Abstract
The present study is designed to assess if exosomes released from Chronic Myelogenous Leukemia (CML) cells may modulate angiogenesis. We have isolated and characterized the exosomes generated from LAMA84 CML cells and demonstrated that addition of exosomes to human vascular endothelial cells (HUVEC) induces an increase of both ICAM-1 and VCAM-1 cell adhesion molecules and interleukin-8 expression. The stimulation of cell-cell adhesion molecules was paralleled by a dose-dependent increase of adhesion of CML cells to a HUVEC monolayer. We further showed that the treatment with exosomes from CML cells caused an increase in endothelial cell motility accompanied by a loss of VE-cadherin and β-catenin from the endothelial cell surface. Functional characterization of exosomes isolated from CML patients confirmed the data obtained with exosomes derived from CML cell line. CML exosomes caused reorganization into tubes of HUVEC cells cultured on Matrigel. When added to Matrigel plugs in vivo, exosomes induced ingrowth of murine endothelial cells and vascularization of the Matrigel plugs. Our results suggest for the first time that exosomes released from CML cells directly affect endothelial cells modulating the process of neovascularization.
Keywords: Exosomes, Chronic Myelogenous Leukemia Cells, Endothelial cells, Tumor Microenvironment
Introduction
Chronic myelogenous leukemia (CML) is a myeloproliferative disorder in which leukemic cells display the Philadelphia (Ph) chromosome generated from a reciprocal t(9:22) (q34:q11) translocation1. This translocation results in the expression of the chimeric Bcr–Abl oncoprotein with constitutive tyrosine kinase activity that, in turn, stimulates a number of downstream signaling cascades2. As a consequence, activated Bcr-Abl leads to increased proliferation, altered cell adhesion, and inhibition of apoptosis3. Bcr-Abl has also been involved in VEGF-mediated angiogenesis in CML4 and evidence indicates that the formation of new vessels plays an important role in the development and progression of CML5, 6.
Exosomes are small vesicles of 40–100 nm diameter that are initially formed within the endosomal compartment and are secreted when a multivesicular body (MVB) fuses with the plasma membrane7. These vesicles are released by many cell types including cancer cells and are considered messengers in intercellular communication8. Biochemical and proteomic analysis of exosomes revealed that these vesicles contain, besides a common set of membrane and cytosolic molecules, cell-type specific proteins that characterize their functional activity9. The exact function of exosomes in malignant cells has yet to be elucidated, but investigation has suggested roles in cell-to-cell communication, tumor-stroma interaction, and antigen presentation, thus potentially affecting cancer progression at different steps10. Exosomes have been shown to interact with endothelial cells11, 12. Although production of exosomes by K562 CML cells13, 14 has been reported, little is known regarding the role of these vesicles in CML biology. Working from these assumptions, the aim of our work was to investigate the role of exosomes released by CML cells in the angiogenic process. Our results indicate LAMA84, a human CML cell line, releases exosomes and that the addition of those microvesicles to human vascular endothelial cells (HUVEC) affects several steps of in vitro angiogenesis including motility, cytokine production, cell adhesion, and cell signalling, as well as stimulation of angiogenesis in a nude mouse assay. Finally, application of exosomes isolated from blood of CML patients confirmed the data obtained with exosomes, derived from LAMA84 cells, suggesting a critical role of exosomes in angiogensis.
Material and Methods
Cell culture, reagents and treatments
HUVEC were obtained from Lonza (Clonetics, Verviers, Belgium) and grown in endothelial growth medium (EGM) according to supplier’s information. LAMA84, chronic myelogenous leukemia, cells were cultured as previously described15. All other reagents were purchased from Sigma (St. Louis, MO, USA), if not otherwise cited.
PBMC isolation
Human blood samples were obtained from healthy donors, after written informed consent was obtained, in accordance with the Declaration of Helsinki guidelines and University of Palermo Ethics committee. Human peripheral blood mononuclear cells (PBMC) were isolated using the Ficoll-Paque (GE Helthcare-Bio Science, Uppsala, Sweden) separation technique.
Exosome isolation and characterization
Exosomes produced by LAMA84 CML cells during a 24h culture period, were isolated from conditioned culture medium supplemented with 10% FBS (previously ultracentrifuged) by differential centrifugation as described by Thery and colleagues16. Exosome protein content was determined by the Bradford method. On average, we obtained 100 µg of exosomes/40ml of LAMA84 conditioned medium similar to the amount recovered from other CML cell lines such as K562 cells13. The activity of acetylcholinesterase, an exosome marker protein, was determined as described by Savina et al13. To further verify the identity of vesicles as exosomes, we isolated exosomes on a 30% sucrose/D2O cushion as described by Lamparski and colleagues17. Vesicles contained in the cushion were recovered, washed several times, ultracentrifuged for 90 min in PBS and collected for use.
Exosomes were next examined by scanning electron microscopy analysis. They were fixed with 2% glutaraldehyde in PBS for 10 min, attached onto stubs, coated with gold in a sputterer (Sputter Coater 150A, Edwards, UK) and observed using a field emission scanning electron microscope (FEG-ESEM QUANTA 200 FEI, USA) at working voltage 30 kV.
Patients
Blood samples were obtained from two newly diagnosed CML patients. Informed consent was obtained from patients, according to the Declaration of Helsinki and with hospital Ethics Committee approval. Whole blood samples were treated with red blood cell lysing buffer (Sigma, St. Louis, MO) for 2 min at room temperature, then centrifuged at 350g for 7 min to recover and discard lysed red cells. The interphase layer containing CML cells was collected, resuspended in PBS and lysated for controls. Exosomes released in fresh patient’s plasma were prepared as described in the previous paragraph.
Flow cytometry
Expression of HUVEC cell surface VCAM-1 was determined by flow cytometry analysis. HUVEC were treated with or without 50 µg/ml of LAMA 84-exosomes in low serum medium (EGM:RPMI, 1:9). 500,000 cells were washed in PBS and incubated with 0.5 µg VCAM-1-FITC (Santa Cruz Biotechnology, Santa Cruz, CA, USA) for 15 min a t 4° C according to manufacturer’s recommendations. Viable cells were gated by forward and side scatter and the analysis was performed on 100,000 acquired events for each sample. Samples were analyzed on a FACS Calibur with the use of the CellQuest software (BD Biosciences).
Western blot and immunoprecipitation assay
Total cell or exosome lysates were subjected to SDS-PAGE electrophoresis and immunoblot as previously described15.
Antibodies used in the experiments were: HSC70, CD63 and VCAM-1 (Santa Cruz Biotechnology, Santa Cruz, CA, USA), actin18, MAPK and phosphoMAPK (Cell Signaling Technology, Beverly, MA). Five million HUVEC were incubated with 50 µg/ml of LAMA 84 exosomes for 6h or with 10 ng/ml TNF α for 2h (positive control) or with low serum medium for 6h (negative control) and processed for immunoprecipitation experiments using precleared lysates as previously described19. Samples were resolved in 8% SDS-PAGE followed by immunoblotting with anti-VCAM1. Aliquots of the precleared cell lysates were resolved independently by 8% SDS-PAGE and examined for actin quantity as a surrogate of IP input (named as starting material or St).
