Induction of endotoxin tolerance in vivo inhibits IRAK4 and increases negative TLR4 regulators.
Keywords: lipopolysaccharide, signal transduction, tolerance/suppression/anergy, cell activation, monocytes/macrophages
Abstract
TLRs mediate host defense against microbial pathogens by eliciting production of inflammatory mediators and activating expression of MHC, adhesion, and costimulatory molecules. Endotoxin tolerance limits excessive TLR-driven inflammation during sepsis and reprograms macrophage responses to LPS, decreasing expression of proinflammatory cytokines without inhibiting anti-inflammatory and antimicrobial mediators. Molecular mechanisms of reprogramming of TLR4 signaling upon in vivo induction of endotoxin tolerance are incompletely understood. We used an in vivo model of endotoxin tolerance, whereby C57BL/6 mice were i.p.-inoculated with LPS or PBS, followed by in vitro challenge of peritoneal or splenic macrophages with LPS to examine activation of IRAK4 and expression of negative regulatory molecules. Administration of LPS in vivo-induced endotoxin tolerance in peritoneal and splenic macrophages, as evidenced by decreased degradation of IκBα, suppressed phosphorylation of p38 and reduced expression of TNF-α, IL-6, and KC mRNA upon in vitro LPS challenge. Macrophages from control and endotoxin-tolerant mice exhibited comparable TLR4 mRNA levels and similar expression of IL-1RA and IL-10 genes. Endotoxin tolerization in vivo blocked TLR4-driven IRAK4 phosphorylation and activation in macrophages, while increasing expression of IRAK-M, SHIP-1, A20 mRNA, and A20 protein. Thus, induction of endotoxin tolerance in vivo inhibits expression of proinflammatory mediators via impaired activation of IRAK4, p38, and NF-κB and increases expression of negative regulators of TLR4 pathways.
Introduction
Host immune defense is initiated via detection of microbial pathogens by PRRs, including TLRs [1, 2], which are present on the cell surface (TLR1, -2, -4, -5, -6, and -10) or in intracellular endosomes (TLR3, -7, -8, and -9) in macrophages, neutrophils, DCs, mast cells, and endothelial cells [1, 2]. All TLRs express an ectodomain involved in ligand recognition, a transmembrane region, and a cytoplasmic tail with a conserved signaling TIR domain [3]. Although endosomal TLRs detect microbial nucleic acids, TLRs expressed on the cell surface sense microbial lipids (e.g., LPS by TLR4) and proteins (e.g., flagellin by TLR5 and toxoplasma profilin-like proteins by TLR11) [3, 4]. TLR sensing of microbial pathogens activates innate host defense and primes adaptive immune responses via up-regulation of MHC and costimulatory molecules and secretion of cytokines [5, 6].
Recognition of LPS initiates TLR4 oligomerization, creating docking platforms in the TIR domain to enable recruitment of TIRAP, MyD88 [7, 8], IRAK4, and IRAK1 [9, 10]. Clustering of IRAK4 molecules induces IRAK4 kinase activity, IRAK4 → IRAK1 phosphorylation, recruitment of TRAF6, and engagement of TAK1 [11–13], which activates MAPKs and transcription factors NF-κB and AP-1, resulting in transcription of genes encoding inflammatory mediators, adhesion, and costimulatory molecules [13]. Following activation of this MyD88-dependent pathway, TLR4 translocates to endosomes, where it associates with TRIF and TRAF3 to signal transcription of type I IFNs via the TBK/IFN regulatory factor 3 signaling axis [14–16].
Septic patients surviving a proinflammatory “cytokine storm” develop a compensatory anti-inflammatory response syndrome that harnesses excessive TLR signaling but renders the host immunocompromised and susceptible to secondary infections [17–20]. Monocytes from immunocompromised septic patients exhibit suppressed LPS-inducible expression of proinflammatory cytokines [17], closely resembling the endotoxin-tolerant phenotype. Endotoxin tolerance was defined initially as a state of hyporesponsiveness to LPS challenge, subsequent to prior exposure to LPS [21–24]. Later studies showed that endotoxin tolerance reprograms TLR4 signaling by suppressing LPS-induced proinflammatory cytokines and chemokines without inhibiting anti-inflammatory cytokines and antimicrobial effectors [25–27]. Although decreased TLR4 expression was suggested to underlie mitigated LPS responses in endotoxin-tolerant mouse macrophages [28, 29], it cannot explain unchanged, LPS-driven expression of anti-inflammatory and antimicrobial mediators in endotoxin-tolerized cells. Subsequent studies showed that endotoxin tolerization in vitro does not inhibit TLR4 expression [30–37] but blocks tyrosine phosphorylation of TLR4 and TIRAP, TLR4-MyD88, and IRAK1-MyD88 assemblies and activation of IRAK4, IRAK1, K63-linked ubiquitination of IRAK1 and TRAF6 and increases expression of negative regulators IRAK-M, SHIP-1, suppressor of cytokine signaling 1, and A20 [30, 32–34, 38–42]. However, the relevance of these in vitro findings to in vivo endotoxin tolerance is poorly understood.
In this study, we used an in vivo model of endotoxin tolerance induced by i.p. administration of LPS to examine TLR4 signaling in peritoneal and splenic macrophages upon in vitro LPS challenge. We found that induction of endotoxin tolerance in vivo reduces LPS-induced activation of IRAK4, degradation of IκBα and phosphorylation of p38, and blocks up-regulation of TNF-α, IL-6, and KC mRNA in peritoneal and spleen macrophages In contrast, similar levels of TLR4 mRNA and anti-inflammatory cytokines IL-1RA and IL-10 were observed in macrophages obtained from control and endotoxin-tolerized mice. Blunted LPS-mediated, proinflammatory responses of macrophages derived from mice developing endotoxin tolerance in vivo coincided with increased expression of negative regulators IRAK-M, SHIP-1, and A20. These findings demonstrate inhibited LPS-mediated activation of IRAK4 and increased expression of IRAK-M, SHIP-1, and A20 as general mechanisms driving endotoxin tolerance and suggest their commonality to endotoxin tolerant-like states observed in immunocompromised septic patients.
MATERIALS AND METHODS
Reagents
Antibodies against IκBα, β-actin, tubulin, and A20 were from Santa Cruz Biotechnology (Santa Cruz, CA, USA); anti-p-p38, anti-total p38, and anti-IRAK4 antibodies were from Cell Signaling Technology (Danvers, MA, USA). Thioglycollate medium was purchased from Remel (Lenexa, KS, USA), and protein G-agarose was from Roche Applied Sciences (Indianapolis, IN, USA). Protein-free, ultrapure LPS from Escherichia coli K235 was kindly provided by Dr. Stefanie N. Vogel (University of Maryland School of Medicine, Baltimore, MD, USA).
