12.1 truncation. Instead, recent studies propose that similar splice-site mutations associated with EA2 result in aberrantly-spliced α12.1 isoforms with unusual domain compositions (Eunson et al., 2005; Wan et al., 2005a). These results demonstrate that some but not all α12.1 truncations perturb Cav2.1 function when co-expressed with wild-type subunits.
Dominant-negative Cav2.1 current suppression by α12.1 truncations requires AID
We hypothesized that the inability of YFP-330X mutants to affect Cav2.1 currents was related to their termination prior to the alpha interaction domain, AID, as all of the reported truncations associated with EA2 predict termination distal to its location (Denier et al., 1999; Jen et al., 1999; van den Maagdenberg et al., 2002; Jen et al., 2004). Membrane translocation and functional expression of Cav α1 subunits depend on β subunit binding to the AID, a 20 amino-acid sequence with 9 conserved residues, located within the α1-subunit domain I–II cytoplasmic loop (Pragnell et al., 1994; Bichet et al., 2000). To determine if α12.1 truncations require the AID to suppress Cav2.1 currents from wild-type subunits, we designed two additional cDNA constructs intended to express mutants terminating directly proximal (YFP-384X) or distal (YFP-402X) to the AID (Figure 1A). Co-expression of YFP-384X truncations with CFP-Wt subunits had no effect on the size of Cav2.1 currents relative to control (Figure 1B, Figure 2A), resulting in a mean peak current density of −85.8 ± 11.2 pA pF−1 at 0 mV (n = 7, p > 0.1; Table 1). However, co-expression of YFP-402X truncations with CFP-Wt subunits caused severely reduced currents across all voltages (Figure 1B, Figure 2A), with a mean peak current density of 25.2 ± 5.9 pA pF−1 at 0 mV (n = 7, p < 0.001; Table 1), similar to the mean current densities following co-expression of the AID-bearing YFP-612X or YFP-1443X truncations. Therefore, co-expression of α12.1 truncations with wild-type subunits was associated with Cav2.1 current densities which were suppressed to less than 40% of control exclusively when truncations contained the AID.
Co-expression of truncated isoforms does not result in wild-type protein instability
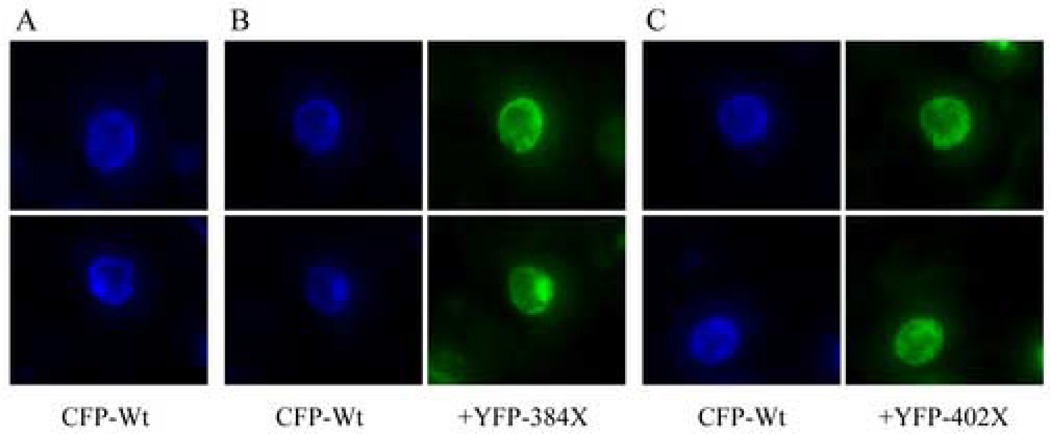
Findings from several other studies, including those reported here, indicate that mutant α12.1 subunits in EA2 likely inhibit the functional impact of wild-type subunits (Jouvenceau et al., 2001; Arikkath et al., 2002; Jeng et al., 2006). However, previous reports also suggest that α12.1 truncations could perturb Cav2.1 currents by impairing translation or stability of wild-type subunits (Raghib et al., 2001; Page et al., 2004). Co-transfection of CFP-Wt and YFP-truncated α12.1 subunit constructs resulted in an abundance of cells with dual CFP and YFP fluorescence across all experiments, indicating ample expression of both the wild-type and mutant isoforms (Figure 3). Nevertheless, to rule out a link between the reduced Cav2.1 current densities observed here and a decrease in wild-type α12.1 protein expression levels previously reported by others, we performed image analyses to compare the relative CFP fluorescence intensity (RF) individual β1-HEK cells treated identically to those used for electrophysiological recordings. Compared to control cells expressing CFP-Wt subunits alone, there was no decrease in mean CFP fluorescence intensity detected in any of the groups co-expressing YFP-truncations (Table 2). In fact, mean CFP-Wt expression levels were remarkably similar across all groups, except for cells co-expressing YFP-1443X truncations, which actually exhibited significantly higher levels of CFP fluorescence intensity (69.2 ± 5.8 RF, n = 17) compared to control cells (50.1 ± 3.8 RF, n = 27; p < 0.005). These results clearly demonstrate that the reduction in whole-cell Cav2.1 currents caused by co-expression of AID-bearing α12.1 truncations with wild-type subunits cannot be explained by a reduction of wild-type subunit translation or stability.
Figure 3. Expression patterns of CFP-tagged wild-type α12.1 subunits and YFP-tagged truncations co-expressed in β1-HEK cells.
Representative epifluorescence images from cells treated identically to those used for electrophysiological analyses. A. Transfecting β1-HEK cells with CFP-tagged wild-type α12.1 subunit cDNA constructs (CFP-Wt) resulted in an abundance of cells with CFP fluorescence distributed in patterns suggesting robust membrane expression. B. and C. Compared to control cells expressing CFP-Wt subunits alone, no changes were discernable to the levels or patterns of CFP fluorescence in cells co-expressing YFP-tagged truncations terminating proximal (YFP-384X) or distal (YFP-402X) to the AID.
