Summary
Cortical processing reflects the interplay of synaptic excitation and synaptic inhibition. Rapidly accumulating evidence is highlighting the crucial role of inhibition in shaping spontaneous and sensory-evoked cortical activity and thus underscores how a better knowledge of inhibitory circuits is necessary for our understanding of cortical function. We discuss current views of how inhibition regulates the function of cortical neurons and point to a number of important open questions.
Excitation and inhibition walk hand in hand
Synaptic excitation and inhibition are inseparable events. Even the simplest sensory stimulus, like a whisker deflection (Swadlow, 2003; Wilent and Contreras, 2005) a brief tone (Tan et al., 2004; Wehr and Zador, 2003; Wu et al., 2008), an odor (Poo and Isaacson, 2009) or an oriented bar in the visual field (Anderson et al., 2000; Monier et al., 2003) lead to the concomitant occurrence of synaptic excitation and inhibition in sensory cortices. This co-occurrence of excitation and inhibition is not limited to activity generated by sensory stimuli but holds true also for spontaneous cortical activity. During spontaneous cortical oscillations (Atallah and Scanziani, 2009) or ”up and down states” (Haider et al., 2006; Okun and Lampl, 2008), for example, excitation and inhibition wax and wane together.
What are the physiological consequences of this co-occurrence of excitation and inhibition; i.e. why should the cortex simultaneously push on the accelerator and on the brake? What cortical circuits regulate the relative magnitude of these two opposing forces and their spatial and temporal relation? The combination of these two synaptic conductances, by impacting the membrane potential and input resistance of the neuron, plays a fundamental role in regulating neuronal output. In other words, these two conductances together govern the computations performed by cortical neurons. Ultimately, the relative strength of these two conductances and their temporal relationship orchestrate cortical function in space and time.
Building blocks
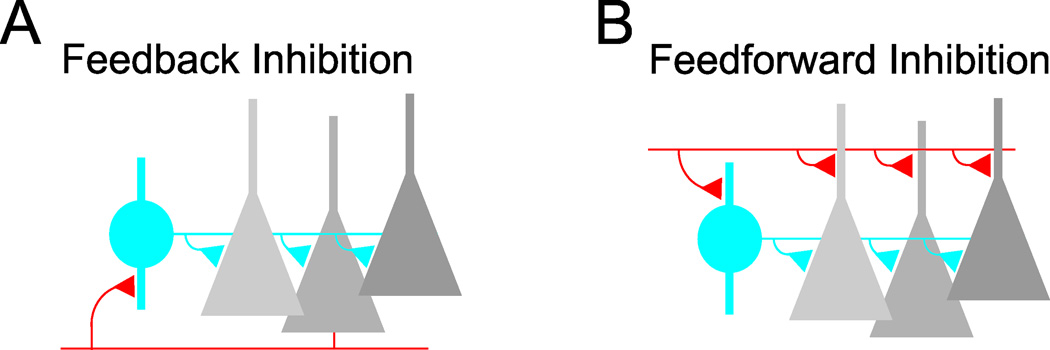
Inhibition in the cortex is generated by neurons that release the transmitter GABA. These neurons comprise approximately 20% of the cortical neuronal population (Meinecke and Peters, 1987) and, in contrast to their counterpart, the excitatory glutamatergic principal cells, don't generally form long range projections with their axon; hence the name local circuit interneurons. The interactions between GABAergic interneurons and glutamatergic principal cells are reciprocal: interneurons inhibit principal cells and are excited by them. In fact the connectivity between these two neuronal classes is quite high: individual interneurons can inhibit >50% of principal cells located within ~100 µm and receive excitatory input from a large fraction of them (Ali et al., 1999; Fino and Yuste, 2011; Glickfeld et al., 2008; Holmgren et al., 2003; Kapfer et al., 2007; Packer and Yuste, 2011; Silberberg and Markram, 2007; Stokes and Isaacson, 2010; Yoshimura and Callaway, 2005). Thus, not only are GABAergic interneurons excited in proportion to the level of local network activity, but they directly influence it through their inhibitory feedback. This simple connectivity pattern is ubiquitous in cortex and forms the basis for so-called feedback or recurrent inhibition (Fig. 1A). Of course, not all cortical excitation received by inhibitory interneurons is locally generated. Cortical cells receive excitatory inputs via long-range axons originating from subcortical nuclei, as well as from different cortical regions and different cortical layers. These excitatory afferent inputs diverge onto both principal cells and interneurons, generating feedforward inhibitory circuits (Fig. 1B) (Buzsaki, 1984). Interestingly, the same afferent fibers make stronger excitatory connections onto interneurons than principal cells ensuring that even minimal levels of afferent input generate inhibition in cortical circuits (Cruikshank et al., 2007; Gabernet et al., 2005; Glickfeld and Scanziani, 2006; Helmstaedter et al., 2008; Hull et al., 2009; Stokes and Isaacson, 2010). Together, these two simple inhibitory circuits, feedback and feedforward, represent fundamental building blocks of cortical architecture and account for the fact that cortical excitation and inhibition are inseparable (van Vreeswijk and Sompolinsky, 1996). GABAergic interneurons will be recruited no matter whether excitation is generated locally or received from distant sites. In addition to principal cells, GABAergic interneurons also make inhibitory contacts onto each other and the connectivity between interneurons is highly reciprocal (Galarreta and Hestrin, 2002; Gibson et al., 1999; Tamas et al., 1998). This mutual connectivity between interneurons is also poised to shape spatial and temporal features of cortical inhibition.
Figure 1.
Feedback and feedfoward circuits are fundamental building blocks of cortical inhibition. A, Feedback inhibition arises when cortical principal cells (grey) make excitatory synaptic contacts (red) on local interneurons (blue) that in turn form inhibitory synaptic contacts (blue triangles) on the principal cell population. B, Feedforward inhibition is generated when long-range excitatory afferent inputs (red) diverge onto both principal cells and local interneurons.
Cortical GABAergic interneurons are a heterogeneous bunch (reviewed in (Ascoli et al., 2008; Freund and Buzsaki, 1996; Kawaguchi and Kondo, 2002; Kawaguchi and Kubota, 1998; Klausberger and Somogyi, 2008; Markram et al., 2004; Monyer and Markram, 2004; Mott and Dingledine, 2003; Somogyi and Klausberger, 2005; Somogyi et al., 1998). One of the most striking features of this group of neurons is their morphological diversity, in particular with regard to their axonal arborization and, as a consequence, their postsynaptic targets. In fact, distinct classes of GABAergic interneurons inhibit particular compartments of principal neurons; "basket" cells, that target the somatic and perisomatic compartment, "chandelier" cells, that selectively inhibit the axon initial segment, or "Martinotti" cells, that preferentially target the apical dendritic tuft are just a few classic examples of this compartmentalization of inhibition. Morphological differences are however not the only properties that contribute to the diversity of cortical inhibitory neurons. Interneurons can be also subdivided based on intrinsic electrophysiological properties, synaptic characteristics, and protein expression patterns. Probably because of the many dimensions that can be used to describe an interneuron, no consensus yet exists with regard to their categorization and several reviews have been written on the topic (Ascoli et al., 2008; Freund and Buzsaki, 1996; Klausberger and Somogyi, 2008; Markram et al., 2004). Strikingly, in contrast to the large amount of information that exists on the properties of the various types of cortical inhibitory neurons, knowledge of the specific role that each one plays in orchestrating cortical activity is still extremely limited. Thus, in this review, unless explicitly mentioned, we remain agnostic as to the specific interneuron subtypes mediating inhibition.
Key question: The specific contribution of different subtypes of interneurons to cortical inhibition is still largely unknown, and is likely to strongly depend on the activity pattern of the network. An important open question is whether specific subtypes of interneurons have unique functional roles in cortical processing.
The "balance" of excitation and inhibition
Through the recruitment of interneurons via feedforward and/or feedback excitatory projections, inhibition generated in cortical networks is somehow proportional to local and/or incoming excitation. This proportionality has been observed in several sensory cortical regions where changes in the intensity or other features of a sensory stimulus lead to concomitant changes in the strength of both cortical excitation and inhibition (Fig. 2A) (Anderson et al., 2000; Poo and Isaacson, 2009; Wehr and Zador, 2003; Wilent and Contreras, 2004; Zhang et al., 2003). In addition, during spontaneous cortical activity, increases in excitation are invariably accompanied by increases in inhibition (Fig. 2B) (Atallah and Scanziani, 2009; Haider et al., 2006; Okun and Lampl, 2008). Furthermore, acute experimental manipulations selectively decreasing either inhibition or excitation shift cortical activity to a hyper-excitable (epileptiform) or silent (comatose) state (Dudek and Sutula, 2007). Thus, not only does excitation and inhibition increase and decrease together during physiological cortical activity (van Vreeswijk and Sompolinsky, 1996), but interference of this relationship appears to be highly disruptive. Highlighting the importance of a proper relationship between excitation and inhibition, is the fact that changes in the weight of excitation or inhibition are accompanied by compensatory effects that preserve the excitability of cortical networks (Turrigiano, 2011).
Figure 2.
Proportionality of excitation and inhibition during stimulus-evoked and spontaneous cortical activity. A, Intracellular recording of responses to drifting gratings of different orientations in cat visual cortex. Peristimulus time histograms of spike rate reveal the strongest increases in firing of the cortical neuron to a stimulus orientated at 90° (“preferred stimulus”). Measurements of changes in excitatory (red) and inhibitory (blue) synaptic conductance from the same recording reveal that both excitation and inhibition are tuned to the same orientation. Modified from (Anderson et al., 2000). B, Simultaneous intracellular recordings of spontaneous synaptic activity from two nearby neurons in rat somatosensory cortex. One cell (red trace) is hyperpolarized at the reversal potential for inhibition to reveal excitatory postsynaptic potentials (EPSPs) and the other (blue trace) is depolarized to reveal inhibitory postsynaptic potentials (IPSPs). Spontaneously occurring EPSPs (monitored in one cell) are accompanied by IPSPs (monitored in the neighboring cell) of co-varying amplitude. Modified from (Okun and Lampl, 2008).
