Abstract
Moderate improvements in cardiac performance have been reported in some clinical settings after delivery of bone marrow mononuclear cells to patients with cardiovascular disease (CVD). However, mechanistic insights into how these cells impact outcomes are lacking. To address this, the NHLBI Cardiovascular Cell Therapy Research Network (CCTRN) established a Biorepository Core for extensive phenotyping and cell function studies, and storing bone marrow and peripheral blood for 10 years. Analyzing cell populations and cell function in the context of clinical parameters and clinical outcomes, after cell or placebo treatment, empower the development of novel diagnostic and prognostics. Developing such biomarkers that define the safety and efficacy of cell therapy is a major Biorepository aim.
Introduction
Cell therapy has emerged as a potential treatment for CVD states ranging from refractory ischemia and acute myocardial infarction (AMI) to left ventricular dysfunction and heart failure (HF) (Figure 1). Unlike current treatments aimed at increasing or preserving myocardial performance, cell therapy has the potential to enhance repair of underlying myocyte, matrix, and/or vascular damage. While few insights about cell function and its optimization have materialized, experience suggests autologous cell therapy is safe and offers potential for moderate improvement in cardiac performance (1-6). However, optimal cell preparation, handling and delivery have not been established (7, 8).
Figure 1.
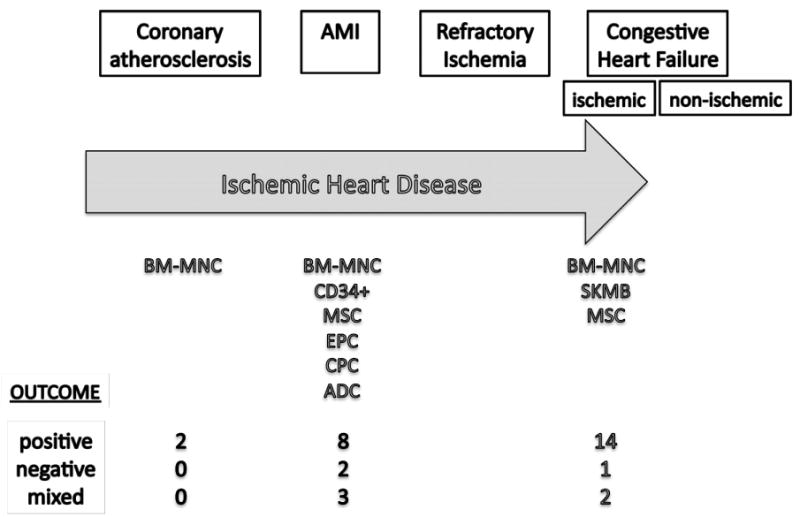
Overview of clinical cell therapy studies in IHD and outcome using different cell types at different clinical stages (BM-MNC, bone marrow mononuclear cells; MSC, mesenchymal cells; EPC, endothelial progenitor cells; CPC, cardiac progenitor cells; ADC, adipose-derived cells; SKMB, skeletal myoblasts)
The most prevalent cell population studied in cardiovascular clinical settings is the bone marrow mononuclear cell fraction (BM-MNC) that contains stem and progenitor cells. Benefit has been suggested with autologous BM-MNCs for acute ST segment elevation myocardial infarction (STEMI) patients after percutaneous reperfusion (1-4, 6, 16-22), but in other patient cohort results have been mixed (Figure 1). Covariates suggested to impact effects of transplanted cells on left ventricular ejection fraction (LVEF) include cell isolation techniques, cell phenotype (i.e. types of cells present), and functional viability of these cells (e.g. ability of cells to form colonies in vitro) (8, 15). The Cardiovascular Cell Therapy Research Network (CCTRN) was established by the National Heart, Lung and Blood Institute (NHLBI) to develop, coordinate, and conduct collaborative trials evaluating effects of stem/progenitor cell therapy on CVD (23). The purpose of this article is to describe the CCTRN Biorepository Core Laboratory, which was designed both to store samples, and to begin to evaluate some of these covariates.
Methods
Details of the trial protocols are published elsewhere (21-24). All studies were conducted according to CCTRN guidelines with approval of Institutional Review Boards of each participating institution, and each patient provided written informed consent.
Collection of bone marrow and peripheral blood for storage and analysis
All patients undergo a standardized bone marrow (BM) aspiration and venipuncture on the day of treatment. Details of cell handling and processing are published elsewhere (25). For patients who provide informed additional consent, after preparation of the treatment product (cell or cell-free) the remaining mononuclear cells and a peripheral blood (PB) specimen are sent to the Biorepository for storage and phenotypic analysis (University of Minnesota) and for functional analysis (University of Florida). The day of BM harvest and study product delivery is designated Day 0. PB is collected at predetermined time points before (Day 0), and after study product delivery (Figure 2).
Figure 2.
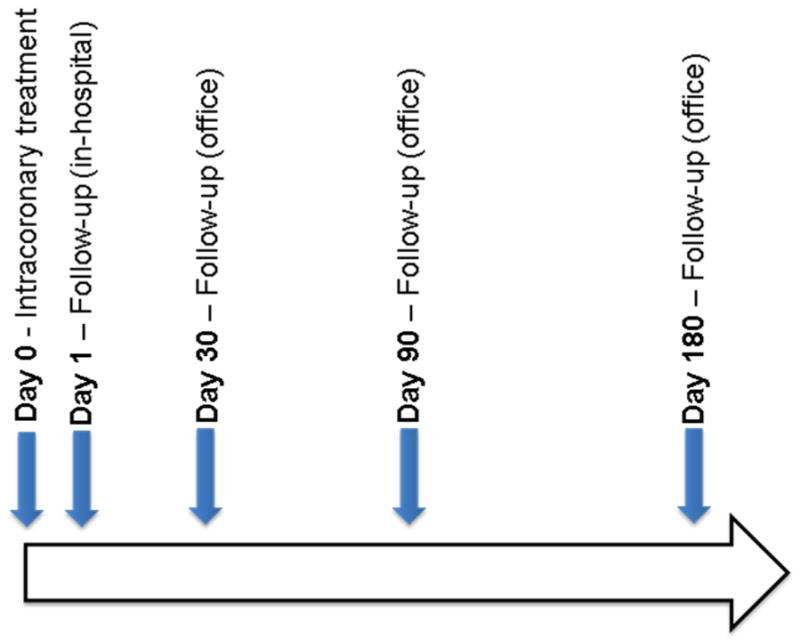
Blood collection across time points in CCTRN TIME, Late-TIME, and FOCUS protocols.
