Abstract
Peroxisome proliferator-activated receptor γ (PPARγ) forms a heterodimeric DNA-binding complex with retinoid X receptors (RXRs). It has been reported that the effect of the PPAR agonist is reduced in hepatocyte RXR-deficient mice. Therefore, it is suggested that the endogenous RXR ligand is involved in the PPARγ agonist-induced anti-inflammatory effect. However, the participation of the RXR ligand in the PPARγ-induced anti-inflammatory effect is unknown. Here, we investigated the influence of RXR antagonist on the anti-inflammatory effect of PPARγ agonist pioglitazone in carrageenan test. In addition, we also examined the influence of PPAR antagonist on the anti-inflammatory effect induced by RXR agonist NEt-3IP. The RXR antagonist suppressed the antiedema effect of PPARγ agonist. In addition, the anti-inflammatory effect of RXR agonist was suppressed by PPARγ antagonist. PPARγ agonist-induced anti-inflammatory effects were reversed by the RXR antagonist. Thus, we showed that the endogenous RXR ligand might contribute to the PPARγ agonist-induced anti-inflammatory effect.
1. Introduction
Peroxisome proliferator-activated receptor (PPAR) is a family comprising 3 different isoforms: PPARα, PPARγ, and PPARδ. PPAR forms a heterodimeric DNA-binding complex with the retinoid X receptor (RXR) and serves as a transcriptional regulator of genes involved in lipid metabolism [1, 2]. In addition, it has been reported that PPARγ is expressed in monocytes and macrophages; therefore, researchers have shown much interest in the involvement of PPARγ in inflammatory processes [3–6]. Studies have shown that the PPARγ agonist is effective in inflammatory models such as intestinal inflammation [7], rheumatoid arthritis [8], inflammatory lung disease [9], and allergic rhinitis [10]. These studies suggested that PPARγ agonist might be a new drug for the treatment of inflammatory disease.
RXR is a member of the nuclear hormone receptor superfamily and is activated by the endogenous agonist 9-cis retinoic acid [11]. RXR functions as a dimer not only with PPAR but also with other nuclear receptor partners such as retinoid acid receptor (RAR), vitamin D receptor (VDR), and liver X receptor (LXR) [2, 12]. Therefore, RXR is closely linked to the function of such partners, and RXR agonists synergistically control the function of RXR heterodimeric partners [13]. Manzano et al. [14] have reported that in human mesangial cells, 9-cis retinoic acid suppressed the expression of vascular cell adhesion molecule (VCAM)-1 and intercellular adhesion molecule (ICAM)-1 that was induced by lipopolysaccharide (LPS), a bacterial endotoxin. In addition, it has been reported that in microglial cells, 9-cis retinoic acid reduced tumour necrosis factor-α (TNF-α)-induced nitric oxide (NO) expression [15]. Therefore, it is suggested that the RXR agonist exerts an anti-inflammatory effect. However, 9-cis retinoic acid activated not only RXR but also RAR. Motomura et al. [16] have reported that the suppressive effect of 9-cis retinoic acid was not reversed by RAR-specific antagonist. Moreover, the RAR-specific agonist Ro 40-6055 did not show the inhibitory effect shown by 9-cis retinoic acid on the increase of NO and TNF-α levels in Kupffer cells [16]. Therefore, it is suggested that the inhibitory effect of 9-cis retinoic acid does not depend on the RAR/RXR signalling pathway but on another RXR heterodimer signalling pathway. Benson et al. [17] have reported that the antiproliferative activity induced by the endogenous PPAR agonist 15 deoxy-Δ12,14-PGJ2 was enhanced by the endogenous RXR agonist 9-cis retinoic acid. In addition, coactivation of the PPARγ agonist troglitazone and the RXR agonist LG100268 resulted in additive effects on glucose and lipid metabolism in skeletal muscles [18]. Moreover, Diab et al. [15] have reported that in microglial cells, 15 deoxy-Δ12,14-PGJ2 and 9-cis retinoic acid individually weakly inhibited NO production but together strongly and synergistically inhibited NO production. Furthermore, it has been reported that PPAR agonist did not inhibit carrageenan-induced paw edema in hepatocyte-specific RXR-deficient mice, whereas PPAR agonist reduced carrageenan-induced paw edema in wild-type mice [19]. Therefore, we hypothesized that the endogenous RXR ligand is involved in the PPAR agonist-induced anti-inflammatory effect. However, it is not known whether PPAR was activated by RXR in a ligand-dependent manner.
Carrageenan-induced paw edema has been increasingly used to test new anti-inflammatory drugs as well as to study the mechanisms involved in inflammation. Therefore, carrageenan-induced local inflammation is a useful model to assess the contribution of mediators involved in vascular changes associated with acute inflammation [20–23]. In the present study, we examined the effect of the RXR antagonist on the anti-inflammatory effect of the PPARγ agonist pioglitazone in order to investigate the participation of RXR in PPARγ activation. Moreover, we examined the effects of PPARα, PPARγ, and PPARδ antagonists on the anti-inflammatory effect induced by the RXR agonist NEt-3IP with the aim of determining the PPAR subtype that is involved in the RXR agonist-induced anti-inflammatory effect.
2. Materials and Methods
2.1. Animals
Five-week-old male ICR mice (body weight, 23–28 g) were purchased from Japan SLC, Shizuoka, Japan. The animals were kept in an air-conditioned room at a controlled temperature (24°C ± 2°C) and humidity (55% ± 15%). They were housed in plastic cages lined with sawdust and kept under a light-dark cycle (lights on from 0700–1900). Food and water were freely available, except during test periods. All procedures involving animals were conducted in accordance with the Guidelines for Animal Experiments at Okayama University Advanced Science Research Center, and all procedures were licensed by the Animal Research Control Committee of Okayama University.
