Abstract
High-dose niacin therapy in humans reduces mortality from cardiovascular disease and may also protect against death from other causes, with benefits apparent more than a decade beyond the therapeutic period. Niacin therapy modulates circulating lipids, raising HDL and lowering LDL, but has the unwanted side effect of inducing skin flushing in response to treatment. Skin flushing results from niacin-induced activation of GPR109A and subsequent release of prostaglandins that promote vasodilation. GPR109A may also mediate HDL elevation. Recent data suggest that high-dose niacin may have benefits beyond improved lipid profiles, such as quelling inflammation, suggesting a potential role in immune cell trafficking. To explore effects of niacin on immune cell trafficking independently of its effects on lipid profiles, we took advantage of the fact that niacin therapy does not raise HDL in wild-type or apoE−/− mouse strains. Wild-type and apoE−/− C57BL/6 mice were fed standard chow or high-fat diets supplemented or not with 1% niacin. Against our predictions, this treatment did not modulate monocyte recruitment to or retention within atherosclerotic plaques. By contrast, stimulating the skin of niacin-treated mice with a contact sensitizer revealed impaired dendritic cell accumulation in draining lymph nodes and associated impaired adaptive immunity. Surprisingly, niacin-mediated impaired dendritic cell mobilization could not be reversed by cyclooxygenase inhibitor treatment nor deletion of the niacin receptor GPR109A, suggesting that the effects of niacin on modulating the migration of dendritic cells are not directly linked to skin flushing. Overall, these data suggest the existence of novel pathways triggered by niacin that, through suppression of dendritic cell migration, might impact adaptive immune responses that participate in sustained therapeutic benefits independent of niacin’s cardioprotective capabilities.
Keywords: nicotinic acid, nicotinamide, arteriosclerosis, macrophage, contact hypersensitivity, PUMA-G, HM74A
Introduction
High dose niacin, or nicotinic acid, has been used as an athero-protective drug for more than 50 years (Carlson, 2005; Offermanns, 2006). When taken in pharmacological doses (>1 gram/day), the nicotinic acid form of niacin modulates plasma lipid profiles including decreasing circulating total cholesterol, plasma LDL, triglycerides, and Lp(a), while increasing plasma HDL (reviewed in (Carlson, 2005; Montecucco et al., 2010) and inhibits lipolysis in adipose tissue (Carlson, 1963; Wahlberg and Walldius, 1993). Clinical trials assessing the cardiovascular benefits of niacin therapy, either alone or in combination with statins or fibrates, demonstrate that high-dose niacin treatment reduces nonfatal acute myocardial infarction (Brown et al., 1990; 1975; Whitney et al., 2005), plaque progression (Blankenhorn et al., 1991; Blankenhorn et al., 1987; Brown et al., 1990; Lee et al., 2009a; Taylor et al., 2009), and overall mortality (Brown et al., 2001; Canner et al., 1986; Carlson and Rosenhamer, 1988; Taylor et al., 2009; 1975; Whitney et al., 2005). Further, niacin therapy can reverse plaque progression in patients with peripheral artery disease and in fact, treatment induces regression in peripheral plaques (Lee et al., 2009b; Ost and Stenson, 1967).
Identification of an orphan G-protein coupled receptor designated GPR109A, or HM74A in humans and PUMA-G in mice (Schaub et al., 2001), that specifically binds the nicotinic acid form of niacin with high affinity (Soga et al., 2003; Tunaru et al., 2003; Wise et al., 2003; Zhang et al., 2005), has shed light on possible mechanisms of niacin-mediated lipid modification (Tunaru et al., 2003; Zhang et al., 2005). These discoveries have also spurred a renewed interest in developing therapeutics that exploit the protection from cardiovascular disease that niacin induces. Emergent questions are whether the protective nature of niacin is solely due to its effects on raising plasma HDL and lowering LDL or whether it has other beneficial properties. The identification of GPR109A as a receptor for nicotinic acid opens up new approaches in addressing these questions.
Humans express two closely related genes, HM74 and HM74A; however, only HM74A (GPR109A) binds niacin with high affinity (Wise et al., 2003; Zhang et al., 2005), and mice express only the high affinity GPR109A protein (Offermanns, 2006). When niacin binds GPR109A, expressed primarily on mononuclear phagocytes, neutrophils, and adipocytes (Tunaru et al., 2003), an intense vasodilation in the skin, often called skin flushing, ensues (Benyo et al., 2005; Tunaru et al., 2003). Skin flushing is the primary reason for noncompliance and discontinuation during niacin therapy (Dunbar and Gelfand, 2010), although there are a variety of symptoms that accompany the flush after ingestion of high-dose niacin. This has lead some investigators to describe the ancillary effects of niacin therapy as skin toxicity rather than skin flushing (Dunbar and Gelfand, 2010). A biphasic model of niacin-induced skin flushing has been described (Hanson et al., 2010). The brief, first phase is dependent on Langerhans cells and their expression of GPR109A, cyclooxygenase (cox)-1 signaling, and prostaglandin D2 (PGD2) release that in turn promotes the vasodilation that characterizes the flush. A second, more sustained phase is mediated by GPR109A expression on keratinocytes, cox-2 signaling, and PGE2 release (Hanson et al., 2010). Considering that PGD2 release in the skin can inhibit the mobilization of antigen-presenting dendritic cells (DCs) to draining lymph nodes (Allan et al., 2006; Angeli et al., 2001), we hypothesized that skin flushing may lead to impaired DC migration. We reasoned that if niacin did inhibit DC migration, in turn, downstream adaptive immunity would likely be altered, and possibly in a way that confers clinical benefit, since atherosclerosis is partly a T cell-driven disease (Robertson and Hansson, 2006). This consideration would be important to address in order to evaluate whether targeted pharmacological inhibition of skin flushing would simultaneously diminish any positive effects of niacin therapy. We therefore set out to examine whether skin flushing mediated by GPR109A altered DC trafficking from skin.
Another possible effect of niacin is in altering immune cell trafficking; as it has been suggested that niacin therapy quells endothelial cell activation (Digby et al., 2010; Ganji et al., 2009) and thereby suppresses inflammatory cell recruitment (Wu et al., 2010). However, the impact of niacin on leukocyte trafficking to sites of inflammation has not been studied in the context of diseases like atherosclerosis where such agonists confer at least partial protection against disease. Monocyte recruitment to atherosclerotic plaques is well established to drive atherosclerosis (Gautier et al., 2009; Glass and Witztum, 2001). Thus, we also assessed how therapeutic levels of niacin affected monocyte recruitment to or egress from atherosclerotic plaques of apoE−/− mice in order to better understand the mechanism of niacin action in the treatment of atherosclerosis.
Materials and Methods
Animals
Animal experiments were conducted in accordance with approvals from the Institutional Animal Care and Use Committee at Mount Sinai School of Medicine. Wild-type C57Bl/6J mice were from The Jackson Laboratory and apoE−/− and apoE−/− PUMA-G−/− (gift from Merck)(Schaub et al., 2001) mice were bred and housed in a specifc pathogen-free environment at Mount Sinai School of Medicine. Wild-type mice were maintained on standard laboratory chow diet (Picolab Rodent Diet #5053, Lab Diet), or fed this same chow diet milled with 1% niacin (wt/wt) for 2 to 4 weeks. Beginning at 5 weeks of age, some apoE−/− mice (where noted) were fed a high fat diet (HFD) (21% milk fat (wt/wt) containing 0.2% cholesterol; TD.88137 Harlan Teklad). Cohorts of HFD-fed mice were subsequently fed niacin-supplemented HFD (1% w/w in 0.2% cholesterol diet; TD.05181, Harlan Teklad) for 2-4 weeks.
Immunofluorescence
Mice were perfused with PBS and the aortic arch and aortic sinus were excised and frozen in OCT TissueTek. Then, 8  m-sections were collected along the entire aortic arch or through the entire sinus and fixed with 4% paraformaldehyde. Immunofluorescence imaging was performed using purified hamster anti-mouse ICAM (BD Biosciences) or rat anti-mouse CD68 (Serotec) followed by secondary fluor-conjugated anti-hamster or anti-rat antibodies, respectively (Jackson ImmunoResearch). Morphometric assessments and staining intensities were quantified with ImageJ software.
m-sections were collected along the entire aortic arch or through the entire sinus and fixed with 4% paraformaldehyde. Immunofluorescence imaging was performed using purified hamster anti-mouse ICAM (BD Biosciences) or rat anti-mouse CD68 (Serotec) followed by secondary fluor-conjugated anti-hamster or anti-rat antibodies, respectively (Jackson ImmunoResearch). Morphometric assessments and staining intensities were quantified with ImageJ software.