Table 2.
Expression levels of α12.1 truncations and wild-type subunits co-expressed in β1-HEK cells
| Wt | + 384X | + 402X | + 612X | + 1443X | |
|---|---|---|---|---|---|
| Cyan RF | 50.1 ± 3.8 (27) |
52.0 ± 3.0 (33) |
50.3 ± 4.7 (18) |
48.4 ± 6.9 (20) |
69.2 ± 5.8a (17) |
| Yellow RF | NA | 82.9 ± 7.0 | 43.0 ± 4.9b | 81.7 ± 12.5 | 66.0 ± 6.5 |
| C:Y Ratio | NA | 0.86 ± 0.12 | 1.4 ± 0.17a | 0.74 ± 0.11 | 1.17 ± 0.10 |
Data are expressed as means ± SEM and tested for significance using ANOVA and post hoc Bonferrroni correction. Conditions were identical to those for physiological experiments. All values calculated for individual cells and then averaged across groups. RF = background-subtracted relative fluorescence levels. (N) = number of cells, C:Y Ratio = ratio of Cyan and Yellow RF levels,
pa < 0.005,
pb < 0.001
Truncation proximal to the AID prevents high-affinity Cav α12.1-β interactions
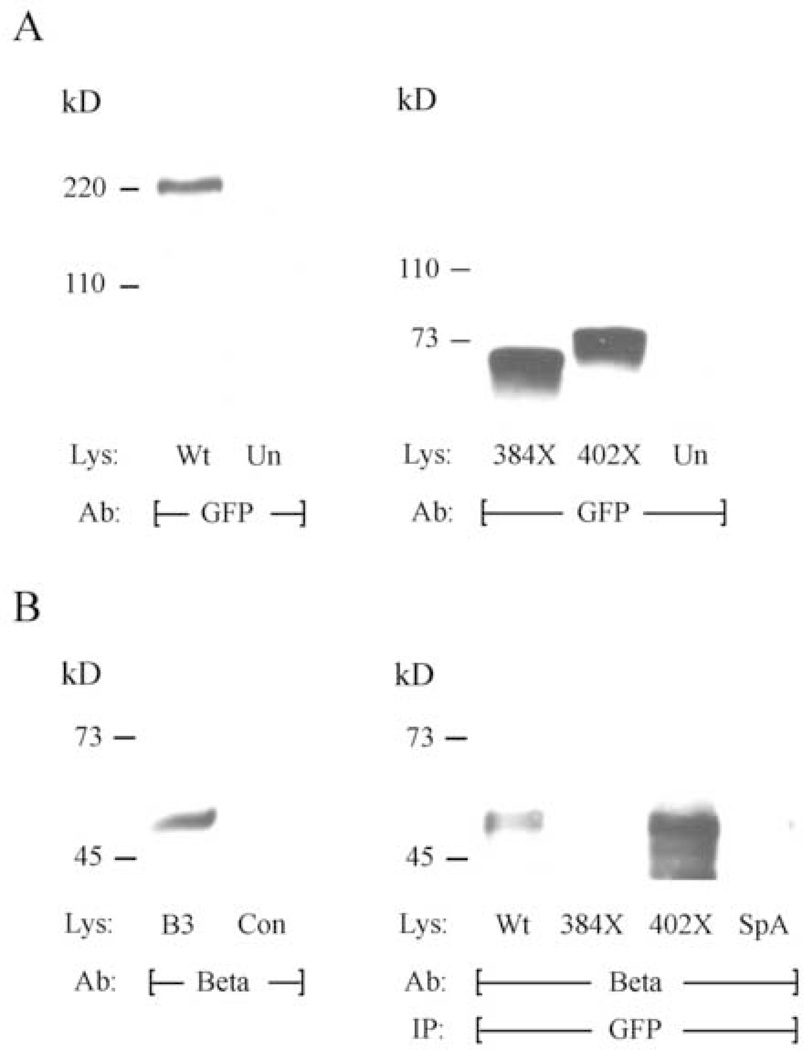
Although necessary for β subunit-mediated α1 membrane translocation, the region of the AID is only one of several known to exhibit affinity for β subunit proteins (Pragnell et al., 1994; De Waard et al., 1996; Brice et al., 1997; Walker et al., 1998; Walker et al., 1999; Bichet et al., 2000; Cornet et al., 2002; Maltez et al., 2005). To examine the role of the AID in facilitating Cav α1-β subunit associations, we tested preparations from HEK cells stably expressing β3 and α2δ auxiliary subunits (β3-HEK) and transiently expressing YFP-384X, YFP-402X, or CFP-Wt α12.1 isoforms for the presence of α12.1-β proteins complexes. When immunoblotted with antisera to the α12.1 N-terminal fluorescent protein tag (anti-GFP), lysates from cells expressing β subunits and either the wild-type or mutant α12.1 proteins were positive for only the expected α12.1 reactive species (Figure 4A). Similarly, when these lysates were immunoblotted with anti-β3 sera, a single approximately 60 kD species was detected, corresponding to the predicted β subunit molecular weight (Figure 4B, left panel). Anti-GFP did not react with lysates prepared from untransfected cells (Figure 4A, left and right panels) nor did anti-β 3 react with lysates prepared from HEK cells not expressing β subunits (Figure 4B, left panel). To determine if the expressed α12.1 proteins form detectable complexes with β subunits, we first immunoprecipitated lysates from transfected cells with anti-GFP and staphylococcal protein A, and then transferred immunoprecipitates to membranes following SDS-PAGE separation. Anti-β3 antibody detected the same β subunit species in immunoprecipitates from cells expressing the CFP-Wt or YFP-402X α12.1 isoforms, but failed to yield detectable levels of the β subunit protein in preparations from cells expressing YFP-384X truncations, terminating proximal to the AID (Figure 4B, right panel). These data suggest that formation of these α12.1-β complexes is largely, if not exclusively, dependent on the amino acid residues contained within the AID, and further implicate α1-β interactions in the suppression of Cav2.1 currents we observed when wild-type α12.1 subunits and truncations terminating distal to the AID were co-expressed.
