Abstract
The possible effect of antihypertensive therapy on Alzheimer's disease (AD) has been studied, and angiotensin II receptor blockers (ARBs) have been suggested to exert an effect on cognitive decline. The purpose of this study is to clarify the functional effects of telmisartan, a long-acting ARB, on AD brain using prospective longitudinal 18F-fluorodeoxyglucose positron emission tomography (FDG-PET) studies. For this purpose, brain glucose metabolism of four hypertensive patients with AD was examined with FDG-PET before and after administration of telmisartan. Studied subjects underwent three FDG-PET studies at intervals of 12 weeks. Antihypertensive treatment except for telmisartan was started after the first FDG-PET and continued for 24 weeks. Then 40–80 mg of telmisartan was added after the second FDG-PET and continued for 12 weeks.Glucose metabolism was significantly decreased during the first 12 weeks without telmisartan use at an area (−10, 21, −22, x, y, z; Z = 3.56) caudal to the left rectal gyrus and the olfactory sulcus corresponding to the left olfactory tract. In contrast, the introduction of telmisartan during the following 12 weeks preserved glucose metabolism at areas (5, 19, −20, x, y, z; Z = 3.09; 6, 19, −22, x, y, z; Z = 2.88) caudal to the bilateral rectal gyri and olfactory sulci corresponding to the bilateral olfactory tracts. No areas showed decreased glucose metabolism after the introduction of telmisartan. In AD, amyloid-β deposition is observed in the anterior olfactory nucleus (AON) of the olfactory tract. Glucose metabolism in AON may be progressively decreased and preserved by telmisartan.
Keywords: Alzheimer's disease (AD), angiotensin II receptor blocker (ARB), telmisartan, 18F-fluorodeoxyglucose positron emission tomography (FDG-PET), anterior olfactory nucleus
Introduction
Many risk factors for dementia have been epidemiologically investigated with the hope of preventing or delaying the onset of Alzheimer's disease (AD; Korczyn and Vakhapova 2007). Hypertension is linked to AD along with smoking, diabetes mellitus, and hypercholesterolemia (Papademetriou 2005; Kehoe and Wilcock 2007). The possible effect of antihypertensive therapy on AD has been studied, and it is suggested that angiotensin-converting enzyme (ACE) inhibitors and angiotensin II receptor blockers (ARBs) exert a greater effect on cognitive decline than other antihypertensive medications (Gard 2002, 2004).
Telmisartan is a long-acting ARB that is effective for early hypertension. It has in addition peroxisome proliferator-activated receptor gamma (PPARγ) agonist effects (Benson et al. 2004; Lacourcire et al. 2004). Henka et al. (2005) reported that treatment with the PPARγ agonist pioglitazone reduces soluble amyloid-β (Aβ)1–42 peptide in mice. It has been shown that mRNA and protein levels of β-secretase or β-site amyloid precursor protein cleaving enzyme is repressed by pioglitazone resulting in reduction of Aβ1–42 (Sastre et al. 2006). Clinically, PPARγ agonists have been reported to act as insulin sensitizers, and to improve cognition and memory in AD patients (Watson et al. 2005; Landreth 2007). Mogi et al (2008) showed that telmisartan prevented cognitive decline partly due to PPARγ activation. Recently PPARγ activation in the brain has been highlighted to prevent AD via enhancement of Aβ clearance (Camacho et al. 2004) and antiinflammatory effects in neurons (Luna–Medina et al. 2005), endothelial cells (Wang et al. 2002), astrocytes and microglia (Klotz et al. 2003), and an increase in neural stem cell proliferation (Wada et al. 2006; Morales–Garcia et al. 2010). From these findings, it is hoped that treatment of blood pressure (BP) with telmisartan may mitigate the cognitive decline in AD. The purpose of the present study is to clarify the functional effects of telmisartan on AD brain using prospective longitudinal 18F-fluorodeoxyglucose positron emission tomography (FDG-PET) studies. In the revised NINCDS-ADRDA criteria, FDG-PET is dealt with as a topographical marker and is described to be more useful than pathological markers when the first cognitive symptoms are manifest in preclinical AD patients (Dubois et al. 2010).
