Abstract
The rising cost of musculoskeletal pathology, disease, and injury creates a pressing need for accurate and reliable methods to quantify 3D musculoskeletal motion, fostering a renewed interest in this area over the past few years. To date, cine-phase contrast (PC) MRI remains the only technique capable of non-invasively tracking in vivo 3D musculoskeletal motion during volitional activity, but current scan times are long on the 1.5T MR platform (~2.5 minutes or 75 movement cycles). With the clinical availability of higher field strength magnets (3.0T) that have increased signal-to-noise ratios, it is likely that scan times can be reduced while improving accuracy. Therefore, the purpose of this study was to validate cine-PC MRI on a 3.0T platform, in terms of accuracy, precision and subject-repeatability, and to determine if scan time could be minimized. On the 3.0T platform it is possible to limit scan time to 2 minutes, with sub-millimeter accuracy (<0.33mm/0.97°), excellent technique precision (<0.18°), and strong subject-repeatability (<0.73 mm/1.10°). This represented a reduction in imaging time by 25% (42 seconds), a 50% improvement in accuracy, and a 72% improvement in technique precision over the original 1.5T platform. Scan time can be reduced to 1 minute (30 movement cycles), but the improvements in accuracy are not as large.
Introduction
The rising cost of musculoskeletal pathologies, disease, and injury creates a pressing need for accurate and reliable methods to quantify 3D musculoskeletal motion, fostering a renewed interest in this area over the past few years. Due to the prevalence of patellofemoral pain syndrome (Iwamoto et al., 2008; Boling et al., 2009), along with the inaccessibility of the patella to external measurement, the accuracy of tracking patellofemoral kinematics has been a focal point of these studies. Differences in validation methodologies can make comparisons between techniques difficult, for example, accuracy (Last, 1995) has been reported in two ways: 1) as a bias (average error) and precision (ASTM, 2010) or 2) as an average absolute or RMS error, which represents the combined effects of bias and precision. Although understanding subject-repeatability is critical for quantifying kinematic changes with time or intervention, few studies have evaluated this parameter.
When applied in the appropriate context, the available techniques for quantifying joint mechanics are capable of providing valuable insights into both healthy and pathological musculoskeletal function. To date, cine-phase contrast (PC) MRI remains the only technique capable of non-invasively tracking in vivo 3D musculoskeletal motion during volitional activity, but current scan times are long (~2.5 minutes or 75 movement cycles). With the clinical availability of higher field strength magnets (3.0T) that have increased signal-to-noise ratios, it is likely that scan times can be reduced while improving accuracy. Therefore, the purpose of this study was to validate cine-PC MRI on 3.0T platform (in terms of accuracy, precision, and subject-repeatability) and to determine if scan time could be minimized.
Methods
To define cine-PC MRI’s accuracy on the 3.0T platform (Philips Medical Systems, Best, NL), a motion phantom, similar to one previously used (Sheehan et al., 1998), was created. Briefly, the phantom was composed of a mechanical housing, gears, and connectors designed for manual cyclical rotation and displacement of a sample-box (63.8×40.5×35.3mm, Figure 1). The phantom was designed to mimic the key in vivo properties observed when tracking musculoskeletal motion. The sample-box contained a mixture of 1:10 of copper sulfate (CuSO4) in a 0.6% agarose gel solution, mimicking the T1-relaxation constant of bone at 3.0T. Four plastic rods were inserted through the box to create visually traceable MRI signal voids (fiducials), providing a secondary (visual) method for tracking motion. For imaging, sample-box movement was confined to the axial plane (as defined by the MRI’s fixed coordinate system) and standard two-element flexible coils were placed left-right and anterior-posterior to the phantom. A pole extended from the phantom, which a single researcher used to move the sample-box in a circuitous path at 30 cycles/min, guided by an auditory metronome.
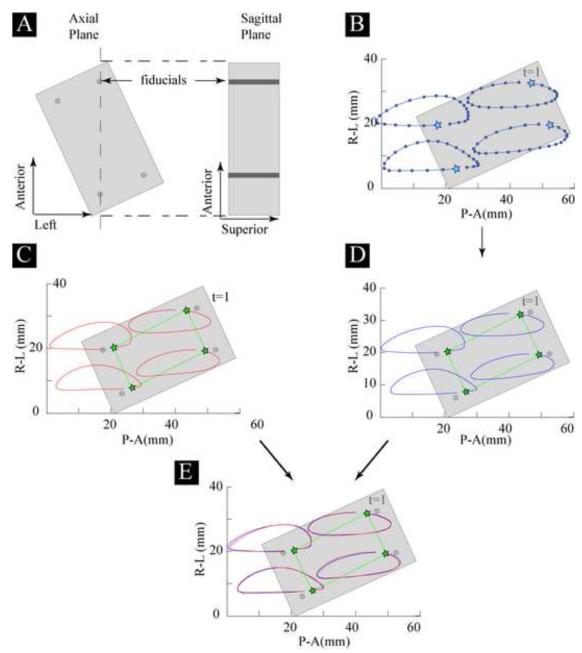
Figure 1.