Animal treatment and macrophage cell culture
Six- to 8-week-old C57BL/6J mice were purchased from the Charles River Laboratories (Wilmington, MA, USA). Animals were maintained in a laminar flow hood in cages fitted with polyester filter hoods and fed standard laboratory food and acid water ad libitum. We adapted a protocol published previously to induce endotoxin tolerance in vivo [43]. In brief, mice were i.p. injected with sterile 3% thioglycolate (2.5 ml/mouse), and 72 h later, i.p. administration of 0.2 ml PBS (control group) or 0.2 ml LPS (endotoxin-tolerant group, 25 μg/mouse) was carried out. After 26 h, peritoneal lavage cells and spleens were isolated, and single-cell splenocytes were obtained by gently pressing spleens through strainers. Thioglycolate-elicited peritoneal exudate cells and splenocytes were plated in six-well plates (4×106 cells/well) in RPMI 1640 (Mediatech, Herndon, VA, USA), supplemented with 2 mM L-glutamine, 10% FBS (HyClone, Logan, UT, USA), and 100 U/ml penicillin and 100 μg/ml streptomycin (Invitrogen, San Diego, CA, USA; complete RPMI). Macrophages were obtained after adherence to plastic for 20 h at 37°C in a 5% CO2 atmosphere, nonadherent cells were removed by extensive washing, and macrophages were stimulated with medium or 100 ng/ml LPS for the indicated times. All animal experiments were conducted with institutional approval.
Preparation of whole cell lysates and Western blot analyses
Macrophages were incubated for 30 min at 4°C with a lysis buffer containing 20 mM Tris-HCl (pH 7.4), 1% Triton X-100, 150 mM NaCl, 12.5 mM β-glycerophosphate, 50 mM sodium fluoride, 1 mM DTT, 1 mM sodium orthovanadate, 2 mM EDTA, 1 mM PMSF, and protease inhibitor cocktail (Roche Applied Sciences). Insoluble debris was removed by centrifugation at 10,000 g for 20 min, and supernatants were collected and frozen at –80°C. Protein concentration was determined by the protein assay kit (Bio-Rad, Hercules, CA, USA); samples were resuspended in Laemmli buffer (50 mM Tris-Cl, pH 6.8/10% glycerol/2% SDS/0.1% bromophenol blue/5% 2-ME) and loaded (20 μg/lane) onto 4–20% polyacrylamide minigels (Invitrogen). Proteins were separated by electrophoresis and electrotransferred to Immobilon-P membranes (Millipore, Bedford, MA, USA). Membranes were blocked and probed with the corresponding antibodies, as described previously [30, 33, 34, 38]. The intensities of the bands were quantified using Quantity One software (Bio-Rad), as reported previously [33, 34].
Isolation of RNA and real-time RT-PCR analysis
Total RNA was isolated with the Trizol reagent (Invitrogen), treated with DNase, and repurified, as recommended by the manufacturer. cDNA was prepared from 1 μg total RNA, using a reverse transcription system (Promega, Madison, WI, USA), and subjected to real-time PCR, using IQ SYBR Green supermix (Bio-Rad) with gene-specific primers. The following primers were used: mouse HPRT: 5′-GCTGACCTGCTGGATTACATT-3′ forward, 5′-GTTGAGAGATCATCTCCACCA-3′ reverse; TNF-α: 5′-ACCCTCACACTCAGATCATCT-3′ forward, 5′-TTGTCTTTGAGATCCATGCCGT-3′ reverse; IL-6: 5′-TCAGGAAATTTGCCTATTGAAAATTT-3′ forward, 5′-GCTTTGTCTTTCTTGTTATCTTTTAAGTTGT-3′ reverse; KC: 5′-TGTCAGTGCCTGCAGACCAT-3′ forward, 5′-GCTATGACTTCGGTTTGGGTG-3′ reverse; TLR4: 5′-GCATGGATCAGAAACTCAGCA-3′ forward, 5′-TCTGGATAGGGTTTCCTGTCA-3′ reverse; IL-1RA: 5′-TGTGCCAAGTCTGGAGATGA-3′ forward, 5′-TTCTTTGTTCCTGCTCAGATCAGT-3′ reverse; IL-10: 5′-ATTTGAATTCCC-TGGGTGAGAAG-3′ forward, 5′-CACAGGGGAGAAATCGATGACA-3′ reverse; IRAK-M: 5′-CATCAACTATGGAGTAAGCTGGAC-3′ forward, 5′-GTCCAGGGTCGTTTTCTCTG-3′ reverse; SHIP-1: 5′-GACATTTAAAACAGTTGCCATCC-3′ forward, 5′-GCCTGTCTTCACGTTGTCAG-3′ reverse; A20: 5′-TACGACACTCGGAACT GGAAT-3′ forward, 5′-TGACAATGATGGGTCTTCTGA-3′ reverse, on a MyIQ real-time PCR detection system (Bio-Rad). Data were analyzed by the 2–ΔΔCT method [44].
In vitro kinase assay
In vitro kinase assays were performed as described [38], using MBP (Sigma-Aldrich, St. Louis, MO, USA) as a substrate. IRAK4 proteins were immunoprecipitated for 20 h at 4°C under rotation with anti-IRAK4 antibody/protein G agarose, and immune complexes were washed twice in a lysis buffer, three times in a kinase buffer containing 20 mM Hepes, pH 7.6, 50 mM NaCl, 10 mM MgCl2, 20 mM β-glycerophosphate, 1 mM sodium orthovanadate, and 1 mM ATP, and then incubated for 25 min at 30°C in a kinase reaction with 1.0 μg MBP, 1 mM ATP, and 5 μCi [32P]ATP (PerkinElmer, Wellesley, MA, USA), made up to a total volume of 20 μl with kinase buffer. Samples were then resolved by SDS-PAGE, dried, exposed to X-ray films, and visualized by autoradiography.
Statistical analysis
Statistical analysis was performed using the GraphPad Prism 5 program for Windows (GraphPad Software, San Diego, CA, USA). Statistical differences were evaluated by the Student's unpaired t test with the confidence interval set at 99%. Values are expressed as mean ± sd.
RESULTS
In vivo LPS administration decreases the ability of macrophages to up-regulate expression of proinflammatory cytokine genes in response to LPS challenge in vitro
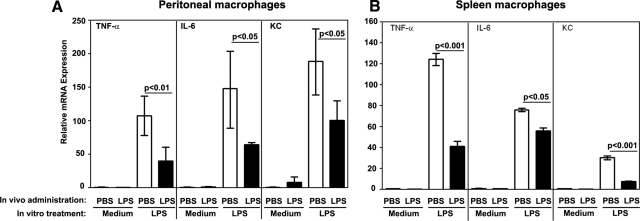
To induce endotoxin tolerance in vivo, we adapted a published protocol [24, 43], whereby mice were i.p.-administered PBS (control) or LPS (endotoxin-tolerant group). Twenty-six hours later, peritoneal and spleen macrophages were treated in vitro with medium or LPS to examine expression of TNF-α, IL-6, and KC cytokine genes, which are tolerized effectively by LPS [25, 27, 35, 45]. To obtain sufficient numbers of peritoneal macrophages, mice were inoculated i.p with thioglycollate, 72 h prior administration of PBS or LPS. Whereas peritoneal macrophages derived from PBS-injected mice showed a 115-, 148-, and 188-fold increase of TNF-α, IL-6, and KC mRNA in response to LPS challenge in vitro, expression of these cytokine genes was inhibited by 47–65% in macrophages from mice inoculated with LPS (Fig. 1A). LPS stimulation of spleen macrophages from PBS-treated mice increased TNF-α, IL-6, and KC mRNA by 124-, 76-, and 34-fold whereas macrophages obtained from LPS-inoculated mice exhibited 67%, 26%, and 74% suppression, respectively, in LPS-induced cytokine genes (Fig. 1B). These data indicate that endotoxin administration in vivo reduces proinflammatory cytokine responses of peritoneal and spleen macrophages upon in vitro restimulation with LPS, indicating the induction of endotoxin tolerance.