Figure 4. Truncation proximal to the AID prevents α12.1-β subunit interactions.
A. Western blotting lysates (Lys) from β3-HEK cells with GFP antibody (Ab) detects the predicted α12.1 isoforms in cells expressing CFP-tagged wild-type α12.1 subunits (Wt) or YFP-tagged 384X or 402X truncations, but GFP Ab does not react with lysates from untransfected (Un) β3-HEK cells. B. Western blotting with β3 Ab (Beta) detects an approximate 60 kD β3 subunit species in lysates from β3-HEK cells (B3) but not control (Con) HEK cells (left panel). Western blotting with β3 Ab following immunoprecipitation (IP) of lysates with GFP Ab detects the same β3 subunit species in preparations from β3-HEK cells expressing CFP-tagged wild-type α12.1 subunits or YFP-tagged 402X truncations but not from cells expressing 384X truncations, which terminate proximal to the AID (right panel).
DISCUSSION
Episodic ataxia type 2 (EA2) is a paroxysmal ion channel disorder or channelopathy arising from one of several mutations within the CACNA1A gene, which encodes the pore-forming α12.1 subunit of Cav2.1 channels (Ophoff et al., 1996). While attacks in patients with EA2 can be somewhat variable in character, it is not surprising that symptoms of cerebellar dysfunction are the predominant feature (Ptacek, 1999; Jen, 2000; Jen et al., 2004), given that cerebellar output through Purkinje cells relies heavily on the Cav2.1 channel subtype (Swensen and Bean, 2003; Womack and Khodakhah, 2004; Walter et al., 2006). Study of the electrophysiological consequences of EA2 mutations has consistently demonstrated that the disorder is associated with a loss of Cav2.1 channel function (Guida et al., 2001; Jen et al., 2001; Jouvenceau et al., 2001; Wappl et al., 2002; Imbrici et al., 2004; Spacey et al., 2004; Imbrici et al., 2005; Wan et al., 2005b; Jeng et al., 2006), but the implications for disease pathogenesis in EA2 are not so clear.
As previously reported for α12.1 subunits harboring EA2 mutations (Jouvenceau et al., 2001; Raghib et al., 2001; Arikkath et al., 2002; Page et al., 2004; Jeng et al., 2006), we routinely observed a dominant-negative suppression of Cav2.1 channel currents when non-conductive α12.1 truncations were co-expressed with wild-type subunits. In the present study we also observed that these effects occurred despite abundant levels of wild-type subunit protein expression, adding to the growing evidence that non-conductive α12.1 mutants in EA2 exert dominant-negative effects on the functional impact rather than on the translation or stability of wild-type subunits (Jouvenceau et al., 2001; Raghib et al., 2001; Arikkath et al., 2002; Page et al., 2004; Jeng et al., 2006). The electrophysiological data presented here are novel in that only a subset of the truncated α12.1 isoforms tested suppressed currents from Cav2.1 channels which were composed of wild-type subunits. Current suppression by truncations depended on whether or not they terminated distal to the AID, implying that α12.1 subunit interactions with auxiliary β subunits were crucial for the effect. This conclusion is supported by our demonstration that α12.1-β subunit heterodimers were detectable by immunoprecipitation only in samples with full-length or truncated α12.1 proteins bearing the AID, which agrees with several previous studies characterizing the role of the AID in the α12.1-β high-affinity interaction (Pragnell et al., 1994; De Waard et al., 1996; Brice et al., 1997; Walker et al., 1998; Walker et al., 1999; Bichet et al., 2000). Overall, our data suggest that AID-mediated interactions with β subunits permit truncated α12.1 subunits in EA2 to perturb the function of normal Cav2.1 channels, a view consistent with the fact that all of the reported EA2 nonsense mutations predict α12.1 subunit truncations to terminate distal to the AID (Ophoff et al., 1996; Denier et al., 1999; van den Maagdenberg et al., 2002; Jen et al., 2004).
A model of EA2 pathophysiology involving Cav auxiliary β subunits is well supported by the literature. The functional density of Cav channels depends on the availability of auxiliary β subunits (Berrow et al., 1995; Gao et al., 1999), which in addition to normalizing channel activity (Lacerda et al., 1991) facilitate α1 subunit membrane translocation by binding to the AID and masking several endoplasmic reticulum (ER) retention signals residing within the α1 subunit domain I–II intracellular loop (Bichet et al., 2000; Cornet et al., 2002). Similar to our results, severely reduced Cav current densities have been observed in situ following exogenous expression of membrane-targeted peptides bearing the AID (Cuchillo-Ibanez et al., 2003). During the present study, we found no indication that expression of AID-bearing α12.1 truncations affected wild-type subunit expression levels. However, the suppression of Cav currents implies that the function of wild-type α12.1 subunits was reduced in β1-HEK cells despite a stable pool of auxiliary β subunits. In agreement with this assessment, titration experiments performed by others show that the concentration of β subunits required to traffic α1 subunits to the plasma membrane is substantially less than that required to modulate channel activity (Canti et al., 2001). Thus, α12.1 mutants in EA2 have the potential to sequester a significant portion of the available pool of β subunits, reducing the overall functional impact of Cav2.1 channels composed of wild-type α12.1 pores.