A. A fixed coordinate system within the MRI was established using three anatomical directions (Left, Anterior, and Superior). The motion phantom was aligned with the MRI’s axial plane (Left-Anterior) and moved such that all motion was confined to this plane. Thus, when a sagittal imaging plane (Superior-Anterior) was used, large out of plane motions occurred B. Based on high resolution cine images, the location of the four fiducials were visually tracked throughout the motion cycle. C. Next, an ROI, defined by a series of vertices, was tracked through integration of the cine-PC MRI data. D. Then these same vertices were tracked based on the kinematics derived through the visual tracking in B. E. Finally, the trajectories of the vertices from the two tracking methods (integration of the cine-PC data and visualization) were compared. The paths within the figure were derived by plotting the visual and integration tracking data.
During movement, cine-PC images were acquired in the axial and sagittal planes (Figure 1) using two signal averages (2NEX) and no data averaging (scan times of 2.06 and 1.08 minutes, respectively). All other imaging parameters [TR(6.8msec), TE(3.4msec), spatial resolution (0.47mm2), slice thickness(10mm), flip angle(20°), temporal resolution(81.6msec), number of views(3)] remained constant. Large out-of-plane motion (18mm in the right-left direction) occurred when data were acquired in the sagittal plane. This motion was greater than expected from any in vivo study, as proper imaging alignment can limit out-of-plane movement (e.g. ~4.5° and ~5.1mm for the patella (Seisler and Sheehan, 2007)). Data were not acquired in the coronal plane, as the out-of-plane motion would have been unrealistically large (25mm in the anterior-posterior direction). Regions of interest (ROIs), similar in size to those used in analyzing in vivo patellar motion, were visually identified within the boundaries of the sample-box, on the first time frame of the temporal series of images. ROI 3D trajectories were then computed through integration of cine-PC velocity data (Zhu et al., 1996).
For comparison, high spatial and temporal resolution cine images (without velocity encoding) were acquired in the axial plane [TR(4.1msec), TE(1.2msec), spatial resolution(0.25mm2), temporal resolution(4.1 msec)]. The centroid of each fiducial was visually identified in each frame using ImageJ (NIH, Bethesda, MD). Accuracy was defined as the absolute difference in the ROI’s path calculated through integration and through visualization, averaged over the 24 time frames.
Twenty-six volunteers (13M/13F, age=24.9±5.1years, height=170.7±8.7cm, mass=72.3±20.7kg, 12/14 for technique precision/subject-repeatability) were recruited from the greater Washington, DC area. All gave informed consent upon entering this IRB approved study and had no MR contraindications or prior history of knee problems. Each volunteer was placed supine in the MR unit. Then a complete cine-PC MR image set (x,y,z velocity and anatomic images), using a sagittal-oblique imaging plane, was acquired during cyclic knee flexion/extension. Cine images were also acquired in three axial planes to establish anatomical coordinate systems (Seisler and Sheehan, 2007). To test subject-repeatability, the movement was repeated and the images were acquired for a second time. Kinematics of the femur, tibia, and patella were quantified via integration of the velocity data. The rotations were defined in terms of an XYZ-body fixed rotation sequence (Sheehan and Mitiguy, 1999). Subject-repeatability was defined as the grand mean of the standard deviation of the average kinematics across trials. Technique precision was calculated for the femur and tibia, as was done previously (Rebmann and Sheehan, 2003).
Results
Applying cine-PC on the 3.0T platform with nearly identical scanning parameters to the original validation on the 1.5T platform (Sheehan et al., 1998) reduced imaging time by 25% (42 seconds) and improved accuracy by 50% (Table 1). Technique precision improved on average by 72% (Table 2). By eliminating data averaging imaging time was reduced by 47%, requiring only 30 movement cycles, and accuracy remained improved (8%-58%) over the 1.5T platform.
Table 1. Accuracies for Cine-PC MRI.