Figure 1. Macrophages from mice i.p. inoculated with LPS show suppressed expression of TNF-α, IL-6, and KC mRNA in response to stimulation with LPS in vitro.
Mice were i.p. administered thioglycollate, followed by i.p. inoculation with PBS or LPS (25 μg/mouse) 48 h post-thioglycollate administration. Twenty-six hours thereafter, macrophages were obtained from peritoneal lavage fluid and spleens by plastic adherence. Peritoneal (A) and spleen (B) macrophages were treated for 3 h with medium or 100 ng/ml LPS, RNA was isolated, reverse-transcribed, and analyzed by real-time PCR with the primers specific for HPRT, TNF-α, IL-6, and KC genes. Data were calculated by the 2-ΔΔCT method [44], with the values in the medium-treated macrophages from PBS-injected mice set as controls. (A) The summary data of three experiments (mean±sd) are presented. (B) Shown are the data (mean±sd) of a representative (n=3) experiment.
The impact of endotoxin tolerization in vivo on expression of TLR4 and anti-inflammatory cytokine mRNA
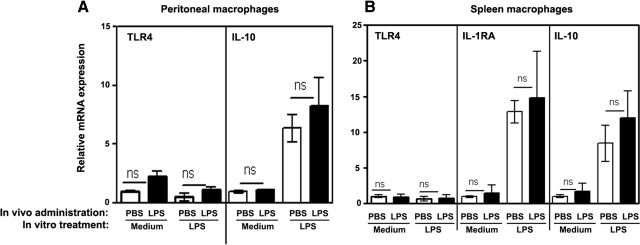
The magnitude of LPS responsiveness has been reported to correlate with relative expression levels of TLR4 [46]. Therefore, we studied whether decreased LPS-inducible expression of proinflammatory cytokines was a result of altered TLR4 expression and determined the impact of endotoxin tolerance on expression of anti-inflammatory cytokine genes. As shown in Fig. 2, peritoneal (Fig 2A) and splenic (Fig. 2B) macrophages obtained from PBS-injected or LPS-administered mice had comparable levels of TLR4 mRNA. Furthermore, in vitro LPS challenge led to similar up-regulation of IL-1RA and IL-10 in macrophages obtained from control or endotoxin-tolerant mice (Fig. 2). Thus, in vivo induction of endotoxin tolerance does not reduce TLR4 gene expression and expression of anti-inflammatory cytokine genes in peritoneal and splenic macrophages.
Figure 2. Expression of TLR4, IL-1RA, and IL-10 mRNA in macrophages obtained from PBS-injected and endotoxin-tolerized mice.
Peritoneal (A) and spleen (B) macrophages were obtained from mice injected with PBS or LPS, as described in Fig. 1. Cells were treated for 3 h with medium or 100 ng/ml LPS, and RNA was isolated, converted to cDNA, and analyzed by real-time PCR with the corresponding gene-specific primers. The data were processed by the 2–ΔΔCT method [44] compared with the values in unstimulated macrophages obtained from mice administered with PBS. (A) The results of a representative experiment (mean±sd) are depicted. (B) Shown are the summary data (mean±sd) of three independent experiments.
Macrophages obtained from mice treated with LPS in vivo exhibit decreased LPS-inducible IκBα degradation and phosphorylation of p38
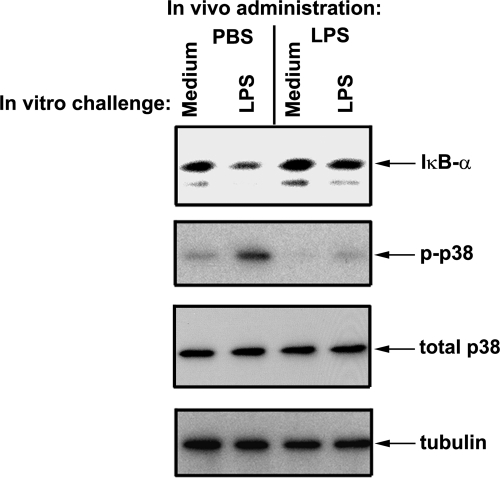
p38 MAPK and NF-κB regulate expression of proinflammatory cytokines and chemokines, including TNF-α, IL-6, and KC [47]. Activation of p38 occurs via phosphorylation, which induces p38 kinase activity [48–50], whereas proteasomal degradation of IκBα enables NF-κB to translocate to the nucleus, switching on transcription of proinflammatory cytokine genes [51]. Therefore, we examined the impact of induction of endotoxin tolerance in vivo on the ability of macrophages to respond to LPS challenge in vitro by phosphorylation of p38 and degradation of IκBα. LPS induced robust phosphorylation of p38 and degradation of IκBα in splenic macrophages obtained from PBS-treated mice, whereas cells from endotoxin-inoculated animals failed to respond to LPS by activation of these signaling events (Fig. 3). Equal levels of total p38 and tubulin were detected in macrophage samples obtained from control and endotoxin-tolerized mice, indicating that differences in phosphorylation of p38 and degradation of IκBα were not a result of variations in total p38 expression or protein loading. Taken collectively, these data indicate that induction of endotoxin tolerance in vivo inhibits LPS-induced p38 phosphorylation and IκBα degradation in peritoneal and splenic macrophages.
Figure 3. Administration of LPS in vivo results in decreased degradation of IκBα and phosphorylation of p38 in spleen macrophages on in vitro LPS challenge.
Mice were administered PBS or LPS (25 μg/mouse), and 26 h later, spleens were isolated and homogenized, and macrophages were obtained by adherence to plastic. Cells were stimulated for 15 min with medium or 100 ng/ml LPS, and cell lysates were prepared and analyzed by Western blotting, using antibodies against IκBα, p-p38, and tubulin. Shown are the results of a representative (n=3) experiment.