2.2. Reagents
λ-Carrageenan (Wako, Osaka, Japan) was dissolved in physiological saline. 6-[N-ethyl-N-(3-isopropoxy-4-isopropylphenyl)-amino] nicotinic acid (NEt-3IP), 6-[N-4-(trifluoromethyl) benzenesulfonyl-N-(5,5,8,8-tetramethyl-5,6,7,8-tetrahydro-2-naphthyl) amino] nicotinic acid (NS-4TF), and 3′-((2-fluoro-4-(trifluoromethyl) benzamido) methyl)-4′-propoxybiphenyl-4-carboxylic acid (JKPL-85) were synthesized at Okayama University [24, 25]. NEt-3IP and pioglitazone (Actos; Takeda Pharmaceutics, Osaka, Japan) were suspended in 0.5% carboxymethylcellulose solution. Bisphenol A diglycidyl ether (BADGE; Sigma, St. Louis, Mo, USA), N-((2S)-2-(((1Z)-1-methyl-3-oxo-3-(4-(trifluoromethyl) phenyl) prop-1-enyl) amino)-3-(4-(2-(5-methyl-2-phenyl-1,3-oxazol-4-yl) ethoxy) phenyl) propyl) propanamide (GW6471; Sigma), 2-chloro-5-nitro-N-phenylbenzamide (GW9662; Sigma), and JKPL-85 were dissolved in physiological saline containing 10% dimethylsulphoxide. Actinomycin D (Sigma) was dissolved in physiological saline containing 10% ethanol.
2.3. Drug Administration
Both pioglitazone (1, 3, and 10 mg/kg) and NEt-3IP (1, 3, and 10 mg/kg) were orally administered 3 h before carrageenan injection. NS-4TF (10 and 30 μg/paw), GW6471 (10 and 30 μg/paw), GW9662 (1, 3, and 10 μg/paw), JKPL-85 (10 and 30 μg/paw), and actinomycin D (3 and 10 μg/paw) were injected into the subplantar region of the hind paw 15 min before carrageenan injection. BADGE (10 and 30 mg/kg) was intraperitoneally injected into mice 30 min before the carrageenan injection.
2.4. Mouse Paw Edema
After carrageenan injection, the hind paw volume was measured at intervals of 1 h for up to 3 h. The paw volume was determined using a plethysmometer (TK-101; UNICOM, Chiba, Japan). The basal volume of the hind paw was determined before the administration of any drug. After determination of the basal volume, the animals were divided into experimental groups in such a way that the mean volumes of the different groups were similar. A 1% solution of λ-carrageenan dissolved in saline (0.05 mL/animal) was injected subcutaneously into the right hind paw of each mouse. Paw edema was determined as the difference in the paw volume before and after carrageenan injection and was expressed as Δ paw volume.
2.5. Statistical Analysis
All data are presented as the mean ± standard error of the mean (S.E.M.). Statistical analysis was performed using one-way analysis of variance (ANOVA) with the Dunnett's test or Student's unpaired t test. When the probability (P) value was less than 0.05, the difference was considered to be significant.
3. Results
3.1. Anti-Inflammatory Effect of PPARγ Agonist
The PPARγ agonist pioglitazone showed anti-inflammatory effect in a dose-dependent manner (Figure 1(a)). Oral administration of pioglitazone at doses of 3 and 10 mg/kg significantly inhibited carrageenan-induced paw edema as compared with that of the control group. Pioglitazone (3 mg/kg) significantly suppressed paw edema at 2 and 3 h after carrageenan injection. Pioglitazone (10 mg/kg) significantly suppressed paw edema at 1, 2, and 3 h after carrageenan injection. Figure 1(b) shows the effect of the PPARγ antagonist GW9662 on the anti-inflammatory effect of pioglitazone. Intraplantar injection of GW9662 at doses of 1 and 3 μg/paw significantly reduced the anti-inflammatory effect of pioglitazone.
Figure 1.
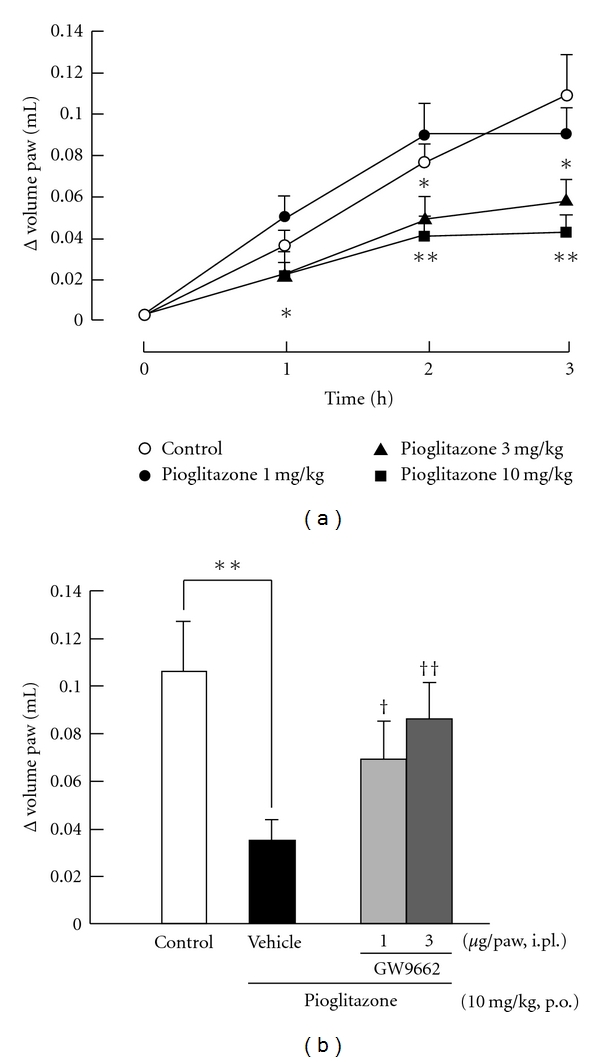
Involvement of PPARγ on carrageenan-induced paw edema in mice. (a) Dose-dependence and time course of PPARγ agonist, pioglitazone on carrageenan-induced paw edema in mice. Pioglitazone was orally administrated 3 h before carrageenan injection at doses of 1, 3, and 10 mg/kg. The control group received 0.5% carboxymethylcellulose. (b) Effect of PPARγ antagonist, GW9662 on pioglitazone induced anti-inflammatory effect. Pioglitazone was orally administrated 3 h before carrageenan injection at a dose of 10 mg/kg. GW9662 was injected 15 min before carrageenan injection at doses of 1 and 3 μg/paw. The vehicle group received physiological saline including 10% dimethylsulphoxide. Each column and vertical bar represents the means ± S.E.M. (n = 7). *,**: Significantly different from the control group at P < 0.05 and P < 0.01, respectively, (Dunnett's test). †,††: Significantly different from the vehicle group at P < 0.05 and P < 0.01, respectively, (Dunnett's test).