Materials and Methods
Subjects
Among hypertensive outpatients with memory impairment with systolic blood pressure (SBP) of ≥140 mmHg or diastolic blood pressure (DBP) of 90 mmHg in the Department of Neurology of Saitama Medical University Hospital, those who were clinically diagnosed with AD according to revised NINCDS-ADRDA criteria, were recruited (Dubois et al. 2010). Patients who met the following criteria were excluded: (1) history of allergy to ARB; (2) SBP of ≥160 mmHg or DBP of ≥100 mmHg; (3) pregnancy; (4) severe biliary excretion dysfunction with serum total bilirubin concentration above 2.0 mg/dL; (5) severe liver dysfunction with serum asparatate aminotransferase (AST) or alanine aminotransferase (ALT) above 100IU/L; (6) severe renal dysfunction with serum Cr level above 3.0 mg/dL; (7) hyperkalemia; (8) continuous administration of ARB, ACE inhibitor, or pioglitazone; (9) other conditions deemed inappropriate for the purposes of this study by the investigators. Seven patients who met these criteria were enrolled. FDG-PET findings of all patients were supportive of AD. Of these seven patients, one experienced digestive tract hemorrhage during the follow-up studies and two refused to continue to participate. Finally four AD patients, two men and two women, aged from 70 to 77 years, finished the present longitudinal study protocol. At each FDG-PET study, mini-mental state examination (MMSE) was administered and BP was measured.
This study was approved by the institutional review board of Saitama Medical University International Medical Center and Saitama Medical University Hospital, and all subjects gave written informed consent to participate.
Study protocol
The subjects underwent three FDG-PET studies at intervals of 12 weeks. Antihypertensive treatment except for telmisartan was started immediately after the first FDG-PET study and continued for 24 weeks. Then 40–80 mg of telmisartan was added immediately after the second FDG-PET study and continued for 12 weeks (Fig. 1).
Figure 1.
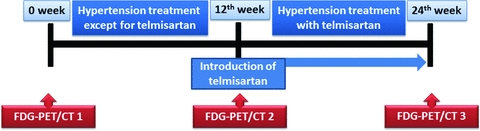
Study protocol. Subjects underwent FDG-PET at three points: the first at entry into this study, the second and third at 12 and 24 weeks after the 1st study, respectively. Telmisartan therapy was started immediately after the second study.
FDG-PET
FDG-PET was performed in the Department of Nuclear Medicine of Saitama Medical University International Medical Center. Before FDG-PET was performed, all subjects had an intravenous line established. Each subject received an intravenous injection of 185 MBq of FDG while lying in the supine position with eyes closed in a dimly lit, quiet room and was kept in the same resting state for at least 20 minutes. Fifty minutes after the injection of FDG, brain PET was performed using PET/Computed Tomography (CT) equipment with high spatial resolution (Biograph 6 Hi-Rez; Siemens Medical Systems, Inc.:Suite, Washington, D.C., United States). The combination of Fourier rebinning and the ordered subsets expectation-maximization at iteration number 4 and subset 16, and Gaussian filter at 6-mm full width at half maximum (FWHM) was used for PET image reconstruction. Attenuation correction was performed using CT data.
Image preprocessing
All FDG-PET images were spatially normalized using statistical parametric mapping 2 (SPM2; http://www.fil.ion.ucl.ac.uk/spm/) to a standardized stereotactic space based on the Talairach and Tournoux (1988) atlas, using 12-parameter linear affine normalization and a further 16 nonlinear iteration algorithms with an original template of FDG (Sakai et al. 2006). Then, isotropic Gaussian smoothing with 12-mm full-width at half maximum was performed.
