Abstract
Aims
Obesity is associated with insulin resistance, liver steatosis, and low-grade inflammation. The role of estrogen in sex differences in the above comorbidities is not fully understood. Our aim was to assess the role estrogen has in modulating adipocyte size, adipose tissue oxidative stress, inflammation, insulin resistance, and liver steatosis.
Methods
To determine the role estrogen has in the above comorbidities related to obesity, we randomize C57BL/6J mice into four groups (15 mice per group): 1) male, 2) non-ovariectomized female (novx), 3) ovariectomized female (ovx), and 4) ovariectomized female mice supplemented with 17β estradiol (ovx-E2). Mice received either a low-fat or a high-fat diet for 10 weeks. Outcomes measured were bodyweight, body fat, adipocyte diameter, adipose tissue lipolysis markers, adipose tissue oxidative stress, inflammation, insulin resistance, and liver steatosis.
Results
Male and ovx female mice consuming the high-fat diet had a higher propensity of gaining weight, specifically in the form of body fat. Estrogen protected female mice from adipocyte hypertrophy and from developing adipose tissue oxidative stress and inflammation. Moreover, novx-female and ovx-female+E mice had higher phosphorylated levels of PKA and HSL, markers associated with lipolysis. Additionally, male and ovx female mice had a higher propensity of developing liver steatosis and insulin resistance. In contrast, estrogen protected female mice from developing liver steatosis and from becoming insulin resistant.
Conclusion
We show that estrogen protects female mice from adipocyte hypertrophy and adipose tissue oxidative stress and inflammation. Furthermore, estrogen prevented female mice from developing liver steatosis and from becoming insulin resistant.
Keywords: Estrogen, sex, obesity, inflammation, adipose tissue, insulin resistance
Introduction
Obesity is a growing global epidemic that increases the risk of diabetes, cardiovascular disease, and metabolic syndrome [1]. Evidence supports the notion that the susceptibility to the above morbidities is modified by sex. However, the specific role estrogen plays in the differential susceptibility to these morbidities between males and females is not well known. Others have established that estrogen protects female mice from becoming obese, and that this protection is mediated through the estrogen receptor-alpha (ERα) [2–4]. On the other hand, it remains to be established if there are differences in adipocyte size, adipose tissue inflammation and oxidative stress between males and females, and more specifically how may modulate these biological parameters [5, 6].
Epidemiological studies show that premenopausal women are less likely to develop inflammation compared to age-matched men, suggesting that estrogen may protect against inflammatory diseases such as cardiovascular events [7, 8]. Moreover, studies show that postmenopausal women have a higher propensity of developing abdominal adiposity, which is associated with increased systemic levels of inflammatory cytokines, thus indicating that estrogen can modulate both body adiposity and systemic inflammation [9]. Evidence also implies that postmenopausal women have enlarged adipocytes and that the lipolytic activity in these adipocytes is high, which may explain why postmenopausal women have higher systemic levels of free fatty acids [10, 11]. Lipolysis is a tightly regulated process, which consists of the activation of key lipases. Upon phosphorylation by protein kinase A (PKA), adipose triglyceride lipase (ATGL) and hormone sensitive lipase (HSL) cleave free fatty acids off triacylglycerides, which are released into the blood. Hyperlipidemia can promote drastic morbidities; for example, excess free fatty acids in the blood can accumulate in the liver and skeletal muscle, which can lead to an inflammatory environment and to the development of liver steatosis and insulin resistance [12, 13]. Excess accumulation of fatty acids in the liver can increase the risk of liver steatosis, which has been suggested to be a risk factor for liver cancer [14]. Differences in liver lipid metabolism between males and females may be one explanation why males have a higher propensity of developing liver cancer. For instance, the global incidence of liver cancer is 2.4 fold greater in males compared to females [15]. Moreover, Naugler et al. showed that estrogen protects female mice from hepatocellular carcinoma by decreasing liver inflammation [16]. Furthermore, estrogen can indirectly protect female mice from liver cancer possibly by altering lipid metabolism. Evidence suggests that estrogen increases fatty acid oxidation, thus decreasing the probability of fatty acids accumulating in skeletal muscle and in the liver [17, 18]. The objective of the present study was to determine if estrogen protects female mice from liver steatosis and insulin resistance by modulating adipocyte biology.
To establish the role estrogen has in the susceptibility to obesity, insulin resistance, and liver steatosis, we randomized the following groups of mice to receive a low- or a high-fat diet: 1) males, 2) non-ovariectomized females (novx), 3) ovariectomized females (ovx), and 4) ovariectomized females supplemented with estrogen (ovx-E2). Male and ovx-female mice had a higher susceptibility of becoming obese compared to novx female mice and ovx-female+E mice. Male and ovx-female mice gained more adipose tissue and had larger adipocytes compared to novx-female and ovx-female+E mice. Moreover, we show that estrogen protects female mice from adipocyte oxidative stress and inflammation. Results also indicate that novx-female mice and ovx-female mice supplemented with estrogen are protected from liver steatohepatitis and insulin resistance. Overall, our results show that estrogen not only protects female mice from obesity but also its co-morbidities, possibly through the modulation of adipose tissue and lipid metabolism.
Methods
Mouse husbandry and diets
A total of 135 pathogen-free C57BL/6J male, non-ovariectomized female, ovariectomized female, and sham-ovariectomized female mice were purchased from Jackson Labs (Bar Harbor, Maine, USA) at 6 weeks of age and housed according to NIH guidelines (National Research Council, 1996) in the Animal Resources Center at the University of Texas at Austin. The animal protocol was approved by the Institutional Animal Care and Use Committee at UT-Austin. The mice were singly housed and maintained on a 12-hour light–dark cycle at a temperature of 22–24°C. After two weeks of acclimation, the mice were randomized, 15 mice per group, to receive a low-fat (LF) diet (10% fat, D12450B), or a high-fat (HF) diet (60% fat, D12492). To control for the effects of surgery on bodyweight, we included sham-ovariectomized female mice who consumed the low-fat diet. All diets were obtained from Research Diets, Inc. (New Brunswick, NJ, USA). A table with detailed information on these diets was previously described [19]. Mice were fed ad libitum; bodyweight, food, and liquid consumption were recorded weekly.
Estrogen supplementation
To further characterize the role estrogen plays in the susceptibility to obesity and insulin resistance, we implanted a 0.72mg 17β estradiol pellet into ovariectomized female mice, which delivered 5µg/d. This dosage protocol is similar to estradiol supplementation used by others and has been shown to re-establish physiological estradiol levels in ovariectomized females [20]. Control mice were implanted with placebo pellets. At 9 weeks of age, ovariectomized mice were randomized to receive either a placebo or an estradiol pellet. Mice were anesthetized with isoflurane, the dorsal area between the ear and shoulder was shaved and sterilized with 70% isopropyl alcohol, and a trochar was used to implant the 4.5 mm pellet subcutaneously.