The data for the 1.5T is derived from a previous publication (Sheehan et al., 1998) and the 3.0T was derived from the current experiment. Sagittal (out-of-plane-motion) indicate the tracking of motion with large (18mm) components of out-of-plane motion.
| Axial (in-plane motion) | Sagittal (out-of-plane-motion) | |||||
|---|---|---|---|---|---|---|
| LR mm |
AP mm |
ROT deg |
LR mm |
AP mm |
ROT deg |
|
| 1.5T | 0.62 ±0.55 | 0.57±0.43 | 0.85±0.52 | 0.34±0.23 | ||
| 3.0T (2 NEX) | 0.16±0.12 | 0.27±0.22 | 0.46±0.39 | 0.33±0.29 | 0.25±0.17 | 0.97±0.70 |
| 3.0T (1 NEX) | 0.57±0.50 | 0.38±0.31 | 0.42±0.24 | 0.36±0.29 | 0.14±0.10 | 1.20±0.76 |
Table 2.
Technique Precision
| Flexion (deg) | Tilt (deg) | Twist (deg) | |
|---|---|---|---|
| Femoral Rotations | 0.091 | 0.175 | 0.083 |
| Tibial Rotations | 0.151 | 0.176 | 0.093 |
| Left (mm) | Anterior (mm) | Superior (mm) | |
|
|
|||
| Femoral Translations | 0.048 | 0.026 | 0.026 |
| Tibial translations | 0.057 | 0.027 | 0.051 |
Subject-repeatability was better for tibiofemoral, as compared to patellofemoral kinematics, and both were slightly better than those previously published (Table 3).
Table 3. Subject-Repeatability.
The top table lists the current and past results for subject-repeatability (patellofemoral joint) in terms of the grand mean across subjects of the standard deviation (SD) of the kinematics across trials. n = number of subjects measures, LM, SI, and AP refer to lateral-medial, inferior-superior, and posterior-anterior displacement, respectively. Flex, tilt, varus, and int rot refer to extension-flexion, lateral-medial tilt, valgus-varus rotation, and external-internal rotation respectively. The SD of the grand mean is given in parentheses. The data from the Rebmann et al. (2003) was modified so that the results are presented as the grand mean of the SD across the two trials, instead of as the absolute average difference across two trials, as was originally reported. The bottom section reports the subject-repeatability for the tibiofemoral joint, comparing the 1.5T and the 3.0T platforms.
| Standard Deviation (Patellofemoral Joint) |
n | LM (mm) |
SI (mm) |
AP (mm) |
Flex (deg) |
Tilt (deg) |
Varus (deg) |
|---|---|---|---|---|---|---|---|
| Current (PF) | 14 | 0.41 (0.14) |
0.73 (0.31) |
0.31 (0.12) |
0.91 (0.41) |
1.10 (0.35) |
0.93 (0.47) |
| Fellows et al. 2005 | 3 | 0.42 (0.23) |
0.81 (0.37) |
0.32 (0.14) |
1.40 (0.29) |
1.04 (0.35) |
1.02 (0.40) |
| von Eisenhart-Roth et al. 2004 | 1 | 0.47 | 3.16 | 0.5 | |||
| Rebmann et al. (1.5T) 2003 | 7 | - | - | _ | 1.12 (0.96) |
1.67 (0.46) |
1.62 (0.64) |
| (Tibiofemoral Joint) | LM (mm) |
SI (mm) |
AP (mm) |
Flex (deg) |
Int Rot (deg) |
Varus (deg) |
|
|
| |||||||
| Current (TF) | 14 | 0.63 (0.35) |
0.48 (0.34) |
0.55 (0.27) |
0.31 (0.58) |
0.78 (0.49) |
0.64 (0.39) |
| Rebmann et al. (1.5T) 2003 | 7 | _ | _ | _ | 1.42 (0.78) |
1.00 (0.30) |
0.67 (0.33) |
Discussion
Given the variety of techniques available for quantifying in vivo joint motion, cine-PC MRI is ideal for this task due to its ability to simultaneously quantify 3D skeletal and muscular motion during dynamic volitional tasks. It is non-invasive, non-ionizing, and maintains the highest accuracy of all currently reported techniques (Table 4). On the 3.0T platform it is possible to limit scan time to 2 minutes, with sub-millimeter accuracy (<0.33mm/0.97°), excellent technique precision <0.18°), and strong subject-repeatability (<0.73 mm/1.10°). With a minor decrease in accuracy, scan time can be reduced to 1 minute. In addition, scanning parameters can be altered for different applications. For example, it is possible to scan at 10 cycle/min, dropping the number of required cycles to 10 (1NEX), and thus permitting the study of musculoskeletal function under much higher loads.
