Abstract
Cells sense oxygen availability using not only the absolute value for cellular oxygen in regard to its energetic and metabolic functions, but also the gradient from the cell surface to the lowest levels in the mitochondria. Signals are used for regulatory purposes locally as well as in the generation of cellular, tissue, and humoral remodeling. Lowered oxygen availability (hypoxia) is theoretically important in the consideration of pharmacology because (1) hypoxia can alter cellular function and thereby the therapeutic effectiveness of the agent, (2) therapeutic agents may potentiate or protect against hypoxia-induced pathology, (3) hypoxic conditions may potentiate or mitigate drug-induced toxicity, (4) hypoxia may alter drug metabolism and thereby therapeutic effectiveness, and (5) therapeutic agents might alter the relative coupling of blood flow and energy metabolism in an organ. The prototypic biochemical effect of hypoxia is related to its known role as a cofactor in a number of enzymatic reactions, e.g., oxidases and oxygenases, which are affected independently from the bioenergetic effect of low oxygen on energetic functions. The cytochrome P-450 family of enzymes is another example. Here, there is a direct effect of oxygen availability on the conformation of the enzyme, thereby altering the metabolism of drug substrates. Indirectly, the NADH/NAD+ ratio is increased with 10% inspired oxygen, leading not only to reduced oxidation of ethanol but also to reduction of azo- and nitro-compounds to amines and disulfides to sulfhydryls. With chronic hypoxia, many of these processes are reversed, suggesting that hypoxia induces the drug-metabolizing systems. Support for this comes from observations that hypoxia can induce the hypoxic inducible factors which in turn alters transcription and function of some but not all cytochrome P-450 isoforms. Hypoxia is identified as a cofactor in cancer expression and metastatic potential. Thus, the effects of hypoxia play an important role in pharmacology, and the signaling pathways that are affected by hypoxia could become new targets for novel therapy or avenues for prevention.
Keywords: Drug metabolism, Hypoxia, Cytochrome c, Hypoxic response elements, Pharmacology
Introduction
Oxygen in the mammalian cell plays a major role in energy production in the mitochondria, an oxygen-using organelle. Some suggest that oxygen is used as an energy source in the development of complex organisms [1, 2], although others think that this is controversial [3]. Whatever the position, oxygen availability has been an inconstant factor over the evolution of unicellular and multicellular organisms [4] and has an inconstant effect with development [5]. As a result, cellular mechanisms which evolved in response to nutrient availability, xenobiotics, and other environmental threats have curious responses to and uses for oxygen [6, 7]. Glycolytic enzyme pathways are regulated by hypoxia [8]. Oxygen interacts with enzymes containing metals, structural motifs, and inorganic molecules which can bind oxygen [9]. Perhaps the best known are oxidase and oxygenase enzymes, which use oxygen as a cofactor. Moreover, oxygen is a molecule whose toxicity as a free biradical is actively regulated by the cell to attack viruses and bacteria, despite its danger for collateral damage to cell membranes and organelles [10].
Historically, various nomenclatures to describe hypoxia were proposed. Prior classifications attempt to distinguish environmentally induced hypoxia from tissue and to cellular hypoxia [11] and from cellular energetic outcomes such as lactate production [12]. Most acknowledge that extreme hypoxia kills cells through a loss of energetic function, while lesser degrees are associated with survival and not only decrements in energetic function but also a number of enzymatic, cellular, and, at an organ system level, tissue compensatory reactions on the timescale of seconds to hours to days [11, 13, 14]. For the purposes of this review, we will not use a classification system but rather discuss the issue in terms of oxygen availability and select examples showing the relationships among oxygen level, biologic functions, temporal plasticity, and effects of pharmacologic agents.
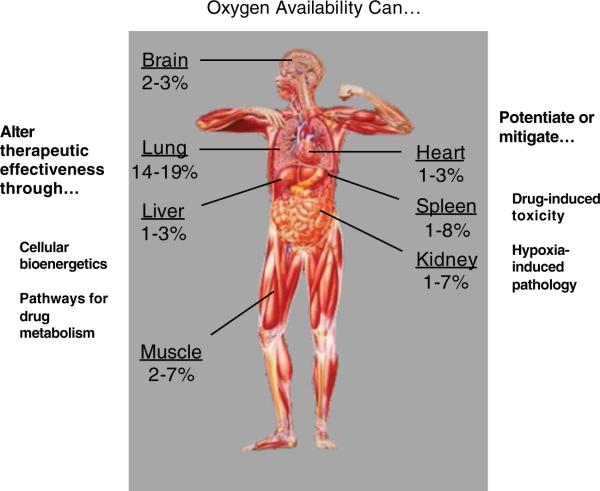
Another factor to consider is the heterogeneity in oxygenation in the human body (Fig. 1) [15, 16]. A cascade of oxygen availably occurs given the gradient from atmospheric oxygen concentration (21%) to the alveoli of the lungs (16–20%) to the extracellular compartment (~1.5–5.5%) and then to mitochondria (~0.05–1.0%) [17, 18]. Oxygen delivery in blood vessels is facilitated by a four-dimer heme protein, hemoglobin, in which the affinity for oxygen is allosterically affected by oxygen availability; however, even in the absence of hemoglobin, there exist complex organisms [19]. Myoglobin in the muscle can be considered the intracellular storage equivalent and contains only one heme unit [19]. The other factor is that, within a given organ, oxygen availability may vary according to blood flow, oxygen extraction, and oxygen dissociation from blood [4, 20, 21]. Hence, based on the cascade, it is difficult to generalize the potential for oxygen effects on micronutrients and xenobiotics.
Fig. 1.
This represents the approximate values for oxygen expressed as percent content in various organ systems in the body and lists the ways that oxygen levels could influence drug action, metabolism, and novel therapeutics
Against this background, it is not surprising that pharmacologic action, metabolism, and catabolism might be affected by oxygen availability. Theoretically then, one can organize the effects of hypoxia into three broad categories. First, low oxygen can effect cellular function and alter drug action or metabolism. Second, hypoxia could potentiate or mitigate drug-induced toxicity. Third, cellular oxygen availability could be a cofactor to manipulate in the treatment of hypoxia-induced pathology.