3.2. Influence of RXR Antagonists on the Anti-Inflammatory Effect Induced by PPARγ Agonist
Figure 2 shows the influence of NS-4TF on the anti-inflammatory effect of pioglitazone. Intraplantar injection of NS-4TF at a dose of 30 μg/paw significantly reduced the anti-inflammatory effect induced by pioglitazone.
Figure 2.
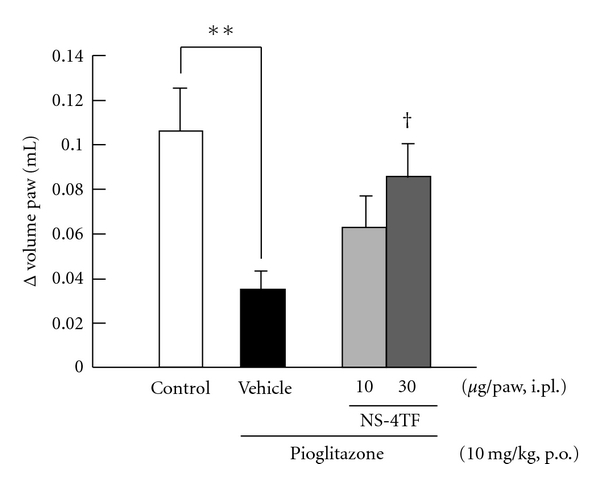
Influence of RXR antagonists on PPARγ agonist-induced anti-inflammatory effects. Pioglitazone was orally administrated 3 h before carrageenan injection at a dose of 10 mg/kg. RXR antagonist, NS-4TF was injected into subplantar 15 min before carrageenan injection at doses of 10 and 30 μg/paw. The vehicle group received physiological saline including 10% dimethylsulphoxide. Each column and vertical bar represents the means ± S.E.M. (n = 7). **: Significantly different from the control group at P < 0.01 (Dunnett's test). †: Significantly different from the vehicle group at P < 0.05 (Dunnett's test).
3.3. Anti-Inflammatory Effect of RXR Agonist
The RXR agonist NEt-3IP showed anti-inflammatory effect in a dose-dependent manner (Figure 3(a)). Oral administration of NEt-3IP at doses of 3 and 10 mg/kg significantly inhibited carrageenan-induced paw edema compared with that of the control group. The control group received 0.5% carboxymethylcellulose solution. NEt-3IP (3 mg/kg) significantly suppressed paw edema at 2 and 3 h after carrageenan injection. NEt-3IP (10 mg/kg) significantly suppressed paw edema at 1, 2, and 3 h after carrageenan injection. Figure 3(b) shows the effect of the RXR antagonist NS-4TF on the anti-inflammatory effect of NEt-3IP. Intraplantar injection of NS-4TF at a dose of 30 μg/paw significantly reduced the anti-inflammatory effect of NEt-3IP.
Figure 3.
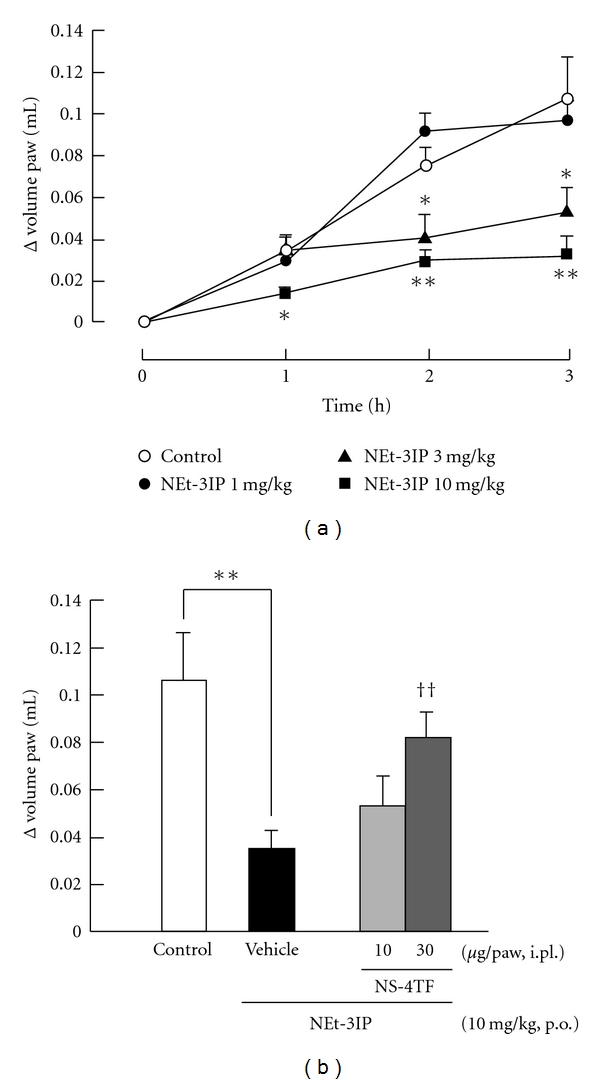
Involvement of RXR on carrageenan-induced paw edema in mice. (a) Dose dependence and time course of RXR agonist, NEt-3IP on carrageenan-induced paw edema in mice. NEt-3IP was orally administrated 3 h before carrageenan injection at doses of 1, 3, and 10 mg/kg. The control group was received 0.5% carboxymethylcellulose. (b) Effect of RXR antagonist, NS-4TF on NEt-3IP induced anti-inflammatory effect. NEt-3IP was orally administrated 3 h before carrageenan injection at a dose of 10 mg/kg. NS-4TF was injected 15 min before carrageenan injection at doses of 10 and 30 μg/paw. The vehicle group received physiological saline including 10% dimethylsulphoxide. Each column and vertical bar represents the means ± S.E.M. (n = 7). *,**: Significantly different from the control group at P < 0.05 and P < 0.01, respectively, (Dunnett's test). ††: Significantly different from the vehicle group at P < 0.01 (Dunnett's test).
