Abstract
The molecular constituents of endogenous cannabinoid (eCB) signaling are abundantly expressed within the mammalian amygdaloid complex, consistent with the robust role of eCB signaling in the modulation of emotional behavior, learning, and stress-response physiology. Here we detail the anatomical distribution of eCB signaling machinery in the amygdala and the role of this system in the modulation of excitatory and inhibitory neuroplasticity in this region. We also summarize recent findings demonstrating dynamic alternations in eCB signaling that occur in response to stress exposure, as well as known behavioral consequences of eCB-mediated modulation of amygdala function. Finally, we discuss how integrating anatomical and physiological data regarding eCB signaling in the amygdala could help elucidate common functional motifs of this system in relation to broader forebrain function.
Keywords: marijuana, CB1 receptor, anxiety, fear conditioning, anatomy, cannabis
1.1 INTRODUCTION
1.1.1 Endocannabinoids
eCBs comprise a family of arachidonic acid-derived lipid molecules synthesized by vertebrate and invertebrate animals (Piomelli, 2003; Di Marzo et al., 2005). eCBs include 2-arachidonoylglycerol (2-AG), anandamide (AEA), noladin ether, and several other candidate molecules. 2-AG is synthesized by conversion of sn-2 arachidonic acid containing diacylglycerols to the monoacylglycerol 2-AG, via an sn-1-specific diacylglycerol lipase (DAGL) (Bisogno et al., 2003) and is degraded primarily by monoacylglycerol lipase (MGL)(Dinh et al., 2002b; Blankman et al., 2007). On the other hand, synthesis of AEA has been demonstrated to occur via multiple pathways but is degraded primarily by fatty acid amide hydrolase (FAAH) (Cravatt and Lichtman, 2002; Ahn et al., 2008; Ueda et al., 2010).
Although many molecular targets of eCBs have been described, the primary targets of these compounds are the cannabinoid receptor types 1 and 2 (CB1 and CB2 respectively). eCBs are produced by almost all cell types that have been examined, and the CB1 and CB2 receptors are present in both nervous and peripheral tissues. Here we will primarily focus on CB1 receptor-mediated eCB signaling as this is most heavily implicated in the synaptic and behavioral functions of cannabinoids (Chevaleyre et al., 2006; Hashimotodani et al., 2007; Heifets and Castillo, 2009; Kano et al., 2009).
Within neurons of the central nervous system, initiation of eCB signaling results in retrograde inhibition of afferent neurotransmission. Specifically, post-synaptic eCB synthesis, initiated via voltage-dependent calcium channel activation or G-protein coupled receptor (GPCR)-dependent mechanisms, results in release and diffusion of eCBs into the synaptic cleft (Kano et al., 2009). Thereafter, eCBs bind to the CB1 receptor localized to axon terminals of glutamatergic and GABAergic neurons, thus activating intracellular signaling downstream of Gi/o proteins and causing either short-term or long-term suppression of vesicular neurotransmitter release (Chevaleyre et al., 2006). This signaling motif has been described in numerous brain regions including the brainstem, midbrain, cerebellum, hippocampus, striatum, and neocortex. The widespread distribution of eCB-mediated synaptic signaling underscores the pleiotropic functions of eCBs in the regulation of motor control, learning and memory, pain, motivation, metabolic integration, and affect regulation (see (Kano et al., 2009)).
1.1.2 The Amygdala
The amygdaloid complex is comprised of several interconnected regions which can be classified into cortical-like nuclei including the basolateral complex (BLA; which is comprised of the lateral (LA), basal (BA) and basomedial (BM) nuclei), as well as the centromedial nuclei, which include the central amygdala (CeA; which is subdivided into the lateral (CeAL) and medial (CeAM) compartments) and the medial amygdala (MeA)(see (Sah et al., 2003; Pape and Pare, 2010) for review). The BLA exhibits a cortical-like structure (i.e. being comprised of principal projection neurons and local interneurons), whereas the CeAL exhibits a striatal-like cytoarchitecture (medium spiny-like, predominantly GABAergic, neurons). Furthermore, it has been suggested that cells of the CeM share significant homology to pallidal neurons based on neurochemical, connectional, and cytoarchitectural evidence (Cassell et al., 1999; McDonald, 2003). Thus, the concept of a cortico-striato-pallidal motif, traditionally conceptualized as a functional unit of forebrain organization relevant to motor/cognitive control, may also be relevant to the fundamental organization of the amygdala (Cassell et al., 1999). There also exists a population of identified amygdalar neurons known as intercalated cell masses (ICMs). These are groups of tightly packed GABAergic interneurons that form islands at the anatomical interface between the BLA and CeA (Royer et al., 1999; Pare et al., 2004). Functionally, these cells are thought to provide feed-forward inhibition onto CeM neurons in response to BLA input (Samson and Pare, 2006; Amano et al., 2010).
The BLA receives massive cortical and thalamic input carrying monomodal and highly processed polymodal sensory information (Sah et al., 2003; Pape and Pare, 2010). The BLA contains glutamatergic pyramidal projection neurons that exhibit very low rates of spontaneous activity due to a high level of tonic GABAergic input supplied by local interneurons. There are also extensive reciprocal, predominantly excitatory, connections between various nuclei within the BLA (Sah et al., 2003; Knapska et al., 2007). Furthermore, the BLA projects to the nucleus accumbens, hippocampus, prefrontal cortex, and the CeA (Sah et al., 2003; Knapska et al., 2007).
The CeAL contains predominantly GABAergic neurons that receive excitatory input from the BLA, cortex, thalamus, and brainstem autonomic nuclei carrying visceral, as well as nociceptive information, and in turn, project to the parabrachial nucleus, the bed nucleus of the stria terminalis, basal forebrain, and CeAM (Knapska et al., 2007). The CeAM is considered the output structure of the amygdala, and receives excitatory input from the cortex, BLA, brainstem, and hypothalamus, and tonic inhibitory input from the CeAL (Sah et al., 2003; Knapska et al., 2007; Ciocchi et al., 2010).
From this anatomical organization, it is clear that the BLA is positioned to receive information regarding salient environmental stimuli and orchestrate instrumental responses to these stimuli via projections to the prefrontal cortex and nucleus accumbens, as well as to the CeAM, which in turn targets hypothalamic, brainstem, and reticular structures. Thus, the BLA and CeA are often considered to comprise a serial pathway, where information from the BLA is transferred to the CeA to modulate specific endocrine, autonomic, and arousal-related responses (Davis, 1997; Davis and Whalen, 2001). However, a parallel processing system, where the BLA and CeA serve distinct functions that operate independently has also been suggested (see (Knapska et al., 2007) for review).
Considerable work has established the amygdala as a key site involved in the generation of fear and anxiety responses, the assignment of emotional salience to external stimuli, the coordination of affective, autonomic, and behavioral responses to such stimuli, as well as Pavlovian learning processes (Davis, 1997; Pare et al., 2004; Pape and Pare, 2010). Significant recent progress has been made to elucidate the complex synaptic connectivity between amygdaloid nuclei and the means by which afferent information is processed within the amygdala to affect behavioral outcomes. Over the past decade, eCB signaling has emerged as a key neuromodulatory system that regulates synaptic efficacy within and between amygdaloid subnuclei. Here we summarize data elaborating the role of eCB signaling in amygdala, the adaptations in this system that occur in response to stress, and the behavioral consequences of eCB-mediated modulation of amygdala function.
1.2 ANATOMY OF eCB SIGNALING IN THE AMYGDALA
1.2.1 CB1 receptor
The CB1 receptor is a membrane associated G-protein coupled receptor that is expressed widely, but not exclusively, within the central nervous system. The CB1 receptor is activated by the principle psychoactive constituent of Cannabis sativa, Δ9-tetrahydrocannabinol (THC), as well as synthetic and endogenous ligands (Pertwee, 2005). In situ hybridization (ISH) studies in developing and adult rodents and humans have repeatedly classified CB1-expressing neurons into two groups: low-expressing and high-expressing. High CB1-expressing cells are sparsely distributed within the BLA and other cortical structures, whereas low CB1-expressing cells are more evenly distributed and found within both the BLA and centromedial nuclei (Mailleux and Vanderhaeghen, 1992; Matsuda et al., 1993; Marsicano and Lutz, 1999; Chhatwal et al., 2005; Hermann and Lutz, 2005; Yoshida et al., 2011).
Marsicano and Lutz provided the first detailed description of CB1 receptor mRNA expression within the mouse amygdala (Marsicano and Lutz, 1999). These authors reported the presence of both high CB1− and low CB1-expressing cells within the BLA and low levels of CB1 mRNA in the central amygdala. These authors showed that ~95% of high CB1-expressing cells co-expressed the GABAergic marker glutamic acid decarboxylase 65 (GAD65). Furthermore, almost all high CB1-expressing cells, and 90% of low CB1-expressing cells, co-express the peptide cholecystokinin (CCK). Subsequent work by this group demonstrated that 38% of CB1-expressing neurons within the BLA co-expresses corticotrophin releasing hormone receptor type-1 (CRHR1) mRNA, and all CRHR1-expressing neurons within the BLA co-express CB1 mRNA (Hermann and Lutz, 2005).
Co-expression of serotonin type 3 receptor (5-HT3) and CB1 has been demonstrated within the BLA (Hermann et al., 2002; Morales and Backman, 2002; Morales et al., 2004). Between 16–36% of CB1-expressing neurons, depending on the subregion of the BLA, express transcript for 5-HT3 receptors. Conversely, 37–55% of 5-HT3 receptor-expressing neurons also express CB1 receptor transcript. These co-expressing neurons correspond to the GABAergic, high CB1-expressing population within the BLA (Morales et al., 2004).
Within the CeA, CB1 mRNA expression has generally been described as low but present (Matsuda et al., 1993; Marsicano and Lutz, 1999; Chhatwal et al., 2005; Hermann and Lutz, 2005). It is, however, unclear from these studies if there are differences in CB1 mRNA expression within subregions of the CeA (Chhatwal et al., 2005).
Immunohistochemical studies have also revealed the presence of CB1 receptor immunoreactivity within the rodent amygdala. The first detailed description by Tsou and co-workers, using an antibody directed against the N-terminal of the CB1 receptor, revealed CB1-immunoreactive (CB1-ir) neurons within both the centromedial nuclei and the BLA (Tsou et al., 1998a). Using this antibody, McDonald and Mascagni found light staining in principal neurons of the BLA, other cortical-like amygdaloid nuclei, CeAL, and anteroventral division of the MeA. In addition, lightly CB1-ir dendrites of pyramidal cells were also observed in all BLA nuclei. Double-labeling studies revealed that between 60–81% of high-CB1 expressing neurons within the BLA co-expressed CCK. Furthermore, all medium to large sized CCK neurons (type L) co-expresses CB1 (100% co-expression of CB1 and CCK in L-type CCK-positive neurons), whereas only a small population of the small CCK-expressing neurons (type S) co-expresses CB1 (10–14% co-localization depending on anatomical subregion) (McDonald and Mascagni, 2001).
Freund and co-workers utilized a CB1 receptor antibody raised against the C-terminal intracellular tail of CB1 receptor to explore its immunohistochemical distribution within the mouse and rat amygdala (Katona et al., 2001). In general, the densest immunoreactivity was found within the BLA and related cortical-like nuclei, whereas the CeA, MeA, and ICMs were not immunoreactive for CB1. The most prominent feature of the CB1 immunostaining in this study was a dense meshwork of varicose axon collaterals. These axon collaterals were observed to form pericellular arrays around immunonegative cell bodies, while no dendritic staining was observed using this antibody. This pattern of staining was also observed by Elphick and co-workers in rats and mice using a C-terminal antibody (Egertova et al., 2003). In line with ISH data, double-labeled immunofluorescence experiments revealed that 88% of CB1-ir neurons co-expressed CCK with only the large CCK expressing neurons co-expressing CB1 (Katona et al., 2001).