Phenotypic analysis by fluorescence activated cell sorting (FACS)
At each time point (Figure 2), 20mL of PB is obtained in two vacutainers (BD Biosciences, Bedford, MA) containing EDTA following venipuncture; tubes are mixed well, and both BM and PB stored at 4°C until packaged shipping. Prior to shipping, tubes are wrapped in absorbent material and bubble wrap, placed in a Biohazard bag, and shipped with two refrigerated (4°C) packs and appropriate forms for next morning delivery. Upon arrival, PB samples are treated with ammonium chloride potassium (ACK) standard red blood cell lysis buffer, centrifuged at 50×g for five minutes at 4°C, and the supernatant aspirated. The pellet is resuspended in ACK lysis buffer, centrifuged at 50×g for five minutes at 4°C, the supernatant aspirated, and cells washed with PBS twice, before resuspension in FACS buffer containing PBS + 2.5% Fetal Bovine Serum. The number and viability of resulting nucleated cells is determined in an automated cell counter using the Guava ViaCount assay (Millipore, Billerica, MA). One to five million cells are stained using antibodies raised against human CD45, CD34, CD31, CD133, VEGFR2, CXCR4, CD3, CD11, CD14, CD19, and their corresponding isotype control. After incubation, cells are washed twice with, and resuspended in FACS buffer for FACS (LSRII cytometer, BD Biosciences, Bedford, MA). Data are analyzed using FlowJo software (Tree Star Inc, Ashland, OR) with standardized guidelines for gating.
Shipping conditions
To ascertain maximal cell viability during shipping, a shipping study was undertaken. Overall PB cell and subpopulation viabilities were quantified after shipping of samples with either refrigerated (4°C) packs or frozen packs. As a control, samples were not shipped but stored at 4°C overnight or left at room temperature. Room temperature storage resulted in with widely disparate sample viabilities (57.7-92.5%) and was not considered optimal for these samples, and thus was not used as a control. Optimal time-to-processing of PB was determined by evaluating viabilities by FACS-sorted PB at 0, 24, 48, or 72 hrs after harvest. Once optimal shipping conditions were determined, SOPs were generated and distributed to cell processing facilities. Standardized sample shipping containers, cold packs and wrapping materials were also provided. After distribution of materials, optimal sample shipping conditions were addressed by phone calls with network investigators and research coordinators. If BM or PB sample viability from a given center drops below 80%m for a single sample, the center is immediately contacted and shipping procedures reviewed.
Bone Marrow and Peripheral Blood Functional Analysis
On Day 0, 30mL of blood is collected in vacutainers containing EDTA following venipuncture as described above. BM and PB mononuclear cells are analyzed for viability using trypan blue staining (Figure 3A) (Cellometer AutoT4, Nexcelom Bioscience, Lawrence, MA). Cells are reserved for endothelial and mesenchymal colony assays. When available, additional BM cells are processed for enrichment of the CD34+ stem and progenitor cell subset by immunomagnetic selection (Miltenyi, Auburn, CA). These CD34+ cells are then tested using assays for migration to stromal cell derived factor 1 (SDF-1). The CD34+ cell population is used as a surrogate for stem and progenitor cell activity in the entire BM-MNC fraction because use of the distinctly heterogeneous population of BM-MNCs generates highly variable migration data that would be difficult to interpret in the context of the CCTRN trials, which seek to recruit less than 100 subjects per trial.
Figure 3. Cell Function Analyses of Bone Marrow and Peripheral Blood Cells From Subjects Enrolled in CCTRN Clinical Trials.

(A) Micrograph of bone marrow cells upon receipt to core laboratory. Trypan blue exclusion staining is used to evaluate viable cell number. (B) Micrograph of a CFU-EC colony. (C) Micrograph of an ECFC colony. (D) Micrograph of a CFU-F colony.
For endothelial colony assays, mononuclear cells are first plated in endothelial growth media-2 (EGM-2, Lonza, Walkersville, MD), plated on fibronectin-coated dishes (BD Biosciences, Bedford, MA) and incubated at 37°C, 5% CO2, > 95% humidity for 48 hrs. Non-adherent cells are removed for colony forming unit Hill (CFU-Hill) assay and adherent cells are trypsinized for endothelial colony forming cell (ECFC) assay, as previously described (26, 27). Media is exchanged every other day and number of colony forming units, types of colonies and confluence are recorded on days 7, 14, 21 and 28 (Figure 3B & 3C). CFU-Hill colonies are defined as a central core of round cells with radiating elongated spindle-like cells at the periphery. ECFC colonies are defined as circumscribed monolayers of cobblestone-appearing cells.
For mesenchymal stem cell colony assay, also known as the CFU-F assay, three concentrations of BM-MNCs and PB-MNCs are plated in Mesencult serum and cytokine containing media (STEMELL Technologies, Vancouver, CA) according to manufacturer's instructions. Number of colony forming units, type of colonies and confluence are recorded on days 7, 14, 21, and 28 (Figure 3D). CFU-F colonies are defined as circumscribed clusters of spindle-like cells typically 1 to 8 mm in diameter.