Table 4. Past Patellofemoral Accuracy Studies.
For a study to be included within the table, the validation of accuracy had to be performed for the patellofemoral joint or be directly applicable to the patellofemoral joint. It was assumed that in-plane motion referred to all motion confined to the sagittal plane and if all three directions of motion were reported, the worst error for the in-plane motion was listed in the tables. Asterisks along with italics denote studies that reported accuracies as an average error. All other studies reported accuracies as an absolute average error or a RMS error.
| Translation (mm) | Rotation (deg) | ||||
|---|---|---|---|---|---|
| In-plane | Out-of- plane |
In-plane | Out-of- plane |
||
| Cine-PC MRI | |||||
|
| |||||
| Current – 3.0T | 0.28(0.22) | 0.33(0.29) | 0.46(0.39) | 0.97(0.70) | |
| Previous – 1.5T | Rebmann et al. 2003 | 0.62(0.55) | 0.85(0.52) | ||
| Intracortical Pins | |||||
|
| |||||
| Lafortune et al. 1992 | 0.5 | ||||
| Fluoroscopy (w/ bone model) | |||||
|
| |||||
| Biplane | Bey et al. 2008 | 0.34 (0.16) | 0.40(0.16) | 0.88 (0.24) | 0.86 (0.16) |
| Single-Plane | Fregly et al.* 2005 | 1.1 (1.7) | 9.5 (16.0) | −0.10 (0.86) | −2.6 (2.8) |
| Ultrasound | |||||
|
| |||||
| Shih et al* 2004 | 0.6 (1.9) | ||||
| Motion Analysis | |||||
|
| |||||
| Magnetic Tracking | Wilson et al. 2009 | 1.04 | 0.77 | 1.12 | 0.97 |
| Magnetic Tracking | Laprade et al.* 2005 | 3 | |||
| MRI | |||||
|
| |||||
| 3D Model | Fellows et al. 2005 | 0.69(0.20) | 0.88(0.09) | 1.75(0.32) | 1.02(0.89) |
| Real Time | Draper et al. 2008 | 2.0 | |||
A potential limitation of this study was that the accuracy validation was not directly based on patellar tracking. This was considered appropriate because the accuracy of cine-PC MRI is independent of the shape of the object being tracked, as tracking is based on the integration of velocity data. This is unlike most other techniques (Bey et al., 2008; Fellows et al., 2005a) for which the size and shape of the object directly impacts accuracy due to a reliance on shape matching or visualization. Further, the phantom was designed to emulate in vivo conditions as closely as possible by mimicking the T1-relaxation properties of bone, including the imprecision of human movement, tracking small ROIs, and incorporating large out-of-plane movement. The accuracy currently reported is applicable to both tracking skeletal and muscle kinematics, as cine-PC MRI has been shown to have similar accuracies for both tasks (Sheehan et al., 1998; Drace and Pelc, 1994).
MRI, fluoroscopy, motion analysis, ultrasound, and CT are the primary categories of techniques available for measuring musculoskeletal motion. Static MRI quantifies static joint poses with excellent accuracy, precision, and subject-repeatability (Fellows et al., 2005b). Cine, real-time, and cine-PC MRI, by sharing the common trait of being cardiac-based techniques, can quantify joint dynamics during volitional tasks. The novelty of real-time MRI (Draper et al., 2008) holds potential in advancing the field, unfortunately its accuracy is not yet on par with other techniques. Further, its validation has not taken into account the errors associated with the imaging plane changing relative to the joint anatomy (Shibanuma et al., 2004). Fluoroscopy allows for full weight-bearing experiments, yet is unable to capture soft tissue, requires ionizing radiation, and the majority of systems can only capture extremely slow motions (Tashman, 2008). Ultrasound has similar advantages to MRI, but is limited in its ability to track bone motion. Static CT has excellent accuracy in defining joint pose (Moore et al., 2002), whereas dynamic CT has yet to report accuracy in quantifying joint motion (Kalia et al., 2009). Recently, near sub-millimeter accuracies have been demonstrated for tracking patellar motion with motion analysis (Wilson et al., 2008).