These authors also investigated the subcellular distribution of the CB1 receptor using electron microscopy (EM)(Katona et al., 2001). Within the BLA, immunogold labeling was observed within intracellular membrane compartments including rough endoplasmic reticulum and golgi. In addition, multivesicular bodies were densely labeled. Furthermore, no staining was observed on either the plasma membrane of cell bodies or proximal dendrites. Densely labeled axon terminals were also observed using immunogold labeling on the intracellular surface. These axon terminals formed symmetric, presumably GABAergic, contacts with somata and dendritic shafts. CB1-expressing terminals were also found to co-express CCK and form exclusively symmetric synapses (Katona et al., 2001).
Ong and Mackie, using an N-terminal CB1 antibody, provided a description of CB1 receptor distribution within the primate brain (Macaca fascicularis)(Ong and Mackie, 1999). Their studies also reported that the amygdala was densely labeled for the CB1 receptor. Within the BLA, both putative pyramidal projection and non-projection neurons were labeled. EM studies revealed large, CB1-ir cell bodies with features of pyramidal projection neurons, as well as labeled smaller neurons with features of GABAergic interneurons. Many densely labeled dendrites were observed in the neuropil of the LA, which formed symmetric contacts with unlabeled axon terminals. In addition, occasionally labeled axon terminals forming asymmetric synapses were also observed (Ong and Mackie, 1999).
Eggan and Lewis utilized C-terminal antibodies to explore the distribution of CB1 receptors within the amygdala of this species as well (Eggan and Lewis, 2007). Their findings were in qualitative agreement with those of Ong and Mackie as they observed a dense meshwork of CB1-ir varicose axons surrounding unstained cell bodies within the BLA; although some differences in the intensity of staining was seen within various subregions of the BLA. No CB1-ir axons were observed within the centromedial nuclei (CeA and MeA). However, light staining above that of the white matter was observed within these nuclei, although this labeling was noted to be morphologically indistinct (Eggan and Lewis, 2007). Though CB1 receptor expression within the amygdala may shed light onto its potential modulatory mechanisms, exploring the expression of the enzymes that are integral to eCB metabolism may further unveil the role of eCB signaling within the amygdala.
1.2.2 FAAH
FAAH is a serine hydrolase that catalyzes the degradation of AEA into arachidonic acid and ethanolamine (Ahn et al., 2008). Initial ISH studies by Cravatt and co-workers revealed an intense ISH signal within the amygdala, and specifically within the BLA (Thomas et al., 1997). These studies were closely followed by immunohistochemical localization of FAAH within the central nervous system using a C-terminal antibody (Tsou et al., 1998b). This study found intracellular punctate staining preferentially localized to large principal neurons of the rat brain, a pattern consistent with intracellular membrane localization of the protein. Within the BLA, FAAH immunoreactivity was described as moderate to strong. However, within the CeA, smaller round neurons also expressed cytoplasmic FAAH immunoreactivity, although to a lesser degree than that of the BLA.
Similarly, Elphick and co-workers described FAAH immunoreactivity within the somata of neurons throughout the BLA (Egertova et al., 2003). Furthermore, Freund and co-workers, using an antibody generated against a native 6X-His tagged truncation of FAAH (Bracey et al., 2002), also published detailed light and EM descriptions of FAAH within the rat and mouse amygdala (Gulyas et al., 2004). At the light microscopy level, a strong cellular (cytoplasmic and proximal dendritic) and granular/reticular neuropil staining was observed within the BLA. In the CeA, only occasional neurons were FAAH immunoreactive (Gulyas et al., 2004). At the EM level, FAAH was present postsynaptically within dendrites and somata of BLA neurons. FAAH was localized to dendritic shafts and spine heads, but not axon terminals (Gulyas et al., 2004).
1.2.3 MGL
In contrast to FAAH, MGL is a serine hydrolase that degrades 2-AG into arachidonic acid and glycerol (Dinh et al., 2002a). MGL has been shown to be an important regulator of brain 2-AG signaling in vivo (Long et al., 2009). ISH studies did not reveal a prominent hybridization signal within the rat amygdala (Dinh et al., 2002a). However, immunohistochemical studies using an N-terminal antibody against rat MGL revealed punctuate staining within the BLA, but not the CeA, of the rat amygdala (Gulyas et al., 2004). This punctuate staining was observed within the neuropil and often surrounded by unstained neurons within the BLA; furthermore, no cytoplasmic staining was observed. At the EM level, MGL was localized to a subset of axon terminals that formed both symmetric and asymmetric synapses onto MGL-negative postsynaptic dendrites (Gulyas et al., 2004). MGL expression in inhibitory axon terminals is higher in the BA than LA (Yoshida et al., 2011).
1.2.4 DAGL
DAGL catalyzes the second step of 2-AG synthesis, liberating 2-AG from diacylglycerol (see above). Recent molecular characterization of an Sn-1 selective DAGL has shed considerable light on the 2-AG-CB1 signaling system (Bisogno et al., 2003). Two isoforms, alpha and beta DAGL, were cloned and appear to have similar enzymatic properties. During embryonic development, DAGLα is localized to axon terminals, whereas during adulthood, a somatic/dendritic localization seems to predominate (Bisogno et al., 2003).
Two recent studies have elucidated the expression pattern of DAGLα in the amygdala. We initially demonstrated a significant heterogeneity in DAGLα expression within the amygdala (Patel et al., 2009). High levels of DAGLα were observed in the BA and dLA, whereas lower levels were observed in the vLA. Within the CeA, the CeAL was more heavily stained than the CeAM. In addition, ICMs were noticeably immunoreactive (Patel et al., 2009). Subsequent studies by Yoshida et al. demonstrated a similar subregional pattern (Yoshida et al., 2011). These authors also showed post-synaptic localization of DAGLα in close apposition to CCK and CB1-ir terminals forming invaginating synapses (Yoshida et al., 2011). These authors suggest that invaginating synapses on BA principal neurons are subject to robust modulation by 2-AG signaling, relative to flat (non-CB1/DAGLα-expressing) synapses.
1.2.5 Summary
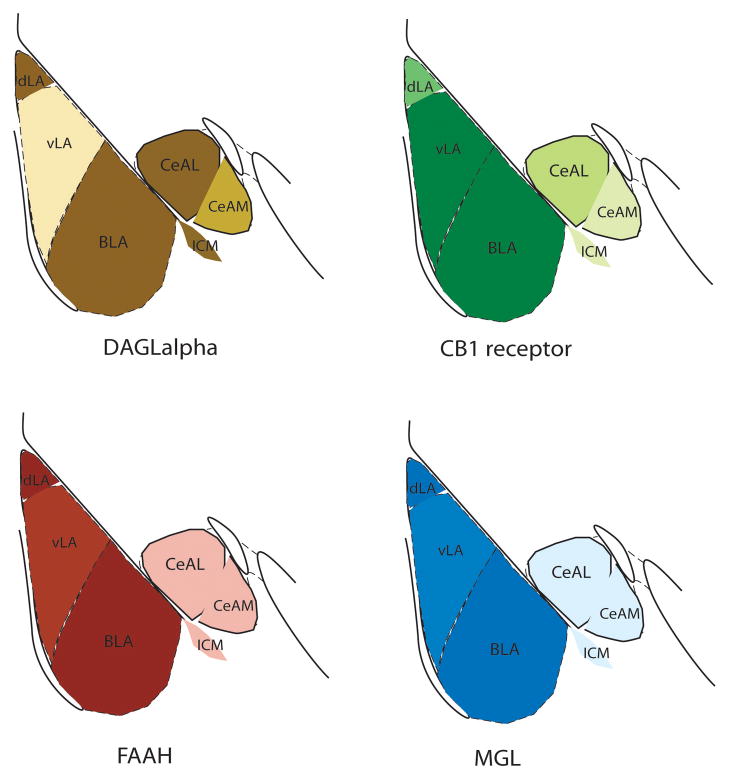
A summary of the regional and subcellular expression of various eCB signaling components is diagramed in figure 1a and b. In general, the BLA expresses eCB signaling molecules in a distribution pattern common to other cortical brain regions. Specifically, the BLA exhibits high CB1-expression by a subset of large CCK+ GABAergic interneurons, and low levels of expression by principal neurons. FAAH and MGL are expressed post-synaptically and presynaptically, respectively. DAGLα is expressed by principal neurons, and appears to cluster at invaginating synapses that receive GABAergic inputs from CB1+/CCK+ interneurons. The CeA exhibits staining more similar to that of the striatum, although more detailed subcellular studies are lacking in this brain region.
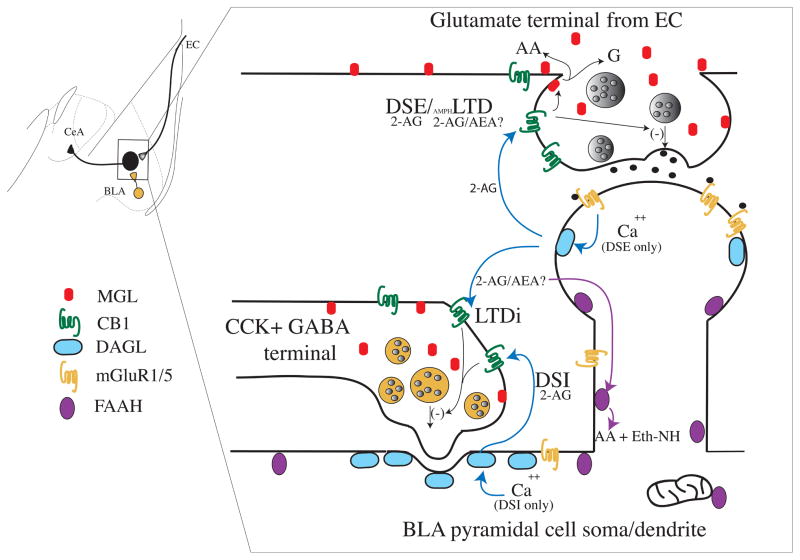
Figure 1.
(a) Schematic diagram depicting the regional heterogeneity in the expression of the molecular components of eCB signaling between different amygdala subnuclei. The localization of DAGLα, CB1 receptors, FAAH and MGL are depicted based on data summarized in the in the text. (b) Left top inset: diagram of the amygdala with simplified cellular organization. BLA projection neuron in black, GABAergic interneuron in yellow, and glutamatergic inputs in grey. Boxed area is expanded in the main figure. Synaptic localization of eCB signaling components in a schematized BLA neuron receiving synaptic inputs from a GABAergic interneuron, and glutamatergic input from the external capsule. Known forms of eCB-mediated synaptic signaling at these synapses are overlaid, and specific eCB ligands noted where data is available.
1.3 SYNAPTIC MODULATION BY eCBs IN THE AMYGDALA
1.3.1 eCBs modulate GABAergic transmission in the BLA
Cannabinoids have been shown to decrease evoked and spontaneous GABAergic synaptic transmission onto BLA principal neurons. Within BA principal neurons, Katona et al. demonstrated that the amplitude of evoked GABA-A receptor-mediated inhibitory post-synaptic currents (eIPSC) are depressed by application of the CB1 agonists Win 55212-2 and CP 55940; effects that were reversed by the CB1 receptor antagonist SR141716 (Katona et al., 2001). Similarly, Win 55212-2 was unable to suppress GABAergic transmission in CB1−/− mice. Moreover, Win 55212-2 also decreased the frequency and conductance of spontaneous, action potential-driven IPSCs (sIPSC). Azad et al. found a similar affect of CB1 receptor activation on eIPSCs recorded from mouse LA principal neurons (Azad et al., 2003).