These observations have led to the concept that the two opposing synaptic conductances balance each other out and that this balance is important for proper cortical function. "Balance" is a useful concept as it qualitatively captures some important properties of excitation and inhibition in the cortex, like the overall proportionality mentioned above and the fact that manipulating one conductance without the other can shift cortical activity to un-physiological extremes. However it is also misleading if taken too literarily: first it should not be understood as excitatory and inhibitory conductances being equal, i.e. canceling each other out. Excitation and inhibition are differentially distributed along the soma, dendrites and axon initial segment of neurons and thus their exact ratio is highly dependent on where it is measured. Furthermore, the concept of balance may lead to the naive view that the main role of cortical inhibition is to prevent epileptiform activity, a notion that is clearly too simplistic. Finally, and most important, despite the overall proportionality of excitation and inhibition, their exact ratio is highly dynamic, as will be detailed below.
Inhibition’s impact on membrane potential and excitability
Cortical transmission is largely mediated by ionotropic neurotransmitter receptors that produce fast (<10 ms) synaptic conductances. Glutamate elicits fast excitation via the activation of cation permeable AMPA and NMDA receptor-mediated conductances, while GABA evokes fast inhibition via anion (Cl− and HCO3−) permeable GABAA receptor-mediated conductances. The possibility of varying the ratio between synaptic excitation and inhibition allows for the shifting of the membrane potential of a neuron towards any arbitrary value in-between the reversal potential of synaptic excitation (around 0 mV for AMPA and NMDA receptors) and synaptic inhibition (typically around −70 to −80 mV for GABAA receptors). Thus, by changing the ratio between synaptic excitation and inhibition, neuronal membranes can be rapidly brought to threshold for action-potential generation, just near threshold or far below threshold in a matter of a few milliseconds (Fig. 3A) (Higley and Contreras, 2006). Furthermore, even a specific ratio between excitation and inhibition can lead to different membrane potentials depending on the absolute magnitude of the two opposing conductances. In fact, since synaptic excitation and inhibition are not the only conductances of a neuron, their contribution to the membrane potential will depend on their magnitude relative to other conductances. Accordingly, the larger their magnitude, the closer the membrane potential of the neuron will approach the equilibrium potential set by the combination of synaptic excitation and inhibition. Finally, because the impact on membrane potential of any current flowing through the membrane is affected in a divisive manner by the conductance of the membrane (Ohm's law), the activation of GABAA receptors, simply by increasing the conductance, can significantly reduce the excitability of a neuron, an effect referred to as "shunting inhibition". This might represent the major inhibitory effect of GABAA receptor activation in those specific cases in which the resting membrane potential is equal to or even more negative than the reversal potential of GABAA receptor-mediated currents. In other words, activation of GABAA receptors may not change the membrane potential or even generate a depolarization and still reduce neuronal excitability. Membrane pumps, by setting intracellular Cl− concentration, play a critical role in regulating the reversal potential of GABAA receptor-mediated currents (Blaesse et al., 2009). In certain instances, for example in immature neurons (Ben-Ari et al., 2007) or in specialized neuronal compartments (Gulledge and Stuart, 2003; Szabadics et al., 2006; Woodruff et al., 2009), the reversal potential for Cl− is so depolarized that it may lead to an excitatory action of GABAA receptors. Although intriguing, still too little is known about how excitatory actions of GABA might impact processing in adult cortex to be discussed here.
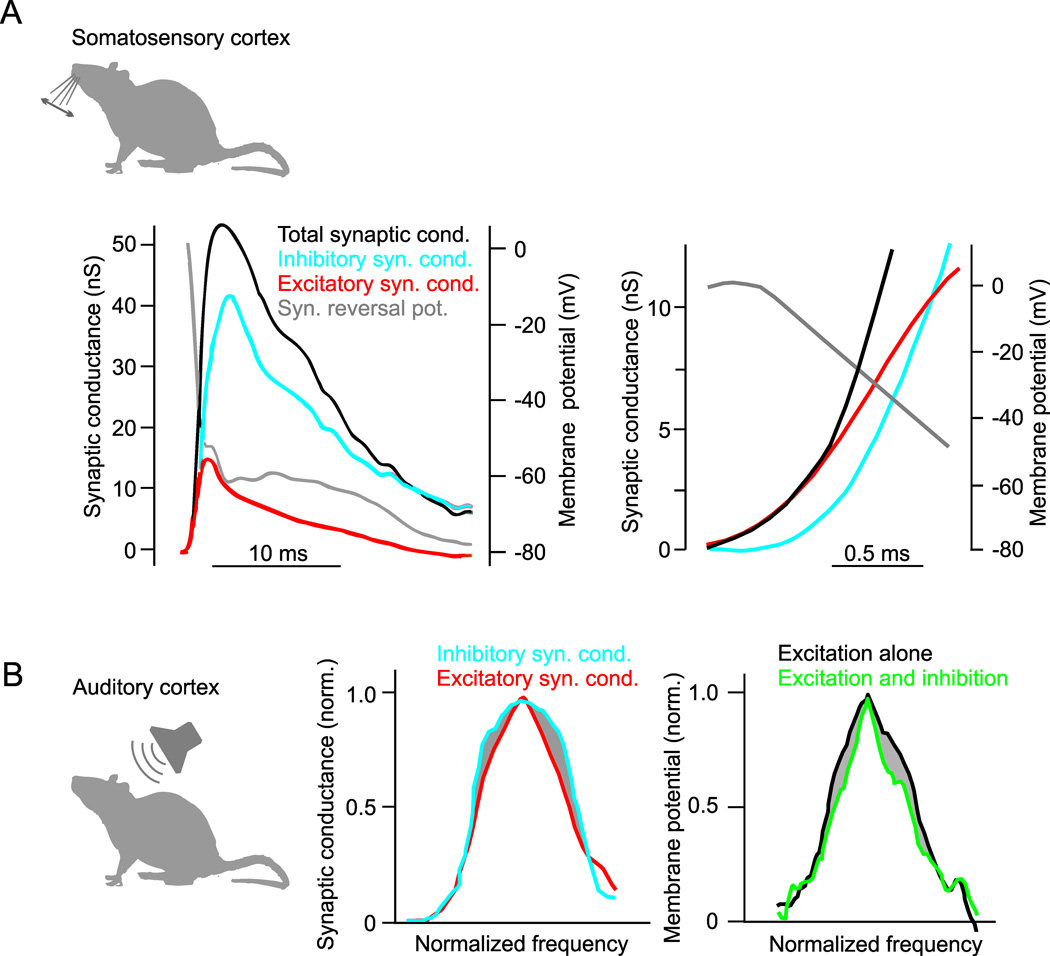
Figure 3.
Dynamics and tuning of cortical excitation and inhibition. A, Intracellular recording of synaptic response to whisker deflection in rat somatosensory (barrel) cortex neuron. Left, Whisker deflection produces an increase in total synaptic conductance (black trace) that is composed of a rapid increase in excitatory conductance (red trace) and a delayed increase in inhibitory conductance (blue trace). The calculated reversal potential of the synaptic response (gray trace) reaches 0 mV (the reversal potential for glutamatergic excitation) immediately after the synaptic conductance begins to rise and becomes hyperpolarized (towards the reversal potential for GABA receptors) as the inhibitory conductance begins. Right, Same as left but on an expanded time-scale. Changes in reversal potential and conductances early in the response show that the onset of excitation precedes inhibition. Modified from (Higley and Contreras, 2006). B, Inhibitory sharpening of frequency tuning in rat auditory cortex. Left, Across a population of recorded cells, the average frequency tuning curve of tone-evoked inhibitory synaptic conductances (blue) is broader than the tuning curve of synaptic excitation (red). Right, Estimated membrane potential tuning curves derived from the same recordings show that inhibition causes a lateral sharpening of tone-evoked responses around the preferred stimulus frequency. Modified from (Wu et al., 2008).
In addition to fast GABAA receptor-mediated conductances, GABA activates G-protein coupled GABAB receptors that cause slow (100–500 ms) postsynaptic inhibition by opening inwardly rectifying K+ (GIRK) channels (Luscher et al., 1997). Postsynaptic GABAB receptors also inhibit voltage-gated calcium channels, thereby, for example, reducing dendritic excitability (Perez-Garci et al., 2006). Furthermore, GABAB receptors are present on both glutamatergic and GABAergic nerve terminals where their activation causes presynaptic inhibition of transmitter release (Bowery, 1993). Curiously, while inhibitory actions of GABAB receptors have been well characterized in brain slices, few in vivo studies have probed the role of slow GABAB receptor mediated transmission in cortical function. Although transgenic mice lacking functional GABAB receptors are prone to spontaneous epileptic seizures (Schuler et al., 2001), the contribution of GABAB receptor signaling to spontaneous or sensory-evoked cortical activity is unclear. It has been suggested that synaptically released GABA from a large number of co-active interneurons must be pooled or accumulated to activate GABAB receptors (Isaacson et al., 1993; Scanziani, 2000). Since the conditions in which spontaneous or sensory-evoked cortical activity in vivo drives GABAB-mediated responses remain to be established, it is simplest to interpret cortical inhibition as largely reflecting the actions of ionotropic GABAA receptors.
Excitation-inhibition ratio in time and in space
Within individual neurons the ratio between incoming excitation and inhibition can change rapidly, on a millisecond basis. In principal neurons of the auditory cortex, for example, brief tones lead to an increase in synaptic excitation that is followed within a couple of milliseconds by a surge in inhibition (Wehr and Zador, 2003; Wu et al., 2008). Similarly, whisker deflections lead to a rapid sequence of excitation followed by inhibition in neurons of the somatosensory "barrel" cortex (Fig. 3A) (Swadlow, 2002; Wilent and Contreras, 2005). Also in the visual cortex, visual stimulation with a light flash triggers excitatory and inhibitory conductances that are staggered by a few milliseconds (Liu et al., 2010). Hence, in these cortical areas, in response to impulse-like sensory stimuli, the ratio between excitation and inhibition is initially tilted towards excitation, and subsequently shifts towards inhibition. These rapid changes in the ratio between excitation and inhibition can have important consequences in tuning cortical neurons to specific stimuli and in shaping their activity pattern in time (see below).