This brief discusses in a nonexhaustive fashion examples of how hypoxia can alter pharmacologic actions either directly or indirectly through effects on metabolism and catabolism. Not only will acute effects on cellular proteins be considered, but also the effects of genomic remodeling. Finally, the elements that confer hypoxic responsiveness will be discussed in regard to therapeutic gain in those conditions, such as cancer, sleep apnea, chronic obstructive pulmonary disease (COPD), and heart failure, where a fall in oxygen availability appears to alter the pathogenesis of diseases.
Acute effects of hypoxia
There are general effects to acute hypoxia (seconds to minutes). Lowering levels of oxygen acutely inside the cell leads to immediate reductions in mitochondrial bioenergetic capacity and the development of a local anaerobic state. A reduction in adenosine triphosphate (ATP) production is estimated to begin at a critical [O2] of 14 Torr, with a half-maximal reduction occurring at ~1 Torr [22, 23]. Thus, drug action that depends on cell energy, for instance, the accumulation of a toxin or drug, will be impaired. In addition, with a shift from aerobic to anaerobic metabolism, there may occur a fall in pH [7]. Local pH conditions in the cell can also contribute to an alteration in clinical effect or metabolism [24, 25].
Many, but not all, enzymatic activities are directly affected by oxygen availability. These effects may be caused either by effect of oxygen on a catalytic domain, as in the case of oxygenases, or in a regulatory domain, as happens in cytochrome c [26]. The effects of oxygen in the latter instance can come into play over minutes and can persist for hours. Cytochrome oxidase has a widespread role in the cellular response to hypoxia such that when its activation state is experimentally manipulated the carotid body discharge rate is immediately increased and a hypoxia inducible factor 1 alpha (HIF-1 alpha) response is initiated [27]. The cellular effect on oxygen consumption is immediately recovered with reoxygenation and independent of an effect on cell survival or apparent injury [28].
Another example of the interplay between oxygen and drug metabolism occurs in the cytochrome P-450 family of enzymes in which in the liver it catalyzes oxidations of drugs having both activating and catabolic effects [9]. The activities in this family of proteins are determined by Heme [29] spin rate which in turn is affected independently by xenobiotics and, as a consequence, drug binding and by oxygen availability [30]. The average hepatic O2 concentration is between 10 and 21 Torr [31]. Affinities [32] of various P-450 isoforms vary not only by substrate but also by oxygen availability so that the range of Km activities of the P-450 class as measured in vitro ranges from 0.7 to 140 Torr [33]. Many other oxidases are oxygen-limited, and in the limited literature with whole animal studies, reducing inspired oxygen from 21% to 10% will reduce activity by ~50% in many oxidases and oxygenases as well [34, 35]. While the biochemical mechanisms on this topic was extensively known by the 1970s, the translational import of these basic functions in terms of drug action and turnover is not well described and its effects on drug efficacy in recent times are not described. New approaches in modeling affinity based on crystalline structure may permit the prediction of its hypoxic effects [36, 37].
The cell and the organism will respond to a slow fall by a conformance of oxygen consumption to oxygen availability. If oxygen is lowered rather slowly over several minutes, mitochondrial oxygen consumption is reduced along with the lowered oxygen uptake [38]. This reduction, if continued for 4–6 h, is immediately reversed upon re-exposure to oxygen. One well-described mechanism for this effect is an allosteric inhibition of mitochondrial cytochrome c oxidase [38, 39]. A second is a reduction in Na//K-ATPase activity [40], by internalization of the surface enzyme as triggered by reactive oxygen radicals [41]. The effect would be to reduce effectiveness of drugs that require energy for efficacy.
Redox-dependent mechanisms that depend upon the NADPH/NADP+ ratio are in turn regulated by oxygen availability. This ratio is reported to be increased as cellular oxygen tension falls [42]. This becomes relevant for alcohol to ketone conversion, which becomes a slower process (well known to mountaineers). Other nitro- and azo-compounds are metabolized in an NADPH-dependent manner, as is glucuronidation. However, these could lead in vivo to paradoxical effects, namely the increase in NADPH/NADP+ ratio would be offset by an inhibition and downregulation of cytochrome P-450 [43].
Hypoxia can augment drug toxicity. Pre-exposure to chronic intermittent hypoxia in mice increases acetaminophen toxicity. As initial metabolism of acetaminophen is achieved via glucuronidation and neutralization of the toxic acetaminophen byproduct, NAPQI, is highly dependent on glutathione, it is hypothesized that the altered redox state of the hepatocytes induced by CIH was responsible for the injury seen in these animals [44]. Paraquat is an insecticide that produces an acute damaging phase in the lung, followed by a reparative phase dominated by an extensive fibrosis. It selectively accumulates through an energy-dependent diamine transport process in the alveolar epithelial and Clara cells of the airway. When accumulated, paraquat undergoes a NADPH-dependent one-electron reduction to its free radical which reacts with molecular oxygen to reform a cation and concomitantly produce a superoxide anion, leading to direct damage of cell membranes. Paraquat will stimulate the pentose phosphate pathway and inhibit synthesis of fatty acids in a dose-dependent manner and increase pulmonary levels of mixed disulfides and reduce NADPH levels in the lung [45]. There is one case report of acute paraquat poisoning that suggests a combination of hypoxic ventilation with 14% oxygen and 86% nitrogen; hemodialysis and forced diuresis led to recovery without lung complications, implying that reducing oxygen under certain conditions can lead to therapeutic benefit [46].
Common drugs can have unexpected effects that serve to mitigate the adverse effects of reduced oxygen availability and support the approach of “chemical preconditioning” to protect the brain [47]. Erythromycin and kanamycin appear to provide such hypoxic tolerance; in models, pretreatment by as much as 6 h will improve recovery to hypoxia in a hippocampal slice preparation (90% vs 30% recovery; p<0.01) [48]. This has implications in terms of being a potentially confounding effect in an in vivo study of hypoxia in the setting of survival surgery. In in vitro studies, aspirin has a neuroprotective effect against hypoxic hypoxia and chemical hypoxia, delaying a decline in intracellular ATP content [49]. Such drugs might be considered for use in preconditioning in humans. Indeed, aspirin has been shown to be efficacious in preventing headaches in those traveling to high altitude [50].