Image analysis
Data were analyzed also using SPM2 program. The SPM2 combines the general linear model and the theory of Gaussian fields to make statistical inferences about regional effects (Friston et al. 1991, 1994). To examine changes in brain glucose metabolism during the 12 weeks without telmisartan, regionally specific differences between the first and second FDG-PET were statistically assessed using a two-tailed paired contrast testing for a decreased probability of glucose metabolism. Then to examine regionally specific differences in glucose metabolism between the two conditions, with and without telmisartan, the first, second, and third FDG-PET were statistically assessed. A two-tailed contrast testing was used for an increased or decreased probability (multiple subjects with different conditions in SPM2). To exclude time effect in voxel intensity across three scans, the term days was included as a nuisance covariate. The analysis was performed with normalization of global glucose metabolism for each subject to the same mean value with proportional scaling to compare two conditions regarding relative FDG distribution. The gray matter threshold was set to 0.8.
The resulting set of values for each contrast constituted a statistical parametric map of the t statistic SPM {t}. The SPM {t} maps were then transformed to the units of normal distribution (SPM {Z}), and height threshold was set to P = 0.005 uncorrected for multiple comparisons with extent threshold to 100 voxels. These areas of significance were visualized as overlaid on a normalized MR image to obtain a clear view of the location of the glucose metabolism changes.
Results
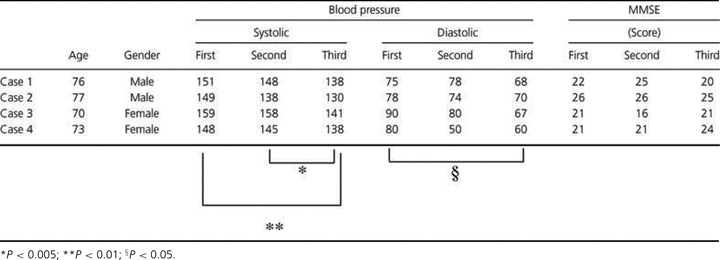
MMSE scores and BP are listed in Table 1. No significant changes in MMSE scores were observed during the time course. SBP declined significantly from the first to third and from the second to third FDG-PET. DBP declined significantly from the first to third FDG-PET.
Table 1.
Subjects' background, blood pressure, and cognitive state
 |
Glucose metabolism was significantly decreased during the first 12 weeks without telmisartan at an area (−10, 21, −22, x, y, z; Z = 3.56) caudal to the left rectal gyrus and the olfactory sulcus corresponding to the left olfactory tract (Fig. 2).
Figure 2.

Statistically significant decrease of glucose metabolism from the first to second FDG-PET studies in an area caudal to the left rectal gyrus and the olfactory sulcus corresponding to the left olfactory tract (P < 0.005). The SPM of the t-statistics is displayed in a standard format as a maximum intensity projection viewed from right-hand side and from the top and the back (top), and as orthogonal sections (bottom).
In contrast, the introduction of telmisartan during the following 12 weeks preserved glucose metabolism at areas (5, 19, −20, x, y, z; Z = 3.09; −6, 19, −22, x, y, z; Z = 2.88) caudal to the bilateral rectal gyri and olfactory sulci corresponding to the bilateral olfactory tracts (Fig. 3). No areas showed decreased glucose metabolism after the introduction of telmisartan.
Figure 3.
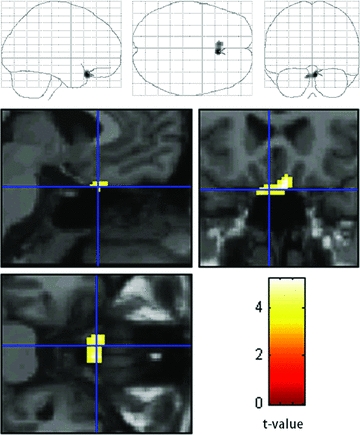
Statistically significant preservation of glucose metabolism by telmisartan from the first and second to third FDG-PET studies in areas caudal to the bilateral rectal gyri and the olfactory sulci corresponding to bilateral olfactory tracts (P < 0.005). The SPM of the t-statistics is displayed in a standard format as a maximum intensity projection viewed from right-hand side and from the top and the back (top) and as orthogonal sections (bottom).
