Abstract
Despite the important role of cannabinoid CB1 receptors (CB1R) in brain development, little is known about their status during adolescence, a critical period for both the development of psychosis and for initiation to substance abuse. In the present study, we assessed the ontogeny of CB1R in adolescent and adult rats in vivo using positron emission tomography with [18F]MK-9470. Analysis of covariance (ANCOVA) to control for body weight that would potentially influence [18F]MK-9470 values between the two groups revealed a main effect of age (F(1,109)=5.0, P = 0.02) on [18F]MK-9470 absolute binding (calculated as percentage of injected dose) with adult estimated marginal means being higher compared to adolescents amongst 11 brain regions. This finding was confirmed using in vitro autoradiography with [3H]CP55,940 (F(10,99)=140.1, P < 0.0001). This ontogenetic pattern, suggesting increase of CB1R during the transition from adolescence to adulthood, is the opposite of most other neuroreceptor systems undergoing pruning during this period.
1. Introduction
The endocannabinoid system is a lipid signalling system [1] that appeared early in evolution [2]. It consists of at least two G-protein-coupled cannabinoid receptors CB1 and CB2 (CB1R and CB2R) [3], their intrinsic ligands (endocannabinoids) such as N-arachidonoyl ethanolamine (anandamide, AEA) [4] and 2-arachidonoyl glycerol (2-AG) [5], and their associated proteins involved in synthesis, transport, and degradation [6].
The CB1R, which mediates the psychoactive effects of marijuana, is widely expressed and is considered one of the most abundant G-protein-coupled receptors in the brain. In the central nervous system, endocannabinoids are released from postsynaptic sites and, by activation of the presynaptically located CB1R [7], inhibit the release of several neurotransmitters such as GABA, glutamate [8], dopamine, and acetylcholine [9]. In vitro immunohistochemical [10] and autoradiography [11] studies in rats have shown that the CB1R is highly expressed in the basal ganglia (lateral caudate-putamen, globus pallidus, entopeduncular nucleus, and substantia nigra pars reticulata), cerebellum (molecular layer), and hippocampus (CA1, CA3, and dentate gyrus molecular layer). Moderate levels are found throughout the cortical regions, whereas low levels are observed in the brainstem (midbrain, pons) and spinal cord.
The CB1R has been shown to be involved in various physiological functions like nociception [12], control of movement [13], memory [14], neuroendocrine regulation [15], brain development, and maturation [16, 17]. Biochemical and functional alterations of CB1R have been shown to be implicated in the pathophysiology of distinct neurological and psychiatric disorders [18] including schizophrenia [19–21]. It is known that cannabis and its derivatives can trigger psychotic-like symptoms in normal individuals [22], and numerous epidemiological studies have demonstrated that consuming cannabis during adolescence (particularly early adolescence) constitutes a risk factor for schizophrenia onset later in life [23–25].
Adolescence is a critical developmental period during the transition from childhood to adulthood. The ages associated with adolescence are commonly considered in humans to be approximately 12 to 20–25 years of age and postnatal day (PND) 28–55 in rodents [26]. The adolescent brain undergoes both progressive and regressive changes providing the biological basis for the unique adolescent behaviors and their associated changes during maturation to adulthood. At the cellular level, these changes correspond to the marked overproduction of axon and synapses in early puberty and rapid pruning in late adolescence [27]. To date, most developmental studies of the cannabinoid system [28–31] have focused on the embryonic and early postnatal stages. In vitro autoradiographic studies have reported a fivefold increase in CB1R density in the brain during postnatal development [32]. CB1R capacity in the striatum was doubled between PND 14 and 21. Significant increases in CB1R density appeared regionally in the developing brain until PND 21 [32] or PND 30 [33], and the maximum adult level was reached at PND 60 [32]. In contrast, Rodriguez de Fonseca et al. [33] reported slight decreases in binding between PND 30 and 40 and adulthood (PND 70).
Recently, the development of new efficient radiotracers has enabled the study of CB1R in vivo using positron emission tomography (PET). Burns et al. [34] demonstrated that the selective, high-affinity inverse agonist for the CB1R, named [18F]MK-9470 had the potential to be a valuable tool for the in vivo study of CB1R biology and pharmacology. Several in vivo preclinical [35–41] and clinical studies [42–45] have used this compound successfully.
We have recently reported higher levels of dopamine D1 and D2 receptors [46], both serotonin 5HT1A receptor binding and mRNA expression [47], and GABAA receptor binding [48] in adolescent rats (PND 39) compared to adults (PND 70), that is, in accordance with the regressive elimination of synapses and receptors that occurs during the transition from adolescence to adulthood [27]. In the present study, we have undertaken two objectives: first, to demonstrate the feasibility of imaging CB1R in vivo in adolescence and adulthood using small animal PET with [18F]MK-9470; second, to compare the level of expression/regional distribution of CB1R in adolescent and adult rats obtained in vivo with PET and in vitro with autoradiography using [3H]CP55,940. The aim was to test the hypothesis whether CB1R pruning occurs during the transition from adolescence to adulthood as it has been indicated for other neuroreceptor systems.
2. Materials and Methods
2.1. Radiochemical Synthesis of [18F]MK-9470
CB1R imaging was performed in all animals using the radioligand [18F]MK-9470 (N-[2-(3-cyanophenyl)-3-(4-(2-[18F]fluoroethoxy)phenyl)-1-methylpropyl]-2-(5-methyl-2-pyridyloxy)-2-methylpropanamide), a high specificity, high-affinity inverse agonist at the CB1R. The precursor for radiotracer synthesis and the authentic [19F]MK-9470 standard were obtained from MERCK Research labs (West Point, Pa, USA). Radiolabelling was performed using a two-step semiautomated procedure following the method outlined by Burns et al. [34] with some modifications. In the first step, 2-Bromo-1[18F]fluoroethane ([18F]BrFE) was synthesised using a Nuclear Interface FDG synthesizer (GE Medical System). 18F-Fluoroalkylation of the MK-9470 precursor was then manually carried out using Cs2CO3 as a base. An aliquot of [18F]BrFE was added, and [18F]MK-9470 was obtained in up to 8% overall yield (not corrected for decay) after high-performance liquid chromatography (HPLC) and Sep-Pak purification. [18F]MK-9470 product was confirmed by coinjection with the [19F]MK-9470 standard. The final product obtained had a radiochemical purity > 95% and specific activity averaging 6000 Ci/mmole (222 GBq/μmole).
2.2. Animals
Male Wistar rats were obtained from the Animal Resource Centre Pty. Ltd (Perth, Australia) and were housed in polyethylene boxes with wire lids (489 × 343 × 240 mm) in groups of two-three per cage. All handling of animals and procedures was carried out in accordance with the guidelines established by the Animal Care and Ethics Committee at the Australian Nuclear Science and Technology Organisation (ANSTO). The animals were kept at a constant temperature of 22 ± 2°C on a 12–12 h light-dark cycle with lights on at 9 am and were handled during the seven days preceding the experiment. Food and water were freely available.
The adult cohort consisted of 6 rats with body weights ranging between 381 ± 22 g at 10 weeks of age (PND 70–72), and the adolescent cohort consisted of 6 rats with body weights ranging between 148 ± 22 g at 7 weeks of age (PND 35–37).