Zuh and Lovinger utilized an isolated neuron/synaptic bouton preparation to examine the effects of CB1 receptor activation on the regulation of GABAergic transmission in BLA principal neurons (Zhu and Lovinger, 2005). Application of Win 55212-2 to this preparation produced a rapidly reversible reduction in the frequency and amplitude of sIPSCs. These authors also found that blockade of the CB1 receptor with either AM 251 or SR141716 increased sIPSC frequency. This effect was eliminated when the post-synaptic neuron was filled with a calcium chelator or extracellular calcium concentrations were reduced, suggesting a tonic CB1 receptor activation via calcium-dependent, post-synaptic eCB production in this preparation. Recent work by Yoshida et al. demonstrated that DSI in the BA is absent in DAGLα knock-out mice, implicating 2-AG as the relevant eCB ligand mediating this form of eCB signaling (Yoshida et al., 2011).
In addition to the modulation of IPSCs by direct CB1 receptor activation, eCB signaling has been demonstrated to mediate long-term depression (LTDi) and depolarization-induced suppression (DSI) of inhibitory synaptic transmission onto principal neurons of the BLA. Initial studies by Marsicano et al., within the BA, showed that LTDi was induced by low frequency (1 Hz) stimulation (LFS) of the external capsule (100 pulses), which produced a decrease in isolated GABA-A receptor-mediated eIPSC amplitude to 66% of control. This reduction in IPSC amplitude was accompanied by an increase in the PPR, indicating a presynaptic expression mechanism. In addition, LFS reduced sIPSC frequency but not amplitude, further suggesting a presynaptic mechanism of action (Azad et al., 2004). Furthermore, LFS-induced LTDi was mediated by eCB signaling, as it was absent in CB1−/− mice and prevented by the CB1 receptor antagonist SR141716.
Subsequent studies by the same authors shed considerable light on the molecular mechanisms subserving eCB-mediated LTDi in the BA. These authors found that LTDi was occluded by Win 55212-2 and did not depend upon post-synaptic elevations in intracellular calcium (Azad et al., 2004). A role for metabotropic glutamate receptors (mGluR) in LTDi was also suggested. The mGluR1/5 agonist, DHPG, suppressed eIPSC amplitude in a manner similar to LTDi in wild-type, but not CB1−/− mice. DHPG also occluded LFS-LTDi, and did not affect eIPSC amplitude after LTDi had been induced. A specific role for mGluR1, rather than mGluR5, was suggested based upon pharmacological evidence. LTDi was also found to be dependent upon post-synaptic G-protein activation and the adenylate cyclase (AC)-protein kinase A (PKA) pathway (Azad et al., 2004). However, LTDi was not dependent upon PLC or DAGL activity. Lastly, FAAH−/− mice (which exhibit elevated brain AEA levels) showed a more pronounced LTDi than wild-type mice. These authors suggest that the G-protein coupled mGluR1, acting via an AC-PKA pathway, is important for LTDi in the BA, and AEA rather than 2-AG may be the relevant eCB subserving this process (Azad et al., 2004).
A role for the presynaptic protein RIM1α has also been demonstrated in BLA LTDi. Specifically, LFS-LTDi and DHPG-LTDi are both absent in RIM1α −/− mice (Chevaleyre et al., 2007). Interestingly, Win 55212-2-induced LTDi is also absent in Rim1α −/− mice; instead, it yielded a reversible depression. These data implicate RIM1α as an important synaptic protein mediating long-term synaptic effects of eCBs in the BLA, and other brain regions such as the hippocampus (Chevaleyre et al., 2007).
eCB signaling has also been explored at the circuit level in vitro. Specifically, eCB-mediated LTDi (see details above) is expected to increase the excitability of BLA projection neurons by decreasing inhibitory tone, and thus increase the activity of BLA target neurons in the CeA, nucleus accumbens, and prefrontal cortex. Using extracellular field potential recordings, Azad et al. found that high frequency stimulation (100 pulses 50 Hz, HFS) of the external capsule (EC) produced a long-term potentiation (LTP) of field potentials recorded from the BLA (Azad et al., 2004). Interestingly, pre-induction of LTDi enhanced the magnitude of HFS-induced LTP; an effect that was abolished by the CB1 receptor antagonist SR141716. To determine whether this enhanced excitability of BLA neurons in response to eCB-mediated LTDi affects the activity of their target neurons, EPSCs from neurons within the CeA were recorded in response to LA stimulation, before and after HFS-LTP induction. Pre-induction of eCB-dependent LTDi enhanced LTP in the LA-CeA pathway, an effect abolished by SR141716 (Azad et al., 2004). These findings suggest eCB-mediated LTDi enhances the excitability of BLA neurons in response to afferent stimulation and thus, increases the activity of the BLA-CeA pathway.
Zhu and Lovinger have also demonstrated a role for eCB signaling in DSI at inhibitory synapses onto BLA principal neurons in mechanically dissociated postsynaptic neuron/synaptic bouton preparations and in acute rat brain slices (Zhu and Lovinger, 2005). In the acute brain slice preparation, post-synaptic depolarization (−60 to 0 mV) of BLA principal neurons for 4 seconds produced a 44% decrease in sIPSC frequency and a 34% decrease in sIPSC amplitude. Chelation of postsynaptic calcium eliminated DSI, however, a slowly developing decrease in sIPSC frequency was still present under these conditions. The mGluR5 receptor antagonist MPEP did not eliminate DSI but did significantly shorten its duration, suggesting that the longer-lasting component of DSI requires mGluR5 receptor activation, rather than increases in intracellular calcium (Zhu and Lovinger, 2005). These data further suggest that mGluR-dependent long-term eCB-mediated depression of GABAergic transmission is calcium independent.
Recently, heterogeneity in the responsivity of GABAergic transmission to eCB signaling within the LA and BA divisions of the BLA has been demonstrated. Specifically, Win 55212-2-induced depression of eIPSCs is significantly greater in the BA than the LA, an effect related to higher expression of the CB1 receptor on inhibitory axon terminals in the BA relative to the LA (Yoshida et al., 2011). As such, this difference in CB1 receptor expression results in an increase in DSI magnitude in the BA compared to LA (Yoshida et al., 2011).
1.3.2 eCBs modulate glutamatergic transmission in the BLA
The effect of CB1 receptor activation on glutamatergic transmission in the BLA has also been extensively studied. When recording from neurons in the LA, and stimulating glutamatergic afferents arising from the EC, Win 55212-2 reduced AMPA-dependent eEPSC amplitude to 52% of control while significantly increasing the PPR, an effect absent in CB1−/− mice. Win 55212-2 also reduced the frequency of sEPSCs and action potential-independent mEPSCs without affecting the amplitude of either response. These data indicate that CB1 receptor activation decreases EC-evoked excitatory glutamatergic transmission onto LA principal neurons via a presynaptic mechanism (Azad et al., 2003). This effect was subsequently confirmed to be CB1 receptor-dependent using mutant mice with selective deletion of CB1 receptors from forebrain glutamatergic neurons (Domenici et al., 2006).
Short-term eCB mediated synaptic plasticity in the form of depolarization-induced suppression of excitation (DSE), has also been described in the LA (Kodirov et al., 2009). Lutz and co-workers found that 10 seconds of depolarization produced a slowly developing, low magnitude depression of EPSCs recorded from LA pyramidal neurons. DSE of sEPSCs was also observed in this study, both of which were mediated via activation of CB1 receptors (Kodirov et al., 2009).
In addition to direct modulation of EPSCs by cannabinoids within the LA, Huang et al. have demonstrated a role for eCB signaling in amphetamine-induced LTD of EC-evoked EPSPs in LA principal neurons (Huang et al., 2003). Application of amphetamine caused a rapid, dose-dependent reduction in eEPSP slope with an early component (acute depression) and late component (LTD). Interestingly, the CB1 receptor antagonist, AM251, blocked the amphetamine-induced acute depression and LTD, while having no effect on eEPSP slope alone. In this preparation, Win 5512-2 produced an initial depression and LTD similar to amphetamine, an effect abolished by AM 251. A presynaptic mechanism of action for both amphetamine and Win 55212-2 was demonstrated by showing an increase in PPR and a reduction in mEPSPs frequency, but not amplitude, for both compounds. Furthermore, Win 55212-2 occluded amphetamine-induced LTD, while amphetamine occluded Win 55212-2-induced LTD. Similarly, blockade of eCB transport with AM404, at low concentrations, mimicked amphetamine and Win 55212-2 induced LTD, while at high concentrations occluded amphetamine-induced LTD. eCB-mediated amphetamine LTD was dependent upon postsynaptic calcium influx and P/Q-type calcium channel activity. Taken together, these data strongly suggest that amphetamine-induced LTD is mediated by eCBs acting at presynaptic CB1 receptors that regulate glutamate release onto LA principle neurons (Huang et al., 2003).
Recently, a role for eCB signaling at thalamic inputs to the LA has also been demonstrated (Shin et al., 2010). In this study, LFS of thalamic inputs to the LA combined with post-synaptic depolarization resulted in LTP that is expressed post-synaptically. This post-LTP requires post-synaptic calcium and CB1 activation to suppress a form of kainate-mediated presynaptic LTP. These authors conclude that combined LFS and post-synaptic depolarization releases eCBs that counteract LFS-induced presynaptic LTP, resulting in an exclusive post-synaptic LTP. These data suggest a hierarchical order of pre- and post-synaptic LTP at thalamo-LA synapses that is regulated by eCB signaling (Shin et al., 2010).
The effects of cannabinoid signaling on neuronal population activity and lateral amygdalar circuits in vivo and in vitro have been explored. Azad et al. explored the effects of cannabinoid signaling on EC stimulation-induced local field potentials within the LA. Win 55212-2 reduced field potential amplitudes to 52% of control (Azad et al., 2003). This effect was eliminated by the application of SR141716 (which had no effect on field potential amplitude alone), as well as the Gi/o protein inhibitor pertussis toxin (PTX), and absent in CB1−/− mice. Furthermore, inhibition of PKA did not affect Win 55212-2-induced reductions in LA field potential amplitude (Azad et al., 2003). These data suggest the AC-PKA pathway does not significantly contribute to the CB1 receptor-mediate reductions in field potential amplitude within the LA.
A role for eCB signaling in synaptic plasticity at the neuronal population level has also been examined. High frequency stimulation (HFS; 5 trains of 100 Hz for 1 second) of the EC induced a long-term potentiation (LTP) of population spike amplitude within the LA (Marsicano et al., 2002). This HFS-induced LTP was significantly enhanced in CB1−/− mice, but not affected by SR141716, suggesting that long-term neural adaptations in these mice could underlie these effects, rather than an acute involvement of eCB signaling. Similarly, LFS (900 pulses at 1 Hz) produced a LTD of population spike amplitude within the LA, which was similar in both wild-type and CB1−/− mice, suggesting no role for eCB signaling in these forms of synaptic plasticity (Marsicano et al., 2002). However, LFS-LTD was prevented by activation of the CB1 receptor specifically located on GABAergic terminals (Azad et al., 2008). Pre-application of either CB1 receptor agonist, Win 55212-2 or THC, prevented LFS-LTD via a mechanisms involving Gi/o proteins, inwardly-rectifying potassium channels, and PKA-dependent regulation of N-type calcium channels. These data provide a novel link between eCB signaling at GABAergic synapses and long-term plasticity at excitatory synapses in the LA (Azad et al., 2008).