Discussion
This short-term study showed a significant decline and preservation of glucose metabolism in a localized area caudal to the rectal gyrus corresponding to the olfactory tract during the first 12 weeks without telmisartan, and during the following 12 weeks with telmisartan, respectively.
The localized area corresponding to the olfactory tract detected by statistical analysis of longitudinal FDG-PET studies contains the anterior olfactory nucleus (AON; Saiz–Sanchez et al. 2010). AON plays a central role in human olfactory processing (Price 2004; Brunjes and Kenerson 2010). Though central olfactory connections are scarcely known in man, AON is assumed to have connections to the piriform cortex, anterior amygdala, periamygdaloid cortex, and the rostal entorhinal cortex (Price 2004). In Parkinson's disease, Lerner and Bagic (2008) proposed that AON is connected to the dorsal motor nucleus of the vagus by three principal pathways: the stria medullaris thalami and habenular nuclei, the amygdala and stria terminalis, and the medial forebrain bundle and hypothalamus. Because of these many pathways, AON is assumed to be rich in dendrites and astrocytes, resulting in abundant glucose consumption in this small region (Iadecola and Nedergaard 2007).
Hyposmia has been suggested to be a diagnostic symptom in early AD (Djordjevic et al. 2008). Li et al. (2010) proposed an objective way to reveal olfactory functional deficits in AD patients using a functional MRI. Olfactory functional impairment may result from early neurodegeneration of olfactory systems including AON (Pearson et al. 1985; Braak and Braak 1991; Price et al. 1991). Kovacs et al. (1999) showed that Aβ deposition and neurofibrillary tangle formation are observed in the olfactory bulb both in aging and AD though more frequently in the latter. Moreover, Saiz-Sanchez et al. (2010) analyzed the AON expression levels of somatostatin in AD versus controls, and found that levels of somatostatin were reduced in AON of AD cases compared to controls. It also has been reported that the reduction in somatostatin induces downregulation of neprylisin, a peptidase that catalyzes the proteolytic degradation of Aβ, and that may be a trigger for Aβ accumulation leading to late-onset sporadic AD (Saito et al. 2005). Decreased somatostatin expression may therefore result in Aβ accumulation. Furthermore, a reduction in the density of axons was observed in the olfactory tract of AD patients (Armstrong et al. 2008). Considering such involvement of the olfactory system in AD (Brunjes and Kenerson 2010), glucose metabolism in AON may be decreased more progressively within a short interval than in any other brain region.
Telmisartan is known to effectively reduce Aβ deposition (Mogi et al. 2008) and to induce PPARγ activation. This PPARγ activation has been reported to prevent brain damage through an antiinflammatory effect, for example in endothelial cells, astrocytes, and microglia (Wang et al. 2002; Klotz et al. 2003; Camacho et al. 2004; Heneka et al. 2005; Luna–Medina et al. 2005; Watson et al. 2005; Sastre et al. 2006; Wada et al. 2006; Landreth 2007; Mogi et al. 2008; Morales–Garcia et al. 2010). Thus, the current study supports the contention that progressive AD pathology in AON may be prevented by telmisartan.
The present study period may be too short to detect cognitive changes. However, this short term may not be inappropriate to observe any early effect of telmisartan on brain glucose metabolism. Although a further study may be necessary in a larger number of subjects, the current well-localized results with statistical significance may help to define the effect of telmisartan on AD brain.
Conclusion
In consideration of the recent many studies on the olfactory systems in AD, high-resolution FDG-PET is quite useful for the functional evaluation of a small area involving AON. Telmisartan therapy may inhibit short-term decline of glucose metabolism in the olfactory tract in AD brain.