Consideration should be taken in respect to each technique’s method of validation. Numerous studies report patellofemoral kinematics, yet validation was done only for the tibiofemoral joint (Fernandez et al., 2008). Other studies did not validate complete 3D kinematics, instead opting to validate for a single degree of freedom (Draper et al., 2008; Laprade and Lee, 2005; Shih et al., 2004). Additionally, techniques validated with in vitro models often utilized an isolated pull from the quadriceps to create tibiofemoral flexion-extension, which likely oversimplified the complicated in vivo patellofemoral motion. Lastly, accuracy has frequently been reported in terms of bias (Fregly et al., 2005; Shih et al., 2004). Although a valid engineering approach (ASTM, 2010), it assumes imprecision can be removed from the final measurements through a recommended average of “30 or more tests” on the identical system. Such multiple measures for the human system can be a challenge to acquire. Therefore, reporting the average absolute or RMS error better represents the accuracy of tracking in vivo musculoskeletal motions.
In conclusion, this validation study of cine-PC MRI on the stronger, more homogeneous 3.0T platform demonstrated improvements in terms of accuracy, precision, and subject-repeatability. Since two different techniques produced very similar results for subject-repeatability (Table 3), it appears as if 0.73mm and 1.10° may be the limit of subject-repeatability for patellofemoral kinematics. Validation with other techniques is needed to confirm this. In comparing subject-repeatability and accuracy, it would appear that subject-repeatability is the rate-limiting step for tracking patellofemoral and tibiofemoral kinematics, yet few studies have quantified this parameter.
ACKNOWLEDGEMENT
This research was supported by the Intramural Research Program of the NIH, and the Clinical Center at the NIH. We thank Sara Sadeghi, Bonnie Damaska, and the Diagnostic Radiology Department at the National Institutes of Health for their support and research time. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of the National Institutes of Health or the US Public Health Service.
Footnotes
CONFLICT OF INTEREST STATEMENT All authors, but D.A Herzka report no potential conflicts. D.A. Herzka was employed during part of this work by Philips Research North America, a subsidiary of Royal Philips Electronics, the parent company of Philips Medical Systems, and the manufacturer of the MR equipment used in the work.
Reference List
- ASTM Standard Practice for Use of the Terms Precision adn Bias in ASTM Test Methods. American Society for Testing and Materials. 2010:E177–08. [Google Scholar]
- Banks SA, Hodge WA. Accurate measurement of three-dimensional knee replacement kinematics using single-plane fluoroscopy. IEEE Trans.Biomed.Eng. 1996;43:638–649. doi: 10.1109/10.495283. [DOI] [PubMed] [Google Scholar]
- Bey MJ, Kline SK, Tashman S, Zauel R. Accuracy of biplane x-ray imaging combined with model-based tracking for measuring in-vivo patellofemoral joint motion. J.Orthop.Surg.Res. 2008;3:38. doi: 10.1186/1749-799X-3-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boling M, Padua D, Marshall S, Guskiewicz K, Pyne S, Beutler A. Gender differences in the incidence and prevalence of patellofemoral pain syndrome. Scand.J.Med.Sci.Sports. 2009 doi: 10.1111/j.1600-0838.2009.00996.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drace JE, Pelc NJ. Tracking the motion of skeletal muscle with velocity-encoded MR imaging. J Magn Reson Imaging. 1994;4:773–8. doi: 10.1002/jmri.1880040606. [DOI] [PubMed] [Google Scholar]
- Draper CE, Santos JM, Kourtis LC, Besier TF, Fredericson M, Beaupre GS, Gold GE, Delp SL. Feasibility of using real-time MRI to measure joint kinematics in 1.5T and open-bore 0.5T systems. J.Magn Reson.Imaging. 2008;28:158–166. doi: 10.1002/jmri.21413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellows RA, Hill NA, Gill HS, Macintyre NJ, Harrison MM, Ellis RE, Wilson DR. Magnetic resonance imaging for in vivo assessment of three-dimensional patellar tracking. J.Biomech. 2005a;38:1643–1652. doi: 10.1016/j.jbiomech.2004.07.021. [DOI] [PubMed] [Google Scholar]