Immunofluorescence and cell cytoskeleton analysis
HUVEC monolayers were grown to confluence on coverslips coated with type I collagen (Calbiochem, Darmstadt, Germany) and were treated with increasing doses of CML exosomes or low serum medium for 6h. After incubation, cells were processed as previously described20. Antibodies used in the experiments were: VE cadherin and β-catenin (1:100; Santa Cruz Biotechnology, Santa Cruz CA, USA). Cells were stained with Texas Red-conjugated secondary anti-mouse antibodies (1:100; Molecular Probe, Eugene, Oregon USA) and analyzed by confocal microscopy (Olympus 1X70 with Melles Griot laser system). Analyses of the actin cytoskeleton were performed as described20. The semi-quantitative analysis of fluorescence intensity was performed using IMAGE-J software (http://rsbweb.nih.gov/ij/)21
RNA extraction and real-time PCR
HUVEC cells were grown to confluence in 6-well plates and incubated for different times with the indicated stimuli. IL-8, VCAM-1, ICAM-1 transcript levels were measured by reverse transcription (RT) and TaqMan real-time quantitative polymerase chain reaction (RT-PCR) and analyzed as described20. The following primers were used: IL-8, HS00174103_m1; VCAM-1, HS 00174239 m1; ICAM-1, HS 00277001_m1; and, GAPDH, Hs99999905 m1 (Applied Biosystems, Foster City, CA, USA). GAPDH was used as the internal control.
Motility assays
Migration assays were performed following two standard protocols, Transwell chemotaxis chambers (NeuroProbe Inc., Cabin John, MD, USA)15 and wound repair assay. Briefly, LAMA84 cells (2×106/ml) were suspended in serum-free RPMI 1640 medium supplemented with 0.1% BSA in transwell chemotaxis above 8 µm pore filters, and exposed to chemoattractants with increased amount of exosomes (10–20–50 µg/ml), 10 ng/ml of recombinant IL8 (Sigma, St. Louis, MO, USA), or neutralizing antibodies anti IL8 (5 µg/ml) (R&D system, Minneapolis, MN) as indicated. Filters were removed after 6h, fixed in ethanol and stained with Diff-Quick (Medion Diagnostics GmbH, Düdingen, Switzerland). Each cell line was tested in three independent experiments; the number of migrating cells in five high-power fields per well were counted at 400X magnification.
For the wound healing assay, a wound was created by manually scraping the confluent endothelial cell monolayer with a p1000 pipette tip. After washing with PBS, cells were incubated for 3h with medium containing exosomes or control medium without exosomes. Images of cell-free spaces were taken with a digital camera at the indicated times and measured manually with the IMAGE-J software (http://rsbweb.nih.gov/ij/)21. The data are reported as the percentage of the distance migrated relative to the control cultures for each experiment.
Adhesion assay
Adhesion assays were performed as previously described19. Briefly, HUVEC monolayer was incubated for 6h with indicated conditions. After treatment, cells were washed with PBS and CML cells were added for 1h at 37°C. Adherent cells were stained with hematoxylin/eosin, each test group was assayed in triplicate; five high power (400X) fields were counted for each condition.
ELISA
HUVEC conditioned medium (CM) was collected from cells stimulated for 6h with indicated treatments. CM aliquots were centrifuged to remove cellular debris and IL-8 protein concentrations were quantified using an ELISA kit (R&D Systems, Minneapolis, USA), according to the manufacturer’s protocol. IL8 was also measured directly in LAMA84 exosomes.
HUVEC tube formation on Matrigel
Matrigel was used to test the effects of exosomes on in vitro vascular tube formation as described22. Exosomes were added to HUVEC plated on Matrigel in endothelial basal medium containing 0.2% of FBS as indicated. Cells were incubated for 6h and then evaluated by phase-contrast microscopy and photographed. The length of the cables was measured manually with the IMAGE-J software (http://rsbweb.nih.gov/ij/)21.
Matrigel plug assay
All animal experiments were conducted in full compliance with University of Palermo and Italian Legislation for Animal Care. Four week old BALB/c nude mice (Charles River Laboratories International, Inc, Wilmington, MA) were injected subcutaneously with 400 µL Matrigel (BD Biosciences Pharmingen, San Diego, CA) containing 100 µg LAMA84-derived exosomes with or without 10 µg/ml non specific anti-actin antibody or anti-IL-8 neutralizing antibody or PBS (negative control). The degree of vascularization was evaluated by determination of hemoglobin content using the Drabkin method (Drabkin’s reagent kit)23
Statistics
Data were expressed as means ± SEMs of the indicated number of experiments. Statistical analysis was performed by using a unpaired Student’s t test. Differences were considered to be significant when P values were < than 0.05.
Results
Exosome vesiscles released by LAMA84 cells
We examined the ability of LAMA84 cells to release exosomes into the culture medium during a 24h period. Scanning electron microscope analysis revealed that the isolated vesicles consisted of membrane particles (figure 1a) with an average diameter of 70 nm ± 10 in agreement with that described by other groups in different cell lines24.
Figure 1. LAMA84 exosome characterization.
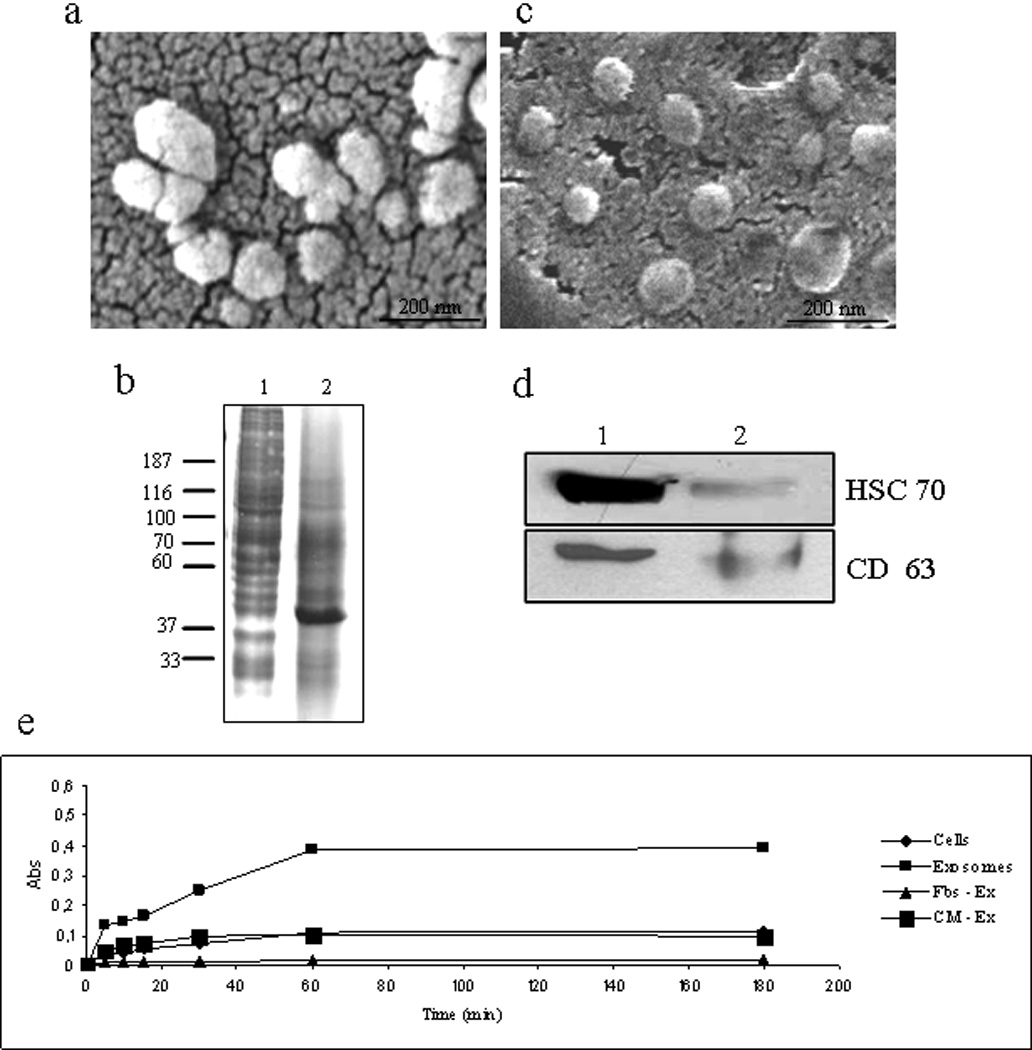
a Exosomes released by LAMA84 cells observed by scanning electron microscopy. b: Coomassie Blue staining of 30 µg of cell lysate (lane 1) and exosome lysate (lane 2) separated on 8% SDS gel. c: Exosomes released by LAMA84 cells and purified on a 30% sucrose/D2O cushion observed by scanning electron microscopy d: Detection of Hsc 70 and CD63 in 30 µg of exosomes purified after ultracentrifugation on 30% sucrose/D2O gradient (lane 1) and 30 µg of cell lysate (lane 2). e: Acetylcholinesterase assay. The activity of acetylcholinesterase, an exosome-specific protein marker, was determined in exosomes (10 µg) (▪), total cell lysates (10 µg) (♦), exosome-deprived Fbs (▲) and exosome-deprived conditioned medium (CM) (■) as negative control.