Body composition
Body composition was assessed using magnetic resonance imaging (MRI), specifically, the EchoMRI QNMR from Jackson Labs (Bar Harbor, Maine, USA). This device allowed us to measure lean mass, percent body fat and water content without sedating the mice.
Measurement adipocyte diameter and quantification of gamma-H2AX
At necropsy, adipose tissue was fixed in 10% neutral buffered formalin for 48 hours and then transferred to 70% ethanol indefinitely. Adipose tissue was paraffin-embedded and cut 5µm thick. For histological analysis, tissues were hematoxylin and eosin-stained. The diameter of the adipocytes was determined using Nikon’s NIS Elements AR software (Melville, N.Y, USA). Eight samples from each group were randomly selected for analysis. In order to determine if estrogen protected female mice from adipocyte oxidative stress, we stained perigonadal adipose tissue with gamma-H2AX (γH2AX). Images were quantified using Nikon's NIS Elements Software at 20x magnification, with six slides taken per group, and two fields per slide. Briefly, the image RGB threshold was set in order to account for the stained proteins of the image. After a set threshold was applied, the area fraction, object count and total area threshold were calculated.
Quantitative real-time PCR (qRT-PCR)
We measured the mRNA levels of CD68, IL6 and TNFα in perigonadal adipose tissue to determine if estrogen protected female mice from adipose tissue inflammation. Total RNA was extracted from frozen white adipose tissue using an RNAeasy Mini Kit (Qiagen, Hilden, Germany) following the manufacturer’s instructions. The amount of RNA was determined by measuring the absorbance at 260 and 280 nm. Reverse transcription was conducted with a High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA), using 2µg of RNA for each reaction. Real time PCR was performed with a SYBR GreenER qPCR kit (Invitrogen, Carslbad, CA) and a Mastercycle Realplex Thermocycler (Eppendorf, Hamburg, Germany). The relative expression level of the target genes was normalized to the endogenous reference control gene 18s rRNA. The primers are available upon request.
Assessment of serum triglycerides and free fatty acids
To determine if estrogen altered serum lipid levels, we measured serum triglycerides and free fatty acids. Mice were fasted three hours prior to necropsy and serum was collected. Serum triglycerides were assessed using a triglyceride assay from Wako Pure Chemical Industries (Osaka, Japan). Serum free fatty acids were detected using TSZ elisa free fatty acid kit (Framingham, MA).
Protein extraction and immunoblotting
To gain a better understanding of the role of estrogen in lipolysis we homogenized perigonadal adipose tissue protein lysates in T-PER Tissue Protein Extraction Reagent (Thermo Scientific, 78510) and prepared according to manufacturer’s instructions. Equal amounts of protein (30µg) were subjected to gel electrophoresis on a 4–12% Bis Tris gradient gel. Proteins were then transferred to PVDF membranes. The membranes were blocked with 5% non-fat dry milk (NFDM) in Tris-buffered saline with 0.1% Tween (TBST) buffer. The membranes were immunoblotted with antibodies from Cell Signaling (P-HSL (ser563), HSL, PPKA, ATGL, and Beta-Actin) at 4°C overnight. Following incubation with the primary antibody, membranes were washed in TBS-T and then exposed to a rabbit secondary antibody conjugated with horseradish peroxidase. After secondary antibody incubation, the membranes were washed again with TBST followed by enhanced chemiluminescence reagent (ECL) (Pierce,Rockford, IL). Bands were quantified using Image J (NIH, Bethesda, MD) as previously described [21]
Liver histology and measurement of serum ALT
In order to assess if estrogen modulated liver biology, we collected liver at the time of necropsy and fixed it in 10% neutral buffered formalin. Liver tissue was paraffin-embedded and cut 5µm thick and then stained with hematoxylin and eosin. Images were taken at a 10× magnification. To establish if estrogen affected liver inflammation, we analyzed levels of serum alanine transaminase (ALT), a biomarker for liver inflammation. Serum was collected from the mice at necropsy and was analyzed using an ALT Elisa kit from Bioo Scientific (Austin, TX).
Insulin tolerance test
To determine if estrogen protected female mice from insulin resistance, we conducted an insulin tolerance test. Ten randomly selected mice were fasted for 7 hours and then intraperitoneally injected with insulin 0.75U/g of bodyweight. Blood glucose was measured using a Glucometer Elite (Bayer, Elkhart, IN). Approximately half a drop of blood was drawn from each mouse tail. Blood glucose levels were measured at 0, 15, 30, 60, and 120 minutes from injection time and area under the curve was calculated.
Statistics
To measure if the effects of both diet and sex were significantly different, results were analyzed by ANOVA with pairwise comparisons and a post-hoc comparison of means using Tukey’s Honestly Significant Difference. All results are presented as mean ± standard error mean (SEM). SPSS version 16.0 for Windows (SPSS Inc., Chicago, IL, USA) was used for all statistical comparisons. P-values ≤ 0.05 were considered statistically significant.
Results
Sex differences in bodyweight and adiposity
Table 1 shows that after 10 weeks of consuming the low-fat diet, male and ovx-female mice gained significantly more weight compared to novx-female and ovx-female+E2 mice (p<0.05). Male mice consuming the high-fat diet gained significantly more body weight than novx-female mice (15.32 vs. 7.59g, p < 0.001). Ovx-female mice mimicked the male mice in their bodyweight change (16.47g), but when supplemented with estrogen, their change in bodyweight was minimal and similar to the novx-female mice (4.07g, p<0.001). This pattern was also seen in total body adiposity levels, Table 2. Total adiposity levels were assessed at baseline, 5 weeks, and 10 weeks. After 10 weeks, there was a significant change in adiposity levels for mice consuming the high-fat diet. Briefly, male mice increased their body adiposity levels by approx 28% and ovx-female mice increased their adiposity by 36%. However, novx-female mice only increased their adiposity by 13%, and ovx-female mice supplemented with estrogen only increased their adiposity by 4%.
Table 1. Bodyweight change.
When exposed to a low-fat and high-fat diet, there was no significant difference in calorie consumption between novx-females and ovx-females.
| Change in Bodyweight (g) | ||||
|---|---|---|---|---|
| Males | Novx-females | Ovx-females | Ovx-females+E | |
| LF | 6.30a | 2.49 | 4.11 | 1.59 |
| HF | 15.32b | 7.59 | 16.47b | 4.07 |
Significantly different compared to ovx-females and ovx-females+E in the low fat diet group.