2.3. In Vivo PET/CT with [18F]MK-9470
2.3.1. Acquisition and Reconstruction
Animals were fasted for at least 6 hours before the start of the experiment. PET imaging with [18F]MK-9470 was performed with a preclinical PET/CT Inveon (Siemens) system [49]. Anaesthesia was induced by exposing rats to 4% isoflurane in oxygen and then maintained by reducing the ratio to 1.5–2.5% for the duration of the studies. Isoflurane anesthesia has been shown not to have any significant effects on absolute [18F]MK-9470 binding as compared to control conditions [36]. The eyes were coated with a lubricating eye ointment (Allergan Inc., Ireland). Body temperature was maintained by a heating pad set at 38°C and monitored rectally. Heart rate (333.2 ± 25.9 beats/min), respiratory rate (41.6 ± 9.2 cycles/min), and saturation in oxygen (>95%) were measured with a pulse oximeter (Starr, Life Sciences Corp, USA). We also monitored the respiratory rate under the CT part of the scanner with a pressure sensor connected to a computer (Biovet, m2m imaging crop, USA). After anaesthesia and placing of the animal in the scanner with the help of laser guidance, a catheter was placed in a lateral tail vein of the rat and connected to an infusion pump (Harvard Apparatus, USA). A 60 min PET scan was started at the same time of the start of the one-minute injection of [18F]MK-9470 at a constant tracer mass (65.2 ± 1.5 pmoles). A 15 min CT scan was systematically performed after the PET scan. Activity volumes were reconstructed with an iterative reconstruction (OSEM/MAP) [50] including attenuation and scatter correction, achieving a reconstructed spatial resolution of 1.5 mm.
2.3.2. Data Analysis
A previously developed magnetic-resonance-imaging- (MRI-)based rat brain atlas was coregistered to the PET volume, using the CT information of the skull (Anatomist/BrainVisa, V3.1.4, http://brainvisa.info/). In detail, all PET acquisitions (12 animals) were coregistered with their respective CT (see Figure 1). All CTs in the adolescent cohort were manually/visually coregistered to one adolescent CT (adolescence reference CT). The same methodology was used in the adult group. Finally, the “reference” CTs were manually/visually coregistered to the MRI-based rat brain atlas encompassing eleven volumes of interest (VOI) (Figure 2(a)). Transformation matrixes were then created from the MRI-based rat brain atlas to each PET image in each group.
Figure 1.
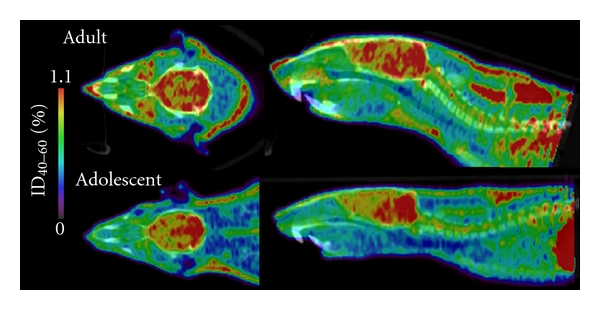
Typical in vivo PET/CT scan images of an adolescent (PND 35–37) and an adult (PND 70–72) Wistar rat in transversal (left) and sagittal (right) planes. For illustration purpose, the absolute binding intensity of [18F]MK-9470 to CB1R (%ID40–60) was increased (Anatomist/BrainVisa, V3.1.4, http://brainvisa.info/) in order to reflect the results expressed in estimated marginal means of %ID40–60, that is, a higher CB1R absolute binding in adults compared to adolescents.
Figure 2.
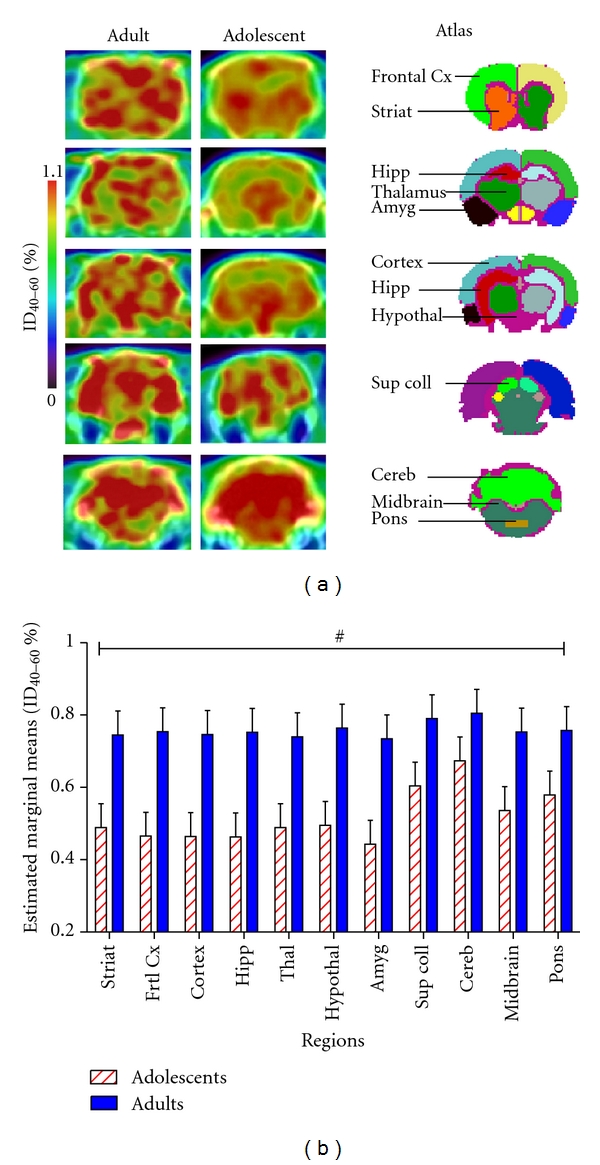
(a) In vivo PET/CT images of [18F]MK-9470 binding (%ID40–60 ± SEM) at 5 different coronal levels in the adolescent and the adult rat brain. The MRI-based atlas of the rat brain with 11 VOI is shown on the right side of the image. (b) Histograms presenting the adjusted absolute [18F]MK-9470 binding intensities (estimated marginal means of %ID40–60 ± SEM) in the adolescent compared to the adult cohort in 11 VOI. Two-way ANCOVA (age × region) controlling for weight was used to assess statistical significant differences in absolute [18F]MK-9470 binding between adulthood and adolescence. Statistical analysis revealed that there was a significant (# P < 0.05 significant main effect) increase in CB1R absolute binding (44.4% calculated over 11 VOI) in adulthood compared to adolescence. Abbreviations: Striat: striatum; Frtl Cx: frontal cortex; Hipp: hippocampus; Thal: thalamus; Hypothal: hypothalamus; Amyg: amygdala; Sup Coll: superior colliculus; Cereb: cerebellum.
Previous studies in rats with [18F]MK-9470 have used the last 20 min of a 60 min acquisition period (40 to 60 min) for quantification purposes [35–37]. In this study, we used percentage of injected dose (activity concentration (MBq/mL) divided by injected dose (MBq)) of the last 20 minutes of acquisition (%ID40–60) as absolute CB1R binding measure.
2.4. In Vitro Autoradiography with [3H]CP55,940
2.4.1. Experiments
Twenty-four hours after in vivo imaging, the animals (6 adolescents and 5 adults) were euthanized, their brain was dissected, frozen in liquid nitrogen, and stored at −80°C. Coronal brain sections (16 μm) were cut with a cryostat and thaw-mounted onto microscope slides.