Both feedforward and feedback inhibitory circuits can generate these rapid sequences of excitation and inhibition. In feedforward circuits, since afferent inputs contact both principal cells and interneurons, the onset of excitation recorded in principal neurons will precede the onset of inhibition by a monosynaptic delay (that can be as brief as one ms) (Gabernet et al., 2005; Pouille and Scanziani, 2001; Stokes and Isaacson, 2010). Feedback circuits also provide inhibition that follows excitation because the firing of local principal neurons will be followed by the recruitment of GABAergic interneurons.
Differences in the timing of excitation and inhibition in response to impulse like stimuli are not the only way in which the ratio of these two opposing conductances is relevant for cortical processing. In some model sensory systems the ratio between excitation and inhibition in a given cortical neuron also depends on the property of the sensory stimulus, like its frequency (for auditory stimuli (Wu et al., 2008)), its position in space or orientation (for visual stimuli (Liu et al., 2011) but see (Tan et al., 2011)), or its chemical composition (for olfactory stimuli (Poo and Isaacson, 2009)). As will be described in more detail below, in these specific systems, sensory stimuli that are optimal for firing a cortical neuron (the "preferred" stimulus) generate an excitation-inhibition ratio that can be different than the ratio generated by sub-optimal stimuli. Thus, in some systems the excitation-inhibition ratio can contribute to shaping the response of a cortical neuron to distinct stimuli. As a consequence, because neighboring principal neurons in several cortical sensory areas are not necessarily tuned to the same stimuli (i.e. the rodent visual cortex with regard to orientation (Ohki et al., 2005); the auditory cortex with regard to frequency (Bandyopadhyay et al., 2010; Rothschild et al., 2010) and the olfactory cortex with regard to odors (Stettler and Axel, 2009) in response to a given stimulus, the ratio between excitation and inhibition may vary significantly between nearby neurons. Thus, differences in the excitation-inhibition ratio between neurons can also shape the activity pattern of a population of cortical neurons in space.
Finally, differences in excitation-inhibition ratio can also direct signal flow within and across cortical layers. Principal neurons in layer 2/3, for example, project their axons horizontally within their own layer, as well as vertically, towards layer 5. The activity of layer 2/3 principal neurons, however, generates an excitation-inhibition ratio that differs between layers: it favors inhibition within its own layer but is biased towards excitation in layer 5 (Adesnik and Scanziani, 2010).
Key questions: What is the relative contribution of excitation and inhibition in firing cortical neurons, for example in response to a sensory stimulus? Despite the simplicity of this question, one factor that has limited our understanding of how the excitation-inhibition ratio influences cortical processing is the paucity of in vivo intracellular recording analyzing the relative contribution of the two opposing conductances during sensory stimulation. High-quality, whole-cell voltage clamp recordings are still the gold standard for distinguishing excitatory and inhibitory conductances within individual cells; further improvements of this method for in vivo studies, particularly in awake, behaving animals, are essential.
Inhibition, Gain Control and Dynamic Range
The rate at which the firing of a neuron increases in response to increasing excitatory input, i.e. the slope of the input-output relationship, is called gain and is a property that describes how neurons integrate incoming signals. This slope is not fixed but can be modulated, a phenomenon that goes under the name of gain control (Carvalho and Buonomano, 2009; Chance et al., 2002; Mitchell and Silver, 2003; Shu et al., 2003). Changes in gain are often referred to as multiplicative (or divisive) because for a pure change in slope the firing probability of the neuron is affected by the same factor across a wide range of inputs. Neurons in the visual cortex offer a classical example of gain modulation, where two independent properties of a visual stimulus, contrast and orientation, interact in a multiplicative manner in generating spike output (Anderson et al., 2000; Carandini and Heeger, 1994; Miller, 2003; Sclar and Freeman, 1982). Specifically, increasing the contrast of the stimulus increases the spike output of the neuron by a given factor, no matter what the orientation of the stimulus is. As a consequence, the stimulus selective output of a neuron for a particular orientation remains the same at each contrast. This illustrates that changes in gain, while modulating the responsiveness of a neuron to a stimulus, do not affect the representation of that stimulus in the cortex. Gain modulation in cortex is a very general phenomenon that is proposed to play a role at every level of sensory processing, including modulation of visual responses by gaze direction (Andersen and Mountcastle, 1983) and attention (Williford and Maunsell, 2006).
Though the precise mechanisms of gain modulation in the cortex still need to be elucidated, several theoretical models and some experimental observations indicate that synaptic inhibition is likely to play a key role. Curiously, adding a tonic inhibitory conductance to a neuron does not affect the gain of the neuron's input-output relationship, if the driving input is a simple depolarizing current step. This may seem counterintuitive but experimental manipulations clearly indicate that decreasing the resistance of a neuron (as happens when adding an inhibitory conductance) does not change the slope of the input-output relationship to depolarizing current steps (Chance et al., 2002; Mitchell and Silver, 2003). Furthermore neuronal models provide a theoretical framework for these observations (Holt and Koch, 1997). However, under physiological conditions, neuronal spike output is driven by the integration of barrages of synaptic inputs rather than depolarizing current steps and voltage noise from transient synaptic conductances contributes to the frequency of spike output. If the opening of a tonic inhibitory conductance occurs in combination with an increase in the variability of driving excitatory input (Mitchell and Silver, 2003) or if a noisy barrage of mixed excitatory and inhibitory synaptic conductances (an increase in background synaptic activity) is added to the driving input (Chance et al., 2002), the slope of the input-output relationship of individual neurons can be changed.
The examples described above consider conditions in which the excitatory input that drives the neuron varies independently of the inhibition received by that same neuron. We know, however, this is not generally the case, as excitation and inhibition appear tightly coupled in cortical networks. Under this condition, gain modulation may be a natural consequence of scaling inhibition with excitation (Pouille et al., 2009; Shadlen and Newsome, 1998). Thus with increasing input strength, it becomes progressively harder for any given quantity of excitation to reach spike threshold because of the concomitant increase in inhibition. If the relationship between excitation and inhibition are chosen properly, models show that the interaction between these two opposing conductances can lead to pure changes in gain (Shadlen and Newsome, 1998).
Synaptic inhibition also helps in solving an important problem relating to dynamic range: how neuronal populations are recruited as the number of active excitatory afferents changes (Pouille et al., 2009; Shadlen and Newsome, 1998). The problem results from two basic connectivity properties of excitatory afferents in cortex; namely, high divergence (each afferent excites many neurons) and weak synapses (the activity of a single afferent is insufficient to depolarize a neuron above spike threshold). Because neurons need the concomitant activity of several afferents to reach spike threshold, yet these afferents diverge onto many neurons, small increases in the number of active excitatory afferents can lead to an explosive, almost all or none recruitment of the entire population. This strongly limits the range or combinations of afferent inputs that can be differentially represented by the firing of neuronal populations. With inhibition increasing concomitantly with the number of active afferents (for example through the progressive recruitment of feedforward inhibitory neurons), on the other hand, the recruitment of the neuronal population occurs in a progressive manner over a much wider range of inputs (Liu et al., 2011; Pouille et al., 2009). Through the concomitant increase of excitation and inhibition, neuronal populations, or individual neurons (Liu et al., 2011) can thus differentially represent a larger range and number of combinations of afferent inputs.
Key questions: Normalization is a basic cortical computation through which the excitability of cortical neurons changes in a manner that is inversely proportional to the overall activity level of the network (Heeger, 1992). It can account for several properties of cortical sensory processing, ranging from cross orientation suppression in the visual system (Freeman et al., 2002), to the modulation of sensory responses with attention (Reynolds and Heeger, 2009). The potential involvement of inhibition in cortical normalization is debated (Katzner et al., 2011) and needs to be elucidated. Furthermore, while the role of inhibition in gain modulation, another basic cortical operation, is better established, the exact contribution of the various inhibitory circuits to this operation still needs to be assessed.
Inhibition sharpens tuning
A basic property of cortical neurons is that particular features of sensory stimuli preferentially drive the spike output of individual cells. For example, neurons in visual cortex can fire selectively to visual stimuli that have a particular orientation or direction (Fig. 2A). Stimulus selective responses are observed in cortical regions devoted to all sensory modalities and understanding the mechanisms governing this tuning of responses to preferred stimuli is critical for unraveling how the cortex represents sensory information. Since the selectivity to certain stimuli (e.g. orientation tuning) emerges for the first time in the cortex, (i.e. it is not present in any of the neurons along the chain that conveys the signal from the sensory interface to the cortex), cortical circuitry must contribute to generating this stimulus selectivity (Hubel and Wiesel, 1962). What role does synaptic inhibition play in the tuning of cortical neurons to sensory stimuli?
Pharmacological blockade of GABAA receptors reduces the stimulus selectivity of neurons in a variety of sensory cortices (Katzner et al., 2011; Kyriazi et al., 1996; Poo and Isaacson, 2009; Sillito, 1979; Wang et al., 2000). However, the mechanisms by which synaptic inhibition regulates cortical tuning have been a source of debate. One popular idea follows from studies of lateral inhibition in the retina, in which stimulation in the receptive field center of a photoreceptor elicits excitation and stimulation in the surround evokes inhibition (Hartline et al., 1956). In terms of the cortex, the strictest form of this lateral inhibition requires a spatial organization in which cortical neurons tuned to the same particular features of sensory stimuli are located near one another. “Lateral” inhibition could occur if adjacent domains of sensory cortex (such as orientation columns within cat visual cortex or whisker maps in rodent barrel cortex) are tuned to different stimulus features—and inhibition in one cortical subregion can be influenced by neighboring domains. While the necessary circuits for such lateral inhibitory interactions exist in cortex (Adesnik and Scanziani, 2010), determining their exact spatial extent and impact on sensory processing will require more work. Furthermore, in the visual, auditory, and olfactory cortices of rodents, stimulus selective responses occur despite the fact that cells tuned to particular stimulus features are spatially intermingled in a “salt and pepper” organization (Ohki et al., 2005; Rothschild et al., 2010; Stettler and Axel, 2009).
