Abstract
Stress and alcohol context cues are each associated with alcohol‐related behaviors, yet neural responses underlying these processes remain unclear. This study investigated the neural correlates of stress and alcohol context cue experiences and examined sex differences in these responses. Using functional magnetic resonance imaging, brain responses were examined while 43 right‐handed, socially drinking, healthy individuals (23 females) engaged in brief guided imagery of personalized stress, alcohol‐cue, and neutral‐relaxing scenarios. Stress and alcohol‐cue exposure increased activity in the cortico–limbic–striatal circuit (P < 0.01, corrected), encompassing the medial prefrontal cortex (mPFC), orbitofrontal cortex (OFC), anterior cingulate cortex (ACC), left anterior insula, striatum, and visuomotor regions (parietal and occipital lobe, and cerebellum). Activity in the left dorsal striatum increased during stress, while bilateral ventral striatum activity was evident during alcohol‐cue exposure. Men displayed greater stress‐related activations in the mPFC, rostral ACC, posterior insula, amygdala, and hippocampus than women, whereas women showed greater alcohol‐cue‐related activity in the superior and middle frontal gyrus (SFG/MFG) than men. Stress‐induced anxiety was positively associated with activity in emotion‐modulation regions, including the medial OFC, ventromedial PFC, left superior‐mPFC, and rostral ACC in men, but in women with activation in the SFG/MFG, regions involved in cognitive processing. Alcohol craving was significantly associated with the striatum (encompassing dorsal, and ventral) in men, supporting its involvement in alcohol “urge” in healthy men. These results indicate sex differences in neural processing of stress and alcohol‐cue experiences and have implications for sex‐specific vulnerabilities to stress‐ and alcohol‐related psychiatric disorders. Hum Brain Mapp, 2011. © 2010 Wiley‐Liss, Inc.
Keywords: sex differences, stress, alcohol cue, reward, brain fMRI, prefrontal cortex
INTRODUCTION
Stress is a key vulnerability factor in psychiatric disorders [Cohen et al.,2007; Sinha,2009b], and sex differences in the prevalence of mood and anxiety disorders and addiction have been documented [Becker et al.,2007; Kessler et al.,1993]. In individuals with alcohol‐use disorders, stress and alcohol‐related cues are important factors increasing alcohol craving and relapse risk [Sinha and Li,2007]. In epidemiological samples, stress increases alcohol consumption [Dawson et al.,2005; Grzywacz and Almeida,2008], and greater alcohol use was also reported after exposure to the 9/11 attacks in New York [Boscarino et al.,2006].
Stress provokes defensive motivation and avoidant behaviors [Nachmias et al.,1996], whereas social alcohol consumption is associated with appetitive motivation and approach behaviors [Lukas et al.,1986; Robinson and Berridge,1993]. The defensive and appetitive systems are two parallel systems with common integrative components [Cacioppo et al.,1999; Carver,2001], suggesting the presence of specific yet overlapping brain systems underlying these two systems.
Neuroimaging studies on stress and aversive processing have identified a specific corticostriatal‐limbic circuitry including the medial prefrontal cortex (mPFC), anterior cingulate cortex (ACC), hippocampus, amygdala, and striatum [Lopez et al.,1999; Sinha et al.,2004; Zhou et al.,2008]. Recent evidence also indicates that alcohol taste cue [Filbey et al.,2008] and intravenous injection of alcohol [Gilman et al.,2008] reliably activate brain reward circuits, specifically, mesocortico‐striatal structures such as the PFC and ventral striatum (VS) in healthy social drinkers. The VS has been associated with reward processing [Schott et al.,2008], and the amygdala is associated with stress response [Zhou et al.,2008] with some evidence in reward processing [Garavan et al.,2001]. This data indicates that the coticostriatal–limbic regions may represent the core circuits involved in both stress and alcohol cue‐related processing, but no previous research has directly compared neural circuits associated with these processes in humans.
Sex differences in stress responses have also been previously reported. Recent neuroimaging evidence indicates that compared to women, men showed greater stress‐related brain responses in fronto‐limbic areas, especially in the medial PFC, ACC, hypothalamus, and amygdala [Goldstein et al.,2010]. Greater physiological responses to stress in men are consistent with behavioral and neuroendocrine studies. For example, men show greater stress‐related negative emotion and aggression responses, more robust fear conditioning, and higher cortisol responses than women [Jackson et al.,2006; Kudielka and Kirschbaum,2005; Verona et al.,2007], whereas women under stress show greater tendencies to rumination and negative cognition [Nolen‐Hoeksema,1987]. Furthermore, men showed greater stress‐related increases in alcohol craving and consumption than women [Lindquist et al.,1997; Tamres et al.,2002]. Although this literature suggests sex differences in responses to stress and alcohol‐related behaviors, and there is some evidence of sex differences in neuroimaging of stress, no previous research has examined sex differences when directly comparing the neural responses to emotional stress and alcohol context cues in healthy individuals.
To clarify sex‐specific neural responses to stress and alcohol context cues, this study used individually calibrated, personally relevant, guided imagery scripts of stress, and alcohol context cue that were compared to personalized neutral relaxing scripts. It is a widely used, ecologically valid method of emotion, stress, and craving provocation in laboratory and in neuroimaging studies (for a review, Sinha [2009a]). On the basis of the previously cited research, we hypothesized that, in healthy individuals, both stress and alcohol cues would activate the prefrontal and ACC regions known to be involved in stress and reward modulation. Further compared to women, men would show greater neural responses to stress in corticostriatal limbic regions, especially in the mPFC, ACC, and amygdala. In terms of self‐reported anxiety and craving, we expected significantly elevated stress‐induced anxiety and alcohol cue‐induced craving from the baseline. However, we did not expect that stress‐induced craving or alcohol‐cue induced anxiety would be increased in healthy individuals based on previous studies [Fox et al.,2008; Sinha et al.,2009]. As the striatum is involved in motivation for alcohol [Heinz et al.,2004; Schneider et al.,2001; Wrase et al.,2007], we expected it to be associated with subjective alcohol craving. We also hypothesized that the stress circuit involving the medial PFC, ACC, and the amygdala would be associated with stress‐induced anxiety.
MATERIALS AND METHODS
Participants
Forty‐three healthy individuals between the ages of 18 and 50 were recruited from the community via newspaper, web advertizing, and flyers. All participants were right‐handed and reported light‐to‐moderate levels of alcohol consumption. Over the course of two to three sessions, participants completed demographic, psychiatric, cognitive, drug use, and self assessments. To ensure healthy physical function, a medical evaluation was conducted including laboratory testing of renal, hepatic, pancreatic, hematopoietic, and thyroid functions. Participants were excluded if they had the following: (a) a history of head trauma, (b) pregnancy, (c) use of psychoactive medication, (d) current or lifetime substance abuse or dependence, and (e) current or lifetime history of neurological or psychiatric disorders (as assessed by the Structured Clinical Interview for DSM‐IV). Women were scheduled to participate in the laboratory sessions during the follicular or luteal phases of their menstrual cycle (as determined by sex steroid hormone measurements) and excluded if they reported irregular menstrual cycle or were taking hormonal birth control, to control for the possible influence of steroid hormonal fluctuations on stress responses [Kirschbaum et al.,1999]. All participants were asked to refrain from alcohol for at least 72 h before the scanning session and breathalyzer, and urine toxicology screening was used to confirm drug and alcohol abstinence for each assessment session and on scanning day. Upon completion of the assessments, they participated in a 1.5‐h functional magnetic resonance imaging (fMRI) session. The Human Investigation Committee at the Yale University School of Medicine approved the study procedures, and all participants signed an informed consent prior to study participation.
Guided Imagery Script Development and Training
During the session before the fMRI scan, six individually tailored imagery scripts were developed from participants' descriptions of two alcohol context cue, two stressful, and two neutral‐relaxing experiences using Scene Construction Questionnaires [Li et al.,2005; Sinha,2009a], based on previously described standardized methods [Sinha,2009a]. For stress scripts, participants identified “a situation that made them sad, mad, upset and which in the moment they could do nothing to change it.” Examples of highly stressful situations include loss of a job, death of or conflict with a significant other, and loss of an important relationship. As a manipulation check, the situations were rated by the participants on a 10‐point Likert scale (1 = not at all stressful and 10 = the most stressful) and only those situations that were rated as eight or above were found appropriate for stimulus provocation and used for script development. Alcohol context cue scripts were developed from individual experiences of alcohol anticipation and consumption (e.g., birthday celebration and meeting friends at a bar), and scenarios occurring in the context of negative affect or psychological distress were excluded. Specifically, the alcohol cue script was developed in response to the query, “please tell us about a recent situation when you really wanted an alcoholic drink and then you went ahead and had one.” Thus, each subject provided their preferred individual situation of wanting and consuming an alcoholic beverage. Neutral scripts were based on the personal experiences of commonly experienced neutral‐relaxing situations, such as lying on the beach or reading at the park. Although individual stimulus and response content specific to an experience were included in each script, the script style, content format, and length were standard across conditions and subjects, as described previously [Sinha,2009a]. Each script was 2 min in length and was audio‐taped in random order for presentation during the scanning session. During the scanning session, all research staff and fMRI technicians were blind to content, order, and type of the script stimuli.