- Fellows RA, Hill NA, Macintyre NJ, Harrison MM, Ellis RE, Wilson DR. Repeatability of a novel technique for in vivo measurement of three-dimensional patellar tracking using magnetic resonance imaging. J.Magn Reson.Imaging. 2005b;22:145–153. doi: 10.1002/jmri.20360. [DOI] [PubMed] [Google Scholar]
- Fernandez JW, Akbarshahi M, Kim HJ, Pandy MG. Integrating modelling, motion capture and x-ray fluoroscopy to investigate patellofemoral function during dynamic activity. Comput.Methods Biomech.Biomed.Engin. 2008;11:41–53. doi: 10.1080/10255840701551046. [DOI] [PubMed] [Google Scholar]
- Fregly BJ, Rahman HA, Banks SA. Theoretical accuracy of model-based shape matching for measuring natural knee kinematics with single-plane fluoroscopy. J.Biomech.Eng. 2005;127:692–699. doi: 10.1115/1.1933949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwamoto J, Takeda T, Sato Y, Matsumoto H. Retrospective case evaluation of gender differences in sports injuries in a Japanese sports medicine clinic. Gend.Med. 2008;5:405–414. doi: 10.1016/j.genm.2008.10.002. [DOI] [PubMed] [Google Scholar]
- Kalia V, Obray RW, Filice R, Fayad LM, Murphy K, Carrino JA. Functional joint imaging using 256-MDCT: Technical feasibility. AJR Am.J.Roentgenol. 2009;192:W295–W299. doi: 10.2214/AJR.08.1793. [DOI] [PubMed] [Google Scholar]
- Laprade J, Lee R. Real-time measurement of patellofemoral kinematics in asymptomatic subjects. Knee. 2005;12:63–72. doi: 10.1016/j.knee.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Last JM. A Dictionary of Epidemiology. Oxford Univeristy Press; New York: 1995. [Google Scholar]
- Moore DC, Hogan KA, Crisco JJ, III, Akelman E, Dasilva MF, Weiss AP. Three-dimensional in vivo kinematics of the distal radioulnar joint in malunited distal radius fractures. J Hand Surg [Am] 2002;27:233–242. doi: 10.1053/jhsu.2002.31156. [DOI] [PubMed] [Google Scholar]
- Rebmann AJ, Sheehan FT. Precise 3D skeletal kinematics using fast phase contrast magnetic resonance imaging. J.Magn Reson.Imaging. 2003;17:206–213. doi: 10.1002/jmri.10253. [DOI] [PubMed] [Google Scholar]
- Seisler AR, Sheehan FT. Normative three-dimensional patellofemoral and tibiofemoral kinematics: a dynamic, in vivo study. IEEE Transactions on Biomedical Engineering. 2007;54 doi: 10.1109/TBME.2007.890735. [DOI] [PubMed] [Google Scholar]
- Sheehan FT, Mitiguy P. In regards to the “ISB recommendations for standardization in the reporting of kinematic data”. J.Biomech. 1999;32:1135–6. doi: 10.1016/s0021-9290(99)00077-9. [DOI] [PubMed] [Google Scholar]
- Sheehan FT, Zajac FE, Drace JE. Using cine phase contrast magnetic resonance imaging to non-invasively study in vivo knee dynamics. J Biomech. 1998;31:21–26. doi: 10.1016/s0021-9290(97)00109-7. [DOI] [PubMed] [Google Scholar]
- Shibanuma N, Sheehan FT, Lipsky P, Stanhope SJ. Sensitivity of Femoral Orientation Estimates to Condylar Surface and MR Image Plane Location. J.Magn Reson.Imaging. 2004 doi: 10.1002/jmri.20106. in press. [DOI] [PubMed] [Google Scholar]
- Shih YF, Bull AM, McGregor AH, Amis AA. Active patellar tracking measurement: a novel device using ultrasound. Am.J.Sports Med. 2004;32:1209–1217. doi: 10.1177/0363546503262693. [DOI] [PubMed] [Google Scholar]
- Tashman S. Comments on “validation of a non-invasive fluoroscopic imaging technique for the measurement of dynamic knee joint motion”. J.Biomech. 2008;41:3290–3291. doi: 10.1016/j.jbiomech.2008.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Eisenhart-Rothe R, Siebert M, Bringmann C, Vogl T, Englmeier KH, Graichen H. A new in vivo technique for determination of 3D kinematics and contact areas of the patello-femoral and tibio-femoral joint. J.Biomech. 2004;37:927–934. doi: 10.1016/j.jbiomech.2003.09.034. [DOI] [PubMed] [Google Scholar]
- Wilson NA, Press JM, Koh JL, Hendrix RW, Zhang LQ. In Vivo and Noninvasive Evaluation of Abnormal Patellar Tracking During Squatting in Patellofemoral Pain. 2008. [DOI] [PMC free article] [PubMed]
- Zhu Y, Drangova M, Pelc NJ. Fourier tracking of myocardial motion using cine-PC data. Magn.Reson.Med. 1996;35:471–80. doi: 10.1002/mrm.1910350405. [DOI] [PubMed] [Google Scholar]