Significantly different compared to novx-females and ovx-females+E in the high fat diet group, p<0.001.
Table 2. Change in Body fat (%).
When exposed to a low-fat and high-fat diet, there was no significant difference in calorie consumption between novx-females and ovx-females.
| Change in Bodyfat (%) | ||||
|---|---|---|---|---|
| Males | Novx-females | Ovx-females | Ovx-females+E | |
| LF | 11.18% | 4.07% | 13.87%a | −2.19% |
| HF | 27.70%b | 12.74% | 36.45%b | 3.40% |
Significantly different compared to ovx-females and ovx-females+E in the low fat diet group.
Significantly different compared to novx-females and ovx-females+E in the high fat diet group, p<0.001.
Sex differences in adipocyte diameter
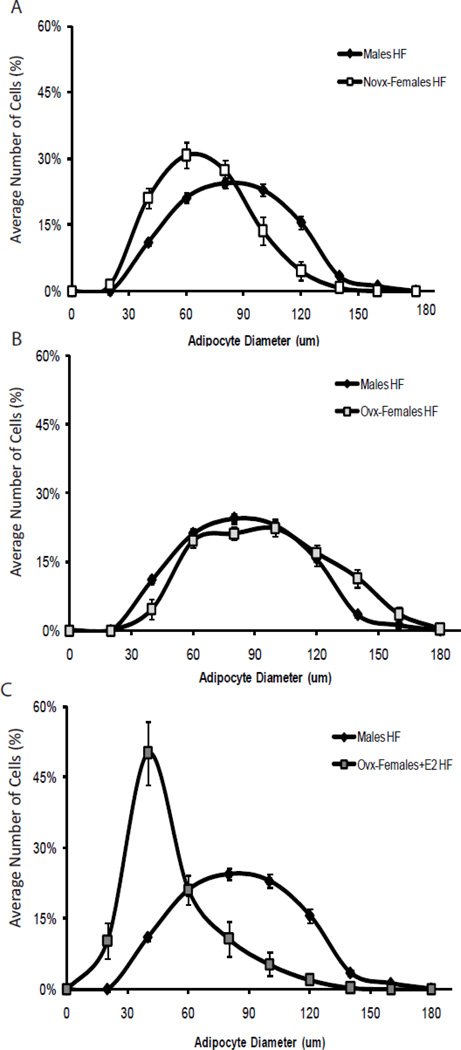
To assess if sex and estrogen influenced the adipocyte morphology, we measured adipocyte diameter. Since the most dramatic changes in body adiposity were observed in the mice consuming the high-fat diet, we only measured adipocyte diameter within the high-fat diet groups. Results in Figure 1 show that male mice had larger adipocytes compared to novx-female mice (75.22±1.16 vs. 59.79±3.23µm, p <0.001). However, adipocyte diameter increased when the ovaries were removed from the female mice (84.88±2.38µm), but decreased when the ovx-female mice were supplemented with estrogen (41.48±4.91µm). Our data suggest that estrogen decreased the number of large adipocytes in female mice.
Figure 1. Sex differences in adipocyte diameter.
Panel A: Mice were randomized to receive either a low- or high-fat diet for 10 weeks (n=15). When exposed to a high-fat diet, males had significantly larger adipocytes compared to novx-females. Panel B shows that when the ovaries were removed from female mice, their adipocyte diameter mimicked that of males. Panel C demonstrates that when estrogen was supplemented to the ovx-females, their overall adipocyte diameter and size decreased to a size similar to that of the novx-females.
Sex differences in adipose tissue oxidative stress and inflammation
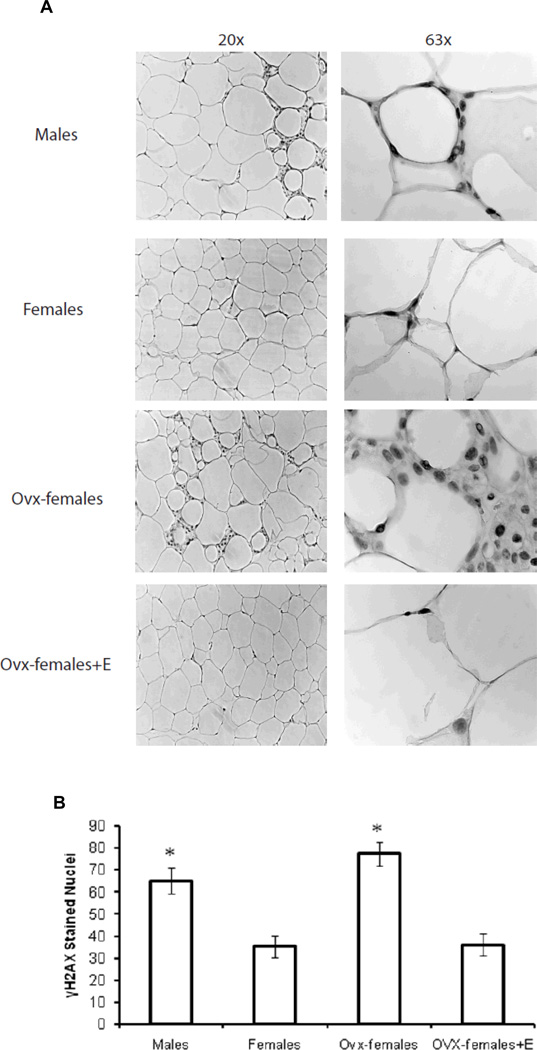
In order to determine if there was a relationship between adipocyte hypertrophy and adipocyte oxidative stress, we stained perigonadal adipose tissue with gamma-H2AX (γH2AX), which has been previously used as a biomarker for oxidative stress [22]. Figure 2A shows images taken at 20× and 63× magnification. Figure 2B shows the quantification of γH2AX stained nuclei for images taken at 20× (6 slides per animal and 2 fields per slide). Briefly, male and ovx-female mice had significantly more γH2AX stained nuclei compared to novx-female and ovx-female+E mice; thus, the data suggest that estrogen protects against the development of adipose tissue oxidative stress.
Figure 2. Sex differences in adipocyte oxidative stress.
Panel A shows perigonadal adipose tissue stained for γH2AX. Images were taken at 20× and 63× magnification. Panel B shows the quantification of γH2AX at 20× magnification. For mice consuming the high-fat diet, males and ovx-females had significantly more γH2AX stained nuclei. Briefly, 6 slides were taken per animal and 2 different images were captured per slide. *Significantly different compared to novx-female and ovx-female+E, p<0.05 (n=6).