Effects of chronic hypoxia
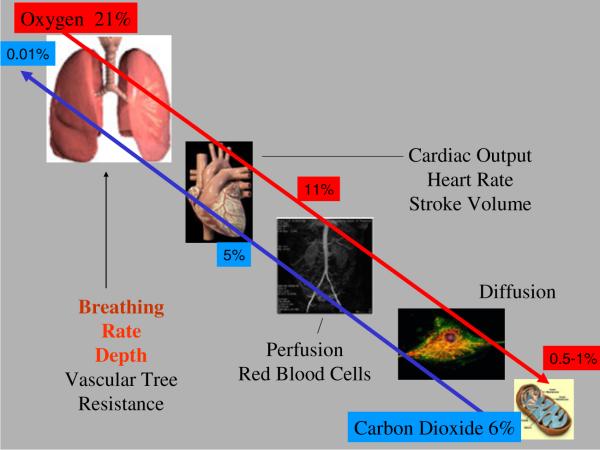
If hypoxia is present over hours-to-days, a number of physiologic and cellular responses that many consider as adaptive, but probably should be considered as a remodeling process in the event that the hypoxic event is not fatal [22], are engaged. Physiologically, oxygen delivery to tissue is part of the same network as that that excretes carbon dioxide (Fig. 2). The gradients are reversed. If hypoxia is encountered, it can be restored through a number of systemic and central reflex adaptations. Some include increased alveolar ventilation mediated by carotid body reflexes, increased red cell volume via increased transcription and translation of erythropoietin, decreased oxygen consumption in some tissues which results in increased mixed venous oxygen, and an increased capillary density resulting in a shorter diffusion length from arterial wall to the cell [51].
Fig. 2.
Oxygen is delivered to tissue through a cascade of physiologic systems starting with ventilation. The excretion of carbon dioxide from the cell uses the same route. Oxygen and carbon dioxide intersect in regard to the actions of pH not only in red blood cells but also in cellular enzymatic pathways
With chronic hypoxia, cellular adaptations will occur and some of these responses will be organ specific. In the brain, there is decreased oxidative metabolic capacity and a decrease in oxidative enzymes, decreased cytochrome c oxidase activity, decreased neuronal mitochondrial density, and increased glycolysis with an upregulation of several glycolytic enzymes [51]. These responses are thought to be appropriate for the maintenance of neuronal activity. In the periphery, hypoxia can upregulate P-450 enzymatic transcription and function and is predicted to affect drug efficacy. For instance, modafinil and its isomer armodafinil upregulate P-450 enzymes, thereby decreasing the effectiveness of birth control hormones [52], in a manner similar to hypoxia. This being the case, there is the theoretical impact of hypoxic conditions in altering hormonal actions in conditions like cystic fibrosis or at altitude when oxygen-regulated P-450 isoforms could be upregulated by hypoxia [53].
One prototypic hypoxic response involves a family of HIFs, namely HIF-1 alpha and beta and HIF-2 alpha. Of these, the story for HIF1 is better developed [54]. In the presence of oxygen, HIF is rapidly degraded, but in the absence of oxygen the mechanism for degradation is impaired, permitting it to move into the nucleus and regulate a number of downstream gene transcriptions, some of which like erythropoietin or vascular endothelial growth factor can be considered as an attempt to restore oxygen delivery. A number of drug enzyme levels are altered by chronic hypoxic exposure through the HIF pathway. These include cytochrome c oxidase [55, 56] and presumably other oxidases as well. The cellular response is an ancient one, occurring in pathways that regulate reactive oxygen species [55].
The discovery of the HIF response pathway may give insight into design of pharmacologic or genomic manipulations that could counter hypoxic pathology or effect change using a hypoxia-mediated trigger [57]. For example, in some but not all epidemiological studies, coffee consumption is associated with decreased colon cancer but increased cardiac mortality [58, 59]. In an in vitro study, pretreatment with caffeine reduced adenosine-induced vascular endothelial growth factor (VEGF) promoter activity, VEGF levels, and interleukin (IL)-8 expression. One mechanism for this effect may be explained by the knowledge that HIF-1 alpha and VEGF levels can be increased through A3 adenosine receptor stimulation, and the effects on IL-8 are mediated via the A2B subtype [60]. A similar effect on VEGF, as well as on other features of acute vascular leakage from subacute hypoxia, is seen with ingestion of seabuckthorn, a natural alternative therapy used for vascular disease [61]. Assuming that this effect is present in vivo, one could suspect that angiogenesis, a potentially deleterious effect in cancer but a beneficial one with vascular disease, is affected by xenobiotics commonly used by a population or group of patients.
Applied hypoxic pharmacology
The integration of these concepts currently finds a home in cancer therapeutics. Studies, using a variety of in vitro methods, have shown that cellular hypoxia produces at a DNA level point mutations, oxidative base damage, double-and single-strand breaks, gene amplification, overreplication, and genetic instability [62]. These mechanisms were present by histochemical probes in the tumor microenvironment [63]. A second factor is the effects of hypoxia on oxygen consumption [64].
Two other lines of research support the concept of manipulation of the tumor oxygen environment as a potential target for therapy, and not surprisingly HIF-1 alpha sits at the center of this research due to its ability to augment tumor angiogenesis, metabolism, proliferation, and metastasis [65]. First, the angiogenic response to hypoxia can be considered an important target for reducing cancer growth and metastasis [66]. This vascular response was well known from studies of systemic exposures, and the HIF-activated protein VEGF was known to be a major factor mediating this response [67, 68]. It became apparent that HIF expression occurred in a number of solid tumors, and furthermore that overexpression of HIF correlated with clinical outcome in terms of mortality, metastasis, and invasion in vivo and in vitro [54, 69]. The microenvironment of the tissue itself could also contribute to metastatic potential [70]; and inhibition of HIF [54] or interruption of signaling downstream from HIF can reverse this process [71]. Second was the evidence that hypoxic cells were more resistant to radiation damage, thus providing an environmental haven for recovery. By 2004, strategies to manipulate the oxygen were articulated [62]. One approach is to improve tumor oxygenation at time of radiation or drug therapy, using strategies to increase tissue oxygen for instance with hyperbaric exposure, compounds that transiently improve oxygenation for instance motexafin gadolinium, and development of allosteric modifiers of hemoglobin–oxygen binding to reduce O2 affinity for efficient O2 discharge at the tissue level. A second approach would be to suppress HIF activation in order to enhance the efficacy of chemotherapy and/or antiangiogenic strategies [72]. A third is to develop agents called hypoxic sensitizers. The class of compounds with this effect are nitroimidazoles, agents with high electron affinity; one such agent (nimorazole) has been shown to have some benefit in head and neck cancer [73]. A fourth class is called hypoxia cytotoxins, bioreductive agents that selectively kill or are activated in the hypoxic regions of tumors. There are a number of enzyme systems being proposed but paramount is the consideration of P-450 isoforms using in silico approaches [37].