To evaluate hematopoietic stem and progenitor cell migration to SDF-1, CD34+ cells are isolated from BM cells using immunomagnetic microbeads conjugated with anti-CD34 antibodies (Miltenyi Biotec Inc., Auburn, CA) and automated magnetic selection (AutoMACS, Miltenyi Biotec Inc.). These CD34+ cells are tested for migration to SDF-1 in a modified Boyden chamber, as previously described (28).
Peripheral blood collection for cytokine or DNA/RNA analysis
At each time point, 10mL of blood are collected in vacutainers containing Heparin Sodium and mixed properly. Tubes are centrifuged within 30 min at 190×g for 10 minutes at 4°C, the top plasma layer is carefully removed for cytokine analysis, without disturbing the middle leukocyte layer. Plasma is aliquoted into cryovials kept at 4°C. For future DNA/RNA analysis, the leukocyte layer is transferred to a separate cryovial avoiding significant erythrocyte contamination. Cryovials are placed in a -80°C freezer within 30 minutes for storage until shipment on dry ice to Biorepository Cell Phenotyping and Storage Core Laboratory (UMN) for centralized storage at -86°C. All samples are handled and disposed of following institutional guidelines, with universal precautions at all times.
Cryopreservation for long-term storage
In addition to plasma and leukocytes, BM and PB from CCTRN subjects are shipped to the Biorepository Cell Phenotyping and Storage Core Laboratory for FACS profiling and long term storage. After BM and PB samples are analyzed by FACS, remaining cells are frozen in 10, 25 and 50 million cell aliquots (50 million cells per mL) in FBS and 10% DMSO according to CCTRN SOPs. Cells are slowly frozen to -80°C in a isopropanol bath apparatus for 24-48 hrs, and transferred to liquid nitrogen tanks for long-term storage (-175°C). Cells are stored for at least ten years so that if any safety questions arise, the initially harvested samples can be made available for analysis. No samples received at the Biorepository Core Laboratory are returned to the sites for clinical application. Remaining sample above those needed for safety studies are available for ancillary studies once they are approved by the CCTRN.
Results
Effect of shipping temperature on cell viability and phenotype
Shipping with refrigerated (4°C) packs maintained both cell number and overall viability at levels similar to control samples stored at 4°C for 24 hours (Figure 4A). The temperature of the samples was: 1) 8°C for 4°C stored samples, 2) 7-14°C for samples shipped with refrigerated packs, 3) variable for samples shipped with frozen packs, usually <5°C, and 4) 19-22°C for room temperature samples. Specimens shipped with frozen packs showed a significant decrease in viability compared to samples shipped with refrigerated packs (p<0.05, Figure 4A), and a significant decrease in TNC per mL of blood (p<0.0001, Figure 4B). The impact of frozen packs was less pronounced when packs were smaller (data not shown). The relative percentage of cell subpopulations determined by FACS (progenitor or inflammatory cells), showed that samples shipped with refrigerated packs were least variable and mostly within a ±10% variability that can be expected for FACS analysis. Specific rare populations (AC133+, CD34+, and VEGFR2+) showed greater variability because changes of just a few cells, result in large relative changes. Samples shipped with frozen packs, or stored at room temperature were more variable for most subpopulations (Figure 4C). Though Seeger et al. showed that BM samples stored at room temperature were more functional (8), we found that PB became sub-optimal when temperatures of the samples exceeded 21°C (data not shown). Based on these data, all samples are routinely shipped with 4°C refrigerated packs for overnight delivery, which maintains the PB at temperatures that are least detrimental to the sample.
Figure 4.
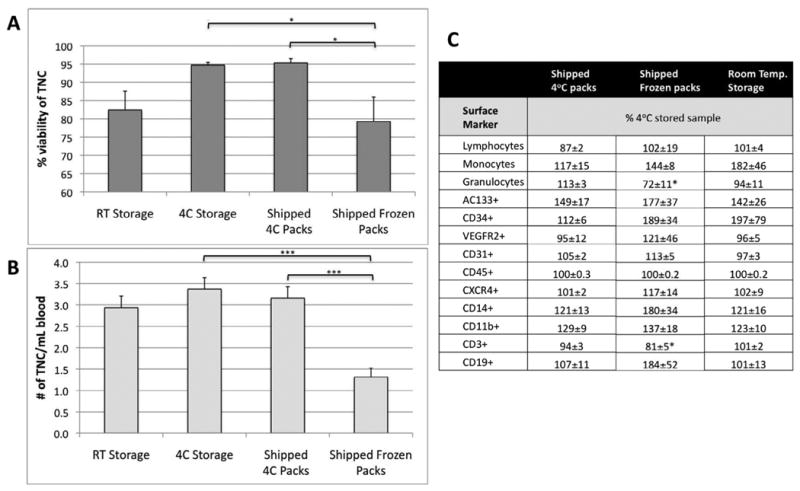
Peripheral blood shipped under different conditions. A) comparison of overall cell viability as determined by Guava count. B) comparison of total nucleated cell (TNC) number per mL of blood. C) Comparison of cell numbers of subpopulations in peripheral blood shipped under different conditions. The MNC subpopulations are determined by surface markers and expressed as a % of the unshipped control. All data are expressed as mean ± standard error. Statistical differences determined by ANOVA, * p<0.05, *** p<0.0001
Effect of time on cell number and viability
Cell viability was highest 24 hours post-draw, and decreased over time (Figure 5A). TNC number per mL of blood, decreased at 48 and 72 hrs post blood draw due to clumping or lysis of cells (Figure 5B). Both TNC number and viability were significantly reduced in samples 48 and 72 hrs post-draw (p<0.01 and P<0.0001, Figure 5A and 5B).