A comparison of the protein profiles of cell lysates and exosomes revealed that lysates of CML cells were different from those of exosomes (figure 1b). Vesicles secreted by CML cells were also purified on a sucrose gradient and analysed by scanning electron microscope (figure 1c) and Western blotting using antibodies specific for HSC 70 and CD63. These proteins were detected in cell lysates and found more expressed in exosome fractions (fig 1d). Acetylcholinesterase activity, a characteristic enzyme localized in exosomes, was found associated with the exosome fraction while negligible amounts were found in conditioned medium deprived of exosomes (Fig.1e).
Exosomes purified from whole blood of 2 patients with chronic myelogenous leukemia displayed same properties of vesicles isolated from LAMA84 cells (Fig. S1)
CML vesicles modulate IL-8 expression by HUVEC
Cytokine expression was evaluated by RT PCR analysis to determine the potential effects of CML exosomes on induction of genes promoting tumor growth and angiogenesis. We evaluated IL-8, IL-6, VEGF, and TGF-β since these cytokines are induced in endothelial cells by several stimuli and are also involved in the angiogenic process. Figure 2a shows that LAMA84-derived exosomes added to HUVEC monolayer caused both a dose- and time-dependent increase in IL-8 transcript quantity. Increased mRNA production was statistically significant and reached approximately a 20-fold induction after 6h of stimulation of the endothelial monolayer with 50 µg/ml of vesicles. The negative control, exosome-deprived medium (CM-ex) did not significantly induce mediator gene expression in the endothelial monolayer. Measurement of IL-8 release into HUVEC conditioned medium confirmed the augmented synthesis of IL-8 in exosome-stimulated endothelial cells (Fig. S2). IL-8 was also found in LAMA84 exosomes (~ 300pg/50 µg exosomes) and no significant induction of IL-8 release was observed when HUVEC were stimulated with exosomes purified from PBMC (Fig. S2). No effect was observed on IL-6, VEGF or TGF-β (data not shown).
Figure 2. Exosome treatment modulates IL8, VCAM1 and ICAM1 mRNA expression in HUVEC cells.
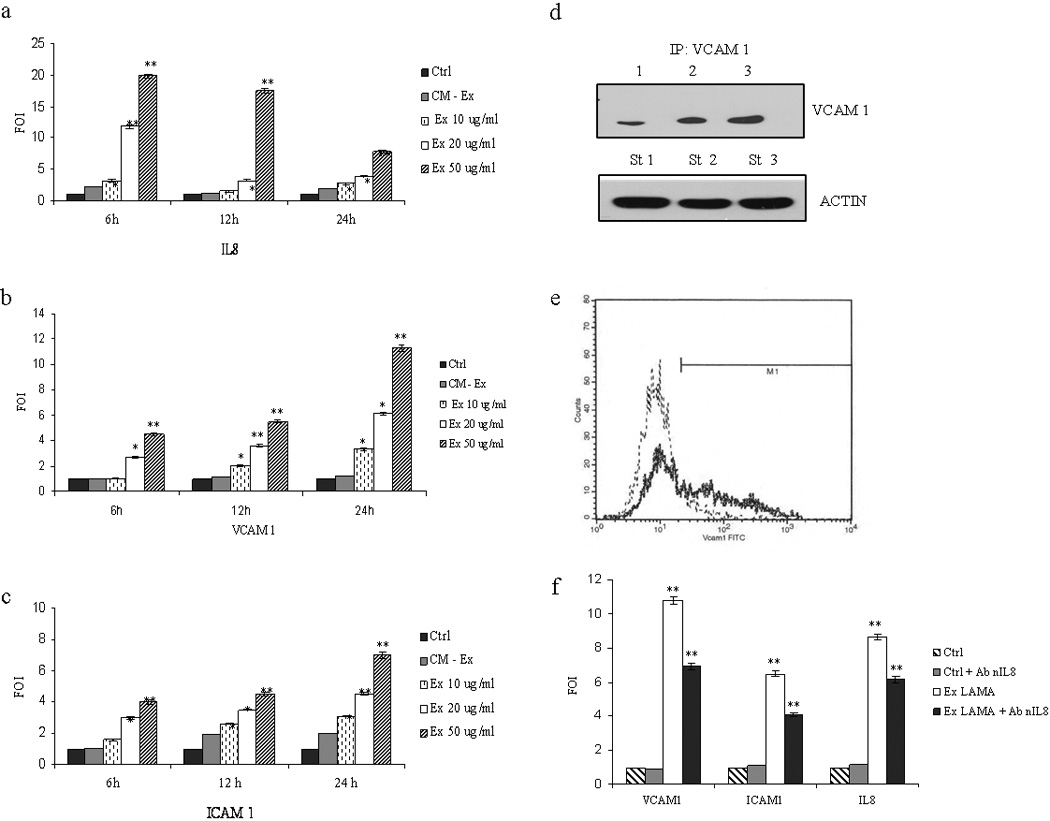
IL8 (a), VCAM1 (b) and ICAM1 (c) mRNA expression increased in a time- and dose-dependent (10, 20, 50 µg/ml) manner after addition of exosomes to endothelial cell monolayer. Exosome-deprived conditioned medium (CM-Ex) and low-serum medium were used as negative controls d: Immunoprecipitation assay with anti-VCAM1 antibody; top panel: HUVEC were incubated for 6h with low-serum medium (lane 1); 50 µg/ml LAMA84 exosomes (lane 2) or 10 ng/ml TNFα (lane 3); results indicate an increased amount of VCAM-1 in exosome-treated cells. Bottom panel: western blot anti-actin performed, as a loading control, on cell lysates before the immunoprecipitation step (starting material, St) e: Representative overlay histogram showing an increase of surface expression of VCAM 1 on HUVEC treated with 50 µg/ml of LAMA84 exosomes (solid line) compared untreated HUVEC, as control (dot line). f: VCAM1, ICAM1 and IL8 mRNA expression in HUVEC treated for 12h with low serum medium (Ctrl), Ctrl plus 10 µg/ml of a neutralizing anti-IL8 antibody, 50 µg/ml exosomes or 50 µg/ml exosomes plus 10 µg/ml of a neutralizing anti-IL8 antibody. Values are representative of three independent experiments. *p ≤ 0.05; **p ≤ 0.01.
Exosomes treatment of HUVECs induces cell-cell adhesion molecules
VCAM-1 and ICAM-1 are expressed by endothelial cells following activation by cytokines released during the inflammatory process25. HUVECs were treated with different amount of exosomes or controls to determine whether LAMA84 exosomes affect cell-cell adhesion molecules mRNA expression. As shown in figures 2b and 2c, respectively, addition of increasing doses of exosomes to the endothelial monolayer caused a dose- and time- dependent increase in VCAM-1 and ICAM-1 mRNA expression (p ≤ 0.01.). More specifically, exosome stimulation for 24h caused a 13- and 6-fold increase in VCAM-1 and ICAM-1 mRNA expression. Figure 2d shows that incubation of HUVEC with increasing doses of LAMA84 exosomes or TNF-α, used as positive control, induced an increase of VCAM-1 protein levels. FACS analysis confirmed that incubation of HUVEC with LAMA84 exosomes resulted in the detection of VCAM-1 on the surface of HUVEC (Fig. 2e); immunoprecipitation and western blotting assays showed that VCAM-1 was undetectable in LAMA84 exosomes (data not shown) and flow cytometry analysis of latex bead-coupled exosomes confirmed the absence of VCAM-1 on membrane particles (Fig. S3). The neutralization of IL-8 inhibited exosome-stimulated increase of ICAM-1 and VCAM-1adhesion molecules (Fig. 2f; p ≤ 0.01).