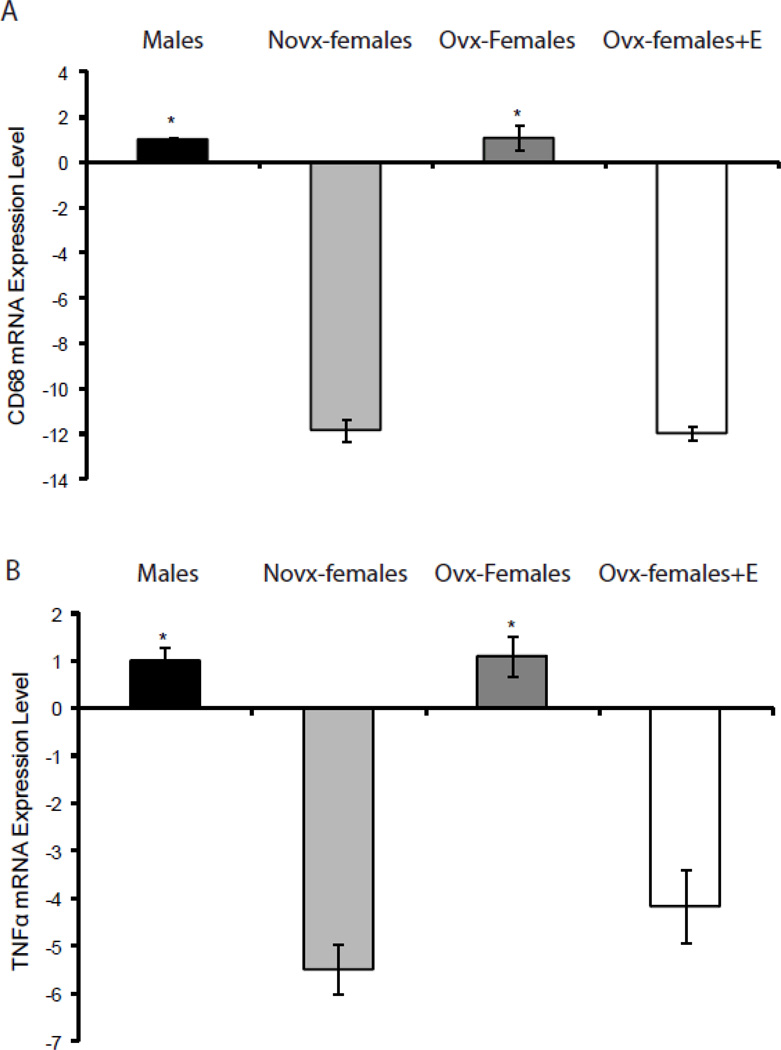
To establish if the sex differences we observed in adipocyte oxidative stress were also reflected in adipose tissue inflammation, we measured mRNA levels of CD68, IL6, and TNFα. CD68 is a glycoprotein that is used to identify macrophages, IL6 and TNFα are inflammatory markers produced by macrophages and adipocytes [23, 24]. Results show that novx-female and ovx-female+E mice have significantly lower CD68 and TNFα mRNA levels compared to male and ovx-female mice (−11.8x and −12x vs. 1.00 and 1.1, p<0.05), Figure 3A and 3B, respectively. Moreover, IL6 mRNA levels were lower in novx-female and ovx-female+E mice; however, they were not significantly different (data not shown). Hence, the data suggest that estrogen protects female mice from adipocyte oxidative stress and inflammation.
Figure 3. Sex differences in adipocyte inflammation.
RNA was isolated from perigonadal adipose tissue and quantified using QRTPCR. Panels A and B show that males and ovx-females had significantly more CD68 and TNFα mRNA levels compared to novx-female and ovx-female+E mice. *Significantly different compared to novx-female and ovx-female+E, p<0.05 (n=6).
Sex differences in serum triglycerides and free fatty acids
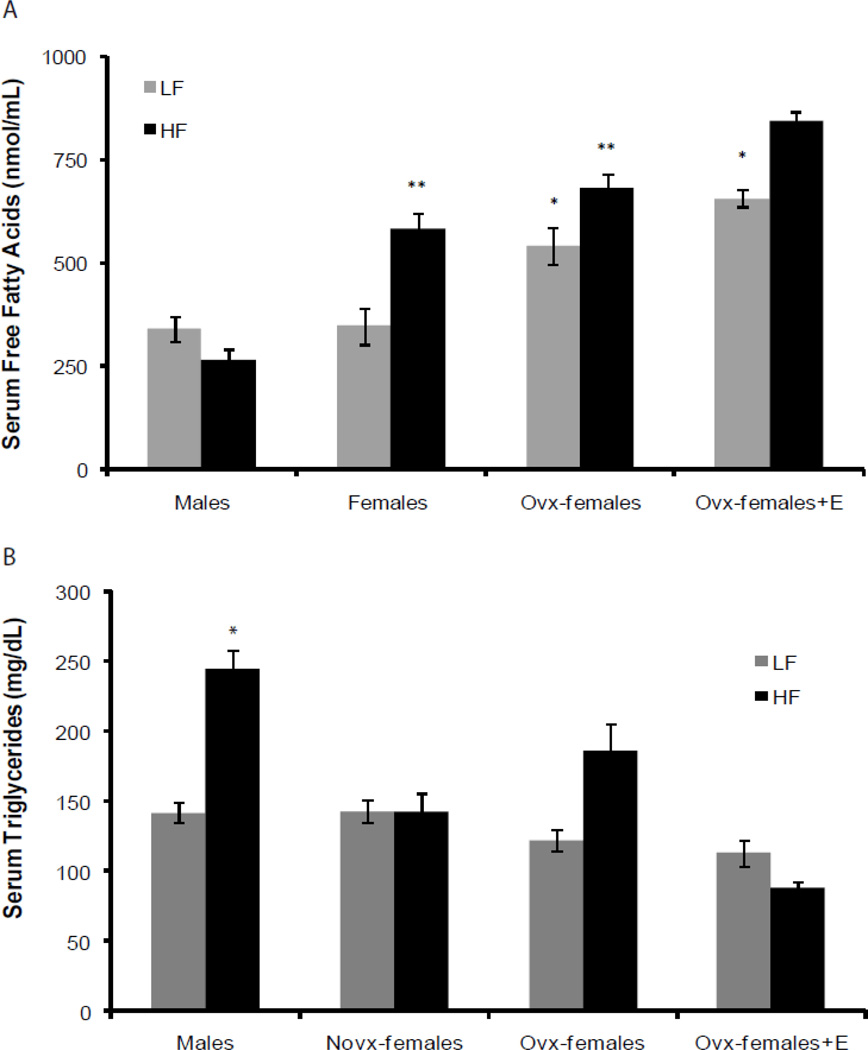
We measured systemic levels of serum triglycerides and free fatty acids to assess if sex and estrogen altered their levels. Figure 4A shows that male mice had significantly higher serum triglyceride levels compared to novx-female and ovx-female+E mice (244±13 vs. 142±13 and 88±5 mg/dL, p<0.05). In addition, ovx-female mice consuming the high-fat diet also had higher serum triglyceride levels compared to novx-female and ovx-female+E mice, but this was not significant (186±19 vs. 142±13 and 88±5 mg/dL). Surprisingly, we found that male mice had significantly lower serum free fatty acids compared to novx-female, ovx-female, and ovx-female+ E mice (264±26 vs. 583±38, 680±37, 845±21 nmol/mL, p<0.001), Figure 4B. Our data suggest that there are possible sex differences in lipid metabolism independent of estrogen.