Figure 5.
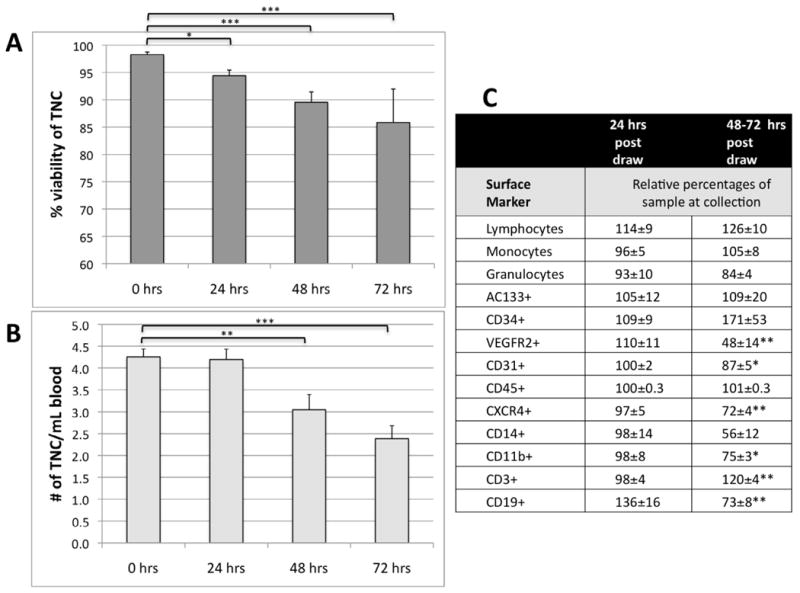
Peripheral blood maintained at 4°C and processed at different times post-draw. A) Comparison of overall viability as determined by Guava count, B) comparison of total nucleated cell number per mL of blood.. C) Comparison of cell numbers of subpopulations in peripheral blood at different time points following collection. The subpopulations were determined by surface markers and expressed relative to percentages at the time of collection. All data are expressed as mean ± standard error. Statistical differences determined by ANOVA, * p<0.05, ** p<0.01, *** p<0.0001
Effect of time on cell phenotype
Progenitor cell populations that contribute to injury and repair were quantified in PB at increasing times after blood draw. All populations except CD19+ changed by less than 10% in the first 24 hrs (Figure 5C). By 48– and 72 hrs, the relative numbers of several subpopulations were significantly changed. (Figure 5C). Based on these subpopulation changes over 72 hours, shipping SOPs were developed for overnight courier delivery. Most samples arrive at the Biorepository Cell Phenotyping and Storage Core Laboratory within 24±2 hours from draw and are processed immediately. Occasionally, due to various circumstances, samples are processed later, and this is recorded.
Effect of shipping time on cell viability and function
BM-MNCs and PB from CCTRN subjects were also shipped to the Biorepository Cell Function Core Laboratory in Florida. Specimens were received in the Cell Function Core Lab within 30.4 ± 17.9 hours from time of harvesting. Cell viability was 84% ± 8% for BM-MNCs and 83% ± 11% for PB-MNCs. We found that the percent of both non-viable BM-MNCs and PB-MNCs did not correlate with the shipping time (Rho=0.04 with P=0.7283 for BM, and Rho=-0.04 with P=0.7147 for PB). The scatter plots of raw data (before transformation) show the same results with the fitted flat regression lines (Figure 7). In a regression analysis there is no bias introduced by uneven distribution across the predictor (X-axis). These data indicate that the shipping time within our established Shipping SOP had an insignificant effect on cell viability. Thus, variability in results from BM and PB specimens received in the Biorepository Cell Function Core Lab is likely related to clinical and biologic factors rather than time out of body.
Figure 7. Effect of Shipping Time on Cell Viability of Bone Marrow and Peripheral Blood Received in the Cell Function Core Laboratory.
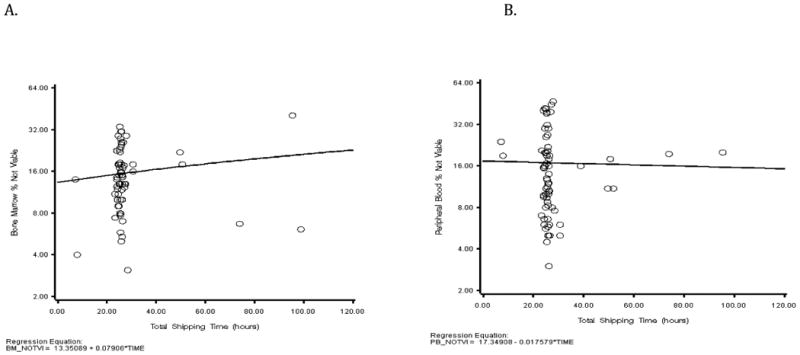
(A) Scatter plot and regression line for percent non-viable BM-MNCs versus Total Shipping Time (hours). There was a slight increase in non-viable cells with increasing shipping time, however the correlation was statistically insignificant (p=0.7283). (B) Scatter plot and regression line for percent non-viable BM-MNCs versus Total Shipping Time (hours). There was a slight decrease in non-viable cells with increasing shipping time, however the correlation was statistically insignificant (p=0.7147).
Learning curve
A significant network “learning curve” existed relative to how best to store and ship both BM and PB samples (Figure 6). This was evidenced by low viabilities and longer draw-to-delivery times for samples from early in our CCTRN experience compared to samples from later in our experience. Sample viability was similar among the centers with the exception of one site for which same day courier delivery was used in place of overnight shipping in which case viability was increased.
Figure 6.