Induction of endotoxin tolerance in vivo suppresses LPS-inducible IRAK4 activation
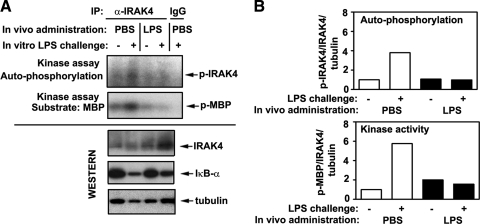
Next, we determined whether endotoxin tolerization in vivo changes the capacity of macrophages to respond to in vitro LPS stimulation by activation of IRAK4, which occurs via its autophosphorylation, as a consequence of clustering of multiple IRAK4 molecules [52]. Therefore, we used an in vitro kinase assay of immunoprecipitated IRAK4 proteins to assess their phosphorylation status and kinase activity toward a substrate MBP, similar to our previous report [38]. Splenic macrophages from PBS-inoculated mice responded to LPS challenge in vitro by robust IRAK4 phosphorylation, induction of kinase activity, and degradation of IκBα (Fig. 4A, upper panels), resulting in a 3.8- and 5.3-fold increase in IRAK4 phosphorylation and activity (Fig. 4B). LPS administration in vivo resulted in the loss of LPS-inducible phosphorylation and activation of IRAK4 in macrophages, correlating with their failure to degrade IκBα (Fig. 4A, lower panels, and B). Comparable levels of total IRAK4 proteins were observed in samples of control macrophages and medium-treated cells obtained from LPS-injected mice, and no reduction of total IRAK4 expression was observed in macrophages from endotoxin-tolerized cells challenged with LPS (Fig. 4A, third panel from the top). In all three experiments, densitometric quantifications of total IRAK4 proteins in splenic macrophages from control versus endotoxin-tolerant mice showed 0.5 ± 0.19 vs. 0.55 ± 0.28 (P>0.05) arbitrary values in medium-treated cells and 0.59 ± 0.35 vs. 0.83 ± 0.5 (P>0.05) values in LPS-stimulated macrophages, indicating no statistically significant differences. LPS-induced IRAK4 phosphorylation and kinase activity in peritoneal macrophages derived from endotoxin-inoculated mice were also inhibited compared with strong responses observed in cells from PBS-injected mice (data not shown). Our data indicate that induction of endotoxin tolerance in vivo blocks activation of IRAK4 in macrophages upon in vitro LPS challenge and that deficient activation of IRAK4 is not a result of its lower expression.
Figure 4. Suppressed LPS-inducible activation of IRAK4 in macrophages obtained from endotoxin-treated mice.
(A) Twenty-six hours after i.p. inoculation of PBS or LPS (25 μg/mouse), spleen were isolated and homogenized, and spleen cells were adhered to plastic to obtain macrophages, followed by stimulation of cells for 15 min with medium or 100 ng/ml LPS. IRAK4 proteins were immunoprecipitated (IP) from cell lysates and subjected to in vitro kinase assays with MBP as a substrate. Cell lysates were analyzed by immunoblotting, using anti-IκBα, anti-IRAK4, and antitubulin antibodies. The results of representative (n=3) experiments are presented. (B) Densitometric quantification of the data shown in A. Intensities of p-IRAK4 and p-MBP bands were measured and normalized to the levels of total IRAK4 and tubulin. Data in the treatment groups were divided by values obtained in medium-treated macrophages obtained from PBS-injected mice and presented as fold induction.
Macrophages obtained from endotoxin-tolerized mice exhibit increased expression of IRAK-M, SHIP-1, and A20
Previous studies by us and others [34, 38] have identified several negative regulators of TLR4 signaling that have been associated with induction of endotoxin tolerance in vitro, including IRAK-M, SHIP-1, Toll-interacting protein, sterile-α and Armadillo motif-containing protein, suppressor of IKK-ε, and A20. To confirm whether this mechanism plays a role on in vivo induction of endotoxin tolerance, we analyzed expression levels of IRAK-M, SHIP-1, and A20 in peritoneal and spleen macrophages obtained from control and PBS-injected mice vs. mice inoculated with LPS. Real-time PCR analyses revealed statistically significant increases in expression of IRAK-M, SHIP-1, and A20 mRNA and levels of A20 proteins in peritoneal (Fig. 5A) and spleen (Fig. 5B and C) macrophages isolated from LPS-inoculated mice, compared with their expression in cells from PBS-treated animals (Fig. 5). The SHIP-1 gene showed the highest up-regulation, followed by IRAK-M and A20 genes. Up-regulation of the A20 protein in splenic macrophages from mice administered LPS in vivo was paralleled by repressed, LPS-inducible phosphorylation of p38, documenting the endotoxin-tolerant phenotype, which was not a result of variations in total levels of p38 or tubulin (Fig. 5C). These data show that endotoxin tolerization in vivo increases expression of IRAK-M, SHIP-1, and A20 in peritoneal and spleen macrophages, suggesting their involvement in reprogramming TLR4 pathways that signal expression of inflammatory mediators.
Figure 5. Endotoxin tolerization in vivo increases expression of negative regulators of TLR4 signaling.
Twenty-six hours after i.p. inoculation of PBS or LPS, spleens were isolated and homogenized, and macrophages were obtained by plastic adherence. Peritoneal (A) and spleen (B) macrophages were treated with Trizol, and RNA was isolated, reverse-transcribed, and subjected to real-time PCR. The data were calculated according to the 2–ΔΔCT method. Shown are the results (mean±sd) of three experiments. (C) Spleen macrophages were treated for 15 min with medium or 100 ng/ml LPS and lysed, and whole cell lysates were analyzed by Western blotting using antibodies against A20, p-p38, total p38, and tubulin.
DISCUSSION
This paper demonstrates that impaired phosphorylation and activation of IRAK4 and up-regulation of negative TLR4 regulators IRAK-M, SHIP-1, and A20 are important mechanisms by which induction of endotoxin tolerance in vivo reprograms TLR macrophage responses. We show that i.p. administration of LPS suppressed expression of proinflammatory cytokines TNF-α, IL-6, and KC mRNA upon in vitro challenge of peritoneal and splenic macrophages with LPS, compared with robust cytokine induction in cells from PBS-inoculated mice. Decreased LPS-inducible, proinflammatory cytokine expression in macrophages from endotoxin-tolerant mice was not a result of decreased TLR4 expression, consistent with similar data obtained for in vitro LPS-tolerized macrophages and monocytes [30–32, 34]. Rather, it was linked to blocked activation of IRAK4, supporting the essential role for IRAK4 kinase activity in triggering proinflammatory cytokines and chemokines [13, 53, 54]. Consistent with the requirement for IRAK4 kinase activity for MAPK and NF-κB stimulation [54, 55], the failure of LPS to activate IRAK4 in macrophages from mice tolerized with endotoxin in vivo correlated with inhibited p38 phosphorylation and IκBα degradation. Inhibition of LPS-mediated IRAK4 activation in macrophages derived from endotoxin-tolerant mice was not a result of protein degradation of IRAK4, confirming similar findings obtained for in vitro LPS-tolerized human monocytes [38] but contrasting reports about degradation of IRAK4 proteins in LPS-tolerant macrophages [56, 57].
Notably, in contrast to IRAK1, IRAK4 lacks proline-glutamic acid-serine-threonine protein degradation motifs [58]. In addition, whereas the exact ubiquitination events regulating IRAK4 activity are currently unknown, IRAK4 could be subject to preferential K63-linked ubiquitination, which is linked to protein activation and signalosome assemblies, rather than K48-linked ubiquitination, which is linked to protein degradation [59]. In this respect, it is interesting that we recently found preferential, LPS-inducible, K63-linked ubiquitination of IRAK-1 in human monocytes, which was down-regulated by endotoxin tolerization in vitro, whereas K48-linked ubiquitination of IRAK1 was very weak and not affected [38]. Future studies are necessary to delineate the exact molecular basis of preserved IRAK4 expression during endotoxin tolerance. Sustained IRAK4 expression was reported on stimulation of mouse macrophages and human embryonic kidney 293 cells via IL-1R or TLRs [60]. Consistent with these data, our results argue against the role for IRAK4 protein degradation in inhibiting LPS-driven IRAK4 activation during endotoxin tolerance.