A less literal form of lateral inhibition that does not require a two dimensional spatial mapping of stimulus features still applies to cortical tuning: namely, that synaptic excitation to a preferred stimulus roughly shapes the tuning of a cell’s spike output and that tuning is further sharpened by robust synaptic inhibition in response to non-preferred stimuli (Priebe and Ferster, 2008). This notion, however, has been challenged by intracellular recording studies in several cortical regions showing that in individual neurons the stimuli that generate the strongest excitation (preferred stimuli) can be the same as those generating the strongest inhibition (Fig. 2A, 3B) (Anderson et al., 2000; Liu et al., 2011; Marino et al., 2005; Martinez et al., 2002; Tan et al., 2004; Tan et al., 2011; Wehr and Zador, 2003; Wilent and Contreras, 2005; Wu et al., 2008; Zhang et al., 2003), but see (Monier et al., 2003). Furthermore, as the stimulus gradually changes away from the preferred feature, both excitation and inhibition decrease. In other words the tuning curves for excitation and for inhibition show considerable overlap.
How then could inhibition sharpen the tuning of cortical neurons to the preferred stimuli? This can happen in several ways. First, it is important to note that the tuning curve determined through the spike output of a neuron is not equal to the tuning curve determined by recording the membrane potential of that neuron. Because only the strongest excitatory input received by a neuron sufficiently depolarizes the membrane to reach threshold for spike generation, (i.e. the "tip" of the tuning curve of the membrane potential), the spike output of the neuron is more sharply tuned than the underlying membrane potential (Fig. 4), a phenomenon appropriately called "iceberg effect" (Carandini and Ferster, 2000; Rose and Blakemore, 1974). In other words, the non-linearity of spike rate versus membrane potential sharpens the tuning of a neuron. The addition of inhibition exacerbates the iceberg effect because it further reduces the amount by which the tip of the iceberg (the membrane potential tuning curve) sticks out of the water surface (the spike threshold) thus further sharpening the tuning of the spike output of the neuron (Fig. 4). Importantly, this effect of inhibition occurs no matter whether inhibition is un-tuned or as equally tuned as stimulus-driven excitation. Indeed, the increased firing rates and reduced stimulus selectivity in visual cortex following pharmacological blockade of inhibition could be explained by a simple spike threshold model in which excitation and inhibition are identically tuned (Katzner et al., 2011). Second, recent studies in auditory (Wu et al., 2008), olfactory cortex (Poo and Isaacson, 2009) and visual cortex (Liu et al., 2011), but see (Tan et al., 2011) of the rodent, reveal that in these model systems the tuning curves of inhibition are actually broader than those of excitation in individual cells (Fig. 3B). As a consequence, non-preferred stimuli generate an excitation inhibition ratio that favors inhibition relative to the preferred stimulus. Here, inhibition contributes to sharpening the tuning not only by exacerbating the iceberg effect, but also by actually narrowing the iceberg (Fig. 3B).
Figure 4.
Inhibition sharpens stimulus selective spike output via the “iceberg effect”. Schematic illustrates hypothetical tuning curves for firing rate (green), membrane potential (black), and excitatory conductance (red) of a cortical neuron to stimulus features (e.g. orientation). Action potential firing occurs only when membrane potential exceeds a fixed spike threshold. Responses are shown in the presence (left) and absence (right) of a weakly tuned inhibitory conductance (blue). Weakly tuned inhibition leads to more narrowly tuned spike output by allowing only the strongest (preferred) excitatory stimuli to drive the membrane potential above spike threshold.
The timing of sensory-evoked inhibition relative to excitation is another factor that could sharpen the tuning of cortical neurons to preferred stimuli. As mentioned above, studies in auditory (Wehr and Zador, 2003; Wu et al., 2006), somatosensory (Wilent and Contreras, 2005), and visual cortex (Liu et al., 2010) indicate that, in response to impulse like stimuli, inhibition follows excitatory input with a brief (few ms) temporal delay (Fig. 3A, Fig. 5). This slight lag between excitation and inhibition enforces a brief window of opportunity for the integration of synaptic excitation and subsequent spike output (Fig. 5) thus making principal cells precise coincidence detectors of afferent input (Luna and Schoppa, 2008; Mittmann et al., 2005; Pouille and Scanziani, 2001). Some experimental observations suggest that the relative timing of excitatory and inhibitory synaptic input contributes to stimulus-selective firing. For example, in response to preferred directions of whisker deflection, excitation precedes inhibition in barrel cortex but the temporal delay between the two synaptic conductances is reduced in response to nonpreferred stimuli (Wilent and Contreras, 2005). Similarly, in neurons of auditory cortex that are tuned to sound intensity, the temporal delay of inhibition relative to excitation becomes smaller as tone intensity increases resulting in a sharpening of intensity tuning (Wu et al., 2006). Thus, stimulus selectivity in the cortex can emerge from a temporal shift in the timing of excitation relative to inhibition.
Figure 5.
Inhibition enforces precise spike timing. Intracellular recording of responses to a brief tone (gray box) from a principal cell in auditory cortex illustrating timing of action potentials (top), subthreshold membrane potential (middle), and the underlying excitatory and inhibitory synaptic conductances (bottom). Action potentials largely occur only in the narrow time window during which excitation precedes inhibition. Modified from (Wehr and Zador, 2003).
All the above observations indicate that inhibition can sharpen the tuning of cortical neurons without being itself tuned oppositely to excitation, but rather by being as equally tuned as excitation, more broadly tuned or not tuned at all. Not surprisingly, the tuning properties of inhibition measured in principal neurons are consistent with the tuning properties of inhibitory interneurons. In some systems, interneurons and principal cells show similar stimulus selectivity in their firing (Cardin et al., 2007; Runyan et al., 2010), while in others cortical inhibitory neurons appear to be less sharply tuned than principal cells (Fig. 6) (Kameyama et al., 2010; Kerlin et al., 2010; Liu et al., 2009; Niell and Stryker, 2008; Poo and Isaacson, 2009; Sohya et al., 2007; Swadlow, 1988). One possibility that would account for the differences in interneuron tuning properties observed in different systems is that they receive convergent excitatory inputs from surrounding principal cells irrespective of their tuning properties (Bock et al., 2011). In other words, the tuning of an interneuron may reflect the average tuning of the network of excitatory neurons it is embedded in. If the surrounding network is homogenously tuned to a specific feature, interneurons inherit that feature selectivity (as for interneurons in an orientation column of the cat (Cardin et al., 2007). If the surrounding network is heterogenous, such as in the rodent visual (Ohki et al., 2005), auditory (Bandyopadhyay et al., 2010; Rothschild et al., 2010) and olfactory cortices (Stettler and Axel, 2009), interneurons will be more broadly tuned.
Figure 6.
Cortical interneurons are more broadly tuned to sensory stimuli than principal cells. A, In vivo 2-photon calcium imaging of activity in visual cortex of transgenic mice expressing GFP in GABAergic interneurons. Cells are loaded with the calcium-sensitive dye fura-2 AM to monitor activity evoked by drifting gratings of different orientations and interneurons (Int) are distinguished from pyramidal cells (Pyr) based on expression of GFP. B, Top, Traces of calcium responses show that while GFP(−) pyramidal cells are highly selective for stimuli of particular orientations, a nearby GFP(+) interneuron is broadly responsive to all stimulus orientations. Bottom, Polar plots of visual responses to the oriented stimuli from the same cells. C, Distributions of orientation selectivity index (range 0=untuned to 1=highly selective) from a number of recordings show that responses in GFP(+) interneurons are less selective to stimulus orientation than pyramidal cells. Modified from (Sohya et al., 2007).
Sir John C. Eccles famously wrote, "I always think that inhibition is a sculpturing process. The inhibition, as it were, chisels away at the … mass of excitatory action and gives a more specific form to the neuronal performance at every stage of synaptic relay" (Eccles, 1977). The evidence listed above suggests either that Eccles attributed too much specificity to inhibition, at least with regard to its possible role in cortical sensory tuning or, more likely, that we haven't yet explored the full parameter space of sensory stimuli (e.g. timing, naturalistic stimuli) in which inhibition exerts its sculpting action. Further work will be needed to elucidate whether indeed particular types of interneurons may play a more specific role in tuning cortical responses to sensory stimuli.
Key questions: Within any given cortical sensory area principal cells are tuned to a large number of spatial and temporal features of the stimulus. It will be important to explore the specific roles played by different subtypes of interneurons (i.e. basket cells, Martinotti cells) in shaping the different tuning properties of cortical principal cells.
Inhibition paces oscillations
A prominent characteristic of cortical activity is the rhythmic and synchronous oscillation of the membrane potential of populations of neurons, a phenomenon that can be detected even with scalp electrodes as a component of the electroencephalogram. Cortical inhibition is an essential element in at least some of the fastest oscillations, occurring in the "beta" and "gamma" frequency range (20–80 Hz) (Atallah and Scanziani, 2009; Cardin et al., 2009; Hasenstaub et al., 2005; Sohal et al., 2009; Traub et al., 1997; Traub et al., 1996; Wang and Buzsaki, 1996). These fast oscillations take place under a variety of behavioral states, either spontaneously or in response to sensory stimuli and are thought to play a role in the transmission of information across cortical areas. Specifically, because excitatory input is more efficient in depolarizing target neurons when they are active synchronously rather than distributed in time (Azouz and Gray, 2000; Pouille and Scanziani, 2001), oscillations enable neurons to cooperate in the depolarization of common downstream targets, and thus in the propagation of neuronal signals. Through this mechanism, gamma oscillations are proposed to contribute to the merging of information processed in distinct cortical regions, for example, by "binding" neuronal ensembles that oscillate in phase.