Stimulated binding of CML cells to HUVEC monolayer
A hallmark feature of leukemia progression is the adhesion of cancer cells to endothelial cells for extramedullary infiltration. We therefore tested the ability of leukemia cells to adhere to an endothelial monolayer to investigate functional effects of the observed increase of ICAM-1 and VCAM-1 expression in exosome-treated HUVECs. Figure 3a shows a dose-dependent increase in leukemia cell adhesion to HUVEC after 6h treatments; figure 3b shows the increase in adhesion of LAMA84 cells (arrows) to HUVEC monolayer after a 6h treatment with 50 µg/ml of exosomes. Figure 3c shows that addition of recombinant IL-8 to endothelial cells causes an increase of CML cells adhesion to HUVEC monolayer similar to that produced by LAMA84 or CML patient exosomes; on the contrary treatment of HUVEC with LAMA84 exosomes plus anti IL-8 neutralizing antibodies or with PBMC exosomes didn’t increase the adhesion of leukemia cells to the endothelial cells.
Figure 3. Adhesion of LAMA84 cells to exosome-treated HUVEC monolayer.

a: Adhesion of LAMA84 cells to endothelial cell monolayer treated for 6h with different amount of LAMA84 exosomes or with EGM, used as positive control. b: Cells (arrows) treated as in a and observed at contrast phase microscopy. c: Adhesion of LAMA84 cells to HUVEC treated with 50 µg/ml of LAMA84 exosomes, 50 µg/ml of exosomes plus antibodies anti actin (5 µg/ml), 10 ng/ml of recombinant IL8, 50 µg/ml of CML patients exosomes, EGM (as positive control), 50 µg/ml of exosomes plus neutralizing antibodies anti IL8 (5 µg/ml), 50 µg/ml of PBMC-exosomes in low serum medium and low serum medium (as negative control). Values are the mean ± SD of 5 fields in three independent experiments CTRL: control. *p ≤ 0.05; **p ≤ 0.01.
CML exosomes promote migration of endothelial cells
Cell migration is critical for many physiologic processes including angiogenesis. Confluent, scrape-wounded endothelial cell monolayers were incubated with various concentrations of CML vesicles, and the percentage of closure was observed after 3h. Figure 4a shows that endothelial cell migration was significantly increased in exosome-treated cultures but not in the control medium. As positive control, EGM-treated cells migrated into the denuded area, almost completely covering the exposed surface after 3h. Measurement of wounded area evidenced, compared to control, a 55% percentage of closure when endothelial cells were treated with the dose of 50 µg/ml of exosomes (fig. 4b). We further analyzed the effects of exosomes on cell migration by Boyden chamber assay. Figure 4c shows that addition of a range of concentrations of vesicles (10–50 µg) to the bottom wells of the chamber caused, after 6h, a dose-dependent increase of CML cell migration. A similar, statistically significant, effect in the stimulation of endothelial cell migration was obtained when recombinant IL-8, CML patients exosomes were added as chemoattractant in the Boyden assay (fig 4d); on the contrary the presence of anti IL-8 neutralizing antibodies or PBMC exosomes in the bottom wells of boyden chamber didn’t increase the motility of leukemia cells (fig 4d).
Figure 4. LAMA84 exosomes promote HUVEC migration.
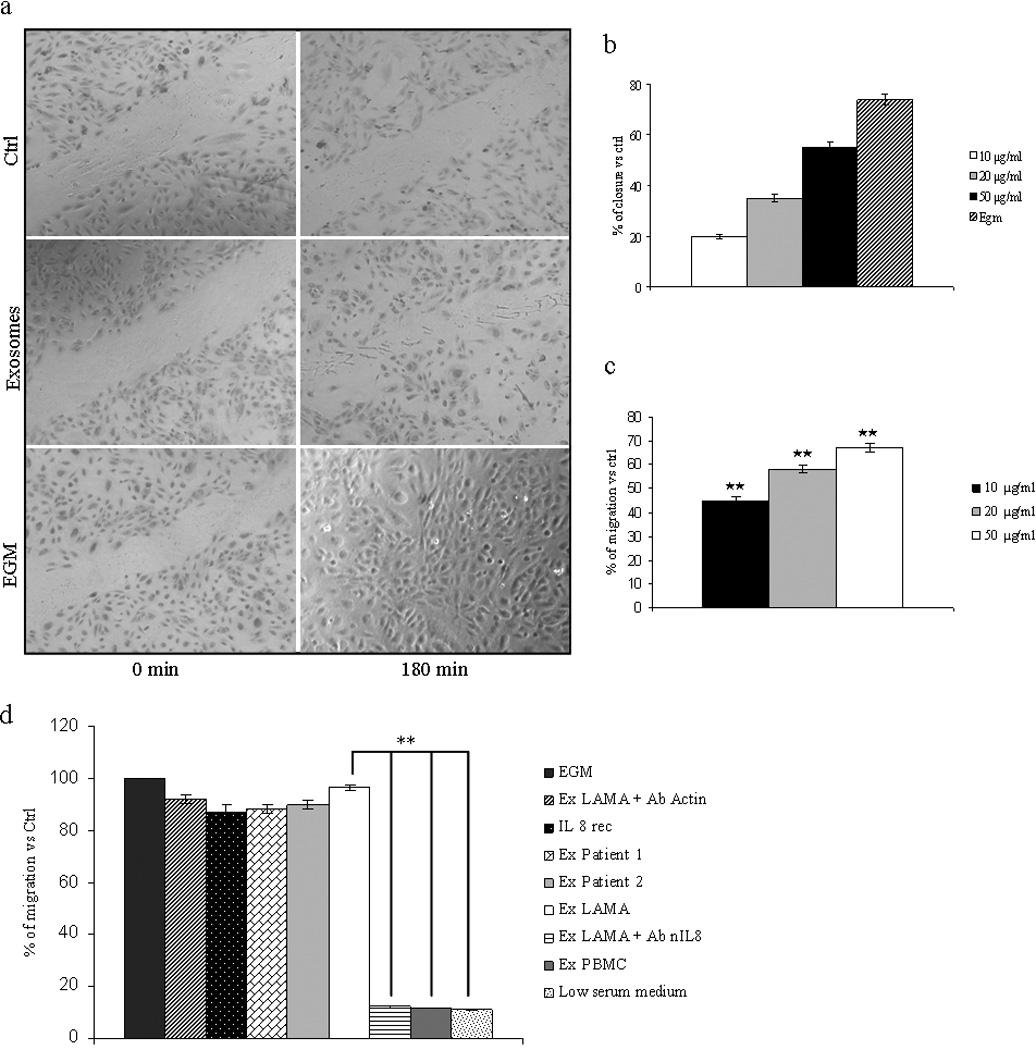
a: Confluent, scrape-wounded endothelial cell monolayer incubated with low serum medium (negative control), 50 µg/ml of LAMA84 exosomes, and EGM medium (positive control), for 3h. b: Percentage of closure of the wounded area measured after addition of different amount of exosomes. c: Effects of exosomes on endothelial cell migration as measured by Boyden chamber assay. Addition of exosomes (10, 20, 50 µg/ml) for 6h to the bottom wells of the chamber induced a dose-dependent increase of HUVEC migration. Values are the mean ± SD of 3 fields in three independent experiments *p ≤ 0.05; **p ≤ 0.01. d: 50 µg/ml of LAMA84 exosomes, 50 µg/ml of LAMA84 exosomes plus antibodies anti actin (5 µg/ml), 10 ng/ml of recombinant IL8, 50 µg/ml of CML patients exosomes, EGM (as positive control), 50 µg/ml of LAMA84 exosomes plus neutralizing antibodies anti IL8 (5 µg/ml), 50 µg/ml of PBMC-exosomes in low serum medium and low serum medium (as negative control) were added as chemoattractants to the bottom wells. Values are the mean ± SD of 3 fields in three independent experiments *p ≤ 0.05; **p ≤ 0.01.