Although the exact mechanism by which endotoxin tolerization ablates LPS-induced IRAK4 activation is unknown, our findings on increased expression of IRAK-M in macrophages obtained from LPS-tolerant animals suggest IRAK-M involvement. IRAK-M−/− mice exhibit enhanced LPS-mediated cytokine production and heightened TLR-mediated inflammatory responses upon bacterial challenge in vivo, and IRAK-M−/− macrophages are deficient in the development of endotoxin tolerance [42]. IRAK-M has been reported to interact with IRAK4, altering assembly of the MyD88-IRAK4-IRAK1 signalosome, inhibiting IRAK4-driven IRAK1 phosphorylation, and preventing IRAK1-TRAF6 interactions [42]. It is plausible that increased IRAK-M expression in endotoxin-tolerant monocytes and macrophages interferes with recruitment, post-translational modifications, or signalosome assembly of IRAK4, contributing to ablated, LPS-induced IRAK4 activation. Interestingly, increased IRAK-M has been found in several models of endotoxin tolerance and in immunocompromised septic patients [34, 41, 42, 61, 62], stressing the importance of IRAK-M for reprogramming TLR4 signaling occurring in these conditions.
This paper shows that in vivo endotoxin tolerization of mice up-regulates expression of SHIP-1 and A20 in peritoneal and spleen macrophages, confirming previous findings of increased expression of these molecules upon induction of in vitro endotoxin tolerance in human monocytes [34]. SHIP-1 and A20 are negative regulators of TLR signaling that act downstream of IRAK4. SHIP-1−/− mice and macrophages exhibit enhanced production of proinflammatory cytokines upon challenge with TLR agonists or bacteria and readily succumb to endotoxin shock, and SHIP-1−/− macrophages and mice fail to develop endotoxin tolerance [40]. SHIP-1 inhibits LPS-mediated expression of proinflammatory cytokines and type I IFNs by affecting signaling pathways triggered by PI3K and TBK1 [63, 64]. A20 is a deubiquitinating enzyme, which removes K63-linked polyubiquitin chains from TRAF6, receptor-interacting protein-1, and IKK-γ, effectively disabling TRAF6-mediated activation of TAK1 and activation of the IKK complex [65]. Recently, we reported that endotoxin-tolerized human monocytes show increased A20 expression and that A20 overexpression suppresses LPS-inducible IκBα degradation and activation of NF-κB within the IRAK-TRAF6-TAK1 signaling axis via interactions with IRAK1, IRAK2, and TRAF6 [38]. Furthermore, A20 knockdown in THP1 cells [38] and primary human enterocytes [66] abolishes the ability of LPS to induce endotoxin tolerance, indicating a mechanistic role for A20. These data show a principal role for A20 in endotoxin tolerizaton of THP1 cells, primary human monocytes, enterocytes, and mouse macrophages. In contrast, A20−/− mouse macrophages pretreated with LPS were reported to develop endotoxin tolerance [67], and A20 gene knockdown in human intestinal epithelial cell lines fails to affect tolerance induction to flagellin [68], arguing that A20 is not involved in TLR tolerance. The reason for contrasting results regarding A20 functions in mediating TLR tolerance is unclear and will require further studies. Our data support involvement of negative regulators IRAK-M, SHIP-1, and A20 in mediating endotoxin tolerance.
Transcription of proinflammatory cytokines requires TLR4-driven activation of p38 kinase [69], controlling induction of activating transcription factor-2 [48–50], and stimulation of NF-κB involving proteasomal IκB degradation, which enables NF-κB nuclear translocation [51]. In line with the involvement of MAPK and NF-κB in initiating transcription of proinflammatory cytokines [70, 71], we found that endotoxin tolerization of mice in vivo decreases LPS-mediated phosphorylation of p38 and degradation of IκBα in macrophages. This inhibition is selective, as it does not alter, or it up-regulates expression of many NF-κB-dependent negative regulators of TLR4, as well as anti-inflammatory cytokines [26, 27, 34, 40–42, 45], including IL-1RA and IL-10 (this paper). It is possible that a different composition of NF-κB subunits is responsible for differential control of promoters of proinflammatory cytokines vs. anti-inflammatory cytokines and negative regulators. We and others [72–75] reported accumulation of p50 and RelB subunits in endotoxin-tolerized mouse macrophages and human monocytes, whereas control cells stimulated with LPS expressed p50-p65 NF-κB dimers. Accumulation of transcription-incompetent p50-p50 dimers can repress transcription of proinflammatory cytokines, whereas heterodimerization of p50 with p52, Bcl3, or Rel-B confers the ability to drive transcription of selective genes [51]. This mechanism could explain persistent expression of anti-inflammatory cytokines and negative regulators of TLR4 signaling in endotoxin-tolerant cells. Studies are in progress to address this possibility.
Collectively, we demonstrate that induction of endotoxin tolerance in vivo blunts LPS-mediated activation of IRAK4, activation of p38 MAPK, and IκBα degradation and induction of proinflammatory cytokine genes in spleen and peritoneal macrophages, while not inhibiting TLR4, IL-1RA, and IL-10 mRNA levels. Furthermore, in vivo endotoxin tolerization elevates expression of negative regulators of TLR4 signaling IRAK-M, SHIP-1, and A20 in mouse macrophages. Combined with previous reports, these findings indicate that dysregulation of LPS-induced activation of IRAK4 and up-regulation of negative TLR4 regulators targeting MyD88- and TRIF-dependent pathways are general molecular determinants of endotoxin tolerance.
ACKNOWLEDGMENTS
This work was supported by NIH grants AI059524 and AI067468 (to A.E.M.).
Footnotes
- CT
- threshold cycle
- HPRT
- hypoxanthine phosphoribosyltransferase
- IL-1RA
- IL-1R antagonist
- IRAK
- IL-1R-associated kinase
- KC
- keratinocyte chemokine
- MBP
- myelin basic protein
- p
- phospho
- TAK1
- TGF-β-activating kinase 1
- TBK
- TRAF family member-associated NF-κB activator-binding kinase
- TIR
- Toll-IL-1R resistance
- TIRAP
- Toll-IL-1R resistance domain-containing adapter protein
- TRIF
- Toll-IL-1R resistance domain-containing adapter-inducing IFN-β
AUTHORSHIP
A.E.M. conceived the idea, designed the experiments, oversaw the entire project, and prepared the manuscript. Y.X. performed inoculation of mice in vivo with PBS, LPS, and thioglycollate; isolated spleen and peritoneal macrophages; carried out all real-time PCR, immunoprecipitation, in vitro kinase assay, and immunoblot analyses; and prepared the figures.