Inhibition is not only directly involved in the generation of these fast oscillations, but also in synchronizing participating neurons, in setting the pace of the oscillations and in maintaining their coherence in space. Among the various types of inhibitory neurons, basket cells play a key role in gamma oscillations (Cardin et al., 2009; Cobb et al., 1995; Sohal et al., 2009). Two important properties of interneurons appear crucial to the generation of synchronized oscillations. First, interneurons are electrically-coupled via gap junctions allowing large populations of interneurons to be synchronized with millisecond precision (Beierlein et al., 2000; Galarreta and Hestrin, 1999, 2001; Gibson et al., 1999; Hestrin and Galarreta, 2005). Second, interneurons make reciprocal synaptic connections onto each other (Bartos et al., 2002; Galarreta and Hestrin, 2002; Gibson et al., 1999; Tamas et al., 1998), a property that models show is important for the robustness of oscillations (Bartos et al., 2007; Vida et al., 2006). Two alternate mechanisms, "PING" (pyramidal-interneuron network gamma oscillations) and "ING" (interneuron network gamma oscillations) have been proposed for the role of inhibitory neurons in the generation of gamma oscillations (Tiesinga and Sejnowski, 2009; Whittington et al., 2000). PING is based on the reciprocal (feedback) connectivity between pyramidal cells and interneurons. Here, the oscillation is generated by the alternation in the firing of interneurons (excited by pyramidal cells) and pyramidal cells (as they re-emerge from the inhibition triggered by interneurons). The fact that individual basket cells contact a very large fraction of neighboring (i.e. within ~100 um) pyramidal cells, and that individual pyramidal cells in turn contact many local inhibitory neurons leads to the synchronous involvement of large populations of neurons in the oscillation. Furthermore, the decay time constant of inhibition of pyramidal cells sets the pace of the oscillation. The alternative mechanism, ING, is solely based on the reciprocal interactions between inhibitory neurons. Basket cells are interconnected via reciprocal inhibitory synapses. Given the right physiological conditions, these synaptically-coupled networks of inhibitory neurons can generate fast synchronous oscillations (Van Vreeswijk et al., 1994). In this model, the entrainment of pyramidal cells to the oscillation is a natural consequence (since interneurons synapse onto pyramidal cells) but not a necessity for their generation.
Several of the properties that characterize the interaction between excitation and inhibition in response to sensory stimuli are also found during beta and gamma oscillations (Fig. 7). During hippocampal gamma oscillations for example, despite the fact that the magnitude of excitation and inhibition can vary on a cycle-by-cycle basis, their overall ratio remains approximately constant (Fig. 7A) (Atallah and Scanziani, 2009). Furthermore, there is a phase difference between the excitatory and inhibitory components of the oscillation. During hippocampal gamma oscillations the inhibitory phase is delayed by 1–2 milliseconds relative to the phase of excitation (Fig. 7B) (Atallah and Scanziani, 2009). Similarly, inhibition has a lag of 5–10 ms relative to excitation during beta frequency oscillations (20 – 40 Hz) in olfactory cortex (Fig. 7C,D) (Poo and Isaacson, 2009). As a consequence, the ratio between excitation and inhibition, favors excitation early during these oscillation cycles while shifting towards inhibition later in the cycle. This sequence of excitation and inhibition leads to relatively narrow time windows for spiking, as is apparent in the tightly phase-locked firing behavior of pyramidal cells relative to the oscillations in the hippocampus and olfactory cortex (Fig. 7B, D) (Atallah and Scanziani, 2009; Poo and Isaacson, 2009).
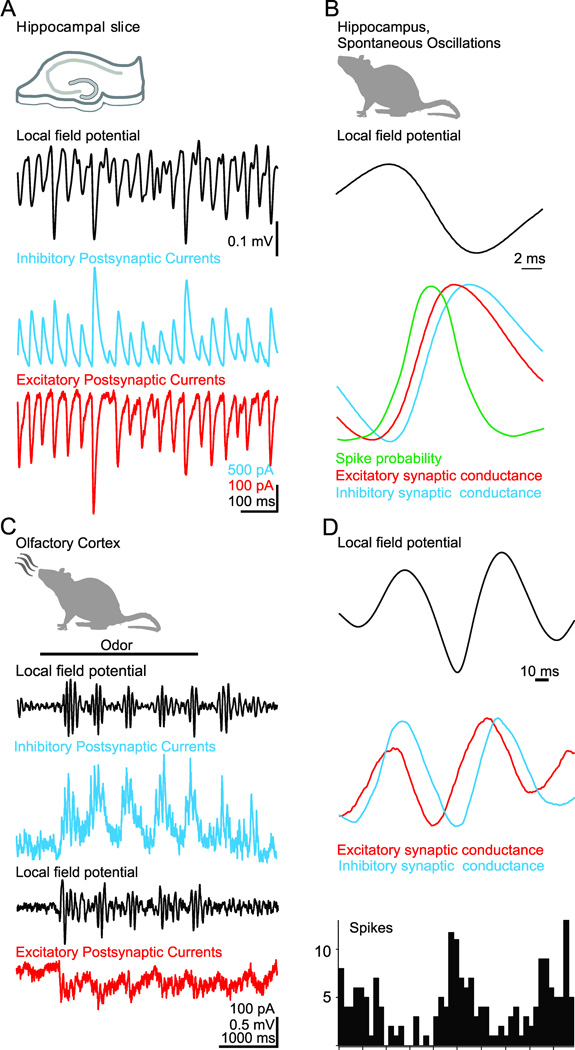
Figure 7.
Inhibition is an essential component of synchronous oscillations in cortical activity. A, Simultaneous recording of gamma frequency (~40 Hz) oscillations in the local field potential (LFP) and the inhibitory (blue) and excitatory (red) postsynaptic currents recorded in two nearby pyramidal cells in hippocampal slices. While the amplitude of the LFP and synaptic currents varies on a cycle-to-cycle basis, the ratio of excitation to inhibition remains constant. B, In vivo recordings of spontaneous gamma oscillations in the hippocampus reveal that the phase-specific firing of action potentials (green) relative to the local field potential coincides with a brief time window during which synaptic excitation (red) precedes inhibition (blue). C, In vivo recordings from olfactory cortex reveal odor-evoked beta frequency (~20 Hz) oscillations in the local field potential and inhibitory and excitatory postsynaptic currents in a pyramidal cell. D, In olfactory cortex, phase-specific firing of action potentials (bottom histogram) relative to the local field potential (top) coincides with the brief time window during which odor-evoked synaptic excitation (red) precedes inhibition (blue). A and B modified from (Atallah and Scanziani, 2009). C and D modified from (Poo and Isaacson, 2009).
Key questions: Does PING or ING predominate during physiological oscillations in the cortex? And what are the exact mechanisms that initiate and terminate oscillations? Do other interneurons beside basket cells contribute to cortical oscillations?
Future studies
Understanding the role of inhibition in cortical function has been a challenge, mainly due to the lack of sufficiently specific tools. The general pharmacological block of inhibition in cortical structures invariably leads to epileptiform activity and thus precludes an accurate assessment of which cortical properties (tuning, receptive field size, etc.) are affected by the absence of inhibition. Thus, many of the reported roles of inhibition rely on correlative evidence substantiated by a great deal of computational models. Despite the relative paucity of functional analysis, however, there has been an explosion in the number of studies reporting on the properties and mechanisms of cortical inhibition. Morphological, physiological, pharmacological, biochemical and genetic properties of cortical inhibitory neurons, the circuits they are embedded in, and the properties of synapses they form are being worked out with unprecedented detail (Ascoli et al., 2008; Freund and Buzsaki, 1996; Kawaguchi and Kondo, 2002; Kawaguchi and Kubota, 1998; Klausberger and Somogyi, 2008; Markram et al., 2004; Monyer and Markram, 2004; Mott and Dingledine, 2003; Somogyi and Klausberger, 2005; Somogyi et al., 1998). We are thus facing a discrepancy between the vast and detailed knowledge of inhibitory mechanisms and properties and our limited understanding of how these mechanisms and properties play together to contribute to cortical function. In other words, we now have more details about interneurons than we know what to do with. A clear example of this discrepancy has been the spectacular and still ongoing characterization of the many types of cortical inhibitory interneurons on one hand and our very poor understanding of what each type contributes to cortical processing on the other hand.
How will further efforts bring us closer to understanding the role of inhibition in cortical function? New methodological approaches offer an unprecedented ability to precisely determine the functional properties of distinct inhibitory circuits. A variety of genetic tools are now available to perturb neuronal activity with exquisite spatial and temporal precision (Fenno et al., 2011; Kim et al., 2009; Magnus et al., 2011; Rogan and Roth, 2011; Tan et al., 2006). However, a critical factor in using these genetic tools to dissect circuit function is the capacity to target them to particular types of neurons using cell-specific promoters. Thankfully, the abundance of studies characterizing biochemical and genetic phenotypes of cortical inhibitory neurons makes this possible. For example, these characterizations have established the foundations for designing a variety of currently available mouse lines in which Cre recombinase can be used to target genetic tools to discrete subtypes of interneurons, such as parvalbumin-expressing basket cells or somatostatin-expressing Martinotti cells (Taniguchi et al., 2011).
The possibility of selectively target and perturb specific inhibitory circuits will lead to a better mechanistic understanding of their exact role in cortical function and help reveal the biological advantage of such a variety of inhibitory processes. Furthermore, identifying the specific role of cortical inhibitory interneurons will help understand their contribution to neurological or cognitive disorders. We look forward to a significant advance in our knowledge of how inhibition shapes cortical activity.