3.4. Influence of PPAR Antagonists on the Anti-Inflammatory Effect Induced by RXR Agonist
Figure 4 shows the influence of GW6471, BADGE, GW9662, and JKPL-85 on the anti-inflammatory effect induced by NEt-3IP. Intraperitoneal injection of the PPARγ antagonist BADGE (30 mg/kg) and intraplantar injection of the PPARγ antagonist GW9662 (3 and 10 μg/paw) significantly reduced the anti-inflammatory effect induced by the RXR agonist. In contrast, intraplantar injection of the PPARα antagonist GW6471 (10 and 30 μg/paw) and the PPARδ antagonist JKPL-85 (10 and 30 μg/paw) did not inhibit the anti-inflammatory effect induced by the RXR agonist. Both antagonists did not affect the paw edema at any time or concentration.
Figure 4.
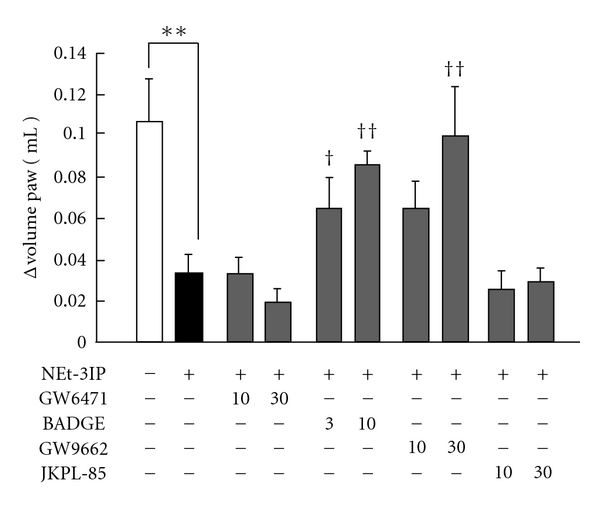
Influence of PPAR antagonists on RXR agonist and PPARγ agonist-induced anti-inflammatory effects. NEt-3IP was orally administrated 3 h before carrageenan injection at a dose of 10 mg/kg. Nonselective PPAR antagonist, BADGE was injected intraperitoneal 30 min before carrageenan injection at doses of 10 and 30 mg/kg. PPARα antagonist, GW6471 was injected into subplantar 15 min before carrageenan injection at doses of 10 and 30 μg/paw. PPARγ antagonist, GW9662 was injected into subplantar 15 min before carrageenan injection at doses of 3 and 10 μg/paw. PPARδ antagonist, JKPL-85 was injected into subplantar 15 min before carrageenan injection at doses of 10 and 30 μg/paw. The vehicle group received physiological saline including 10% dimethylsulphoxide. Each column and vertical bar represents the means ± S.E.M. (n = 7). **: Significantly different from the control group at P < 0.01 (Dunnett's test). †,††: Significantly different from the vehicle group at P < 0.05 and P < 0.01, respectively (Dunnett's test).
3.5. The Combination Effect of RXR Agonist and PPARγ Agonist on Carrageenan-Induced Paw Edema
Figure 5 shows the combination effect of pioglitazone and NEt-3IP on carrageenan-induced paw edema. Coadministration of pioglitazone (1 mg/kg) and NEt-3IP (1 mg/kg) suppressed carrageen-induced paw edema as compared to that of the control group and the groups treated with pioglitazone (1 mg/kg) and NEt-3IP (1 mg/kg).
Figure 5.
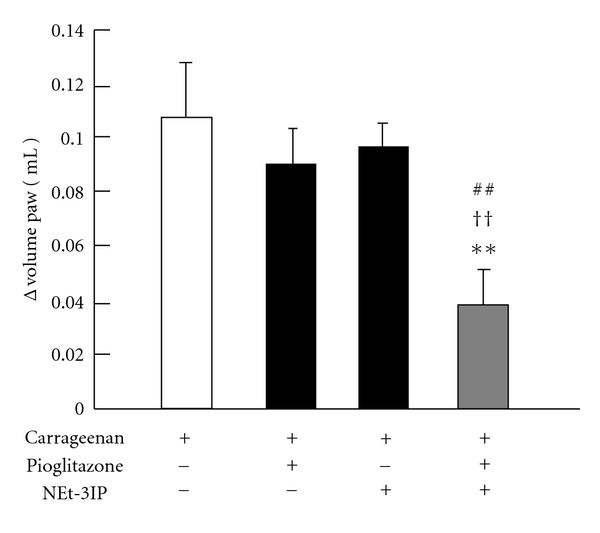
Effect of combination of RXR agonist and PPARγ agonist on carrageenan-induced paw edema. Coadministration of pioglitazone (1 mg/kg) and NEt-3IP (1 mg/kg), which showed no inhibition each alone, suppressed carrageenan-induce paw edema compared with control group, pioglitazone (1 mg/kg) and NEt-3IP (1 mg/kg) treated group, respectively. The control group received 0.5% carboxymethylcellulose solution. Each column and vertical bar represents the means ± S.E.M. (n = 7). **: Significantly different from the control group at P < 0.01 (Dunnett's test). ††: Significantly different from the pioglitazone treated group at P < 0.01 (Dunnett's test). ##: Significantly different from the NEt-3IP treated group at P < 0.01 (Dunnett's test).
3.6. Influence of Actinomycin D on the Anti-Inflammatory Effect Induced by RXR Agonist and PPARγ Agonist
Figures 6(a) and 6(b) show the influence of the RNA polymerase inhibitor actinomycin D on the anti-inflammatory effect of pioglitazone and NEt-3IP. Intraplantar injection of actinomycin D at doses of 3 and 10 μg/paw significantly reduced the anti-inflammatory effect induced by PPARγ and RXR agonist, respectively, compared with that in vehicle-treated mice.
Figure 6.