[3H]CP55,940 autoradiography was carried out based on the method previously described in Dalton et al. [51]. All sections were processed simultaneously to minimize experimental variance. On the day of the experiment, sections were taken out of the −80°C freezer and allowed to come to room temperature for approximately 60 min or until dry. Sections were preincubated for 30 min at room temperature in 50 mM Tris-HCl (pH 7.4) containing 5% bovine serum albumin (BSA) in order to equilibrate the tissue to the assay conditions and remove any endogenous ligand. Radioligand binding was measured using single-point saturation analysis which provides a good estimate of receptor density. The Kd of rat brain CB1R has been evaluated at 5.2 nM [11]. In order to ensure saturation of CB1R, sections were then incubated for 2 h at room temperature in the same buffer as preincubation with the addition of 10 nM [3H]CP55,940 (specific activity 139.6 Ci/mmole, Perkin Elmer, USA). Nonspecific binding was determined by incubating adjacent sections in the presence of 10 μM CP55,940. The concentration of [3H]CP55,940 was measured in 10 μL aliquots taken from the incubation mixture. After the incubation, sections were washed for 1 h at 4°C in 50 mM Tris-HCl (pH 7.4) containing 1% BSA, and a second wash was then carried out for 3 h in the same buffer at 4°C. The third wash was in 50 mM Tris-HCl (pH 7.4) for 5 min at 4°C. Sections were then dipped briefly in ice cold distilled water and then dried. Dried sections were apposed to Kodak Biomax MR films, together with autoradiographic tritium standards ([3H] microscales from Amersham), in X-ray film cassettes. Films were developed after 35 days using Kodak GBX developer and fixed with Kodak GBX fixer.
2.4.2. Data Analysis
Films were analysed using a computer-assisted image analysis system, Multianalyst, connected to a GS-690 Imaging Densitometer (Bio-Rad, USA). Eleven brain regions of interest (ROI) were manually drawn with the help of a stereotaxic atlas of the rat brain [52] and corresponded to the 11 VOI analysed in vivo (Figure 4). Quantification of receptor binding in each brain region was performed by measuring the average optical density in adjacent brain sections. Nonspecific binding was subtracted to total binding to give a value for specific binding. Optical density measurements for specific binding were then converted into fmoles of [3H]CP55,940 per mg of tissue equivalent (fmol/mg TE) according to the calibration curve obtained from the [3H]-labelled standards.
Figure 4.
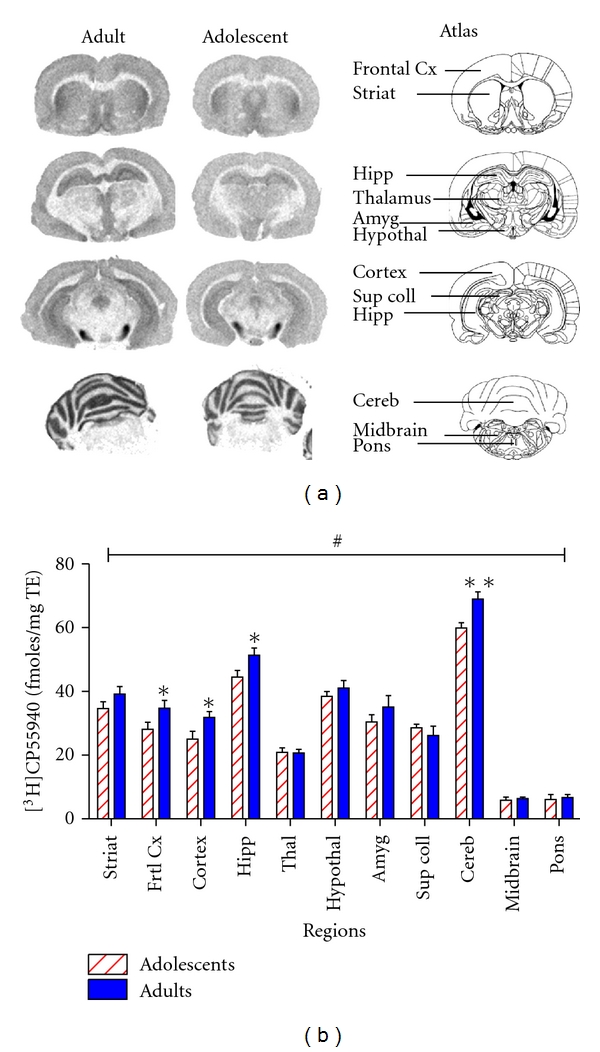
(a) In vitro autoradiographies of total [18F]MK-9470 binding intensity in coronal sections in an adolescent and an adult rat brain. The atlas of Paxinos and Watson [52] serves as a visual anatomical reference of the 11 brain regions analysed. (b) Histograms of the in vitro specific binding intensities of [18F]MK-9470 (fmoles/mg TE ± SEM) in the adolescent compared to the adult rat brain. Eleven regions of interest were analysed and assessed for statistical significant difference between adolescence and adulthood with two-way ANOVA (age × region) followed by LSD post hoc tests (*P < 0.05; **P < 0.01). Statistical analysis revealed a significant main effect (# P < 0.05) of age with adults having higher CB1R densities than adolescents. The frontal cortex, the cortex, the hippocampus, and the cerebellum showed a statistically significant increase in adults compared to adolescence in the post hoc analysis. Abbreviations: Striat: striatum; Frtl Cx: frontal cortex; Hipp: hippocampus; Thal: thalamus; Hypothal: hypothalamus; Amyg: amygdala; Sup Coll: superior colliculus; Cereb: cerebellum.
2.5. Statistical Analysis
Statistical tests were performed using PASW Statistics (Version 18.0.0) and Graphpad Prism (Version 5.04). Data were analysed for significant outliers (±2 SD), and none were detected. The Kolmogorov-Smirnov test was used to test normality of the data. Parametric tests were used in subsequent analysis since data were normally distributed. The mass and injected dose of [18F]MK-9470 between the adolescent and adult cohorts were compared using unpaired Student's t-tests. Pearson correlations were used to examine the relationship between %ID40–60 and body weight and between [18F]MK-9470 CB1R binding in vivo and [3H]CP55,940 CB1R binding in vitro. Analysis of covariance (ANCOVA) controlling for body weight was used to determine if there was an effect of age and/or region on CB1R absolute binding measured in vivo. In vitro data were analysed using two-way ANOVA (age × region) followed by least significant difference (LSD) tests. Significance was set at P ≤ 0.05.
3. Results
3.1. In Vivo PET with [18F]MK-9470
Adolescent rats showed the regional distribution that corresponds to the previously published regional distribution of CB1R [11, 53], but adult rats unexpectedly demonstrated a more uniform regional distribution of the PET radioligand (Figures 1 and 2). Cerebellum, striatum, cortical regions, and (moderately) hippocampus showed higher in vivo CB1R absolute binding compared to other brain regions. Regions known to have fewer CB1R like the thalamus and especially the brainstem (midbrain, pons) presented relatively high CB1R absolute binding in vivo (Figure 2).
Time-activity curves (expressed in %ID40–60) showed that [18F]MK-9470 entered the brain with a slow kinetic and reached a peak at approximately 20 min after-injection.