Monocyte subset labeling; analysis of monocyte entry and egress from plaque
Bead labeling of monocyte subsets in male or female apoE−/− mice was performed as previously described (Tacke et al., 2007; Tacke et al., 2006) with the exception that 1-μM beads were used to label each subset. Monocyte recruitment to plaques was determined by bead-labeling classical or nonclassical monocyte subsets in separate experiments, sacrificing the animals on day 2 or 5 after labeling, and quantifying the number of beads in plaque as described (Tacke et al., 2007).
Egress of monocyte-derived cells from plaques was determined by bead labeling classical or nonclassical monocyte subsets in separate experiments. Animals were sacrificed at baseline day 5 or treated with niacin or control chow and then sacrificed 15 days after these treatments, on day 20 after monocyte labeling, and the number of beads in plaques was quantified. We determined empirically that counting the number of beads per section in every fifth section gave a reliable quantification of the average number of beads per section over the entire length anatomical site being assessed. Egress was determined by a decrease in the number of beads/section as compared to the number of beads present in baseline animals sacrificed 5 days after labeling (Randolph, 2008).
Thioglycollate-induced peritonitis
C57BL/6J or apoE−/− mice were injected intraperitoneally with 1mL of a sterile 3% thioglycollate solution (Sigma). Animals were sacrificed 1 day post-injection. Elicited cells were recovered by injection of 4 mL of PBS supplemented with 2.5 mM EDTA. Cells were counted and stained for flow cytometry. Data were acquired on a BD FACS Canto Flow Cytometer or a BD LSR II Flow Cytometer (BD Biosciences) and analyzed using FlowJo software (Treestar).
“FITC painting” DC migration assay
DC migration assays were performed as previously described (Robbiani et al., 2000), with the following modifications. One 25 μL application of fluorescein isothiocyanate (FITC) (Sigma), 8 mg/mL dissolved in a 1:1 mixture of dibutyl phthalate and acetone, was applied epicutaneously to the shaved back skin on each side in the scapular region of avertin (2-2-2 tribromoethanol)-anesthetized wild-type, apoE−/−, or apoE−/− PUMA-G−/− mice. 18 h after FITC application, brachial and axillary lymph nodes were removed and pooled, keeping lymph nodes from left and right sides of the mouse separated. Total lymph node cells were counted and stained with mAbs to CD24, I-A/I-E (MHC II), CD11b, CD11c, and CD103 (all eBioscience). Data were acquired on a BD FACS Canto Flow Cytometer or a BD LSR II Flow Cytometer (BD Biosciences) and analyzed using FlowJo software (Treestar). For cyclooxygenase inhibition experiments, 15 mg/kg naproxen, dissolved in PBS, was administered intraperitoneally 30 minutes prior to FITC application to the back skin. To assess whether the dosage of naproxen was effective, the same dosing protocol was used to block ear swelling upon application of arachidonic acid (AA, 2 mg/10 μL in acetone) (Langenbach et al., 1995). AA was applied to the left ears while acetone was applied to the right ears of wild-type mice. Ear thickness was measured with an ABSOLUTE Digimatic Caliper (Miyutoyo). DC migration results are displayed as the percentage and total number of FITC+ cells within the total lymph node cell population. Statistical significance between two groups was determined using unpaired T-tests.
Contact Hypersensitivity assay
Contact hypersensitivity was determined using the mouse ear swelling test (Garrigue et al., 1994). Briefly, wild-type mice were fed control chow or 1% niacin-supplemented chow for 2 weeks. One application of fluorescein isothiocyanate (FITC), 8 mg/mL dissolved in a 1:1 mixture of dibutyl phthalate and acetone, was applied epicutaneously to the shaved back skin on each side in the scapular region of avertin (2-2-2 tribromoethanol)-anesthetized mice. One week later, the same mixture was applied to each side of the ears. Ear thickness was measured using ABSOLUTE Digimatic Caliper (Miyutoyo) to quantify the magnitude of the effector response.
Lung DC migration assay
Lung DC migration was assessed as described previously (Jakubzick et al., 2008). Briefly, 30 μL FITC-labeled ovalabumin (5 mg/mL, Invitrogen), spiked with 1 μg LPS (Sigma) was delivered intranasally to avertin (2-2-2 tribromoethanol)-anesthetized wild-type mice, fed control chow or 1% niacin-supplemented chow for 2 weeks prior to the assay. Mice were sacrificed 24 hours later and the mediastinal lymph node excised and digested for flow cytometric analysis. Total lymph node cells were counted and stained with mAbs to I-A/I-E (MHC II), CD11b, CD11c, and CD103 (all eBiosciences). Data were acquired on a BD FACS Canto Flow Cytometer or a BD LSR II Flow Cytometer (BD Biosciences) and analyzed using FlowJo software (Treestar).
Results
Niacin treatment does not alter monocyte subset migration into or out of atherosclerotic plaques of apoE−/− mice
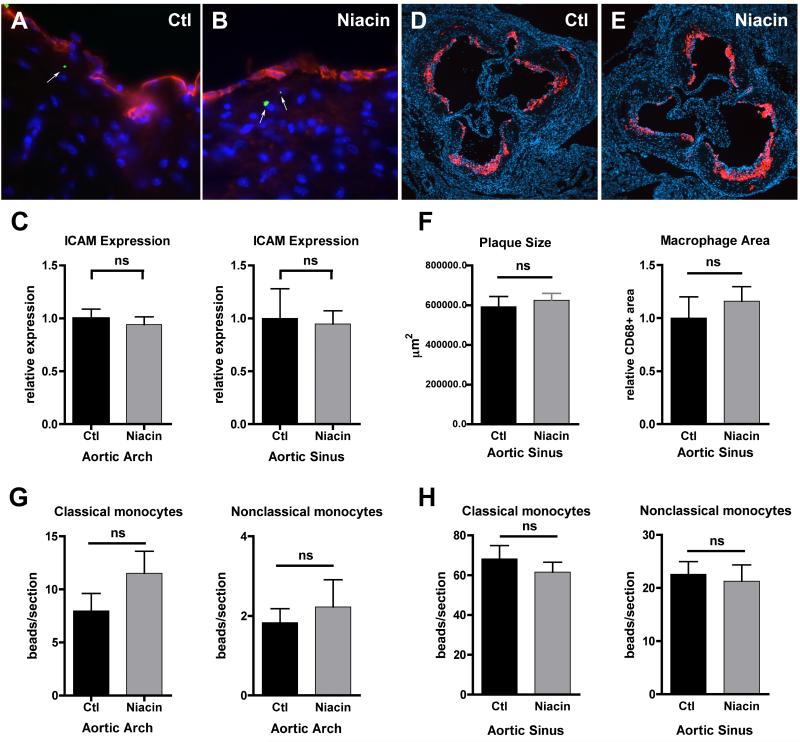
Atherosclerosis is a prevalent inflammatory disease characterized by the accumulation of monocyte-derived cells and modified lipoproteins beneath the inflamed endothelial lining of arteries. Recently, Wu et al. reported that feeding rabbits for two weeks with high-dose niacin decreases vascular inflammation, as measured by a reduction in ICAM-1 and VCAM-1 on the endothelium that results in a subsequent decrease in neutrophil recruitment 24 hours after collar insertion at the carotid artery (Wu et al., 2010). Thus, we predicted that monocyte migration into plaques would be decreased in niacin-treated mice as compared to control-treated mice. However, we observed no changes in the expression of ICAM-1 in the aortic arch or sinus near plaques (Fig. 1A-C,) at two or four weeks following niacin feeding of apoE−/− mice with advanced atherosclerotic lesions. Additionally, niacin feeding did not induce any gross morphometric changes in plaque size or CD68+ macrophage area after four weeks of treatment (Fig. 1D-F). To measure recruitment of classical or nonclassical blood monocytes to atherosclerotic lesions, we utilized a technique previously employed in our laboratory to intravenously label and track the two monocyte subsets, substituting 1-μm fluorescent latex spheres for the 0.5-μm size used previously (Tacke et al., 2007; Tacke et al., 2006). At 10 or 25 days prior to monocyte labeling, one cohort of apoE−/− mice were transitioned from HFD to 1% niacin-supplemented HFD, while the remaining mice were maintained throughout the experiment on control HFD. Then monocytes were labeled in all mice and animals were sacrificed 2 or 5 days later. The number of fluorescent tracer particles carried by classical or nonclassical monocytes into lesions of the aortic arches (Fig. 1G) or sinuses (Fig. 1H) was unaffected by niacin feeding, suggesting that niacin treatment did not alter the recruitment of either monocyte subset to plaques. To extend these studies to other scenarios of inflammation, we quantified leukocyte recruitment to the inflamed peritoneum 24 hours after intraperitoneal injection of thiogylcollate. Cohorts of wild-type or apoE−/− mice were transitioned to 1% niacin supplemented-chow or remained on control diets for two weeks prior to initiation of the experiment. Here, as in our atherosclerosis studies, we failed to observe an impact of niacin on the magnitude of leukocyte recruitment to the inflamed peritoneum (Supplemental Fig. 1).