Acknowledgements
We thank Dr. Matteo Carandini for helpful comments. Work in the authors’ labs supported by R01DC04682 (J.S.I.) and the Howard Hughes Medical Institute and Gatsby Foundation (M.S.)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adesnik H, Scanziani M. Lateral competition for cortical space by layer-specific horizontal circuits. Nature. 2010;464:1155–1160. doi: 10.1038/nature08935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali AB, Bannister AP, Thomson AM. IPSPs elicited in CA1 pyramidal cells by putative basket cells in slices of adult rat hippocampus. The European journal of neuroscience. 1999;11:1741–1753. doi: 10.1046/j.1460-9568.1999.00592.x. [DOI] [PubMed] [Google Scholar]
- Andersen RA, Mountcastle VB. The influence of the angle of gaze upon the excitability of the light-sensitive neurons of the posterior parietal cortex. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1983;3:532–548. doi: 10.1523/JNEUROSCI.03-03-00532.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson JS, Carandini M, Ferster D. Orientation tuning of input conductance, excitation, and inhibition in cat primary visual cortex. Journal of neurophysiology. 2000;84:909–926. doi: 10.1152/jn.2000.84.2.909. [DOI] [PubMed] [Google Scholar]
- Ascoli GA, Alonso-Nanclares L, Anderson SA, Barrionuevo G, Benavides-Piccione R, Burkhalter A, Buzsaki G, Cauli B, Defelipe J, Fairen A, et al. Petilla terminology: nomenclature of features of GABAergic interneurons of the cerebral cortex. Nat Rev Neurosci. 2008;9:557–568. doi: 10.1038/nrn2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atallah BV, Scanziani M. Instantaneous modulation of gamma oscillation frequency by balancing excitation with inhibition. Neuron. 2009;62:566–577. doi: 10.1016/j.neuron.2009.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azouz R, Gray CM. Dynamic spike threshold reveals a mechanism for synaptic coincidence detection in cortical neurons in vivo. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:8110–8115. doi: 10.1073/pnas.130200797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandyopadhyay S, Shamma SA, Kanold PO. Dichotomy of functional organization in the mouse auditory cortex. Nature neuroscience. 2010;13:361–368. doi: 10.1038/nn.2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartos M, Vida I, Frotscher M, Meyer A, Monyer H, Geiger JR, Jonas P. Fast synaptic inhibition promotes synchronized gamma oscillations in hippocampal interneuron networks. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:13222–13227. doi: 10.1073/pnas.192233099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartos M, Vida I, Jonas P. Synaptic mechanisms of synchronized gamma oscillations in inhibitory interneuron networks. Nature reviews. Neuroscience. 2007;8:45–56. doi: 10.1038/nrn2044. [DOI] [PubMed] [Google Scholar]
- Beierlein M, Gibson JR, Connors BW. A network of electrically coupled interneurons drives synchronized inhibition in neocortex. Nat Neurosci. 2000;3:904–910. doi: 10.1038/78809. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y, Gaiarsa JL, Tyzio R, Khazipov R. GABA: a pioneer transmitter that excites immature neurons and generates primitive oscillations. Physiol Rev. 2007;87:1215–1284. doi: 10.1152/physrev.00017.2006. [DOI] [PubMed] [Google Scholar]
- Blaesse P, Airaksinen MS, Rivera C, Kaila K. Cation-chloride cotransporters and neuronal function. Neuron. 2009;61:820–838. doi: 10.1016/j.neuron.2009.03.003. [DOI] [PubMed] [Google Scholar]
- Bock DD, Lee WC, Kerlin AM, Andermann ML, Hood G, Wetzel AW, Yurgenson S, Soucy ER, Kim HS, Reid RC. Network anatomy and in vivo physiology of visual cortical neurons. Nature. 2011;471:177–182. doi: 10.1038/nature09802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowery NG. GABAB receptor pharmacology. Annu Rev Pharmacol Toxicol. 1993;33:109–147. doi: 10.1146/annurev.pa.33.040193.000545. [DOI] [PubMed] [Google Scholar]
- Buzsaki G. Feed-forward inhibition in the hippocampal formation. Prog Neurobiol. 1984;22:131–153. doi: 10.1016/0301-0082(84)90023-6. [DOI] [PubMed] [Google Scholar]
- Carandini M, Ferster D. Membrane potential and firing rate in cat primary visual cortex. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2000;20:470–484. doi: 10.1523/JNEUROSCI.20-01-00470.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carandini M, Heeger DJ. Summation and division by neurons in primate visual cortex. Science. 1994;264:1333–1336. doi: 10.1126/science.8191289. [DOI] [PubMed] [Google Scholar]
- Cardin JA, Carlen M, Meletis K, Knoblich U, Zhang F, Deisseroth K, Tsai LH, Moore CI. Driving fast-spiking cells induces gamma rhythm and controls sensory responses. Nature. 2009;459:663–667. doi: 10.1038/nature08002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardin JA, Palmer LA, Contreras D. Stimulus feature selectivity in excitatory and inhibitory neurons in primary visual cortex. J Neurosci. 2007;27:10333–10344. doi: 10.1523/JNEUROSCI.1692-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho TP, Buonomano DV. Differential effects of excitatory and inhibitory plasticity on synaptically driven neuronal input-output functions. Neuron. 2009;61:774–785. doi: 10.1016/j.neuron.2009.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chance FS, Abbott LF, Reyes AD. Gain modulation from background synaptic input. Neuron. 2002;35:773–782. doi: 10.1016/s0896-6273(02)00820-6. [DOI] [PubMed] [Google Scholar]
- Cobb SR, Buhl EH, Halasy K, Paulsen O, Somogyi P. Synchronization of neuronal activity in hippocampus by individual GABAergic interneurons. Nature. 1995;378:75–78. doi: 10.1038/378075a0. [DOI] [PubMed] [Google Scholar]
- Cruikshank SJ, Lewis TJ, Connors BW. Synaptic basis for intense thalamocortical activation of feedforward inhibitory cells in neocortex. Nat Neurosci. 2007;10:462–468. doi: 10.1038/nn1861. [DOI] [PubMed] [Google Scholar]
- Dudek FE, Sutula TP. Epileptogenesis in the dentate gyrus: a critical perspective. Progress in brain research. 2007;163:755–773. doi: 10.1016/S0079-6123(07)63041-6. [DOI] [PubMed] [Google Scholar]
- Eccles JC. The understanding of the brain. 2nd edn. New York: McGraw-Hill; 1977. [Google Scholar]
- Fenno L, Yizhar O, Deisseroth K. The development and application of optogenetics. Annual review of neuroscience. 2011;34:389–412. doi: 10.1146/annurev-neuro-061010-113817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fino E, Yuste R. Dense inhibitory connectivity in neocortex. Neuron. 2011;69:1188–1203. doi: 10.1016/j.neuron.2011.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman TC, Durand S, Kiper DC, Carandini M. Suppression without inhibition in visual cortex. Neuron. 2002;35:759–771. doi: 10.1016/s0896-6273(02)00819-x. [DOI] [PubMed] [Google Scholar]
- Freund TF, Buzsaki G. Interneurons of the hippocampus. Hippocampus. 1996;6:347–470. doi: 10.1002/(SICI)1098-1063(1996)6:4<347::AID-HIPO1>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Gabernet L, Jadhav SP, Feldman DE, Carandini M, Scanziani M. Somatosensory integration controlled by dynamic thalamocortical feed-forward inhibition. Neuron. 2005;48:315–327. doi: 10.1016/j.neuron.2005.09.022. [DOI] [PubMed] [Google Scholar]
- Galarreta M, Hestrin S. A network of fast-spiking cells in the neocortex connected by electrical synapses. Nature. 1999;402:72–75. doi: 10.1038/47029. [DOI] [PubMed] [Google Scholar]
- Galarreta M, Hestrin S. Spike transmission and synchrony detection in networks of GABAergic interneurons. Science. 2001;292:2295–2299. doi: 10.1126/science.1061395. [DOI] [PubMed] [Google Scholar]
- Galarreta M, Hestrin S. Electrical and chemical synapses among parvalbumin fast-spiking GABAergic interneurons in adult mouse neocortex. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:12438–12443. doi: 10.1073/pnas.192159599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson JR, Beierlein M, Connors BW. Two networks of electrically coupled inhibitory neurons in neocortex. Nature. 1999;402:75–79. doi: 10.1038/47035. [DOI] [PubMed] [Google Scholar]
- Glickfeld LL, Atallah BV, Scanziani M. Complementary modulation of somatic inhibition by opioids and cannabinoids. J Neurosci. 2008;28:1824–1832. doi: 10.1523/JNEUROSCI.4700-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glickfeld LL, Scanziani M. Distinct timing in the activity of cannabinoid-sensitive and cannabinoid-insensitive basket cells. Nat Neurosci. 2006;9:807–815. doi: 10.1038/nn1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulledge AT, Stuart GJ. Excitatory actions of GABA in the cortex. Neuron. 2003;37:299–309. doi: 10.1016/s0896-6273(02)01146-7. [DOI] [PubMed] [Google Scholar]
- Haider B, Duque A, Hasenstaub AR, McCormick DA. Neocortical network activity in vivo is generated through a dynamic balance of excitation and inhibition. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2006;26:4535–4545. doi: 10.1523/JNEUROSCI.5297-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartline HK, Wagner HG, Ratliff F. Inhibition in the eye of Limulus. J Gen Physiol. 1956;39:651–673. doi: 10.1085/jgp.39.5.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasenstaub A, Shu Y, Haider B, Kraushaar U, Duque A, McCormick DA. Inhibitory postsynaptic potentials carry synchronized frequency information in active cortical networks. Neuron. 2005;47:423–435. doi: 10.1016/j.neuron.2005.06.016. [DOI] [PubMed] [Google Scholar]
- Heeger DJ. Normalization of cell responses in cat striate cortex. Vis Neurosci. 1992;9:181–197. doi: 10.1017/s0952523800009640. [DOI] [PubMed] [Google Scholar]
- Helmstaedter M, Staiger JF, Sakmann B, Feldmeyer D. Efficient recruitment of layer 2/3 interneurons by layer 4 input in single columns of rat somatosensory cortex. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008;28:8273–8284. doi: 10.1523/JNEUROSCI.5701-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hestrin S, Galarreta M. Electrical synapses define networks of neocortical GABAergic neurons. Trends in neurosciences. 2005;28:304–309. doi: 10.1016/j.tins.2005.04.001. [DOI] [PubMed] [Google Scholar]