REFERENCES
- 1. Kawai T., Akira S. (2010) The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat. Immunol. 11, 373–384 [DOI] [PubMed] [Google Scholar]
- 2. Doyle S. L., O'Neill L. A. (2006) Toll-like receptors: from the discovery of NF-κB to new insights into transcriptional regulations in innate immunity. Biochem. Pharmacol. 72, 1102–1113 [DOI] [PubMed] [Google Scholar]
- 3. Beutler B. (2009) Microbe sensing, positive feedback loops, and the pathogenesis of inflammatory diseases. Immunol. Rev. 227, 248–263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Carpenter S., O'Neill L. A. (2007) How important are Toll-like receptors for antimicrobial responses? Cell. Microbiol. 9, 1891–1901 [DOI] [PubMed] [Google Scholar]
- 5. Pasare C., Medzhitov R. (2005) Toll-like receptors: linking innate and adaptive immunity. Adv. Exp. Med. Biol. 560, 11–18 [DOI] [PubMed] [Google Scholar]
- 6. Iwasaki A., Medzhitov R. (2010) Regulation of adaptive immunity by the innate immune system. Science 327, 291–295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Medzhitov R., Preston-Hurlburt P., Kopp E., Stadlen A., Chen C., Ghosh S., Janeway C. A., Jr. (1998) MyD88 is an adaptor protein in the hToll/IL-1 receptor family signaling pathways. Mol. Cell 2, 253–258 [DOI] [PubMed] [Google Scholar]
- 8. Horng T., Barton G. M., Medzhitov R. (2001) TIRAP: an adapter molecule in the Toll signaling pathway. Nat. Immunol. 2, 835–841 [DOI] [PubMed] [Google Scholar]
- 9. Burns K., Janssens S., Brissoni B., Olivos N., Beyaert R., Tschopp J. (2003) Inhibition of interleukin 1 receptor/Toll-like receptor signaling through the alternatively spliced, short form of MyD88 is due to its failure to recruit IRAK-4. J. Exp. Med. 197, 263–268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lin S. C., Lo Y. C., Wu H. (2010) Helical assembly in the MyD88-IRAK4-IRAK2 complex in TLR/IL-1R signaling. Nature 465, 885–890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kawagoe T., Sato S., Matsushita K., Kato H., Matsui K., Kumagai Y., Saitoh T., Kawai T., Takeuchi O., Akira S. (2008) Sequential control of Toll-like receptor-dependent responses by IRAK1 and IRAK2. Nat. Immunol. 9, 684–691 [DOI] [PubMed] [Google Scholar]
- 12. O'Neill L. A. (2008) When signaling pathways collide: positive and negative regulation of Toll-like receptor signal transduction. Immunity 29, 12–20 [DOI] [PubMed] [Google Scholar]
- 13. Li X. (2008) IRAK4 in TLR/IL-1R signaling: possible clinical applications. Eur. J. Immunol. 38, 614–618 [DOI] [PubMed] [Google Scholar]
- 14. Kagan J. C., Su T., Horng T., Chow A., Akira S., Medzhitov R. (2008) TRAM couples endocytosis of Toll-like receptor 4 to the induction of interferon-β. Nat. Immunol. 9, 361–368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fitzgerald K. A., Rowe D. C., Barnes B. J., Caffrey D. R., Visintin A., Latz E., Monks B., Pitha P. M., Golenbock D. T. (2003) LPS-TLR4 signaling to IRF-3/7 and NF-κB involves the Toll adapters TRAM and TRIF. J. Exp. Med. 198, 1043–1055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fitzgerald K. A., McWhirter S. M., Faia K. L., Rowe D. C., Latz E., Golenbock D. T., Coyle A. J., Liao S. M., Maniatis T. (2003) IKKε and TBK1 are essential components of the IRF3 signaling pathway. Nat. Immunol. 4, 491–496 [DOI] [PubMed] [Google Scholar]
- 17. Munoz C., Carlet J., Fitting C., Bisset B., Bleriot J-P., Cavaillon J. M. (1991) Dysregulation of in vitro cytokine production by monocytes during sepsis. J. Clin. Invest. 88, 1747–1754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Schefold J. C., Hasper D., Volk H. D., Reinke P. (2008) Sepsis: time has come to focus on the later stages. Med. Hypotheses 71, 203–208 [DOI] [PubMed] [Google Scholar]
- 19. van der Poll T., Meijers J. C. (2010) Systemic inflammatory response syndrome and compensatory anti-inflammatory response syndrome in sepsis. J. Innate Immun. 2, 379–380 [DOI] [PubMed] [Google Scholar]
- 20. Adib-Conquy M., Cavaillon J. M. (2009) Compensatory anti-inflammatory response syndrome. Thromb. Haemost. 101, 36–47 [PubMed] [Google Scholar]
- 21. Greisman S. E., Woodward W. E. (1965) Mechanisms of endotoxin tolerance. III. The refractory state during continuous intravenous infusions of endotoxin. J. Exp. Med. 121, 911–933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mengozzi M., Ghezzi P. (1993) Cytokine down-regulation in endotoxin tolerance. Eur. Cytokine Netw. 4, 89–98 [PubMed] [Google Scholar]
- 23. Madonna G. S., Vogel S. N. (1986) Induction of early-phase endotoxin tolerance in athymic (nude) mice, B cell-deficient (XID) mice, and splenectomized mice. Infect. Immun. 53, 707–710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Karp C. L., Wysocka M., Ma X., Marovich M., Factor R. E., Nutman T., Armant M., Wahl L., Cuomo P., Trinchieri G. (1998) Potent suppression of IL-12 production from monocytes and dendritic cells during endotoxin tolerance. Eur. J. Immunol. 28, 3128–3136 [DOI] [PubMed] [Google Scholar]
- 25. Cavaillon J. M., Adib-Conquy M. (2006) Bench-to-bedside review: endotoxin tolerance as a model of leukocyte reprogramming in sepsis. Crit. Care 10, 233–245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Medvedev A. E., Sabroe I., Hasday J. D., Vogel S. N. (2006) Tolerance to microbial TLR ligands: molecular mechanisms and relevance to disease. J. Endotoxin Res. 12, 133–150 [DOI] [PubMed] [Google Scholar]
- 27. Biswas S. K., Lopez-Collazo E. (2009) Endotoxin tolerance: new mechanisms, molecules and clinical significance. Trends Immunol. 30, 475–487 [DOI] [PubMed] [Google Scholar]
- 28. Nomura F., Akashi S., Sakao Y., Sato S., Kawai T., Matsumoto M., Nakanishi K., Kimoto M., Miyake K., Takeda K., Akira S. (2000) Cutting edge: endotoxin tolerance in mouse peritoneal macrophages correlates with down-regulation of surface Toll-like receptor 4 expression. J. Immunol. 164, 3476–3479 [DOI] [PubMed] [Google Scholar]
- 29. Sato S., Nomura F., Kawai T., Takeuchi O., Muhlradt P. F., Takeda K., Akira S. (2000) Synergy and crosstolerance between Toll-like receptor (TLR) 2- and TLR4-mediated signaling pathways. J. Immunol. 165, 7096–7101 [DOI] [PubMed] [Google Scholar]