Figure 1.
Effect of niacin treatment on monocyte entry into advanced atherosclerotic lesions. A cohort of apoE−/− mice was transitioned to a HFD supplemented with 1% niacin (Niacin) for two weeks while the other half remained on control HFD (Ctl). (A-B) Representative images of aortic sinuses from mice with green microbead-labeled nonclassical monocytes (green microspheres, white arrows) and stained with anti-ICAM antibody (red) and DAPI (blue), 63X magnification. (C) Graphs depict quantification of anti-ICAM antibody staining in the aortic arch and sinus. (D-E) Representative images of aortic sinuses stained with anti-CD68 antibody (red) to depict macrophages and DAPI (blue), 10X magnification. (F) Graphs depict quantification of plaque size and macrophage area in aortic sinuses. (G-H) Classical or nonclassical monocytes were labeled intravenously following niacin treatment. Mice were sacrificed 2-5 days post-labeling, aortic arches and hearts removed and sectioned and beads per section in the (G) aortic arch and (H) sinus enumerated. There were no statistically significant differences (ns), as determined by an unpaired T-test, in ICAM staining, morphometric measurements, or the number of beads in any treatment group (Ctl vs Niacin) in any experiments. N=4-9/group, each experiment repeated 2-4 times.
We also monitored the possibility that migratory egress of monocyte-derived cells from plaques was induced after niacin feeding by tracking the persistence of the bead label in plaques with or without niacin feeding for two weeks relative to a baseline value assessed prior to niacin feeding. Egress from plaques in experimental models is associated with a loss of beads from lesions, since phagocytes bearing beads can exit with beads in tow (Feig et al., 2010; Llodra et al., 2004; Potteaux et al., 2011; Randolph, 2009). However, migratory egress has so far only been demonstrated in a surgical model of plaque regression (Feig et al., 2010; Llodra et al., 2004) and is not observed in apoE−/− mice in the absence of surgery, even under conditions of plaque regression and robust macrophage removal (Potteaux et al., 2011). Accordingly, we did not observe induction of migratory egress in response to two-week niacin treatment (Supplemental Fig. 2). Together, these data indicate that, at least in the absence of raising HDL, niacin did not decrease monocyte recruitment into or retention within mouse atherosclerotic plaques.
Niacin therapy inhibits skin DC accumulation in the draining lymph node
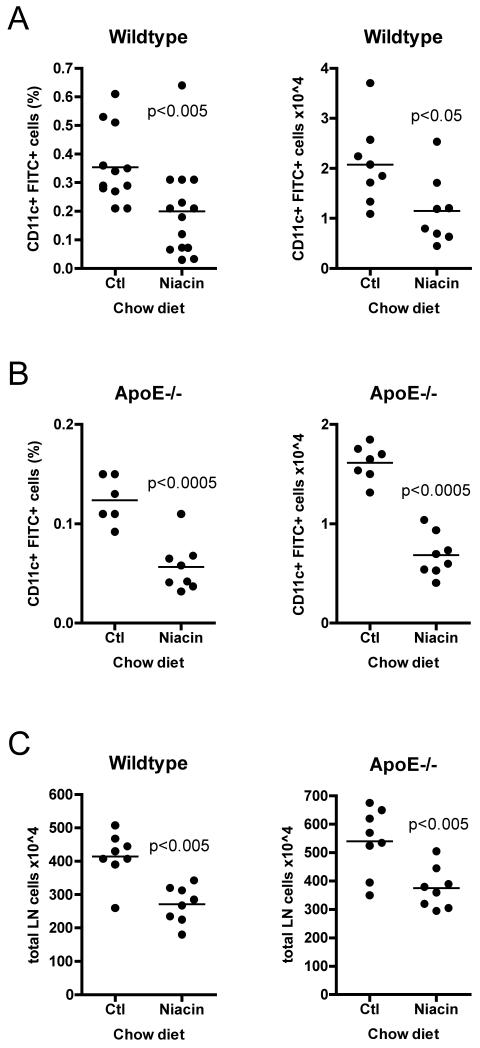
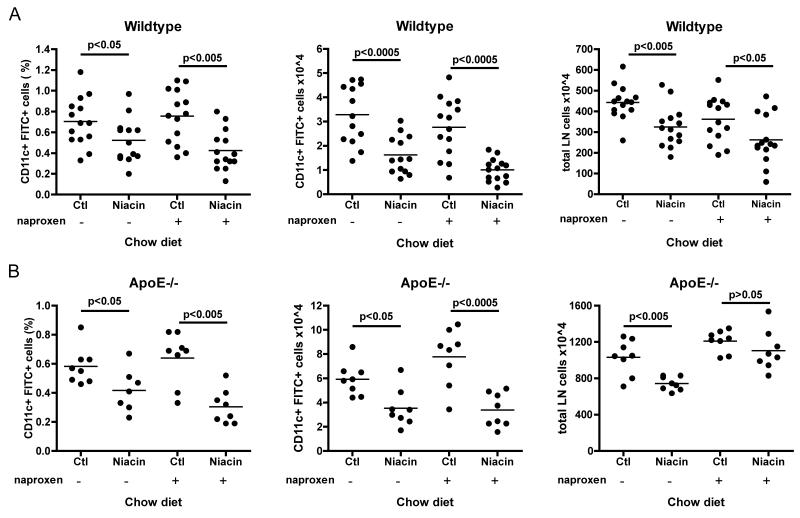
Given that niacin had no effect on monocyte trafficking in plaques, we wanted to confirm that niacin delivered in chow or high fat diet was bio-available. As per the following rationale, we hypothesized that niacin should have a negative effect on DC mobilization to skin draining lymph nodes. At therapeutic doses, niacin causes acute flushing in the skin of both mice and humans (Benyo et al., 2006; Benyo et al., 2005; Maciejewski-Lenoir et al., 2006). This uncomfortable reaction is caused by vasodilation that is triggered by the ligation of the niacin receptor GPR109A that in turn induces the release of prostaglandins, including PGD2 and PGE2, from Langerhans cells and keratinocytes (Benyo et al., 2006; Benyo et al., 2005; Hanson et al., 2010; Maciejewski-Lenoir et al., 2006). It is known that PGD2 release in the skin can inhibit DC mobilization from skin to skin-draining lymph nodes in a DP1-dependent manner, thereby impeding presentation of antigen in draining lymph nodes that drives T cell-mediated immunity (Allan et al., 2006; Angeli et al., 2001). Therefore, since niacin causes PGD2 release in skin (Benyo et al., 2006; Benyo et al., 2005; Maciejewski-Lenoir et al., 2006), we reasoned that the skin flushing reaction would subsequently inhibit the migration of DCs to draining lymph nodes. To address this question, we carried out the classical “FITC painting” assay, used to study DC migration from skin, in wild-type and apoE−/− mice. We applied a fluorescent tracer dissolved in contact-sensitizing agents to the shaved back skin of wild-type mice fed one of four diets for 2 weeks: (1) control chow, (2) 1% niacin-supplemented chow, (3) control HFD, or (4) 1% niacin-supplemented HFD. Eighteen hours later, at the peak time of DC emigration to lymph nodes from skin (Robbiani et al., 2000), we enumerated the number of FITC+ DCs in the brachial and axillary lymph nodes. Indeed, niacin feeding decreased the percentage and absolute number of DCs, by an average of 44 percent, that accumulated in the skin-draining lymph nodes of wild-type mice fed niacin-supplemented chow as compared to control chow fed animals (Fig. 2A). Previously, we reported that DC migration is decreased in older (≥ 16 weeks of age) apoE−/− mice (Angeli et al., 2004). Here, we observed that feeding apoE−/− mice niacin-supplemented chow (Fig. 2B) further reduced both the percentage and the absolute number of FITC+ DCs in skin-draining lymph nodes, by 54 and 58 percent respectively, in response to FITC painting. That the proportion of FITC+ DCs in lymph nodes was decreased by niacin was especially remarkable considering that niacin feeding generally and reproducibly reduced lymph node cellularity (Fig. 2C), by 35% in wild-type and 31% in apoE−/− mice. We observed similar results in wild-type and apoE−/− mice fed a niacin-supplemented HFD (data not shown). These data demonstrate that niacin treatment negatively impacted DC accumulation in skin-draining lymph nodes. Given the paradigm that the number of antigen-presenting DCs is proportional to the magnitude of a T cell response (Martin-Fontecha et al., 2003), decreased accumulation of antigen-bearing DCs in skin-draining lymph nodes after treatment with niacin might be expected to negatively affect antigen presentation and induction of adaptive immunity in these lymph nodes.