There were no statistically significant differences in the mass of [18F]MK-9470 injected between the adolescent and adult cohort (mean ± SEM: 64.8 ± 1.5 pM and 66.2 ± 2.9 pM, resp., t(10) = 0.73, P = 0.48). No statistical differences were found in the injected doses (ID) (t(10) = 0.56, P = 0.59) between the adolescents (8.22 ± 2.07 MBq) and the adults (7.02 ± 0.59 MBq). Animal weights were found to be significantly different (t(10) = 18.16, P < 0.0001) between adolescent (148 ± 9 g) and adult animals (382 ± 9 g), and Pearson's correlation showed that weight was strongly and negatively correlated to %ID40–60 (r = −0.921, P < 0.0001). Two-way ANCOVA (age × region) controlling for weight showed a significant main effect of age (F(1,109) = 4.95, P = 0.028) with adults having higher CB1R absolute binding compared to adolescents (+44.4% over 11 VOI) (Figure 2(b)). A significant effect of region was also found (F(10,109) = 2.41, P = 0.012). No interaction was observed between age and region (F(10,109) = 0.84, P = 0.59). Table 1 presents CB1R absolute binding levels in adolescents and adults before (unadjusted values) and after controlling for animal body weight (adjusted values).
Table 1.
CB1 receptor in vivo binding levels ([18F]MK-9470) in adolescents and adults rats.
| Adolescents | Adults | % change (adjusted) | |||
|---|---|---|---|---|---|
| Unadjusted | Adjusted | Unadjusted | Adjusted | ||
| Striatum | 0.86 ± 0.07 | 0.49 ± 0.07 | 0.38 ± 0.03 | 0.74 ± 0.07 | 50.3 |
| Frontal cortex | 0.83 ± 0.06 | 0.47 ± 0.07 | 0.38 ± 0.03 | 0.75 ± 0.07 | 59.8 |
| Cortex | 0.83 ± 0.07 | 0.47 ± 0.07 | 0.38 ± 0.03 | 0.74 ± 0.07 | 58.5 |
| Hippocampus | 0.83 ± 0.06 | 0.47 ± 0.07 | 0.38 ± 0.03 | 0.75 ± 0.07 | 59.9 |
| Thalamus | 0.86 ± 0.06 | 0.49 ± 0.07 | 0.37 ± 0.03 | 0.74 ± 0.07 | 49.1 |
| Hypothalamus | 0.86 ± 0.06 | 0.50 ± 0.07 | 0.40 ± 0.03 | 0.76 ± 0.07 | 52.0 |
| Amygdala | 0.81 ± 0.06 | 0.45 ± 0.07 | 0.36 ± 0.03 | 0.73 ± 0.07 | 63.1 |
| Superior colliculus | 0.97 ± 0.07 | 0.61 ± 0.07 | 0.42 ± 0.03 | 0.79 ± 0.07 | 29.2 |
| Cerebellum | 1.04 ± 0.09 | 0.68 ± 0.07 | 0.44 ± 0.03 | 0.80 ± 0.07 | 18.2 |
| Midbrain | 0.90 ± 0.06 | 0.54 ± 0.07 | 0.38 ± 0.02 | 0.75 ± 0.07 | 38.6 |
| pons | 0.95 ± 0.07 | 0.58 ± 0.07 | 0.39 ± 0.03 | 0.75 ± 0.07 | 29.0 |
Unadjusted values are mean %ID40–60 ± SEM; adjusted values are estimated marginal means %ID40–60 ± SEM.
Two-way ANCOVA controlling for weight was performed (n = 6 per group).
Covariates appearing in the ANCOVA model are evaluated at the following values: weight = 264.9142.
3.2. In Vitro [3H]CP55,940 Autoradiography
Two-way ANOVA (age × region) showed a statistically significant main effect of age (F(1,99) = 17.323, P < 0.0001) with the adults having higher CB1R binding than the adolescents (Figure 4). A significant main effect of region (F(10,99) = 140.1, P < 0.0001) was also found. No interaction between age and region (F(10,99) = 1.62, P = 0.113) was observed. The significant main effect of age was further analysed by LSD post hoc tests revealing that CB1R-specific binding was significantly higher in the adults compared to adolescents in the frontal cortex (+23.4%; P = 0.024), the cortex (+27.1%; P = 0.020), the hippocampus (+15.4%; 0.018), and the cerebellum (+15.2%, P = 0.002) (Table 2 and Figure 4).
Table 2.
CB1 receptor in vitro binding levels ([3H]CP55,940) in adolescents and adults rats.
| Adolescents | Adults | % change | P value | |
|---|---|---|---|---|
| Striatum | 34.62 ± 2.18 | 39.13 ± 2.38 | 13.0 | 0.117 |
| Frontal cortex | 28.12 ± 2.21 | 34.68 ± 2.51 | 23.4 | 0.024 |
| Cortex | 25.02 ± 2.49 | 31.81 ± 1.86 | 27.1 | 0.020 |
| Hippocampus | 44.51 ± 2.03 | 51.36 ± 2.26 | 15.4 | 0.018 |
| Thalamus | 20.90 ± 1.41 | 20.70 ± 1.16 | −0.9 | 0.946 |
| Hypothalamus | 38.46 ± 1.53 | 41.01 ± 2.40 | 6.6 | 0.376 |
| Amygdala | 30.41 ± 2.29 | 35.09 ± 3.62 | 15.4 | 0.106 |
| Superior colliculus | 28.58 ± 1.12 | 26.16 ± 2.93 | −8.5 | 0.400 |
| Cerebellum | 59.89 ± 1.66 | 68.98 ± 2.23 | 15.2 | 0.002 |
| Midbrain | 5.86 ± 0.96 | 6.35 ± 0.51 | 8.4 | 0.864 |
| Pons | 6.12 ± 1.50 | 6.70 ± 0.92 | 9.5 | 0.840 |
Two-way ANOVA followed by LSD post hoc test.
Data expressed as mean fmol/mg TE ± SEM; n = 6 per group.
3.3. Correlation between [18F]MK-9470 CB1R Binding In Vivo and [3H]CP55,940 CB1R Binding In Vitro
Correlations were not statistically significant between absolute CB1R binding evaluated with [18F]MK-9470 in vivo and specific CB1R binding calculated with [3H]CP55,940 in vitro, (r = 0.1816, P = 0.41).
4. Discussion
In the present study, we used two complementary techniques to examine potential developmental differences in CB1R binding in the brain of adolescent and adult rats. After controlling for body weight, CB1R absolute binding measured in vivo with PET and [18F]MK-9470 was significantly higher in the adult animals compared to adolescents over 11 brain regions. This finding was confirmed in vitro with autoradiography and [3H]CP55,940.
Noteworthy, the percentage of increase observed in the adult compared to the adolescent cohort with the 2 complementary techniques was not of the same magnitude (44% in vivo versus 11% in vitro over the 11 regions of interest), and no significant correlation was found between the data obtained with the two techniques. The comparison between in vitro and in vivo results (no correlation) and the apparent discrepancies may relate to a number of factors.
