Abstract
Preclinical investigations and selected clinical observational studies support an association between higher vitamin D intake and 25-hydroxyvitamin D levels with lower breast cancer risk. However, the recently updated report from the Institute of Medicine concluded that, for cancer and vitamin D, the evidence was 'inconsistent and insufficient to inform nutritional requirements'. Against this background, reports examining vitamin D intake, 25-hydroxyvitamin D levels and breast cancer incidence and outcome were reviewed. Current evidence supports the pursuit of several research questions but not routine 25-hydroxyvitamin D monitoring and vitamin D supplementation to reduce breast cancer incidence or improve breast cancer outcome.
Introduction
The role of vitamin D in relation to breast cancer incidence and outcome is controversial. Evidence from in vitro studies [1,2], animal studies [3,4], and selected clinical observational studies [5,6] has generally supported an association between higher vitamin D intakes and levels with lower breast cancer risk, but the results have not been consistent. Nonetheless, intervention strategies based on monitoring of vitamin D status with 25-hydroxyvitamin D (25(OH)D) levels and supplementation with vitamin D have been proposed for implementation in breast cancer clinical practice [6-8].
In contrast are findings from the 2011 report on dietary requirements for calcium and vitamin D from the Institute of Medicine (IOM) [9,10]. For cancer outcomes, the report concluded that 'the evidence was inconsistent, inconclusive as to causality, and insufficient to inform nutritional requirements' [10]. Against this background, current evidence regarding vitamin D and breast cancer was reviewed to inform clinical practice and identify potential research directions.
Identification of studies
A literature search identified observational studies and randomized clinical trials assessing associations among vitamin D intake and/or serum 25(OH)D levels and breast cancer incidence and outcome. We searched the PubMed and EMBASE databases and the American Society of Clinical Oncology and the San Antonio Breast Cancer Symposium proceedings through 31 January 2011 for relevant reports. Search terms included vitamin D, 25-hydroxyvitamin D, 1,25-hydroxyvitamin D, and clinical breast cancer incidence and outcome. The same source literature was searched for review articles addressing optimal and recommended vitamin D intake and 25(OH)D levels and determinants of 25(OH)D levels. Cross-referencing was used to complement relevant report identification. Titles and abstracts were reviewed for relevance. The full text was reviewed for those articles with relevant relationships.
Vitamin D intake and breast cancer incidence
Vitamin D intake (from diet and supplements) and breast cancer incidence have been examined in 10 case-control studies [11-20] and in 10 studies in cohorts with mixed results [5,21-29].
A meta-analyses of five case-control studies reported no overall association between vitamin D intake and breast cancer risk (relative risk = 0.95, 95% confidence interval (CI) = 0.69 to 1.32), but an analysis limited to premenopausal/perimenopausal women demonstrated a significant association (relative risk = 0.83, 95% CI = 0.73 to 0.95) [30]. Other case-control studies identified significant associations in subgroups. In one study, vitamin D exposure mainly early in life (ages 10 to 19, based on outdoor activities) was strongly related to subsequent breast cancer risk (low to high quartile, odds ratio = 0.65, 95% CI = 0.50 to 0.85) [17]. Similar to the studies by Abbas and colleagues [14] and Lin and colleagues [24], two recent studies found significant associations between vitamin D exposure and breast cancer incidence only in premenopausal women [18,19].
While a significant inverse association between vitamin D intake and breast cancer risk was seen in a meta-analysis of six of the cohort studies (relative risk = 0.90, 95% CI = 0.83 to 0.98) [30], this analysis did not include two recent, large, well-conducted, completely negative Scandinavian reports or the negative report from a large European cohort [26-28]. In the recent French E3N cohort report, only in regions with the highest ultraviolet solar radiance was high vitamin D intake associated with lower breast cancer risk (hazard ratio (HR) = 0.68, 95% CI = 0.54 to 0.85) [29].
25-Hydroxyvitamin D concentration and breast cancer incidence
Concentration of 25(OH)D is a generally accepted biomarker for determining vitamin D status [31], and studies of 25(OH)D and breast cancer incidence also provide mixed results. Four case-control studies significantly associated lower 25(OH)D levels with higher breast cancer incidence [32-36]. In these studies, however, the 25(OH)D levels were obtained at some interval following breast cancer diagnoses with potential alterations by cancer therapy or its sequellae. For example, women with lower physical activity have lower 25(OH)D levels, and physical activity is consistently decreased for years following a breast cancer diagnosis [37,38]. Positive associations may therefore not be reliable as only one case-control study adjusted for physical activity [33].
Six prospective nested case-control studies, which should provide more reliable findings, have examined 25(OH)D levels and subsequent breast cancer incidence [39-45]. In contrast to case-control studies, only one of these cohort studies that measure 25(OH)D before diagnosis reported a significant association between 25(OH) D levels and breast cancer incidence (Table 1) [45], while one study showed a borderline association [39]. In the positive French E3N cohort, the odds ratio was 0.73 (95% CI = 0.55 to 0.96, Ptrend = 0.02) and the association was stronger in younger women (age <53 years) [45]. Finally, in a relatively small cohort of female participants in the Third National and Nutritional Examination Survey, no association was seen between 25(OH)D levels and breast cancer mortality [46].
Table 1.
25-Hydroxyvitamin D and breast cancer incidence: nested case-control studies in cohorts
| Cohort | Lead author | Cohort (n) | Case patients (n) | Control subjects (n) | P trend a |
|---|---|---|---|---|---|
| Cancer Prevention Study II Nutrition Cohort | McCullough | 21,965 | 516 | 516 | 0.60 |
| Malmo Diet and Cancer Study | Almquist | 53,000 | 764 | 764 | NS |
| Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial | Freedman | 38,660 | 1,005 | 1,005 | 0.81 |
| Women's Health Initiative | Chlebowski | 32,826 | 895 | 898 | 0.20 |
| Nurses' Health Study | Bertone-Johnson | 32,826 | 701 | 724 | 0.06 |
| French E3N Cohort | Engel | 17,391 | 636 | 1,272 | 0.02b |
aPtrend for analyses comparing breast cancer incidence in low versus high 25-hydroxyvitamin D groups. bFindings driven by results in women <53 years old at sampling.
The importance of incorporating physical activity as a covariate is illustrated in findings from the prospective case-control study nested in the Women's Health Initiative (WHI) cohort [42]. In analyses without body mass index and physical activity measures, a statistically significant association was seen between lower 25(OH)D levels and higher breast cancer incidence. The finding was attenuated, however, and became nonsignificant with inclusion of these factors in the analytic model [42].