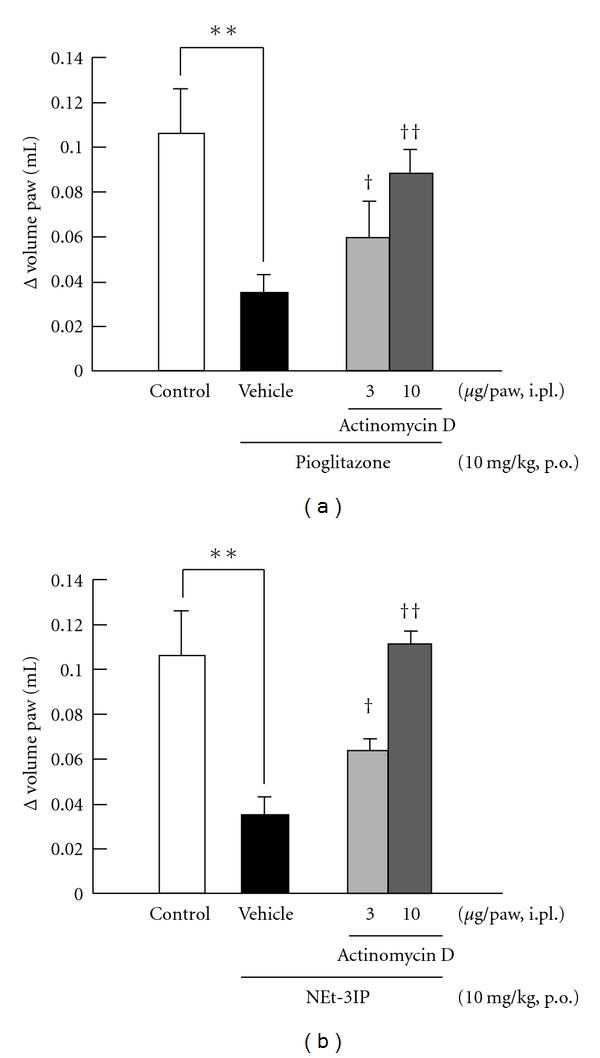
Influence of RNA polymerase inhibitor on PPARγ agonist and RXR agonist-induced anti-inflammatory effects. (a) Effect of RNA polymerase inhibitor, actinomycin D on pioglitazone-induced anti-inflammatory effect. Pioglitazone was orally administrated 3 h before carrageenan injection at a dose of 10 mg/kg. Actinomycin D was injected 15 min before carrageenan injection at doses of 3 and 10 μg/paw. The vehicle group received physiological saline including 10% dimethylsulphoxide. (b) Effect of actinomycin D on NEt-3IP-induced anti-inflammatory effect. NEt-3IP was orally administrated 3 h before carrageenan injection at a dose of 10 mg/kg. Actinomycin D was injected 15 min before carrageenan injection at doses of 3 and 10 μg/paw. The vehicle group received physiological saline including 10% dimethylsulphoxide. Each column and vertical bar represents the means ± S.E.M. (n = 7). **: Significantly different from the control group at P < 0.01 (Dunnett's test). †,††: Significantly different from the vehicle group at P < 0.05 and P < 0.01, respectively, (Dunnett's test).
3.7. Influence of Reagents on Edema Induced by Carrageenan
Figure 7 shows the influence of reagents on edema induced by carrageenan. NS-4TF (30 μg/paw), GW6471 (30 μg/paw), BADGE (30 mg/kg), GW9662 (10 μg/paw), and JKPL-85 (30 μg/paw) have no influence on edema induced by carrageenan.
Figure 7.
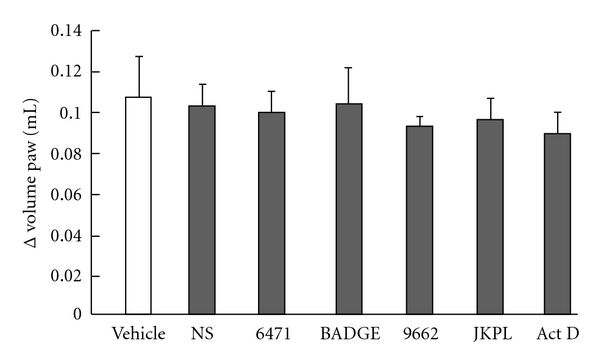
Influence of reagents on edema induced by carrageenan. BADGE was injected intraperitoneal 30 min before carrageenan injection at doses of 30 mg/kg. NS-4TF (30 μg/paw), GW6471 (30 μg/paw), BADGE (30 mg/kg), GW9662 (10 μg/paw) and JKPL-85 (30 μg/paw), were injected into subplantar 15 min before carrageenan injection. The vehicle group received physiological saline including 10% dimethylsulphoxide. Each column and vertical bar represents the means ± S.E.M. (n = 7).
4. Discussion
In the present study, the PPARγ agonist pioglitazone significantly inhibited carrageenan-induced paw edema in mice, and PPARγ antagonist significantly inhibited this suppressive effect. In addition, the effect of PPARγ agonist was prevented by an inhibitor of RNA synthesis. Studies have reported that rosiglitazone or pioglitazone showed anti-inflammatory effect via PPARγ in the carrageenan test [22, 26, 27]; these findings are similar to the findings of our study. A previous study found that the pioglitazone dose showing a significant anti-inflammatory effect was less than that of another study [28]. Therefore, it is suggested that that oral administration of pioglitazone at 3 h before carrageenan injection is a better pathway of administration.
Wan et al. [29] have reported that fasting-induced PPARα activation was strongly inhibited in the absence of hepatocyte RXRα. In addition, Wan and Badr [19] have also reported that PPARα agonist did not inhibit carrageenan-induced paw edema in hepatocyte-specific RXRα-deficient mice, whereas PPARα agonist reduced carrageenan-induced paw edema in wild-type mice. These results indicate that RXRα plays a central role in PPARα-induced effects. Therefore, we hypothesize that the endogenous RXR ligand is involved in PPAR activation. Consequently, we used an RXR antagonist to ignore the influence of the endogenous RXR ligand. As described in the results section, the RXR antagonist significantly reduced the anti-inflammatory effect of the PPARγ agonist. Therefore, we suggest that the endogenous RXR ligand may contribute to PPARγ activation. On the basis of these findings, we speculate that the endogenous RXR ligand and synthetic PPARγ agonist may function synergistically.