Figure 2.
Effect of niacin therapy on the migration of DCs from skin to draining lymph nodes. Mice were fed control chow (Ctl) or 1% niacin-supplemented chow (Niacin) for two weeks. 18 hours after FITC-containing contact sensitizer solution was applied to the shaved flanks of anesthetized mice, animals were sacrificed, brachial and axillary lymph nodes were removed and pooled by side, and FITC+ migrated DCs quantified by flow cytometry in (A) wild-type or (B) apoE−/− mice. (C) Total lymph node cellularity was quantified by manual counting. Each dot represents a pool of brachial and axillary lymph node from one side of a mouse (two dots per mouse). Each experimental condition was repeated 3-6 times with 3-5 mice per condition. P-values, depicted within each graph, were determined with an unpaired T-test.
Niacin-mediated impairment of DC accumulation is restricted to the skin
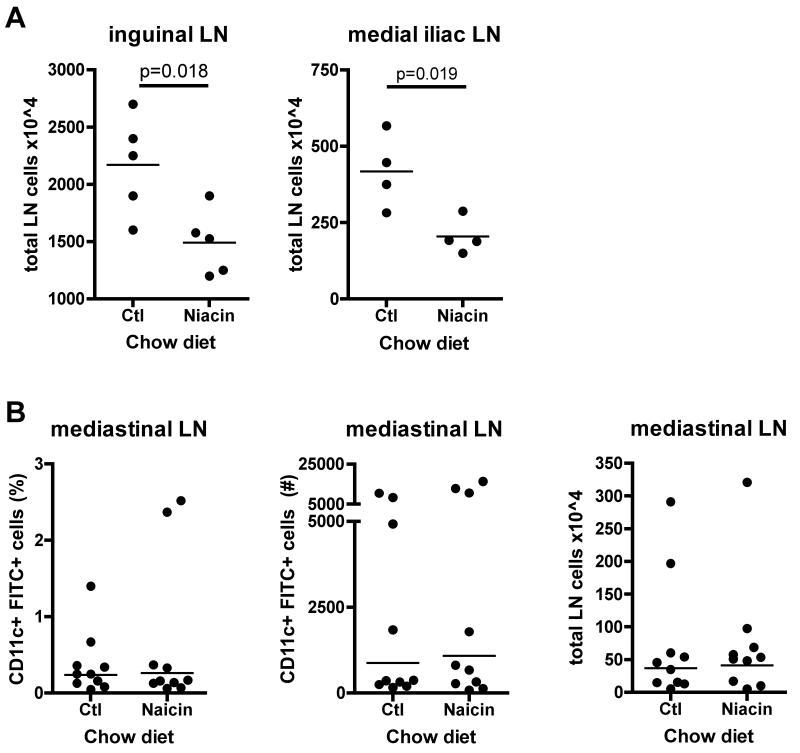
Niacin feeding reduced the total cellularity in the brachial and axillary lymph nodes measured in the FITC painting assay (Fig. 2C). To determine if this decrease was restricted to immunized lymph nodes, we measured cellularity of the non-immunized inguinal lymph node in apoE−/− mice and the medial iliac lymph node, located at the aortic bifurcation, in wild-type mice fed niacin or control chow for 2 weeks. In all cases, cellularity was similarly decreased in the niacin-fed animals (Fig. 3A). These data suggest that niacin therapy broadly affects lymph node cellularity. However, cellularity of the mediastinal lymph node was unaffected by niacin treatment. Likewise, when we measured DC migration from inflamed lungs to the mediastinal lymph node, using FITC-conjugated OVA (Jakubzick et al., 2008), we observed no significant differences in the percentage or number of FITC+ migrated DCs, raising the possibility that niacin’s effect on DC mobilization is restricted to the skin (Fig. 3B).
Figure 3.
Effect of niacin therapy on the migration of DCs from lung to draining lymph nodes. ApoE−/− or wild-type mice were fed control chow (Ctl) or 1% niacin-supplemented chow (Niacin) for two weeks. (A) Total lymph node cellularity was quantified by manual counting of the inguinal lymph node in ApoE−/− mice and the medial iliac lymph node in wild-type mice. (B) 24 hours after a solution of FITC-ovalbumin and LPS was delivered intranasally to anesthetized mice, animals were sacrificed, mediastinal lymph nodes were removed, and FITC+ migrated DCs quantified by flow cytometry in wild-type. Each dot represents a mouse. Each experimental condition was repeated 2 times with 3-5 mice per condition. P-values, depicted within each graph, were determined with an unpaired T-test.
Niacin-mediated impairment of DC migration decreases contact hypersensitivity responses in skin
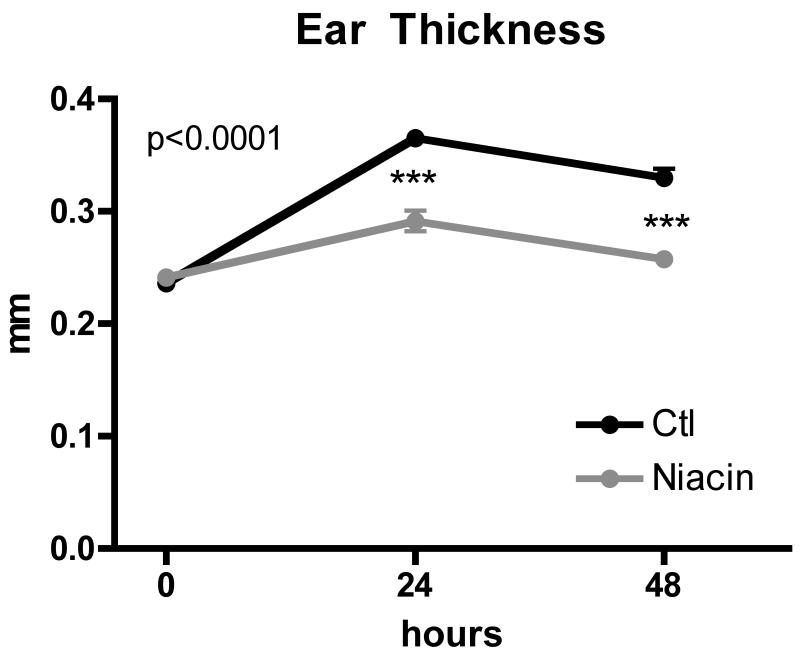
If the inhibition of DC accumulation in lymph nodes in response to niacin were functionally meaningful, we would expect to observe a diminished adaptive immune response. Thus, we evaluated contact hypersensitivity mediated by memory T cells and NK cells (Askenase, 2001; O’Leary et al., 2006; Paust et al., 2010). Wild-type mice were fed control or niacin-supplemented chow for 2 weeks, and then skin contact sensitizer with fluorescent tracer was applied to the shaved flank skin. One week later, the same contact sensitizer solution was applied to the ears of the previously treated mice. Ear swelling in mice treated with niacin was reduced by 61% at day 1 and 83% at day 2 post-sensitizer application (Fig. 4). Thus, impairment of DC migration to the draining lymph nodes in response to niacin therapy was correlated with impaired adaptive immunity.
Figure 4.
Effect of niacin feeding on contact hypersensitivity responses in skin. Wild-type mice were fed control chow (Ctl) or 1%-niacin supplemented chow (Niacin) for 2 weeks. FITC-containing skin contact sensitization solution was applied bilaterally to shaved flanks. One week later the same solution was applied to both sides of the ears. Graph depicts ear thickness measured prior to application and one and two days post application. Experiment was repeated 2 times, with 4 mice per condition in each experiment. P-value determined by one-way ANOVA.
Niacin-mediated impairment of DC migration cannot be reversed with naproxen treatment
Based on evidence that PGD2 binding to DP1 inhibits DC migration (Allan et al., 2006; Angeli et al., 2001), we hypothesized that the impairment of DC migration was most likely a result of increased niacin-mediated local prostaglandin release (Benyo et al., 2006; Benyo et al., 2005; Hanson et al., 2010; Maciejewski-Lenoir et al., 2006) and would therefore most likely be reversed by inhibition of prostanoid generation. To test this hypothesis, we treated mice with the cyclooxygenase inhibitor naproxen prior to inducing DC migration by FITC painting. Mice treated or not with niacin for 2 weeks were injected intraperitoneally with a single dose of naproxen 30 minutes prior to FITC skin painting. Against our expectations, naproxen treatment failed to reverse the inhibition of DC migration induced by niacin treatment in wild-type (Fig. 5A) or apoE−/− mice (Fig. 5B). To confirm that the dose of naproxen we chose was sufficient to reduce or block prostaglandin production, we applied arachidonic acid (AA) to the left ears of wild-type mice treated or not with the same dose of naproxen used in the previous DC migration assays 30 minutes prior to AA application. Right ears were treated with the vehicle, acetone. AA application results in a rapid ear swelling that is cyclooxygenase 1-dependent (Langenbach et al., 1995). Indeed, naproxen treatment quelled AA-induced ear swelling as compared to untreated mice (Supplemental Fig. 3). Together, these results suggested that niacin-induced production of prostaglandins was not the underlying mechanism of inhibited DC migration from the skin.