25-Hydroxyvitamin D concentration in breast cancer patients
Several uncontrolled studies have reported a high frequency of low 25(OH)D levels in breast cancer patients [7,8,47,48]. In one study that identified 74% of breast cancer patients deficient for 25(OH)D (defined as <20 ng/ml or <50 nmol/l), despite a recommendation to take 400 IU vitamin D with calcium daily, few patients (<15%) achieved 25(OH)D levels >30 ng/ml (75 nmol/l) [47]. In a retrospective study of 500 newly diagnosed breast cancer patients, 69% were deficient for 25(OH)D (defined as <32 ng/ml or <80 nmol/l) and were supplemented with 8,000 IU vitamin D3 daily (from 4,200 IU D3 capsules). The subsequent 25(OH)D values were increased (19.7 (8.0) ng/ml vs. 37.6 (16.8) ng/ml, respectively; P < 0.01) but many remained <32 ng/ml [49].
Based on such findings, some studies have suggested routine monitoring of 25(OH)D and supplemental vitamin D use for those identified at low levels [7,47]. Others note that these uncontrolled observational study reports have not linked 25(OH)D to breast cancer outcomes [50,51]. In addition, the recent IOM report now recommends a lower 25(OH)D level than those used in several of these reports as being sufficient (>20 ng/ml or >50 nmol/l) [9,10].
25-Hydroxyvitamin D levels and breast cancer recurrence
Three studies have examined the association between 25(OH)D levels at diagnosis and subsequent breast cancer outcome (Table 2). Goodwin and colleagues followed a cohort of 522 early-stage breast cancer patients for a mean of 11.6 years [51]. Women were sampled postoperatively before initiation of systemic adjuvant therapy. Those women with deficient 25(OH)D levels (defined as <50 nmol/l or <20 ng/ml), compared with those women with sufficient levels (>72 nmol/l), had a higher risk of distant recurrence (HR = 1.94, 95% CI = 1.16 to 3.25, P < 0.01) and of death (HR = 1.73, 95% CI = 1.05 to 2.86, P < 0.01). The associations were attenuated, however, and became non significant after multivariate analysis adjusting for traditional prognostic factors [51].
Table 2.
25-Hydroxyvitamin D concentration and subsequent breast cancer outcome in patients with resected early-stage disease
| Adjuvant therapy | ||||||
|---|---|---|---|---|---|---|
| Lead author | n | Category | Hormonal therapy | Chemotherapy | Mean follow-up (years) | Study outcome |
| Goodwin | 512 | Early breast cancer, resected | Tamoxifen per clinical decision | Varies per clinical decision | 11.6 | Deficient (<50 nmol/l) vs. sufficient (>72 nmol/l) 25(OH)D levels, in multivariant adjusted analysesa |
| Cohort | Distant recurrence HR = 1.71 95% CI = 1.02 to 2.86a | |||||
| Premenopausal and postmenopausal | Survival HR = 1.60, 95% CI = 0.96 to 2.64 | |||||
| Piura | 622 | Early breast cancer, resected | Tamoxifen for 53 years vs. tamoxifen for 53 years + octreotide for 2 years (per protocol) | Varies per clinical decision | 7.9 | No significant association with event-free survival or relapse-free survival with 25(OH)D level |
| Cohort within a randomized clinical trial Postmenopausal | ||||||
| Jacobs | 1,024 | Early breast cancer, resected entered within 43 years from diagnosis Nested case-control within a randomized clinical trial | Varies per clinical decision | Varies per clinical decision | 7.3 | No significant association with breast cancer recurrence (local, regional, or distant) or death with 25(OH)D level |
| Premenopausal and postmenopausal | ||||||
CI, confidence interval; HR, hazard ratio; 25(OH)D, 25-hydroxyvitamin D. aFindings were statistically significant in analyses adjusted for age and tumor stage.
Piura and colleagues examined the same association in 607 postmenopausal women with early-stage, hormone-receptor-positive breast cancer participating in a randomized, controlled adjuvant trial in which all patients received tamoxifen with or without octreotide [52]. In this setting, no association between baseline 25(OH)D levels and relapse-free survival or relapse at any site was seen [52].
Finally, a nested case-control analysis was conducted in the 3,085 early-stage, resected breast cancer patients participating in the Women's Healthy Eating and Living study [53]. Women in this study evaluating a dietary intervention were re-consented within 4 years of early-stage breast cancer diagnosis and were recurrence free at entry. In 512 matched pairs of breast cancer patients who had experienced cancer recurrence and control subjects who were recurrence-free at a comparable follow-up period, no association between 25(OH)D levels at baseline and subsequent breast cancer recurrence was observed. Taken together, these three studies provide mixed findings and no compelling evidence of an association between lower 25(OH)D levels and adverse breast cancer clinical outcome.
The feasibility of conducting a randomized trial of vitamin D supplementation in adjuvant breast cancer has been explored recently. In women with early-stage, resected breast cancer, more than 80% were found to be already using vitamin D supplements at a median daily dose >1,200 IU/day and the median 25(OH)D levels were above 34.3% ng/ml (85.5 nmol/l), exceeding the sufficient level (20 ng/ml or 50 nmol/l). Considering such findings, a phase III trial was not judged to be feasible [54].
Vitamin D and arthralgias in breast cancer patients
Low 25(OH)D levels have been associated with musculo-skeletal disorders [55]. More recently, Chlebowski and colleagues found significantly higher joint pain with extremely low 25(OH)D levels (<29 nmol/l or 12 ng/ml) in 1,993 postmenopausal women [56]. In postmenopausal breast cancer patients, aromatase inhibitors are not uncommonly associated with limiting arthralgias [57], which have been described as greater in those with low 25(OH)D levels [58,59]. Currently, several prospective but nonrandomized trials evaluating higher dose vitamin D regimens have reported less joint pain in women who achieved relatively higher target 25(OH)D levels of 403 ng/ml (100 nmol/l) [59] and 66 ng/ml (218 nmol/l on vitamin D supplementation) [60]. As the recent IOM report has identified concerns about higher clinical risks at 25(OH)D levels >50 ng/ml (125 nmol/l) [9,10] and observations in a breast cancer cohort suggest survival may be optimal for women with 25(OH)D levels <44 ng/ml (110 nmol/l) [51], such high-dose vitamin D strategies require careful clinical trial evaluation before implementation in general practice.
Vitamin D, 25-hydroxyvitamin D levels and mammogram breast density
Reports of associations among vitamin D intake, 25(OH) D levels and mammographic breast density have been mixed. Early reports associated higher vitamin D intake with lower mammographic breast density [61], perhaps especially in premenopausal women [62,63]. A series of more recent studies, however, reports no such association in either premenopausal women [64,65] or postmenopausal women [64-68].