1.3.3 eCB modulation of synaptic signaling in the CeA
The first study that examined CB1 receptor signaling in the CeA found no effect of Win 55212-2 on GABAergic transmission in this region (Katona et al., 2001). However a subsequent study by Roberto et al. examined eIPSPs from neurons within the CeAM, and found that Win 55212 caused a ~50% depression in eIPSP amplitude (Roberto et al., 2010). Interestingly, blockade of CB1 receptors with SR141716 caused a ~130% increase in eIPSP amplitude, suggesting a significant tonic eCB-mediated suppression GABA release at these synapses. The SR141716-induced increase in eIPSP amplitude was abolished by postsynaptic calcium chelation, supporting the notion of enhanced eCB tone, rather than effects due to the inverse-agonist properties of SR141716. In this study, Win 55212-2 was able to reverse the effects of ethanol to enhance eIPSP amplitude (Roberto et al., 2010).
In addition to CB1 agonist-induced synaptic suppression, DSI and DSE have both been demonstrated within the CeAM. Kamprath et al. recently demonstrated that GABAergic inputs from the CeAL to the CeAM were susceptible to DSI (Kamprath et al., 2011). In fact, depolarizations as short as 100 ms induced DSI in these neurons. Similarly, excitatory BLA inputs to CeAM were susceptible to DSE: however much longer depolarization times were required (up to 10 seconds). Moreover, both DSI and DSE were blocked by CB1 receptor antagonists and absent in CB1−/− mice and showed rapid adaptations to acute foot-shock stress as will be discussed below (Kamprath et al., 2011). Taken together, these data indicate a high level of eCB tone regulating GABAergic transmission in the CeAM, and excitatory synapses are less susceptible than GABAergic synapses to eCB-mediated retrograde signaling. Whether these differences are due to either reduced CB1 receptor function at glutamatergic synapses (relative to GABAergic synapses) or differences in eCB production at these different synapses remains to be determined.
1.3.4 In vivo electrophysiological studies
Several studies have investigated the effects of cannabinoid signaling on amygdalar neuocircuitry in vivo. Pistis et al. studied the effect of systemically administered CB1 receptor agonists on the activity of nucleus accumbens neurons receiving monosynaptic inputs from BLA projection neurons, using single-unit extracellular recordings (Pistis et al., 2002). Stimulation currents were adjusted such that 50% of the stimuli delivered to the BLA (at 1 Hz) resulted in an action potential in the nucleus accumbens neuron being recorded. Systemic administration of the CB1 receptor agonists Win 55212-2, HU-210, and THC all decreased BLA-stimulation evoked spike probability in nucleus accumbens neurons, an effect reversed by the CB1 receptor antagonist SR141716, which when administered alone, had no effect (Pistis et al., 2002). These data suggest that CB1 receptor activation modulates glutamate release from BLA projection neurons, likely at the level of axon terminals that innervate target structures such as the nucleus accumbens.
Studies by Pistis et al., using extracellular single-unit recordings, explored the effect of CB1 receptor activation on the spontaneous activity of BLA principal neurons and local GABAergic interneurons within the BLA (Pistis et al., 2004). Administration of the CB1 receptor agonist HU-210 decreased BLA interneuron spike frequency, an effect reversed by the CB1 receptor antagonists AM251 and SR141716. Win 55212-2 had a bi-phasic effect in interneuron firing rate, as a low dose (0.125 mg/kg i.v.) stimulated firing, while higher doses (0.5–1 mg/kg i.v.) inhibited firing. Both effects blocked by the CB1 receptor antagonists AM251 and SR141716. Furthermore, both Win 55212-2 and HU-210 inhibited firing rate of antidromically identified BLA projection neurons. This effect of Win 55212-2 was reversed by the CB1 receptor antagonist SR141716 and the vanilloid receptor antagonist capsazepine, but not the CB1 receptor antagonist, AM251 (Pistis et al., 2004). Interestingly, AM251 not only failed to reverse the inhibitory effect of Win 55212-2, it actually strongly potentiated it. Neither SR141716 nor AM251 administered alone affected BLA projection neuron firing rate (Pistis et al., 2004).
These authors also studied the effect of cannabinoid receptor activity on medial prefrontal cortex (mPFC) stimulation-induced BLA neuron responses (Pistis et al., 2004). Stimulation current was adjusted such that 50% of stimuli produced spiking activity in BLA neurons. Win 55212-2 reduced mPFC stimulation-induced spike probability in BLA neurons to 48% of control; an effect that was reversed by SR141716, but not AM251. HU-210 did not affect mPFC stimulation-induced spike probability in BLA neurons (Pistis et al., 2004).
Recently, CB1 receptor-mediated signaling within the BLA-mPFC circuit was explored in the context of a Pavlovian conditioning paradigm. The mPFC–amygdala circuit has been shown to play a critical role in the processing and integration of emotionally salient sensory information and learning (Milad and Quirk, 2002), however, a mechanism by which cannabinoid signaling within this circuit could influence emotional associative learning at the level of the single neuron was unknown. To this end, Laviolette and Grace carried out single unit extracellular recordings of pyramidal, BLA-responsive mPFC neurons during a Pavlovian odor fear-conditioning procedure in anesthetized rodents (Laviolette and Grace, 2006b). This procedure involved the acquisition of an association between an aversive foot-shock and an odor cue, via repeated contiguous presentation. During the testing phase, animals were presented with either a footshock-paired odor (CS+) or a non-paired odor (CS−).
In this paradigm, post-conditioning CS+ presentation caused a stimulus-locked discharge in BLA-responsive mPFC neurons, which was absent upon CS− presentation. Systemic CB1 receptor activation by WIN 55,212-2 (0.5m/kg; i.v.), before conditioning, dramatically potentiated neuronal associative learning to the CS+ as demonstrated by both increased neuronal burst frequency and spikes within each burst event compared to saline treatment (Laviolette and Grace, 2006b). This effect was blocked by systemic co-administration of the CB1 receptor antagonist, AM251 (1.0mg/kg; i.v.). Given that cortical neuronal bursting has been hypothesized to represent the processing of reward-related learning cues, memory encoding, as well as decision-making, these results suggest that CB1 receptor signaling modulates the active encoding and expression of associative learning in BLA-responsive mPFC neurons (Laviolette and Grace, 2006b).
Laviolette and co-workers also investigated the role of cannabinoid signaling in LTP. LTP was examined using the functional connections between the BA and the prelimbic cortex (Tan et al., 2010). In vivo, tetanic stimulation (HFS, 3 sets of 10 trains,100 Hz) of the ipsilateral BA produced a robust LTP of field potentials within the prelimbic cortex of control rats. This effect was dose-dependently blocked by systemic pretreatment with the CB1 antagonist, AM251, thereby demonstrating that long-term synaptic plasticity along the BA-prelimbic cortex pathway is dependent on CB1 receptor signaling (Tan et al., 2010).
To further explore CB1 receptor-mediated signaling with the BA-prelimbic cortex circuit, this group also utilized single-unit in vivo extracellular recordings to determine how intra BA CB1-receptor activity could remotely control neuronal activity patterns within subpopulations of prelimbic neurons (Tan et al., 2011). These data indicate that intra-BA microinfusion of WIN 55,212-2 caused an increase in neuronal activity (as measured by the spontaneous firing rate, neuronal bursting activity and interspike intervals) of recorded prelimbic neurons. Conversely, intra-BA CB1 receptor blockade by treatment with AM251 caused a decrease in spontaneous prelimbic cortex neuronal activity. Interestingly, under the highest tested doses of both intra-BA treatment conditions, a small population of prelimbic cortex neurons demonstrated the opposite response patterns, i.e., a decrease or increase in prelimbic cortex neuronal activity in response to intra-BA CB1 receptor activation or inhibition respectively, possibly demonstrating the bidirectional functional connectivity between these two regions (Tan et al., 2011). These authors demonstrate that intra-BLA CB1 receptor can strongly modulate neuronal activity within a subpopulation of prelimbic cortex neurons (Tan et al., 2011).
A role for eCB signaling in alcohol-induced suppression of BA stimulated activation of nucleus accumbens neurons has also been demonstrated (Perra et al., 2005). Alcohol was shown to depressed BA-evoked spike probability in nucleus accumbens neurons, an effect reversed by SR141716 which, when administered alone had no effect on BA-evoked spike probability in nucleus accumbens neurons. The FAAH inhibitor URB597 also inhibited the alcohol-induced suppression of BLA-evoked spike probability in nucleus accumbens neurons. Interestingly, treatment with the CB1 receptor agonist Win 55212-2 15–20 hours before alcohol treatment reduced the alcohol-induced suppression of BLA-stimulated spike probability in nucleus accumbens neurons. This effect was not due to a functional tolerance as the dose-response curves for Win 55212-2-induced suppression of BLA-evoked spike probability in nucleus accumbens neurons was not different between control rats and rats treated with Win 55212-2 15–20 hours earlier (Perra et al., 2005). These data suggest a role for eCB signaling in alcohol-induced suppression of BLA-evoked spike probability in nucleus accumbens neurons and further suggest that CB1 receptor activity can modulate BA efferent neurotransmission.
1.3.5 Summary
eCB signaling robustly modulates GABAergic signaling in the BLA (see figure 1b). DSI and LTDi are expressed by BLA pyramidal neurons. While DSI is mediated via 2-AG, the identity of the eCB ligand subserving LTDi remains controversial. Circuit levels analyses reveal that LTDi in the BA facilitates activation of BA-CeA pathway. At glutamatergic synapses onto BLA neurons, 2-AG mediates DSE, and eCB ligand mediating amphetamine-LTD is not known. In general, the BA exhibits more robust eCB signaling than the LA. eCB signaling also modulates excitatory transmission between the BA and efferent targets, including the nucleus accumbens and prefrontal cortex. Within the CeAM, eCB-mediated DSI and DSE have also been demonstrated
1.4 STRESS-INDUCED ADAPTATIONS IN eCB SIGNALING IN THE AMYGDALA
1.4.1 Biochemical adaptations
We initially investigated the effects of acute and repeated restraint stress on levels of 2-AG and AEA in the amygdala of mice (Patel et al., 2004; Patel et al., 2005; Patel and Hillard, 2008). With regard to stress effects on 2-AG levels, we observed a progressive increase in tissue 2-AG levels, which was greater after 10, as compared to 5 or 1 consecutive days of restraint stress for 30 minutes per day. We subsequently showed that this increase in 2-AG was observable within the BLA specifically and was also associated with increases in two diacylglycerol precursors of 2-AG (Patel et al., 2009). Similar effects were observed in rats exposed to 10 days of consecutive restraint stress (Hill et al., 2010b). The stress-induced increase in 2-AG clearly demonstrates sensitization, however, the increase in 2-AG levels in response to repeated restraint stress are short-lived. Indeed, 2-AG levels peak at 20 minutes following the start of the 10th restraint exposure and, by 60 minutes, are almost back to control levels (Patel et al., 2009). Similar effects were observed in rats, where 24 hours after the 10th restraint exposure, 2-AG levels are not different from control (Hill et al., 2010b). These data suggest that levels of 2-AG surge in response to stress, and that if the stress is repeated in a predictable fashion, each surge gets progressively larger. However, between surges, 2-AG levels return to baseline. In addition to restraint stress, administration of chronic corticosterone to rats increases levels of 2-AG in the amygdala (Hill et al., 2005), nonetheless, acute corticosterone treatment produced only a non-significant trend toward 2-AG elevation (Hill et al., 2010a).
Another consistent finding in rats and mice is that restraint stress and chronic unpredictable stress produce a profound and sustained reduction in AEA levels within the amygdala (Patel et al., 2005; Hill et al., 2009). In contrast to 2-AG, reductions in AEA are not related to subsequent stress exposure as 24 hours after the last stress exposure, AEA levels are still profoundly reduced in many brain regions (Hill et al., 2009). In some cases, chronic stress produces an up-regulation of FAAH activity, suggesting increased tonic AEA degradation as a mechanism subserving the sustained reductions in AEA levels after repeated stress (Rademacher et al., 2008; Hill et al., 2009). In contrast to 2-AG, acute corticosterone produces a rapid (within 10 min) elevation of AEA in the amygdala.