Figure 5.
Effect of naproxen on DC migration in mice treated with niacin. Cohorts of 6-8 week old (A) wild-type or (B) apoE−/− mice were fed control chow (Ctl) or 1%-niacin supplemented chow (Niacin) for 2 weeks and DC migration was subsequently assessed by FITC painting assay. Half of the mice from each diet condition were treated 30 minutes prior to contact sensitizer application with 15 mg/kg naproxen. 18 hours post sensitizer application, migration was quantified by flow cytometry. Each dot represents a pool of brachial and axillary lymph node from one side of a mouse (two dots per mouse), graphs in (A) represent two experiments compiled. Each experimental condition was repeated 3 times with 3-5 mice per condition. P-values, depicted within each graph, were determined with an unpaired T-test.
Niacin-mediated impairment of DC migration is not GPR109A-dependent
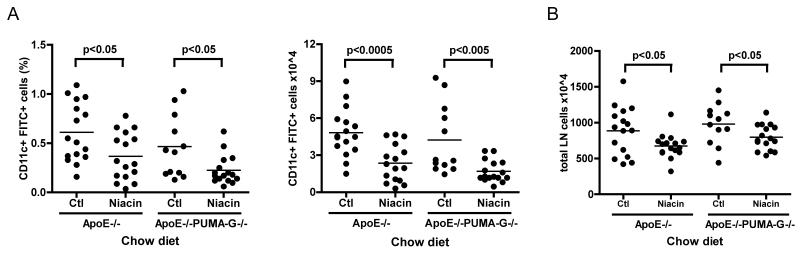
We next wondered whether GPR109A (PUMA-G in mice), the receptor that niacin binds to trigger prostanoid release and vasodilation in the skin (Benyo et al., 2005; Tunaru et al., 2003), was required for impaired DC migration. We compared DC migration in apoE−/− mice to that in apoE−/− PUMA-G−/− double knockout mice. In these experiments, as in Fig. 5, we assayed migration in younger apoE−/− and apoE−/−PUMA-G−/− mice; they do not exhibit suppression of DC migration seen in older apoE−/− mice of the same genotype (Angeli et al., 2004). ApoE−/− or apoE−/−PUMA-G−/− knockout mice, aged 6-8 weeks old, were fed either a control chow diet or a 1% niacin-supplemented diet for 10-14 days, and FITC-containing skin sensitizer was applied epicutaneously as in earlier experiments. As shown previously, apoE−/− mice fed niacin demonstrated significantly decreased DC migration (40% and 51% reduction in percentage and absolute number of migrated DCs, respectively) as compared to control chow-fed animals (Fig. 5A, 6A). Unexpectedly, apoE−/−PUMA-G−/− animals exhibited similarly decreased percentage and absolute number of migrated DCs, 51 and 60 percent, respectively, in response to niacin feeding compared to littermates fed control chow (Fig. 6A). Total lymph node cell counts were reduced in both genotypes of niacin-treated animals by 24 and 19 percent as compared to the control diet-fed apoE−/− or apoE−/−GPR109A−/− mice, respectively (Fig. 6B). Thus, the niacin receptor GPR109A was not necessary for niacin-mediated inhibition of DC migration. Taking our findings together, these data indicated that niacin had effects on the adaptive immune response unrelated to the pathways that mediate skin flushing. It is possible that these effects impact the therapeutic benefits of niacin to treat cardiovascular disease.
Figure 6.
Impact of GPR109A deficiency on niacin-mediated impairment of DC migration. Cohorts of 6-8 week old apoE−/− or apoE−/− PUMA-G−/− mice were fed control chow (Ctl) or 1%-niacin supplemented chow (Niacin) for 10-14 days and (A) DC migration was assessed by skin contact sensitization assay and quantified by flow cytometry while (B) total lymph node cells were quantified by manual counting. Each dot represents a pool of brachial and axillary lymph node from one side of a mouse (two dots per mouse), graphs represent two experiments compiled with 4 mice per condition/experiment. P-values, depicted within each graph, were determined with an unpaired T-test.
Discussion
The marked cardiovascular benefits of high-dose niacin have been recognized for more than fifty years (Carlson, 2005), but the phenomenon of skin flushing has limited the therapeutic use of niacin. Understanding the mechanisms of flushing, including downstream signaling pathways, may lead to drug discovery that targets the lipid modulating capabilities of niacin without triggering complications like skin toxicity (Walters et al., 2009). Remarkably, niacin therapy confers lasting protection against mortality from cardiovascular disease in men at high-risk (Canner et al., 1986). Furthermore, the same study, in which men taking niacin for 6 years were studied in a follow-up analysis 15 years post-treatment, suggested that niacin confers a protection against mortality from all causes (Canner et al., 1986), raising the possibility that niacin therapy confers beneficial effects beyond action in the cardiovascular system. The explanation for a lasting benefit still measurable 15 years after treatment may lie in the ability of niacin to raise HDL, which in turn may remodel atherosclerotic plaques to a long-term stable phenotype. However, it is also possible that other mechanisms contribute to these outcomes. Long-term protection from disease is a well-known feature of immunological memory. Our present study reveals that niacin treatment affects adaptive immunity, raising the possibility that part of its beneficial therapeutic effect results from immunomodulation.
Specifically, in this study, we took advantage of the fact that mice do not raise HDL in response to niacin therapy to search for possible HDL-independent effects of niacin beyond induction of skin flushing. We first investigated whether niacin would have an effect on plaque composition or monocyte recruitment and retention in atherosclerotic plaques. We also investigated whether skin flushing associated with niacin therapy would have an impact on the mobilization of DCs from skin and their subsequent accumulation in lymph nodes, given that niacin-mediated flushing is the result of prostaglandin release in both humans (Morrow et al., 1992; Morrow et al., 1989) and mice (Benyo et al., 2006; Hanson et al., 2010; Maciejewski-Lenoir et al., 2006). Prostaglandin D2 has been shown to inhibit DC migration to lymph nodes (Allan et al., 2006; Angeli et al., 2001).
We found that up to 4 weeks of high-dose niacin therapy did not have an impact on gross morphological changes in plaque and did not affect the magnitude of monocytes recruited to plaque (Fig. 1). These findings are consistent with Declercq et al., who did not assess monocyte trafficking but found no changes in plaque size after 14 weeks of niacin feeding in an apoE−/− mouse model of atherosclerosis (Declercq et al., 2005). However, our data contrast to a more recent study from Offermanns and colleagues (Lukasova et al., 2011). These investigators reported decreased lipid accumulation and reduced lesion area, downregulation of VCAM-1 and P-selectin mRNA expression in aortae, and decreased macrophage accumulation in plaques after niacin therapy. This study also found that monocyte recruitment to sites of acute inflammation, such as the peritoneum, was decreased by niacin treatment and that this recruitment, as well as that to atherosclerotic plaques, was dependent upon GPR109A. One difference between our findings and those of Lukasova et al. is that their study was carried out in LDLR−/− mice. However, this difference cannot explain discrepancies in trafficking to the inflamed peritoneum, as for this assay, we both studied wild-type C57/BL6 mice. Here, we carried out a typical peritoneal inflammation assessment, enumerating leukocytes 24 h after thioglycollate administration, when recruited monocytes dominate the infiltrate, in mice fed niacin or control diet for 2 weeks. Lukasova et al. utilized an unusual protocol in which the impact of niacin was not assessed until 4 days after thioglycollate instillation, a timepoint when recruited monocytes have already accumulated and differentiated to macrophages. At this timepoint, they administered one dose of niacin (or saline as a control) into the peritoneum along with CCL-2 (MCP-1) to assess macrophage recruitment. Given that niacin treatment was not administered until after the peak accumulation of monocytes, induced by thiogylcollate injection, we suggest that many interpretations unrelated to monocyte recruitment could be applied, including niacin-induced local macrophage death or adhesion. In their assessment of recruitment to atherosclerotic plaques, 4-day thioglycollate-elicited peritoneal macrophages, rather than monocytes that normally infiltrate plaques, were adoptively transferred and their accumulation in plaques was assessed. The sensitivity of their assay was approximately 10-fold lower than our bead assay tracking endogenous monocytes (7 labeled macrophages per mm2 of plaque using their approach versus 70 labeled macrophages per mm2 of plaque using ours) (Potteaux et al., 2011).