- 30. Medvedev A. E., Piao W., Shoenfelt J., Rhee S. H., Chen H., Basu S., Wahl L. M., Fenton M. J., Vogel S. N. (2007) Role of TLR4 tyrosine phosphorylation in signal transduction and endotoxin tolerance. J. Biol. Chem. 282, 16042–16053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Medvedev A. E., Vogel S. N. (2003) Overexpression of CD14, TLR4, and MD-2 in HEK 293T cells does not prevent induction of in vitro endotoxin tolerance. J. Endotoxin Res. 9, 60–64 [DOI] [PubMed] [Google Scholar]
- 32. Medvedev A. E., Lentschat A., Wahl L. M., Golenbock D. T., Vogel S. N. (2002) Dysregulation of LPS-induced Toll-like receptor 4-MyD88 complex formation and IL-1 receptor-associated kinase 1 activation in endotoxin-tolerant cells. J. Immunol. 169, 5209–5216 [DOI] [PubMed] [Google Scholar]
- 33. Piao W., Song C., Chen H., Wahl L. M., Fitzgerald K. A., O'Neill L. A., Medvedev A. E. (2008) Tyrosine phosphorylation of MyD88 adapter-like (Mal) is critical for signal transduction and blocked in endotoxin tolerance. J. Biol. Chem. 283, 3109–3119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Piao W., Song C., Chen H., Diaz M. Q., Wahl L. M., Fitzgerald K. A., Li L., Medvedev A. E. (2009) Endotoxin tolerance dysregulates MyD88- and Toll/IL-1R domain-containing adapter inducing IFN-β-dependent pathways and increases expression of negative regulators of TLR signaling. J. Leukoc. Biol. 86, 863–875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Adib-Conquy M., Cavaillon J. M. (2002) γ Interferon and granulocyte/monocyte colony-stimulating factor prevent endotoxin tolerance in human monocytes by promoting interleukin-1 receptor-associated kinase expression and its association to MyD88 and not by modulating TLR4 expression. J. Biol. Chem. 277, 27927–27934 [DOI] [PubMed] [Google Scholar]
- 36. Wittebole X., Coyle S. M., Kumar A., Goshima M., Lowry S. F., Calvano S. E. (2005) Expression of tumor necrosis factor receptor and Toll-like receptor 2 and 4 on peripheral blood leukocytes of human volunteers after endotoxin challenge: a comparison of flow cytometric light scatter and immunofluorescence gating. Clin. Exp. Immunol. 141, 99–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mendes M. E., Baggio-Zappia G. L., Brunialti M. K., Fernandes Mda L., Rapozo M. M., Salomao R. (2011) Differential expression of Toll-like receptor signaling cascades in LPS-tolerant human peripheral blood mononuclear cells. Immunobiology 216, 285–295 [DOI] [PubMed] [Google Scholar]
- 38. Xiong Y., Qiu F., Piao W., Song C., Wahl L. M., Medvedev A. E. (2011) Endotoxin tolerance impairs IL-1 receptor-associated kinase (IRAK) 4 and TGF-{β}-activated kinase 1 activation, K63-linked polyubiquitination and assembly of IRAK1, TNF receptor-associated factor 6, and I{κ}B kinase {γ} and increases A20 expression. J. Biol. Chem. 286, 7905–7916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kinjyo I., Hanada T., Inagaki-Ohara K., Mori H., Aki D., Ohishi M., Yoshida H., Kubo M., Yoshimura A. (2002) SOCS1/JAB is a negative regulator of LPS-induced macrophage activation. Immunity 17, 583–591 [DOI] [PubMed] [Google Scholar]
- 40. Sly L. M., Rauh M. J., Kalesnikoff J., Song C. H., Krystal G. (2004) LPS-induced upregulation of SHIP is essential for endotoxin tolerance. Immunity 21, 227–239 [DOI] [PubMed] [Google Scholar]
- 41. van 't Veer C., van den Pangaart P. S., van Zoelen M. A., de Kruif M., Birjmohun R. S., Stroes E. S., de Vos A. F., van der Poll T. (2007) Induction of IRAK-M is associated with lipopolysaccharide tolerance in a human endotoxemia model. J. Immunol. 179, 7110–7120 [DOI] [PubMed] [Google Scholar]
- 42. Kobayashi K., Hernandez L. D., Galán J. E., Janeway C. A., Jr., Medzhitov R., Flavell R. A. (2002) IRAK-M is a negative regulator of Toll-like receptor signaling. Cell 110, 191–202 [DOI] [PubMed] [Google Scholar]
- 43. Wysocka M., Robertson S., Riemann H., Caamano J., Hunter C., Mackiewicz A., Montaner L. J., Trinchieri G., Karp C. L. (2001) IL-12 suppression during experimental endotoxin tolerance: dendritic cell loss and macrophage hyporesponsiveness. J. Immunol. 166, 7504–7513 [DOI] [PubMed] [Google Scholar]
- 44. Livak K. J., Schmittgen T. D. (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Δ Δ C(T)) method. Methods 25, 402–408 [DOI] [PubMed] [Google Scholar]
- 45. Foster S. L., Hargreaves D. C., Medzhitov R. (2007) Gene-specific control of inflammation by TLR-induced chromatin modifications. Nature 447, 972–978 [DOI] [PubMed] [Google Scholar]
- 46. Du X., Poltorak A., Silva M., Beutler B. (1999) Analysis of Tlr4-mediated LPS signal transduction in macrophages by mutational modification of the receptor. Blood Cells Mol. Dis. 25, 328–338 [DOI] [PubMed] [Google Scholar]
- 47. O'Neill L. A. (2006) How Toll-like receptors signal: what we know and what we don't know. Curr. Opin. Immunol. 18, 3–9 [DOI] [PubMed] [Google Scholar]
- 48. Hirose N., Maekawa T., Shinagawa T., Ishii S. (2009) ATF-2 regulates lipopolysaccharide-induced transcription in macrophage cells. Biochem. Biophys. Res. Commun. 385, 72–77 [DOI] [PubMed] [Google Scholar]
- 49. Song H., Ki S. H., Kim S. G., Moon A. (2006) Activating transcription factor 2 mediates matrix metalloproteinase-2 transcriptional activation induced by p38 in breast epithelial cells. Cancer Res. 66, 10487–10496 [DOI] [PubMed] [Google Scholar]
- 50. Ardeshna K. M., Pizzey A. R., Devereux S., Khwaja A. (2000) The PI3 kinase, p38 SAP kinase, and NF-κB signal transduction pathways are involved in the survival and maturation of lipopolysaccharide-stimulated human monocyte-derived dendritic cells. Blood 96, 1039–1046 [PubMed] [Google Scholar]
- 51. Hayden M. S., Ghosh S. (2008) Shared principles of NF-κ B signaling. Cell 132, 344–362 [DOI] [PubMed] [Google Scholar]
- 52. Cheng H., Addona T., Keshishian H., Dahlstrand E., Lu C., Dorsch M., Li Z., Wang A., Ocain T. D., Li P., Parsons T. F., Jaffee B., Xu Y. (2007) Regulation of IRAK-4 kinase activity via autophosphorylation within its activation loop. Biochem. Biophys. Res. Commun. 352, 609–616 [DOI] [PubMed] [Google Scholar]