Firstly, methodological issues of data analysis should be considered. The percentage of injected dose (% ID) that we used here gives an absolute index of binding in vivo. This means that it reflects specific and nonspecific binding in the brain, radioligand present in the brain blood circulation, and possible radioactive metabolites crossing the blood-brain barrier. We did not calculate standardised uptake values (absolute index normalising for weight) because it would have biased our results as our two groups had significantly different weight means. Also, the absence of a brain region devoid of CB1R prevented us from implementing a simplified reference tissue model. We chose to use an atlas-based analysis of our data, with predefined VOI, over a statistical parametric mapping approach because we wanted to compare the same regions with the in vivo and in vitro methodologies. To our knowledge, the metabolism of [18F]MK-9470 in the male (adult and adolescent) rat brain has not been assessed; therefore, the presence of active metabolites that cross the blood-brain barrier cannot be ruled out. Indeed, a metabolite is likely to cross the blood-brain barrier of adult female Wistar rats (Casteels et al., oral communication). Radiometabolites produced in adults (but not in adolescents) could potentially cross the blood brain barrier, affect the %ID we calculated, and in turn contribute to the uniform regional distribution of the PET radioligand we observed in adults compared to adolescents (Figures 1 and 2). Differences in radioligand present in the brain blood circulation (e.g., difference in blood flow) between the adolescent and adult cohorts that would have affected our measures cannot be ruled out either. To be in line with previous studies in rats with [18F]MK-9470 [35–37], we have used the last 20 min of a 60 min acquisition period (40 to 60 min) for quantification purposes. A recent study however indicated that the distribution volume (V T) of [18F]MK-9470 as quantitative outcome evaluated by full kinetic modelling was reasonably correlated with standardised uptake values between 60 and 80 min (Casteels et al., oral communication, 2011). Longer acquisitions periods (at least 80 min) in future studies using this radioligand would ensure that equilibrium is reached.
The second factor that could explain discrepancies between in vitro and in vivo results is the drug phenotype. Indeed, [18F]MK-9470 is an inverse agonist at CB1R [34], whereas [3H]CP55,940 is an agonist at both CB1R and CB2R [54, 55]. The concentration of CB2R in the rat brain is supposed to be small in comparison to CB1R [3, 56, 57]. Thus, [3H]CP55,940 binding in the brain will mainly reflect CB1R. Inverse agonists will preferentially bind to receptors uncoupled from their G-protein, whereas agonists will preferentially bind to receptors that are coupled to their G-protein [58]. This means that in vivo we would have preferentially bound CB1R uncoupled to their G-protein, whereas in vitro the G-protein-coupled ones would have been targeted. In vitro assays typically reflect all receptors that are available to bind to radioligand, whereas in vivo, only a subset of these receptors are available to bind to radioligand since some may be compartmentalised, some in a low affinity state and some occupied by endogenous ligand [59].
Finally, another factor affecting the comparison between in vitro and in vivo measures is the difference in concentration of the radioligand used. Theory of PET experiment is based upon the injection of a radioligand at tracer concentration that is not supposed to trigger any biological effect. In order to meet this requirement, the radioligand should not bind to more than 5–10% of the total receptors concentration (B max) [59]. Based on previously reported B max in rat brain (0.5–1.1 pmol/mg prot) [11], we calculated that the mass of ligand needed to be approximately 0.1–0.7 nmoles. On the other hand, quantitative in vitro autoradiography studies need saturation of the available binding sites (at least 3 times greater than the K d). Thus, by saturating a different proportion of receptors in vivo and in vitro, differential outcomes must be cautiously interpreted.
Our main results, showing an increase in CB1R in adults (PND 70–72) compared to adolescents (PND 35–37) in vivo and in vitro, are in accordance with in vitro studies that have looked at CB1R expression over time in development. Belue and collaborators [32] have found significant regional increases in the numbers of CB1R (B max) in the developing rat brain (PND 0, 7, 14, 21, and 60) using in vitro autoradiography with [3H]CP55,940. Although CB1R density was not measured during adolescence, this study suggested that CB1R binding continuously increased until the maximum adult level was reached at PND 60. They observed that cortical regions (mainly posterior cortex) and hippocampus showed a statistically significant increase in binding between PND 21 and PND 60 [32]. According to the authors, the increase in CB1R could be an indication of either an increased differentiation of neurones into cells harbouring CB1R or an induction of the expression of CB1R in cells already differentiated. Another in vitro study using the same radioligand ([3H]CP55,940) showed that CB1Rs are transiently expressed in white matter areas during embryonic and early postnatal periods, progressively “shift” to their adult localization at PND 30, and increase between PND 30 and adulthood in the hippocampus, nucleus accumbens, and cerebral cortex [53]. In addition, Ellgren et al. [60] reported an increase in CB1R protein expression in the nucleus accumbens shell and no changes in prefrontal cortex between mid-(PND 38) and late-(PND 49) adolescence.
In humans, an in vitro study found an increase in CB1R density between children/infant age (n = 5, 3 months to 8 years old) and adults (n = 5, 22 to 73 years old) in frontal cortex, hippocampus CA1 and DG, caudate putamen, globus pallidus, and cerebellum [61]. Interestingly, a recent PET study using [18F]MK-9470 found an increase in CB1R binding in the basal ganglia, lateral temporal cortex, and limbic system of aged female but not male humans [62]. Another PET study using [11C]OMAR in healthy males showed an age-associated decline in CB1R volume of distribution that was significant in globus pallidus only [63]. To allow comparison with other studies from our group [46, 51], we chose to evaluate the ontogeny of CB1R in adolescent and adult male rodents. Recent experiments have shown that female Wistar rats presented a high ~35–39% intersubject variability in CB1R binding evaluated as [18F]MK-9470 standard uptake values between 60 and 80 minutes (Casteels et al., oral communication, 2011). Intersubject variability in our study with males only was of 17% in the adult group and 18% in the adolescent group. Future in vivo animal studies looking at the ontogeny of CB1R in female rats as well as during aging would help in clarifying the relationships between gender, aging, and the endocannabinoid system (Figure 3).
Figure 3.
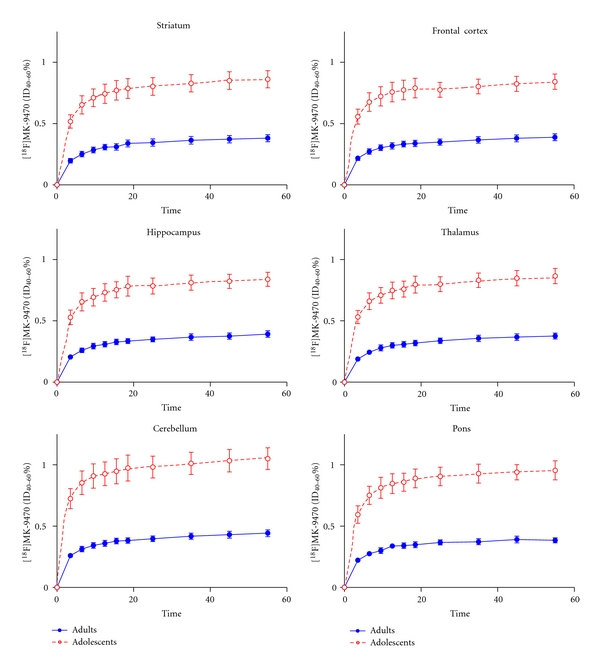
Time-activity kinetic curves of [18F]MK-9470 expressed in %ID40–60 ± SEM in 6 volumes of interest (VOI), in the adolescent (n = 6) (dotted line in red) and the adult (n = 6) (plain line in blue) cohort. Note that adolescents' kinetic curves appear higher compared to adults' kinetic curve because values are expressed as %ID40–60 not taking into account weight as covariate. Estimated marginal means of %ID40–60 were evaluated in the ANCOVA and showed higher [18F]MK-9470 absolute binding in adults compared to adolescents.