Figure 4. Sex differences in serum triglycerides and free fatty acids.
Serum was collected at the time of necropsy after a 3hr fast and was analyzed for serum triglycerides and free fatty acids. Briefly, males had significantly higher serum triglycerides compared to novx-female and ovx-female+E; however, males had significantly lower serum free fatty acids compared to all other groups. Panel A: *Significantly different compared to novx-female and ovx-female+E2 within the high-fat diet groups, p<0.05 (n=8). Panel B: *Significantly different compared to novx-female and ovx-female+E within the low-fat diet groups, p<0.001 (n=8). **Significantly different compared to all others within the high-fat diet groups, p<0.001 (n=8).
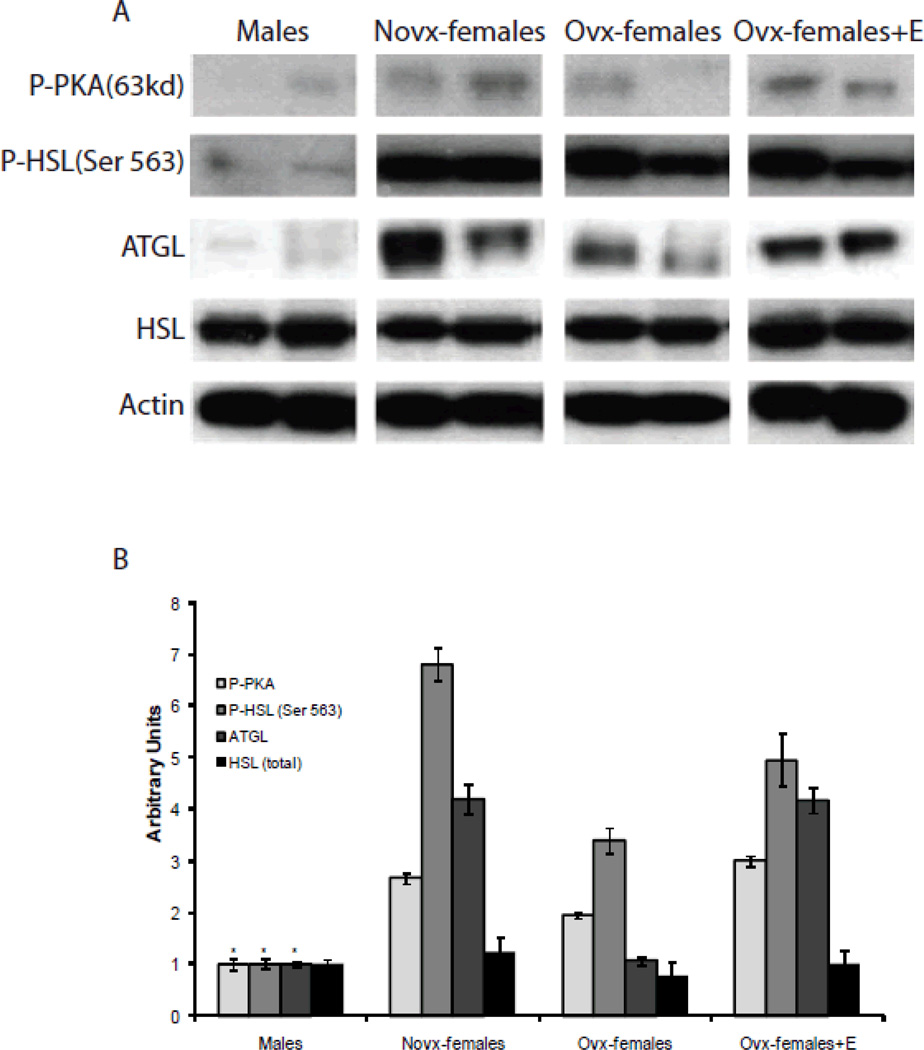
Sex differences in lipolysis
To determine the sex differences in lipolysis we assessed protein expression and activity in perigonadal adipose tissue in mice consuming the high-fat diet. As shown in Figure 5A, novx-female and ovx-female+E mice had significantly more phosphorylated PKA compared to males. Additionally, novx-female and ovx-female+E mice also had significantly more phosphorylated HSL at ser563, which is known to be phosphorylated by PKA [25]. Interestingly, ovx-female mice also had an increase in phosphorylation of PKA and HSL compared to male mice, although this was not significant, it suggests that ovx-female mice may have increased lipolysis. Protein expression of ATGL was also increased in the novx-female and ovx-female+E mice. Our data suggests that the presence of estrogen is activating lipolysis in the adipocytes of female mice. Furthermore, independent of estrogen, ovx-female mice are also experiencing lipolysis, which is contributing to the elevated serum free fatty acids.
Figure 5. Sex differences in lipolysis.