Efficacy of Script‐Driven Imagery Manipulation
The following procedures were implemented to ensure efficacy of the imagery manipulation. First, all participants completed the Questionnaire on Mental Imagery [Sheehan,1967] that measures individual difference in mental imagery ability, and individuals reporting average or above levels of imagery ability were included [Sinha,2009a]. There were no statistical differences in scores of QMI between male (M = 70.4, SD = 20.2) and female (M = 65.2, SD = 22.3) participants. Additionally, the scores of Toronto Alexithymia Scale [Bagby et al.,1988] indicated that there were no statistical differences between men (M = 58.1, SD = 9.6) and women (M = 58.6, SD = 15.4), suggesting that men and women were equivalent in ability to reflect upon and rate their emotions.
Second, a structured relaxation and imagery‐training procedure, known to minimize variability in imagery ability (see Sinha [2009a] for details), was implemented before the scanning session. Finally, each participant rated imagery vividness on a 10‐point Likert scale (1 = cannot visualize the image and 10 = extremely clear, “as if” it were happening right now) following each stress, alcohol cue, and neutral trial, and there were no significant differences between men and women, or across conditions in vividness of the imagery for each trial (Men: M = 8.2, SD = 1.3, Women: M = 8.8, SD = 1.0).
The stress scripts were rated for the type of stress as either: Interpersonal (men: 72%, women: 72.5%; personal violation, relationship, and betrayal), Environmental (men: 9%, women: 2.5%; housing, legal, and financial), Achievement (men: 17%, women: 22.5%; job and career), and Medical (men: 2%, women: 2.5%; injury and illness). There were no differences in type of stressor by gender, χ2 = 1.71, P = NS.
fMRI Acquisition
The 3‐T Siemens Trio MRI system equipped with a single‐channel, standard‐quadrature head coil collected the images using T2*‐sensitive gradient‐recalled single‐shot echo‐planar pulse sequence. Anatomical images were acquired with spin‐echo imaging in the axial‐plane parallel to the AC–PC line [repetition time (TR) = 300 ms, echo time (TE) = 2.5 ms, bandwidth = 300 Hz/pixel, flip angle = 60°, field‐of‐view = 220 × 220 mm, matrix = 256 × 256, 32 slices, slice thickness = 4 mm, no gap]. Functional images were obtained using a single‐shot gradient echo‐planar imaging sequence with 32 axial slices parallel to the AC–PC line covering whole brain (TR = 2,000 ms, TE = 25 m, bandwidth = 2004 Hz/pixel, flip angle = 85°, field of view = 220 × 220 mm, matrix = 64 × 64, slice thickness = 4 mm with no gap, and 190 measurements). Once the functional images were collected, a high‐resolution 3D Magnetization Prepared Rapid Gradient‐Echo sequence was used to acquire sagittal images for multisubject registration. (TR = 2,530 ms; TE = 3.34 ms; bandwidth = 180 Hz/pixel; flip angle = 7°; slice thickness = 1 mm; field‐of‐view = 256 × 256 mm; matrix = 256 × 256).
fMRI Trials
A block design was used where each block comprised a 5.5‐min (min) fMRI run that entailed a 1.5‐min quiet baseline period followed by a 2.5‐min imagery period (2 min of read‐imagery and 0.5 min of quiet‐imagery) and a 1‐min quiet recovery period. During the baseline period, participants were asked to stay still in the scanner without engaging in any mental activity. During the recovery period, participants were instructed to stop imagining and lay still for another minute.
The order of all three script types were counterbalanced and randomized across subjects. Scripts from the same condition were never presented consecutively, and each script was presented only once, such that different scripts were used per trial.
Behavioral Ratings Pre‐ and Posttrials
Before and after each BOLD trial, participants were instructed to rate anxiety and craving levels using a 10‐point verbal analog scale (VAS: 1 = not at all, 10 = extremely high). Anxiety rating refers to how “tense, anxious and/or jittery” they felt, and craving ratings indicate their “desire to drink alcohol at that moment.” To decrease and normalize any residual anxiety or craving from prior trials, participants engaged in a brief exposure of progressive relaxation between fMRI trials for 2 min. Participants were instructed to progressively relax muscles in each part of the body (e.g., arm, leg, and stomach muscles). This technique is mainly focused on relaxing physiological muscle tension and does not involve mental relaxation or imagery. After relaxation, anxiety and craving ratings returned back to baseline, and there were no baseline differences across trials in these ratings.
fMRI Analysis
The raw imaging data were converted from Digital Imaging and Communication in Medicine format to Analyze format using XMedCon [Nolfe,2003]. To achieve a steady‐state equilibrium between radio‐frequency pulsing and relaxation, the first 10 images were discarded from the beginning of each functional run, leaving 180 measurements. Images were motion‐corrected for three translational and three rotational directions [Friston et al.,1996] discarding any trial with linear motion exceeding 1.5 mm or having a rotation greater than 2°. At the individual level, General Linear Model on each voxel in the entire brain volume was used with a regressor (time during imagery) for each trial per condition, and the baseline for each trial was included separately as a regressor. The recovery period was excluded from the data analysis and was not used as the baseline regressor, due to the possibility of carryover effects from the imagery period. Each functional trial was spatially smoothed using a 6‐mm Gaussian kernel and individually normalized to create β‐maps (3.44 mm × 3.44 mm × 4 mm).
To adjust for individual anatomical differences, three registrations were sequentially conducted using the Yale BioImage Suite software package [Duncan et al.,2004; Papademetris,2006]; linear registration of raw data into 2D anatomical image, the 2D–3D (1 × 1 × 1 mm) linear registration, a nonlinear registration to a reference 3D image. The reference image was the Colin27 Brain [Holmes et al.,1998] in the Montreal Neurological Institute (MNI) space [Evans et al.,1993].
The second‐level group analysis was conducted with Analysis of Functional NeuroImages software [Cox,1996] using random mixed effects models. A 2 × 3 ANOVA (sex by condition) was carried out with sex as the between‐subjects fixed‐effect factor, condition (neutral/alcohol cue/stress) as the within‐subjects fixed‐effect factor, and subject (N = 43) as the random‐effect factor. A Family Wise Error rate (FWE) correction for multiple comparisons was applied using Monte Carlo simulations [Xiong et al.,1995] conducted with AlphaSim in AFNI [Cox,1996] and set at P < 0.01 for the overall factorial analysis (main effects and interaction terms), and at P < 0.05 (FWE corrected) for simple effect analysis to understand source of significant main effects and interactions.
Whole‐brain correlation analyses with anxiety and alcohol craving were conducted using BioImage Suite [Papademetris,2006] with the application of AFNI AlphaSim FWE correction for multiple comparisons. To reduce the influence of any possible extreme values (outliers) in the correlation analyses, the Winsorization method [Chen and Dixon,1972; Dixon,1960] for values with a Cook's Distance score greater than one was used. The correlation r value was presented after extreme values were reigned into the value of the next highest score to reduce their influences. However, scatter plots (see Fig. 4) displayed all values without the Winsorization to show the overall patterns of the data.
Figure 4.

Whole‐brain voxel‐based correlation and corresponding scatter plots for (A) alcohol cue‐induced craving ratings with neural responses in males as well as (B) stress‐induced anxiety ratings with neural response in males and females (P < 0.05, whole‐brain FWE corrected). A: In males, elevated alcohol craving ratings were associated with increased activity in the striatum cluster (r = 0.74) that encompassed ventral and dorsal striatum, including the left nucleus accumbens (X = −13, Y = 12, Z = −12). B1: In males, enhanced stress‐induced anxiety ratings were associated with increased brain activity in a medial prefrontal cortex cluster that included the ACC, ventromedal PFC, and medial OFC (r = 0.59). B2: In females, stress‐induced anxiety ratings were positively correlated with bilateral brain activity in superior/middle frontal gyrus (winsorized r = 0.62). Coordinates are given in MNI space. R, right; L, left; PFC, prefrontal cortex; ACC, anterior cingulated cortex; MFG, middle frontal gyrus; SFG, superior frontal gyrus. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
RESULTS
Demographics
Table I presents demographic characteristics and current levels of alcohol consumption in men and women. There were no significant differences in age, education, and race by gender. As expected and consistent with general trends [Kessler et al.,1994; Office for National Statistics,2006], women drank less than men on average, but there were no significant differences between genders in current levels of alcohol consumption.
Table I.