Exosome treatment alters VE-cadherin and β-catenin localization
We determined the effects of exosomes on expression of VE-cadherin, an endothelial specific transmembrane adhesion molecule to investigate if alteration of cell junctional components could be responsible for the increase in cell motility. Control cells had continuous peripheral VE-cadherin staining (fig. 5a). VE-cadherin staining decreased in intensity and became patchy at the membrane concomitant with the appearance of a granular cytoplasmic staining in HUVEC treated with 50 µg/ml of LAMA84-exosomes (fig. 5a, arrows). Immunolocalization of β-catenin a protein known to interact with VE-cadherin, revealed a reduction of membrane immunostaining in the treated HUVEC monolayer compared to control cells after a 6h incubation (fig 5b). Treatment of the HUVEC monolayer with exosomes caused a translocation of β–catenin from the plasma membrane to the cytoplasm and nucleus (fig 5c). The images shown in figures 5b (sections at 2 µm from the surface) and 5c (sections at 4 µm from the surface) were the cells acquired at different confocal planes. Semi-quantitative analysis of fluorescence intensity confirmed both VE-cadherin delocalization and β-catenin translocation (Figure 5e, f). Furthermore, staining of actin filaments with r hodamine-conjugated phalloidin confirmed the alteration of endothelial integrity when exosomes are added to HUVEC monolayer (fig 5d).
Figure 5. Alteration of HUVEC monolayer after addition of LAMA84 exosomes.
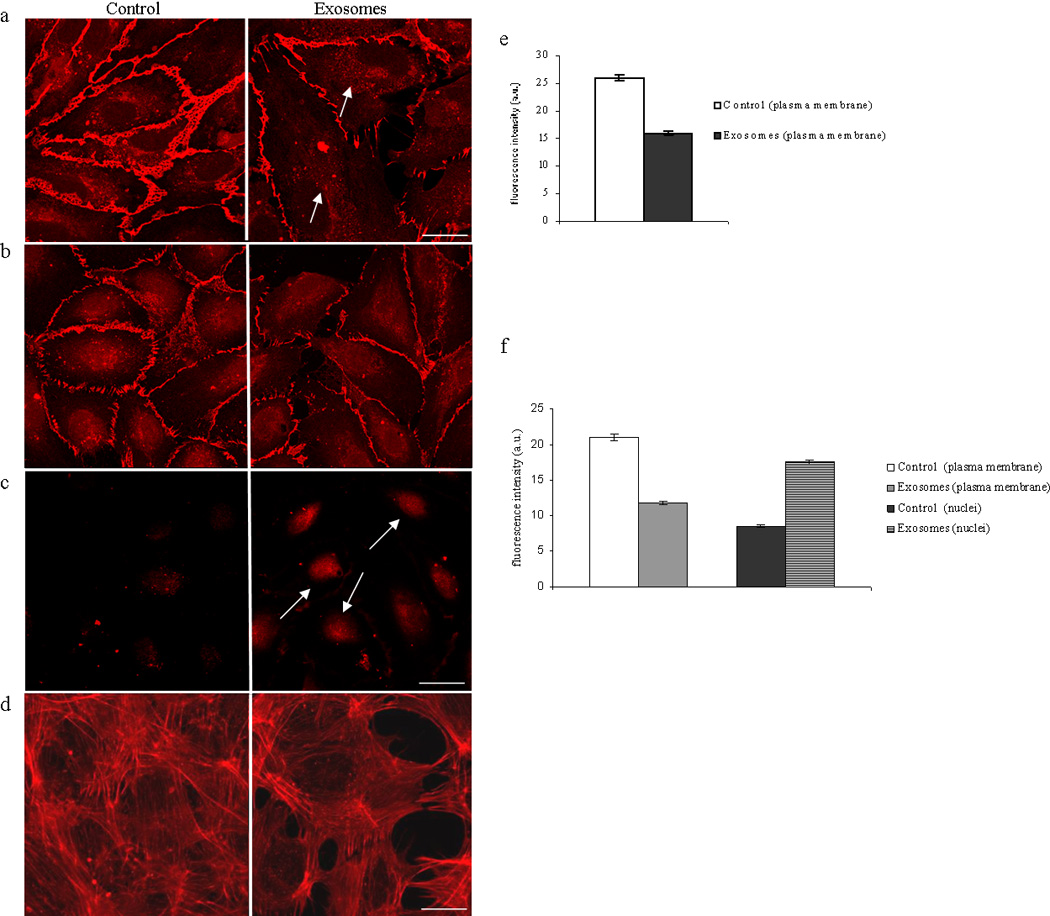
a: Analysis at confocal microscopy of VE Cadherin localization in HUVEC cells treated with LAMA84 exosomes revealed a decrease of immunostaining compared to untreated cells (control). b: Decrease of immunostaining for β catenin in cell membranes was revealed after 6h incubation of HUVEC with 50 µg/ml of LAMA84 exosomes compared to control cells (section at 2 µm from the cell surface). c: figure shows the translocation of β catenin in the cytoplasm and nucleus compared to control (section at 4 µm from the cell surface). d: modification of cytoskeletal structures as observed with actin localization in HUVEC monolayer treated with 50 µg/ml of exosomes compared to control cells. These fields are representative of three independent experiments. Scale bar = 10 µm. e: Semi-quantitative analysis of VE-Cadherin fluorescence intensity in the plasma membrane of HUVEC treated with 50 µg/ml of LAMA84 exosomes compared to control cells. f: Semi-quantitative analysis of β-Catenin fluorescence intensity in the plasma membrane and nuclei of HUVEC treated with 50 µg/ml of LAMA84 exosomes compared to control cells.
CML exosomes stimulate in vitro and in vivo tube formation
We analyzed the differentiation of HUVECs into capillary-like structures when plated on Matrigel as a model of angiogenesis26. HUVECs maintained in medium containing 0.2% serum were almost unable to form a tube network (fig 6a). Addition of LAMA84 exosomes (fig 6a), recombinant IL-8 (Fig. S4a) or exosomes from CML patients (Fig. S4a) resulted in increased cellularity of the network formation compared to cells treated with PBMC exosomes (Fig. S4a). The anti-IL8 neutralizing antibody inhibited exosome-induced tube formation while treatment of cells with an anti-actin antibody had no effect (fig 6a). Measurements of the length of tubular connections showed a 3-fold increase in cellular projections interconnecting HUVECs when treated with LAMA84 exosomes (fig 6b), recombinant IL-8 or exosomes from CML patients (Fig. S4b) compared to controls. The angiogenic potential of LAMA84 exosomes was then assayed in vivo by examining the recruitment of vasculature into subcutaneously implanted Matrigel plugs containing exosomes. Figure 6c shows that the plugs containing LAMA84 exosomes and LAMA84 exosomes plus a non specific antibody (anti-actin antibody), became more vascularized than implants with PBS control or with exosomes plus an anti-IL8 neutralizing antibody. This suggests that IL-8 is critical for vascular recruitment and organization in this model. This is supported by the increased haemoglobin concentration in the exosomes-containing Matrigel (table I). Activation of MAPK signalling pathway has been demonstrated to play an important role in angiogenis27. We next investigated whether addition of LAMA84 exosomes to endothelial cells triggers phosphorylation of MAPK p42/44. Figure 6d shows stimulation of HUVEC with 50 µg/ml exosomes caused a time-dependent phosphorylation of MAPK indicating that exosomes interact with HUVECs and act as a regulators of signal transduction in endothelial cells.