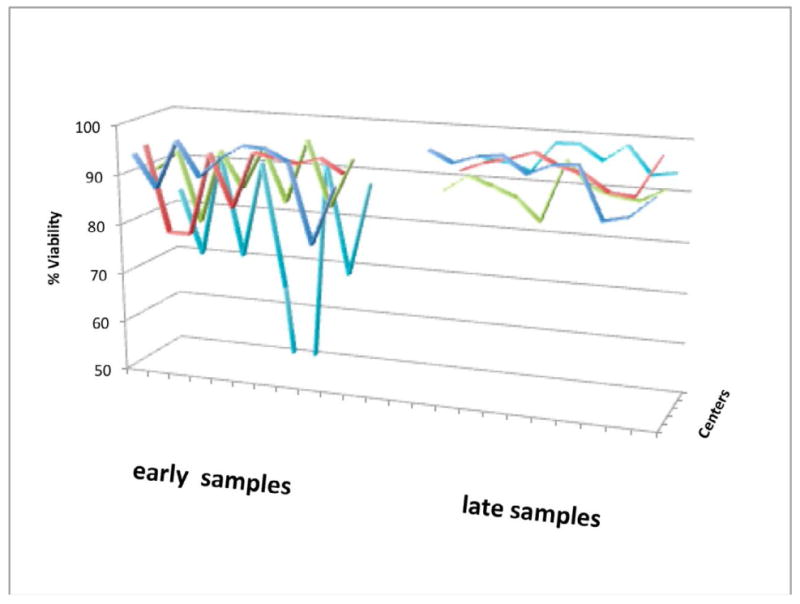
Comparison of viabilities for each center for the first 10 peripheral blood samples shipped at the beginning of the studies, and 10 peripheral blood samples for each center shipped at least one year later.
Effects of cryopreservation on cell phenotype
A qualification of the Biorepository Cell Phenotyping and Storage Core Laboratory is long-term cell preservation, and thus evaluation of the impact of freezing on cell phenotype is important. Comparison of BM and PB cells before and after freezing showed that recoveries ranged between 32-60% for PB-MNC and 41-55% for BM-MNC. Whereas stem, progenitor, myeloid and T lymphoid cells remained well preserved with excellent post-thaw viability, the B lymphocyte subset which made up the majority of collected MNCs was primarily responsible for the overall lower post-thaw viability (Table 1).
Table 1.
Cell viabilities after thawing bone marrow (BM) and peripheral blood (PB) specimens in comparison with the cell viabilities prior to cryopreservation. Values reported are post-thaw cell viability divided by pre-thaw cell viability multiplied times 100 and expressed as percentage (%) ± standard error (n=3 each).
| BM | PB | |
|---|---|---|
| Surface Marker | % of pre freezing | % of pre freezing |
| AC133+ | 115±9 | 97±17 |
| CD34+ | 108±5 | nd |
| VEGFR2+ | 82±12 | 114±45 |
| CD31+ | 111±13 | 104±3 |
| CD45+ | 97±3 | 100±0 |
| CXCR4+ | 146±33 | 100±3 |
| CD14+ | 203±100 | 190±34 |
| CDllb+ | 118±16 | 132±20 |
| CD3+ | 85±12 | 81±8 |
| CD19+ | 14±6 | 11±11 |
Discussion
One of the most valuable assets in the CV field is the Framingham Heart Study data set from which several important diagnostic and prognostic markers have emerged over the past 60 years (29). Yet to-date, it is not possible to correlate the important prognostic and diagnostic data gathered from Framingham patients with one of the more potent therapies envisioned – stem cell-mediated recovery after myocardial or vascular injury. The heterogeneity of clinical outcomes after cell therapy (Figure 1) suggests that important variables in cells, target tissue, or both exist. Understanding if those variables are a result of clinical disease, patient demographics (e.g. age, sex) or cell processing is critical if the field of cell-based therapy and diagnostics is to advance. For example, others showed that storage of patient BM cells over 24 hours could alter function (8); however, our preclinical data suggest that sex and age impact phenotype and function of BM-MNC (30, 31). By evaluating the number and function of cells in patients undergoing CV cell therapy, we have an opportunity to generate a reference database for patients undergoing such therapy. Understanding relationships between cell phenotype of BM-MNC, circulating cells, and function is crucial to optimize future cell therapy treatments. In addition, by storing BM, PB and serum, the CCTRN has the ability to bring both relevant clinical and specimen biologic data together in order to address patient safety questions.
In addition, our results show that BM and PB can be collected at various sites, shipped, and analyzed in a central core – making evaluation of cell phenotype and potency feasible. Preliminary findings suggest progenitor cell populations differ both in composition and function as time elapses after AMI.
Our work demonstrates that proper education of site coordinators on a validated shipping SOP, appropriate documentation, and ongoing communication between the core lab and clinical sites is essential for reproducible, consistent data over time. Since analysis of FACS data by gating for subpopulations can be subjective, standardized guidelines are required for data accuracy. Likewise, evaluations of colony formations and cell confluence should involve a visual atlas to verify accuracy of assessments. The development of cryopreservation methods that allow phenotypic and other analyses of frozen samples with minimal cell loss and minimal changes is of longstanding need, and is being pursued by the Biorepository Cell Phenotyping and Storage Core Laboratory.
Central core labs do not come without a cost. They can be expensive; and burdens imposed by complicated shipping procedures are considerable. However, in a centralized core lab with SOPs tailored to the studies, you have the advantage of fewer personnel to reduce operator variability, the quality of the data are superior, less variable, and more precise, and interactions with a centralized data coordinating center for statistical analysis are simplified. In addition, centralized core labs have access to a variety of data for controls or to patients with various other cardiovascular diseases to which study data can be compared and contrasted.