Additionally, we investigated the participation of PPAR in the action of RXR. First, we evaluated the effect of the RXR agonist NEt-3IP on carrageenan-induced paw edema. We found that the RXR agonist significantly inhibited carrageenan-induced paw edema in mice and that this effect was significantly inhibited by both RXR antagonist and actinomycin D, an inhibitor of RNA synthesis. RXR binds the nuclear factor kappa B (NF-κB) components p50 and p65 and also inhibits NF-κB transactivation [30]. Moreover, Uchimura et al. [31] have showed that the synthetic RXR agonist Ro47-5944 suppressed LPS-induced inducible nitric oxide synthesis (iNOS) and TNF-α mRNA expression. In addition, they also reported that Ro47-5944 suppressed the promoter activity of NF-κB in RAW 264.7 cells. Studies have reported that nuclear translocation of NF-κB was activated by carrageenan injection and that the expression of iNOS and cyclooxigenase-2 (COX-2) observed in paw exudates was induced by carrageenan via NF-κB signalling [21, 23]. Therefore, in the present study, the anti-inflammatory effect of the RXR agonist may be caused via inhibition of NF-κB.
It has been reported that the PPAR/RXR heterodimer can be activated by both RXR and PPAR agonists, either independently or together to cause a synergistic activation [32–34]. Therefore, it is thought that PPAR is involved in the RXR-induced anti-inflammatory effect. However, it is unclear which subtypes of PPAR contribute to the anti-inflammatory effect of RXR agonist. Therefore, we confirmed the effect of subtype-selective PPAR antagonist on RXR agonist-induced anti-inflammatory effect. Our data showed that the PPARγ-selective antagonist, BADGE and GW9662 significantly inhibited the anti-inflammatory effect of the RXR agonist. In contrast, the PPARα-selective antagonist GW6471 and the PPARδ-selective antagonist JKPL-85 did not inhibit the suppressive effect of the RXR agonist. These results confirm that the anti-inflammatory effect of the RXR agonist occurs partially though PPARγ activation. In addition, it is suggested that the endogenous PPARγ ligand may contribute to RXR activation. However, the PPAR/RXR heterodimer can be activated only by RXR agonists via permissive mechanisms [35]. Furthermore, Ijpenberg et al. [36] reported that the RXR/9-cis retinoic acid signalling pathway could selectively bind to peroxisome proliferator response element (PPRE) function and PPAR response element and induce transactivation. Therefore, it is necessary to perform a more detailed in vitro investigation of these functions.
In the case of the PPAR/RXR heterodimer, the binding of the ligand of either receptor can activate the complex, yet simultaneous binding of both ligands is more potent [37, 38]. This raises the question whether the coadministration of PPAR and RXR ligands further enhances the anti-inflammatory effect of either ligand in the carrageenan test. Therefore, we studied whether the PPAR and RXR agonists show synergistic function in the carrageenan test. Administration of either pioglitazone (1 mg/kg) or NEt-3IP (1 mg/kg) showed no effect on carrageenan-induced paw edema; however, the combined administration of these 2 compounds resulted in significant inhibition of carrageenan-induced paw edema. Studies have reported the combined effect of PPARγ and RXR agonists on the chronic inflammatory phase. Desreumaux et al. [39] also reported that simultaneous treatment with the PPARγ agonist rosiglitazone and the RXR agonist LG101305 enhanced the levels of TNF-α and interleukin (IL)-1β mRNA in the mouse colon. Diab et al. [15] reported that 9-cis retinoic acid showed an effect on experimental autoimmune encephalomyelitis and that this effect was enhanced by the endogenous PPAR agonist 15 deoxy-Δ12,14-PGJ2. Additionally, Burrage et al. [40] reported that combinatorial treatment with rosiglitazone and the RXR agonist LG100268 inhibits IL-1β-induced expression of MMP-1 more effectively than treatment with either individual compound. We showed that RXR and PPARγ agonists could exert a synergistic anti-inflammatory effect during the acute-phase response in vivo.
Wang et al. [41] have reported that LPS, TNF-α, and IL-1β caused RXR downregulation in mouse kidney cells during the acute-phase response. In addition, Harada et al. [42] have reported that Th1 cytokine induced PPARγ downregulation in human biliary cells. Moreover, it has been reported that RXR and PPAR are suppressed in the liver and heart during the acute-phase response [43]. Furthermore, Wan et al. [44] reported that the expression levels of both PPAR and RXR mRNA decrease in tissues, including the animal model of liver inflammation. These findings indicate that each agonist of PPARγ and RXR may be important for the action of therapeutic drugs on inflammatory diseases.
In conclusion, we found that PPARγ agonist-induced anti-inflammatory effects were reversed by RXR antagonist. Thus, we showed that the endogenous RXR ligand might contribute to the PPARγ agonist-induced anti-inflammatory effect.