Demographic characteristics of men and women
| Subject variable | Women (N = 23) | Men (N = 20) |
|---|---|---|
| Age | 30.87 (8.37) | 32.5 (10.18) |
| Years of education | 14.87 (2.1) | 14.7 (1.84) |
| Race (N %) | ||
| Caucasian (N % Caucasian) | 10 (43.48%) | 12 (60%) |
| Alcohol use measures | ||
| Age of onset of alcohol use | 16.22 (5.35) | 16.6 (2.44) |
| range | 7–32 | 13–24 |
| Number of days of alcohol used/week | 0.61 (0.68) | 1.39 (1.82) |
| range | 0–2 | 0–7 |
| Average alcohol drinks/occasion | 1.72 (1.28) | 2.3 (1.8) |
| range | 0–5 | 0–6 |
Note: No significant difference between men and women in any measures.
Anxiety and Craving Ratings
Condition and sex effects on self‐report measures of craving and anxiety in each were assessed using linear mixed models with Condition (stress, alcohol, and neutral) and Time‐point (baseline, imagery) as within‐subjects factors and Sex as a between‐subjects factor (see Fig. 1). Post hoc t‐tests were corrected for multiple comparisons using a modified Bonferroni procedure [Hochberg,1988].
Figure 1.
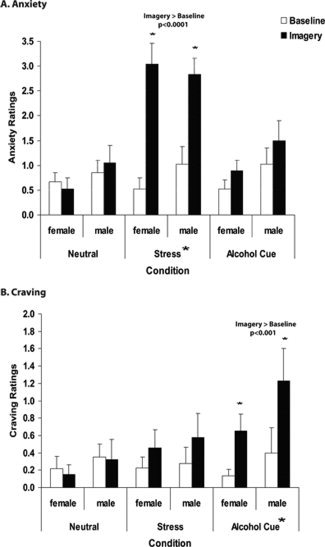
Mean and standard error (SEM) in healthy men and women for verbal analog scales (VAS, 0–10) assessing (A) subjective anxiety and (B) alcohol craving ratings averaged across stress, alcohol cue, and neutral‐relaxing imagery trials. A: During stress imagery, anxiety increased significantly from baseline (P < 0.0001*) for both men and women. Anxiety in the stress imagery was significantly greater than anxiety responses in the neutral (P < 0.0001) and alcohol‐cue (P < 0.001) imagery. B: During alcohol‐cue imagery, alcohol craving was significantly elevated from the baseline (P < 0.001*) for both men and women. Craving response during alcohol‐cue imagery was significantly greater than craving response during neutral (P < 0.001) and stress (P < 0.05) imagery. All P values are Bonferroni corrected. No significant main effects of sex or sex × condition interactions were observed.
For the anxiety ratings, significant main effects of Condition [F(2,82) = 26.6, P < 0.0001], Time‐point [F(1,41) = 58.04, P < 0.0001], and a Condition × Time‐point interaction [F(2,80) = 33.37, P < 0.0001] were observed. No other effects were significant including Sex main effect, Sex × Condition, and Sex × Condition × Time‐point interactions. There were no differences in anxiety ratings across the baseline period in each trial. However, as expected, in the imagery period, anxiety during stress was greater than in the neutral [t = 10.33, P < 0.0001] and in the alcohol [t = 8.36, P < 0.001] conditions. When the imagery period was compared to the baseline period, anxiety ratings during imagery were significantly elevated from baseline only in the stress condition [t = 10.98, P < 0.0001] and not in the neutral and alcohol cue conditions.
For the craving ratings, significant main effects of Condition [F(2,82) = 4.24, P < 0.05], Time‐point [F(1,41) = 15.52, P < 0.001], and a Condition × Time‐point [F(2,80) = 10.3, P < 0.0001] interaction were observed. No other effects were significant including Sex main effect, Sex × Condition, and Sex × Condition × Time‐point interactions. There were no differences in craving ratings across the baseline period in each trial. However, during the imagery period, craving in the alcohol‐cue condition was greater than in the neutral (t = 5.24, P < 0.001) and stress (t = 3.16, P < 0.05) conditions. When the imagery period was compared to the baseline period, craving during imagery was greater than the baseline for the alcohol cue condition (t = 5.59, P < 0.001) and not during the neutral and stress conditions.
fMRI Results
Main effect of condition: Brain activity during stress and alcohol cue conditions
A significant condition main effect (P < 0.01, whole‐brain FWE corrected) on brain activation was observed for the medial and lateral orbitofrontal cortex (OFC), ventromedial PFC, lateral PFC, superior/middle frontal gyrus (SFG/MFG), anterior and posterior cingulate cortex (ACC and PCC), and left anterior insula. Additionally, activation in specific areas of superior/middle/inferior temporal lobe, superior/inferior parietal lobe, angular gyrus, occipital lobe, and cerebellum were observed (see Fig. 2). These regions were strongly activated for both Stress‐Neutral and Alcohol cue‐Neutral contrasts (see Table II). Activation in the dorsal striatum was present only during stress exposure, whereas activations in the VS, occipital lobe and bilateral MFG/precentral gyrus were evident only during the alcohol‐cue exposure (see Table II). Greater activity during alcohol‐cue relative to stress was seen bilaterally in the pre‐/postcentral gyrus at P < 0.01, whole‐brain corrected (Left: t = 2.81, 1,592 mm3, X = −52, Y = −6, Z = 35; Right: t = 2.97, 1,967 mm3, X = 62, Y = −11, Z = 25).
Figure 2.
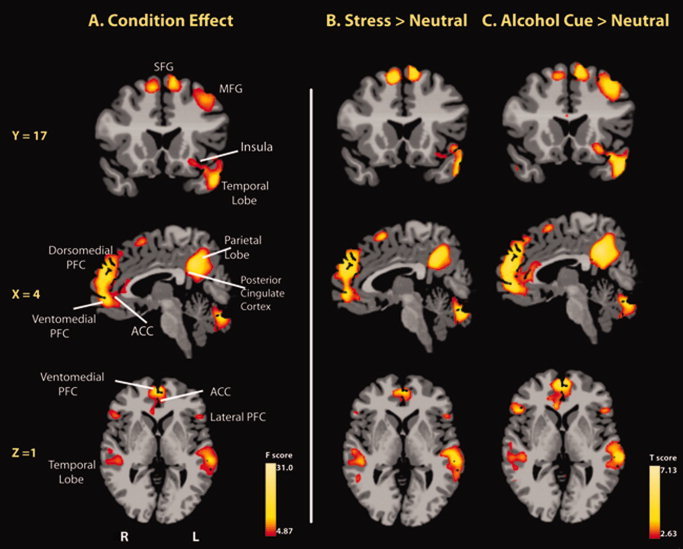
Whole‐brain voxel‐based analyses showing (A) main effect of condition, and (B) stress and (C) alcohol‐cue induced increases in fMRI signal relative to neural responses in the neutral‐relaxing condition (P < 0.01, whole‐brain FWE corrected). Selected areas of the prefrontal cortex (PFC: lateral, dorsomedial, medial, and ventromedial), ACC, left anterior insula, temporal lobe as well as visuomotor perception areas (parietal lobe and cerebellum) were activated in the stress and in the alcohol cue conditions. Note: activation in the dorsal striatum was present only during stress exposure and activation in the ventral striatum was present only during alcohol‐cue exposure at P < 0.05 level (whole‐brain FWE corrected). R, right; L, left; PFC, prefrontal cortex; ACC, anterior cingulate cortex; MFG, middle frontal gyrus; SFG, superior frontal gyrus. All P values are using two‐tailed tests. Coordinates are given in MNI space. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Table II.