Nonetheless, the mechanisms of EA2 pathogenesis remain controversial due to several contradictory reports in the literature. Still under debate is whether EA2 arises from a simple haplo-insufficiency of wild-type Cav2.1 channels (Guida et al., 2001; Wappl et al., 2002; Imbrici et al., 2004), or if non-conductive α12.1 mutants cause disease by exerting dominant-negative effects on the function of Cav2.1 channels composed of wild-type subunits (Jouvenceau et al., 2001; Raghib et al., 2001; Page et al., 2004; Jeng et al., 2006). The discrepancies in the EA2 literature may be related to the differences in Cav channel heterologous expression systems and α12.1 isoforms employed by individual groups. As demonstrated by Jeng et al, α12.1 truncation mutants are more pathogenic when co-expressed with wild-type isoforms bearing extended C-termini, which are abundant in cerebellum (Restituito et al., 2000; Jeng et al., 2006; Kordasiewicz et al., 2006). We therefore devised a strategy to easily record whole-cell Cav2.1 currents from mammalian cells expressing a reliably mixed population of mutant and wild-type α12.1 pores by voltage-clamping cells stably expressing Cav auxiliary β and α2δ subunits and co-transfected with fluorescent-tagged α12.1 subunit truncations and wild-type isoforms with extended C-termini.
Including the findings presented here, of the eight known studies where non- or hypo-conductive α1 subunit mutants were co-expressed with wild-type subunits, six report a dominant-negative suppression of Cav currents (Jouvenceau et al., 2001; Raghib et al., 2001; Arikkath et al., 2002; Wappl et al., 2002; Imbrici et al., 2004; Page et al., 2004; Jeng et al., 2006). Moreover, of the five groups that describe current suppression, three, including our own, found evidence implicating Cav auxiliary β subunits in the EA2 disease process (Jouvenceau et al., 2001; Arikkath et al., 2002; Page et al., 2004; Jeng et al., 2006). Similar to the findings presented here, Arrikkath et al. used a cell line stably expressing α12.1 subunits and auxiliary β subunits to co-express a two-domain α12.1 truncation, and observed that the current-suppressing effects of the truncated isoform were completely abolished by mutations to amino acid residues within the AID that are critical for the high-affinity α1-β interaction (Pragnell et al., 1994; De Waard et al., 1996; Arikkath et al., 2002). More recently, it was reported that the suppressive effects of EA2 truncations on exogenous Cav2.1 currents from wild-type α12.1 subunits were significantly alleviated by increasing the availability of β subunits (Jeng et al., 2006). However, the same approach failed to even modestly reverse the dominant-negative effects exerted by EA2 missense mutants (Jeng et al., 2006), which in addition to hypo-conduction exhibit defective intracellular trafficking (Wan et al., 2005b). These latter findings suggest that α12.1 missense mutants may be more pathogenic than truncations.
While ample data argue for an EA2 model in which the disease process is facilitated by Cav auxiliary β subunits, the behavior of α12.1 mutants in neurons in vivo is presumably more complex than that suggested by studies performed in heterologous expression systems. Recently, Cao et al reported that exogenous over-expression of wild-type α12.1 subunits in neurons in situ increased Cav2.1 current density 5-fold without affecting synaptic transmission, while expression of non-conductive α12.1 mutants under the same conditions had no effect on the density of currents, but reduced the contribution of the endogenous Cav2.1 currents to the release of neurotransmitter (Cao et al., 2005; Cao and Tsien, 2005). These findings suggest that neurons, at least in culture, are able to functionally express α12.1 subunits much more densely than normal, but that expression of such a mixed population of wild-type subunits and non-conductive mutants causes dominant-negative defects in neurotransmission unrelated to whole-cell current density. Throughout the nervous system, synaptic transmission relies heavily on the activity of Cav2.1 channels, a reliance that can be a attributed in part to the targeted trafficking of α12.1 subunits to nerve terminals by Cav auxiliary β subunits (Wittemann et al., 200; Brice and Dolphin, 1999; Mochida et al., 2003a). However, Cav2.1 channel regulation of synaptic transmission also depends on the high-affinity interactions between the SNARE proteins of the synaptic release machinery and the synprint region of α12.1 subunits, located within the domain II–III cytoplasmic loop (Martin-Moutot et al., 1996; Mochida et al., 1996; Mochida et al., 2003b). Similar to the effects on Cav current density following the exogenous expression of membrane-targeted AID peptides (Cuchillo-Ibanez et al., 2003), synprint peptides exogenously expressed in neurons in situ cause a severe suppression of synaptic transmission (Mochida et al., 1996). Therefore, the results presented here and elsewhere suggest that the same protein-protein interactions which are crucial for α12.1 subunit trafficking and regulation of neurotransmission may also permit α12.1 mutants to act as decoys, entrapping a portion of the limited pool of regulatory proteins and decreasing the overall contributions of wild-type Cav2.1 currents to the maintenance of normal neuronal function.
Additional studies are needed to discern the discreet molecular events leading to a suppression of normal Cav2.1 function by mutant α12.1 subunits in neurons in EA2. Moreover, a detailed analysis of the kinetic consequences of wild type and truncated α12.1 subunit co-expression may uncover additional insights regarding the severity of the dysfunction. Furthermore, determining how a chronic reduction of Cav2.1 current magnitude triggers compensatory changes in calcium homeostasis will elucidate the etiology of the ataxic episodes in EA2. In turn, understanding the pathophysiology driving attacks in EA2 will have broader implications for a variety of other paroxysmal neurological disorders, including migraine, dystonia and epilepsy.