References
- 1.Kliewer SA, Forman BM, Blumberg B, et al. Differential expression and activation of a family of murine peroxisome proliferator-activated receptors. Proceedings of the National Academy of Sciences of the United States of America. 1994;91(15):7355–7359. doi: 10.1073/pnas.91.15.7355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mangelsdorf DJ, Thummel C, Beato M, et al. The nuclear receptor super-family: the second decade. Cell. 1995;83(6):835–839. doi: 10.1016/0092-8674(95)90199-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ricote M, Li AC, Willson TM, Kelly CJ, Glass CK. The peroxisome proliferator-activated receptor-γ is a negative regulator of macrophage activation. Nature. 1998;391(6662):79–82. doi: 10.1038/34178. [DOI] [PubMed] [Google Scholar]
- 4.Moraes LA, Piqueras L, Bishop-Bailey D. Peroxisome proliferator-activated receptors and inflammation. Pharmacology and Therapeutics. 2006;110(3):371–385. doi: 10.1016/j.pharmthera.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 5.Xu J, Barger SW, Drew PD. The PPAR-γ agonist 15-deoxy-Δ 12, 14-prostaglandin J2 attenuates microglial production of IL-12 family cytokines: potential relevance to Alzheimer's disease. PPAR Research. 2008;2008 doi: 10.1155/2008/349185. Article ID 349185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang XY, Wang LH, Farrar WL. A role for PPARγ in the regulation of cytokines in immune cells and cancer. PPAR Research. 2008;2008 doi: 10.1155/2008/961753. Article ID 961753. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 7.Nakajima A, Wada K, Miki H, et al. Endogenous PPARγ mediates anti-inflammatory activity in murine ischemia-reperfusion injury. Gastroenterology. 2001;120(2):460–469. doi: 10.1053/gast.2001.21191. [DOI] [PubMed] [Google Scholar]
- 8.Shiojiri T, Wada K, Nakajima A, et al. PPARγ ligands inhibit nitrotyrosine formation and inflammatory mediator expressions in adjuvant-induced rheumatoid arthritis mice. European Journal of Pharmacology. 2002;448(2-3):231–238. doi: 10.1016/s0014-2999(02)01946-5. [DOI] [PubMed] [Google Scholar]
- 9.Belvisi MG, Mitchell JA. Targeting PPAR receptors in the airway for the treatment of inflammatory lung disease. British Journal of Pharmacology. 2009;158(4):994–1003. doi: 10.1111/j.1476-5381.2009.00373.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jeon EJ, Lee SK, Park YS, Kim DH, Yum JH, Park CS. The effects of Peroxisome Proliferator-Activated Receptor-γ agonist on a murine model of experimental allergic rhinitis. Otolaryngology—Head and Neck Surgery. 2008;139(1):124–130. doi: 10.1016/j.otohns.2008.03.030. [DOI] [PubMed] [Google Scholar]
- 11.Mangelsdorf DJ, Borgmeyer U, Heyman RA, et al. Characterization of three RXR genes that mediate the action of 9-cis retinoic acid. Genes and Development. 1992;6(3):329–344. doi: 10.1101/gad.6.3.329. [DOI] [PubMed] [Google Scholar]
- 12.Kizaki M, Ikeda Y, Tanosaki R, et al. Effects of novel retinoic acid compound, 9-cis-retinoic acid, on proliferation, differentiation, and expression of retinoic acid receptor-α and retinoid X receptor-α RNA by HL-60 cells. Blood. 1993;82(12):3592–3599. [PubMed] [Google Scholar]
- 13.Mangelsdorf DJ, Evans RM. The RXR heterodimers and orphan receptors. Cell. 1995;83(6):841–850. doi: 10.1016/0092-8674(95)90200-7. [DOI] [PubMed] [Google Scholar]
- 14.Manzano VM, Muñoz JCS, Jiménez JR, et al. Human renal mesangial cells are a target for the anti-inflammatory action of 9-cis retinoic acid. British Journal of Pharmacology. 2000;131(8):1673–1683. doi: 10.1038/sj.bjp.0703728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Diab A, Hussain RZ, Lovett-Racke AE, Chavis JA, Drew PD, Racke MK. Ligands for the peroxisome proliferator-activated receptor-γ and the retinoid X receptor exert additive anti-inflammatory effects on experimental autoimmune encephalomyelitis. Journal of Neuroimmunology. 2004;148(1-2):116–126. doi: 10.1016/j.jneuroim.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 16.Motomura K, Sakai H, Isobe H, Nawata H. Effects of retinoids on the production of tumour necrosis factor-α and nitric oxide by lipopolysaccharide stimulated rat Kupffer cells in vitro: evidence for participation of retinoid X receptor signalling pathway. Cell Biochemistry and Function. 1997;15(2):95–101. doi: 10.1002/(SICI)1099-0844(19970601)15:2<95::AID-CBF727>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 17.Benson S, Padmanabhan S, Kurtz TW, Pershadsingh HA. Ligands for the peroxisome proliferator-activated receptor-γ and the retinoid X receptor-α exert synergistic antiproliferative effects on human coronary artery smooth muscle cells. Molecular Cell Biology Research Communications. 2000;3(3):159–164. doi: 10.1006/mcbr.2000.0209. [DOI] [PubMed] [Google Scholar]
- 18.Cha BS, Ciaraldi TP, Carter L, et al. Peroxisome proliferator-activated receptor (PPAR) γ and retinoid X receptor (RXR) agonists have complementary effects on glucose and lipid metabolism in human skeletal muscle. Diabetologia. 2001;44(4):444–452. doi: 10.1007/s001250051642. [DOI] [PubMed] [Google Scholar]
- 19.Wan YJ, Badr MZ. Inhibition of carrageenan-induced cutaneous inflammation by PPAR agonists is dependent on hepatocyte-specific retinoid X receptor α . PPAR Research. 2006;2006:1–6. doi: 10.1155/PPAR/2006/96341. Article ID 96341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cuzzocrea S, Pisano B, Dugo L, et al. Rosiglitazone, a ligand of the peroxisome proliferator-activated receptor-γ, reduces acute inflammation. European Journal of Pharmacology. 2004;483(1):79–93. doi: 10.1016/j.ejphar.2003.10.056. [DOI] [PubMed] [Google Scholar]
- 21.Posadas I, Bucci M, Roviezzo F, et al. Carrageenan-induced mouse paw oedema is biphasic, age-weight dependent and displays differential nitric oxide cyclooxygenase-2 expression. British Journal of Pharmacology. 2004;142(2):331–338. doi: 10.1038/sj.bjp.0705650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ramos GC, Fernandes D, Charão CT, Souza DG, Teixeira MM, Assreuy J. Apoptotic mimicry: phosphatidylserine liposomes reduce inflammation through activation of peroxisome proliferator-activated receptors (PPARs) in vivo. British Journal of Pharmacology. 2007;151(6):844–850. doi: 10.1038/sj.bjp.0707302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.D'Agostino G, La Rana G, Russo R, et al. Acute intracerebroventricular administration of palmitoylethanolamide, an endogenous peroxisome proliferator-activated receptor-α agonist, modulates carrageenan-induced paw edema in mice. Journal of Pharmacology and Experimental Therapeutics. 2007;322(3):1137–1143. doi: 10.1124/jpet.107.123265. [DOI] [PubMed] [Google Scholar]