Panel A: Adipose tissue was collected from the perigonadal fat pad and 30µg of protein was extracted for western blot analysis. Images represent 3 independent experiments (n=6). Panel B: Images were quantified using Image J. * Significantly different compared to novx-female and ovx-female+E, p<0.05 (n=6)
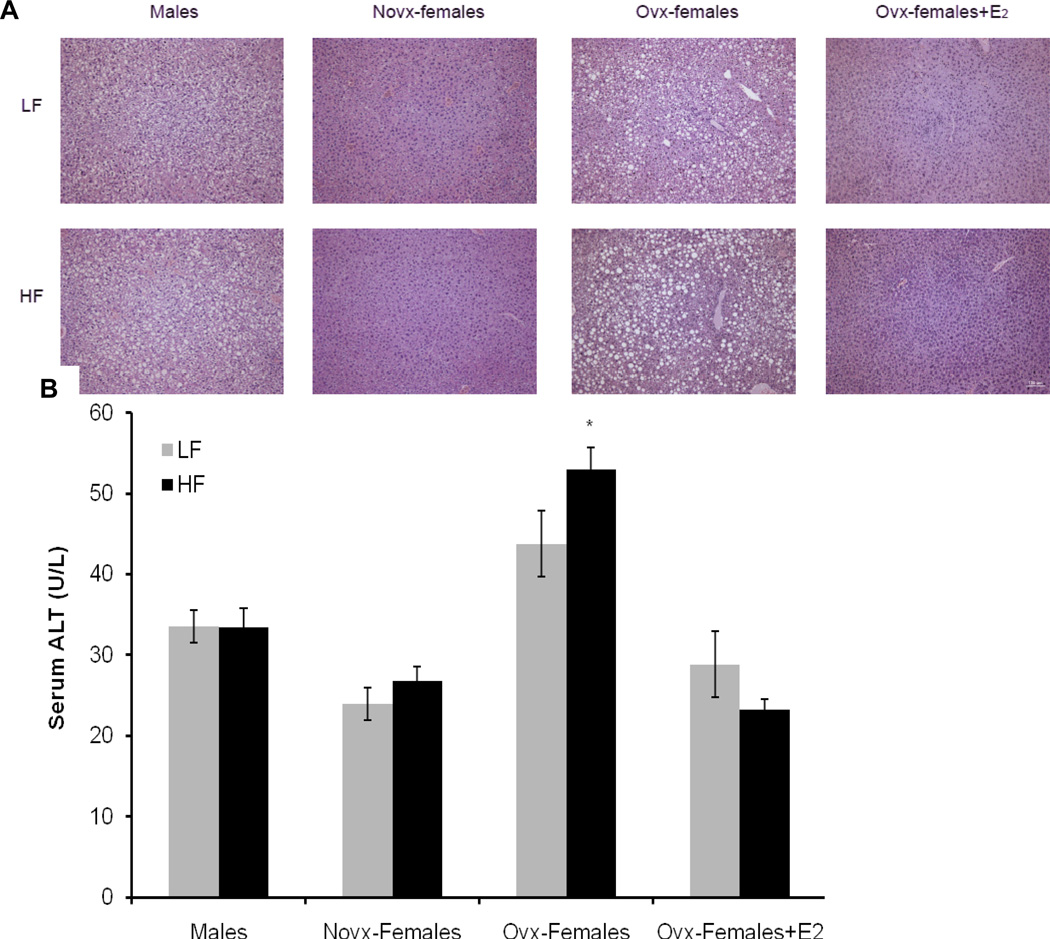
Sex differences in liver biology
To gain a better understanding of the role of estrogen and sex in liver biology, we collected and hematoxylin- and eosin-stained liver tissue from each group. Results indicate that male mice and ovx-female mice have higher incidence of steatohepatitis. However, steatohepatitis was absent in novx-female and ovx-female+E mice, suggesting that estrogen protects female mice from the development of fatty liver (Figure 5A). Serum ALT is a serum marker for liver injury; our data show that ovx-female mice had significantly higher levels of serum ALT compared to novx-female and ovx-female+E mice (52.9 vs. 26.8 and 23.3 U/L, respectively, p<0.05), Figure 5B. ALT levels were also elevated in male mice compared to novx-female mice and ovx-female+E mice (33.4 vs. 26.8 and 23.3 U/L, respectively) (not significant).
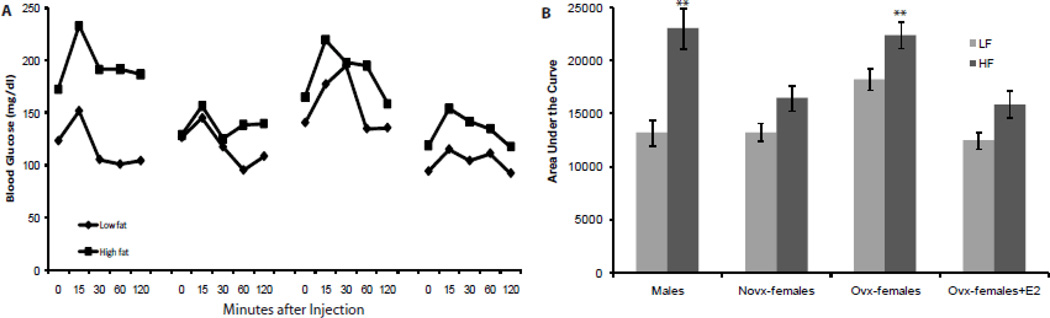
Sex differences in insulin resistance
Both obesity and steatohepatitis have been strongly correlated with insulin resistance; therefore, to determine if there is a relationship between steatohepatitis and insulin resistance in regards to sex, we measured insulin sensitivity. Our results demonstrate that estrogen improved insulin sensitivity in female mice. Briefly, when exposed to a high-fat diet, male mice were more insulin resistant compared to novx-female mice (Figure 6A). Removal of the ovaries caused the female mice to mimic the males’ insulin resistance; however, supplementation with estrogen to the ovx-female mice improved their insulin sensitivity to levels similar to novx-female mice. Figure 6B is a graphical representation of the calculated area under the curve.
Figure 6. Sex differences in liver biology and inflammation.
Panel A: Liver tissue was collected and fixed in 10% neutral buffered formalin and then stained with hematoxylin and eosin. Images were taken at 20× magnification, scale bar equals 100µm. Panel B: Serum was collected 3 hours after fasting and was analyzed for alanine transaminase. Briefly, ovx-females had significantly higher serum ALT levels compared to novx-female and ovx-female+E. *Significantly different compared to novx-female and ovx-female+E, p<0.001 (n=8).
Discussion
Obesity is associated with numerous co-morbidities, such as insulin resistance and chronic adipocyte inflammation [26]. Our results show that estrogen protects female mice from the susceptibility to obesity and its related morbidities: insulin resistance, inflammation, and liver steatosis. We demonstrated that estrogen protects female mice from adipocyte hypertrophy, adipocyte inflammation, and adipocyte oxidative stress. Since estrogen protects female mice by modulating adipocyte biology, it has an indirect and/or direct beneficial effect on insulin sensitivity, possibly due to alterations in liver biology and adipocyte inflammation. Moreover, we show that male and ovx-female mice experience more adipocyte oxidative stress and possibly increased DNA damage due to diet-induced obesity. As a result, male and ovx-female mice have enhanced adipocyte inflammation and systemic insulin resistance in addition to altered lipid metabolism.