ACKNOWLEDGEMENTS
We thank Dr. Paul Mermelstein at the University of Minnesota for β1-HEK cells and technical assistance, Dr. Edward Perez-Reyes at the University of Virginia for β3 and α2δ cDNA clones, and Dr. Henry Colecraft at Johns Hopkins University for assistance with data analysis. This work was supported by NIH NS38332 and by the Dystonia Medical Research Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Arikkath J, Campbell KP. Auxiliary subunits: essential components of the voltage-gated calcium channel complex. Curr. Opin. Neurobiol. 2003;13:298–307. doi: 10.1016/s0959-4388(03)00066-7. [DOI] [PubMed] [Google Scholar]
- Arikkath J, Felix R, Ahern C, Chen CC, Mori Y, Song I, Shin HS, Coronado R, Campbell KP. Molecular characterization of a two-domain form of the neuronal voltage-gated P/Q-type calcium channel alpha(1)2.1 subunit. FEBS Lett. 2002;532:300–308. doi: 10.1016/s0014-5793(02)03693-1. [DOI] [PubMed] [Google Scholar]
- Barrett CF, Cao YQ, Tsien RW. Gating deficiency in a familial hemiplegic migraine type 1 mutant P/Q-type calcium channel. J Biol Chem. 2005;280:24064–24071. doi: 10.1074/jbc.M502223200. [DOI] [PubMed] [Google Scholar]
- Battistini S, Stenirri S, Piatti M, Gelfi C, Righetti PG, Rocchi R, Giannini F, Battistini N, Guazzi GC, Ferrari M, Carrera P. A new CACNA1A gene mutation in acetazolaminde-responsive familial hemiplegic migraine and ataxia. Neurology. 1999;53:38–43. doi: 10.1212/wnl.53.1.38. [DOI] [PubMed] [Google Scholar]
- Berrow NS, Campbell V, Fitzgerald EM, Brickley K, Dolphin AC. Antisense depletion of beta-subunits modulates the biophysical and pharmacological properties of neuronal calcium channels. J. Physiol. 1995;482:481–491. doi: 10.1113/jphysiol.1995.sp020534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bichet D, Veronique C, Sandrine G, Carlier E, Volsen S, Hoshi T, Mori Y, De Waard M. The I–II loop of the calcium channel alpha1 subunit contains an endoplasmic reticulum retention signal antagonized by the beta subunit. Neuron. 2000;25:177–190. doi: 10.1016/s0896-6273(00)80881-8. [DOI] [PubMed] [Google Scholar]
- Brice NL, Dolphin AC. Differential plasma membrane targeting of voltage-dependent calcium channel subunits expressed in a polarized epithelial cell line. Physiol. 1999;515:685–694. doi: 10.1111/j.1469-7793.1999.685ab.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brice NL, Berrow NS, Campbell V, Page KM, Brickley K, Tedder I, Dolphin AC. Importance of the different beta subunits in the membrane expression of the alpha1A and alpha2 calcium channel subunits: studies using a depolarization-sensitive alpha1A antibody. Eur J Neurosci. 1997;9:749–759. doi: 10.1111/j.1460-9568.1997.tb01423.x. [DOI] [PubMed] [Google Scholar]
- Canti C, Davies A, Berrow NS, Butcher AJ, Page KM, Dolphin AC. Evidence for two concentration-dependent processes for beta-subunit effects on alpha1B calcium channels. Biopys. J. 2001;81:1439–1451. doi: 10.1016/S0006-3495(01)75799-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao YQ, Tsien RW. Effects of familial hemiplegic migraine type 1 mutations on neuronal P/Q-type Ca2+ channel activity and inhibitory synaptic transmission. Proc. Natl. Acad. Sci. USA. 2005;102:2590–2595. doi: 10.1073/pnas.0409896102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao YQ, Piedras-Renteria ES, Smith GB, Chen G, Harata NC, Tsien RW. Presynaptic Ca2+ channels compete for channel type-preferring slots in altered neurotransmission arising from Ca2+ channelopathy. Neuron. 2005;43:387–400. doi: 10.1016/j.neuron.2004.07.014. [DOI] [PubMed] [Google Scholar]
- Catterall WA, Perez-Reyes E, Snutch TP, Striessnig J. International Union of Pharmacology. XLVIII. Nomenclature and structure-function relationships of voltage-gated calcium channels. Pharmacol. Rev. 2005;57:411–425. doi: 10.1124/pr.57.4.5. [DOI] [PubMed] [Google Scholar]
- Cornet V, Bichet D, Sandoz G, Marty I, Brocard J, Bourinet E, Mori Y, Villaz M, De Waard M. Multiple determinants in voltage-dependent P/Q calcium channels control their retention in the endoplasmic reticulum. Eur. J. Neurosci. 2002;16:883–895. doi: 10.1046/j.1460-9568.2002.02168.x. [DOI] [PubMed] [Google Scholar]
- Cuchillo-Ibanez I, Aldea M, Brocard J, Albillos A, Weiss N, Garcia AG, De Waard M. Inhibition of voltage-gated calcium channels by sequestration of beta subunits. Biochem. Biophys. Res. Commun. 2003;311:1000–1007. doi: 10.1016/j.bbrc.2003.10.102. [DOI] [PubMed] [Google Scholar]
- De Waard M, VES S, Pragnell M, Campbell KP. Identification of critical amino acids involved in alpha-beta interaction in voltage dependent Ca2+ channels. FEBS Lett. 1996;380 doi: 10.1016/0014-5793(96)00007-5. [DOI] [PubMed] [Google Scholar]