- 24.Takamatsu K, Takano A, Yakushiji N, et al. The first potent subtype-selective retinoid X receptor (RXR) agonist possessing a 3-isopropoxy-4-isopropylphenylamino moiety, NEt-3IP (RXRα/β-dual agonist) ChemMedChem. 2008;3(5):780–787. doi: 10.1002/cmdc.200700313. [DOI] [PubMed] [Google Scholar]
- 25.Morishita KI, Yakushiji N, Ohsawa F, et al. Replacing alkyl sulfonamide with aromatic sulfonamide in sulfonamide-type RXR agonists favors switch towards antagonist activity. Bioorganic and Medicinal Chemistry Letters. 2009;19(3):1001–1003. doi: 10.1016/j.bmcl.2008.11.086. [DOI] [PubMed] [Google Scholar]
- 26.Taylor BK, Dadia N, Yang CB, Krishnan S, Badr M. Peroxisome proliferator-activated receptor agonists inhibit inflammatory edema and hyperalgesia. Inflammation. 2002;26(3):121–127. doi: 10.1023/a:1015500531113. [DOI] [PubMed] [Google Scholar]
- 27.Youssef J, Badr M. Role of peroxisome proliferator-activated receptors in inflammation control. Journal of Biomedicine and Biotechnology. 2004;2004(3):156–166. doi: 10.1155/S1110724304308065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oliveira AC, Bertollo CM, Rocha LT, Nascimento EB, Costa KA, Coelho MM. Antinociceptive and antiedematogenic activities of fenofibrate, an agonist of PPAR α, and pioglitazone, an agonist of PPAR γ . European Journal of Pharmacology. 2007;561(1–3):194–201. doi: 10.1016/j.ejphar.2006.12.026. [DOI] [PubMed] [Google Scholar]
- 29.Wan YJ, Cai Y, Lungo W, et al. Peroxisome proliferator-activated receptor α-mediated pathways are altered in hepatocyte-specific retinoid x receptor α-deficient mice. Journal of Biological Chemistry. 2000;275(36):28285–28290. doi: 10.1074/jbc.M000934200. [DOI] [PubMed] [Google Scholar]
- 30.Na SY, Kang BY, Chung SW, et al. Retinoids inhibit interleukin-12 production in macrophages through physical associations of retinoid X receptor and NFκB. Journal of Biological Chemistry. 1999;274(12):7674–7680. doi: 10.1074/jbc.274.12.7674. [DOI] [PubMed] [Google Scholar]
- 31.Uchimura K, Nakamuta M, Enjoji M, et al. Activation of retinoic X receptor and peroxisome proliferator-activated receptor-γ inhibits nitric oxide and tumor necrosis factor-α production in rat Kupffer cells. Hepatology. 2001;33(1):91–99. doi: 10.1053/jhep.2001.21145. [DOI] [PubMed] [Google Scholar]
- 32.Shulman AI, Mangelsdorf DJ. Retinoid X receptor heterodimers in the metabolic syndrome. New England Journal of Medicine. 2005;353(6):604–615. doi: 10.1056/NEJMra043590. [DOI] [PubMed] [Google Scholar]
- 33.Chan LS, Wells RA. Cross-talk between PPARs and the partners of RXR: a molecular perspective. PPAR Research. 2009;2009 doi: 10.1155/2009/925309. Article ID 925309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shimizu M, Moriwaki H. Synergistic effects of PPARγ ligands and retinoids in cancer treatment. PPAR Research. 2008;2008 doi: 10.1155/2008/181047. Article ID 181047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shulman AI, Larson C, Mangelsdorf DJ, Ranganathan R. Structural determinants of allosteric ligand activation in RXR heterodimers. Cell. 2004;116(3):417–429. doi: 10.1016/s0092-8674(04)00119-9. [DOI] [PubMed] [Google Scholar]
- 36.Ijpenberg A, Tan NS, Gelman L, et al. In vivo activation of PPAR target genes by RXR homodimers. EMBO Journal. 2004;23(10):2083–2091. doi: 10.1038/sj.emboj.7600209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kersten S, Desvergne B, Wahli W. Roles of PPARS in health and disease. Nature. 2000;405(6785):421–424. doi: 10.1038/35013000. [DOI] [PubMed] [Google Scholar]
- 38.Wu Y, Guo SW. Peroxisome proliferator-activated receptor-γ and retinoid X receptor agonists synergistically suppress proliferation of immortalized endometrial stromal cells. Fertility and Sterility. 2009;91(5):2142–2147. doi: 10.1016/j.fertnstert.2008.04.012. [DOI] [PubMed] [Google Scholar]
- 39.Desreumaux P, Dubuquoy L, Nutten S, et al. Attenuation of colon inflammation through activators of the retinoid X receptor (RXR)/peroxisome proliferator-activated receptor γ (PPARγ) heterodimer. A basis for new therapeutic strategies. Journal of Experimental Medicine. 2001;193(7):827–838. doi: 10.1084/jem.193.7.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Burrage PS, Schmucker AC, Ren Y, Sporn MB, Brinckerhoff CE. Retinoid X receptor and peroxisome proliferator-activated receptor-γ agonists cooperate to inhibit matrix metalloproteinase gene expression. Arthritis Research and Therapy. 2008;10(6, article R139) doi: 10.1186/ar2564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang Y, Moser AH, Shigenaga JK, Grunfeld C, Feingold KR. Downregulation of liver X receptor-α in mouse kidney and HK-2 proximal tubular cells by LPS and cytokines. Journal of Lipid Research. 2005;46(11):2377–2387. doi: 10.1194/jlr.M500134-JLR200. [DOI] [PubMed] [Google Scholar]
- 42.Harada K, Isse K, Kamihira T, Shimoda S, Nakanuma Y. Th1 cytokine-induced downregulation of PPARγ in human biliary cells relates to cholangitis in primary biliary cirrhosis. Hepatology. 2005;41(6):1329–1338. doi: 10.1002/hep.20705. [DOI] [PubMed] [Google Scholar]
- 43.Beigneux AP, Moser AH, Shigenaga JK, Grunfeld C, Feingold KR. The acute phase response is associated with retinoid X receptor repression in rodent liver. Journal of Biological Chemistry. 2000;275(21):16390–16399. doi: 10.1074/jbc.M000953200. [DOI] [PubMed] [Google Scholar]
- 44.Wan YJ, An D, Cai Y, et al. Hepatocyte-specific mutation establishes retinoid X receptor α as a heterodimeric integrator of multiple physiological processes in the liver. Molecular and Cellular Biology. 2000;20(12):4436–4444. doi: 10.1128/mcb.20.12.4436-4444.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]