It is beyond the scope of this review to go into more detail on this active area in research; however, we point to three examples as potential models for application to chronic medical diseases. First, there are agents which are activated in hypoxic regions. Such “prodrugs” would be selectively metabolized by oxygen-dependent enzymes from an innocuous agent to one that targets and damages DNA, leading to cell death. A hypoxia-activated prodrug, AQ4N, delays tumor growth and metastasis in a preclinical model of pancreatic cancer; but drugs in this class have in general had mixed results [74, 75]. Nevertheless, such a drug approach might be considered in diseases like sleep apnea, where transient hypoxia occurs, with the intent to reduce the presence or effects of reactive oxygen species. Second, it is proposed to use combination therapy to alter hypoxic effects in the tumor environment that might reduce killing of cancer in the setting of immunotherapy. For example, lowered oxygen availability would inhibit natural killer cells by several mechanisms. Antihypoxia adenosinergic therapy and immunotherapy are proposed to target the detrimental effects of high extracellular adenosine produced by inhibition of ATP synthesis and a reduced interferon response in response to HIF activation [76, 77]. This effect could operate to limit effectiveness of vaccines in high-altitude populations or in those with diseases like COPD where there is systemic hypoxemia. Third, one could utilize the HIF upstream regulators in a gene construct for an enzyme, perhaps even a novel enzyme, that would serve to convert a protein or prodrug into a locally cytotoxic agent [78]. Another is to use inhibition of HIF in certain pathways [54]. Any number of questions can be generated in considering these approaches. While the initial risk–reward might be higher in malignant than in nonmalignant disease, the issues of how one would construct such agents and utilize cellular promoters for therapeutic advantage in pathologic conditions where hypoxia has a causative or supportive role is of much interest.
If hypoxic effects are beneficial as in the instance of increasing oxygen-carrying capacity, one can pharmacologically elicit the same effect and potentially treat disease [79, 80]. The proof of principle has been shown in cell culture [81], but in vivo inhibition of prolyl hydroxylase can work in vivo to inhibit ischemic injury [82].
Issues and complications
We would be remiss to not mention the problems and confounding factors in these various research models and clinical applications. Some are obvious, namely cellular in vitro models are limited to the study of metabolism and cellular actions and results are often collected under cell suspension and/or without direct measures of local oxygen conditions. Dramatic differences in oxygen environments are used, assuming that such differences must have an impact on cellular oxygen, and are limited to one or another molecular without consideration of confounding effects of acidosis, enzymatic processes, etc. In addition, generalizations are difficult not only because tissues differ in the oxygen history and utilization, for instance comparisons between pulmonary and arterial endothelium, but also cells within a tissue differ in oxygen history and responses. In addition, results are independent of the effects of ventilation, flow, or tissue heterogeneity.
Exposure paradigms are not standardized. While methods could be better defined, a lack of common protocols does not permit comparisons between laboratories. The use of a common expression profiling platform could help adjust for differences in laboratory conditions, but often these are the same genes that are under study or subject to change in the intervention.
In regard to animal models, the systemic responses to hypoxia include not only the cardiopulmonary adaptations but hormonal and neural responses. For instance, hypoxic exposure activates carotid body afferent fibers not only to enhance ventilation and sympathetic activity but also to promote release of hypothalamic ACTH and increase cortisol levels [83, 84]. In the setting of chronic exposures, there are genomic adaptations that either mitigate or enhance pathology [85]. These and other factors might explain the paradox between the occurrence of lower oxygen consumption in cellular models of hypoxia and the increased oxygen consumption measured in humans ascending to altitude. Second, the animal model of hypoxia by causing a change in inspired oxygen level, may not model all disease states where cellular hypoxia is thought to operate in disease. Third, the paradigm of hypoxia forcing in steady-state (chronic) hypoxia is different to that in intermittent hypoxia when the intermittent exposures are spaced such as to assure full reoxygenation [86].
Conclusions
From this brief review, it is apparent that the pharmacology of a wide variety of drugs is or can be dependent on local levels of oxygen availability. Mechanisms include both direct and indirect immediate effects on enzymes, energy-dependent ion pumps, and mitochondrial functions. There may occur genomic remodeling in a number of pharmacologic metabolic pathways. Some of these pathways are exaggerated in tumors and are associated with poor outcomes. From this literature on cancer therapy, it is clear that the oxygen environment of the cell can be manipulated for therapeutic gain.
There are in our opinion opportunities for research and discovery, under the heading of “Hypoxic Pharmacology.” We can conceive a field devoted to clinically relevant outcomes and development of models for translational research. The applied chemistry and biology “model” is borrowed from the strategy used for cancer therapeutics. Pharmacology of hypoxia has been proposed for chronic diseases where cellular hypoxia is thought to increase or decrease disease progression [87, 88]. These include COPD, sleep apnea, heart failure, angina, and stroke. In addition, one could apply these concepts to altitude or other hypoxic exposures, including a consideration of drug effectiveness, cancer risk, probiotics, etc.
Acknowledgements
We thank Nikolaus Netzer MD for his suggestion to review this topic for the conference. We thank the conference members and the Division of Pulmonary, Critical Care, and Sleep Medicine at University Hospitals of Cleveland, for comments and suggestions on the topic and the presentation.