- 53. Kim T. W., Staschke K., Bulek K., Yao J., Peters K., Oh K. H., Vandenburg Y., Xiao H., Qian W., Hamilton T., Min B., Sen G., Gilmour R., Li X. (2007) A critical role for IRAK4 kinase activity in Toll-like receptor-mediated innate immunity. J. Exp. Med. 204, 1025–1036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Lye E., Dhanji S., Calzascia T., Elford A. R., Ohashi P. S. (2008) IRAK-4 kinase activity is required for IRAK-4-dependent innate and adaptive immune responses. Eur. J. Immunol. 38, 870–876 [DOI] [PubMed] [Google Scholar]
- 55. Fraczek J., Kim T. W., Xiao H., Yao J., Wen Q., Li Y., Casanova J. L., Pryjma J., Li X. (2008) The kinase activity of IL-1 receptor-associated kinase 4 is required for interleukin-1 receptor/Toll-like receptor-induced TAK1-dependent NFκB activation. J. Biol. Chem. 283, 31697–31705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Hatao F., Muroi M., Hiki N., Ogawa T., Mimura Y., Kaminishi M., Tanamoto K. (2004) Prolonged Toll-like receptor stimulation leads to down-regulation of IRAK-4 protein. J. Leukoc. Biol. 76, 904–908 [DOI] [PubMed] [Google Scholar]
- 57. De Nardo D., Nguyen T., Hamilton J. A., Scholz G. M. (2009) Down-regulation of IRAK-4 is a component of LPS- and CpG DNA-induced tolerance in macrophages. Cell. Signal. 21, 246–252 [DOI] [PubMed] [Google Scholar]
- 58. Janssens S., Beyaert R. (2003) Functional diversity and regulation of different interleukin-1 receptor-associated kinase (IRAK) family members. Mol. Cell 11, 293–302 [DOI] [PubMed] [Google Scholar]
- 59. Wullaert A., Heyninck K., Janssens S., Beyaert R. (2006) Ubiquitin: tool and target for intracellular NF-κB inhibitors. Trends Immunol. 27, 533–540 [DOI] [PubMed] [Google Scholar]
- 60. Jiang Z., Johnson H. J., Nie H., Qin J., Bird T. A., Li X. (2003) Pellino 1 is required for interleukin-1 (IL-1)-mediated signaling through its interaction with the IL-1 receptor-associated kinase 4 (IRAK4)-IRAK-tumor necrosis factor receptor-associated factor 6 (TRAF6) complex. J. Biol. Chem. 278, 10952–10956 [DOI] [PubMed] [Google Scholar]
- 61. Lyn-Kew K., Rich E., Zeng X., Wen H., Kunkel S. L., Newstead M. W., Bhan U., Standiford T. J. (2010) IRAK-M regulates chromatin remodeling in lung macrophages during experimental sepsis. PLoS ONE 5, e11145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Wiersinga W. J., van't Veer C., van den Pangaart P. S., Dondorp A. M., Day N. P., Peacock S. J., van der Poll T. (2009) Immunosuppression associated with interleukin-1R-associated-kinase-M upregulation predicts mortality in Gram-negative sepsis (melioidosis). Crit. Care Med. 37, 569–576 [DOI] [PubMed] [Google Scholar]
- 63. Gabhann J. N., Higgs R., Brennan K., Thomas W., Damen J. E., Ben Larbi N., Krystal G., Jefferies C. A. (2010) Absence of SHIP-1 results in constitutive phosphorylation of TANK-binding kinase 1 and enhanced TLR3-dependent IFN-β production. J. Immunol. 184, 2314–2320 [DOI] [PubMed] [Google Scholar]
- 64. Sly L. M., Hamilton M. J., Kuroda E., Ho V. W., Antignano F. L., Omeis S. L., van Netten-Thomas C. J., Wong D., Brugger H. K., Williams O., Feldman M. E., Houseman B. T., Fiedler D., Shokat K. M., Krystal G. (2009) SHIP prevents lipopolysaccharide from triggering an antiviral response in mice. Blood 113, 2945–2954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Coornaert B., Carpentier I., Beyaert R. (2009) A20: central gatekeeper in inflammation and immunity. J. Biol. Chem. 284, 8217–8221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Wang J., Ouyang Y., Guner Y., Ford H. R., Grishin A. V. (2009) Ubiquitin-editing enzyme A20 promotes tolerance to lipopolysaccharide in enterocytes. J. Immunol. 183, 1384–1392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Boone D. L., Turer E. E., Lee E. G., Ahmad R. C., Wheeler M. T., Tsui C., Hurley P., Chien M., Chai S., Hitotsumatsu O., McNally E., Pickart C., Ma A. (2004) The ubiquitin-modifying enzyme A20 is required for termination of Toll-like receptor responses. Nat. Immunol. 5, 1052–1060 [DOI] [PubMed] [Google Scholar]
- 68. Oshima N., Ishihara S., Rumi M. A., Aziz M. M., Mishima Y., Kadota C., Moriyama I., Ishimura N., Amano Y., Kinoshita Y. (2010) A20 is an early responding negative regulator of Toll-like receptor 5 signaling in intestinal epithelial cells during inflammation. Clin. Exp. Immunol. 159, 185–198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Espelin C. W., Goldsipe A., Sorger P. K., Lauffenburger D. A., de Graaf D., Hendriks B. S. (2010) Elevated GM-CSF and IL-1β levels compromise the ability of p38 MAPK inhibitors to modulate TNFα levels in the human monocytic/macrophage U937 cell line. Mol. Biosyst. 6, 1956–1972 [DOI] [PubMed] [Google Scholar]
- 70. Cloutier A., Ear T., Blais-Charron E., Dubois C. M., McDonald P. P. (2007) Differential involvement of NF-κB and MAP kinase pathways in the generation of inflammatory cytokines by human neutrophils. J. Leukoc. Biol. 81, 567–577 [DOI] [PubMed] [Google Scholar]
- 71. Mäkelä S. M., Strengell M., Pietilä T. E., Osterlund P., Julkunen I. (2009) Multiple signaling pathways contribute to synergistic TLR ligand-dependent cytokine gene expression in human monocyte-derived macrophages and dendritic cells. J. Leukoc. Biol. 85, 664–672 [DOI] [PubMed] [Google Scholar]
- 72. Bohuslav J., Kravchenko V. V., Parry G. C., Erlich J. H., Gerondakis S., Mackman N., Ulevitch R. J. (1998) Regulation of an essential innate immune response by the p50 subunit of NF-κB. J. Clin. Invest. 102, 1645–1652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Porta C., Rimoldi M., Raes G., Brys L., Ghezzi P., Di Liberto D., Dieli F., Ghisletti S., Natoli G., De Baetselier P., Mantovani A., Sica A. (2009) Tolerance and M2 (alternative) macrophage polarization are related processes orchestrated by p50 nuclear factor κB. Proc. Natl. Acad. Sci. USA 106, 14978–14983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Kastenbauer S., Ziegler-Heitbrock H. W. (1999) NF-κB1 (p50) is upregulated in lipopolysaccharide tolerance and can block tumor necrosis factor gene expression. Infect. Immun. 67, 1553–1559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Medvedev A. E., Kopydlowski K. M., Vogel S. N. (2000) Inhibition of lipopolysaccharide-induced signal transduction in endotoxin-tolerized mouse macrophages: dysregulation of cytokine, chemokine, and Toll-like receptor 2 and 4 gene expression. J. Immunol. 164, 5564–5574 [DOI] [PubMed] [Google Scholar]