Conclusions
Creation of a Biorepository Core Laboratory for characterization of biologics is feasible in a network. Within the US, samples can be shipped nationally and physiologically relevant measurements made in a timely manner from shipped samples. To maximize the utility of these cell samples they should be shipped at 4°C to avoid freezing, and elapsed time from blood draw to processing should be 24-48 hours. Adhering to these established SOPs, reduces variability in cell phenotype and function assessments.
Stored biologic samples from cell therapy clinical trial patients provide a safety net should therapeutic questions occur. They allow for future analyses as new assays are developed and hypotheses generated, and provide a platform to address novel questions arising in treatment of cardiovascular disease. No samples received and evaluated in the Biorepository Core Laboratory are returned to the clinical sites or used for subject treatment. Instead, research samples are used to characterize injected cell phenotype and function, and stored for future Network-approved ancillary studies and safety analyses, if needed.
Acknowledgments
The authors wish to thank the CCTRN site principal investigators, the site research coordinators and the patients. The authors also wish to thank Paul Champoux at the University of Minnesota for his flow cytometry support and advice.
Funding Sources: This study was supported by research grants from the NIH NHLBI (U01 HL087318-01) and by the Production Assistance for Cellular Therapies (PACT), N01-HB-37164.
The University of Minnesota flow cytometer, a Masonic Cancer Center shared resource, was supported in part by grant NIH P30 CA77598.
Footnotes
Authorship: The authors are solely responsible for the design and conduct of this study, all study analyses, the drafting and editing of the paper and its final contents.
Conflict of Interest Disclosure: The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Abdel-Latif A, Bolli R, Tleyjeh IM, Montori VM, Perin EC, Hornung CA, Zuba-Surma EK, Al-Mallah M, Dawn B. Adult bone marrow-derived cells for cardiac repair: a systematic review and meta-analysis. Arch Intern Med. 2007;167(10):989–997. doi: 10.1001/archinte.167.10.989. [DOI] [PubMed] [Google Scholar]
- 2.Lipinski MJ, Biondi-Zoccai GG, Abbate A, Khianey R, Sheiban I, Bartunek J, Vanderheyden M, Kim HS, Kang HJ, Strauer BE, Vetrovec GW. Impact of intracoronary cell therapy on left ventricular function in the setting of acute myocardial infarction: a collaborative systematic review and meta-analysis of controlled clinical trials. J Am Coll Cardiol. 2007;50(18):1761–1767. doi: 10.1016/j.jacc.2007.07.041. [DOI] [PubMed] [Google Scholar]
- 3.Losordo DW, Schatz RA, White CJ, Udelson JE, Veereshwarayya V, Durgin M, Poh KK, Weinstein R, Kearney M, Chaudhry M, Burg A, Eaton L, Heyd L, Thorne T, Shturman L, Hoffmeister P, Story K, Zak V, Dowling D, Traverse JH, Olson RE, Flanagan J, Sodano D, Murayama T, Kawamoto A, Kusano KF, Wollins J, Welt F, Shah P, Soukas P, Asahara T, Henry TD. Intramyocardial transplantation of autologous CD34+ stem cells for intractable angina: a phase I/IIa double-blind, randomized controlled trial. Circulation. 2007;115(25):3165–3172. doi: 10.1161/CIRCULATIONAHA.106.687376. [DOI] [PubMed] [Google Scholar]
- 4.Schächinger V, Erbs S, Elsässer A, Haberbosch W, Hambrecht R, Hölschermann H, Yu J, Corti R, Mathey DG, Hamm CW, Süselbeck T, Werner N, Haase J, Neuzner J, Germing A, Mark B, Assmus B, Tonn T, Dimmeler S, Zeiher AM REPAIR-AMI Investigators. Improved clinical outcome after intracoronary administration of bone-marrow-derived progenitor cells in acute myocardial infarction: final 1-year results of the REPAIR-AMI trial. Eur Heart J. 2006;27(23):2775–2783. doi: 10.1093/eurheartj/ehl388. [DOI] [PubMed] [Google Scholar]
- 5.van Ramshorst J, Bax JJ, Beeres SL, Dibbets-Schneider P, Roes SD, Stokkel MP, de Roos A, Fibbe WE, Zwaginga JJ, Boersma E, Schalij MJ, Atsma DE. Intramyocardial bone marrow cell injection for chronic myocardial ischemia: a randomized controlled trial. JAMA. 2009;301(19):1997–2004. doi: 10.1001/jama.2009.685. [DOI] [PubMed] [Google Scholar]
- 6.Wollert KC, Meyer GP, Lotz J, Ringes-Lichtenberg S, Lippolt P, Breidenbach C, Fichtner S, Korte T, Hornig B, Messinger D, Arseniev L, Hertenstein B, Ganser A, Drexler H. Intracoronary autologous bone-marrow cell transfer after myocardial infarction: the BOOST randomised controlled clinical trial. Lancet. 2004;364(9429):141–148. doi: 10.1016/S0140-6736(04)16626-9. [DOI] [PubMed] [Google Scholar]