With regard to changes in CB1 receptor expression, chronic corticosterone does not alter number or affinity of CB1 receptors in the amygdala of rats (Hill et al., 2005). Furthermore, neither chronic restraint stress in mice (Rademacher et al., 2008) nor chronic unpredictable stress in rats alters CB1 receptor number or affinity in the amygdala (Hill et al., 2008).
1.4.2 Synaptic adaptations
Several recent studies have begun to describe synaptic adaptations in eCB signaling in the BLA and CeA in response to both acute and chronic stress. We found that CB1-mediated suppression of GABAergic transmission is reduced in the BA following 10 days of restraint stress, suggesting a small but significant desensitization of CB1 receptors by chronic stress (Patel et al., 2009). In contrast, DSI is prolonged in mice exposed to 10, but not 1 day, of restraint stress (Patel et al., 2009). These data indicate eCB signaling at GABAergic synapses in the BLA is enhanced by chronic stress exposure, while CB1 receptors exhibit a small measure of functional desensitization.
Within the CeA, Pape and co-workers showed that 24 hours after a single tone-foot shock pairing, the magnitude of DSI and DSE was enhanced in neurons from the CeAM (Kamprath et al., 2011). This effect was not observed 3 days after foot-shock stress. These findings suggest that strong acute stressors can cause transient enhancements in short-term eCB signaling in the CeAM (Kamprath et al., 2011). The biochemical mechanisms subserving these adaptations require further investigation.
1.5 BEHAVIORAL CONSEQUENCES OF CANNABINOID-MEDIATED MODULATION OF AMYGDALA FUNCTION
1.5.1 Behavioral effects of eCB signaling in the BLA
eCB signaling within the BLA has been implicated in stress-induced analgesia, stress-induced neuroendocrine activation, and associative learning. This section will highlight some of these key behavioral roles subserved by eCB signaling in the BLA.
Bilateral microinjection of the CB1 agonist, Win 55212-2, produced antinociceptive effects in the tail-flick assay, which was blocked by a CB1, but not CB2 receptor antagonist, suggesting that activation of CB1 receptors in this region may be an important anatomical site for the production of cannabinoid antinociception (Hasanein et al., 2007). Interestingly, stress-induced analgesia is partially blocked by administration of the CB1 receptor antagonist SR141716 into the BLA (Connell et al., 2006). However, blockade of CB1 receptors in the right BLA did not alter fear-conditioning-induced analgesia in rats (Roche et al., 2007).
eCB signaling in the BLA has also been demonstrated to play an important role in stress-induced neuroendocrine activation. In response to acute restraint stress, AEA levels are robustly decreased in the BLA, and levels of AEA are inversely correlated with plasma corticosterone levels measures after stress exposure (Hill et al., 2009). Intra-BLA infusion of a FAAH inhibitor, or a direct CB1 agonist, reduced stress-induced corticosterone release, whereas infusion of the CB1 antagonist AM251 increased corticosterone release in response to stress (Hill et al., 2009). In addition, intra-BLA infusions of Win 55212-2 blocked elevated platform-stress-induced increase in plasma corticosterone in rats (Ganon-Elazar and Akirav, 2009). These studies suggest that decreased AEA signaling in the BLA facilitates stress-induced neuroendocrine activation. With regard to long-term adaptation of neuroendocrine stress responses, Hill et al. showed that 2-AG levels in the amygdala, following repeated restraint stress, are inversely correlated with plasma corticosterone levels measured after the last restraint session (Hill et al., 2010b). Importantly, bilateral intra-BLA infusions of the CB1 antagonist, AM251, prior to the last restraint session prevented the normal habituation of the corticosterone response (Hill et al., 2010b). These data link 2-AG-CB1 signaling in the BLA with functional habituation of the neuroendocrine response to repeated homotypic stressors.
With regard to unconditioned anxiety behaviors, unilateral intra-BLA infusions of THC produced an anxiogenic effect in rats measured using the elevated plus-maze (Rubino et al., 2008). In contrast, neither bilateral intra-BLA Win 55212-2, nor intra-BLA AM251 had any effect on locomotor behavior or anxiety measures in the open field assay (Ganon-Elazar and Akirav, 2009).
Using aversive associative learning paradigms, it has been shown that post-training blockade of CB1 receptors in the right BLA increased freezing behavior and ultrasonic vocalizations in a contextual fear-conditioning paradigm in rats (Roche et al., 2007). Along similar lines, Kamprath et al. recently showed that post-training intra-BLA infusions of the CB1 antagonist SR141716 impaired long-term extinction of conditioned freezing in a cue fear-conditioning paradigm in mice (Kamprath et al., 2011). Similarly, Ganon-Elazar and Akirav recently showed that intra-BLA infusion of AM251 either prior to conditioning or prior to extinction training, impaired extinction of inhibitory avoidance learning in rats (Ganon-Elazar and Akirav, 2009). In contrast, these authors found that intra-BLA infusion of Win 55212-2, prior to conditioning, did not affect acquisition or extinction of inhibitory avoidance learning in rats (Ganon-Elazar and Akirav, 2009). Interestingly, intra-BLA Win 55212-2 infusion did prevent: 1) the enhancing effects of platform stress on the acquisition of inhibitory avoidance learning when administered prior to conditioning and 2) the impairment in extinction caused by pre-extinction stress exposure. By means of a similar olfactory-fear conditioning paradigm, Laviolette and co-workers also demonstrated that asymmetrical, interhemispheric injection of AM251 in the BLA-mPFC pathway prior to conditioning, dose dependently prevented the encoding of associative fear memory as demonstrated by decreased percentage time spent freezing and increased spontaneous exploratory activity upon CS+ presentation (Laviolette and Grace, 2006a). These data indicate that eCB signaling in the BLA is critical for extinction of aversive conditioned responses but could also be important for the acquisition of these responses.
In contrast to aversive associative learning, little is known regarding the role of BLA eCB signaling in appetitively-motivated learning. Parsons and coworkers investigated the role of eCB signaling in the BLA in appetitive instrumental learning (Alvarez-Jaimes et al., 2008). They utilized a heroine self-administration paradigm to study the role eCB signaling in cue-induced relapse behavior and found that blockade of CB1 receptors in the prefrontal cortex and nucleus accumbens prevented cue-induced drug seeking behavior, whereas blockade of CB1 receptors in the BLA had no effect (Alvarez-Jaimes et al., 2008).
1.5.2 Behavioral effects of eCB signaling in the CeA
Initial studies into the role of cannabinoid signaling in the CeA by Onaivi and coworkers described an anxiogenic effect of THC (100 and 150 μg per injection) bilaterally administered into the CeA of mice using the elevated plus-maze test (Onaivi et al., 1995). However, a more recent study demonstrated that the CB1 agonist ACPA (1.25 and 5 ng/rat) caused an anxiolytic effect in rats when administered directly into the CeA using this same assay (Zarrindast et al., 2008). The differences in these studies could be related to the well-known bi-phasic effects of cannabinoids on anxiety, with low doses producing anxiolytic effects, while higher doses produce anxiogenic effects, since the former study used doses of THC significantly higher than doses of ACPA used in the latter. Interestingly, intra-CeA blockade of the CB1 receptor, using AM251, caused a decrease in exploratory activity without changing any anxiety measures (Zarrindast et al., 2008). In addition to these studies on unconditioned fear, the expression of conditioned fear is also modulated by eCB signaling in the CeA. Specifically, post-training infusion of the CB1 antagonist SR141716 into the CeA causes impairment within session (short-term) extinction of conditioned freezing responses to CS+ presentation (Kamprath et al., 2011). Taken together these data suggest that eCB signaling in the CeA serves to facilitate active/exploratory behaviors, and suppress passive/freezing coping responses to salient aversive stimuli.
With regard to motivational behavior, intra-CeA administration of the CB1 antagonist, AM251, produced place aversion and blocked the place preference induced by morphine (Rezayof et al., 2011). In contrast, activation of CB1 receptors with ACPA produced place preference when administered alone and enhanced place preference induced by morphine (Rezayof et al., 2011). One limitation of these studies is the lack of distinction between the CeAL and CeAM; due to the close proximity of these nuclei, it will be challenging to dissociate functional roles of these regions using microinjection techniques.
1.5.3 Summary
Taken together, these data indicate a prominent role for eCB signaling in the BLA in the acquisition/expression of aversive associative memories, as well as in the long-term (over periods of days) extinction and/or habituation of conditioned fear responses. In addition, eCB signaling within the BLA is important for the deleterious effect of stress on associative learning and extinction. eCB signaling in the BLA is also important for stress-induced neuroendocrine activation, and for the habituation of neuroendocrine response to repeated homotypic stress. Lastly, eCB signaling in the BLA contributes to descending pain modulation. Within the CeA, available data suggest that eCB signaling is important for short-term extinction of conditioned fear responses, facilitates exploratory behavior, and produces a reinforcing motivational state.
1.6 FUTURE DIRECTIONS
Although the past work summarized here has provided substantial insights into the role of eCB signaling in the amygdala, as well as the behavioral consequences of pharmacological and genetic modulation of this system, several key questions remain open for experimental analysis.
One important question is whether there are conditions under which physiologically released AEA and 2-AG exert actions over distinct synapses in the amygdala, especially since some types of stress appear to differentially regulate these two ligands (see above). For example, do AEA and 2-AG regulate retrograde signaling at different synapses based on chemical characteristics, i.e. AEA modulating glutamate release while 2-AG modulates GABA release? Alternatively, is differential signaling spatially regulated, with perisomatic synapses being regulated by one ligand regardless of the neurotransmitter being affected, while distal dendritic synapses being regulated by the other or both? Additionally, determining whether there are pathological conditions under which this type of regulation is perturbed could facilitate rational development of eCB-based treatment for these conditions.
Determining the adaptations in eCB synaptic signaling that occur after in vivo treatment with eCB modulating ligands with anxiolytic/antidepressant actions are important experiments that could shed light on fundamental aspects of the pathology of anxiety and depressive disorders. Some initial studies have suggested that chronic MGL inhibition with JZL184 treatment (which increases 2-AG levels and has anxiolytic effects in animal models) down-regulates CB1 receptor function after chronic treatment and, thus, impairs eCB retrograde signaling in some brain regions (Schlosburg et al., 2010). Therefore, if pathological conditions exist where eCB signaling is deleteriously enhanced, chronic, high-dose JZL184 administration may provide therapeutic benefits by “tuning down” eCB signaling in brain regions with high 2-AG turnover. In contrast, if pathological conditions can be identified where eCB signaling is reduced, low-doses of eCB degradation inhibitors could be beneficial by enhancing or prolonging eCB-mediated synaptic signaling in brain regions with eCB deficits. This hypothesis suggests that determining the relationship between tissue content of eCBs and retrograde signaling capacity is a critical question, as is determining the relationship between eCB signaling at different synapses in the amygdala and the expression of anxiety-related behavior and stress responses. This could allow us to determine whether eCB modulation of glutamatergic signaling in the amygdala is relevant for some aspects of behavior independent of modulation of GABAergic transmission, and vice versa, or are the coordinated effects of eCB on both neurotransmitters more relevant?
Moreover, understanding the relationship between adaptations in eCB signaling in models of affective disorders and during behavioral dysregulation will be critical to development of eCB-based therapeutics. Along these lines, Kamparath et al., have recently shown that fear-conditioned mice exhibit increased DSI/DSE in the central amygdala (see above), and that blockade of the CB1 receptor in this region impairs short-term behavioral adaptation to conditioned fear stimuli, suggesting these synaptic adaptations are a beneficial compensatory response aimed to moderate fear responses. Additional work, conceptually modeled after these types of studies, will be of significant benefit in developing a more detailed scheme illuminating the role of eCB signaling in the modulation of anxiety behaviors.