Interestingly, we found sex differences in serum free fatty acids and triglycerides possibly due to differences in adipocyte lipolysis Briefly, our results indicate that male mice have higher serum triglyceride levels but lower serum free fatty acid levels. However, ovx-female mice have high levels of both serum triglycerides and free fatty acids, thus suggesting that there are sex differences in lipogensis and/or lipolysis independent of estrogen. Even though they may differ in lipolytic activity, male and ovx-female mice have similar adipocyte size. Furthermore, others have shown that ovx-female mice and postmenopausal women have large adipocytes despite their high lipolytic activity (10,11). Novx-female and ovx-female+E mice also had elevated levels of serum free fatty acids, which might be explained by the significantly increased activity of key lipolytic enzymes (PKA and HSL) that we observed in these mice. However, the increase in serum free fatty acids observed in female mice may be subjected to different metabolic pathways. For example, unlike the novx-female and ovx-female+E mice, ovx-females showed signs of liver steatosis and had elevated levels of serum ALT, thus suggesting that the high circulating serum free fatty acids in the ovx-female mice were deposited in their liver but not in the livers of novx-female and ovx-female+E mice. Serum free fatty acids in novx-female and ovx-female+E mice might be used for energy through fatty acid oxidation occurring in the liver and skeletal muscle. In fact, studies have shown that estrogen increases the expression of genes involved in fatty acid oxidation, suggesting that estrogen stimulates the use of fatty acids for energy (16). Moreover, results by others have established that ovariectomy reduces energy expenditure and that the loss of ERα in the ventromedial nucleus of the hypothalamus results in a decrease in energy expenditure [27, 28]. Thus, estrogen may help prevent obesity and its co-morbidities by affecting lipid metabolism.
Evidence shows that free fatty acids can promote insulin resistance by inducing an inflammatory response via the TLR4 pathway [29]. In our studies, we show that ovx-female mice have high systemic levels of free fatty acids and are insulin resistant; thus, it is feasible that the insulin resistance observed in our ovx-female mice is due to the activation of the TLR4 pathway and inflammation. In fact, we show that the adipose tissue of both ovx-female and male mice contains high levels of inflammatory markers, as evidenced by the high Cd68 and TNFα mRNA levels compared to novx-female and ovx-female+E mice. CD68 is a glycoprotein highly expressed in tissue macrophages (22); thus, its presence indicates that male and ovx-female mice consuming the high-fat diet may have enhanced macrophage infiltration in their adipose tissue. Results also show that novx-female and ovx-female+E mice are protected from insulin resistance when consuming the high-fat diet and that inflammation markers are drastically reduced by the presence of estrogen. Thus, estrogen may protect against insulin resistance by decreasing adipose tissue inflammation. Alternatively, it is feasible that estrogen may improve insulin sensitivity via other pathways. For example, estrogen may improve insulin sensitivity by decreasing hepatic gluconeogenesis and glycogenolysis or by increasing the production of insulin by the pancreas [30, 31].
It has been well established that as people become obese, they experience adipocyte hypertrophy [32]. We show that male and ovx-female mice have much larger adipocytes compared to novx-female and ovx-female+E. An increase in adipocyte size can eventually lead to a hypoxic environment. This hypoxic setting can worsen an already inflamed environment and augment adipocyte oxidative stress, leading to the production of reactive oxygen species, which can lead to DNA damage [33]. The biomarker γH2AX can be used as an indicator for oxidative stress, since H2AX phosphorylation occurs following the initiation of DNA double strand breaks (21). Oxidative stress has been linked to metabolic syndrome and cancer, so it is of great importance [34]. Our results show that obese male and ovx-female mice have more γH2AX stained nuclei compared to novx-female and ovx-female+E mice. Thus, our data suggest that estrogen prevents adipocyte oxidative stress.
In conclusion, obesity is a global growing epidemic that contributes to the development of insulin resistance, liver steatosis, and chronic low-grade inflammation. Here, we show that estrogen modulates bodyweight by altering adipocyte size. This change in adipocyte biology has beneficial effects on adipocyte inflammation, oxidative stress, liver steatosis, and insulin resistance.
Figure 7. Sex differences in insulin resistance.
On the 10th week, ITT was performed after a 6hr fast. Mice were injected with 0.75U/g of bodyweight of insulin, and a small drop of blood was collected from the tail to measure blood glucose levels at 0, 15, 30, 60, 120 minutes after injection. Panel A: After 10 weeks of consuming a high-fat diet, male and ovx-female mice experience significantly more insulin resistance compared to novx-female and ovx-female+E mice. Area under the curve was calculated and is graphically shown in Panel B. ** Significantly different compared to novx-female and ovx-female+E consuming the high fat diet, p<0.001 (n=10).
Acknowledgements
This work was supported by American Cancer Society grant ACS RSG CNE-113703 and by grants from the National Institutes of Health: National Cancer Society grant NCI 1K22CA127519-01A1 and National Institute of Environmental Health Sciences Center grants ES09145 and ES007784
Footnotes
Competing Interests
The authors have nothing to disclose.
Authors Contribution
RS-Experimental design and execution and writing of manuscript
VH-Experimental design and execution
JH-Experimental design and execution
NN-Experimental design and writing of manuscript
All authors read and approved the final manuscript
References
- 1.Ogden CLCM, McDowell MA, Flegal KM. Obesity among adults in the United States—no change since 2003–2004. NCHS data brief no 1. Hyattsville, MD: National Center for Health Statistics; 2007. [PubMed] [Google Scholar]
- 2.Jones ME, Thorburn AW, Britt KL, et al. Aromatase-deficient (ArKO) mice accumulate excess adipose tissue. J Steroid Biochem Mol Biol. 2001;79:3–9. doi: 10.1016/s0960-0760(01)00136-4. [DOI] [PubMed] [Google Scholar]
- 3.Hao L, Wang Y, Duan Y, Bu S. Effects of treadmill exercise training on liver fat accumulation and estrogen receptor alpha expression in intact and ovariectomized rats with or without estrogen replacement treatment. Eur J Appl Physiol. 2010;109:879–886. doi: 10.1007/s00421-010-1426-6. [DOI] [PubMed] [Google Scholar]