- 7.Assmus B, Tonn T, Seeger FH, Yoon CH, Leistner D, Klotsche J, Schächinger V, Seifried E, Zeiher AM, Dimmeler S. Red blood cell contamination of the final cell product impairs the efficacy of autologous bone marrow mononuclear cell therapy. J Am Coll Cardiol. 2010;55(13):1385–1394. doi: 10.1016/j.jacc.2009.10.059. [DOI] [PubMed] [Google Scholar]
- 8.Seeger FH, Tonn T, Krzossok N, Zeiher AM, Dimmeler S. Cell isolation procedures matter: a comparison of different isolation protocols of bone marrow mononuclear cells used for cell therapy in patients with acute myocardial infarction. Eur Heart J. 2007;28(6):766–772. doi: 10.1093/eurheartj/ehl509. [DOI] [PubMed] [Google Scholar]
- 9.Fuchs S, Baffour R, Zhou YF, Shou M, Pierre A, Tio FO, Weissman NJ, Leon MB, Epstein SE, Kornowski R. Transendocardial delivery of autologous bone marrow enhances collateral perfusion and regional function in pigs with chronic experimental myocardial ischemia. J Am Coll Cardiol. 2001;37(6):1726–1732. doi: 10.1016/s0735-1097(01)01200-1. [DOI] [PubMed] [Google Scholar]
- 10.Hamano K, Li TS, Kobayashi T, Hirata K, Yano M, Kohno M, Matsuzaki M. Therapeutic angiogenesis induced by local autologous bone marrow cell implantation. Ann Thorac Surg. 2002;73(4):1210–1215. doi: 10.1016/s0003-4975(02)03373-8. [DOI] [PubMed] [Google Scholar]
- 11.Kamihata H, Matsubara H, Nishiue T, Fujiyama S, Tsutsumi Y, Ozono R, Masaki H, Mori Y, Iba O, Tateishi E, Kosaki A, Shintani S, Murohara T, Imaizumi T, Iwasaka T. Implantation of bone marrow mononuclear cells into ischemic myocardium enhances collateral perfusion and regional function via side supply of angioblasts, angiogenic ligands, and cytokines. Circulation. 2001;104(9):1046–1052. doi: 10.1161/hc3501.093817. [DOI] [PubMed] [Google Scholar]
- 12.Kocher AA, Schuster MD, Szabolcs MJ, Takuma S, Burkhoff D, Wang J, Homma S, Edwards NM, Itescu S. Neovascularization of ischemic myocardium by human bone-marrow-derived angioblasts prevents cardiomyocyte apoptosis, reduces remodeling and improves cardiac function. Nat Med. 2001;7(4):430–436. doi: 10.1038/86498. [DOI] [PubMed] [Google Scholar]
- 13.Gunsilius E, Duba HC, Petzer AL, Kähler CM, Grünewald K, Stockhammer G, Gabl C, Dirnhofer S, Clausen J, Gastl G. Evidence from a leukaemia model for maintenance of vascular endothelium by bone-marrow-derived endothelial cells. Lancet. 2000;355(9216):1688–1691. doi: 10.1016/S0140-6736(00)02241-8. [DOI] [PubMed] [Google Scholar]
- 14.Orlic D, Kajstura J, Chimenti S, Jakoniuk I, Anderson SM, Li B, Pickel J, McKay R, Nadal-Ginard B, Bodine DM, Leri A, Anversa P. Bone marrow cells regenerate infarcted myocardium. Nature. 2001;410(6829):701–705. doi: 10.1038/35070587. [DOI] [PubMed] [Google Scholar]
- 15.Tomita S, Li RK, Weisel RD, Mickle DA, Kim EJ, Sakai T, Jia ZQ. Autologous transplantation of bone marrow cells improves damaged heart function. Circulation. 1999;100(19 Suppl):II247–256. doi: 10.1161/01.cir.100.suppl_2.ii-247. [DOI] [PubMed] [Google Scholar]
- 16.Assmus B, Schächinger V, Teupe C, Britten M, Lehmann R, Döbert N, Grünwald F, Aicher A, Urbich C, Martin H, Hoelzer D, Dimmeler S, Zeiher AM. Transplantation of Progenitor Cells and Regeneration Enhancement in Acute Myocardial Infarction (TOPCARE-AMI) Circulation. 2002;106(24):3009–3017. doi: 10.1161/01.cir.0000043246.74879.cd. [DOI] [PubMed] [Google Scholar]
- 17.Erbs S, Linke A, Schächinger V, Assmus B, Thiele H, Diederich KW, Hoffmann C, Dimmeler S, Tonn T, Hambrecht R, Zeiher AM, Schuler G. Restoration of microvascular function in the infarct-related artery by intracoronary transplantation of bone marrow progenitor cells in patients with acute myocardial infarction: the Doppler Substudy of the Reinfusion of Enriched Progenitor Cells and Infarct Remodeling in Acute Myocardial Infarction (REPAIR-AMI) trial. Circulation. 2007;116(4):366–374. doi: 10.1161/CIRCULATIONAHA.106.671545. [DOI] [PubMed] [Google Scholar]
- 18.Meyer GP, Wollert KC, Lotz J, Steffens J, Lippolt P, Fichtner S, Hecker H, Schaefer A, Arseniev L, Hertenstein B, Ganser A, Drexler H. Intracoronary bone marrow cell transfer after myocardial infarction: eighteen months' follow-up data from the randomized, controlled BOOST (BOne marrOw transfer to enhance ST-elevation infarct regeneration) trial. Circulation. 2006;113(10):1287–1294. doi: 10.1161/CIRCULATIONAHA.105.575118. [DOI] [PubMed] [Google Scholar]
- 19.Schächinger V, Erbs S, Elsässer A, Haberbosch W, Hambrecht R, Hölschermann H, Yu J, Corti R, Mathey DG, Hamm CW, Süselbeck T, Assmus B, Tonn T, Dimmeler S, Zeiher AM REPAIR-AMI Investigators. Intracoronary bone marrow-derived progenitor cells in acute myocardial infarction. N Engl J Med. 2006;355(12):1210–1221. doi: 10.1056/NEJMoa060186. [DOI] [PubMed] [Google Scholar]