- Denier C, Ducros A, Durr A, Eymard B, Chassande B, Tournier-Lasserve E. Missense CACNA1A mutation causing episodic ataxia type 2. Arch. Neurol. 2001;58:292–295. doi: 10.1001/archneur.58.2.292. [DOI] [PubMed] [Google Scholar]
- Denier C, Ducros A, Vahedi K, Joutel A, Thierry P, Ritz A, Castelnovo G, Deonna T, Gerard P, Devoize JL, Gayou A, Perrouty B, Soisson T, Autret A, Warter JM, Vighetto A, Van Bogaert P, Alamowitch S, Roullet E, Tournier-Lasserve E. High prevalence of CACNA1A truncations and broader clinical spectrum in episodic ataxia type 2. Neurology. 1999;52:1816–1821. doi: 10.1212/wnl.52.9.1816. [DOI] [PubMed] [Google Scholar]
- Ertel EA, Campbell KP, Harpold MM, Hofmann F, Mori Y, Perez-Reyes E, Schwartz A, Snutch TP, Tanabe T, Birnbaumer L, Tsien RW, Catterall WA. Nomenclature of voltage-gated calcium channels. Neuron. 2000;25:533–535. doi: 10.1016/s0896-6273(00)81057-0. [DOI] [PubMed] [Google Scholar]
- Eunson LH, Graves TD, Hanna MG. New calcium channel mutations predict aberrant RNA splicing in episodic ataxia. Neurology. 2005;65:308–310. doi: 10.1212/01.wnl.0000169020.82223.dd. [DOI] [PubMed] [Google Scholar]
- Gao T, Chien AJ, Hosey MM. Complexes of the alpha1C and beta subunits generate the necessary signal for membrane targeting of class C L-type calcium channels. J. Biol. Chem. 1999;274:2137–2144. doi: 10.1074/jbc.274.4.2137. [DOI] [PubMed] [Google Scholar]
- Guida S, Trettel F, Pagnutti S, Mantuano E, Tottene A, Veneziano L, Fellin T, Spadaro M, Stauderman K, Williams M, Volsen S, Ophoff R, Frants R, Jodice C, Frontali M, Pietrobon D. Complete loss of P/Q calcium channel activity caused by CACNA1A missense mutation carried by patients with episodic ataxia type 2. AmJ. Hum. Genet. 2001;68:759–764. doi: 10.1086/318804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imbrici P, Eunson LH, Graves TD, Bhatia KP, Wadia NH, Kullmann DM, Hanna MG. Late-onset episodic ataxia type 2 due to an in-frame insertion in CACNA1A. Neurology. 2005;65:944–946. doi: 10.1212/01.wnl.0000176069.64200.28. [DOI] [PubMed] [Google Scholar]
- Imbrici P, Jaffe SL, Eunson LH, Davies NP, Herd C, Robertson R, Kullmann DM, Hanna MG. Dysfunction of the brain calcium channel CaV2.1 in absence epilepsy and episodic ataxia. Brain. 2004;127:2682–2692. doi: 10.1093/brain/awh301. [DOI] [PubMed] [Google Scholar]
- Jen J. Calcium channelopathies in the central nervous system. Curr Opin Neurobiol. 1999;9:274–280. doi: 10.1016/s0959-4388(99)80040-3. [DOI] [PubMed] [Google Scholar]
- Jen J. Familial Episodic Ataxias and Related Ion Channel Disorders. Curr Treat Options Neurol. 2000;2:429–431. doi: 10.1007/s11940-000-0041-y. [DOI] [PubMed] [Google Scholar]
- Jen J, Kim GW, Baloh RW. Clinical Spectrum of episodic ataxia type 2. Neurology. 2004;62:17–22. doi: 10.1212/01.wnl.0000101675.61074.50. [DOI] [PubMed] [Google Scholar]
- Jen J, Yue Q, Nelson SF, Yu H, Litt M, Nutt J, Baloh RW. A novel nonsense mutation in CACNA1A causes episodic ataxia and hemiplegia. Neurology. 1999;53:34–37. doi: 10.1212/wnl.53.1.34. [DOI] [PubMed] [Google Scholar]
- Jen J, Wan J, Graves M, Yu H, Mock AF, Coulin CJ, Kim G, Yue Q, Papazian DM, Baloh RW. Loss-of-function EA2 mutations are associated with impaired neuromuscular transmission. Neurology. 2001;57:1843–1848. doi: 10.1212/wnl.57.10.1843. [DOI] [PubMed] [Google Scholar]
- Jeng CJ, Chen YT, Chen YW, Tang CY. Dominant-Negative Effects of Human P/Q-type Ca2+ Channel Mutations Associated with Episodic Ataxia Type 2. Am. J. Physiol. Cell Physiol. 2006;290:1209–1220. doi: 10.1152/ajpcell.00247.2005. [DOI] [PubMed] [Google Scholar]
- Jouvenceau A, Eunson LH, Spauschus A, Ramesh V, Zuberi SM, Kullmann DM, Hanna MG. Human epilepsy associated with dysfunction of the brain P/Q-type calcium channel. Lancet. 2001;358:801–807. doi: 10.1016/S0140-6736(01)05971-2. [DOI] [PubMed] [Google Scholar]
- Kordasiewicz HB, Thompson RM, Clark HB, Gomez CM. Carboxyl termini of P/Q-type Ca2+ channel {alpha}1A subunits translocate to nuclei and promote polyglutmine-mediated toxicity. Hum. Mol. Genet. 2006;15:1587–1599. doi: 10.1093/hmg/ddl080. [DOI] [PubMed] [Google Scholar]
- Lacerda AE, Kim HS, Ruth P, Perez-Reyes E, Flockerzi V, Hofmann F, Birnbaumer L, Brown AM. Normalization of current kinetics by interaction between the alpha 1 and beta subunits of the skeletal muscle dihydropyridine-sensitive Ca2+ channel. Nature. 1991;352:527–530. doi: 10.1038/352527a0. [DOI] [PubMed] [Google Scholar]
- Maltez JM, Nunziato DA, Kim J, Pitt GS. Essential Ca(V)beta modulatory properties are AID-independent. Nat. Struct. Mol. Biol. 2005;12:372–377. doi: 10.1038/nsmb909. [DOI] [PubMed] [Google Scholar]