Acknowledgments
We are thankful to the radiology technicians of the Department of Nuclear Medicine of Saitama Medical University International Medical Center for their technical support and to Prof. John Gelblum for proofreading this manuscript.
References
- Armstrong RA, Syed AB, Smith CU. Density and cross-sectional areas of axons in the olfactory tract in control subjects and Alzheimer's disease: an image analysis study. Neurol. Sci. 2008;29:23–27. doi: 10.1007/s10072-008-0854-0. [DOI] [PubMed] [Google Scholar]
- Benson SC, Pershadsingh HA, Ho CI, Chittiboyina A, Desai P, Pravenec M, Qi N, Wang J, Avery MA, Kurtz TW. Identification of telmisartan as a unique angiotensin II receptor antagonist with selective PPARgamma-modulating activity. Hypertension. 2004;43:993–1002. doi: 10.1161/01.HYP.0000123072.34629.57. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E. Neuropathological staging of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- Brunjes PC, Kenerson MC. The anterior olfactory nucleus: quantitative study of dendritic morphology. J. Comp. Neurol. 2010;1:1603–1616. doi: 10.1002/cne.22293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camacho IE, Serneels L, Spittaels K, Merchiers P, Dominguez D, De Strooper B. Peroxisome-proliferator-activated receptor gamma induces a clearance mechanism for the amyloid-beta peptide. J. Neurosci. 2004;1:10908–10917. doi: 10.1523/JNEUROSCI.3987-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djordjevic J, Jones-Gotman M, De Sousa K, Chertkow H. Olfaction in patients with mild cognitive impairment and Alzheimer's disease. Neurobiol. Aging. 2008;29:693–706. doi: 10.1016/j.neurobiolaging.2006.11.014. [DOI] [PubMed] [Google Scholar]
- Dubois B, Feldman HH, Jacova C, Cummings JL, Dekosky ST, Barberger-Gateau P, Delacourte A, Frisoni G, Fox NC, Galasko D, et al. Revising the definition of Alzheimer's disease: a new lexicon. Lancet Neurol. 2010;9:1118–1127. doi: 10.1016/S1474-4422(10)70223-4. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Frith CD, Liddle PF, Frackowiak RSJ. Comparing functional (PET) images: the assessment of significant change. Cereb. Blood Flow Metab. 1991;11:690–699. doi: 10.1038/jcbfm.1991.122. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Worsley KJ, Frackowiak RSJ, Mazziotta JC, Evans AC. Assessing the significance of focal activations using their spatial extent. Hum. Brain Mapping. 1993;1:210–220. doi: 10.1002/hbm.460010306. [DOI] [PubMed] [Google Scholar]
- Gard PR. The role of angiotensin II in cognition and behaviour. Eur. J. Pharmacol. 2002;1:1–14. doi: 10.1016/s0014-2999(02)01283-9. [DOI] [PubMed] [Google Scholar]
- Gard PR. Angiotensin as a target for the treatment of Alzheimer's disease, anxiety and depression. Expert Opin. Ther. Targets. 2004;8:7–14. doi: 10.1517/14728222.8.1.7. [DOI] [PubMed] [Google Scholar]
- Heneka MT, Sastre M, Dumitrescu-Ozimek L, Hanke A, Dewachter I, Kuiperi C, O'Banion K, Klockgether T, Van Leuven F, Landreth GE. Acute treatment with the PPARgamma agonist pioglitazone and ibuprofen reduces glial inflammation and Abeta1–42 levels in APPV717I transgenic mice. Brain. 2005;128:1442–53. doi: 10.1093/brain/awh452. [DOI] [PubMed] [Google Scholar]