References
- 1.Koch LG, Britton SL. Aerobic metabolism underlies complexity and capacity. J Physiol. 2008;586:83–95. doi: 10.1113/jphysiol.2007.144709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Britton SL, Koch LG. Animal models of complex diseases: an initial strategy. IUBMB Life. 2005;57:631–638. doi: 10.1080/15216540500251684. [DOI] [PubMed] [Google Scholar]
- 3.Budd GE. The earliest fossil record of the animals and its significance. Philos Trans R Soc Lond B Biol Sci. 2008;363:1425–1434. doi: 10.1098/rstb.2007.2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hochachka PW, Land SC, Buck LT. Oxygen sensing and signal transduction in metabolic defense against hypoxia: lessons from vertebrate facultative anaerobes. Comp Biochem Physiol A Physiol. 1997;118:23–29. doi: 10.1016/s0300-9629(96)00372-6. [DOI] [PubMed] [Google Scholar]
- 5.Webster WS, Abela D. The effect of hypoxia in development. Birth Defects Res C Embryo Today. 2007;81:215–228. doi: 10.1002/bdrc.20102. [DOI] [PubMed] [Google Scholar]
- 6.Koch LG, Britton SL. Evolution, atmospheric oxygen, and complex disease. Physiol Genomics. 2007;30:205–208. doi: 10.1152/physiolgenomics.00043.2007. [DOI] [PubMed] [Google Scholar]
- 7.Hochachka PW. Mechanisms and evolution of hypoxiatolerance in humans. J Exp Biol. 1998;201:1243–1254. doi: 10.1242/jeb.201.8.1243. [DOI] [PubMed] [Google Scholar]
- 8.Baldwin JE, Krebs H. The evolution of metabolic cycles. Nature. 1981;291:381–382. doi: 10.1038/291381a0. [DOI] [PubMed] [Google Scholar]
- 9.Karlin KD. Metalloenzymes, structural motifs, and inorganic models. Science. 1993;261:701–708. doi: 10.1126/science.7688141. [DOI] [PubMed] [Google Scholar]
- 10.Dowling DK, Simmons LW. Reactive oxygen species as universal constraints in life-history evolution. Proc Biol Sci. 2009;276:1737–1745. doi: 10.1098/rspb.2008.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beilman GJ, Cerra FB. The future. Monitoring cellular energetics. Crit Care Clin. 1996;12:1031–1042. doi: 10.1016/s0749-0704(05)70291-8. [DOI] [PubMed] [Google Scholar]
- 12.Duke T. Dysoxia and lactate. Arch Dis Child. 1999;81:343–350. doi: 10.1136/adc.81.4.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Caille V, Squara P. Oxygen uptake-to-delivery relationship: a way to assess adequate flow. Crit Care. 2006;10(Suppl 3):4. doi: 10.1186/cc4831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Levy RJ, Deutschman CS. Cytochrome c oxidase dysfunction in sepsis. Crit Care Med. 2007;35:S468–475. doi: 10.1097/01.CCM.0000278604.93569.27. [DOI] [PubMed] [Google Scholar]
- 15.Kunze K. Significance of oxygen pressure field measurements in human muscle, with special reference on PO2 micro-needle electrodes. Progr Respir Res. 1969;3:153–157. [Google Scholar]
- 16.Van Den Brenk HA, Jamieson D. Potentiation by anaesthetics of brain damage due to breathing high-pressure oxygen in mammals. Nature. 1962;194:777–778. doi: 10.1038/194777a0. [DOI] [PubMed] [Google Scholar]
- 17.Gnaiger E, Lassnig B, Kuznetsov A, Rieger G, Margreiter R. Mitochondrial oxygen affinity, respiratory flux control and excess capacity of cytochrome c oxidase. J Exp Biol. 1998;201:1129–1139. doi: 10.1242/jeb.201.8.1129. [DOI] [PubMed] [Google Scholar]
- 18.Gnaiger E, Steinlechner-Maran R, Mendez G, Eberl T, Margreiter R. Control of mitochondrial and cellular respiration by oxygen. J Bioenerg Biomembr. 1995;27:583–596. doi: 10.1007/BF02111656. [DOI] [PubMed] [Google Scholar]
- 19.Garofalo F, Pellegrino D, Amelio D, Tota B. The Antarctic hemoglobinless icefish, fifty five years later: a unique cardiocirculatory interplay of disaptation and phenotypic plasticity. Comp Biochem Physiol A Mol Integr Physiol. 2009;154:10–28. doi: 10.1016/j.cbpa.2009.04.621. [DOI] [PubMed] [Google Scholar]
- 20.Gonzalez NC, Howlett RA, Henderson KK, Koch LG, Britton SL, Wagner HE, Favret F, Wagner PD. Systemic oxygen transport in rats artificially selected for running endurance. Respir Physiol Neurobiol. 2006;151:141–150. doi: 10.1016/j.resp.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 21.Zuurbier CJ, van Iterson M, Ince C. Functional heterogeneity of oxygen supply-consumption ratio in the heart. Cardiovasc Res. 1999;44:488–497. doi: 10.1016/s0008-6363(99)00231-x. [DOI] [PubMed] [Google Scholar]
- 22.Prabhakar NR. O2 sensing at the mammalian carotid body: why multiple O2 sensors and multiple transmitters? Exp Physiol. 2006;91:17–23. doi: 10.1113/expphysiol.2005.031922. [DOI] [PubMed] [Google Scholar]
- 23.Liss B, Roeper J. Molecular physiology of neuronal K-ATP channels (review) Mol Membr Biol. 2001;18:117–127. [PubMed] [Google Scholar]
- 24.Taburet AM, Tollier C, Richard C. The effect of respiratory disorders on clinical pharmacokinetic variables. Clin Pharmacokinet. 1990;19:462–490. doi: 10.2165/00003088-199019060-00004. [DOI] [PubMed] [Google Scholar]
- 25.Richer M, Lam YW. Hypoxia, arterial pH and theophylline disposition. Clin Pharmacokinet. 1993;25:283–299. doi: 10.2165/00003088-199325040-00004. [DOI] [PubMed] [Google Scholar]