Randomized trials of calcium and vitamin D supplementation and breast cancer incidence
The WHI randomized 36,282 postmenopausal women to placebo or supplementation with calcium (1,000 mg/day) plus vitamin D3 (400 IU/day), with hip fracture as the primary outcome and colorectal cancer and breast cancer as secondary outcomes [69,70]. After 7 years of intervention, there was no difference in invasive breast cancer incidence (528 vs. 546 breast cancers, respectively; HR = 0.96, 95% CI = 0.85 to 1.09) between the randomization groups. In subgroup analyses, women in the highest vitamin D intake quintile at entry (≥600 IU/day) actually had a higher breast cancer incidence with supplemental vitamin D use (HR = 1.34, 95% CI = 1.01 to 1.78) [42]. In the case-control analyses nested in this trial, the mean 25(OH)D level was 50 ± 21 nmol/l among the 895 participants who subsequently were diagnosed with breast cancer, with a closely comparable level of 52 ± 21 nmol/l in the 898 matched controls who did not develop breast cancer [42].
One other clinical trial has evaluated calcium plus vitamin D influence on cancer risk in a smaller study using a larger vitamin D dose. In 1,179 postmenopausal women randomized to placebo, to calcium alone (1,400 to 1,500 mg/day) or to calcium plus 1,100 IU vitamin D3/day in a 1:2:2 ratio [71], there were fewer total cancers in the calcium plus vitamin D supplement compared with the placebo group (2.9% vs. 6.9%, P < 0.05). This finding was based on the distribution of a total of 33 cancer cases but, as only 13 breast cancers were diagnosed, meaningful interpretation regarding breast cancer influence is precluded.
Randomized clinical trials of vitamin D and total mortality in general populations
Vitamin D supplementation has been evaluated in a number of full-scale randomized, clinical trials, with or without calcium, mostly with fracture as the major endpoint. These trials have generally reported few details of clinical outcomes other than fractures or provided systematic evaluation of causes of death. Interest in the potential benefit of vitamin D supplementation on a range of clinical outcomes and overall health, however, prompted interest in examining mortality in these randomized trials.
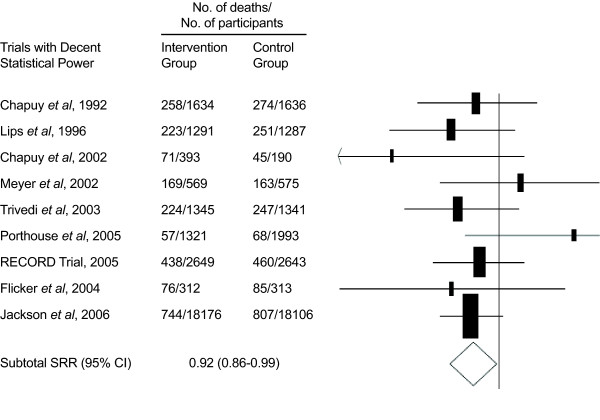
A meta-analysis of nine larger trials (all entering >582 participants) incorporating 57,311 participants (including 36,282 from the WHI trial [72]) identified 4,777 deaths during a median 5.7 years of follow-up [73]. The trial size-adjusted mean vitamin D3 dose was a relatively modest 528 IU/day. Total mortality was 8% lower in the vitamin D supplement group, a finding of borderline significance (HR = 0.92, 95% CI = 0.86 to 0.99, P < 0.05) (Figure 1) [73]. A subsequent analysis suggested lower mortality when vitamin D was given with calcium supplementation [74]. These results should not be simply extrapolated to a 'more is better' concept since an observational study has suggested a U-shaped curve with lowest mortality risk at moderate 25(OH)D levels and increased mortality risk at both low and high levels of 25(OH)D [75].
Figure 1.
Vitamin D and total mortality in a meta-analysis of randomized controlled trials. Meta-analysis of randomized, controlled trials evaluating supplementation with vitamin D alone or in combination with calcium compared with placebo or no intervention on total mortality. The size of the box indicates the number of deaths, and the horizontal lines indicate the 95% confidence interval (CI). Adapted with permission from Autier and Gandini [73]. RR, relative risk.
Further attempts to clarify this potential survival influence of supplemental vitamin D in a conventional dose should be pursued with additional follow-up of existing conventional dose trials. In addition, there is an ongoing full-scale randomized trial evaluating supplemental vitamin D in a higher daily dose (2,000 IU D3) plus omega 3 fatty acids (1,000 mg/day) versus placebo in a large population of about 20,000 otherwise healthy men and women [76]. This trial has begun but results are not expected for several years. Additionally, the Vitamin D and Longevity trial is examining an intermittent high-dose vitamin D regimen in the United Kingdom [77].
Supplemental vitamin D adverse effects
While vitamin D is relatively safe, a review of randomized or quasi-randomized trials found adverse effects of hypercalcemia, gastrointestinal symptoms and renal disease significantly increased by vitamin D administration in conventional dosage (<1,000 IU/day) [43], the latter being of importance given the prevalence of renal deficiency in breast cancer patients [78]. While several pilot studies of short-term, parental high-dose vitamin D on safety have been reported [60,79], the side efforts of high-dose regimens for long duration use are unknown. Finally, the IOM report has identified safety concerns potentially associated with 25(OH)D levels >503 ng/ml (>125 nmol/l) [9,10].
Factors influencing 25-hydroxyvitamin D levels, vitamin D and breast cancer
It is not commonly recognized that factors other than sunlight exposure and vitamin D intake (both dietary and supplement) make a substantial contribution to 25(OH)D levels. In a pooling cohort consortium with 4,723 samples from 10 cohorts, statistically significant positive correlates of 25(OH)D included male sex, summer sample, physical activity and multivitamin use. Significant negative correlates were body mass index, winter and spring samples, diabetes, sedentary behavior, smoking and Black race/ethnicity [80]. The findings of relatively low 25(OH)D levels in Black women compared with White women have led to speculation regarding the potential role of low 25(OH)D contributing to the observed ethnic disparity in breast cancer outcome [81,82].
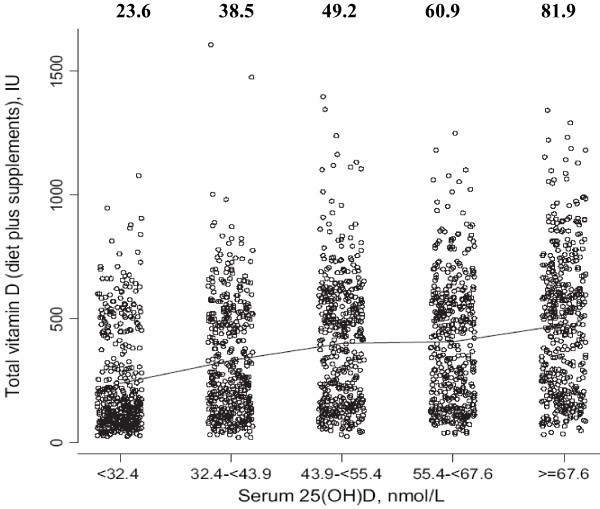
In randomized trials, an inconsistent relation has been observed between total vitamin D intake (diet plus supplement) and subsequent 25(OH)D levels [83]. In the WHI cohort, when 25(OH)D levels were compared with total vitamin D intake (dietary and supplement) [42], the difference in median vitamin D intake comparing low (deficient, 243 nmol/l) with high (optimal, 82 nmol/l) quintiles was only 238 IU daily, about one-half of the usual multivitamin tablet. In addition, only 3% of those in the highest quintile had vitamin D intakes >1,000 IU/day (Figure 2). Compared with vitamin D intake, stronger associations with 25(OH)D were seen for body mass index and physical activity with leaner, more physically active women having significantly higher levels (P3 < 0.0001) [42]. Failure to control for these two factors could thus potentially confound observational studies of 25(OH)D and breast cancer.