We did, however, observe that niacin treatment impaired DC accumulation in skin-draining lymph nodes (Fig. 2). Attenuated adaptive immunity, assessed using a contact hypersensitivity assay (Fig. 4), was observed, as would be expected from impaired DC accumulation in lymph nodes, though other mechanisms may also contribute to reduced hypersensitivity. Surprisingly, impaired DC accumulation was not reversed by inhibition of prostaglandin synthesis (Fig. 5) or genetic deficiency of GPR109A (Fig. 6). Collectively, these results imply that niacin-mediated reductions in DC accumulation within lymph nodes must be due to a mechanism of action altogether separate from skin flushing and other activities mediated by GPR109A (Offermanns, 2006). Importantly, DC migration was significantly suppressed in both apoE−/− and wild-type mice fed high fat or chow diets supplemented with niacin as compared to appropriate control animals, suggesting migration suppression is a universal phenomenon unrelated to underlying atherosclerosis per se. Walters et al. recently described that niacin signaling via a beta-arrestin 1 dependent pathway induces release of prostaglandin precursors leading to flushing (Walters et al., 2009). Interestingly, however, beta-arrestin null mice still retain anti-lipolytic activity, consistent with the idea that niacin can lead to activation of more than one signaling pathway (Walters et al., 2009).
The downstream mediators that account for how niacin impairs accumulation of lymph-trafficking DCs in skin-draining lymph nodes remain to be elucidated. Feeding niacin in the manner that we have used supplies high levels of both major forms of niacin, nicotinic acid and nicotinamide. Only nicotinic acid raises HDL levels (Altschul et al., 1955), but it remains possible that nicotinamide contributes to other protective effects when niacin is used clinically. In future studies, it will be important to determine if impaired DC mobilization is a consequence of other forms of niacin, especially nicotinamide, rather than nicotinic acid that binds to GPR109A. For example, nicotinamide inhibits NFκB and MAPK activation in skin keratinocytes in vitro (Grange et al., 2009) and may underlie the effectiveness of nicotinamide in treating acne. As NFκB signaling drives DC migration, its inhibition in vivo would be expected to produce the results we observed.
In summary, we have identified a novel action of high-dose niacin therapy in mice—the inhibition of DC accumulation in lymph nodes after their mobilization through lymphatic vessels. We furthermore demonstrated that this action was independent of the known receptor for nicotinic acid GPR109A. Though we did not observe that niacin impacted atherosclerotic directly in the same setting where it inhibits DC mobilization, we nonetheless suggest that it is possible that longer term analyses might reveal an impact on plaques or that disease protection that persists after therapy is lifted (Canner et al., 1986) might be related to immune modulation. Thus, it is now of interest to determine if this novel action of niacin is relevant in humans and if it confers protection in cardiovascular disease or in autoimmune diseases wherein overzealous DC activation of T cells has been observed. Conversely, it is possible that this action is detrimental and restoring DC migration during treatment would advance the efficacy of niacin in treating disease.
Supplementary Material
Supplemental Figure 1. Effect of niacin on the recruitment of monocytes to the inflamed peritoneum. Wild-type or apoE−/− mice were fed niacin-supplemented chow for 2 weeks prior to intraperitoneal injection of 3% thioglycollate. 24 hours post injection, infiltrated cells were recovered and counted, and the percentage and number of monocytes were determined by flow cytometric analysis. There are no statistically significant differences in the percentage (data not shown) or number of infiltrating monocytes in the niacin treated group as compared to the chow fed animals. Graph is representative of 3 experiments, n=3-5 animals per group.
Supplemental Figure 2. Effect of niacin treatment on monocyte exit from advanced atherosclerotic lesions. ApoE−/− mice were transitioned to a HFD at 5 weeks of age to induce disease progression. After 12 weeks of feeding, classical or nonclassical monocytes were labeled. 5 days after labeling, a baseline (Bl) group of mice from each labeling protocol was sacrificed and the aortic arches removed and sectioned, and bead number per section enumerated to establish initial monocyte entry into plaques. One half of the remaining mice from each labeling group were then fed 1% niacin-supplemented HFD (niacin) while the second half were maintained on the control (ctl) HFD for 15 days. Graphs depict the number of beads per section in the aortic arches when classical or nonclassical monocytes were labeled. There are no statistically significant differences in the number of remaining beads in the niacin treated group as compared to the baseline (BL) in these experiments. N= 4-9/group.
Supplemental Figure 3. Naproxen suppresses ear swelling induced by arachidonic acid. Naproxen, 15 mg/kg, was injected intraperitoneally 30 minutes prior to application of arachidonic acid or acetone vehicle to the insides of mouse ears. Ear swelling was measured at 2 hours post application. N=5, p-values were determined with an unpaired T-test.
Acknowledgements
We are grateful to Andrew Taggart at Merck for many helpful discussions and the gift of apoE−/− GPR109A−/− mice. We thank Andrew Platt for critical reading and discussion of the manuscript.
This work was supported by the Cordaptive Investigator-Initiated Study Program (IISP) from Merck and Co., Inc., and NIH grant AI061741 to G.J.R, a Ruth L. Kirschstein National Research Service Award F32HL096291 to M.A.I. S.P. was supported in part by Fondation pour la Recherche Medicale (France) and a 2007 Norman Alpert Visiting Scientist Award from the European Society of Cardiology/American Heart Association.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allan RS, Waithman J, Bedoui S, Jones CM, Villadangos JA, Zhan Y, Lew AM, Shortman K, Heath WR, Carbone FR. Migratory dendritic cells transfer antigen to a lymph node-resident dendritic cell population for efficient CTL priming. Immunity. 2006;25:153–162. doi: 10.1016/j.immuni.2006.04.017. [DOI] [PubMed] [Google Scholar]
- Altschul R, Hoffer A, Stephen JD. Influence of nicotinic acid on serum cholesterol in man. Arch Biochem. 1955;54:558–559. doi: 10.1016/0003-9861(55)90070-9. [DOI] [PubMed] [Google Scholar]
- Angeli V, Faveeuw C, Roye O, Fontaine J, Teissier E, Capron A, Wolowczuk I, Capron M, Trottein F. Role of the parasite-derived prostaglandin D2 in the inhibition of epidermal Langerhans cell migration during schistosomiasis infection. J Exp Med. 2001;193:1135–1147. doi: 10.1084/jem.193.10.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angeli V, Llodra J, Rong JX, Satoh K, Ishii S, Shimizu T, Fisher EA, Randolph GJ. Dyslipidemia associated with atherosclerotic disease systemically alters dendritic cell mobilization. Immunity. 2004;21:561–574. doi: 10.1016/j.immuni.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Askenase PW. Yes T cells, but three different T cells (αβ, γδ and NK T cells), and also B-1 cells mediate contact sensitivity. Clinical & Experimental Immunology. 2001;125:345–350. doi: 10.1046/j.1365-2249.2001.01619.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benyo Z, Gille A, Bennett CL, Clausen BE, Offermanns S. Nicotinic acid-induced flushing is mediated by activation of epidermal langerhans cells. Mol Pharmacol. 2006;70:1844–1849. doi: 10.1124/mol.106.030833. [DOI] [PubMed] [Google Scholar]
- Benyo Z, Gille A, Kero J, Csiky M, Suchankova MC, Nusing RM, Moers A, Pfeffer K, Offermanns S. GPR109A (PUMA-G/HM74A) mediates nicotinic acid-induced flushing. J Clin Invest. 2005;115:3634–3640. doi: 10.1172/JCI23626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blankenhorn DH, Azen SP, Crawford DW, Nessim SA, Sanmarco ME, Selzer RH, Shircore AM, Wickham EC. Effects of colestipol-niacin therapy on human femoral atherosclerosis. Circulation. 1991;83:438–447. doi: 10.1161/01.cir.83.2.438. [DOI] [PubMed] [Google Scholar]