1.7 COMPARING eCB MODULATION OF AMYGDALA NEUROCIRCUITRY TO OTHER FOREBRAIN CIRCUITS
Although significant progress has been made in elucidating the molecular determinants of eCB signing in the brain (Kano et al., 2009), global functional properties have been scantly discussed in the literature. Here we suggest a structural framework, derived from both anatomical and physiological data, for describing what we consider to be a fundamental functional motif of the eCB signaling system that modulates forebrain cortico-striato-pallidal circuits (Fig. 2).
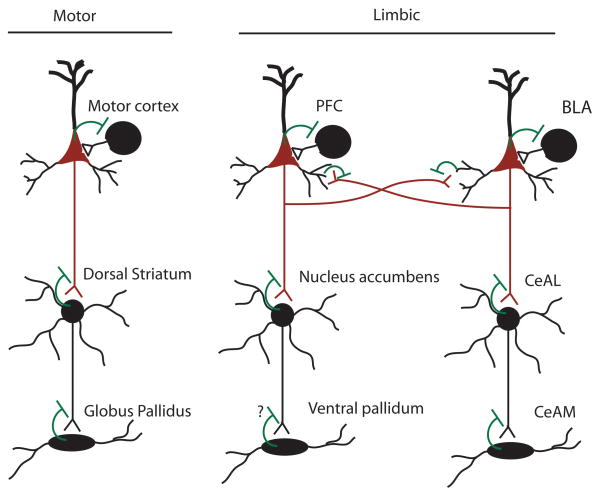
Figure 2.
A highly simplified schematic diagram of a common eCB signaling motif expressed across different cortical territories and their associated parallel cortico-striato-pallidal circuits. In “motor” circuits, cortical glutamatergic pyramidal cells (red), synapse onto striatal neurons, and are subject to eCB-mediated retrograde suppression of glutamate release (indicated by green line). GABAergic (black) striatal neurons send projections to the globus pallidus, which is also subject to eCB-mediated retrograde suppression of GABA release. Thus, each stage of the motor circuit is modulated by eCB signaling. A similar motif is evident within “limbic” cortical regions such as the PFC, and we suggest a similar motif may exist within the amygdala circuit. These limbic parallel circuits also exhibit a high degree of interaction, as indicated by the cross projections between and from, the PFC and BLA. (?) Indicates lack of experimental data.
Within neocortical brain regions, eCB signaling modulates GABA release from a subset of GABAergic interneurons, and modulates cortico-cortico and some thalamocortical glutamatergic inputs to cortical pyramidal neurons (Auclair et al., 2000; Bodor et al., 2005; Hill et al., 2007; Lafourcade et al., 2007). Within the striatum, eCB signaling modulates cortical glutamatergic inputs to medium spiny neurons, as well as recurrent collateral GABAergic transmission onto these cells (Gerdeman and Lovinger, 2001; Uchigashima et al., 2007). Within the globus pallidus, eCB signaling modulates GABA release from striatal neuron terminals onto pallidal neurons (Engler et al., 2006). Based on the anatomical and physiological data on the role of eCB signaling within the amygdala summarized above, a similar pattern can be observed in the amygdaloid complex. The BLA, a cortical-like structure (see introduction), exhibits functional eCB signaling essentially identical to other cortical regions, i.e. eCB modulation of local GABAergic transmission and afferent glutamatergic inputs. Within the CeAL, which exhibits homology to the striatum, eCBs modulate glutamatergic inputs to this region, while eCB signaling modulates GABAergic transmission from the CeAL to the CeAM (which shows homology to the globus pallidus). From these anatomical and physiological similarities arise several testable hypotheses. First, the functional role of eCB signaling, from an information processing perspective, is likely to be similar across homologous structures (i.e. PFC, sensory cortex, and BLA; striatum and CeAL; globus pallidus and CeAM). Second, the net modulation of circuit output is a function of the combined modulation at each step of the cortico-striato-pallidal circuit. Third, regulatory mechanisms that govern adaptations of eCB signaling may be similar across anatomical domains (i.e. cortex and BLA, striatum and CeAL).
Conceptualization of eCB signaling within the forebrain as being overlaid upon parallel but interactive cortico-striato-pallidal loops, with each node in the circuit subject to the same functional modulation across cortical subdomains (motor and limbic; see Fig. 2), could allow for a more integrated understanding of the role of eCB signaling on behavioral output. This conceptualization yields testable hypotheses that could drive future research into the role of eCB signaling in the modulation of forebrain function from both a systems and computational perspective.
Highlights.
eCB signaling components are highly expressed within the amygdala with considerable variability between different subnuclei.
eCBs modulate both GABAergic and glutamatergic transmission in the basolateral and central amygdala.
eCB signaling in the amygdala plays an important role in the modulation of stress-induced neuroendocrine activation and aversive associative learning.
ABBREVIATIONS
- 2-AG
2-arachidonoylglycerol
- AC
adenylate cyclase
- AEA
anandamide
- BA
basal amygdala
- BLA
basolateral amygdaloid complex
- BMA
basomedial amygdaloid nucleus
- CB1
cannabinoid receptor type-1
- CB1ir
cannabinoid receptor type-1 immunoreactive
- CeA
central amygdala
- CeAL
central amygdala lateral
- CeAM
central amygdala medial
- CRHR1
corticotrophin releasing hormone receptor type-1
- CCK
cholecystokinin
- dLA
dorsal lateral amygdala
- DSE
depolarization-induced suppression of excitation
- DSI
depolarization-induced suppression of inhibition
- DAGLα
diacylglycerol lipase alpha
- eCB
endocannabinoid
- EM
electron microscopy
- EPSC
excitatory post-synaptic current
- EPSP
excitatory post-synaptic potential
- EC
external capsule
- FAAH
fatty acid amide hydrolase
- GPCR
G-protein coupled receptor
- GAD65
glutamic acid decarboxylase 65
- IPSC
inhibitory post-synaptic current
- IPSP
inhibitory post-synaptic potential
- ISH
in situ hybridization
- ICMs
intercalated cell masses
- LA
lateral amygdala
- LTD
long-term depression
- LTDi
long-term depression of inhibitory transmission
- LTP
long-term potentiation
- LFS
low-frequency stimulation
- MeA
medial amygdala
- MGL
monoacylglycerol lipase
- PPR
paired pulse ratio
- PLC
phospholipase C
- PKA
protein kinase A
- 5-HT 3
serotonin type 3 receptor
- THC
Δ9-tetrahydrocannabinol
- vLA
ventral lateral amygdala
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahn K, McKinney MK, Cravatt BF. Enzymatic pathways that regulate endocannabinoid signaling in the nervous system. Chem Rev. 2008;108:1687–1707. doi: 10.1021/cr0782067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Jaimes L, Polis I, Parsons LH. Attenuation of cue-induced heroin-seeking behavior by cannabinoid CB1 antagonist infusions into the nucleus accumbens core and prefrontal cortex, but not basolateral amygdala. Neuropsychopharmacology. 2008;33:2483–2493. doi: 10.1038/sj.npp.1301630. [DOI] [PubMed] [Google Scholar]
- Amano T, Unal CT, Pare D. Synaptic correlates of fear extinction in the amygdala. Nat Neurosci. 2010;13:489–494. doi: 10.1038/nn.2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auclair N, Otani S, Soubrie P, Crepel F. Cannabinoids modulate synaptic strength and plasticity at glutamatergic synapses of rat prefrontal cortex pyramidal neurons. J Neurophysiol. 2000;83:3287–3293. doi: 10.1152/jn.2000.83.6.3287. [DOI] [PubMed] [Google Scholar]
- Azad SC, Eder M, Marsicano G, Lutz B, Zieglgansberger W, Rammes G. Activation of the cannabinoid receptor type 1 decreases glutamatergic and GABAergic synaptic transmission in the lateral amygdala of the mouse. Learning & memory (Cold Spring Harbor, NY. 2003;10:116–128. doi: 10.1101/lm.53303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azad SC, Kurz J, Marsicano G, Lutz B, Zieglgansberger W, Rammes G. Activation of CB1 specifically located on GABAergic interneurons inhibits LTD in the lateral amygdala. Learning & memory (Cold Spring Harbor, NY. 2008;15:143–152. doi: 10.1101/lm.741908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azad SC, Monory K, Marsicano G, Cravatt BF, Lutz B, Zieglgansberger W, Rammes G. Circuitry for associative plasticity in the amygdala involves endocannabinoid signaling. J Neurosci. 2004;24:9953–9961. doi: 10.1523/JNEUROSCI.2134-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisogno T, Howell F, Williams G, Minassi A, Cascio MG, Ligresti A, Matias I, Schiano-Moriello A, Paul P, Williams EJ, Gangadharan U, Hobbs C, Di Marzo V, Doherty P. Cloning of the first sn1-DAG lipases points to the spatial and temporal regulation of endocannabinoid signaling in the brain. The Journal of cell biology. 2003;163:463–468. doi: 10.1083/jcb.200305129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blankman JL, Simon GM, Cravatt BF. A comprehensive profile of brain enzymes that hydrolyze the endocannabinoid 2-arachidonoylglycerol. Chem Biol. 2007;14:1347–1356. doi: 10.1016/j.chembiol.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodor AL, Katona I, Nyiri G, Mackie K, Ledent C, Hajos N, Freund TF. Endocannabinoid signaling in rat somatosensory cortex: laminar differences and involvement of specific interneuron types. J Neurosci. 2005;25:6845–6856. doi: 10.1523/JNEUROSCI.0442-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bracey MH, Hanson MA, Masuda KR, Stevens RC, Cravatt BF. Structural adaptations in a membrane enzyme that terminates endocannabinoid signaling. Science (New York, NY. 2002;298:1793–1796. doi: 10.1126/science.1076535. [DOI] [PubMed] [Google Scholar]
- Cassell MD, Freedman LJ, Shi C. The intrinsic organization of the central extended amygdala. Ann N Y Acad Sci. 1999;877:217–241. doi: 10.1111/j.1749-6632.1999.tb09270.x. [DOI] [PubMed] [Google Scholar]
- Chevaleyre V, Heifets BD, Kaeser PS, Sudhof TC, Castillo PE. Endocannabinoid-mediated long-term plasticity requires cAMP/PKA signaling and RIM1alpha. Neuron. 2007;54:801–812. doi: 10.1016/j.neuron.2007.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevaleyre V, Takahashi KA, Castillo PE. Endocannabinoid-mediated synaptic plasticity in the CNS. Annual review of neuroscience. 2006;29:37–76. doi: 10.1146/annurev.neuro.29.051605.112834. [DOI] [PubMed] [Google Scholar]
- Chhatwal JP, Davis M, Maguschak KA, Ressler KJ. Enhancing cannabinoid neurotransmission augments the extinction of conditioned fear. Neuropsychopharmacology. 2005;30:516–524. doi: 10.1038/sj.npp.1300655. [DOI] [PubMed] [Google Scholar]
- Ciocchi S, Herry C, Grenier F, Wolff SB, Letzkus JJ, Vlachos I, Ehrlich I, Sprengel R, Deisseroth K, Stadler MB, Muller C, Luthi A. Encoding of conditioned fear in central amygdala inhibitory circuits. Nature. 2010;468:277–282. doi: 10.1038/nature09559. [DOI] [PubMed] [Google Scholar]
- Connell K, Bolton N, Olsen D, Piomelli D, Hohmann AG. Role of the basolateral nucleus of the amygdala in endocannabinoid-mediated stress-induced analgesia. Neuroscience letters. 2006;397:180–184. doi: 10.1016/j.neulet.2005.12.008. [DOI] [PubMed] [Google Scholar]