Brain activations during stress and alcohol‐cue exposures
| Regions of activation | Lat | BA | Stress > neutral | Alcohol cue > neutral | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coordinates | Volume (mm3) | t | Coordinates | Volume (mm3) | t | |||||||
| X | Y | Z | X | Y | Z | |||||||
| Superior medial frontal gyrus | B | 6, 8 | −1 | 29 | 51 | 3.15 | 15,754 | −2 | 27 | 51 | 3.37 | 20,284 |
| Middle frontal gyrus, precentral gyrus | L | 6, 8 | — | — | — | — | — | −41 | 7 | 46 | 3.11 | 11,259 |
| R | 6, 4 | — | — | — | — | — | 52 | −4 | 38 | 2.9 | 3,994 | |
| Medial frontal gyrus | B | 9 | −1 | 50 | 29 | 3.72 | 13,017 | −1 | 49 | 32 | 3.9 | 14,553 |
| Medial frontal gyrus (VmPFC) | B | 10 | 2 | 54 | 7 | 3.31 | 7,447 | 2 | 54 | 9 | 3.8 | 14,468 |
| Inferior frontal gyrus | L | 44, 45 | −53 | 21 | 7 | 2.85 | 624 | −50 | 22 | 9 | 2.9 | 904 |
| R | 44, 45 | 54 | 26 | −2 | 2.79 | 245 | 52 | 26 | 3 | 3.09 | 1,564 | |
| Orbitofrontal gyrus and insula cluster | L | 47 | −44 | 25 | −13 | 3.35 | 5,390 | −40 | 25 | −13 | 3.2 | 5,365 |
| Anterior insula | L | — | −32 | 18 | −13 | 3.18 | 387 | −27 | 19 | −12 | 3.04 | 909 |
| Orbitofrontal gyrus | R | 47 | 51 | 28 | −11 | 2.89 | 1,172 | 48 | 27 | −12 | 3.05 | 2,307 |
| Medial orbitofrontal gyrus | B | 11 | 0 | 48 | −15 | 3.03 | 761 | 1 | 43 | −16 | 2.97 | 1,310 |
| Dorsal/rostral anterior cingulate gyrus | B | 24, 32 | −5 | 44 | 10 | 3.17 | 5,160 | 1 | 39 | 7 | 3.15 | 10,807 |
| Posterior cingulate gyrus | B | 23, 31 | −2 | −49 | 33 | 3.46 | 8,839 | −2 | −44 | 28 | 3.97 | 9,619 |
| Superior/middle/inferior temporal gyrus | L | 20, 21,22, 38, 39 | −56 | −26 | −6 | 3.51 | 31,855 | −54 | −29 | −3 | 3.7 | 36,993 |
| R | 20, 21,22, 38, 39 | 57 | −34 | −1 | 3.06 | 10,247 | 56 | −28 | −3 | 3.22 | 20,872 | |
| Superior/inferior parietal lobule, angular gyrus | B | 7, 31, 39 | −1 | −59 | 43 | 3.39 | 6,782 | −4 | −60 | 39 | 3.68 | 15,362 |
| Middle occipital gyrus | B | 7, 31 | — | — | — | — | — | −3 | −67 | 28 | 3.19 | 2,481 |
| Cerebellum | B | — | 6 | −74 | −31 | 3.43 | 44,668 | 8 | −72 | −33 | 3.22 | 34,134 |
| Dorsal striatum* | L | — | −11 | 17 | 6 | 2.2 | 342 | — | — | — | — | — |
| Ventral striatum* | B | — | — | — | — | — | — | −6 | 15 | −5 | 2.22 | 2,381 |
Note: Significant activations at P < 0.01 (whole‐brain FWE corrected). Asterisks (*) indicate brain regions that additionally activated at P < 0.05 (whole‐brain FWE corrected). Lat, laterality; B, bilateral; L, left; R, right; BA, Brodmann's area; VmPFC, ventromedial prefrontal cortex. Montreal Neurological Institute (MNI) coordinates were used.
Sex by Condition Interactions
A significant Sex × Condition interaction was evident in several regions of the cortico–striatal–limbic circuit (P < 0.01, whole‐brain FWE corrected) (see Table III and Fig. 3). In the Stress‐Neutral condition, men showed greater activations in brain regions involved in emotional modulation compared to women. These regions included the mPFC (dorsomedial and ventromedial), rostral ACC, posterior insula, putamen, as well as limbic regions such as the amygdala, hippocampus, and parahippocampal gyrus. Additionally, selected areas of superior/middle/inferior temporal lobe, superior parietal lobe, lingual gyrus, and cerebellum were more activated in men compared to women. In the Alcohol cue‐Neutral contrast, women displayed greater activation in the left SFG/MFG (BA 6) compared to men (see Table III and Fig. 3).
Table III.
Sex differences in brain activations during stress and alcohol‐cue exposures
| Sex × task | Stress‐neutral | Alcohol cue‐neutral | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coordinates | Coordinates | Coordinates | |||||||||||||||
| X | Y | Z | X | Y | Z | ||||||||||||
| Regions of activation | Lat | BA | X | Y | Z | Volume (mm3) | F | Male > Female | Volume (mm3) | t | Female > Male | Volume (mm3) | t | ||||
| Superior/middle frontal gyrus | L | 6 | −19 | −8 | 65 | 2,056 | 5.98 | — | — | — | — | — | −15 | −12 | 62 | 2,704 | 2.33 |
| Medial frontal gyrus | B | 9, 10 | −1 | 48 | 21 | 1,595 | 5.63 | 2 | 46 | 23 | 6,878 | 2.5 | — | — | — | — | — |
| Rostral anterior cingulate gyrus | B | 32 | 6 | 39 | 16 | 1,051 | 5.79 | 4 | 40 | 14 | 2,667 | 2.4 | — | — | — | — | — |
| Posterior insula | L | — | −42 | −6 | −7 | 422 | 5.54 | −37 | −6 | −9 | 641 | 2.26 | — | — | — | — | — |
| R | — | 43 | 6 | −4 | 3,432 | 6.04 | 40 | 7 | −13 | 1,381 | 2.24 | — | — | — | — | — | |
| Amygdala | L | — | −23 | −1 | −15 | 906 | 5.66 | −23 | −3 | −17 | 1,803 | 2.53 | — | — | — | — | — |
| R | — | 27 | −3 | −14 | 633 | 5.51 | 26 | −2 | −17 | 1,780 | 2.41 | — | — | — | — | — | |
| Hippocampus | L | — | −29 | −22 | −13 | 919 | 5.47 | −28 | −19 | −16 | 3,095 | 2.5 | — | — | — | — | — |
| R | — | 24 | −15 | −15 | 408 | 5.38 | 28 | −16 | −16 | 1,957 | 2.46 | — | — | — | — | — | |
| Parahippocampal gyrus | R | — | 22 | −21 | −16 | 166 | 5.35 | 21 | −29 | −14 | 1,774 | 2.39 | — | — | — | — | — |
| Putamen | L | — | −24 | 7 | −11 | 387 | 5.78 | −25 | 4 | −9 | 796 | 2.29 | — | — | — | — | — |
| Superior/middle TG | L | 21, 22, 38 | −44 | 0 | −18 | 992 | 5.92 | −36 | 1 | −28 | 1,989 | 2.26 | — | — | — | — | — |
| Superior/middle/inferior TG | R | 20, 21, 22, 37, 39 | 50 | −38 | −4 | 8,061 | 6.29 | 52 | −43 | −4 | 24,088 | 2.47 | — | — | — | — | — |
| Superior parietal lobe | B | 7 | 12 | −66 | 55 | 4,372 | 5.88 | 8 | −62 | 51 | 10,384 | 2.46 | — | — | — | — | — |
| Lingual gyrus (occipital lobe) | L | 19 | −14 | −54 | −2 | 1,340 | 5.81 | −15 | −55 | 0 | 3,085 | 2.46 | — | — | — | — | — |
| Cerebellum | B | — | 17 | −53 | −19 | 4,058 | 6.06 | 10 | −52 | −21 | 7,688 | 2.47 | — | — | — | — | — |
Whole‐brain FWE corrected thresholds at P < 0.01 for Sex × Task and at P < 0.05 for Stress > Neutral and Alcohol Cue > Neutral. Lat, laterality; B, bilateral; L, left; R, right; BA, Brodmann's area; TG, temporal gyrus. No differences in female > male for the Stress‐Neutral and in male > female for the Alcohol Cue‐Neutral survived FWE correction.
Figure 3.
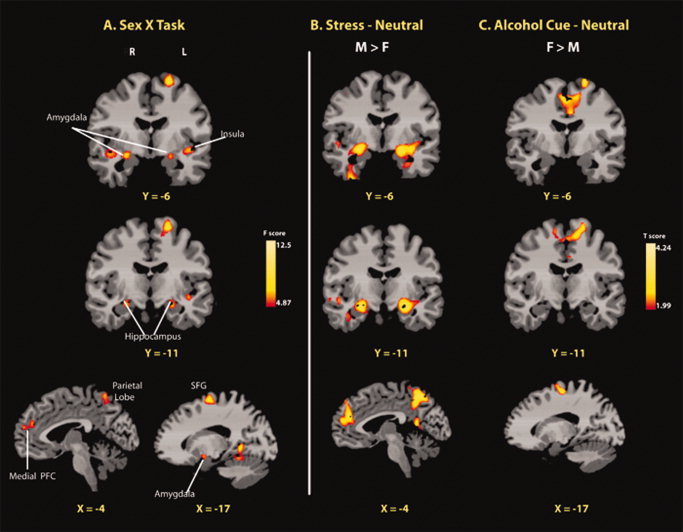
Whole‐brain voxel‐based fMRI images showing a sex × condition interaction and corresponding activations in the Stress‐Neutral and Alcohol Cue‐Neutral contrasts for males (M) and females (F). A: The sex × condition interaction effect was significant in regions of the MFG/SFG, mPFC (dorsomedial and ventromedial), rostral ACC, emotion limbic regions (posterior insula, putamen, amygdala, hippocampus, and parahippocampal gyrus), temporal lobe, and visuomotor perception areas (pariental lobe, occipital lobe, and cerebellum) (P < 0.01 whole‐brain FWE corrected). To elucidate the source of the interaction, male versus female contrasts were conducted for, (B) stress relative to neutral, and (C) alcohol cue relative to neutral brain responses at the P < 0.05 whole‐brain FWE corrected. Significantly, greater M > F stress‐induced activity in the mPFC and limbic regions was observed. Alcohol cue‐induced activity in the MFG/SFG was significantly higher in women than men. No differences in F > M for the Stress‐Neutral and in M > F contrast for the Alcohol cue‐Neutral survived whole‐brain correction. Coordinates are given in MNI space. R, right; L, left; M, male; F, female; PFC, prefrontal cortex. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Secondary analyses including days of alcohol used per week (frequency) and amount of current alcohol use as covariates were also conducted, and the above results were unchanged.