Figure 2.
Total vitamin D intake and serum 25-hydroxyvitamin D by quintile. Individual total vitamin D intake (diet plus supplementation) and serum 25-hydroxyvitamin D (25(OH)D) levels at baseline. Serum 25(OH)D levels from 1,067 women identified as control subjects from a nested case-control study performed in the Women's Health Initiative trial evaluating calcium and vitamin D. Daily intakes of dietary and supplemental vitamin D were determined from self-report. The range of vitamin D intakes substantially overlaps in each 25(OH)D quintile. Line segments connect the mean vitamin D intake level in each quintile, which was 23.6, 38.5, 49.2, 60.9, and 81.9 nmol/l, respectively. Adapted with permission from Chlebowski and colleagues [42].
In the WHI cohort, a multivariant predictive model could account for only 21% of the differences in 25(OH)D levels between individuals in a random sample of 3,055 postmenopausal women [84]. This finding is consistent with other reports in which a substantial proportion of 25(OH)D difference between individuals is probably genetically determined [85,86]. The largely unexplained factors influencing differences in 25(OH)D levels between individuals complicate understanding of associations with disease states and development of rationale therapeutic strategies.
Conclusions
The recent IOM report on calcium and vitamin D requirements provides an authoritative base for consideration of vitamin D and breast cancer issues. For vitamin D, the IOM recommendations are based primarily on bone health outcomes. The total recommended daily vitamin D intake for women <71 years old is 600 IU/day. For those 71 years or older, an intake of 800 IU/day - corresponding to a serum 25(OH)D level of 20 ng/ml (503 nmol/l) - is recommended. These levels and cutoff points are lower than proposed by some in the current literature but the IOM committee did not judge higher level recommendations to be justified by available evidence [9,10]. Randomized clinical trial evidence indicates that vitamin D supplementation (at a dose of about 400 to 800 IU/day), together with supplemental calcium, results in a modest decrease in fracture risk for women at higher fracture risk [87]. As many breast cancer patients are at fracture risk based on age and effects of cancer therapy (such as oophorectomy, chemotherapy-associated amenorrhea, and aromatase inhibitors), use of vitamin D supplements (400 to 800 IU/day) plus calcium in those at increased fracture risk can be recommended. For early-stage breast cancer patients, suggestions regarding routine monitoring of 25(OH)D levels and vitamin D supplementation to some target level are inferential and based on mixed observational study results.
Current evidence is sufficient to support further study of factors influencing 25(OH)D levels, associations between 25(OH)D levels and breast cancer in premenopausal and Black women, moderate dose (≤2,000 IU D3/day) supplemental vitamin D use and breast cancer incidence, and observational studies evaluating whether a threshold higher 25(OH)D level is associated with adverse clinical outcome in women with breast cancer. Before routine clinical application of any strategies targeting vitamin D status for breast cancer prevention or therapy are undertaken, the limitations of the current evidence should be considered.
Abbreviations
CI: confidence interval; HR: hazard ratio; 25(OH)D: 25-hydroxyvitamin D; IOM: Institute of Medicine; WHI: Women's Health Initiative.
Competing interests
The author declares that they have no competing interests.
Acknowledgements
The described studies in the Women's Health Initiative were supported by the National Heart, Lung and Blood Institute, US Department of Health and Human Services.
References
- Deeb KK, Trump DL, Johnson CS. Vitamin D signaling pathways in cancer: potential for anticancer therapeutics. Nat Rev Cancer. 2007;7:684–700. doi: 10.1038/nrc2196. [DOI] [PubMed] [Google Scholar]
- James SY, Mackay AG, Colston KW. Effects of 1,25 dihydroxyvitamin D3 and its analogues on induction of apoptosis in breast cancer cells. J Steroid Biochem Mol Biol. 1996;58:395–401. doi: 10.1016/0960-0760(96)00048-9. [DOI] [PubMed] [Google Scholar]
- Matthews D, LaPorta E, Zinser GM, Narvaez CJ, Welsh J. Genomic vitamin D signaling in breast cancer: insights from animal models and human cells. J Steroid Biochem Mol Biol. 2010;121:362–367. doi: 10.1016/j.jsbmb.2010.03.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh J. Vitamin D and breast cancer: insights from animal models. Am J Clin Nutr. 2004;80:1721S–1724S. doi: 10.1093/ajcn/80.6.1721S. [DOI] [PubMed] [Google Scholar]
- John EM, Schwartz GG, Dreon DM, Koo J. Vitamin D and breast cancer risk: the NHANES I Epidemiologic follow-up study, 1971-1975 to 1992. National Health and Nutrition Examination Survey. Cancer Epidemiol Biomarkers Prev. 1999;8:399–406. [PubMed] [Google Scholar]
- Garland CF, Gorham ED, Mohr SB, Garland FC. Vitamin D and cancer prevention: global perspective. Ann Epidemiol. 2009;19:468–483. doi: 10.1016/j.annepidem.2009.03.021. [DOI] [PubMed] [Google Scholar]
- Palmieri C, MacGregor T, Girgis S, Vigushin D. Serum 25-hydroxyvitamin D levels in early and advanced breast cancer. J Clin Pathol. 2006;59:1334–1336. doi: 10.1136/jcp.2006.042747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuhouser ML, Sorensen B, Hollis BW, Ambs A, Ulrich CM, McTiernan A, Bernstein L, Wayne S, Gilliland F, Baumgartner K, Baumgartner R, Ballard- Barbash R. Vitamin D insufficiency in a multiethnic cohort of breast cancer survivors. Am J Clin Nutr. 2008;88:133–139. doi: 10.1093/ajcn/88.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Institute of Medicine. Dietary Reference Intakes for Calcium and Vitamin D. Washington, DC: National Academies Press; 2011. [PubMed] [Google Scholar]