Figure 6. LAMA84 exosomes stimulate in vitro and in vivo angiogenesis.

Panel a: Phase contrast micrographs showing that exosomes induce an endothelial network formation on matrigel. No tube formation is observed when HUVEC are plated in low-serum medium (upper left panel) or in the presence of 50 µg/ml of exosomes plus neutralizing antibody against IL8 (upper right panel); the addition to HUVEC cells of 50 µg/ml of LAMA84 exosomes (lower right panel) or 50 µg/ml of exosomes plus a non specific antibody against actin (lower left panel) caused the formation of capillary-like structures. Panel b: Measurement of the cables length by ImageJ software. Panel c: Matrigel plug containing LAMA84 exosomes stimulate angiogenesis in nude mice. Ctrl: Negative control (Matrigel plus PBS), Ex + Ab nIL8: LAMA84 exosomes (100 µg) plus 10 µg/ml of an antibody neutralizing anti-IL8, Ex + Ab Actin: LAMA84 exosomes (100 µg) plus 10 µg/ml of a non specific antibody against actin, Ex LAMA84: LAMA84 exosomes (100 µg). Panel d: Western blot analysis of pMAPK and MAPK in HUVEC treated with 50 µg of LAMA 84 exosomes. HUVEC were starvated for 3h with serum free medium and then treated with 50 µg of LAMA 84 exosomes for 15 min (lane 3) and 30 min (lane 4), or with low serum medium alone for 15 min (lane 1) and 30 min (lane 2) as control.
Table 1.
| ABS | |
|---|---|
| Ctrl | 0.040 |
| Ex+ Ab nIL8 | 0.070 |
| Ex+ Ab actin | 0.247 |
| Ex LAMA84 | 0.378 |
Discussion
The current study provides insights into the role of exosomes in angiogenesis stimulated by chronic myelogenous leukemia cells. There are increasing data indicating that angiogenesis plays an important role in the development and progression of CML5. The bone marrow of patients with CML exhibit marked neovascularization and increased number of endothelial cells28; however, little is known about how CML cells induce the angiogenic phenotype.
A number of studies have recently described exosomes as new components that modulate the tumor microenvironment, promoting angiogenesis and tumor progression29, 30. We show here that exosomes released by LAMA84 CML cells “in vitro” and by patient’s leukemia cells in blood have the potential to affect in vitro and in vivo angiogenesis. One of the initial findings of our study was the confirmation that CML cells secrete exosomes with morphological and biochemical properties in agreement with that described by other groups in different cell lines7 including CML cells13. Cell–cell interaction mediated through cell-adhesion molecules occurs following endothelial activation in angiogenesis. Our data showed that the amount of ICAM1 and VCAM1 was significantly higher in cells treated with increasing doses of LAMA84 exosomes and of patients with Chronic Myelogenous Leukemia than the untreated control or PBMC exosomes-treated endothelial cells. Apart from their role in stabilizing cell–cell contact during angiogenesis, an increased expression of cell-cell adhesion molecules on endothelial cells may also be associated with an augmented dissemination of leukemia blast cells to extramedullary sites and associated leukostasis31. Adhesion studies performed under static conditions have shown that myeloblast adhesion to cytokine-activated endothelium is mediated by ICAM-1 and VCAM-132. Accordingly, we show that treatment of an endothelial monolayer with CML exosomes caused a dose-dependent increased adhesion of LAMA84 cells.
Exosomes released from leukemia cells in close proximity of endothelial cells may also contribute in the exacerbation of endothelium activation and following migration of endothelial cells during angiogenesis. Our data demonstrate that LAMA84 exosomes and CML patients exosomes induced the motility of HUVEC cells in a dose-dependent manner both in a Boyden chamber and in a wound healing assay. Loss of cell–cell contacts is consistently observed during migration of endothelial cells or during transendothelial migration of tumor cells33, 34. Endothelial cell adherence junctions are composed of transmembrane adhesive proteins belonging to the cadherin family, with vascular endothelial (VE)-cadherin the most important35. This protein interacts with cytoplasmic catenins, in particular, β-catenin, to link cadherins to the actin cytoskeleton. β-catenin distribution is generally related to the maintenance of cell–cell contacts and regulation of the intracellular signalling pathways that are involved in tissue morphogenesis36. Cai et al. showed a rapid redistribution of VE-cadherin following adherence of MDA MB231 breast cancer cells to HUVECs resulting in its transient loss from regions of endothelial cell-cell contact34. Treatment of HUVECs with LAMA84 exosomes induced a cytoskeletal reorganization with a concomitant translocation of VE-cadherin and β-catenin from cell surface to cytoplasm and nuclei. Overall, these results indicate that addition of exosomes to endothelial cells reduced intercellular adhesion, as a biological consequence of loss of zonulae adherens components, VE-cadherin and β-catenin. These reduced and focal endothelial cell junctions may be responsible for increased motility as well as the leaky vasculature observed in solid tumors37. Additional changes after endothelial cell activation, for example, loss of vascular integrity, and increased endothelial cell production of cytokines, may also contribute to the angiogenic process. We demonstrated increased mRNA and protein expression of interleukin-8 in exosomes-stimulated HUVEC cells. IL-8, a potent proangiogenic factor, is a member of the CXC family of chemokines38 and its plasma concentration are significantly increased in patients with CML6, 39. We demonstrated IL8 was important in the exosome-mediated increase of ICAM1 and VCAM1 by using recombinant IL8 and IL-8 neutralizing antibodies.
We employed both in vitro and in vivo Matrigel assays to further evaluate the angiogenic potential of CML exosomes. We found that exosomes from LAMA84 cells, or those isolated from CML patients or rIL8 induced tubular differentiation of HUVECs and stimulated vascularization of Matrigel plugs implanted into nude mice. The addition of IL-8 neutralizing antibodies to CML exosomes in both assays inhibited the process of angiogenesis thus reinforcing the role of IL-8 in exosomes-induced vascularization.
We analyzed MAPK signalling following interaction between endothelial cells and purified exosomes to begin to understand the molecular pathways through which exosomes affect angiogenesis. MAPK is a key signalling pathway activated in endothelial cells after binding of angiogenic factors40. Cross-talk between MAPK and other signalling pathways can further stimulate angiogenesis41. The strong activation of ERK1/2 in endothelial cells early after the exosomes addition suggest that these exosomes exert a specific stimulus for endothelial cell function. The results here described contribute to highlight the intricate tumor-host interactions in CML. For the first time, exosomes released from CML cells have been involved as important components leading to endothelium activation and angiogenesis.
Recent reports support the role of exosomes in modulation of angiogenesis during tumor progression. Skog and colleagues have showed that glioblastoma tumor cells release different types of microvesicles including exosomes that contain mRNA, miRNA and proteins that may stimulate endothelial cells to acquire an angiogenic phenotype42. It has been demonstrated that exosomes of of human SW480 colon carcinoma cells are enriched in cell cycle-related mRNAs that promote proliferation of endothelial cells, suggesting that microvesicles from cancer cells can be involved in tumor growth and metastasis by facilitating angiogenesis-related processes43. The role of exosomes in metastasis has been also demonstrated in melanoma cells in a study by Hood and collaborators. They found that exosomes modulated both angiogenic and immunological cytokine signalling, thus serving as paracrine nanocarriers that might prepare distal sites for the arrest of metastatic cells44.