- 20.Strauer BE, Brehm M, Zeus T, Köstering M, Hernandez A, Sorg RV, Kögler G, Wernet P. Repair of infarcted myocardium by autologous intracoronary mononuclear bone marrow cell transplantation in humans. Circulation. 2002;106(15):1913–1918. doi: 10.1161/01.cir.0000034046.87607.1c. [DOI] [PubMed] [Google Scholar]
- 21.Traverse JH, Henry TD, Vaughan DE, Ellis SG, Pepine CJ, Willerson JT, Zhao DX, Piller LB, Penn MS, Byrne BJ, Perin EC, Gee AP, Hatzopoulos AK, McKenna DH, Forder JR, Taylor DA, Cogle CR, Baraniuk MS, Olson RE, Jorgenson BC, Sayre SL, Vojvodic RW, Gordon DJ, Skarlatos SI, Moyé LA, Simari RD for the Cardiovascular Cell Therapy Research Network (CCTRN) LateTIME: A Phase-II, Randomized, Double-Blind, Placebo-Controlled, Pilot Trial Evaluating the Safety and Effect of Administration of Bone Marrow Mononuclear Cells 2 to 3 Weeks after Acute Myocardial Infarction. Texas Heart Journal. 2010 Aug;37(4):412–420. [PMC free article] [PubMed] [Google Scholar]
- 22.Traverse JH, Henry TD, Vaughan DE, Ellis SG, Pepine CJ, Willerson JT, Zhao DX, Piller LB, Penn MS, Byrne BJ, Perin EC, Gee AP, Hatzopoulos AK, McKenna DH, Forder JR, Taylor DA, Cogle CR, Olson RE, Jorgenson BC, Sayre SL, Vojvodic RW, Gordon DJ, Skarlatos SI, Moyé LA, Simari RD Cardiovascular Cell Therapy Research Network (CCTRN) Rationale and design for TIME: A phase II, randomized, double-blind, placebo-controlled pilot trial evaluating the safety and effect of timing of administration of bone marrow mononuclear cells after acute myocardial infarction. Am Heart J. 2009;158(3):356–363. doi: 10.1016/j.ahj.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Simari RD, Moyé LA, Skarlatos SI, Ellis SG, Zhao DX, Willerson JT, Henry TD, Pepine CJ. Development of a Network to Test Strategies in Cardiovascular Cell Delivery: The NHLBI-sponsored Cardiovascular Cell Therapy Research Network (CCTRN) J Cardiovasc Transl Res. 2010;3:30–36. doi: 10.1007/s12265-009-9160-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Willerson JT, Perin EC, Ellis SG, Pepine CJ, Henry TD, Zhao DX, Lai D, Penn MS, Byrne BJ, Silva G, Gee A, Traverse JH, Hatzopoulos AK, Forder JR, Martin D, Kronenberg M, Taylor DA, Cogle CR, Baraniuk S, Westbrook L, Sayre SL, Vojvodic RW, Gordon DJ, Skarlatos SI, Moyé LA, Simari RD for the Cardiovascular Cell Therapy Research Network (CCTRN) Rationale and Design for the Intramyocardial Injection of Autologous Bone Marrow Mononuclear Cells for Patients with Chronic Ischemic Heart Disease and Left Ventricular Dysfunction Trial (FOCUS) Am Heart J. 2010 Aug;160(2):215–223. doi: 10.1016/j.ahj.2010.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gee AP, Richman S, Durett A, McKenna D, Traverse J, Henry T, Fisk D, Pepine C, Bloom J, Willerson J, Prater K, Zhao D, Koç JR, Ellis S, Taylor D, Cogle C, Moyé L, Simari R, Skarlatos S. Multicenter Cell Processing for Cardiovascular Regenerative Medicine Applications - The Cardiovascular Cell Therapy Research Network (CCTRN) Experience. Cytotherapy. 2010 Sep;12(5):684–91. doi: 10.3109/14653249.2010.487900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hill JM, Zalos G, Halcox JPJ, Schenke WH, Waclawiw MA, Quyyumi AA, Finkel Toren. Circulating Endothelial Progenitor Cells, Vascular Function, and Cardiovascular Risk. NEJM. 2003;348(7):593–600. doi: 10.1056/NEJMoa022287. [DOI] [PubMed] [Google Scholar]
- 27.Yoder MC, Mead LE, Prater D, Krier TR, Mroueh KN, Li F, Krasich R, Temm CJ, Prchal JT, Ingram DA. Redefining endothelial progenitor cells via clonal analysis and hematopoietic stem/progenitor cell principals. Blood. 2007;109(5):1801–1809. doi: 10.1182/blood-2006-08-043471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Segal MS, Shah R, Afzal A, Perrault CM, Chang K, Schuler A, Beem E, Shaw LC, Calzi SL, Harrison JK, Tran-Son-Tay R, Grant MB. Nitric Oxide Cytoskeletal-Induced Alterations Reverse the Endothelial Progenitor Cell Migratory Defect Associated with Diabetes. Diabetes. 2006;55(1):102–109. [PubMed] [Google Scholar]
- 29.National Heart Lung and Blood Institute. Framingham Heart Study. 2010 June 29; Retrieved September 09/07/2010, 2010, from Framingham Heart study. http://www.framinghamheartstudy.org/
- 30.Nelson WD, Zenovich AG, Ott HC, Stolen C, Caron GJ, Panoskaltsis-Mortari A, Barnes SA, 3rd, Xin X, Taylor DA. Sex-dependent attenuation of plaque growth after treatment with bone marrow mononuclear cells. Circ Res. 2007;101(12):1319–1327. doi: 10.1161/CIRCRESAHA.107.155564. Epub 2007 Oct 1318. [DOI] [PubMed] [Google Scholar]
- 31.Rauscher FM, Goldschmidt-Clermont PJ, Davis BH, Wang T, Gregg D, Ramaswami P, Pippen AM, Annex BH, Dong C, Taylor DA. Aging, progenitor cell exhaustion, and atherosclerosis. Circulation. 2003;108(4):457–463. doi: 10.1161/01.CIR.0000082924.75945.48. [DOI] [PubMed] [Google Scholar]