- Iadecola C, Nedergaard M. Glial regulation of the cerebral microvasculature. Nat. Neurosci. 2007;10:1369–1376. doi: 10.1038/nn2003. [DOI] [PubMed] [Google Scholar]
- Kehoe PG, Wilcock GK. Is inhibition of the renin-angiotensin system a new treatment option for Alzheimer's disease? Lancet Neurol. 2007;6:373–378. doi: 10.1016/S1474-4422(07)70077-7. [DOI] [PubMed] [Google Scholar]
- Klotz L, Sastre M, Kreutz A, Gavrilyuk V, Klockgether T, Feinstein DL, Heneka MT. Noradrenaline induces expression of peroxisome proliferator activated receptor gamma (PPARgamma) in murine primary astrocytes and neurons. J. Neurochem. 2003;86:907–916. doi: 10.1046/j.1471-4159.2003.01909.x. [DOI] [PubMed] [Google Scholar]
- Korczyn AD, Vakhapova V. The prevention of the dementia epidemic. J. Neurol. Sci. 2007;15:2–4. doi: 10.1016/j.jns.2007.01.081. [DOI] [PubMed] [Google Scholar]
- Kovacs T, Cairns NJ, Lantos PL. b-Amyloid deposition and neurofibrillary tangle formation in the olfactory bulb in ageing and Alzheimer's disease. Neuropathol. Appl. Neurobiol. 1999;25:481–491. doi: 10.1046/j.1365-2990.1999.00208.x. [DOI] [PubMed] [Google Scholar]
- Lacourcière Y, Krzesinski JM, White WB, Davidai G, Schumacher H. Sustained antihypertensive activity of telmisartan compared with valsartan. Blood Press. Monit. 2004;9:203–210. doi: 10.1097/00126097-200408000-00005. [DOI] [PubMed] [Google Scholar]
- Landreth G. Therapeutic use of agonists of the nuclear receptor PPARgamma in Alzheimer's disease. Curr. Alzheimer Res. 2007;4:159–164. doi: 10.2174/156720507780362092. [DOI] [PubMed] [Google Scholar]
- Lerner A, Bagic A. Olfactory pathogenesis of idiopathic Parkinson disease revisited. Mov. Disord. 2008;15:1076–1084. doi: 10.1002/mds.22066. [DOI] [PubMed] [Google Scholar]
- Li W, Howard JD, Gottfried JA. Disruption of odour quality coding in piriform cortex mediates olfactory deficits in Alzheimer's disease. Brain. 2010;133:2714–2126. doi: 10.1093/brain/awq209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luna-Medina R, Cortes-Canteli M, Alonso M, Santos A, Martínez A, Perez-Castillo A. Regulation of inflammatory response in neural cells in vitro by thiadiazolidinones derivatives through peroxisome proliferator-activated receptor gamma activation. J. Biol. Chem. 2005;3:21453–21462. doi: 10.1074/jbc.M414390200. [DOI] [PubMed] [Google Scholar]
- Mogi M, Li JM, Tsukuda K, Iwanami J, Min LJ, Sakata A, Fujita T, Iwai M, Horiuchi M. Telmisartan prevented cognitive decline partly due to PPAR-gamma activation. Biochem. Biophys. Res. Commun. 2008;24:446–449. doi: 10.1016/j.bbrc.2008.08.032. [DOI] [PubMed] [Google Scholar]
- Morales-Garcia JA, Luna-Medina R, Alfaro-Cervello C, Cortes-Canteli M, Santos A, Garcia-Verdugo JM, Perez-Castillo A. Peroxisome proliferator-activated receptor γ ligands regulate neural stem cell proliferation and differentiation in vitro and in vivo. Glia. 2010;59:293–307. doi: 10.1002/glia.21101. [DOI] [PubMed] [Google Scholar]
- Papademetriou V. Hypertension and cognitive function. Blood pressure regulation and cognitive function: a review of the literature. Geriatrics. 2005;60:20–24. [PubMed] [Google Scholar]