- 26.Burke PV, Poyton RO. Structure/function of oxygenregulated isoforms in cytochrome c oxidase. J Exp Biol. 1998;201:1163–1175. doi: 10.1242/jeb.201.8.1163. [DOI] [PubMed] [Google Scholar]
- 27.Lahiri S, Antosiewicz J, Pokorski M. A common oxygen sensor regulates the sensory discharge and glomus cell HIF-1alpha in the rat carotid body. J Physiol Pharmacol. 2007;58(Suppl 5):327–333. [PubMed] [Google Scholar]
- 28.Acker T, Acker H. Cellular oxygen sensing need in CNS function: physiological and pathological implications. J Exp Biol. 2004;207:3171–3188. doi: 10.1242/jeb.01075. [DOI] [PubMed] [Google Scholar]
- 29.Mironov V, Hritz MA, LaManna JC, Hudetz AG, Harik SI. Architectural alterations in rat cerebral microvessels after hypobaric hypoxia. Brain Res. 1994;660:73–80. doi: 10.1016/0006-8993(94)90840-0. [DOI] [PubMed] [Google Scholar]
- 30.Lahiri S, Roy A, Li J, Baby SM, Mokashi A, Di Giulio C. Role of Fe2+ in oxygen sensing in the carotid body. Adv Exp Med Biol. 2004;551:59–64. doi: 10.1007/0-387-27023-x_10. [DOI] [PubMed] [Google Scholar]
- 31.Jamieson D, van den Brenk HA. Electrode size and tissue pO2 measurement in rats exposed to air or high pressure oxygen. J Appl Physiol. 1965;20:514–518. doi: 10.1152/jappl.1965.20.3.514. [DOI] [PubMed] [Google Scholar]
- 32.Cramer T, Yamanishi Y, Clausen BE, Forster I, Pawlinski R, Mackman N, Haase VH, Jaenisch R, Corr M, Nizet V, Firestein GS, Gerber HP, Ferrara N, Johnson RS. HIF-1alpha is essential for myeloid cell-mediated inflammation. Cell. 2003;112:645–657. doi: 10.1016/s0092-8674(03)00154-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Korzeniewski B. Is it possible to predict any properties of oxidative phosphorylation in a theoretical way? Mol Cell Biochem. 1998;184:345–358. [PubMed] [Google Scholar]
- 34.Keevil T, Mason HS. Molecular oxygen in biological oxidations—an overview. Meth Enzymol. 1978;52:3–40. doi: 10.1016/s0076-6879(78)52003-x. [DOI] [PubMed] [Google Scholar]
- 35.Jones DP, Aw TY, Shan X. Drug metabolism and toxicity during hypoxia. Drug Metab Rev. 1989;2–4:247–260. doi: 10.3109/03602538909103540. [DOI] [PubMed] [Google Scholar]
- 36.Feiters MC. Mimicking biological electron transfer and oxygen activation involving iron and copper proteins: a bio(in) organic supramolecular approach. Met Ions Biol Syst. 2001;38:461–655. [PubMed] [Google Scholar]
- 37.Sun H, Scott DO. Structure-based drug metabolism predictions for drug design. Chem Biol Drug Des. 2010;75:3–17. doi: 10.1111/j.1747-0285.2009.00899.x. [DOI] [PubMed] [Google Scholar]
- 38.Schumacker PT, Chandel N, Agusti AG. Oxygen conformance of cellular respiration in hepatocytes. Am J Physiol. 1993;265:L395–402. doi: 10.1152/ajplung.1993.265.4.L395. [DOI] [PubMed] [Google Scholar]
- 39.Subramanian RM, Chandel N, Budinger GR, Schumacker PT. Hypoxic conformance of metabolism in primary rat hepatocytes: a model of hepatic hibernation. Hepatology. 2007;45:455–464. doi: 10.1002/hep.21462. [DOI] [PubMed] [Google Scholar]
- 40.Heerlein K, Schulze A, Hotz L, Bartsch P, Mairbaurl H. Hypoxia decreases cellular ATP demand and inhibits mitochondrial respiration of a549 cells. Am J Respir Cell Mol Biol. 2005;32:44–51. doi: 10.1165/rcmb.2004-0202OC. [DOI] [PubMed] [Google Scholar]
- 41.Hsu T, Adereth Y, Kose N, Dammai V. Endocytic function of von Hippel-Lindau tumor suppressor protein regulates surface localization of fibroblast growth factor receptor 1 and cell motility. J Biol Chem. 2006;281:12069–12080. doi: 10.1074/jbc.M511621200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wolin MS, Ahmad M, Gao Q, Gupte SA. Cytosolic NAD (P)H regulation of redox signaling and vascular oxygen sensing. Antioxid Redox Signal. 2007;9:671–678. doi: 10.1089/ars.2007.1559. [DOI] [PubMed] [Google Scholar]
- 43.Fradette C, Du Souich P. Effect of hypoxia on cytochrome P450 activity and expression. Curr Drug Metab. 2004;5:257–271. doi: 10.2174/1389200043335577. [DOI] [PubMed] [Google Scholar]
- 44.Savransky V, Reinke C, Jun J, Bevans-Fonti S, Nanayakkara A, Li J, Myers AC, Torbenson MS, Polotsky VY. Chronic intermittent hypoxia and acetaminophen induce synergistic liver injury in mice. Exp Physiol. 2009;94:228–239. doi: 10.1113/expphysiol.2008.044883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Smith LL. Mechanism of paraquat toxicity in lung and its relevance to treatment. Hum Toxicol. 1987;6:31–36. doi: 10.1177/096032718700600105. [DOI] [PubMed] [Google Scholar]
- 46.Demeere JL. Paraquat toxicity. The use of hypoxic ventilation. Acta Anaesthesiol Belg. 1984;35:219–230. [PubMed] [Google Scholar]
- 47.Riepe MW, Esclaire F, Kasischke K, Schreiber S, Nakase H, Kempski O, Ludolph AC, Dirnagl U, Hugon J. Increased hypoxic tolerance by chemical inhibition of oxidative phosphorylation: “chemical preconditioning”. J Cereb Blood Flow Metab. 1997;17:257–264. doi: 10.1097/00004647-199703000-00002. [DOI] [PubMed] [Google Scholar]
- 48.Huber R, Kasischke K, Ludolph AC, Riepe MW. Increase of cellular hypoxic tolerance by erythromycin and other antibiotics. NeuroReport. 1999;10:1543–1546. doi: 10.1097/00001756-199905140-00027. [DOI] [PubMed] [Google Scholar]