In the mammalian brain, synapses and receptors within most regions are overproduced and eliminated during two phases of life. The first one occurs just before birth, after completion of the brain innervation, and witnesses the apoptosis (programmed cell death) of 50% of neurones in order to increase efficiency of synaptic transmission [64, 65]. The second one occurs during the periadolescence period with a tremendous overproduction of synapses and receptors followed by their progressive elimination or pruning [27]. This pattern of expression—overproduction followed by elimination—is shared among mammalian brains and part of a fundamental developmental strategy called “functional validation” [27]. Teicher et al. [66] reported an overproduction of D1 and D2 from PND 25 to 40 followed by a pruning to reach adulthood [66]. We have recently reported higher levels of dopamine D1 and D2 receptors [46], both serotonin 5HT1A receptor binding and mRNA expression [47], and GABAA receptor binding [48] in adolescent rats (PND 39) compared to adults (PND 70) that is in line with the regressive elimination of synapses and receptors that occurs during the transition from adolescence to adulthood. In contrast, the results of the present study indicate that CB1Rs are not undergoing a dramatic elimination between adolescence (PND 35–37) and adulthood (PND 70–72) and continue to increase, at least until PND 70–72. Our study does not rule out the possibility that the CB1Rs are undergoing pruning at a later developmental “aging” stage. Possible explanations for the observed upregulation in adult rats can be hypothesised. Since a homeostatic and modulatory role is attributed to endocannabinoids [57], the CB1R upregulation could be related to a compensation of functional losses in other monaminergic or GABAergic systems. In addition, changes in CB1R may reflect changes in endocannabinoid markers such as AEA and 2-AG. Limited information is available regarding endogenous cannabinoid ligands levels during the transition from adolescence to adulthood; however, a recent study has shown an increase of AEA but not 2-AG levels from early to late adolescence in the prefrontal cortex of the rats [60]. Studies looking at the developmental profile of endocannabinoid ligands in different brain regions and their correlations with CB1R levels would help in elucidating the developmental and morphogenic roles of this system during the transition from adolescence to adulthood.
5. Conclusion
The present study demonstrated the feasibility of imaging CB1R in vivo with PET and [18F]MK-9470 in adolescent and adult rats. Our results suggest that CB1Rs are increased during the transition from adolescence (PND 35–37) to adulthood (PND 70–72), a pattern that is opposite of most other neuroreceptor systems that have already started undergoing pruning during this time window. Availability of new radioligands such as [18F]MK-9470 in combination with PET would offer a unique opportunity to gain insights into the role of the endocannabinoid system during critical stages of development using longitudinal and within-subjects experimental designs and understand the consequences of its alterations after pharmacological challenges as well as in neurodevelopmental animal models of psychosis.
Acknowledgments
Merck & Co., Inc. is acknowledged for the availability of the [18F]MK-9470 precursor and standard. This work is supported by the Schizophrenia Research Institute (SRI), Australia, utilizing infrastructure funding from NSW Health and by an internal Australian Nuclear Science and Technology Organisation Senior Research Fellowship to K. Zavitsanou.
References
- 1.Di Marzo V, Fontana A, Cadas H, et al. Formation and inactivation of endogenous cannabinoid anandanide in central neurons. Nature. 1994;372(6507):686–691. doi: 10.1038/372686a0. [DOI] [PubMed] [Google Scholar]
- 2.McPartland JM. Phylogenomic and chemotaxonomic analysis of the endocannabinoid system. Brain Research Reviews. 2004;45(1):18–29. doi: 10.1016/j.brainresrev.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 3.Howlett AC, Barth F, Bonner TI, et al. International union of pharmacology. XXVII. Classification of cannabinoid receptors. Pharmacological Reviews. 2002;54(2):161–202. doi: 10.1124/pr.54.2.161. [DOI] [PubMed] [Google Scholar]
- 4.Devane WA, Hanus L, Breuer A, et al. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992;258(5090):1946–1949. doi: 10.1126/science.1470919. [DOI] [PubMed] [Google Scholar]
- 5.Mechoulam R, Ben-Shabat S, Hanuš L, et al. Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochemical Pharmacology. 1995;50(1):83–90. doi: 10.1016/0006-2952(95)00109-d. [DOI] [PubMed] [Google Scholar]
- 6.Piomelli D. The molecular logic of endocannabinoid signalling. Nature Reviews Neuroscience. 2003;4(11):873–884. doi: 10.1038/nrn1247. [DOI] [PubMed] [Google Scholar]
- 7.Wilson RI, Nicoll RA. Neuroscience: endocannabinoid signaling in the brain. Science. 2002;296(5568):678–682. doi: 10.1126/science.1063545. [DOI] [PubMed] [Google Scholar]
- 8.Azad SC, Eder M, Marsicano G, Lutz B, Zieglgänsberger W, Rammes G. Activation of the cannabinoid receptor type 1 decreases glutamatergic and GABAergic synaptic transmission in the lateral amygdala of the mouse. Learning and Memory. 2003;10(2):116–128. doi: 10.1101/lm.53303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gessa GL, Melis M, Muntoni A, Diana M. Cannabinoids activate mesolimbic dopamine neurons by an action on cannabinoid CB1 receptors. European Journal of Pharmacology. 1998;341(1):39–44. doi: 10.1016/s0014-2999(97)01442-8. [DOI] [PubMed] [Google Scholar]
- 10.Tsou K, Mackie K, Sañudo-Peña MC, Walker JM. Cannabinoid CB1 receptors are localized primarily on cholecystokinin-containing GABAergic interneurons in the rat hippocampal formation. Neuroscience. 1999;93(3):969–975. doi: 10.1016/s0306-4522(99)00086-x. [DOI] [PubMed] [Google Scholar]
- 11.Herkenham M, Lynn AB, Johnson MR, Melvin LS, De Costa BR, Rice KC. Characterization and localization of cannabinoid receptors in rat brain: a quantitative in vitro autoradiographic study. Journal of Neuroscience. 1991;11(2):563–583. doi: 10.1523/JNEUROSCI.11-02-00563.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Di Marzo V, Petrocellis LD. Plant, synthetic, and endogenous cannabinoids in medicine. Annual Review of Medicine. 2006;57:553–574. doi: 10.1146/annurev.med.57.011205.135648. [DOI] [PubMed] [Google Scholar]
- 13.Fernandez-Ruiz J, Gonzales S. Cannabinoid control of motor function at the basal ganglia. Handbook of Experimental Pharmacology. 2005;(168):479–507. doi: 10.1007/3-540-26573-2_16. [DOI] [PubMed] [Google Scholar]
- 14.Riedel G, Davies SN. Cannabinoid function in learning, memory and plasticity. Handbook of Experimental Pharmacology. 2005;(168):445–477. doi: 10.1007/3-540-26573-2_15. [DOI] [PubMed] [Google Scholar]