- Cravatt BF, Lichtman AH. The enzymatic inactivation of the fatty acid amide class of signaling lipids. Chemistry and physics of lipids. 2002;121:135–148. doi: 10.1016/s0009-3084(02)00147-0. [DOI] [PubMed] [Google Scholar]
- Davis M. Neurobiology of fear responses: the role of the amygdala. The Journal of neuropsychiatry and clinical neurosciences. 1997;9:382–402. doi: 10.1176/jnp.9.3.382. [DOI] [PubMed] [Google Scholar]
- Davis M, Whalen PJ. The amygdala: vigilance and emotion. Mol Psychiatry. 2001;6:13–34. doi: 10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- Di Marzo V, De Petrocellis L, Bisogno T. The biosynthesis, fate and pharmacological properties of endocannabinoids. Handbook of experimental pharmacology. 2005:147–185. doi: 10.1007/3-540-26573-2_5. [DOI] [PubMed] [Google Scholar]
- Dinh TP, Carpenter D, Leslie FM, Freund TF, Katona I, Sensi SL, Kathuria S, Piomelli D. Brain monoglyceride lipase participating in endocannabinoid inactivation. Proceedings of the National Academy of Sciences of the United States of America. 2002a;99:10819–10824. doi: 10.1073/pnas.152334899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinh TP, Freund TF, Piomelli D. A role for monoglyceride lipase in 2-arachidonoylglycerol inactivation. Chemistry and physics of lipids. 2002b;121:149–158. doi: 10.1016/s0009-3084(02)00150-0. [DOI] [PubMed] [Google Scholar]
- Domenici MR, Azad SC, Marsicano G, Schierloh A, Wotjak CT, Dodt HU, Zieglgansberger W, Lutz B, Rammes G. Cannabinoid receptor type 1 located on presynaptic terminals of principal neurons in the forebrain controls glutamatergic synaptic transmission. J Neurosci. 2006;26:5794–5799. doi: 10.1523/JNEUROSCI.0372-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egertova M, Cravatt BF, Elphick MR. Comparative analysis of fatty acid amide hydrolase and cb(1) cannabinoid receptor expression in the mouse brain: evidence of a widespread role for fatty acid amide hydrolase in regulation of endocannabinoid signaling. Neuroscience. 2003;119:481–496. doi: 10.1016/s0306-4522(03)00145-3. [DOI] [PubMed] [Google Scholar]
- Eggan SM, Lewis DA. Immunocytochemical distribution of the cannabinoid CB1 receptor in the primate neocortex: a regional and laminar analysis. Cereb Cortex. 2007;17:175–191. doi: 10.1093/cercor/bhj136. [DOI] [PubMed] [Google Scholar]
- Engler B, Freiman I, Urbanski M, Szabo B. Effects of exogenous and endogenous cannabinoids on GABAergic neurotransmission between the caudate-putamen and the globus pallidus in the mouse. The Journal of pharmacology and experimental therapeutics. 2006;316:608–617. doi: 10.1124/jpet.105.092718. [DOI] [PubMed] [Google Scholar]
- Ganon-Elazar E, Akirav I. Cannabinoid receptor activation in the basolateral amygdala blocks the effects of stress on the conditioning and extinction of inhibitory avoidance. J Neurosci. 2009;29:11078–11088. doi: 10.1523/JNEUROSCI.1223-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerdeman G, Lovinger DM. CB1 cannabinoid receptor inhibits synaptic release of glutamate in rat dorsolateral striatum. J Neurophysiol. 2001;85:468–471. doi: 10.1152/jn.2001.85.1.468. [DOI] [PubMed] [Google Scholar]
- Gulyas AI, Cravatt BF, Bracey MH, Dinh TP, Piomelli D, Boscia F, Freund TF. Segregation of two endocannabinoid-hydrolyzing enzymes into pre- and postsynaptic compartments in the rat hippocampus, cerebellum and amygdala. The European journal of neuroscience. 2004;20:441–458. doi: 10.1111/j.1460-9568.2004.03428.x. [DOI] [PubMed] [Google Scholar]
- Hasanein P, Parviz M, Keshavarz M, Javanmardi K. CB1 receptor activation in the basolateral amygdala produces antinociception in animal models of acute and tonic nociception. Clinical and experimental pharmacology & physiology. 2007;34:439–449. doi: 10.1111/j.1440-1681.2007.04592.x. [DOI] [PubMed] [Google Scholar]
- Hashimotodani Y, Ohno-Shosaku T, Kano M. Endocannabinoids and synaptic function in the CNS. Neuroscientist. 2007;13:127–137. doi: 10.1177/1073858406296716. [DOI] [PubMed] [Google Scholar]
- Heifets BD, Castillo PE. Endocannabinoid signaling and long-term synaptic plasticity. Annu Rev Physiol. 2009;71:283–306. doi: 10.1146/annurev.physiol.010908.163149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann H, Lutz B. Coexpression of the cannabinoid receptor type 1 with the corticotropin-releasing hormone receptor type 1 in distinct regions of the adult mouse forebrain. Neuroscience letters. 2005;375:13–18. doi: 10.1016/j.neulet.2004.10.080. [DOI] [PubMed] [Google Scholar]
- Hermann H, Marsicano G, Lutz B. Coexpression of the cannabinoid receptor type 1 with dopamine and serotonin receptors in distinct neuronal subpopulations of the adult mouse forebrain. Neuroscience. 2002;109:451–460. doi: 10.1016/s0306-4522(01)00509-7. [DOI] [PubMed] [Google Scholar]
- Hill EL, Gallopin T, Ferezou I, Cauli B, Rossier J, Schweitzer P, Lambolez B. Functional CB1 receptors are broadly expressed in neocortical GABAergic and glutamatergic neurons. J Neurophysiol. 2007;97:2580–2589. doi: 10.1152/jn.00603.2006. [DOI] [PubMed] [Google Scholar]
- Hill MN, Carrier EJ, McLaughlin RJ, Morrish AC, Meier SE, Hillard CJ, Gorzalka BB. Regional alterations in the endocannabinoid system in an animal model of depression: effects of concurrent antidepressant treatment. J Neurochem. 2008;106:2322–2336. doi: 10.1111/j.1471-4159.2008.05567.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill MN, Ho WS, Meier SE, Gorzalka BB, Hillard CJ. Chronic corticosterone treatment increases the endocannabinoid 2-arachidonylglycerol in the rat amygdala. Eur J Pharmacol. 2005;528:99–102. doi: 10.1016/j.ejphar.2005.10.058. [DOI] [PubMed] [Google Scholar]
- Hill MN, Karatsoreos IN, Hillard CJ, McEwen BS. Rapid elevations in limbic endocannabinoid content by glucocorticoid hormones in vivo. Psychoneuroendocrinology. 2010a;35:1333–1338. doi: 10.1016/j.psyneuen.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill MN, McLaughlin RJ, Bingham B, Shrestha L, Lee TT, Gray JM, Hillard CJ, Gorzalka BB, Viau V. Endogenous cannabinoid signaling is essential for stress adaptation. Proceedings of the National Academy of Sciences of the United States of America. 2010b;107:9406–9411. doi: 10.1073/pnas.0914661107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill MN, McLaughlin RJ, Morrish AC, Viau V, Floresco SB, Hillard CJ, Gorzalka BB. Suppression of amygdalar endocannabinoid signaling by stress contributes to activation of the hypothalamic-pituitary-adrenal axis. Neuropsychopharmacology. 2009;34:2733–2745. doi: 10.1038/npp.2009.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YC, Wang SJ, Chiou LC, Gean PW. Mediation of amphetamine-induced long-term depression of synaptic transmission by CB1 cannabinoid receptors in the rat amygdala. J Neurosci. 2003;23:10311–10320. doi: 10.1523/JNEUROSCI.23-32-10311.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamprath K, Romo-Parra H, Haring M, Gaburro S, Doengi M, Lutz B, Pape HC. Short-term adaptation of conditioned fear responses through endocannabinoid signaling in the central amygdala. Neuropsychopharmacology. 2011;36:652–663. doi: 10.1038/npp.2010.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kano M, Ohno-Shosaku T, Hashimotodani Y, Uchigashima M, Watanabe M. Endocannabinoid-mediated control of synaptic transmission. Physiological reviews. 2009;89:309–380. doi: 10.1152/physrev.00019.2008. [DOI] [PubMed] [Google Scholar]
- Katona I, Rancz EA, Acsady L, Ledent C, Mackie K, Hajos N, Freund TF. Distribution of CB1 cannabinoid receptors in the amygdala and their role in the control of GABAergic transmission. J Neurosci. 2001;21:9506–9518. doi: 10.1523/JNEUROSCI.21-23-09506.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapska E, Radwanska K, Werka T, Kaczmarek L. Functional internal complexity of amygdala: focus on gene activity mapping after behavioral training and drugs of abuse. Physiol Rev. 2007;87:1113–1173. doi: 10.1152/physrev.00037.2006. [DOI] [PubMed] [Google Scholar]
- Kodirov SA, Jasiewicz J, Amirmahani P, Psyrakis D, Bonni K, Wehrmeister M, Lutz B. Endogenous cannabinoids trigger the depolarization-induced suppression of excitation in the lateral amygdala. Learning & memory (Cold Spring Harbor, NY. 2009;17:43–49. doi: 10.1101/lm.1663410. [DOI] [PubMed] [Google Scholar]
- Lafourcade M, Elezgarai I, Mato S, Bakiri Y, Grandes P, Manzoni OJ. Molecular components and functions of the endocannabinoid system in mouse prefrontal cortex. PLoS ONE. 2007;2:e709. doi: 10.1371/journal.pone.0000709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laviolette SR, Grace AA. Cannabinoids Potentiate Emotional Learning Plasticity in Neurons of the Medial Prefrontal Cortex through Basolateral Amygdala Inputs. J Neurosci. 2006a;26:6458–6468. doi: 10.1523/JNEUROSCI.0707-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laviolette SR, Grace AA. The roles of cannabinoid and dopamine receptor systems in neural emotional learning circuits: implications for schizophrenia and addiction. Cell Mol Life Sci. 2006b;63:1597–1613. doi: 10.1007/s00018-006-6027-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long JZ, Li W, Booker L, Burston JJ, Kinsey SG, Schlosburg JE, Pavon FJ, Serrano AM, Selley DE, Parsons LH, Lichtman AH, Cravatt BF. Selective blockade of 2-arachidonoylglycerol hydrolysis produces cannabinoid behavioral effects. Nat Chem Biol. 2009;5:37–44. doi: 10.1038/nchembio.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mailleux P, Vanderhaeghen JJ. Distribution of neuronal cannabinoid receptor in the adult rat brain: a comparative receptor binding radioautography and in situ hybridization histochemistry. Neuroscience. 1992;48:655–668. doi: 10.1016/0306-4522(92)90409-u. [DOI] [PubMed] [Google Scholar]
- Marsicano G, Lutz B. Expression of the cannabinoid receptor CB1 in distinct neuronal subpopulations in the adult mouse forebrain. The European journal of neuroscience. 1999;11:4213–4225. doi: 10.1046/j.1460-9568.1999.00847.x. [DOI] [PubMed] [Google Scholar]
- Marsicano G, Wotjak CT, Azad SC, Bisogno T, Rammes G, Cascio MG, Hermann H, Tang J, Hofmann C, Zieglgansberger W, Di Marzo V, Lutz B. The endogenous cannabinoid system controls extinction of aversive memories. Nature. 2002;418:530–534. doi: 10.1038/nature00839. [DOI] [PubMed] [Google Scholar]
- Matsuda LA, Bonner TI, Lolait SJ. Localization of cannabinoid receptor mRNA in rat brain. The Journal of comparative neurology. 1993;327:535–550. doi: 10.1002/cne.903270406. [DOI] [PubMed] [Google Scholar]