- Ross CA, Manson JE, Abrams SA, Aloia JF, Brannon PM, Clinton SK, Durazo- Arvizu RA, Gallagher JC, Gallo RL, Jones G, Kovacs CS, Mayne ST, Rosen CJ, Shapses SA. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab. 2011;96:53–58. doi: 10.1210/jc.2010-2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simard A, Vobecky J, Vobecky JS. Vitamin D deficiency and cancer of the breast: an unprovocative ecological hypothesis. Can J Public Health. 1991;82:300–303. [PubMed] [Google Scholar]
- Potischman N, Swanson CA, Coates RJ, Gammon MD, Brogan DR, Curtin J, Brinton LA. Intake of food groups and associated micronutrients in relation to risk of early-stage breast cancer. Int J Cancer. 1999;82:315–321. doi: 10.1002/(SICI)1097-0215(19990730)82:3<315::AID-IJC1>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Levi F, Pasche C, Lucchini F, La Vecchia C. Dietary intake of selected micronutrients and breast cancer risk. Int J Cancer. 2001;91:260–263. doi: 10.1002/1097-0215(200002)9999:9999<::AID-IJC1041>3.3.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Abbas S, Linseisen J, Chang-Chaude J. Dietary vitamin D and calcium intake and premenopausal breast cancer risk in a German case-control study. Nutr Cancer. 2007;59:54–61. doi: 10.1080/01635580701390223. [DOI] [PubMed] [Google Scholar]
- Rossi M, McLaughlin JK, Lagiou P, Bosetti C, Talamini R, Lipworth L, Giacosa A, Montella M, Franceschi S, Negri E, La Vecchia C. Vitamin D intake and breast cancer risk: a case-control study in Italy. Ann Oncol. 2009;20:374–378. doi: 10.1093/annonc/mdn550. [DOI] [PubMed] [Google Scholar]
- Anderson LN, Cotterchio M, Vieth R, Knight JA. Vitamin D and calcium intakes and breast cancer risk in pre and postmenopausal women. Am J Clin Nutr. 2010;91:1699–1707. doi: 10.3945/ajcn.2009.28869. [DOI] [PubMed] [Google Scholar]
- Knight JA, Lesosky M, Barnett H, Raboud JM, Veith R. Vitamin D and reduced risk of breast cancer: a population-based case-control study. Cancer Epidemiol Biomarkers Prev. 2007;16:422–429. doi: 10.1158/1055-9965.EPI-06-0865. [DOI] [PubMed] [Google Scholar]
- Kawase T, Matsuo K, Suzuki T, Hirose T, Hirose D, Hosono S, Watanabe M, Inagaki M, Iwata H, Tanaka H, Tajima K. Association between vitamin D and calcium intake and breast cancer risk according to menopausal status and receptor status in Japan. Cancer Sci. 2010;101:1234–1240. doi: 10.1111/j.1349-7006.2010.01496.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MS, Huang YC, Wahlqvist ML, Wu TY, Chou YC, Wu MH, Yu JC, Sun CA. Vitamin D decreases risk of breast cancer in premenopausal women of normal weight in subtropical Taiwan. J Epidemiol. 2011;21:87–94. doi: 10.2188/jea.JE20100088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackmore KM, Lesosky M, Barnett H, Raboud JM, Vieth R, Knight JA. Vitamin D from dietary intake and sunlight exposure and the risk of hormonereceptor- defined breast cancer. Am J Epidemiol. 2008;168:915–924. doi: 10.1093/aje/kwn198. [DOI] [PubMed] [Google Scholar]
- Shin MH, Holmes MD, Hankinson SE, Wu K, Colditz GA, Willett WC. Intake of dairy products, calcium, and vitamin D and risk of breast cancer. J Natl Cancer Inst. 2002;94:1301–1311. doi: 10.1093/jnci/94.17.1301. [DOI] [PubMed] [Google Scholar]
- Frazier AL, Li L, Cho E, Willet WC, Colditz GA. Adolescent diet and risk of breast cancer. Cancer Causes Control. 2004;15:73–82. doi: 10.1023/B:CACO.0000016617.57120.df. [DOI] [PubMed] [Google Scholar]
- McCullough ML, Rodriguez C, Diver WR, Feigelson HS, Stevens VL, Thun MJ, Calle EE. Dairy, calcium and vitamin D intake and postmenopausal breast cancer risk in the Cancer Prevention Study II Nutrition Cohort. Cancer Epidemiol Biomarkers Prev. 2005;14:2898–2904. doi: 10.1158/1055-9965.EPI-05-0611. [DOI] [PubMed] [Google Scholar]
- Lin J, Manson JE, Lee IM, Cook NR, Buring JE, Zhang SM. Intakes of calcium and vitamin D and breast cancer risk in women. Arch Intern Med. 2007;167:1050–1059. doi: 10.1001/archinte.167.10.1050. [DOI] [PubMed] [Google Scholar]
- Robien K, Cutler GJ, Lazovich D. Vitamin D intake and breast cancer risk in postmenopausal women: the Iowa Women's Health Study. Cancer Causes Control. 2007;18:775–782. doi: 10.1007/s10552-007-9020-x. [DOI] [PubMed] [Google Scholar]
- Kuper H, Yang L, Sandin S, Lof M, Adam HO, Weiderpass E. Prospective study of solar exposure, dietary vitamin D intake, and risk of breast cancer among middle-aged women. Cancer Epidemiol Biomarkers Prev. 2009;18:2558–2561. doi: 10.1158/1055-9965.EPI-09-0449. [DOI] [PubMed] [Google Scholar]
- Edvardsen K, Veierod MB, Brustad M, Braaten T, Engelsen O, Lund E. Vitamin D effecitve solar UV radiation, dietary vitamin D and breast cáncer risk. Int J Cancer. 2011;128:1425–1433. doi: 10.1002/ijc.25463. [DOI] [PubMed] [Google Scholar]
- Gonzalez CA, Riboli E. Diet and cancer prevention: contributions from the European Prospective Investigation into Cancer and Nutrition (EPIC) study. Eur J Cancer. 2010;46:2555–2562. doi: 10.1016/j.ejca.2010.07.025. [DOI] [PubMed] [Google Scholar]
- Engel P, Fagherazzi G, Mesrine S, Boutron-Ruault MC, Clavel-Chapelon F. Joint effects of dietary vitamin D and sun exposure on breast cancer risk: results from the French E3N cohort. Cancer Epidemiol Biomarkers Prev. 2011;20:187–198. doi: 10.1158/1055-9965.EPI-10-1039. [DOI] [PubMed] [Google Scholar]