- 15.Tasker J. Endogenous cannabinoids take the edge off neuroendocrine responses to stress. Endocrinology. 2004;145(12):5429–5430. doi: 10.1210/en.2004-1218. [DOI] [PubMed] [Google Scholar]
- 16.Fernandez-Ruiz JJ, Berrendero F, Hernandez ML, Romero J, Ramos JA. Role of endocannabinoids in brain development. Life Sciences. 1999;65(6-7):725–736. doi: 10.1016/s0024-3205(99)00295-7. [DOI] [PubMed] [Google Scholar]
- 17.Harkany T, Keimpema E, Barabas K, Mulder J. Endocannabinoid functions controlling neuronal specification during brain development. Molecular and Cellular Endocrinology. 2008;286(1-2, supplement 1):S84–S90. doi: 10.1016/j.mce.2008.02.011. [DOI] [PubMed] [Google Scholar]
- 18.Parolaro D, Realini N, Vigano D, Guidali C, Rubino T. The endocannabinoid system and psychiatric disorders. Experimental Neurology. 2010;224(1):3–14. doi: 10.1016/j.expneurol.2010.03.018. [DOI] [PubMed] [Google Scholar]
- 19.Dean B, Sundram S, Bradbury R, Scarr E, Copolov DD. Studies on [3H]CP-55940 binding in the human central nervous system: regional specific changes in density of cannabinoid-1 receptors associated with schizophrenia and cannabis use. Neuroscience. 2001;103(1):9–15. doi: 10.1016/s0306-4522(00)00552-2. [DOI] [PubMed] [Google Scholar]
- 20.Zavitsanou K, Garrick T, Huang XF. Selective antagonist [3H]SR141716A binding to cannabinoid CB1 receptors is increased in the anterior cingulate cortex in schizophrenia. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2004;28(2):355–360. doi: 10.1016/j.pnpbp.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 21.Dalton VS, Long LE, Weickert CS, Zavitsanou K. Paranoid schizophrenia is characterized by increased CB 1 receptor binding in the dorsolateral prefrontal cortex. Neuropsychopharmacology. 2011;36(8):1620–1630. doi: 10.1038/npp.2011.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Malone DT, Hill MN, Rubino T. Adolescent cannabis use and psychosis: epidemiology and neurodevelopmental models. British Journal of Pharmacology. 2010;160(3):511–522. doi: 10.1111/j.1476-5381.2010.00721.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arseneault L, Cannon M, Poulton R, Murray R, Caspi A, Moffitt TE. Cannabis use in adolescence and risk for adult psychosis: iongitudinal prospective study. British Medical Journal. 2002;325(7374):1212–1213. doi: 10.1136/bmj.325.7374.1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zammit S, Allebeck P, Andreasson S, Lundberg I, Lewis G. Self reported cannabis use as a risk factor for schizophrenia in Swedish conscripts of 1969: historical cohort study. British Medical Journal. 2002;325(7374):1199–1201. doi: 10.1136/bmj.325.7374.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stefanis NC, Delespaul P, Henquet C, Bakoula C, Stefanis CN, Van Os J. Early adolescent cannabis exposure and positive and negative dimensions of psychosis. Addiction. 2004;99(10):1333–1341. doi: 10.1111/j.1360-0443.2004.00806.x. [DOI] [PubMed] [Google Scholar]
- 26.Spear LP. The adolescent brain and age-related behavioral manifestations. Neuroscience and Biobehavioral Reviews. 2000;24(4):417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- 27.Andersen SL. Trajectories of brain development: point of vulnerability or window of opportunity? Neuroscience and Biobehavioral Reviews. 2003;27(1-2):3–18. doi: 10.1016/s0149-7634(03)00005-8. [DOI] [PubMed] [Google Scholar]
- 28.Berrendero F, Sepe N, Ramos JA, Di Marzo V, Fernandez-Ruiz JJ. Analysis of cannabinoid receptor binding and mRNA expression and endogenous cannabinoid contents in the developing rat brain during late gestation and early postnatal period. Synapse. 1999;33(3):181–191. doi: 10.1002/(SICI)1098-2396(19990901)33:3<181::AID-SYN3>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 29.Fernandez-Ruiz J, Berrendero F, Hernandez ML, Ramos JA. The endogenous cannabinoid system and brain development. Trends in Neurosciences. 2000;23(1):14–20. doi: 10.1016/s0166-2236(99)01491-5. [DOI] [PubMed] [Google Scholar]
- 30.Perez-Rosado A, Manzanares J, Fernandez-Ruiz J, Ramos JA. Prenatal Δ9-tetrahydrocannabinol exposure modifies proenkephalin gene expression in the fetal rat brain: sex-dependent differences. Brain Research Developmental Brain Research. 2000;120(1):77–81. doi: 10.1016/s0165-3806(99)00170-4. [DOI] [PubMed] [Google Scholar]
- 31.Ade KK, Lovinger DM. Anandamide regulates postnatal development of long-term synaptic plasticity in the rat dorsolateral striatum. Journal of Neuroscience. 2007;27(9):2403–2409. doi: 10.1523/JNEUROSCI.2916-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Belue RC, Howlett AC, Westlake TM, Hutchings DE. The ontogeny of cannabinoid receptors in the brain of postnatal and aging rats. Neurotoxicology and Teratology. 1995;17(1):25–30. doi: 10.1016/0892-0362(94)00053-g. [DOI] [PubMed] [Google Scholar]
- 33.Rodriguez de Fonseca F, Ramos JA, Bonnin A, Fernandez-Ruiz JJ. Presence of cannabinoid binding sites in the brain from early postnatal ages. NeuroReport. 1993;4(2):135–138. doi: 10.1097/00001756-199302000-00005. [DOI] [PubMed] [Google Scholar]
- 34.Burns HD, Van Laere K, Sanabria-Bohorquez S, et al. [18F]MK-9470, a positron emission tomography (PET) tracer for in vivo human PET brain imaging of the cannabinoid-1 receptor. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(23):9800–9805. doi: 10.1073/pnas.0703472104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goffin K, Bormans G, Casteels C, et al. An in vivo [18F]MK-9470 microPET study of type 1 cannabinoid receptor binding in Wistar rats after chronic administration of valproate and levetiracetam. Neuropharmacology. 2008;54(7):1103–1106. doi: 10.1016/j.neuropharm.2008.02.018. [DOI] [PubMed] [Google Scholar]
- 36.Casteels C, Bormans G, Van Laere K. The effect of anaesthesia on [18F]MK-9470 binding to the type 1 cannabinoid receptor in the rat brain. European Journal of Nuclear Medicine and Molecular Imaging. 2010;37(6):1164–1173. doi: 10.1007/s00259-010-1383-7. [DOI] [PubMed] [Google Scholar]
- 37.Casteels C, Vanbilloen B, Vercammen D, et al. Influence of chronic bromocriptine and levodopa administration on cerebral type 1 cannabinoid receptor binding. Synapse. 2010;64(8):617–623. doi: 10.1002/syn.20769. [DOI] [PubMed] [Google Scholar]
- 38.Casteels C, Lauwers E, Baitar A, Bormans G, Baekelandt V, Van Laere K. In vivo type 1 cannabinoid receptor mapping in the 6-hydroxydopamine lesion rat model of Parkinson’s disease. Brain Research. 2010;1316:153–162. doi: 10.1016/j.brainres.2009.12.026. [DOI] [PubMed] [Google Scholar]
- 39.Gerard N, Ceccarini J, Bormans G, et al. Influence of chronic nicotine administration on cerebral type 1 cannabinoid receptor binding: an in vivo micro-pet study in the rat using [18F]MK-9470. Journal of Molecular Neuroscience. 2010;42(2):162–167. doi: 10.1007/s12031-010-9340-2. [DOI] [PubMed] [Google Scholar]
