Abstract
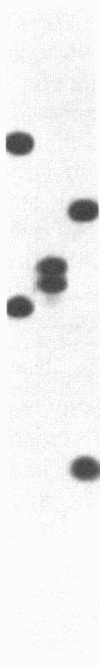
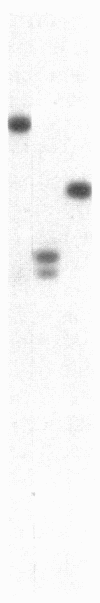
The polypeptide composition of the phycobilisome, the major light-harvesting complex of prokaryotic cyanobacteria and certain eukaryotic algae, can be modulated by different light qualities in cyanobacteria exhibiting chromatic adaptation. We have identified genomic fragments encoding a cluster of phycobilisome polypeptides (phycobiliproteins) from the chromatically adapting cyanobacterium Fremyella diplosiphon using previously characterized DNA fragments of phycobiliprotein genes from the eukaryotic alga Cyanophora paradoxa and from F. diplosiphon. Characterization of two lambda-EMBL3 clones containing overlapping genomic fragments indicates that three sets of phycobiliprotein genes--the alpha- and beta-allophycocyanin genes plus two sets of alpha- and beta-phycocyanin genes--are clustered within 13 kilobases on the cyanobacterial genome and transcribed off the same strand. The gene order (alpha-allophycocyanin followed by beta-allophycocyanin and beta-phycocyanin followed by alpha-phycocyanin) appears to be a conserved arrangement found previously in a eukaryotic alga and another cyanobacterium. We have reported that one set of phycocyanin genes is transcribed as two abundant red light-induced mRNAs (1600 and 3800 bases). We now present data showing that the allophycocyanin genes and a second set of phycocyanin genes are transcribed into major mRNAs of 1400 and 1600 bases, respectively. These transcripts are present in RNA isolated from cultures grown in red and green light, although lower levels of the 1600-base phycocyanin transcript are present in cells grown in green light. Furthermore, a larger transcript of 1750 bases hybridizes to the allophycocyanin genes and may be a precursor to the 1400-base species.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Bryant D. A., Cohen-Bazire G. Effects of chromatic illumination on cyanobacterial phycobilisomes. Evidence for the specific induction of a second pair of phycocyanin subunits in Pseudanabaena 7409 grown in red light. Eur J Biochem. 1981 Oct;119(2):415–424. doi: 10.1111/j.1432-1033.1981.tb05624.x. [DOI] [PubMed] [Google Scholar]
- Bryant D. A., de Lorimier R., Lambert D. H., Dubbs J. M., Stirewalt V. L., Stevens S. E., Jr, Porter R. D., Tam J., Jay E. Molecular cloning and nucleotide sequence of the alpha and beta subunits of allophycocyanin from the cyanelle genome of Cyanophora paradoxa. Proc Natl Acad Sci U S A. 1985 May;82(10):3242–3246. doi: 10.1073/pnas.82.10.3242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church G. M., Gilbert W. Genomic sequencing. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conley P. B., Lemaux P. G., Grossman A. R. Cyanobacterial light-harvesting complex subunits encoded in two red light-induced transcripts. Science. 1985 Nov 1;230(4725):550–553. doi: 10.1126/science.3931221. [DOI] [PubMed] [Google Scholar]
- Curtis S. E., Haselkorn R. Isolation and sequence of the gene for the large subunit of ribulose-1,5-bisphosphate carboxylase from the cyanobacterium Anabaena 7120. Proc Natl Acad Sci U S A. 1983 Apr;80(7):1835–1839. doi: 10.1073/pnas.80.7.1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLange R. J., Williams L. C., Glazer A. N. The amino acid sequence of the beta subunit of allophycocyanin. J Biol Chem. 1981 Sep 25;256(18):9558–9566. [PubMed] [Google Scholar]
- Frank G., Sidler W., Widmer H., Zuber H. The complete amino acid sequence of both subunits of C-phycocyanin from the cyanobacterium Mastigocladus laminosus. Hoppe Seylers Z Physiol Chem. 1978 Nov;359(11):1491–1507. doi: 10.1515/bchm2.1978.359.2.1491. [DOI] [PubMed] [Google Scholar]
- Frischauf A. M., Lehrach H., Poustka A., Murray N. Lambda replacement vectors carrying polylinker sequences. J Mol Biol. 1983 Nov 15;170(4):827–842. doi: 10.1016/s0022-2836(83)80190-9. [DOI] [PubMed] [Google Scholar]