- Blankenhorn DH, Nessim SA, Johnson RL, Sanmarco ME, Azen SP, Cashin-Hemphill L. Beneficial effects of combined colestipol-niacin therapy on coronary atherosclerosis and coronary venous bypass grafts. JAMA. 1987;257:3233–3240. [PubMed] [Google Scholar]
- Brown BG, Zhao XQ, Chait A, Fisher LD, Cheung MC, Morse JS, Dowdy AA, Marino EK, Bolson EL, Alaupovic P, et al. Simvastatin and niacin, antioxidant vitamins, or the combination for the prevention of coronary disease. N Engl J Med. 2001;345:1583–1592. doi: 10.1056/NEJMoa011090. [DOI] [PubMed] [Google Scholar]
- Brown G, Albers JJ, Fisher LD, Schaefer SM, Lin JT, Kaplan C, Zhao XQ, Bisson BD, Fitzpatrick VF, Dodge HT. Regression of coronary artery disease as a result of intensive lipid-lowering therapy in men with high levels of apolipoprotein B. N Engl J Med. 1990;323:1289–1298. doi: 10.1056/NEJM199011083231901. [DOI] [PubMed] [Google Scholar]
- Canner PL, Berge KG, Wenger NK, Stamler J, Friedman L, Prineas RJ, Friedewald W. Fifteen year mortality in Coronary Drug Project patients: long-term benefit with niacin. J Am Coll Cardiol. 1986;8:1245–1255. doi: 10.1016/s0735-1097(86)80293-5. [DOI] [PubMed] [Google Scholar]
- Carlson LA. Studies on the effect of nicotinic acid on catecholamine stimulated lipolysis in adipose tissue in vitro. Acta Med Scand. 1963;173:719–722. doi: 10.1111/j.0954-6820.1963.tb17457.x. [DOI] [PubMed] [Google Scholar]
- Carlson LA. Nicotinic acid: the broad-spectrum lipid drug. A 50th anniversary review. J Intern Med. 2005;258:94–114. doi: 10.1111/j.1365-2796.2005.01528.x. [DOI] [PubMed] [Google Scholar]
- Carlson LA, Rosenhamer G. Reduction of mortality in the Stockholm Ischaemic Heart Disease Secondary Prevention Study by combined treatment with clofibrate and nicotinic acid. Acta Med Scand. 1988;223:405–418. doi: 10.1111/j.0954-6820.1988.tb15891.x. [DOI] [PubMed] [Google Scholar]
- Declercq V, Yeganeh B, Moshtaghi-Kashanian GR, Khademi H, Bahadori B, Moghadasian MH. Paradoxical effects of fenofibrate and nicotinic acid in apo E-deficient mice. J Cardiovasc Pharmacol. 2005;46:18–24. doi: 10.1097/01.fjc.0000162764.12309.25. [DOI] [PubMed] [Google Scholar]
- Digby JE, McNeill E, Dyar OJ, Lam V, Greaves DR, Choudhury RP. Anti-inflammatory effects of nicotinic acid in adipocytes demonstrated by suppression of fractalkine, RANTES, and MCP-1 and upregulation of adiponectin. Atherosclerosis. 2010;209:89–95. doi: 10.1016/j.atherosclerosis.2009.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunbar RL, Gelfand JM. Seeing red: flushing out instigators of niacin-associated skin toxicity. J Clin Invest. 2010;120:2651–2655. doi: 10.1172/JCI44098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feig JE, Pineda-Torra I, Sanson M, Bradley MN, Vengrenyuk Y, Bogunovic D, Gautier EL, Rubinstein D, Hong C, Liu J, et al. LXR promotes the maximal egress of monocyte-derived cells from mouse aortic plaques during atherosclerosis regression. J Clin Invest. 2010;120:4415–4424. doi: 10.1172/JCI38911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganji SH, Qin S, Zhang L, Kamanna VS, Kashyap ML. Niacin inhibits vascular oxidative stress, redox-sensitive genes, and monocyte adhesion to human aortic endothelial cells. Atherosclerosis. 2009;202:68–75. doi: 10.1016/j.atherosclerosis.2008.04.044. [DOI] [PubMed] [Google Scholar]
- Garrigue JL, Nicolas JF, Fraginals R, Benezra C, Bour H, Schmitt D. Optimization of the mouse ear swelling test for in vivo and in vitro studies of weak contact sensitizers. Contact Dermatitis. 1994;30:231–237. doi: 10.1111/j.1600-0536.1994.tb00650.x. [DOI] [PubMed] [Google Scholar]
- Gautier EL, Jakubzick C, Randolph GJ. Regulation of the migration and survival of monocyte subsets by chemokine receptors and its relevance to atherosclerosis. Arterioscler Thromb Vasc Biol. 2009;29:1412–1418. doi: 10.1161/ATVBAHA.108.180505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass CK, Witztum JL. Atherosclerosis. the road ahead. Cell. 2001;104:503–516. doi: 10.1016/s0092-8674(01)00238-0. [DOI] [PubMed] [Google Scholar]
- Grange PA, Raingeaud J, Calvez V, Dupin N. Nicotinamide inhibits Propionibacterium acnes-induced IL-8 production in keratinocytes through the NF-kappaB and MAPK pathways. J Dermatol Sci. 2009;56:106–112. doi: 10.1016/j.jdermsci.2009.08.001. [DOI] [PubMed] [Google Scholar]
- Hanson J, Gille A, Zwykiel S, Lukasova M, Clausen BE, Ahmed K, Tunaru S, Wirth A, Offermanns S. Nicotinic acid- and monomethyl fumarate-induced flushing involves GPR109A expressed by keratinocytes and COX-2-dependent prostanoid formation in mice. J Clin Invest. 2010;120:2910–2919. doi: 10.1172/JCI42273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakubzick C, Helft J, Kaplan TJ, Randolph GJ. Optimization of methods to study pulmonary dendritic cell migration reveals distinct capacities of DC subsets to acquire soluble versus particulate antigen. J Immunol Methods. 2008;337:121–131. doi: 10.1016/j.jim.2008.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langenbach R, Morham SG, Tiano HF, Loftin CD, Ghanayem BI, Chulada PC, Mahler JF, Lee CA, Goulding EH, Kluckman KD, et al. Prostaglandin synthase 1 gene disruption in mice reduces arachidonic acid-induced inflammation and indomethacin-induced gastric ulceration. Cell. 1995;83:483–492. doi: 10.1016/0092-8674(95)90126-4. [DOI] [PubMed] [Google Scholar]
- Lee JM, Robson MD, Yu LM, Shirodaria CC, Cunnington C, Kylintireas I, Digby JE, Bannister T, Handa A, Wiesmann F, et al. Effects of high-dose modified-release nicotinic acid on atherosclerosis and vascular function: a randomized, placebo-controlled, magnetic resonance imaging study. J Am Coll Cardiol. 2009a;54:1787–1794. doi: 10.1016/j.jacc.2009.06.036. [DOI] [PubMed] [Google Scholar]
- Lee JMS, Robson MD, Yu L-M, Shirodaria CC, Cunnington C, Kylintireas I, Digby JE, Bannister T, Handa A, Wiesmann F, et al. Effects of High-Dose Modified-Release Nicotinic Acid on Atherosclerosis and Vascular Function: A Randomized, Placebo-Controlled, Magnetic Resonance Imaging Study. Journal of the American College of Cardiology. 2009b;54:1787–1794. doi: 10.1016/j.jacc.2009.06.036. [DOI] [PubMed] [Google Scholar]
- Llodra J, Angeli V, Liu J, Trogan E, Fisher EA, Randolph GJ. Emigration of monocyte-derived cells from atherosclerotic lesions characterizes regressive, but not progressive, plaques. Proc Natl Acad Sci U S A. 2004;101:11779–11784. doi: 10.1073/pnas.0403259101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukasova M, Malaval C, Gille A, Kero J, Offermanns S. Nicotinic acid inhibits progression of atherosclerosis in mice through its receptor GPR109A expressed by immune cells. J Clin Invest. 2011;121:1163–1173. doi: 10.1172/JCI41651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maciejewski-Lenoir D, Richman JG, Hakak Y, Gaidarov I, Behan DP, Connolly DT. Langerhans cells release prostaglandin D2 in response to nicotinic acid. J Invest Dermatol. 2006;126:2637–2646. doi: 10.1038/sj.jid.5700586. [DOI] [PubMed] [Google Scholar]
- Martin-Fontecha A, Sebastiani S, Hopken UE, Uguccioni M, Lipp M, Lanzavecchia A, Sallusto F. Regulation of dendritic cell migration to the draining lymph node: impact on T lymphocyte traffic and priming. J Exp Med. 2003;198:615–621. doi: 10.1084/jem.20030448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montecucco F, Quercioli A, Dallegri F, Viviani GL, Mach FX. New evidence for nicotinic acid treatment to reduce atherosclerosis. Expert Rev Cardiovasc Ther. 2010;8:1457–1467. doi: 10.1586/erc.10.116. [DOI] [PubMed] [Google Scholar]
- Morrow JD, Awad JA, Oates JA, Roberts LJ., 2nd Identification of skin as a major site of prostaglandin D2 release following oral administration of niacin in humans. J Invest Dermatol. 1992;98:812–815. doi: 10.1111/1523-1747.ep12499963. [DOI] [PubMed] [Google Scholar]