- 40.Casteels C, Vandeputte C, Rangarajan JR, et al. Metabolic and type 1 cannabinoid receptor imaging of a transgenic rat model in the early phase of Huntington disease. Experimental Neurology. 2011;229(2):440–449. doi: 10.1016/j.expneurol.2011.03.014. [DOI] [PubMed] [Google Scholar]
- 41.Casteels C, Martinez E, Bormans G, et al. Type 1 cannabinoid receptor mapping with [18F]MK-9470 PET in the rat brain after quinolinic acid lesion: a comparison to dopamine receptors and glucose metabolism. European Journal of Nuclear Medicine and Molecular Imaging. 2010;37(12):2354–2363. doi: 10.1007/s00259-010-1574-2. [DOI] [PubMed] [Google Scholar]
- 42.Van Laere K, Koole M, Bohorquez SMS, et al. Whole-body biodistribution and radiation dosimetry of the human cannabinoid type-1 receptor ligand18F-MK-9470 in healthy subjects. Journal of Nuclear Medicine. 2008;49(3):439–445. doi: 10.2967/jnumed.107.047290. [DOI] [PubMed] [Google Scholar]
- 43.Sanabria-Bohorquez SM, Hamill TG, Goffin K, et al. Kinetic analysis of the cannabinoid-1 receptor PET tracer [ 18F]MK-9470 in human brain. European Journal of Nuclear Medicine and Molecular Imaging. 2010;37(5):920–933. doi: 10.1007/s00259-009-1340-5. [DOI] [PubMed] [Google Scholar]
- 44.Van Laere K, Goffin K, Bormans G, et al. Relationship of type 1 cannabinoid receptor availability in the human brain to novelty-seeking temperament. Archives of General Psychiatry. 2009;66(2):196–204. doi: 10.1001/archgenpsychiatry.2008.530. [DOI] [PubMed] [Google Scholar]
- 45.Gerard N, Pieters G, Goffin K, Bormans G, Van Laere K. Brain type 1 cannabinoid receptor availability in patients with anorexia and bulimia nervosa. Biological Psychiatry. 2011;70(8):777–784. doi: 10.1016/j.biopsych.2011.05.010. [DOI] [PubMed] [Google Scholar]
- 46.Dalton VS, Zavitsanou K. Differential treatment regimen-related effects of cannabinoids on D1 and D2 receptors in adolescent and adult rat brain. Journal of Chemical Neuroanatomy. 2010;40(4):272–280. doi: 10.1016/j.jchemneu.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 47.Zavitsanou K, Wang H, Dalton VS, Nguyen V. Cannabinoid administration increases 5HT1A receptor binding and mRNA expression in the hippocampus of adult but not adolescent rats. Neuroscience. 2010;169(1):315–324. doi: 10.1016/j.neuroscience.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 48.Verdurand M, Dalton VS, Zavitsanou K. GABAA receptor density is altered by cannabinoid treatment in the hippocampus of adult but not adolescent rats. Brain Research. 2010;1351:238–245. doi: 10.1016/j.brainres.2010.06.032. [DOI] [PubMed] [Google Scholar]
- 49.Bao Q, Newport D, Chen M, Stout DB, Chatziioannou AF. Perfrmance evalution of the inveon dedicated PET preclinical tomograph based on the NEMA NU-4 standards. Journal of Nuclear Medicine. 2009;50(3):401–408. doi: 10.2967/jnumed.108.056374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Qi J, Leahy RM, Cherry SR, Chatziioannou A, Farquhar TH. High-resolution 3D bayesian image reconstruction using the microPET small-animal scanner. Physics in Medicine and Biology. 1998;43(4):1001–1013. doi: 10.1088/0031-9155/43/4/027. [DOI] [PubMed] [Google Scholar]
- 51.Dalton VS, Wang H, Zavitsanou K. HU210-induced downregulation in cannabinoid CB1 receptor binding strongly correlates with body weight loss in the adult rat. Neurochemical Research. 2009;34(7):1343–1353. doi: 10.1007/s11064-009-9914-y. [DOI] [PubMed] [Google Scholar]
- 52.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Vol. 6. Academic Press; 2007. [DOI] [PubMed] [Google Scholar]
- 53.Romero J, Garcia-Palomero E, Berrendero F, et al. Atypical location of cannabinoid receptors in white matter areas during rat brain development. Synapse. 1997;26(3):317–323. doi: 10.1002/(SICI)1098-2396(199707)26:3<317::AID-SYN12>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 54.Pertwee RG. Pharmacology of cannabinoid receptor ligands. Current Medicinal Chemistry. 1999;6(8):635–664. [PubMed] [Google Scholar]
- 55.Wiley JL, Barrett RL, Lowe J, Balster RL, Martin BR. Discriminative stimulus effects of CP 55,940 and structurally dissimilar cannabinoids in rats. Neuropharmacology. 1995;34(6):669–676. doi: 10.1016/0028-3908(95)00027-4. [DOI] [PubMed] [Google Scholar]
- 56.Atwood BK, MacKie K. CB2: a cannabinoid receptor with an identity crisis. British Journal of Pharmacology. 2010;160(3):467–479. doi: 10.1111/j.1476-5381.2010.00729.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Di Marzo V, Bifulco M, De Petrocellis L. The endocannabinoid system and its therapeutic exploitation. Nature Reviews Drug Discovery. 2004;3(9):771–784. doi: 10.1038/nrd1495. [DOI] [PubMed] [Google Scholar]
- 58.Negus SS. Some implications of receptor theory for in vivo assessment of agonists, antagonists and inverse agonists. Biochemical Pharmacology. 2006;71(12):1663–1670. doi: 10.1016/j.bcp.2005.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Innis RB, Cunningham VJ, Delforge J, et al. Consensus nomenclature for in vivo imaging of reversibly binding radioligands. Journal of Cerebral Blood Flow and Metabolism. 2007;27(9):1533–1539. doi: 10.1038/sj.jcbfm.9600493. [DOI] [PubMed] [Google Scholar]
- 60.Ellgren M, Artmann A, Tkalych O, et al. Dynamic changes of the endogenous cannabinoid and opioid mesocorticolimbic systems during adolescence: THC effects. European Neuropsychopharmacology. 2008;18(11):826–834. doi: 10.1016/j.euroneuro.2008.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mato S, Del Olmo E, Pazos A. Ontogenetic development of cannabinoid receptor expression and signal transduction functionality in the human brain. European Journal of Neuroscience. 2003;17(9):1747–1754. doi: 10.1046/j.1460-9568.2003.02599.x. [DOI] [PubMed] [Google Scholar]
- 62.Van Laere K, Goffin K, Casteels C, et al. Gender-dependent increases with healthy aging of the human cerebral cannabinoid-type 1 receptor binding using [18F]MK-9470 PET. NeuroImage. 2008;39(4):1533–1541. doi: 10.1016/j.neuroimage.2007.10.053. [DOI] [PubMed] [Google Scholar]
- 63.Wong DF, Kuwabara H, Horti AG, et al. Quantification of cerebral cannabinoid receptors subtype 1 (CB1) in healthy subjects and schizophrenia by the novel PET radioligand [11C]OMAR. NeuroImage. 2010;52(4):1505–1513. doi: 10.1016/j.neuroimage.2010.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Changeux JP, Danchin A. Selective stabilisation of developing synapses as a mechanism for the specification of neuronal networks. Nature. 1976;264(5588):705–712. doi: 10.1038/264705a0. [DOI] [PubMed] [Google Scholar]
- 65.Purves D, Lichtman JW. Elimination of synapses in the developing nervous system. Science. 1980;210(4466):153–157. doi: 10.1126/science.7414326. [DOI] [PubMed] [Google Scholar]
- 66.Teicher MH, Andersen SL, Hostetter JC. Evidence for dopamine receptor pruning between adolescence and adulthood in striatum but not nucleus accumbens. Developmental Brain Research. 1995;89(2):167–172. doi: 10.1016/0165-3806(95)00109-q. [DOI] [PubMed] [Google Scholar]