- Mantuano E, Veneziano L, Spadaro M, Giunti P, Guida S, MG L, Verriello L, Wood N, Jodice C, Frontali M. Clusters of non-truncating mutations of P/Q type Ca2+ channel subunit Ca(v)2.1 causing episodic ataxia 2. J. Med. Genet. 2004;41:1–6. doi: 10.1136/jmg.2003.015396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Moutot N, Charvin N, Leveque C, Satom K, Nishiki T, Kozaki S, Takahashi M, Seagar M. Interaction of SNARE complexes with P/Q-type calcium channels in rat cerebellar synaptosomes. J. Biol. Chem. 1996;271:6567–6570. doi: 10.1074/jbc.271.12.6567. [DOI] [PubMed] [Google Scholar]
- Mochida S, Sheng ZH, Baker C, Kobayashi H, Catterall WA. Inhibition of neurotransmission by peptides containing the synaptic protein interaction site of N-type Ca2+ channels. Neuron. 1996;17:781–788. doi: 10.1016/s0896-6273(00)80209-3. [DOI] [PubMed] [Google Scholar]
- Mochida S, Westenbroek RE, Yokoyama CT, Itoh K, Catterall WA. Subtype-selective reconstitution of synaptic transmission in sympathetic ganglion neurons by expression of exogenous calcium channels. Proc. Natl. Acad. Sci. USA. 2003a;100:2813–2818. doi: 10.1073/pnas.262787299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochida S, Westenbroek RE, Yokoyama CT, Zhong H, Myers SJ, Scheuer T, Itoh K, Catterall WA. Requirement for the synaptic protein interaction site for reconstitution of synaptic transmission by P/Q-type calcium channels. Proc. Natl. Acad. Sci. USA. 2003b;100:2819–2824. doi: 10.1073/pnas.262787699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ophoff RA, Terwindt GM, Vergouwe MN, van Eijk R, Oefner PJ, Hoffman SM, Lamerdin JE, Mohrenweiser HW, Bulman DE, Ferrari M, Haan J, Lindhout D, van Ommen GJ, Hofker MH, Ferrari MD, Frants RR. Familial hemiplegic migraine and episodic ataxia type-2 are caused by mutations in the Ca2+ channel gene CACNL1A4. Cell. 1996;87:543–552. doi: 10.1016/s0092-8674(00)81373-2. [DOI] [PubMed] [Google Scholar]
- Page KM, Heblich F, Davies A, Butcher AJ, Leroy J, Bertaso F, Pratt WS, Dolphin AC. Dominant-negative calcium channel suppression by truncated constructs involves a kinase implicated in the unfolded protein response. J. Neurosci. 2004;24:5400–5409. doi: 10.1523/JNEUROSCI.0553-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piedras-Renteria ES, Watase K, Harata N, Zhuchenko O, Zoghbi HY, Lee CC, Tsien RW. Increased expression of alpha 1A Ca2+ channel currents arising from expanded trinucleotide repeats in spinocerebellar ataxia type 6. J. Neurosci. 2001;21:9185–9193. doi: 10.1523/JNEUROSCI.21-23-09185.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pragnell M, De Waard M, Mori Y, Tanabe T, Snutch TP, Campbell KP. Calcium channel beta-subunit binds to a conserved motif in the I–II cytoplasmic linker of the alpha 1-subunit. Nature. 1994;368:67–70. doi: 10.1038/368067a0. [DOI] [PubMed] [Google Scholar]
- Ptacek LJ. The place of migraine as a channelopathy. Curr Opin Neurol. 1998;11:217–226. doi: 10.1097/00019052-199806000-00005. [DOI] [PubMed] [Google Scholar]
- Ptacek LJ. Ion channel diseases: episodic disorders of the nervous system. Semin Neurol. 1999;19:363–369. [PubMed] [Google Scholar]
- Raghib A, Bertaso F, Davies A, Page KM, Meir A, Bogdanov Y, Dolphin AC. Dominant-negative suppression of voltage-gated calcium channel Cav2.2 induced by truncated constructs. J Neurosci. 2001;21:8495–8504. doi: 10.1523/JNEUROSCI.21-21-08495.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Restituito S, Thompson R, Eliet J, Raike R, Riedle M, Charnet P, Gomez CM. The polyglutamine expansion in spinocerebellar ataxia type 6 causes a beta subunit-specific enhanced activation of P/Q-type calcium channels in Xenopus oocytes. J. Neurosci. 2000;20:6394–6403. doi: 10.1523/JNEUROSCI.20-17-06394.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock DM, Horne WA, Stoehr SJ, Hashimoto C, Zhou M, Cong R, Palma A, Hidayetoglu D, Offord J. Does alpha1E code for T-type calcium channels? A comparison of recombinant alpha1E calcium channels with GH3 pituitary T-type and recombinant alpha1B calcium channels. In: Tsien RW, Clozel JP, Nargeot J, editors. Low-voltage-activated T-type calcium channels. Adis International; 1989. pp. 279–289. [Google Scholar]
- Scoggan KA, Friedman JH, Bulman DE. CACNA1A mutation in a EA-2 patient responsive to acetazolamide and valproic acid. CanJ. Neurol. Sci. 2006;33:68–72. doi: 10.1017/s0317167100004728. [DOI] [PubMed] [Google Scholar]
- Singer D, Biel M, Lotan I, Flockerzi V, Hofmann F, Dascal N. The roles of the subunits in the function of the calcium channel. Science. 1991;253:1553–1557. doi: 10.1126/science.1716787. [DOI] [PubMed] [Google Scholar]