Correlational Analysis
For stress‐induced anxiety, whole‐brain correlation analysis revealed differential associations for men and women (P < 0.05, whole‐brain FWE corrected). In men, significant positive correlations with anxiety were observed in the medial OFC, ventromedial PFC, left superior‐mPFC, and rostral ACC. In women, stress‐induced anxiety was significantly associated with brain activity in the middle and superior frontal regions (see Table IV and Fig. 4). There were no outliers in the relation between brain activity and stress‐induced anxiety in men. In women, there was one extreme value (greater than one using Cook's distance) in the relationship between female anxiety and SFG/MFG activity. The correlation was still significant even after removing this value. To reduce the influence of this value, the r value was presented after the Winsorization in Table IV (r = 0.62).
Table IV.
Neural correlates of alcohol cue‐induced craving and stress‐induced anxiety
| Regions of activation | Lat | BA | Coordinates | Volume (mm3) | r | ||
|---|---|---|---|---|---|---|---|
| X | Y | Z | |||||
| Alcohol cue‐induced craving | Alcohol cue > neutral | ||||||
| Male | |||||||
| Striatum cluster (dorsal, ventral) | B | — | −3 | 16 | −3 | 2652 | 0.74 |
| Inferior frontal gyrus (DLPFC, LPFC) | R | 45, 46 | 35 | 32 | 12 | 653 | 0.56 |
| Rostral/dorsal anterior cingulate gyrus | B | 24 | −7 | 34 | 0 | 230 | 0.57 |
| Middle/inferior temporal gyrus | L | 20, 37 | −56 | −42 | −22 | 695 | 0.63 |
| Stress‐induced anxiety | Stress > neutral | ||||||
| Male | |||||||
| Superior medial frontal gyrus | L | 8, 9 | −10 | 49 | 42 | 2591 | 0.61 |
| Medial PFC/ACC cluster (medial OFC, VmPFC, and rostral ACC) | B | 10, 11, 32 | −4 | 47 | 1 | 4413 | 0.59 |
| Female | |||||||
| Superior/middle frontal gyrus | B | 6, 8 | 8 | 6 | 61 | 2919 | 0.62 |
Note: Significant correlations at P < 0.05 (whole‐brain FWE corrected). Lat, laterality; B, bilateral; L, left; R, right; BA, Brodmann's area PFC, prefrontal cortex; ACC, anterior cingulate cortex; VmPFC, ventromedial PFC; OFC, orbitofrontal cortex; DLPFC, dorsolateral PFC; LPFC, lateral PFC. MNI coordinates were used.
For alcohol cue‐induced craving, male alcohol craving was positively associated with activity in the striatum, right dorsolateral and lateral PFC, ACC, and middle/inferior temporal lobe (P < 0.05, whole‐brain corrected; see Table IV and Fig. 4). The striatum cluster encompassing both the ventral and dorsal portion was significantly positively correlated with alcohol craving (see Fig. 4). No outlier scores were present in associations between craving and the striatum as well as other brain regions (Table IV). There were no brain regions associated with female alcohol craving that survived multiple comparisons.
DISCUSSION
The current findings indicate overlapping neural responses to stress and alcohol context cues in healthy individuals with increased activation of the cortico‐striatal–limbic circuit encompassing the medial and lateral PFC, ACC, PCC, anterior insula, and striatum, areas known to be involved in processing of emotion, stress, and rewarding stimuli. Furthermore, significant sex differences in neural processing of stress and alcohol context cues were observed, suggesting sex‐specific functional responses to stress and alcohol cues. These differences in neural responses likely contribute to the well‐known sex differences in stress‐related coping and in vulnerabilities to stress‐related psychiatric disorders.
During stress and alcohol‐cue exposure, strong medial and lateral prefrontal activation along with increased activity in ACC was evident. The PFC is involved in executive control and emotion regulation [Ochsner et al.,2004], and the ACC is associated with online conflict monitoring [MacDonald et al.,2000]. Both these regions were active during stress and alcohol‐cue exposures, indicating activation of neuroregulatory systems for online modulation of stress and alcohol cue experiences. Furthermore, the anterior insula was activated, which is known to be involved in interoceptive processing of emotion, pain, and reward [Harris et al.,2009; Hollander et al.,2008]. VS activation was seen during alcohol‐cue exposure, while activity in the left dorsal striatum increased during stress exposure. Increases in dorsal striatal activity during stress have been reported previously [Sinha and Li,2007], and chronic stress is known to alter dorsal striatal projections to the frontal cortex [Rossi et al.,2009], supporting the notion that stress influences habit‐based decision making involving fronto‐striatal pathways [Dias‐Ferreira et al.,2009]. The VS has been associated with reward processing [Haber and Knutson,2010], and our findings of specific activation in the VS during alcohol context cue exposure are consistent with this observation and previous studies showing VS activation with an alcohol taste cue [Filbey et al.,2008] and intravenous injection of alcohol [Gilman et al.,2008].
In comparing the alcohol cue with the stress condition, greater activity in the premotor region was seen in the alcohol cue relative to the stress condition, suggesting that action‐specific components of emotion may be more activated during this condition. This is consistent with previous research indicating that pleasant emotion facilitates approach‐related behaviors and psychomotor activation, whereas unpleasant and stressful emotion promotes defensive urges [Lang,2000; Lang et al.,1997]. Alcohol is also shown to generate positive appetitive states in healthy social drinkers [Lukas et al.,1986], and it is suggested that motor control is an essential part of appetitive processing [Haber and Knutson,2010].
Sex differences in neural responses were also observed. During stress, men showed greater activation in brain regions including the ventromedial PFC, rostral ACC, posterior insula, amygdala, and hippocampus than women. The neural circuit connecting the medial PFC, rostral ACC, and amygdala is regarded as the core circuit for emotional modulation [Davidson et al.,2000; Ochsner et al.,2004], especially in stress experiences [Li and Sinha,2008; Sinha et al.,2006]. Male‐specific hyperactivity in emotion‐related brain regions are consistent with prior research indicating greater emotional and physiological responses in men following stress compared to women, including higher cortisol levels [Kudielka and Kirschbaum,2005] and diastolic blood pressure responses [Chaplin et al.,2008] and greater negative and aggressive emotions [Verona et al.,2007]. Greater sensitivity to stress‐related conditioning effects has also been reported in males relative to females in animal [Wood and Shors,1998; Wood et al.,2001] and in human studies [Jackson et al.,2006]. In contrast, females showed greater activity in the SFG and MFG during alcohol‐cue exposures. The SFG/MFG is involved in high‐level cognitive processing such as reasoning [Goel and Dolan,2003], working memory [Smith and Jonides,1999], and attention during language processing [Cabeza and Nyberg,2000]. Greater activations in these regions during alcohol cue reflect that healthy women may use attention and language‐based processing of alcohol context cues. Interestingly, these sex differences in neural responses to stress and alcohol cues are consistent with previous psychobiological and learning studies indicating a tendency in males toward using interoceptive and associative cues to identify emotions and in learning tasks, whereas females using external, context‐dependent stimuli to identify their emotions and in learning tasks [Herman and Wallen,2007; Roberts and Pennebaker,1995; Sandstrom,2007; Sandstrom et al.,1998]. Thus, males and females appear to rely on different types of information in emotion, stress, and learning contexts, which, in turn, are likely to activate different brain regions in a sex‐specific manner, as observed in the current data.
Sex‐specific patterns were also evident in the relationship between brain activity and stress‐induced anxiety ratings. In men, stress‐induced anxiety was positively correlated with activity in the ventromedial PFC, medial OFC, left superior‐mPFC, and ACC, areas showing greater stress‐related activation in men. Associations with these emotion modulatory regions [Davidson et al.,2000; Ochsner et al.,2004] suggest more emotion‐focused processing in males while experiencing stress‐induced anxiety. In females, SFG/MFG activity was positively correlated with stress‐induced anxiety. The SFG/MFG is involved in cognitive processing [Cabeza and Nyberg,2000; Goel and Dolan,2003] and also closely interacts with anterior parts of PFC to guide emotional behaviors [Koechlin et al.,2000]. Recent evidence indicates that the SFG/MFG contributes to response correction during reasoning processes [Kalbfleisch et al.,2007], and activity in these regions increased in individuals with anxious tendencies [Karch et al.,2008]. These findings suggest greater utilization of cognitive processes in experiencing stress‐induced anxiety in women.