- Higley MJ, Contreras D. Balanced excitation and inhibition determine spike timing during frequency adaptation. J Neurosci. 2006;26:448–457. doi: 10.1523/JNEUROSCI.3506-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmgren C, Harkany T, Svennenfors B, Zilberter Y. Pyramidal cell communication within local networks in layer 2/3 of rat neocortex. J Physiol. 2003;551:139–153. doi: 10.1113/jphysiol.2003.044784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt GR, Koch C. Shunting inhibition does not have a divisive effect on firing rates. Neural Comput. 1997;9:1001–1013. doi: 10.1162/neco.1997.9.5.1001. [DOI] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN. Receptive fields, binocular interaction and functional architecture in the cat's visual cortex. The Journal of physiology. 1962;160:106–154. doi: 10.1113/jphysiol.1962.sp006837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hull C, Isaacson JS, Scanziani M. Postsynaptic mechanisms govern the differential excitation of cortical neurons by thalamic inputs. J Neurosci. 2009;29:9127–9136. doi: 10.1523/JNEUROSCI.5971-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacson JS, Solis JM, Nicoll RA. Local and diffuse synaptic actions of GABA in the hippocampus. Neuron. 1993;10:165–175. doi: 10.1016/0896-6273(93)90308-e. [DOI] [PubMed] [Google Scholar]
- Kameyama K, Sohya K, Ebina T, Fukuda A, Yanagawa Y, Tsumoto T. Difference in binocularity and ocular dominance plasticity between GABAergic and excitatory cortical neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30:1551–1559. doi: 10.1523/JNEUROSCI.5025-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapfer C, Glickfeld LL, Atallah BV, Scanziani M. Supralinear increase of recurrent inhibition during sparse activity in the somatosensory cortex. Nat Neurosci. 2007;10:743–753. doi: 10.1038/nn1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzner S, Busse L, Carandini M. GABAA Inhibition Controls Response Gain in Visual Cortex. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:5931–5941. doi: 10.1523/JNEUROSCI.5753-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi Y, Kondo S. Parvalbumin, somatostatin and cholecystokinin as chemical markers for specific GABAergic interneuron types in the rat frontal cortex. J Neurocytol. 2002;31:277–287. doi: 10.1023/a:1024126110356. [DOI] [PubMed] [Google Scholar]
- Kawaguchi Y, Kubota Y. Neurochemical features and synaptic connections of large physiologically-identified GABAergic cells in the rat frontal cortex. Neuroscience. 1998;85:677–701. doi: 10.1016/s0306-4522(97)00685-4. [DOI] [PubMed] [Google Scholar]
- Kerlin AM, Andermann ML, Berezovskii VK, Reid RC. Broadly tuned response properties of diverse inhibitory neuron subtypes in mouse visual cortex. Neuron. 2010;67:858–871. doi: 10.1016/j.neuron.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JC, Cook MN, Carey MR, Shen C, Regehr WG, Dymecki SM. Linking genetically defined neurons to behavior through a broadly applicable silencing allele. Neuron. 2009;63:305–315. doi: 10.1016/j.neuron.2009.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klausberger T, Somogyi P. Neuronal diversity and temporal dynamics: the unity of hippocampal circuit operations. Science. 2008;321:53–57. doi: 10.1126/science.1149381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyriazi HT, Carvell GE, Brumberg JC, Simons DJ. Quantitative effects of GABA and bicuculline methiodide on receptive field properties of neurons in real and simulated whisker barrels. Journal of neurophysiology. 1996;75:547–560. doi: 10.1152/jn.1996.75.2.547. [DOI] [PubMed] [Google Scholar]
- Liu BH, Li P, Li YT, Sun YJ, Yanagawa Y, Obata K, Zhang LI, Tao HW. Visual receptive field structure of cortical inhibitory neurons revealed by two-photon imaging guided recording. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:10520–10532. doi: 10.1523/JNEUROSCI.1915-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu BH, Li P, Sun YJ, Li YT, Zhang LI, Tao HW. Intervening inhibition underlies simple-cell receptive field structure in visual cortex. Nature neuroscience. 2010;13:89–96. doi: 10.1038/nn.2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu BH, Li YT, Ma WP, Pan CJ, Zhang LI, Tao HW. Broad inhibition sharpens orientation selectivity by expanding input dynamic range in mouse simple cells. Neuron. 2011;71:542–554. doi: 10.1016/j.neuron.2011.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luna VM, Schoppa NE. GABAergic circuits control input-spike coupling in the piriform cortex. J Neurosci. 2008;28:8851–8859. doi: 10.1523/JNEUROSCI.2385-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luscher C, Jan LY, Stoffel M, Malenka RC, Nicoll RA. G protein-coupled inwardly rectifying K+ channels (GIRKs) mediate postsynaptic but not presynaptic transmitter actions in hippocampal neurons. Neuron. 1997;19:687–695. doi: 10.1016/s0896-6273(00)80381-5. [DOI] [PubMed] [Google Scholar]
- Magnus CJ, Lee PH, Atasoy D, Su HH, Looger LL, Sternson SM. Chemical and genetic engineering of selective ion channel-ligand interactions. Science. 2011;333:1292–1296. doi: 10.1126/science.1206606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marino J, Schummers J, Lyon DC, Schwabe L, Beck O, Wiesing P, Obermayer K, Sur M. Invariant computations in local cortical networks with balanced excitation and inhibition. Nature neuroscience. 2005;8:194–201. doi: 10.1038/nn1391. [DOI] [PubMed] [Google Scholar]
- Markram H, Toledo-Rodriguez M, Wang Y, Gupta A, Silberberg G, Wu C. Interneurons of the neocortical inhibitory system. Nat Rev Neurosci. 2004;5:793–807. doi: 10.1038/nrn1519. [DOI] [PubMed] [Google Scholar]
- Martinez LM, Alonso JM, Reid RC, Hirsch JA. Laminar processing of stimulus orientation in cat visual cortex. The Journal of physiology. 2002;540:321–333. doi: 10.1113/jphysiol.2001.012776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinecke DL, Peters A. GABA immunoreactive neurons in rat visual cortex. The Journal of comparative neurology. 1987;261:388–404. doi: 10.1002/cne.902610305. [DOI] [PubMed] [Google Scholar]
- Miller KD. Understanding layer 4 of the cortical circuit: a model based on cat V1. Cerebral cortex. 2003;13:73–82. doi: 10.1093/cercor/13.1.73. [DOI] [PubMed] [Google Scholar]
- Mitchell SJ, Silver RA. Shunting inhibition modulates neuronal gain during synaptic excitation. Neuron. 2003;38:433–445. doi: 10.1016/s0896-6273(03)00200-9. [DOI] [PubMed] [Google Scholar]
- Mittmann W, Koch U, Hausser M. Feed-forward inhibition shapes the spike output of cerebellar Purkinje cells. The Journal of physiology. 2005;563:369–378. doi: 10.1113/jphysiol.2004.075028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monier C, Chavane F, Baudot P, Graham LJ, Fregnac Y. Orientation and direction selectivity of synaptic inputs in visual cortical neurons: a diversity of combinations produces spike tuning. Neuron. 2003;37:663–680. doi: 10.1016/s0896-6273(03)00064-3. [DOI] [PubMed] [Google Scholar]
- Monyer H, Markram H. Interneuron Diversity series: Molecular and genetic tools to study GABAergic interneuron diversity and function. Trends in neurosciences. 2004;27:90–97. doi: 10.1016/j.tins.2003.12.008. [DOI] [PubMed] [Google Scholar]
- Mott DD, Dingledine R. Interneuron Diversity series: Interneuron research--challenges and strategies. Trends in neurosciences. 2003;26:484–488. doi: 10.1016/S0166-2236(03)00200-5. [DOI] [PubMed] [Google Scholar]
- Niell CM, Stryker MP. Highly selective receptive fields in mouse visual cortex. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008;28:7520–7536. doi: 10.1523/JNEUROSCI.0623-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohki K, Chung S, Ch'ng YH, Kara P, Reid RC. Functional imaging with cellular resolution reveals precise micro-architecture in visual cortex. Nature. 2005;433:597–603. doi: 10.1038/nature03274. [DOI] [PubMed] [Google Scholar]
- Okun M, Lampl I. Instantaneous correlation of excitation and inhibition during ongoing and sensory-evoked activities. Nature neuroscience. 2008;11:535–537. doi: 10.1038/nn.2105. [DOI] [PubMed] [Google Scholar]
- Packer AM, Yuste R. Dense, unspecific connectivity of neocortical parvalbumin-positive interneurons: a canonical microcircuit for inhibition? The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:13260–13271. doi: 10.1523/JNEUROSCI.3131-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Garci E, Gassmann M, Bettler B, Larkum ME. The GABAB1b isoform mediates long-lasting inhibition of dendritic Ca2+ spikes in layer 5 somatosensory pyramidal neurons. Neuron. 2006;50:603–616. doi: 10.1016/j.neuron.2006.04.019. [DOI] [PubMed] [Google Scholar]
- Poo C, Isaacson JS. Odor representations in olfactory cortex: "sparse" coding, global inhibition, and oscillations. Neuron. 2009;62:850–861. doi: 10.1016/j.neuron.2009.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouille F, Marin-Burgin A, Adesnik H, Atallah BV, Scanziani M. Input normalization by global feedforward inhibition expands cortical dynamic range. Nature neuroscience. 2009;12:1577–1585. doi: 10.1038/nn.2441. [DOI] [PubMed] [Google Scholar]
- Pouille F, Scanziani M. Enforcement of temporal fidelity in pyramidal cells by somatic feed-forward inhibition. Science. 2001;293:1159–1163. doi: 10.1126/science.1060342. [DOI] [PubMed] [Google Scholar]
- Priebe NJ, Ferster D. Inhibition, spike threshold, and stimulus selectivity in primary visual cortex. Neuron. 2008;57:482–497. doi: 10.1016/j.neuron.2008.02.005. [DOI] [PubMed] [Google Scholar]
- Reynolds JH, Heeger DJ. The normalization model of attention. Neuron. 2009;61:168–185. doi: 10.1016/j.neuron.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogan SC, Roth BL. Remote control of neuronal signaling. Pharmacol Rev. 2011;63:291–315. doi: 10.1124/pr.110.003020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose D, Blakemore C. Effects of bicuculline on functions of inhibition in visual cortex. Nature. 1974;249:375–377. doi: 10.1038/249375a0. [DOI] [PubMed] [Google Scholar]
- Rothschild G, Nelken I, Mizrahi A. Functional organization and population dynamics in the mouse primary auditory cortex. Nature neuroscience. 2010;13:353–360. doi: 10.1038/nn.2484. [DOI] [PubMed] [Google Scholar]