- Pearson RC, Esiri MM, Hiorns RW, Wilcock GK, Powell TP. Anatomical correlates of the distribution of the pathological changes in the neocortex in Alzheimer's disease. Proc. Natl. Acad. Sci. USA. 1985;82:4531–4534. doi: 10.1073/pnas.82.13.4531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price JL. Olfactory system. In: Paxinos GR, editor. The human nervous system 2nd. New York: Academic Press; 2004. pp. 1197–1211. [Google Scholar]
- Price JL, Davis PB, Morris JC, White DL. The distribution of tangles, plaques and related immunohistochemical markers in healthy aging and Alzheimer's disease. Neurobiol. 1991;12:295–312. doi: 10.1016/0197-4580(91)90006-6. [DOI] [PubMed] [Google Scholar]
- Saito T, Iwata N, Tsubuki S, Takaki Y, Takano J, Huang SM, Suemoto T, Higuchi M, Saido TC. Somatostatin regulates brain amyloid beta peptide Abeta42 through modulation of proteolytic degradation. Nat. Med. 2005;11:434–439. doi: 10.1038/nm1206. [DOI] [PubMed] [Google Scholar]
- Saiz-Sanchez D, Ubeda-Bañon I, de la Rosa-Prieto C, Argandoña-Palacios L, Garcia-Muñozguren S, Insausti R, Martinez-Marcos A. Somatostatin, tau, and beta-amyloid within the anterior olfactory nucleus in Alzheimer disease. Exp. Neurol. 2010;223:347–350. doi: 10.1016/j.expneurol.2009.06.010. [DOI] [PubMed] [Google Scholar]
- Sakai Y, Kumano H, Nishikawa M, Sakano Y, Kaiya H, Imabayashi E, Ohnishi T, Matsuda H, Yasuda A, Sato A. Changes in cerebral glucose utilization in patients with panic disorder treated with cognitive-behavioral therapy. Neuroimage. 2006;33:218–226. doi: 10.1016/j.neuroimage.2006.06.017. [DOI] [PubMed] [Google Scholar]
- Sastre M, Dewachter I, Rossner S, Bogdanovic N, Rosen E, Borghgraef P, Evert BO, Dumitrescu-Ozimek L, Thal DR, Landreth G. Nonsteroidal anti-inflammatory drugs repress beta-secretase gene promoter activity by the activation of PPARgamma. Proc. Natl. Acad. Sci. USA. 2006;10:443–448. doi: 10.1073/pnas.0503839103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotactic atlas of the human brain. 1st ed. Stuttgart, Germany: Thieme; 1988. pp. 37–110. [Google Scholar]
- Wada K, Nakajima A, Katayama K, Kudo C, Shibuya A, Kubota N, Terauchi Y, Tachibana M, Miyoshi H, Kamisaki Y. Peroxisome proliferator-activated receptor gamma-mediated regulation of neural stem cell proliferation and differentiation. J. Biol. Chem. 2006;5:12673–12681. doi: 10.1074/jbc.M513786200. [DOI] [PubMed] [Google Scholar]
- Wang N, Verna L, Chen NG, Li H, Forman BM, Stemerman MB. Constitutive activation of peroxisome proliferator-activated receptor-gamma suppresses pro-inflammatory adhesion molecules in human vascular endothelial cells. J. Biol. Chem. 2002;13:34176–34181. doi: 10.1074/jbc.M203436200. [DOI] [PubMed] [Google Scholar]
- Watson GS, Cholerton BA, Reger MA, Baker LD, Plymate SR, Asthana S, Fishel MA, Kulstad JJ, Green PS, Cook DG. Preserved cognition in patients with early Alzheimer disease and amnestic mild cognitive impairment during treatment with rosiglitazone: a preliminary study. Am. J. Geriatr. Psychiatry. 2005;13:950–958. doi: 10.1176/appi.ajgp.13.11.950. [DOI] [PubMed] [Google Scholar]