For alcohol‐cue‐induced craving, significant neural correlates of male craving were found dominantly in the striatum and also selected regions of right dorsolateral/lateral PFC, ACC, and middle/inferior temporal lobe. The association of striatal activity with craving is consistent with previous studies with alcohol‐dependent individuals [Heinz et al.,2004; Modell and Mountz,1995; Myrick et al.,2004] as well as with other addictive disorders [Rothemund et al.,2007; Vanderschuren et al.,2005; Volkow et al.,2006]. We found significant correlations between craving and brain activity in both ventral and dorsal striatum. In reinforcement learning, the VS is thought to be engaged in learning reward values, and the dorsal striatum maintains reward values to guide decision and behaviors [Kahnt et al.2009]. Studies showed that the dorsal striatum is specifically involved in action initiation and in habit learning, including chronic drug‐use habits [Kahnt et al.2009; Porrino et al.2004; Volkow et al.2006]. It is also known that the dorsal striatum is associated with craving when conditioned stimuli (e.g., cues) are presented to human subjects [Volkow et al. 2006]. Concurrent correlated activity of the ventral and dorsal striatum with craving in our data suggests that desire for alcohol involves processing and actively pursuing reward values in the presence of alcohol context cues. It is notable that we found this association in healthy men, because previous studies have shown correlations between striatal activity and craving in individuals with addictive disorders or in heavy drinkers, but not in healthy individuals. This suggests the involvement of the striatum in motivational aspects of wanting in both healthy and in clinical samples.
In addition, the correlation with alcohol craving was found in neural circuit of reward regulation, connecting DLPFC, ACC, and the striatum. The DLPFC and ACC are regarded as key regulatory regions for reward processing, with the dorsal ACC showing an involvement in monitoring reward and the DLPFC evaluating these stimuli for reward‐based decision‐making [Haber and Knutson,2010]. The DLPFC is also involved in context‐dependent processing associated with drug/reward cues [Wilson et al.,2004]. Individuals having alcohol use disorder showed increased activity in DLPFC [George et al.,2001] compared to healthy social drinkers. Research suggests that altered functional connectivity between DLPFC and striatum significantly contributes to increased craving in individuals with alcohol dependence [Park et al.,2010]. Furthermore, increased activity in the ACC and striatum was associated with high levels of alcohol craving in alcoholic participants [Myrick et al.,2004]. These results, along with our finding, suggest that the neural circuit connecting the striatum, ACC, and DLPFC plays an important role in the modulation of alcohol‐cue‐induced motivation.
Unlike in men, we did not find significant association of brain activity with alcohol craving that survived whole‐brain correction in women. This result is similar to previous studies showing alcohol‐cue induced craving only in men, but not in healthy socially drinking women [Willner et al.,1998]. As alcohol craving is known to be influenced by hormonal and mood fluctuations [Epstein et al.,2006; Kraus et al.,2004; Rubonis et al.,1994] in women, it is possible that these factors affected the lack of strong neural associations with craving in women. Furthermore, women were drinking at lower levels than men, albeit not significantly less so, and hence recruitment of women with moderate to heavy drinking habits in future studies would be important to identify neural correlates of alcohol craving in women.
CONCLUSION
Taken together, the current findings provide important neural insights into sex differences in stress‐related coping and alcohol‐related behaviors. Previous research indicates that men show more automatic and behaviorally oriented emotional expression and instrumental coping responses, whereas women tend to verbally express their emotion and use verbal coping strategies [Barrett et al.,2000; Brody and Hall,1993]. Furthermore, women are more likely to engage in conversation, prayer, and rumination [Nolen‐Hoeksema et al.,1999; Tamres et al.,2002] following stress consistent with current data showing engagement of cognitive and verbal brain regions in female stress‐induced anxiety. Men's tendencies of instrumental and action‐oriented stress coping, such as smoking and drinking [Lindquist et al.,1997; Tamres et al.,2002], are consistent with the current data on significant association between striatal activity and alcohol craving as well as greater reactivity in the emotion‐action brain regions during stress. It further supports research showing greater male‐specific vulnerability for developing alcohol use disorders [Kessler et al.,1994] and greater female‐specific vulnerability to rumination and mood disorders [Kessler et al.,1993]. Thus, the current data on sex‐specific neural response to stress and alcohol context cues provide novel insights for understanding sex differences in stress‐related vulnerabilities in the development of stress‐related disorders such as depression and alcohol use disorders.
Acknowledgements
We also thank Adam K. Hong for his technical assistance for this study. All authors do not have direct or indirect financial or personal relationships, interests, and affiliations relevant to the subject matter of the manuscript that have occurred over the last 2 years, nor that are expected in the foreseeable future. Dr. Sinha is on the Scientific Advisory Board for Embera Neurotherapeutics and is also a consultant for Glaxo‐Smith Kline, Pharmaceuticals.
REFERENCES
- Bagby RM, Taylor GJ, Parker JD ( 1988): Construct validity of the Toronto Alexithymia Scale. Psychother Psychosom 50: 29–34. [DOI] [PubMed] [Google Scholar]
- Barrett L, Lane R, Sechrest L, Schwartz G ( 2000): Sex differences in emotional awareness. Person Social Psychol Bull 26: 1027–1035. [Google Scholar]
- Becker JB, Monteggia LM, Perrot‐Sinal TS, Romeo RD, Taylor JR, Yehuda R, Bale TL ( 2007): Stress and disease: Is being female a predisposing factor? J Neurosci 27: 11851–11855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boscarino JA, Adams RE, Galea S ( 2006): Alcohol use in New York after the terrorist attacks: A study of the effects of psychological trauma on drinking behavior. Addict Behav 31: 606–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody LR, Hall J ( 1993): Gender and emotion In: Lewis M, Haviland J, editors. Handbook of Emotions. . N.Y.: Guilford Press; pp 447–460. [Google Scholar]
- Cabeza R, Nyberg L ( 2000): Imaging cognition II: An empirical review of 275 PET and fMRI studies. J Cogn Neurosci 12: 1–47. [DOI] [PubMed] [Google Scholar]
- Cacioppo JT, Gardner WL, Berntson GG ( 1999): The affect system has parallel and integrative processing components: Form follows function. J Person Social Psychol 76: 839–855. [Google Scholar]
- Carver CS ( 2001): Affect and the functional bases of behavior: On the dimensional structure of affective experience. Person Social Psychol Rev 5: 345–356. [Google Scholar]
- Chaplin TM, Hong K, Bergquist K, Sinha R ( 2008): Gender differences in response to emotional stress: An assessment across subjective, behavioral, and physiological domains and relations to alcohol craving. Alcohol Clin Exp Res 32: 1242–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen EH, Dixon WJ ( 1972): Estimates of parameters of a censored regression sample. J Am Stat Assoc 67: 664–671. [Google Scholar]
- Cohen S, Janicki‐Deverts D, Miller GE ( 2007): Psychological stress and disease. JAMA 298: 1685–1687. [DOI] [PubMed] [Google Scholar]
- Cox RW ( 1996): AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res 29: 162–173. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Putnam KM, Larson CL ( 2000): Dysfunction in the neural circuitry of emotion regulation—A possible prelude to violence. Science 289: 591–594. [DOI] [PubMed] [Google Scholar]
- Dawson DA, Grant BF, Ruan WJ ( 2005): The association between stress and drinking: modifying effects of gender and vulnerability. Alcohol Alcohol 40: 453–460. [DOI] [PubMed] [Google Scholar]
- Dias‐Ferreira E, Sousa JC, Melo I, Morgado P, Mesquita AR, Cerqueira JJ, Costa RM, Sousa N ( 2009): Chronic stress causes frontostriatal reorganization and affects decision‐making. Science 325: 621–625. [DOI] [PubMed] [Google Scholar]
- Dixon WJ ( 1960): Simplified estimation from censored normal samples. Ann Math Stat 31: 385–391. [Google Scholar]
- Duncan JS, Papademetris X, Yang J, Jackowski M, Zeng X, Staib LH ( 2004): Geometric strategies for neuroanatomic analysis from MRI. Neuroimage 23 ( Suppl 1): S34–S45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein EE, Rhines KC, Cook S, Zdep‐Mattocks B, Jensen NK, Mccrady BS ( 2006): Changes in alcohol craving and consumption by phase of menstrual cycle in alcohol dependent women. J Substance Use 11: 323–332. [Google Scholar]
- Evans A, Collins D, Mills S, Brown E, Kelly R, Peters T ( 1993): 3D statistical neuroanatomical models from 305 MRI volumes. Proceedings of the Nuclear Science Symposium and Medical Imaging Conference, Vol. 3, San Francisco, CA, USA. pp 1813–1817.