- 4.Heine P, Taylor J, Iwamoto G, Lubahn D, Cooke P. Increased adipose tissue in male and female estrogen receptor-alpha knockout mice. Proc Natl Acad Sci U S A. 2000;97:12729–12734. doi: 10.1073/pnas.97.23.12729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Riant E, Waget A, Cogo H, Arnal JF, Burcelin R, Gourdy P. Estrogens protect against high-fat diet-induced insulin resistance and glucose intolerance in mice. Endocrinology. 2009;150:2109–2117. doi: 10.1210/en.2008-0971. [DOI] [PubMed] [Google Scholar]
- 6.Straub RH. The complex role of estrogens in inflammation. Endocr Rev. 2007;28:521–574. doi: 10.1210/er.2007-0001. [DOI] [PubMed] [Google Scholar]
- 7.Regitz-Zagrosek V, Lehmkuhl E, Weickert MO. Gender differences in the metabolic syndrome and their role for cardiovascular disease. Clin Res Cardiol. 2006;95:136–147. doi: 10.1007/s00392-006-0351-5. [DOI] [PubMed] [Google Scholar]
- 8.Störk S, van der Schouw YT, Grobbee DE, Bots ML. Estrogen, inflammation and cardiovascular risk in women: a critical appraisal. Trends Endocrinol Metab. 2004;15:66–72. doi: 10.1016/j.tem.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 9.Lobo R. Metabolic syndrome after menopause and the role of hormones. Maturitas. 2008;60:10–18. doi: 10.1016/j.maturitas.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 10.D'Eon T, Souza S, Aronovitz M, Obin M, Fried S, Greenberg A. Estrogen regulation of adiposity and fuel partitioning. Evidence of genomic and non-genomic regulation of lipogenic and oxidative pathways. J Biol Chem. 2005;280:35983–35991. doi: 10.1074/jbc.M507339200. [DOI] [PubMed] [Google Scholar]
- 11.Nicklas BJ, Rogus EM, Colman EG, Goldberg AP. Visceral adiposity, increased adipocyte lipolysis, and metabolic dysfunction in obese postmenopausal women. Am J Physiol. 1996;270:E72–E78. doi: 10.1152/ajpendo.1996.270.1.E72. [DOI] [PubMed] [Google Scholar]
- 12.Schmitz-Peiffer C. Signalling aspects of insulin resistance in skeletal muscle: mechanisms induced by lipid oversupply. Cell Signal. 2000;12:583–594. doi: 10.1016/s0898-6568(00)00110-8. [DOI] [PubMed] [Google Scholar]
- 13.Boden G, She P, Mozzoli M, et al. Free fatty acids produce insulin resistance and activate the proinflammatory nuclear factor-kappaB pathway in rat liver. Diabetes. 2005;54:3458–3465. doi: 10.2337/diabetes.54.12.3458. [DOI] [PubMed] [Google Scholar]
- 14.Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. J Clin Invest. 2006;116:1793–1801. doi: 10.1172/JCI29069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 16.Naugler WE, Sakurai T, Kim S, et al. Gender disparity in liver cancer due to sex differences in MyD88-dependent IL-6 production. Science. 2007;317:121–124. doi: 10.1126/science.1140485. [DOI] [PubMed] [Google Scholar]
- 17.Campbell SE, Mehan KA, Tunstall RJ, Febbraio MA, Cameron-Smith D. 17beta-estradiol upregulates the expression of peroxisome proliferator-activated receptor alpha and lipid oxidative genes in skeletal muscle. J Mol Endocrinol. 2003;31:37–45. doi: 10.1677/jme.0.0310037. [DOI] [PubMed] [Google Scholar]
- 18.Rogers N, Witczak C, Hirshman M, Goodyear L, Greenberg A. Estradiol stimulates Akt, AMP-activated protein kinase (AMPK) and TBC1D1/4, but not glucose uptake in rat soleus. Biochem Biophys Res Commun. 2009;382:646–650. doi: 10.1016/j.bbrc.2009.02.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Núñez N, Carpenter C, Perkins S, et al. Extreme obesity reduces bone mineral density: complementary evidence from mice and women. Obesity (Silver Spring) 2007;15:1980–1987. doi: 10.1038/oby.2007.236. [DOI] [PubMed] [Google Scholar]
- 20.Karas RH, Schulten H, Pare G, et al. Effects of estrogen on the vascular injury response in estrogen receptor alpha, beta (double) knockout mice. Circ Res. 2001;89:534–539. doi: 10.1161/hh1801.097239. [DOI] [PubMed] [Google Scholar]
- 21.Nahant L. In: Quantifying western blots without expensive commercial quantification software. Nahant L, editor. 2007. [Google Scholar]
- 22.Li Z, Yang J, Huang H. Oxidative stress induces H2AX phosphorylation in human spermatozoa. FEBS Lett. 2006;580:6161–6168. doi: 10.1016/j.febslet.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 23.Falini B, Flenghi L, Pileri S, et al. PG-M1: a new monoclonal antibody directed against a fixative-resistant epitope on the macrophage-restricted form of the CD68 molecule. Am J Pathol. 1993;142:1359–1372. [PMC free article] [PubMed] [Google Scholar]
- 24.Xu H, Barnes GT, Yang Q, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821–1830. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Anthonsen MW, Rönnstrand L, Wernstedt C, Degerman E, Holm C. Identification of novel phosphorylation sites in hormone-sensitive lipase that are phosphorylated in response to isoproterenol and govern activation properties in vitro. J Biol Chem. 1998;273:215–221. doi: 10.1074/jbc.273.1.215. [DOI] [PubMed] [Google Scholar]
- 26.de Ferranti S, Mozaffarian D. The perfect storm: obesity, adipocyte dysfunction, and metabolic consequences. Clin Chem. 2008;54:945–955. doi: 10.1373/clinchem.2007.100156. [DOI] [PubMed] [Google Scholar]
- 27.Rogers N, Perfield Jn, Strissel K, Obin M, Greenberg A. Reduced energy expenditure and increased inflammation are early events in the development of ovariectomy-induced obesity. Endocrinology. 2009;150:2161–2168. doi: 10.1210/en.2008-1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Musatov S, Chen W, Pfaff D, et al. Silencing of estrogen receptor alpha in the ventromedial nucleus of hypothalamus leads to metabolic syndrome. Proc Natl Acad Sci U S A. 2007;104:2501–2506. doi: 10.1073/pnas.0610787104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shi H, Kokoeva MV, Inouye K, Tzameli I, Yin H, Flier JS. TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest. 2006;116:3015–3025. doi: 10.1172/JCI28898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ahmed-Sorour H, Bailey CJ. Role of ovarian hormones in the long-term control of glucose homeostasis, glycogen formation and gluconeogenesis. Ann Nutr Metab. 1981;25:208–212. doi: 10.1159/000176496. [DOI] [PubMed] [Google Scholar]
- 31.Nadal A, Alonso-Magdalena P, Soriano S, Ropero AB, Quesada I. The role of oestrogens in the adaptation of islets to insulin resistance. J Physiol. 2009;587:5031–5037. doi: 10.1113/jphysiol.2009.177188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jo J, Gavrilova O, Pack S, et al. Hypertrophy and/or Hyperplasia: Dynamics of Adipose Tissue Growth. PLoS Comput Biol. 2009;5:e1000324. doi: 10.1371/journal.pcbi.1000324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Furukawa S, Fujita T, Shimabukuro M, et al. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest. 2004;114:1752–1761. doi: 10.1172/JCI21625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414:813–820. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]