- Runyan CA, Schummers J, Van Wart A, Kuhlman SJ, Wilson NR, Huang ZJ, Sur M. Response features of parvalbumin-expressing interneurons suggest precise roles for subtypes of inhibition in visual cortex. Neuron. 2010;67:847–857. doi: 10.1016/j.neuron.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scanziani M. GABA spillover activates postsynaptic GABA(B) receptors to control rhythmic hippocampal activity. Neuron. 2000;25:673–681. doi: 10.1016/s0896-6273(00)81069-7. [DOI] [PubMed] [Google Scholar]
- Schuler V, Luscher C, Blanchet C, Klix N, Sansig G, Klebs K, Schmutz M, Heid J, Gentry C, Urban L, et al. Epilepsy, hyperalgesia, impaired memory, and loss of pre- and postsynaptic GABA(B) responses in mice lacking GABA(B(1)) Neuron. 2001;31:47–58. doi: 10.1016/s0896-6273(01)00345-2. [DOI] [PubMed] [Google Scholar]
- Sclar G, Freeman RD. Orientation selectivity in the cat's striate cortex is invariant with stimulus contrast. Exp Brain Res. 1982;46:457–461. doi: 10.1007/BF00238641. [DOI] [PubMed] [Google Scholar]
- Shadlen MN, Newsome WT. The variable discharge of cortical neurons: implications for connectivity, computation, and information coding. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1998;18:3870–3896. doi: 10.1523/JNEUROSCI.18-10-03870.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu Y, Hasenstaub A, Badoual M, Bal T, McCormick DA. Barrages of synaptic activity control the gain and sensitivity of cortical neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2003;23:10388–10401. doi: 10.1523/JNEUROSCI.23-32-10388.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silberberg G, Markram H. Disynaptic inhibition between neocortical pyramidal cells mediated by Martinotti cells. Neuron. 2007;53:735–746. doi: 10.1016/j.neuron.2007.02.012. [DOI] [PubMed] [Google Scholar]
- Sillito AM. Inhibitory mechanisms influencing complex cell orientation selectivity and their modification at high resting discharge levels. The Journal of physiology. 1979;289:33–53. doi: 10.1113/jphysiol.1979.sp012723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohal VS, Zhang F, Yizhar O, Deisseroth K. Parvalbumin neurons and gamma rhythms enhance cortical circuit performance. Nature. 2009;459:698–702. doi: 10.1038/nature07991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohya K, Kameyama K, Yanagawa Y, Obata K, Tsumoto T. GABAergic neurons are less selective to stimulus orientation than excitatory neurons in layer II/III of visual cortex, as revealed by in vivo functional Ca2+ imaging in transgenic mice. J Neurosci. 2007;27:2145–2149. doi: 10.1523/JNEUROSCI.4641-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somogyi P, Klausberger T. Defined types of cortical interneurone structure space and spike timing in the hippocampus. The Journal of physiology. 2005;562:9–26. doi: 10.1113/jphysiol.2004.078915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somogyi P, Tamas G, Lujan R, Buhl EH. Salient features of synaptic organisation in the cerebral cortex. Brain Res Brain Res Rev. 1998;26:113–135. doi: 10.1016/s0165-0173(97)00061-1. [DOI] [PubMed] [Google Scholar]
- Stettler DD, Axel R. Representations of odor in the piriform cortex. Neuron. 2009;63:854–864. doi: 10.1016/j.neuron.2009.09.005. [DOI] [PubMed] [Google Scholar]
- Stokes CC, Isaacson JS. From dendrite to soma: dynamic routing of inhibition by complementary interneuron microcircuits in olfactory cortex. Neuron. 2010;67:452–465. doi: 10.1016/j.neuron.2010.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swadlow HA. Efferent neurons and suspected interneurons in binocular visual cortex of the awake rabbit: receptive fields and binocular properties. Journal of neurophysiology. 1988;59:1162–1187. doi: 10.1152/jn.1988.59.4.1162. [DOI] [PubMed] [Google Scholar]
- Swadlow HA. Thalamocortical control of feed-forward inhibition in awake somatosensory 'barrel' cortex. Philos Trans R Soc Lond B Biol Sci. 2002;357:1717–1727. doi: 10.1098/rstb.2002.1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swadlow HA. Fast-spike interneurons and feedforward inhibition in awake sensory neocortex. Cerebral cortex. 2003;13:25–32. doi: 10.1093/cercor/13.1.25. [DOI] [PubMed] [Google Scholar]
- Szabadics J, Varga C, Molnar G, Olah S, Barzo P, Tamas G. Excitatory effect of GABAergic axo-axonic cells in cortical microcircuits. Science. 2006;311:233–235. doi: 10.1126/science.1121325. [DOI] [PubMed] [Google Scholar]
- Tamas G, Somogyi P, Buhl EH. Differentially interconnected networks of GABAergic interneurons in the visual cortex of the cat. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1998;18:4255–4270. doi: 10.1523/JNEUROSCI.18-11-04255.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan AY, Zhang LI, Merzenich MM, Schreiner CE. Tone-evoked excitatory and inhibitory synaptic conductances of primary auditory cortex neurons. J Neurophysiol. 2004;92:630–643. doi: 10.1152/jn.01020.2003. [DOI] [PubMed] [Google Scholar]
- Tan AYY, Brown BD, Scholl B, Mohanty D, Priebe NJ. Orientation Selectivity of Synaptic Input to Neurons in Mouse and Cat Primary Visual Cortex. Journal of Neuroscience. 2011;31:12339–12350. doi: 10.1523/JNEUROSCI.2039-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan EM, Yamaguchi Y, Horwitz GD, Gosgnach S, Lein ES, Goulding M, Albright TD, Callaway EM. Selective and quickly reversible inactivation of mammalian neurons in vivo using the Drosophila allatostatin receptor. Neuron. 2006;51:157–170. doi: 10.1016/j.neuron.2006.06.018. [DOI] [PubMed] [Google Scholar]
- Taniguchi H, He M, Wu P, Kim S, Paik R, Sugino K, Kvitsani D, Fu Y, Lu J, Lin Y, et al. A Resource of Cre Driver Lines for Genetic Targeting of GABAergic Neurons in Cerebral Cortex. Neuron. 2011;71:995–1013. doi: 10.1016/j.neuron.2011.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiesinga P, Sejnowski TJ. Cortical enlightenment: are attentional gamma oscillations driven by ING or PING? Neuron. 2009;63:727–732. doi: 10.1016/j.neuron.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traub RD, Jefferys JG, Whittington MA. Simulation of gamma rhythms in networks of interneurons and pyramidal cells. J Comput Neurosci. 1997;4:141–150. doi: 10.1023/a:1008839312043. [DOI] [PubMed] [Google Scholar]
- Traub RD, Whittington MA, Stanford IM, Jefferys JG. A mechanism for generation of long-range synchronous fast oscillations in the cortex. Nature. 1996;383:621–624. doi: 10.1038/383621a0. [DOI] [PubMed] [Google Scholar]
- Turrigiano G. Too many cooks? Intrinsic and synaptic homeostatic mechanisms in cortical circuit refinement. Annual review of neuroscience. 2011;34:89–103. doi: 10.1146/annurev-neuro-060909-153238. [DOI] [PubMed] [Google Scholar]
- Van Vreeswijk C, Abbott LF, Ermentrout GB. When inhibition not excitation synchronizes neural firing. J Comput Neurosci. 1994;1:313–321. doi: 10.1007/BF00961879. [DOI] [PubMed] [Google Scholar]
- van Vreeswijk C, Sompolinsky H. Chaos in neuronal networks with balanced excitatory and inhibitory activity. Science. 1996;274:1724–1726. doi: 10.1126/science.274.5293.1724. [DOI] [PubMed] [Google Scholar]
- Vida I, Bartos M, Jonas P. Shunting inhibition improves robustness of gamma oscillations in hippocampal interneuron networks by homogenizing firing rates. Neuron. 2006;49:107–117. doi: 10.1016/j.neuron.2005.11.036. [DOI] [PubMed] [Google Scholar]
- Wang J, Caspary D, Salvi RJ. GABA-A antagonist causes dramatic expansion of tuning in primary auditory cortex. Neuroreport. 2000;11:1137–1140. doi: 10.1097/00001756-200004070-00045. [DOI] [PubMed] [Google Scholar]
- Wang XJ, Buzsaki G. Gamma oscillation by synaptic inhibition in a hippocampal interneuronal network model. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1996;16:6402–6413. doi: 10.1523/JNEUROSCI.16-20-06402.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehr M, Zador AM. Balanced inhibition underlies tuning and sharpens spike timing in auditory cortex. Nature. 2003;426:442–446. doi: 10.1038/nature02116. [DOI] [PubMed] [Google Scholar]
- Whittington MA, Traub RD, Kopell N, Ermentrout B, Buhl EH. Inhibition-based rhythms: experimental and mathematical observations on network dynamics. Int J Psychophysiol. 2000;38:315–336. doi: 10.1016/s0167-8760(00)00173-2. [DOI] [PubMed] [Google Scholar]
- Wilent WB, Contreras D. Synaptic responses to whisker deflections in rat barrel cortex as a function of cortical layer and stimulus intensity. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2004;24:3985–3998. doi: 10.1523/JNEUROSCI.5782-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilent WB, Contreras D. Dynamics of excitation and inhibition underlying stimulus selectivity in rat somatosensory cortex. Nat Neurosci. 2005;8:1364–1370. doi: 10.1038/nn1545. [DOI] [PubMed] [Google Scholar]
- Williford T, Maunsell JH. Effects of spatial attention on contrast response functions in macaque area V4. Journal of neurophysiology. 2006;96:40–54. doi: 10.1152/jn.01207.2005. [DOI] [PubMed] [Google Scholar]
- Woodruff A, Xu Q, Anderson SA, Yuste R. Depolarizing effect of neocortical chandelier neurons. Front Neural Circuits. 2009;3:15. doi: 10.3389/neuro.04.015.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu GK, Arbuckle R, Liu BH, Tao HW, Zhang LI. Lateral sharpening of cortical frequency tuning by approximately balanced inhibition. Neuron. 2008;58:132–143. doi: 10.1016/j.neuron.2008.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu GK, Li P, Tao HW, Zhang LI. Nonmonotonic synaptic excitation and imbalanced inhibition underlying cortical intensity tuning. Neuron. 2006;52:705–715. doi: 10.1016/j.neuron.2006.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura Y, Callaway EM. Fine-scale specificity of cortical networks depends on inhibitory cell type and connectivity. Nat Neurosci. 2005;8:1552–1559. doi: 10.1038/nn1565. [DOI] [PubMed] [Google Scholar]
- Zhang LI, Tan AY, Schreiner CE, Merzenich MM. Topography and synaptic shaping of direction selectivity in primary auditory cortex. Nature. 2003;424:201–205. doi: 10.1038/nature01796. [DOI] [PubMed] [Google Scholar]