- Chen P, Hy P, Xie D, Qin Y, Wang F, Wang H. Meta-analysis of vitamin D, calcium and the prevention of breast cancer. Breast Cancer Res Treat. 2010;121:469–477. doi: 10.1007/s10549-009-0593-9. [DOI] [PubMed] [Google Scholar]
- Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- Lowe LC, Guy M, Mansi JL, Peckitt C, Bliss J, Wilson RG, Colston KW. Plasma 25-hydroxyvitamin D concentrations, vitamin D receptor genotype and breast cancer risk in a UK Caucasian population. Eur J Cancer. 2005;41:1164–1196. doi: 10.1016/j.ejca.2005.01.017. [DOI] [PubMed] [Google Scholar]
- Abbas S, Linseisen J, Slanger T, Kropp S, Mutschelknauss EJ, Flesch-Janys D, Chang-Claude J. Serum 25-hydroxyvitamin D and risk of post-menopausal breast cancer: results of a large case-control study. Carcinogenesis. 2008;29:93–99. doi: 10.1093/carcin/bgm240. [DOI] [PubMed] [Google Scholar]
- Crew KD, Gammon MD, Steck SE, Hershman DL, Cremers S, Dworakowski E, Shane E, Terry MB, Desai M, Teitelbaum SL, Neugut AI, Santella RM. Association between plasma-25-hydroxyvitamin D and breast cancer risk. Cancer Prev Res (Phila) 2009;2(6):598–604. doi: 10.1158/1940-6207.CAPR-08-0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandini S, Boniol M, Haukka J, Byrnes G, Cox B, Sneyd MJ, Mullie P, Autier P. Meta-analysis of observational studies of serum 25-hydroxyvitamin D levels and colorectal, breast and prostate cancer and colorectal adenoma. Int J Cancer. 2011;128:1414–1424. doi: 10.1002/ijc.25439. [DOI] [PubMed] [Google Scholar]
- Yin L, Grandi N, Rawm E, Haug U, Arndt V, Brenner H. Meta-analysis: serum vitamin D and breast cancer risk. Eur J Cancer. 2010;46:2196–2205. doi: 10.1016/j.ejca.2010.03.037. [DOI] [PubMed] [Google Scholar]
- Irwin ML, McTiernan A, Bernstein L, Gilliland FD, Baumgartner R, Baumgartner K, Ballard-Barbash R. Physical activity levels among breast cancer survivors. Med Sci Sports Exerc. 2004;36:1484–1491. [PMC free article] [PubMed] [Google Scholar]
- Chlebowski RT, Ligibel JA. In: Diseases of the Breast. 3. Harris JR, Lippman ME, Morrow M, et al, editor. Philadelphia, PA: Lippincott Williams and Wilkins; 2009. Lifestyle issues in breast cancer survivors. [Google Scholar]
- Bertone-Johnson ER, Chen WY, Holick MF, Hollis BW, Colditz GA, Willett WC, Hankinson SE. Plasma 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D and risk of breast cancer. Cancer Epidemiol Biomarkers Prev. 2005;14:1991–1997. doi: 10.1158/1055-9965.EPI-04-0722. [DOI] [PubMed] [Google Scholar]
- McCullough ML, Stevens VL, Patel R, Jacobs EJ, Bain EB, Horst RL, Gapstur SM, Thun MJ, Calle EE. Serum 25-hydroxyvitamin D concentrations and postmenopausal breast cancer risk: a nested case control study in the Cancer Prevention Study-II Nutrition Cohort. Breast Cancer Res. 2009;11:R64. doi: 10.1186/bcr2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman DM, Chang SC, Falk RT, Purdue MP, Huang WY, McCarty CA, Hollis BW, Graubard BI, Berg CD, Ziegler RG. Serum levels of vitamin D metabolites and breast cancer risk in the prostate, lung, colorectal, and ovarian cancer screening trial. Cancer Epidemiol Biomarkers Prev. 2008;17:889–894. doi: 10.1158/1055-9965.EPI-07-2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chlebowski RT, Johnson KC, Kooperberg C, Pettinger M, Wactawski-Wende J, Rohan T, Rossouw J, Lane D, O'Sullivan MJ, Yasmeen S, Hiatt RA, Shikany JM, Vitolins M, Khandekar J, Hubbell FA. Women's Health Initiative Investigators. Calcium plus vitamin D supplementation and the risk of breast cancer. J Natl Cancer Inst. 2008;100:1581–1591. doi: 10.1093/jnci/djn360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avenell A, Gillespie WJ, Gillespie LD, O'Connell D. Vitamin D and vitamin D analogues for preventing fractures associated with involutional and postmenopausal osteoporosis. Cochrane Database Syst Rev. 2009;2:CD000227. doi: 10.1002/14651858.CD000227.pub3. [DOI] [PubMed] [Google Scholar]
- Almquist M, Bondeson AG, Bondeson L, Malm J, Manjer J. Serum levels of vitamin D, PTH and calcium and breast cancer risk - a prospective nested case-control study. Int J Cancer. 2010;127:2159–2168. doi: 10.1002/ijc.25215. [DOI] [PubMed] [Google Scholar]
- Engel P, Fagherazzi G, Boutten A, Dupré T, Mesrine S, Boutron-Ruault MC, Clavel-Chapelon F. Serum 25(OH) vitamin D and risk of breast cancer: a nested case-control study from the French E3N cohort. Cancer Epidemiol Biomarkers Prev. 2010;19:2341–2350. doi: 10.1158/1055-9965.EPI-10-0264. [DOI] [PubMed] [Google Scholar]
- Freedman DM, Looker AC, Abnet CC, Linet MS, Graubard BI. Serum 25-hydroxyvitamin D and cancer mortality in the NHANES III study (1988- 2006) Cancer Res. 2010;70:8587–8597. doi: 10.1158/0008-5472.CAN-10-1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crew KD, Shane E, Cremers S, McMahon DJ, Irani D, Hershman DL. High prevalence of vitamin D deficiency despite supplementation in premenopausal women with breast cancer undergoing adjuvant chemotherapy. J Clin Oncol. 2009;27:2151–2156. doi: 10.1200/JCO.2008.19.6162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napoli N, Vattikuti S, Ma C, Rastelli A, Rayani A, Donepudi R, Asadfard M, Yarramaneni J, Ellis M, Armamento-Villareal R. High prevalence of low vitamin D and musculoskeletal complaints in women with breast cancer. Breast J. 2010;16:609–616. doi: 10.1111/j.1524-4741.2010.01012.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vashi PG, Trukova K, Lammersfeld CA, Braun DP, Gupta D. Impact of oral vitamin D supplementation on serum 25-hydroxyvitamin D levels in oncology. Nutr J. 2010;9:60–69. doi: 10.1186/1475-2891-9-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chlebowski RT. Caution regarding 25-hydroxyvitamin D monitoring in women with breast cancer. J Clin Oncol. 2009;27:e72–e73. doi: 10.1200/JCO.2009.23.8576. [DOI] [PubMed] [Google Scholar]