- Glazer A. N., Lundell D. J., Yamanaka G., Williams R. C. The structure of a "simple" phycobilisome. Ann Microbiol (Paris) 1983 Jul-Aug;134B(1):159–180. doi: 10.1016/s0769-2609(83)80103-3. [DOI] [PubMed] [Google Scholar]
- Glazer A. N. Structure and molecular organization of the photosynthetic accessory pigments of cyanobacteria and red algae. Mol Cell Biochem. 1977 Dec 29;18(2-3):125–140. doi: 10.1007/BF00280278. [DOI] [PubMed] [Google Scholar]
- Gold L., Pribnow D., Schneider T., Shinedling S., Singer B. S., Stormo G. Translational initiation in prokaryotes. Annu Rev Microbiol. 1981;35:365–403. doi: 10.1146/annurev.mi.35.100181.002053. [DOI] [PubMed] [Google Scholar]
- HUGHES E. O., GORHAM P. R., ZEHNDER A. Toxicity of a unialgal culture of Microcystis aeruginosa. Can J Microbiol. 1958 Jun;4(3):225–236. doi: 10.1139/m58-024. [DOI] [PubMed] [Google Scholar]
- Lemaux P. G., Grossman A. R. Major light-harvesting polypeptides encoded in polycistronic transcripts in a eukaryotic alga. EMBO J. 1985 Aug;4(8):1911–1919. doi: 10.1002/j.1460-2075.1985.tb03870.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemaux P. G., Grossman A. Isolation and characterization of a gene for a major light-harvesting polypeptide from Cyanophora paradoxa. Proc Natl Acad Sci U S A. 1984 Jul;81(13):4100–4104. doi: 10.1073/pnas.81.13.4100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundell D. J., Williams R. C., Glazer A. N. Molecular architecture of a light-harvesting antenna. In vitro assembly of the rod substructures of Synechococcus 6301 phycobilisomes. J Biol Chem. 1981 Apr 10;256(7):3580–3592. [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Norrander J., Kempe T., Messing J. Construction of improved M13 vectors using oligodeoxynucleotide-directed mutagenesis. Gene. 1983 Dec;26(1):101–106. doi: 10.1016/0378-1119(83)90040-9. [DOI] [PubMed] [Google Scholar]
- Offner G. D., Brown-Mason A. S., Ehrhardt M. M., Troxler R. F. Primary structure of phycocyanin from the unicellular rhodophyte Cyanidium caldarium. I. Complete amino acid sequence of the alpha subunit. J Biol Chem. 1981 Dec 10;256(23):12167–12175. [PubMed] [Google Scholar]
- Offner G. D., Troxler R. F. Primary structure of allophycocyanin from the unicellular rhodophyte, Cyanidium caldarium. The complete amino acid sequences of the alpha and beta subunits. J Biol Chem. 1983 Aug 25;258(16):9931–9940. [PubMed] [Google Scholar]
- Pilot T. J., Fox J. L. Cloning and sequencing of the genes encoding the alpha and beta subunits of C-phycocyanin from the cyanobacterium Agmenellum quadruplicatum. Proc Natl Acad Sci U S A. 1984 Nov;81(22):6983–6987. doi: 10.1073/pnas.81.22.6983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shine J., Dalgarno L. The 3'-terminal sequence of Escherichia coli 16S ribosomal RNA: complementarity to nonsense triplets and ribosome binding sites. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1342–1346. doi: 10.1073/pnas.71.4.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troxler R. F., Ehrhardt M. M., Brown-Mason A. S., Offner G. D. Primary structure of phycocyanin from the unicellular rhodophyte Cyanidium caldarium. II. Complete amino acid sequence of the beta subunit. J Biol Chem. 1981 Dec 10;256(23):12176–12184. [PubMed] [Google Scholar]
- Troxler R. F., Foster J. A., Brown A. S., Franzblau C. The alpha and beta subunits of Cyanidium caldarium phycocyanin: properties and amino acid sequences at the amino terminus. Biochemistry. 1975 Jan 28;14(2):268–274. doi: 10.1021/bi00673a012. [DOI] [PubMed] [Google Scholar]
- de Lorimier R., Bryant D. A., Porter R. D., Liu W. Y., Jay E., Stevens S. E., Jr Genes for the alpha and beta subunits of phycocyanin. Proc Natl Acad Sci U S A. 1984 Dec;81(24):7946–7950. doi: 10.1073/pnas.81.24.7946. [DOI] [PMC free article] [PubMed] [Google Scholar]