- Morrow JD, Parsons WG, 3rd, Roberts LJ., 2nd Release of markedly increased quantities of prostaglandin D2 in vivo in humans following the administration of nicotinic acid. Prostaglandins. 1989;38:263–274. doi: 10.1016/0090-6980(89)90088-9. [DOI] [PubMed] [Google Scholar]
- O’Leary JG, Goodarzi M, Drayton DL, von Andrian UH. T cell- and B cell-independent adaptive immunity mediated by natural killer cells. Nat Immunol. 2006;7:507–516. doi: 10.1038/ni1332. [DOI] [PubMed] [Google Scholar]
- Offermanns S. The nicotinic acid receptor GPR109A (HM74A or PUMA-G) as a new therapeutic target. Trends Pharmacol Sci. 2006;27:384–390. doi: 10.1016/j.tips.2006.05.008. [DOI] [PubMed] [Google Scholar]
- Ost CR, Stenson S. Regression of peripheral atherosclerosis during therapy with high doses of nicotinic acid. Scand J Clin Lab Invest Suppl. 1967;99:241–245. [PubMed] [Google Scholar]
- Paust S, Gill HS, Wang B-Z, Flynn MP, Moseman EA, Senman B, Szczepanik M, Telenti A, Askenase PW, Compans RW, von Andrian UH. Critical role for the chemokine receptor CXCR6 in NK cell-mediated antigen-specific memory of haptens and viruses. Nat Immunol. 2010;11:1127–1135. doi: 10.1038/ni.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potteaux S, Gautier EL, Hutchison SB, van Rooijen N, Rader DJ, Thomas MJ, Sorci-Thomas MG, Randolph GJ. Suppressed monocyte recruitment drives macrophage removal from atherosclerotic plaques of Apoe−/− mice during disease regression. J Clin Invest. 2011;121:2025–2036. doi: 10.1172/JCI43802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randolph GJ. Emigration of monocyte-derived cells to lymph nodes during resolution of inflammation and its failure in atherosclerosis. Curr Opin Lipidol. 2008;19:462–468. doi: 10.1097/MOL.0b013e32830d5f09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randolph GJ. The fate of monocytes in atherosclerosis. J Thromb Haemost. 2009;7(Suppl 1):28–30. doi: 10.1111/j.1538-7836.2009.03423.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbiani DF, Finch RA, Jager D, Muller WA, Sartorelli AC, Randolph GJ. The leukotriene C(4) transporter MRP1 regulates CCL19 (MIP-3beta, ELC)-dependent mobilization of dendritic cells to lymph nodes. Cell. 2000;103:757–768. doi: 10.1016/s0092-8674(00)00179-3. [DOI] [PubMed] [Google Scholar]
- Robertson AK, Hansson GK. T cells in atherogenesis: for better or for worse? Arterioscler Thromb Vasc Biol. 2006;26:2421–2432. doi: 10.1161/01.ATV.0000245830.29764.84. [DOI] [PubMed] [Google Scholar]
- Schaub A, Futterer A, Pfeffer K. PUMA-G, an IFN-gamma-inducible gene in macrophages is a novel member of the seven transmembrane spanning receptor superfamily. Eur J Immunol. 2001;31:3714–3725. doi: 10.1002/1521-4141(200112)31:12<3714::aid-immu3714>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Soga T, Kamohara M, Takasaki J, Matsumoto S, Saito T, Ohishi T, Hiyama H, Matsuo A, Matsushime H, Furuichi K. Molecular identification of nicotinic acid receptor. Biochem Biophys Res Commun. 2003;303:364–369. doi: 10.1016/s0006-291x(03)00342-5. [DOI] [PubMed] [Google Scholar]
- Tacke F, Alvarez D, Kaplan TJ, Jakubzick C, Spanbroek R, Llodra J, Garin A, Liu J, Mack M, van Rooijen N, et al. Monocyte subsets differentially employ CCR2, CCR5, and CX3CR1 to accumulate within atherosclerotic plaques. J Clin Invest. 2007;117:185–194. doi: 10.1172/JCI28549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tacke F, Ginhoux F, Jakubzick C, van Rooijen N, Merad M, Randolph GJ. Immature monocytes acquire antigens from other cells in the bone marrow and present them to T cells after maturing in the periphery. J Exp Med. 2006;203:583–597. doi: 10.1084/jem.20052119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor AJ, Villines TC, Stanek EJ, Devine PJ, Griffen L, Miller M, Weissman NJ, Turco M. Extended-release niacin or ezetimibe and carotid intima-media thickness. N Engl J Med. 2009;361:2113–2122. doi: 10.1056/NEJMoa0907569. [DOI] [PubMed] [Google Scholar]
- The Coronary Drug Project Research Group Clofibrate and niacin in coronary heart disease. JAMA. 1975;231:360–381. [PubMed] [Google Scholar]
- Tunaru S, Kero J, Schaub A, Wufka C, Blaukat A, Pfeffer K, Offermanns S. PUMA-G and HM74 are receptors for nicotinic acid and mediate its anti-lipolytic effect. Nat Med. 2003;9:352–355. doi: 10.1038/nm824. [DOI] [PubMed] [Google Scholar]
- Wahlberg G, Walldius G. Effects of nicotinic acid treatment on glyceride formation and lipolysis in adipose tissue of hyperlipidemic patients. Int J Clin Lab Res. 1993;23:88–94. doi: 10.1007/BF02592289. [DOI] [PubMed] [Google Scholar]
- Walters RW, Shukla AK, Kovacs JJ, Violin JD, DeWire SM, Lam CM, Chen JR, Muehlbauer MJ, Whalen EJ, Lefkowitz RJ. beta-Arrestin1 mediates nicotinic acid-induced flushing, but not its antilipolytic effect, in mice. J Clin Invest. 2009;119:1312–1321. doi: 10.1172/JCI36806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitney EJ, Krasuski RA, Personius BE, Michalek JE, Maranian AM, Kolasa MW, Monick E, Brown BG, Gotto AM., Jr. A randomized trial of a strategy for increasing high-density lipoprotein cholesterol levels: effects on progression of coronary heart disease and clinical events. Ann Intern Med. 2005;142:95–104. doi: 10.7326/0003-4819-142-2-200501180-00008. [DOI] [PubMed] [Google Scholar]
- Wise A, Foord SM, Fraser NJ, Barnes AA, Elshourbagy N, Eilert M, Ignar DM, Murdock PR, Steplewski K, Green A, et al. Molecular identification of high and low affinity receptors for nicotinic acid. J Biol Chem. 2003;278:9869–9874. doi: 10.1074/jbc.M210695200. [DOI] [PubMed] [Google Scholar]
- Wu BJ, Yan L, Charlton F, Witting P, Barter PJ, Rye KA. Evidence that niacin inhibits acute vascular inflammation and improves endothelial dysfunction independent of changes in plasma lipids. Arterioscler Thromb Vasc Biol. 2010;30:968–975. doi: 10.1161/ATVBAHA.109.201129. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Schmidt RJ, Foxworthy P, Emkey R, Oler JK, Large TH, Wang H, Su EW, Mosior MK, Eacho PI, Cao G. Niacin mediates lipolysis in adipose tissue through its G-protein coupled receptor HM74A. Biochem Biophys Res Commun. 2005;334:729–732. doi: 10.1016/j.bbrc.2005.06.141. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Effect of niacin on the recruitment of monocytes to the inflamed peritoneum. Wild-type or apoE−/− mice were fed niacin-supplemented chow for 2 weeks prior to intraperitoneal injection of 3% thioglycollate. 24 hours post injection, infiltrated cells were recovered and counted, and the percentage and number of monocytes were determined by flow cytometric analysis. There are no statistically significant differences in the percentage (data not shown) or number of infiltrating monocytes in the niacin treated group as compared to the chow fed animals. Graph is representative of 3 experiments, n=3-5 animals per group.
Supplemental Figure 2. Effect of niacin treatment on monocyte exit from advanced atherosclerotic lesions. ApoE−/− mice were transitioned to a HFD at 5 weeks of age to induce disease progression. After 12 weeks of feeding, classical or nonclassical monocytes were labeled. 5 days after labeling, a baseline (Bl) group of mice from each labeling protocol was sacrificed and the aortic arches removed and sectioned, and bead number per section enumerated to establish initial monocyte entry into plaques. One half of the remaining mice from each labeling group were then fed 1% niacin-supplemented HFD (niacin) while the second half were maintained on the control (ctl) HFD for 15 days. Graphs depict the number of beads per section in the aortic arches when classical or nonclassical monocytes were labeled. There are no statistically significant differences in the number of remaining beads in the niacin treated group as compared to the baseline (BL) in these experiments. N= 4-9/group.
Supplemental Figure 3. Naproxen suppresses ear swelling induced by arachidonic acid. Naproxen, 15 mg/kg, was injected intraperitoneally 30 minutes prior to application of arachidonic acid or acetone vehicle to the insides of mouse ears. Ear swelling was measured at 2 hours post application. N=5, p-values were determined with an unpaired T-test.