- McDonald AJ. Is there an amygdala and how far does it extend? An anatomical perspective. Annals of the New York Academy of Sciences. 2003;985:1–21. doi: 10.1111/j.1749-6632.2003.tb07067.x. [DOI] [PubMed] [Google Scholar]
- McDonald AJ, Mascagni F. Localization of the CB1 type cannabinoid receptor in the rat basolateral amygdala: high concentrations in a subpopulation of cholecystokinin-containing interneurons. Neuroscience. 2001;107:641–652. doi: 10.1016/s0306-4522(01)00380-3. [DOI] [PubMed] [Google Scholar]
- Milad MR, Quirk GJ. Neurons in medial prefrontal cortex signal memory for fear extinction. Nature. 2002;420:70–74. doi: 10.1038/nature01138. [DOI] [PubMed] [Google Scholar]
- Morales M, Backman C. Coexistence of serotonin 3 (5-HT3) and CB1 cannabinoid receptors in interneurons of hippocampus and dentate gyrus. Hippocampus. 2002;12:756–764. doi: 10.1002/hipo.10025. [DOI] [PubMed] [Google Scholar]
- Morales M, Wang SD, Diaz-Ruiz O, Jho DH. Cannabinoid CB1 receptor and serotonin 3 receptor subunit A (5-HT3A) are co-expressed in GABA neurons in the rat telencephalon. The Journal of comparative neurology. 2004;468:205–216. doi: 10.1002/cne.10968. [DOI] [PubMed] [Google Scholar]
- Onaivi ES, Chakrabarti A, Gwebu ET, Chaudhuri G. Neurobehavioral effects of delta 9-THC and cannabinoid (CB1) receptor gene expression in mice. Behav Brain Res. 1995;72:115–125. doi: 10.1016/0166-4328(96)00139-8. [DOI] [PubMed] [Google Scholar]
- Ong WY, Mackie K. A light and electron microscopic study of the CB1 cannabinoid receptor in primate brain. Neuroscience. 1999;92:1177–1191. doi: 10.1016/s0306-4522(99)00025-1. [DOI] [PubMed] [Google Scholar]
- Pape HC, Pare D. Plastic synaptic networks of the amygdala for the acquisition, expression, and extinction of conditioned fear. Physiological reviews. 2010;90:419–463. doi: 10.1152/physrev.00037.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pare D, Quirk GJ, Ledoux JE. New vistas on amygdala networks in conditioned fear. Journal of neurophysiology. 2004;92:1–9. doi: 10.1152/jn.00153.2004. [DOI] [PubMed] [Google Scholar]
- Patel S, Hillard CJ. Adaptations in endocannabinoid signaling in response to repeated homotypic stress: a novel mechanism for stress habituation. The European journal of neuroscience. 2008;27:2821–2829. doi: 10.1111/j.1460-9568.2008.06266.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel S, Kingsley PJ, Mackie K, Marnett LJ, Winder DG. Repeated homotypic stress elevates 2-arachidonoylglycerol levels and enhances short-term endocannabinoid signaling at inhibitory synapses in basolateral amygdala. Neuropsychopharmacology. 2009;34:2699–2709. doi: 10.1038/npp.2009.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel S, Roelke CT, Rademacher DJ, Cullinan WE, Hillard CJ. Endocannabinoid signaling negatively modulates stress-induced activation of the hypothalamic-pituitary-adrenal axis. Endocrinology. 2004;145:5431–5438. doi: 10.1210/en.2004-0638. [DOI] [PubMed] [Google Scholar]
- Patel S, Roelke CT, Rademacher DJ, Hillard CJ. Inhibition of restraint stress-induced neural and behavioural activation by endogenous cannabinoid signalling. The European journal of neuroscience. 2005;21:1057–1069. doi: 10.1111/j.1460-9568.2005.03916.x. [DOI] [PubMed] [Google Scholar]
- Perra S, Pillolla G, Melis M, Muntoni AL, Gessa GL, Pistis M. Involvement of the endogenous cannabinoid system in the effects of alcohol in the mesolimbic reward circuit: electrophysiological evidence in vivo. Psychopharmacology (Berl) 2005;183:368–377. doi: 10.1007/s00213-005-0195-0. [DOI] [PubMed] [Google Scholar]
- Pertwee RG. Pharmacological actions of cannabinoids. Handbook of experimental pharmacology. 2005:1–51. doi: 10.1007/3-540-26573-2_1. [DOI] [PubMed] [Google Scholar]
- Piomelli D. The molecular logic of endocannabinoid signalling. Nature reviews. 2003;4:873–884. doi: 10.1038/nrn1247. [DOI] [PubMed] [Google Scholar]
- Pistis M, Muntoni AL, Pillolla G, Gessa GL. Cannabinoids inhibit excitatory inputs to neurons in the shell of the nucleus accumbens: an in vivo electrophysiological study. The European journal of neuroscience. 2002;15:1795–1802. doi: 10.1046/j.1460-9568.2002.02019.x. [DOI] [PubMed] [Google Scholar]
- Pistis M, Perra S, Pillolla G, Melis M, Gessa GL, Muntoni AL. Cannabinoids modulate neuronal firing in the rat basolateral amygdala: evidence for CB1− and non-CB1-mediated actions. Neuropharmacology. 2004;46:115–125. doi: 10.1016/j.neuropharm.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Rademacher DJ, Meier SE, Shi L, Ho WS, Jarrahian A, Hillard CJ. Effects of acute and repeated restraint stress on endocannabinoid content in the amygdala, ventral striatum, and medial prefrontal cortex in mice. Neuropharmacology. 2008;54:108–116. doi: 10.1016/j.neuropharm.2007.06.012. [DOI] [PubMed] [Google Scholar]
- Rezayof A, Sardari M, Zarrindast MR, Nayer-Nouri T. Functional interaction between morphine and central amygdala cannabinoid CB1 receptors in the acquisition and expression of conditioned place preference. Behav Brain Res. 2011;220:1–8. doi: 10.1016/j.bbr.2011.01.023. [DOI] [PubMed] [Google Scholar]
- Roberto M, Cruz M, Bajo M, Siggins GR, Parsons LH, Schweitzer P. The endocannabinoid system tonically regulates inhibitory transmission and depresses the effect of ethanol in central amygdala. Neuropsychopharmacology. 2010;35:1962–1972. doi: 10.1038/npp.2010.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roche M, O’Connor E, Diskin C, Finn DP. The effect of CB(1) receptor antagonism in the right basolateral amygdala on conditioned fear and associated analgesia in rats. The European journal of neuroscience. 2007;26:2643–2653. doi: 10.1111/j.1460-9568.2007.05861.x. [DOI] [PubMed] [Google Scholar]
- Royer S, Martina M, Pare D. An inhibitory interface gates impulse traffic between the input and output stations of the amygdala. J Neurosci. 1999;19:10575–10583. doi: 10.1523/JNEUROSCI.19-23-10575.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubino T, Guidali C, Vigano D, Realini N, Valenti M, Massi P, Parolaro D. CB1 receptor stimulation in specific brain areas differently modulate anxiety-related behaviour. Neuropharmacology. 2008;54:151–160. doi: 10.1016/j.neuropharm.2007.06.024. [DOI] [PubMed] [Google Scholar]
- Sah P, Faber ES, Lopez De Armentia M, Power J. The amygdaloid complex: anatomy and physiology. Physiol Rev. 2003;83:803–834. doi: 10.1152/physrev.00002.2003. [DOI] [PubMed] [Google Scholar]
- Samson RD, Pare D. A spatially structured network of inhibitory and excitatory connections directs impulse traffic within the lateral amygdala. Neuroscience. 2006;141:1599–1609. doi: 10.1016/j.neuroscience.2006.04.077. [DOI] [PubMed] [Google Scholar]
- Schlosburg JE, Blankman JL, Long JZ, Nomura DK, Pan B, Kinsey SG, Nguyen PT, Ramesh D, Booker L, Burston JJ, Thomas EA, Selley DE, Sim-Selley LJ, Liu QS, Lichtman AH, Cravatt BF. Chronic monoacylglycerol lipase blockade causes functional antagonism of the endocannabinoid system. Nature neuroscience. 2010;13:1113–1119. doi: 10.1038/nn.2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin RM, Tully K, Li Y, Cho JH, Higuchi M, Suhara T, Bolshakov VY. Hierarchical order of coexisting pre- and postsynaptic forms of long-term potentiation at synapses in amygdala. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:19073–19078. doi: 10.1073/pnas.1009803107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan H, Lauzon NM, Bishop SF, Bechard MA, Laviolette SR. Integrated cannabinoid CB1 receptor transmission within the amygdala-prefrontal cortical pathway modulates neuronal plasticity and emotional memory encoding. Cereb Cortex. 2010;20:1486–1496. doi: 10.1093/cercor/bhp210. [DOI] [PubMed] [Google Scholar]
- Tan H, Lauzon NM, Bishop SF, Chi N, Bechard M, Laviolette SR. Cannabinoid transmission in the basolateral amygdala modulates fear memory formation via functional inputs to the prelimbic cortex. J Neurosci. 2011;31:5300–5312. doi: 10.1523/JNEUROSCI.4718-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas EA, Cravatt BF, Danielson PE, Gilula NB, Sutcliffe JG. Fatty acid amide hydrolase, the degradative enzyme for anandamide and oleamide, has selective distribution in neurons within the rat central nervous system. Journal of neuroscience research. 1997;50:1047–1052. doi: 10.1002/(SICI)1097-4547(19971215)50:6<1047::AID-JNR16>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Tsou K, Brown S, Sanudo-Pena MC, Mackie K, Walker JM. Immunohistochemical distribution of cannabinoid CB1 receptors in the rat central nervous system. Neuroscience. 1998a;83:393–411. doi: 10.1016/s0306-4522(97)00436-3. [DOI] [PubMed] [Google Scholar]
- Tsou K, Nogueron MI, Muthian S, Sanudo-Pena MC, Hillard CJ, Deutsch DG, Walker JM. Fatty acid amide hydrolase is located preferentially in large neurons in the rat central nervous system as revealed by immunohistochemistry. Neuroscience letters. 1998b;254:137–140. doi: 10.1016/s0304-3940(98)00700-9. [DOI] [PubMed] [Google Scholar]
- Uchigashima M, Narushima M, Fukaya M, Katona I, Kano M, Watanabe M. Subcellular arrangement of molecules for 2-arachidonoyl-glycerol-mediated retrograde signaling and its physiological contribution to synaptic modulation in the striatum. J Neurosci. 2007;27:3663–3676. doi: 10.1523/JNEUROSCI.0448-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda N, Tsuboi K, Uyama T. Enzymological studies on the biosynthesis of N-acylethanolamines. Biochimica et biophysica acta. 2010;1801:1274–1285. doi: 10.1016/j.bbalip.2010.08.010. [DOI] [PubMed] [Google Scholar]
- Yoshida T, Uchigashima M, Yamasaki M, Katona I, Yamazaki M, Sakimura K, Kano M, Yoshioka M, Watanabe M. Unique inhibitory synapse with particularly rich endocannabinoid signaling machinery on pyramidal neurons in basal amygdaloid nucleus. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:3059–3064. doi: 10.1073/pnas.1012875108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarrindast MR, Sarahroodi S, Arzi A, Khodayar MJ, Taheri-Shalmani S, Rezayof A. Cannabinoid CB1 receptors of the rat central amygdala mediate anxiety-like behavior: interaction with the opioid system. Behavioural pharmacology. 2008;19:716–723. doi: 10.1097/FBP.0b013e3283123c83. [DOI] [PubMed] [Google Scholar]
- Zhu PJ, Lovinger DM. Retrograde endocannabinoid signaling in a postsynaptic neuron/synaptic bouton preparation from basolateral amygdala. J Neurosci. 2005;25:6199–6207. doi: 10.1523/JNEUROSCI.1148-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]