- 49.Riepe MW, Kasischke K, Raupach A. Acetylsalicylic acid increases tolerance against hypoxic and chemical hypoxia. Stroke. 1997;28:2006–2011. doi: 10.1161/01.str.28.10.2006. [DOI] [PubMed] [Google Scholar]
- 50.Burtscher M, Likar R, Nachbauer W, Philadelphy M. Aspirin for prophylaxis against headache at high altitudes: randomised, double blind, placebo controlled trial. Bmj. 1998;316:1057–1058. doi: 10.1136/bmj.316.7137.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.LaManna JC, Chavez JC, Pichiule P. Structural and functional adaptation to hypoxia in the rat brain. J Exp Biol. 2004;207:3163–3169. doi: 10.1242/jeb.00976. [DOI] [PubMed] [Google Scholar]
- 52.Garnock-Jones KP, Dhillon S, Scott LJ. Armodafinil. CNS Drugs. 2009;23:793–803. doi: 10.2165/11203290-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 53.Fradette C, du Souich P. Hypoxia-inducible factor-1 and activator protein-1 modulate the upregulation of CYP3A6 induced by hypoxia. Br J Pharmacol. 2003;140:1146–1154. doi: 10.1038/sj.bjp.0705543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Semenza GL. Evaluation of HIF-1 inhibitors as anticancer agents. Drug Discov Today. 2007;12:853–859. doi: 10.1016/j.drudis.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 55.Semenza GL. Oxygen-dependent regulation of mitochondrial respiration by hypoxia-inducible factor 1. Biochem J. 2007;405:1–9. doi: 10.1042/BJ20070389. [DOI] [PubMed] [Google Scholar]
- 56.Beauvoit B, Rigoulet M. Regulation of cytochrome c oxidase by adenylic nucleotides. Is oxidative phosphorylation feedback regulated by its end-products? IUBMB Life. 2001;52:143–152. doi: 10.1080/152165401317316545. [DOI] [PubMed] [Google Scholar]
- 57.Maxwell PH. The HIF pathway in cancer. Semin Cell Dev Biol. 2005;16:523–530. doi: 10.1016/j.semcdb.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 58.Tavani A, La Vecchia C. Coffee, decaffeinated coffee, tea and cancer of the colon and rectum: a review of epidemiological studies, 1990–2003. Cancer Causes Control. 2004;15:743–757. doi: 10.1023/B:CACO.0000043415.28319.c1. [DOI] [PubMed] [Google Scholar]
- 59.LaCroix AZ, Mead LA, Liang KY, Thomas CB, Pearson TA. Coffee consumption and the incidence of coronary heart disease. N Engl J Med. 1986;315:977–982. doi: 10.1056/NEJM198610163151601. [DOI] [PubMed] [Google Scholar]
- 60.Merighi S, Benini A, Mirandola P, Gessi S, Varani K, Simioni C, Leung E, Maclennan S, Baraldi PG, Borea PA. Caffeine inhibits adenosine-induced accumulation of hypoxia-inducible factor-1alpha, vascular endothelial growth factor, and interleukin-8 expression in hypoxic human colon cancer cells. Mol Pharmacol. 2007;72:395–406. doi: 10.1124/mol.106.032920. [DOI] [PubMed] [Google Scholar]
- 61.Purushothaman J, Suryakumar G, Shukla D, Jayamurthy H, Kasiganesan H, Kumar R, Sawhney RC. Modulation of hypoxia-induced pulmonary vascular leakage in rats by seabuckthorn (Hippophae rhamnoides L.) Evid Based Complement Alternat Med. 2010 doi: 10.1093/ecam/nep199. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Brown JM, Wilson WR. Exploiting tumour hypoxia in cancer treatment. Nat Rev Cancer. 2004;4:437–447. doi: 10.1038/nrc1367. [DOI] [PubMed] [Google Scholar]
- 63.Reynolds TY, Rockwell S, Glazer PM. Genetic instability induced by the tumor microenvironment. Cancer Res. 1996;56:5754–5757. [PubMed] [Google Scholar]
- 64.Chen Y, Cairns R, Papandreou I, Koong A, Denko NC. Oxygen consumption can regulate the growth of tumors, a new perspective on the Warburg effect. PLoS ONE. 2009;4:e7033. doi: 10.1371/journal.pone.0007033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kimura K, Iwano M, Higgins DF, Yamaguchi Y, Nakatani K, Harada K, Kubo A, Akai Y, Rankin EB, Neilson EG, Haase VH, Saito Y. Stable expression of HIF-1alpha in tubular epithelial cells promotes interstitial fibrosis. Am J Physiol Ren Physiol. 2008;295:F1023–1029. doi: 10.1152/ajprenal.90209.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Otrock ZK, Hatoum HA, Awada AH, Ishak RS, Shamseddine AI. Hypoxia-inducible factor in cancer angiogenesis: structure, regulation and clinical perspectives. Crit Rev Oncol Hematol. 2009;70:93–102. doi: 10.1016/j.critrevonc.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 67.Maxwell PH. Oxygen homeostasis and cancer: insights from a rare disease. Clin Med. 2002;2:356–362. doi: 10.7861/clinmedicine.2-4-356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Loges S, Roncal C, Carmeliet P. Development of targeted angiogenic medicine. J Thromb Haemost. 2009;7:21–33. doi: 10.1111/j.1538-7836.2008.03203.x. [DOI] [PubMed] [Google Scholar]
- 69.Sasabe E, Zhou X, Li D, Oku N, Yamamoto T, Osaki T. The involvement of hypoxia-inducible factor-1alpha in the susceptibility to gamma-rays and chemotherapeutic drugs of oral squamous cell carcinoma cells. Int J Cancer. 2007;120:277. doi: 10.1002/ijc.22294. [DOI] [PubMed] [Google Scholar]