- Spacey SD, Materek LA, Szczygielski BI, Bird TD. Two novel CACNA1A gene mutations associated with episodic ataxia type 2 and interictal dystonia. Arch. Neurol. 2005;62:314–316. doi: 10.1001/archneur.62.2.314. [DOI] [PubMed] [Google Scholar]
- Spacey SD, Hildebrand ME, Materek LA, Bird TD, Snutch TP. Functional implications of a novel EA2 mutation in the P/Q-type calcium channel. Ann. Neurol. 2004;56:213–220. doi: 10.1002/ana.20169. [DOI] [PubMed] [Google Scholar]
- Stea A, Tomlinson WJ, Soong TW, Bourinet E, Dubel SJ, Vincent SR, Snutch TP. Localization and functional properties of a rat brain alpha 1A calcium channel reflect similarities to neuronal Q- and P-type channels. Proc. Natl. Acad. Sci. USA. 1994;91:10576–10580. doi: 10.1073/pnas.91.22.10576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramony SH, Schott K, Raike RS, Callahan J, Langford LR, Christova PS, Anderson JH, Gomez CM. Novel CACNA1A mutation causes febrile episodic ataxia with interictal cerebellar deficits. Annal. Neurol. 2003;54:725–731. doi: 10.1002/ana.10756. [DOI] [PubMed] [Google Scholar]
- Swensen AM, Bean BP. Ionic mechanisms of burst firing in dissociated Purkinje neurons. J. Neurosci. 2003;23:9650–9663. doi: 10.1523/JNEUROSCI.23-29-09650.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usowicz MM, Sugimori M, Cherksey B, Llinas R. P-type calcium channels in the somata and dendrites of adult cerebellar Purkinje cells. Neuron. 1992;9:1185–1199. doi: 10.1016/0896-6273(92)90076-p. [DOI] [PubMed] [Google Scholar]
- van den Maagdenberg AM, Kors EE, Brunt ER, van Paesschen W, Pascual J, Ravine D, Keeling S, Vanmolkot KR, Vermeulen FL, Terwindt GM, Haan J, Frants RR, Ferrari MD. Episodic ataxia type 2:Three novel truncating mutations and one novel missense mutation in the CACNA1A gene. J. Neurol. 2002;249:1515–1519. doi: 10.1007/s00415-002-0860-8. [DOI] [PubMed] [Google Scholar]
- Walker D, Bichet D, Campbell KP, De Waard M. A beta 4 isoform-specific interaction site in the carboxyl-terminal region of the voltage-dependent Ca2+ channel alpha 1A subunit. J. Biol. Chem. 1998;273:2361–2367. doi: 10.1074/jbc.273.4.2361. [DOI] [PubMed] [Google Scholar]
- Walker D, Bichet D, Geib S, Mori E, Cornet V, Snutch TP, Mori Y, De Waard M. A new beta subtype-specific interaction in alpha1a subunit controls P/Q-type Ca2+ channel activation. J. Biol. Chem. 1999;274:12383–12390. doi: 10.1074/jbc.274.18.12383. [DOI] [PubMed] [Google Scholar]
- Walter JT, Alvina K, Womack MD, Chevez C, Khodakhah K. Decreases in the precision of Purkinje cell pacemaking cause cerebellar dysfunction and ataxia. Nat. Neurosci. 2006;9:389–397. doi: 10.1038/nn1648. [DOI] [PubMed] [Google Scholar]
- Wan J, Carr JR, Baloh RW, Jen JC. Nonconsensus intronic mutations cause episodic ataxia. Ann. Neurol. 2005a;57:131–135. doi: 10.1002/ana.20343. [DOI] [PubMed] [Google Scholar]
- Wan J, Khanna R, Sandusky M, Papazian DM, Jen JC, Baloh RW. CACNA1A mutations causing episodic and progressive ataxia alter channel trafficking and kinetics. Neurology. 2005b;64:2090–2097. doi: 10.1212/01.WNL.0000167409.59089.C0. [DOI] [PubMed] [Google Scholar]
- Wappl E, Koschak A, Poteser M, Sinnegger MJ, Walter D, Eberhart A, Groschner K, Glossmann H, Kraus RL, Grabner M, Striessnig J. Functional consequences of P/Q-type Ca2+ channel Cav2.1 missense mutations associated with episodic ataxia type 2 and progressive ataxia. J. Biol. Chem. 2002;277:6960–6966. doi: 10.1074/jbc.M110948200. [DOI] [PubMed] [Google Scholar]
- Westenbroek RE, Sakurai T, Elliott EM, Hell JW, Starr TV, Snutch TP, Catterall WA. Immunochemical identification and subcellular distribution of the alpha 1A subunits of brain calcium channels. J. Neurosci. 1995;15:6403–6418. doi: 10.1523/JNEUROSCI.15-10-06403.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittemann S, Mark MD, Rettig J, Herlitze S. Synaptic localization and presynaptic function of calcium channel beta 4-subunits in cultured hippocampal neurons. J. Biol. Chem. 275:37807–37814. doi: 10.1074/jbc.M004653200. 200. [DOI] [PubMed] [Google Scholar]
- Womack MD, Khodakhah K. Dendritic control of spontaneous bursting in cerebellar Purkinje cells. J. Neurosci. 2004;24:3511–3521. doi: 10.1523/JNEUROSCI.0290-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue Q, Jen JC, Thwe MM, Nelson SF, Baloh RW. De novo mutation in CACNA1A caused acetazolamide-responsive episodic ataxia. Am. J. Med. Genet. 1998;77:298–301. doi: 10.1002/(sici)1096-8628(19980526)77:4<298::aid-ajmg9>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]