- Filbey FM, Claus E, Audette AR, Niculescu M, Banich MT, Tanabe J, Du YP, Hutchison KE ( 2008): Exposure to the taste of alcohol elicits activation of the mesocorticolimbic neurocircuitry. Neuropsychopharmacology 33: 1391–1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox HC, Hong KI, Siedlarz K, Sinha R ( 2008): Enhanced sensitivity to stress and drug/alcohol craving in abstinent cocaine‐dependent individuals compared to social drinkers. Neuropsychopharmacology 33: 796–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Williams S, Howard R, Frackowiak RS, Turner R ( 1996): Movement‐related effects in fMRI time‐series. Magn Reson Med 35: 346–355. [DOI] [PubMed] [Google Scholar]
- Garavan H, Pendergrass JC, Ross TJ, Stein EA, Risinger RC ( 2001): Amygdala response to both positively and negatively valenced stimuli. Neuroreport 12: 2779–2783. [DOI] [PubMed] [Google Scholar]
- George MS, Anton RF, Bloomer C, Teneback C, Drobes DJ, Lorberbaum JP, Nahas Z, Vincent DJ ( 2001): Activation of prefrontal cortex and anterior thalamus in alcoholic subjects on exposure to alcohol‐specific cues. Arch Gen Psychiatry 58: 345–352. [DOI] [PubMed] [Google Scholar]
- Gilman JM, Ramchandani VA, Davis MB, Bjork JM, Hommer DW ( 2008): Why we like to drink: A functional magnetic resonance imaging study of the rewarding and anxiolytic effects of alcohol. J Neurosci 28: 4583–4591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goel V, Dolan RJ ( 2003): Reciprocal neural response within lateral and ventral medial prefrontal cortex during hot and cold reasoning. Neuroimage 20: 2314–2321. [DOI] [PubMed] [Google Scholar]
- Goldstein JM, Jerram M, Abbs B, Whitfield‐Gabrieli S, Makris N ( 2010): Sex differences in stress response circuitry activation dependent on female hormonal cycle. J Neurosci 30: 431–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grzywacz JG, Almeida DM ( 2008): Stress and binge drinking: A daily process examination of stressor pile‐up and socioeconomic status in affect regulation. Int J Stress Manag 15: 364–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN, Knutson B ( 2010): The reward circuit: Linking primate anatomy and human imaging. Neuropsychopharmacology 35: 4–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris RE, Sundgren PC, Craig AD, Kirshenbaum E, Sen A, Napadow V, Clauw DJ ( 2009): Elevated insular glutamate in fibromyalgia is associated with experimental pain. Arthritis Rheum 60: 3146–3152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinz A, Siessmeier T, Wrase J, Hermann D, Klein S, Grusser SM, Flor H, Braus DF, Buchholz HG, Grunder G, Schreckenberger M, Smolka MN, Rosch F, Mann K, Bartenstein P ( 2004): Correlation between dopamine D2 receptors in the ventral striatum and central processing of alcohol cues and craving. Am J Psychiatry 161: 1783–1789. [DOI] [PubMed] [Google Scholar]
- Herman RA, Wallen K ( 2007): Cognitive performance in rhesus monkeys varies by sex and prenatal androgen exposure. Horm Behav 51: 496–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochberg Y ( 1988): A sharper Bonferroni procedure for multiple tests of significance. Biometrika 75: 800–802. [Google Scholar]
- Hollander JA, Lu Q, Cameron MD, Kamenecka TM, Kenny PJ ( 2008): Insular hypocretin transmission regulates nicotine reward. Proc Natl Acad Sci USA 105: 19480–19485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes CJ, Hoge R, Collins L, Woods R, Toga AW, Evans AC ( 1998): Enhancement of MR images using registration for signal averaging. J Comput Assist Tomogr 22: 324–333. [DOI] [PubMed] [Google Scholar]
- Jackson ED, Payne JD, Nadel L, Jacobs WJ ( 2006): Stress differentially modulates fear conditioning in healthy men and women. Biol Psychiatry 59: 516–522. [DOI] [PubMed] [Google Scholar]
- Kahnt T, Park SQ, Cohen MX, Beck A, Heinz A, Wrase J ( 2009): Dorsal striatal‐midbrain connectivity in humans predicts how reinforcements are used to guide decisions. J Cogn Neurosci 21: 1332–1345. [DOI] [PubMed] [Google Scholar]
- Kalbfleisch ML, Van Meter JW, Zeffiro TA ( 2007): The influences of task difficulty and response correctness on neural systems supporting fluid reasoning. Cogn Neurodyn 1: 71–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karch S, Jager L, Karamatskos E, Graz C, Stammel A, Flatz W, Lutz J, Holtschmidt‐Taschner B, Genius J, Leicht G, Pogarell O, Born C, Moller HJ, Hegerl U, Reiser M, Soyka M, Mulert C ( 2008): Influence of trait anxiety on inhibitory control in alcohol‐dependent patients: Simultaneous acquisition of ERPs and BOLD responses. J Psychiatr Res 42: 734–745. [DOI] [PubMed] [Google Scholar]
- Kessler RC, McGonagle KA, Swartz M, Blazer DG, Nelson CB ( 1993): Sex and depression in the National Comorbidity Survey. I. Lifetime prevalence, chronicity and recurrence. J Affect Disord 29: 85–96. [DOI] [PubMed] [Google Scholar]
- Kessler RC, McGonagle KA, Zhao S, Nelson CB, Hughes M, Eshleman S, Wittchen HU, Kendler KS ( 1994): Lifetime and 12‐month prevalence of DSM‐III‐R psychiatric disorders in the United States. Results from the National Comorbidity Survey. Arch Gen Psychiatry 51: 8–19. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Kudielka BM, Gaab J, Schommer NC, Hellhammer DH ( 1999): Impact of gender, menstrual cycle phase, and oral contraceptives on the activity of the hypothalamus‐pituitary‐adrenal axis. Psychosom Med 61: 154–162. [DOI] [PubMed] [Google Scholar]
- Koechlin E, Corrado G, Pietrini P, Grafman J ( 2000): Dissociating the role of the medial and lateral anterior prefrontal cortex in human planning. Proc Natl Acad Sci USA 97: 7651–7656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus T, Reulbach U, Bayerlein K, Mugele B, Hillemacher T, Sperling W, Kornhuber J, Bleich S ( 2004): Leptin is associated with craving in females with alcoholism. Addict Biol 9: 213–219. [DOI] [PubMed] [Google Scholar]
- Kudielka BM, Kirschbaum C ( 2005): Sex differences in HPA axis responses to stress: A review. Biol Psychol 69: 113–132. [DOI] [PubMed] [Google Scholar]
- Lang PJ. ( 2000): Emotion and motivation: Attention, perception, and action. J Sport Exercise Psychol 22: S122–S140. [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert MM. ( 1997): Motivated Attention: Affect, Activation and Action. Hillsdale, NJ: Lawrence Erlbaum Associates; 39 p. [Google Scholar]
- Li CS, Kosten TR, Sinha R ( 2005): Sex differences in brain activation during stress imagery in abstinent cocaine users: A functional magnetic resonance imaging study. Biol Psychiatry 57: 487–494. [DOI] [PubMed] [Google Scholar]
- Li CS, Sinha R ( 2008): Inhibitory control and emotional stress regulation: Neuroimaging evidence for frontal‐limbic dysfunction in psycho‐stimulant addiction. Neurosci Biobehav Rev 32: 581–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist TL, Beilin LJ, Knuiman MW ( 1997): Influence of lifestyle, coping, and job stress on blood pressure in men and women. Hypertension 29( 1, Pt 1): 1–7. [DOI] [PubMed] [Google Scholar]
- Lopez JF, Akil H, Watson SJ ( 1999): Neural circuits mediating stress. Biol Psychiatry 46: 1461–1471. [DOI] [PubMed] [Google Scholar]
- Lukas SE, Mendelson JH, Benedikt RA, Jones B ( 1986): EEG alpha activity increases during transient episodes of ethanol‐induced euphoria. Pharmacol Biochem Behav 25: 889–895. [DOI] [PubMed] [Google Scholar]
- MacDonald AW, III , Cohen JD, Stenger VA, Carter CS ( 2000): Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science 288: 1835–1838. [DOI] [PubMed] [Google Scholar]
- Modell JG, Mountz JM ( 1995): Focal cerebral blood flow change during craving for alcohol measured by SPECT. J Neuropsychiatry Clin Neurosci 7: 15–22. [DOI] [PubMed] [Google Scholar]
- Myrick H, Anton RF, Li X, Henderson S, Drobes D, Voronin K, George MS ( 2004): Differential brain activity in alcoholics and social drinkers to alcohol cues: Relationship to craving. Neuropsychopharmacology 29: 393–402. [DOI] [PubMed] [Google Scholar]
- Nachmias M, Gunnar M, Mangelsdorf S, Parritz RH, Buss K ( 1996): Behavioral inhibition and stress reactivity: The moderating role of attachment security. Child Dev 67: 508–522. [PubMed] [Google Scholar]
- Nolen‐Hoeksema S ( 1987): Sex differences in unipolar depression: Evidence and theory. Psychol Bull 101: 259–282. [PubMed] [Google Scholar]
- Nolen‐Hoeksema S, Larson J, Grayson C ( 1999): Explaining the gender difference in depressive symptoms. J Pers Soc Psychol 77: 1061–1072. [DOI] [PubMed] [Google Scholar]
- Nolf E, Voet T, Jacobs F, Dierckx R, Lemahieu I. (2003): XMedCon—An open‐source medical image conversion toolkit. Eur J Nucl Med 30, S246, http://xmedcon.sf.net [Google Scholar]
- Ochsner KN, Ray RD, Cooper JC, Robertson ER, Chopra S, Gabrieli JD, Gross JJ ( 2004): For better or for worse: Neural systems supporting the cognitive down‐ and up‐regulation of negative emotion. Neuroimage 23: 483–499. [DOI] [PubMed] [Google Scholar]
- Office for National Statistics ( 2006): Smoking and drinking among adults. General Household Survey 2005. http://www.statistics.gov.uk/ghs.