In this context, the inhibition of either exosomes shedding or modulation of exosome function has been proposed as an approach to cancer therapy45. The molecular basis of endothelial cell stimulation by exosomes is under investigation. A potential working hypothesis involves a direct physical association of membrane microparticles with the endothelial cell surface followed by transmembrane signal transduction and de novo gene expression. It has been proposed that the acid microenvironment of the tumor host interface may facilitate the vesicles lysis, releasing their contents in close proximity to a cellular target46. Alternatively, vesicles can move between cells using specialized structures called nanotubes47. Our data do not go as far as defining which of these hypotheses may be correct. However we have shown that exosomes released from CML cells activate, in endothelial cells, signal transduction pathways leading to the release of IL-8 that ultimately causes, by an autocrine mechanisms, the activation of an angiogenic phenotype. In fact, blocking with anti IL-8 neutralizing antibodies the interaction of the cytokine with endothelial cell surface, causes the inhibition of angiogenic process. Our findings suggest that targeting the release of exosomes could be a rationale approach for CML therapy.
Supplementary Material
Acknowledgments
Author thank Dr Rosalinda Inguanta, Department of Engineering Chemistry Processes and Materials for helping in scanning electron microscopy photographs; Mr Salvatore Agnello for help in confocal microscopy at the STEMBIO department.
This work was supported by Italian Association for Cancer Research (AIRC) to G.D.L. and R.A., University of Palermo (International Cooperation) to R.A.; ex 60% MURST to R.A., AF., S.F. and to G.D.L. Dr. Kohn is supported by the Intramural Program of the Center for Cancer Research, National Cancer Institute, National Institutes of Health, USA.
References
- 1.Rowley J. A new consistent chromosomal abnormality in chronic myelogenous leukaemia identified by quinacrine fluorescence and Giemsa staining. Nature. 1973;243:290–293. doi: 10.1038/243290a0. [DOI] [PubMed] [Google Scholar]
- 2.Perrotti D, Jamieson C, Goldman J, Skorski T. Chronic myeloid leukemia: mechanisms of blastic transformation. J Clin Invest. 2010;120:2254–2264. doi: 10.1172/JCI41246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jabbour E, Cortes JE, Ghanem H, O'Brien S, Kantarjian HM. Targeted therapy in chronic myeloid leukemia. Expert Rev Anticancer Ther. 2008;8:99–110. doi: 10.1586/14737140.8.1.99. [DOI] [PubMed] [Google Scholar]
- 4.Legros L, Bourcier C, Jacquel A, Mahon FX, Cassuto JP, Auberger P, Pages G. Imatinib mesylate (STI571) decreases the vascular endothelial growth factor plasma concentration in patients with chronic myeloid leukemia. Blood. 2004;104:495–501. doi: 10.1182/blood-2003-08-2695. [DOI] [PubMed] [Google Scholar]
- 5.Aguayo A, Kantarjian H, Manshouri T, Gidel C, Estey E, Thomas D, Koller C, Estrov Z, O'Brien S, Keating M, Freireich E, Albitar M. Angiogenesis in acute and chronic leukemias and myelodysplastic syndromes. Blood. 2000;96:2240–2245. [PubMed] [Google Scholar]
- 6.Sillaber C, Mayerhofer M, Aichberger KJ, Krauth M, Valent P. Expression of angiogenic factors in chronic myeloid leukaemia: role of the bcr/abl oncogene, biochemical mechanisms, and potential clinical implications. Eur J Clin Invest. 2004;34 Suppl 2:2–11. doi: 10.1111/j.0960-135X.2004.01365.x. [DOI] [PubMed] [Google Scholar]
- 7.Schorey JS, Bhatnagar S. Exosome function: from tumor immunology to pathogen biology. Traffic. 2008;9:871–881. doi: 10.1111/j.1600-0854.2008.00734.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Camussi G, Deregibus M, Bruno S, Cantaluppi V, Biancone L. Exosomes/microvesicles as a mechanism of cell-to-cell communication. Kidney Int. 2010 doi: 10.1038/ki.2010.278. In Press. [DOI] [PubMed] [Google Scholar]
- 9.Mathivanan S, Ji H, Simpson RJ. Exosomes: extracellular organelles important in intercellular communication. J Proteomics. 2010;73:1907–1920. doi: 10.1016/j.jprot.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 10.Iero M, Valenti R, Huber V, Filipazzi P, Parmiani G, Fais S, Rivoltini L. Tumour-released exosomes and their implications in cancer immunity. Cell Death Differ. 2008;15:80–88. doi: 10.1038/sj.cdd.4402237. [DOI] [PubMed] [Google Scholar]
- 11.Nazarenko I, Rana S, Baumann A, McAlear J, Hellwig A, Trendelenburg M, Lochnit G, Preissner K, Zöller M. Cell surface tetraspanin Tspan8 contributes to molecular pathways of exosome-induced endothelial cell activation. Cancer Res. 2010;70:1668–1678. doi: 10.1158/0008-5472.CAN-09-2470. [DOI] [PubMed] [Google Scholar]
- 12.Sheldon H, Heikamp E, Turley H, Dragovic R, Thomas P, Oon C, Leek R, Edelmann M, Kessler B, Sainson R, Sargent I, Li J, et al. New mechanism for Notch signaling to endothelium at a distance by Delta-like 4 incorporation into exosomes. Blood. 2010;116:2385–2394. doi: 10.1182/blood-2009-08-239228. [DOI] [PubMed] [Google Scholar]
- 13.Savina A, Furlan M, Vidal M, Colombo MI. Exosome release is regulated by a calcium-dependent mechanism in K562 cells. J Biol Chem. 2003;278:20083–20090. doi: 10.1074/jbc.M301642200. [DOI] [PubMed] [Google Scholar]
- 14.Abache T, Le Naour F, Planchon S, Harper F, Boucheix C, Rubinstein E. The transferrin receptor and the tetraspanin web molecules CD9, CD81, and CD9P-1 are differentially sorted into exosomes after TPA treatment of K562 cells. J Cell Biochem. 2007;102:650–664. doi: 10.1002/jcb.21318. [DOI] [PubMed] [Google Scholar]
- 15.Alessandro R, Fontana S, Giordano M, Corrado C, Colomba P, Flugy AM, Santoro A, Kohn EC, De Leo G. Effects of carboxyamidotriazole on in vitro models of imatinib-resistant chronic myeloid leukemia. J Cell Physiol. 2008;215:111–121. doi: 10.1002/jcp.21290. [DOI] [PubMed] [Google Scholar]
- 16.Théry C, Amigorena S, Raposo G, Clayton A. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr Protoc Cell Biol. 2006 doi: 10.1002/0471143030.cb0322s30. Unit 3.22. [DOI] [PubMed] [Google Scholar]
- 17.Lamparski HG, Metha-Damani A, Yao JY, Patel S, Hsu DH, Ruegg C, Le Pecq JB. Production and characterization of clinical grade exosomes derived from dendritic cells. J Immunol Methods. 2002;270:211–226. doi: 10.1016/s0022-1759(02)00330-7. [DOI] [PubMed] [Google Scholar]
- 18.Alessandro R, Gallo A, Barranca M, Principe S, Taverna S, Duro G, Cassata G, Becchi M, Fontana S, De Leo G. Production of an egg yolk antibody against Parietaria judaica 2 allergen. Poult Sci. 2009;88:1773–1778. doi: 10.3382/ps.2009-00054. [DOI] [PubMed] [Google Scholar]
- 19.Alessandro R, Flugy AM, Russo D, Stassi G, De Leo A, Corrado C, Alaimo G, De Leo G. Identification and phenotypic characterization of a subpopulation of T84 human colon cancer cells, after selection on activated endothelial cells. J Cell Physiol. 2005;203:261–272. doi: 10.1002/jcp.20236. [DOI] [PubMed] [Google Scholar]