- Goodwin PJ, Ennis M, Pritchard KI, Koo J, Hood N. Prognostic effects of 25-hydroxyvitamin D levels in early breast cancer. J Clin Oncol. 2009;27:3757–3763. doi: 10.1200/JCO.2008.20.0725. [DOI] [PubMed] [Google Scholar]
- Piura E, Chapman JW, Lipton A, Zhu L, Leitzel K, Wilson CF, Pritchard KI, Pollak MN. Serum 1-OH vitamin D and prognosis of postmenopausal breast cancer patients: NCIC-CTG MA14 trial [abstract] J Clin Oncol. 2009;27:15s. doi: 10.1200/JCO.2008.21.7695. [DOI] [Google Scholar]
- Jacobs ET, Thomson CA, Flat SW, Al-Delaimy WK, Hibler EA, Jones LA, Leroy EC, Newman VA, Parker BA, Rock CL, Pierce JP. Vitamin D and breast cancer recurrence in the Women's Healthy Eating and Living (WHEL) study. Am J Clin Nutr. 2011;93:108–117. doi: 10.3945/ajcn.2010.30009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cescon DW, Ganz PA, Hallak S, Ennis M, Mills BK, Goodwin PJ. Feasibility of a randomized controlled trial of vitamin d vs. placebo in recently diagnosed breast cancer patients [abstract P5-13-09] Cancer Res. 2011;70:P5-13-09. doi: 10.1007/s10549-012-2120-7. [DOI] [PubMed] [Google Scholar]
- McAlindon TE, Felson DT, Zhang Y, Hannan MT, Aliabadi P, Weissman B, Rush D, Wilson PW, Jacques P. Relation of dietary intake and serum levels of vitamin D to progression of osteoarthritis of the knee among participants in the Framingham Study. Ann Intern Med. 1996;125:353–359. doi: 10.7326/0003-4819-125-5-199609010-00001. [DOI] [PubMed] [Google Scholar]
- Chlebowski RT, Johnson KC, Lane D, Pettinger M, Kooperberg CL, Wactawski- Wende J, Rohan T, O'Sullivan MJ, Yasmeen S, Hiatt RA, Shikany JM, Vitolins M, Khandekar J, Hubbell FA. 25-Hydroxyvitamin D concentration, vitamin D intake and joint symptoms in postmenopausal women. Maturitas. 2010;68:73–78. doi: 10.1016/j.maturitas.2010.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chlebowski RT. Aromatase inhibitor-associated arthralgias. J Clin Oncol. 2009;27:4932–4934. doi: 10.1200/JCO.2009.23.3270. [DOI] [PubMed] [Google Scholar]
- Waltman NL, Ott CD, Twiss JJ, Gross GJ, Lindsey AM. Vitamin D insufficiency and musculoskeletal symptoms in breast cancer survivors on aromatase inhibitor therapy. Cancer Nurs. 2009;32:143–150. doi: 10.1097/01.NCC.0000339262.44560.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prieto-Alhambra D, Javaid MK, Servitja S, Arden NK, Martinez-Garcia M, Diez-Perez A, Albanell J, Tusquets I, Nogues X. Vitamin D threshold to prevent aromatase inhibitor-induced arthralgia: a prospective cohort study. Breast Cancer Res Treat. 2011;125:869–878. doi: 10.1007/s10549-010-1075-9. [DOI] [PubMed] [Google Scholar]
- Khan QJ, Reddy PS, Kimler BF, Sharma P, Baxa SE, O'Dea AP, Klemp JR, Fabian CJ. Effect of vitamin D supplementation on serum 25-hydroxy vitamin D levels, joint pain, and fatigue in women starting adjuvant letrozole treatment for breast cancer. Breast Cancer Res Treat. 2010;119:111–118. doi: 10.1007/s10549-009-0495-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vachon CM, Kushi LH, Cerhan JR, Kuni CC, Sellers TA. Association of diet and mammographic breast density in the Minnesota Breast Cancer Family Cohort. Cancer Epidemiol Biomarkers Prev. 2000;9:151–160. [PubMed] [Google Scholar]
- Bérubé S, Diorio C, Verhoek-Oftedahl W, Brisson J. Vitamin D, calcium and mammographic breast densities. Cancer Epidemiol Biomarkers Prev. 2004;13:1466–1472. [PubMed] [Google Scholar]
- Bérubé S, Diorio C, Mâsse B, Hébert-Croteau N, Byrne C, Côté G, Pollak M, Yaffe M, Brisson J. Vitamin D and calcium intakes from food or supplements and mammographic breast density. Cancer Epidemiol Biomarkers Prev. 2005;14(7):1652–1659. doi: 10.1158/1055-9965.EPI-05-0068. [DOI] [PubMed] [Google Scholar]
- Knight JA, Vachon CM, Vierkant RA, Vieth R, Cerhan JR, Sellers TA. No association between 25-hydroxyvitamin D and mammographic density. Cancer Epidemiol Biomarkers Prev. 2006;15:1988–1992. doi: 10.1158/1055-9965.EPI-06-0241. [DOI] [PubMed] [Google Scholar]
- Chai W, Maskarinec G, Cooney RV. Serum 25-hydroxyvitamin D levels and mammographic density among premenopausal women in a multiethnic population. Eur J Clin Nutr. 2010;64:652–654. doi: 10.1038/ejcn.2010.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green AK, Hankinson SE, Bertone-Johnson ER, Tamimi RM. Mammographic density, plasma vitamin D levels and risk of breast cancer in postmenopausal women. Int J Cancer. 2010;127:667–674. doi: 10.1002/ijc.25075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuhouser ML, Bernstein L, Hollis BW, Xiao L, Ambs A, Baumgartner K, Baumgartner R, McTiernan A, Ballard-Barbash R. Serum vitamin D and breast density in breast cancer survivors. Cancer Epidemiol Biomarkers Prev. 2010;19:412–417. doi: 10.1158/1055-9965.EPI-09-0774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertone-Johnson ER, Chlebowski RT, Manson JE, McTiernan A, Wastawski- Wende J, Aragaki AK, Tamimi R, Thomson C, Rohan TE, Rexrode KM, Peck JD, Pissano ED, Martin CF, Sarto G. Dietary vitamin D and calcium intake and mammographic density in postmenopausal women. Menopause. 2010;17:1152–1160. doi: 10.1097/gme.0b013e3181e102d9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson RD, LaCroix AZ, Gass M, Wallace RB, Robbins J, Lewis CE, Bassford T, Beresford SA, Black HR, Blanchette P, Bonds DE, Brunner RL, Brzyski RG, Caan B, Cauley JA, Chlebowski RT, Cummings SR, Granek I, Hays J, Heiss G, Hendrix SL, Howard BV, Hsia J, Hubbell FA, Johnson KC, Judd H, Kotchen JM, Kuller LH, Langer RD, Lasser NL. et al. Calcium plus vitamin D supplementation and the risk of fractures. N Engl J Med. 2006;354:669–683. doi: 10.1056/NEJMoa055218. [DOI] [PubMed] [Google Scholar]