- 70.Bedogni B, Welford SM, Cassarino DS, Nickoloff BJ, Giaccia AJ, Powell MB. The hypoxic microenvironment of the skin contributes to Akt-mediated melanocyte transformation. Cancer Cell. 2005;8:443–454. doi: 10.1016/j.ccr.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 71.Bedogni B, O'Neill MS, Welford SM, Bouley DM, Giaccia AJ, Denko NC, Powell MB. Topical treatment with inhibitors of the phosphatidylinositol 3′-kinase/Akt and Raf/mitogen-activated protein kinase kinase/extracellular signal-regulated kinase pathways reduces melanoma development in severe combined immunodeficient mice. Cancer Res. 2004;64:2552–2560. doi: 10.1158/0008-5472.can-03-3327. [DOI] [PubMed] [Google Scholar]
- 72.McCarty MF, Barroso-Aranda J, Contreras F. Practical strategies for suppressing hypoxia-inducible factor activity in cancer therapy. Med Hypotheses. 2010;74:789–797. doi: 10.1016/j.mehy.2009.12.022. [DOI] [PubMed] [Google Scholar]
- 73.Henk JM, Bishop K, Shepherd SF. Treatment of head and neck cancer with CHART and nimorazole: phase II study. Radiother Oncol. 2003;66:65–70. doi: 10.1016/s0167-8140(02)00284-0. [DOI] [PubMed] [Google Scholar]
- 74.Reddy SB, Williamson SK. Tirapazamine: a novel agent targeting hypoxic tumor cells. Expert Opin Investig Drugs. 2009;18:77–87. doi: 10.1517/13543780802567250. [DOI] [PubMed] [Google Scholar]
- 75.Albertella MR, Loadman PM, Jones PH, Phillips RM, Rampling R, Burnet N, Alcock C, Anthoney A, Vjaters E, Dunk CR, Harris PA, Wong A, Lalani AS, Twelves CJ. Hypoxia-selective targeting by the bioreductive prodrug AQ4N in patients with solid tumors: results of a phase I study. Clin Cancer Res. 2008;14:1096–1104. doi: 10.1158/1078-0432.CCR-07-4020. [DOI] [PubMed] [Google Scholar]
- 76.Sitkovsky MV. T regulatory cells: hypoxia-adenosinergic suppression and re-direction of the immune response. Trends Immunol. 2009;30:102–108. doi: 10.1016/j.it.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 77.Sitkovsky MV, Kjaergaard J, Lukashev D, Ohta A. Hypoxia-adenosinergic immunosuppression: tumor protection by T regulatory cells and cancerous tissue hypoxia. Clin Cancer Res. 2008;14:5947–5952. doi: 10.1158/1078-0432.CCR-08-0229. [DOI] [PubMed] [Google Scholar]
- 78.Chen L, Waxman DJ. Cytochrome P450 gene-directed enzyme prodrug therapy (GDEPT) for cancer. Curr Pharm Des. 2002;8:1405–1416. doi: 10.2174/1381612023394566. [DOI] [PubMed] [Google Scholar]
- 79.McDonough MA, McNeill LA, Tilliet M, Papamicael CA, Chen QY, Banerji B, Hewitson KS, Schofield CJ. Selective inhibition of factor inhibiting hypoxia-inducible factor. J Am Chem Soc. 2005;127:7680–7681. doi: 10.1021/ja050841b. [DOI] [PubMed] [Google Scholar]
- 80.Asikainen TM, Ahmad A, Schneider BK, Ho WB, Arend M, Brenner M, Gunzler V, White CW. Stimulation of HIF-1alpha, HIF-2alpha, and VEGF by prolyl 4-hydroxylase inhibition in human lung endothelial and epithelial cells. Free Radic Biol Med. 2005;38:1002–1013. doi: 10.1016/j.freeradbiomed.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 81.Zaman K, Ryu H, Hall D, O'Donovan K, Lin KI, Miller MP, Marquis JC, Baraban JM, Semenza GL, Ratan RR. Protection from oxidative stress-induced apoptosis in cortical neuronal cultures by iron chelators is associated with enhanced DNA binding of hypoxia-inducible factor-1 and ATF-1/CREB and increased expression of glycolytic enzymes, p21(waf1/cip1), and erythropoietin. J Neurosci. 1999;19:9821–9830. doi: 10.1523/JNEUROSCI.19-22-09821.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Siddiq A, Ayoub IA, Chavez JC, Aminova L, Shah S, LaManna JC, Patton SM, Connor JR, Cherny RA, Volitakis I, Bush AI, Langsetmo I, Seeley T, Gunzler V, Ratan RR. Hypoxiainducible factor prolyl 4-hydroxylase inhibition. A target for neuroprotection in the central nervous system. J Biol Chem. 2005;280:41732–41743. doi: 10.1074/jbc.M504963200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Raff H, Tzankoff SP, Fitzgerald RS. Chemoreceptor involvement in cortisol responses to hypoxia in ventilated dogs. J Appl Physiol. 1982;52:1092–1096. doi: 10.1152/jappl.1982.52.4.1092. [DOI] [PubMed] [Google Scholar]
- 84.Raff H, Shinsako J, Dallman MF. Renin and ACTH responses to hypercapnia and hypoxia after chronic carotid chemodenervation. Am J Physiol. 1984;247:R412–417. doi: 10.1152/ajpregu.1984.247.3.R412. [DOI] [PubMed] [Google Scholar]
- 85.Zinkernagel AS, Johnson RS, Nizet V. Hypoxia inducible factor (HIF) function in innate immunity and infection. J Mol Med. 2007;85:1339–1346. doi: 10.1007/s00109-007-0282-2. [DOI] [PubMed] [Google Scholar]
- 86.Semenza GL, Prabhakar NR. HIF-1-dependent respiratory, cardiovascular, and redox responses to chronic intermittent hypoxia. Antioxid Redox Signal. 2007;9:1391–1396. doi: 10.1089/ars.2007.1691. [DOI] [PubMed] [Google Scholar]
- 87.Fraisl P, Aragones J, Carmeliet P. Inhibition of oxygen sensors as a therapeutic strategy for ischaemic and inflammatory disease. Nat Rev Drug Discov. 2009;8:139–152. doi: 10.1038/nrd2761. [DOI] [PubMed] [Google Scholar]
- 88.Myllyharju J. Prolyl 4-hydroxylases, key enzymes in the synthesis of collagens and regulation of the response to hypoxia, and their roles as treatment targets. Ann Med. 2008;40:402–417. doi: 10.1080/07853890801986594. [DOI] [PubMed] [Google Scholar]