- 20.Taverna S, Flugy A, Colomba P, Barranca M, De Leo G, Alessandro R. Effects of Parietaria judaica pollen extract on human microvascular endothelial cells. Biochem Biophys Res Commun. 2008;372:644–649. doi: 10.1016/j.bbrc.2008.05.118. [DOI] [PubMed] [Google Scholar]
- 21.Rodriguez LG, Wu X, Guan JL. Wound-healing assay. Methods Mol Biol. 2005;294:23–29. doi: 10.1385/1-59259-860-9:023. [DOI] [PubMed] [Google Scholar]
- 22.Kohn EC, Alessandro R, Spoonster J, Wersto RP, Liotta LA. Angiogenesis: role of calcium-mediated signal transduction. Proc Natl Acad Sci U S A. 1995;92:1307–1311. doi: 10.1073/pnas.92.5.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wysoczynski M, Ratajczak M. Lung cancer secreted microvesicles: Underappreciated modulators of microenvironment in expanding tumors. International Jounal of Cancer. 2009;125:1595–1603. doi: 10.1002/ijc.24479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thery C, Zitvogel L, Amigorena S. Exosomes: composition, biogenesis and function. Nat Rev Immunol. 2002;2:569–579. doi: 10.1038/nri855. [DOI] [PubMed] [Google Scholar]
- 25.Muller WA. Mechanisms of transendothelial migration of leukocytes. Circ Res. 2009;105:223–230. doi: 10.1161/CIRCRESAHA.109.200717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aranda E, Owen G. A semi-quantitative assay to screen for angiogenic compounds and compounds with angiogenic potential using the EA.hy926 endothelial cell line. Biol Res. 2009;42:377–389. [PubMed] [Google Scholar]
- 27.Huang D, Ding Y, Luo WM, Bender S, Qian CN, Kort E, Zhang ZF, VandenBeldt K, Duesbery NS, Resau JH, Teh BT. Inhibition of MAPK kinase signaling pathways suppressed renal cell carcinoma growth and angiogenesis in vivo. Cancer Res. 2008;68:81–88. doi: 10.1158/0008-5472.CAN-07-5311. [DOI] [PubMed] [Google Scholar]
- 28.Kvasnicka HM, Thiele J, Staib P, Schmitt-Graeff A, Griesshammer M, Klose J, Engels K, Kriener S. Reversal of bone marrow angiogenesis in chronic myeloid leukemia following imatinib mesylate (STI571) therapy. Blood. 2004;103:3549–3551. doi: 10.1182/blood-2003-08-2734. [DOI] [PubMed] [Google Scholar]
- 29.Hendrix A, Westbroek W, Bracke M, De Wever O. An ex(o)citing machinery for invasive tumor growth. Cancer Res. 2010;70:9533–9537. doi: 10.1158/0008-5472.CAN-10-3248. [DOI] [PubMed] [Google Scholar]
- 30.Zhang H, Grizzle W. Exosomes and cancer: a newly described pathway of immune suppression. Clin Cancer Res. 2011;17:959–964. doi: 10.1158/1078-0432.CCR-10-1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leis JF, Primack SL, Schubach SE, Curtin PT, Druker BJ, Maziarz RT. Management of life-threatening pulmonary leukostasis with single agent imatinib mesylate during CML myeloid blast crisis. Haematologica. 2004;89 ECR30. [PubMed] [Google Scholar]
- 32.Cavenagh JD, Gordon-Smith EC, Gibson FM, Gordon MY. Acute myeloid leukaemia blast cells bind to human endothelium in vitro utilizing E-selectin and vascular cell adhesion molecule-1 (VCAM-1) Br J Haematol. 1993;85:285–291. doi: 10.1111/j.1365-2141.1993.tb03168.x. [DOI] [PubMed] [Google Scholar]
- 33.Dejana E, Spagnuolo R, Bazzoni G. Interendothelial junctions and their role in the control of angiogenesis, vascular permeability and leukocyte transmigration. Thromb Haemost. 2001;86:308–315. [PubMed] [Google Scholar]
- 34.Cai J, Jiang WG, Mansel RE. Phosphorylation and disorganization of vascular-endothelial cadherin in interaction between breast cancer and vascular endothelial cells. Int J Mol Med. 1999;4:191–195. doi: 10.3892/ijmm.4.2.191. [DOI] [PubMed] [Google Scholar]
- 35.Rudini N, Dejana E. Adherens junctions. Curr Biol. 2008;18:R1080–R1082. doi: 10.1016/j.cub.2008.09.018. [DOI] [PubMed] [Google Scholar]
- 36.Dejana E. Endothelial cell–cell junctions: happy together. Nat Rev Mol Cell Biol. 2004;4:261–270. doi: 10.1038/nrm1357. [DOI] [PubMed] [Google Scholar]
- 37.Cai J, Jiang W, Mansel R. Inhibition of the Expression of VE-Cadherin/Catenin Complex by Gamma Linolenic Acid in Human Vascular Endothelial Cells, and Its Impact on Angiogenesis. Biochemical and Biophysical Research Communications. 1999;258:113–118. doi: 10.1006/bbrc.1999.0596. [DOI] [PubMed] [Google Scholar]
- 38.Waugh DJ, Wilson C. The interleukin-8 pathway in cancer. Clin Cancer Res. 2008;14:6735–6741. doi: 10.1158/1078-0432.CCR-07-4843. [DOI] [PubMed] [Google Scholar]
- 39.Negaard HF, Iversen N, Bowitz-Lothe IM, Sandset PM, Steinsvik B, Ostenstad B, Iversen PO. Increased bone marrow microvascular density in haematological malignancies is associated with differential regulation of angiogenic factors. Leukemia. 2009;23:162–169. doi: 10.1038/leu.2008.255. [DOI] [PubMed] [Google Scholar]
- 40.Sebolt-Leopold JS, Herrera R. Targeting the mitogen-activated protein kinase cascade to treat cancer. Nat Rev Cancer. 2004;4:937–947. doi: 10.1038/nrc1503. [DOI] [PubMed] [Google Scholar]
- 41.Jiang BH, Liu LZ. PI3K/PTEN signaling in angiogenesis and tumorigenesis. Adv Cancer Res. 2009;102:19–65. doi: 10.1016/S0065-230X(09)02002-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Skog J, Wurdinger T, van Rijn S, Meijer DH, Gainche L, Sena-Esteves M, Curry WT, Jr, Carter BS, Krichevsky AM, Breakefield XO. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat Cell Biol. 2008;10:1470–1476. doi: 10.1038/ncb1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hong BS, Cho JH, Kim H, Choi EJ, Rho S, Kim J, Kim JH, Choi DS, Kim YK, Hwang D, Gho YS. Colorectal cancer cell-derived microvesicles are enriched in cell cycle-related mRNAs that promote proliferation of endothelial cells. BMC Genomics. 2009;10:556. doi: 10.1186/1471-2164-10-556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hood JL, Pan H, Lanza GM, Wickline SA. Paracrine induction of endothelium by tumor exosomes. Lab Invest. 2009;89:1317–1328. doi: 10.1038/labinvest.2009.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Viaud S, Théry C, Ploix S, Tursz T, Lapierre V, Lantz O, Zitvogel L, Chaput N. Dendritic cell-derived exosomes for cancer immunotherapy: what's next? Cancer Res. 2010;70:1281–1285. doi: 10.1158/0008-5472.CAN-09-3276. [DOI] [PubMed] [Google Scholar]
- 46.Taraboletti G, D'Ascenzo S, Giusti I, Marchetti D, Borsotti P, Millimaggi D, Giavazzi R, Pavan A, Dolo V. Bioavailability of VEGF in tumor-shed vesicles depends on vesicle burst induced by acidic pH. Neoplasia. 2006;8:96–103. doi: 10.1593/neo.05583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rustom A, Saffrich R, Markovic I, Walther P, Gerdes HH. Nanotubular highways for intercellular organelle transport. Science. 2004;303:1007–1010. doi: 10.1126/science.1093133. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