- Wactawski-Wende J, Kotchen JM, Anderson GL, Assaf AR, Brunner RL, O'Sullivan MJ, Margolis KL, Ockene JK, Phillips L, Pottern L, Prentice RL, Robbins J, Rohan TE, Sarto GE, Sharma S, Stefanick ML, Van Horn L, Wallace RB, Whitlock E, Bassford T, Beresford SA, Black HR, Bonds DE, Brzyski RG, Caan B, Chlebowski RT, Cochrane B, Garland C, Gass M, Hays J. et al. Calcium plus vitamin D supplementation and the risk of colorectal cancer. N Engl J Med. 2006;354:684–696. doi: 10.1056/NEJMoa055222. [DOI] [PubMed] [Google Scholar]
- Lappe JM, Travers-Gustafson D, Davies KM, Recker RR, Heaney RP. Vitamin D and calcium supplementation reduces cancer risk: results of a randomized trial. Am J Clin Nutr. 2007;85:1586–1591. doi: 10.1093/ajcn/85.6.1586. [DOI] [PubMed] [Google Scholar]
- LaCroix AZ, Kotchen J, Anderson G, Brzyski R, Cauley JA, Cummings SR, Gass M, Johnson KC, Ko M, Larson J, Manson JE, Stefanick ML, Wactawski-Wende J. Calcium plus vitamin D supplementation and mortality in postmenopausal women: the Women's Health Initiative calcium-vitamin D randomization controlled trial. J Gerontol A Biol Sci Med Sci. 2009;64:559–567. doi: 10.1093/gerona/glp006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Autier P, Gandini S. Vitamin D supplementation and total mortality: a metaanalysis of randomized controlled trials. Arch Intern Med. 2007;167:1730–1737. doi: 10.1001/archinte.167.16.1730. [DOI] [PubMed] [Google Scholar]
- Avenell A, Gillespie WJ, Gillespie LD, O'Connell D. Vitamin D and vitamin D analogues for preventing fractures associated with involutional and postmenopausal osteoporosis. Cochrane Database Syst Rev. 2009. p. CD000227. [DOI] [PubMed]
- Melamed ML, Michos ED, Post W, Aston B. 25-Hydroxyvitamin D levels and the risk of mortality in the general population. Arch Intern Med. 2008;168:1629–1637. doi: 10.1001/archinte.168.15.1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Largest Study of Vitamin D and Omega-3s Set to Begin Soon at Brigham and Women's Hospital. http://www.brighamandwomens.org/about_bwh/publicaffairs/news/pressreleases/PressRelease.aspx?PageID=508
- National Institute of Health Research. http://www.hta.ac.uk/funding/clinicaltrials/index.shtml
- Launay-Vacher V, Gligorov J, Le Tourneau C, Janus N, Spano JP, Ray-Coquard I, Oudard S, Pourrat X, Morere JF, Deray G, Beuzeboc P. Renal Insufficiency and Anticancer Medications Study Group. Prevalence of renal insufficiency in breast cancer patients and related pharmacological issues. Breast Cancer Res Treat. 2010;124:745–753. doi: 10.1007/s10549-008-0131-1. [DOI] [PubMed] [Google Scholar]
- Amir E, Simmons CE, Freedman OC, Dranitsaris G, Cole DE, Vieth R, Ooi WS, Clemons M. A phase 2 trial exploring the effects of high dose (10,000 IU/day) vitamin D(3) in breast patients with bone metastases. Cancer. 2010;116:184–191. doi: 10.1002/cncr.24749. [DOI] [PubMed] [Google Scholar]
- McCullough ML, Weinstein SJ, Freedman DM, Helzlsouer K, Flanders WD, Koenig K, Kolonel L, Laden F, Le Marchand L, Purdue M, Snyder K, Stevens VL, Stolzenberg-Solomon R, Virtamo J, Yang G, Yu K, Zheng W, Albanes D, Ashby J, Bertrand K, Cai H, Chen Y, Gallicchio L, Giovannucci E, Jacobs EJ, Hankinson SE, Hartge P, Hartmuller V, Harvey C, Hayes RB. et al. Correlates of circulating 25-hydroxyvitamin D: Cohort Consortium Vitamin D Pooling Project of Rarer Cancers. Am J Epidemiol. 2010;172:21–35. doi: 10.1093/aje/kwq113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan KM, Signorello LB, Munro HM, Hargreaves MK, Hollis BW, Blot WJ. Vitamin D insufficiency among African-Americans in the southeastern United States: implications for cancer disparities (United States) Cancer Causes Control. 2008;19:527–535. doi: 10.1007/s10552-008-9115-z. [DOI] [PubMed] [Google Scholar]
- Nesby-O'Dell S, Scanlon KS, Cogswell ME, Gillespie C, Hollis BW, Looker AC, Allen C, Doughertly C, Gunter EW, Bowman BA. Hypovitaminosis D prevalence and determinants among African American and white women of reproductive age: Third National Health and Nutrition Examination Survey, 1988-1994. Am J Clin Nutr. 2002;76:187–192. doi: 10.1093/ajcn/76.1.187. [DOI] [PubMed] [Google Scholar]
- Cranney A, Horsley T, O'Donnell S, Weiler H, Puil L, Ooi D, Atkinson S, Ward L, Moher D, Hanley D, Fang M, Yazdi F, Garritty C, Sampson M, Barrowman N, Tsertsvadze A, Mamaladze V. Effectiveness and safety of vitamin D in relation to bone health. Evid Rep Technol Assess (Full Rep) 2007. pp. 1–235. [PMC free article] [PubMed]
- Millen AE, Wactawski-Wende J, Pettinger M, Melamed ML, Tylavsky FA, Liu S, Robbins J, LaCroix AZ, LeBoff MS, Jackson RD. Predictors of serum 25-hydroxyvitamin D concentrations among postmenopausal women: the Women's Health Initiative calcium plus vitamin D clinical trial. Am J Clin Nutr. 2010;91:1324–1335. doi: 10.3945/ajcn.2009.28908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas JA, Bolland MJ, Grey AB, Ames RW, Mason BH, Horne AM, Gamble GD, Reid IR. Determinants of vitamin D status in older women living in a subtropical climate. Osteoporos Int. 2005;16:1641–1648. doi: 10.1007/s00198-005-1888-2. [DOI] [PubMed] [Google Scholar]
- Lappe JM, Davies KM, Travers-Gustafson D, Heaney RP. Vitamin D status in a rural postmenopausal female population. J Am Coll Nutr. 2006;25:395–402. doi: 10.1080/07315724.2006.10719551. [DOI] [PubMed] [Google Scholar]
- Rosen CJ. Vitamin D insufficiency. N Engl J Med. 2011;364:248–254. doi: 10.1056/NEJMcp1009570. [DOI] [PubMed] [Google Scholar]