- Papademetris X ( 2006): BioImage Suite: An Intergrated Medical Image Analysis Suite [database on the Internet] http://bioimagesuite.org. New Haven: Section of Bioimaging Sciences, Deptartment of Diagnostic Radiology, Yale School of Medicine; [Google Scholar]
- Park SQ, Kahnt T, Beck A, Cohen MX, Dolan RJ, Wrase J, Heinz A ( 2010): Prefrontal cortex fails to learn from reward prediction errors in alcohol dependence. J Neurosci 30: 7749–7753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porrino LJ, Lyons D, Smith HR, Daunais JB, Nader MA ( 2004): Cocaine self‐administration produces a progressive involvement of limbic, association, and sensorimotor striatal domains. J Neurosci 24: 3554–3562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts TA, Pennebaker JW ( 1995): Women's and men's strategies in perceiving internal state In: Zanna IM, editor. Advances in Experimental Social Psychology. New York: Academic Press; pp 143–176. [Google Scholar]
- Robinson TE, Berridge KC ( 1993): The neural basis of drug craving: An incentive‐sensitization theory of addiction. Brain Res Brain Res Rev 18: 247–291. [DOI] [PubMed] [Google Scholar]
- Rossi S, De Chiara V, Musella A, Mataluni G, Sacchetti L, Bernardi G, Usiello A, Centonze D ( 2009): Adaptations of striatal endocannabinoid system during stress. Mol Neurobiol 39: 178–184. [DOI] [PubMed] [Google Scholar]
- Rothemund Y, Preuschhof C, Bohner G, Bauknecht HC, Klingebiel R, Flor H, Klapp BF ( 2007): Differential activation of the dorsal striatum by high‐calorie visual food stimuli in obese individuals. Neuroimage 37: 410–421. [DOI] [PubMed] [Google Scholar]
- Rubonis AV, Colby SM, Monti PM, Rohsenow DJ, Gulliver SB, Sirota AD ( 1994): Alcohol cue reactivity and mood induction in male and female alcoholics. J Stud Alcohol 55: 487–494. [DOI] [PubMed] [Google Scholar]
- Sandstrom NJ ( 2007): Sex differences in use of visual cues by rhesus monkeys performing a spatial learning task: Comment on “Cognitive performance in rhesus monkeys varies by sex and prenatal androgen exposure” by Herman and Wallen. Horm Behav 52: 139–142. [DOI] [PubMed] [Google Scholar]
- Sandstrom NJ, Kaufman J, Huettel SA ( 1998): Males and females use different distal cues in a virtual environment navigation task. Brain Res Cogn Brain Res 6: 351–360. [DOI] [PubMed] [Google Scholar]
- Schneider F, Habel U, Wagner M, Franke P, Salloum JB, Shah NJ, Toni I, Sulzbach C, Honig K, Maier W, Gaebel W, Zilles K ( 2001): Subcortical correlates of craving in recently abstinent alcoholic patients. Am J Psychiatry 158:1075–1083. [DOI] [PubMed] [Google Scholar]
- Schott BH, Minuzzi L, Krebs RM, Elmenhorst D, Lang M, Winz OH, Seidenbecher CI, Coenen HH, Heinze HJ, Zilles K, Duzel E, Bauer A ( 2008): Mesolimbic functional magnetic resonance imaging activations during reward anticipation correlate with reward‐related ventral striatal dopamine release. J Neurosci 28: 14311–14319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan P ( 1967): A shortened version of the Bett's questionnaire upon mental imagery. J Clin Psychol 23: 386–389. [DOI] [PubMed] [Google Scholar]
- Sinha R ( 2009a) Modeling stress and drug craving in the laboratory: Implications for addiction treatment development. Addict Biol 14: 84–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R. ( 2009b) Stress and addiction: A dynamic interplay of genes, environment, and drug intake. Biol Psychiatry 66: 100–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R, Fox HC, Hong KA, Bergquist K, Bhagwagar Z, Siedlarz KM ( 2009): Enhanced negative emotion and alcohol craving, and altered physiological responses following stress and cue exposure in alcohol dependent individuals. Neuropsychopharmacology 34: 1198–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R, Garcia M, Paliwal P, Kreek MJ, Rounsaville BJ ( 2006): Stress‐induced cocaine craving and hypothalamic‐pituitary‐adrenal responses are predictive of cocaine relapse outcomes. Arch Gen Psychiatry 63: 324–331. [DOI] [PubMed] [Google Scholar]
- Sinha R, Lacadie C, Skudlarski P, Wexler BE ( 2004): Neural circuits underlying emotional distress in humans. Ann NY Acad Sci 1032: 254–257. [DOI] [PubMed] [Google Scholar]
- Sinha R, Li CS ( 2007): Imaging stress‐ and cue‐induced drug and alcohol craving: Association with relapse and clinical implications. Drug Alcohol Rev 26: 25–31. [DOI] [PubMed] [Google Scholar]
- Smith EE, Jonides J ( 1999): Storage and executive processes in the frontal lobes. Science 283: 1657–1661. [DOI] [PubMed] [Google Scholar]
- Tamres LK, Janicki D, Helgeson VS ( 2002): Sex differences in coping behavior: A meta‐analytic review and an examination of relative coping. Person Social Psychol Rev 6: 2–30. [Google Scholar]
- Vanderschuren LJ, Di Ciano P, Everitt BJ ( 2005): Involvement of the dorsal striatum in cue‐controlled cocaine seeking. J Neurosci 25: 8665–8670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verona E, Reed A II, Curtin JJ, Pole M ( 2007): Gender differences in emotional and overt/covert aggressive responses to stress. Aggress Behav 33: 261–271. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Telang F, Fowler JS, Logan J, Childress AR, Jayne M, Ma Y, Wong C ( 2006): Cocaine cues and dopamine in dorsal striatum: Mechanism of craving in cocaine addiction. J Neurosci 26: 6583–6588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willner P, Field M, Pitts K, Reeve G ( 1998): Mood, cue and gender influences on motivation, craving and liking for alcohol in recreational drinkers. Behav Pharmacol 9: 631–642. [DOI] [PubMed] [Google Scholar]
- Wilson SJ, Sayette MA, Fiez JA ( 2004): Prefrontal responses to drug cues: A neurocognitive analysis. Nat Neurosci 7: 211–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood GE, Beylin AV, Shors TJ ( 2001): The contribution of adrenal and reproductive hormones to the opposing effects of stress on trace conditioning in males versus females. Behav Neurosci 115: 175–187. [DOI] [PubMed] [Google Scholar]
- Wood GE, Shors TJ ( 1998): Stress facilitates classical conditioning in males, but impairs classical conditioning in females through activational effects of ovarian hormones. Proc Natl Acad Sci USA 95: 4066–4071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrase J, Schlagenhauf F, Kienast T, Wustenberg T, Bermpohl F, Kahnt T, Beck A, Strohle A, Juckel G, Knutson B, Heinz A ( 2007): Dysfunction of reward processing correlates with alcohol craving in detoxified alcoholics. Neuroimage 35: 787–794. [DOI] [PubMed] [Google Scholar]
- Xiong J, Gao J‐H, Lancaster JL, Fox PT ( 1995): Clustered pixels analysis for functional MRI activation studies of the human brain. Human Brain Mapp 3: 287–301. [Google Scholar]
- Zhou Z, Zhu G, Hariri AR, Enoch MA, Scott D, Sinha R, Virkkunen M, Mash DC, Lipsky RH, Hu XZ, Hodgkinson CA, Xu K, Buzas B, Yuan Q, Shen PH, Ferrell RE, Manuck SB, Brown SM, Hauger RL, Stohler CS, Zubieta JK, Goldman D ( 2008): Genetic variation in human NPY expression affects stress response and emotion. Nature 452: 997–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]


