FOXP3+ and CTLA-4+ Tregs may modulate CD4+ T-cell activation and homing receptor expression in children.
Keywords: immune regulation, CD45RA, CD45RO, infants, homing receptors, multivariate factor analysis, prospective cohort study
Abstract
In adults, a majority of FOXP3+ Tregs expresses CTLA-4, and this costimulatory molecule is essential to control the expansion of other T cells. However, it remains to be investigated whether FOXP3+ and/or CTLA-4+ Tregs are associated with the expression of memory markers and homing receptors on CD4+ T cells. Thus, in a prospective newborn-infant cohort study, we examined the proportions of FOXP3+ and CTLA-4+ Tregs within the CD4+CD25+ T cell population and the fractions of CD4+ T cells that expressed CD45RA, CD45RO, HLA-DR, α4β7, CD62L, and CCR4 at several time-points during the first 3 years of life using flow cytometry. With the use of multivariate factor analysis, we found that a high proportion of FOXP3+ or CTLA-4+ Tregs during the first 18 months of life was associated positively with the fraction of T cells that expressed a naïve phenotype (CD45RA and α4β7) and inversely related to the fraction of T cells that expressed a memory phenotype (CD45RO and CCR4) later in childhood. In conclusion, FOXP3+ or CTLA-4+ Tregs may modulate CD4+ T cell activation and homing receptor expression in children.
Introduction
Tregs are crucial in maintenance of self-tolerance and immune homeostasis. Mice that lack Tregs suffer from severe autoimmune and inflammatory conditions [1, 2]. In humans, Tregs isolated from cord blood, thymus, and peripheral blood suppress proliferation and cytokine production of other T cells in response to self and environmental antigens [3–5]. Tregs are found within the CD4+CD25+ T cell population and express high levels of the transcription factor FOXP3 [6]. Children born with a dysfunctional foxp3 gene develop immunodysregulation, polyendocrinopathy, enteropathy, X-linked/X-linked autoimmune-allergic dysregulation, a syndrome characterized by organ-specific autoimmune diseases, severe dermatitis, and enterocolitis with food allergy [7, 8]. In adults, a majority of FOXP3+ Tregs expresses intracellular CTLA-4 [9], which has been proven to be essential for Tregs to control activation and expansion of other T cells [10–14]. The importance of CTLA-4 in immune regulation is also shown in CTLA-4-deficient mice that develop a fatal autoimmune disorder, characterized by infiltration of CD4+ T cells in several nonlymphoid tissues [15, 16]. Not only FOXP3+ Tregs but also newly activated CD4+ T cells express CTLA-4, and these cells have been shown to have regulatory functions in vitro [13]. Furthermore, CTLA-4 expressed in non-Tregs prevents the migration and accumulation of autoantigen-specific T cells into target tissue in mice [14, 17]. It was demonstrated recently that CTLA-4, expressed on Tregs or non-Tregs, captures CD80 or CD86 on APCs by transendocytosis, a process that prevents T cell activation [18]. Although FOXP3 and CTLA-4 expression in CD4+CD25+ T cells have been widely studied in adults, it is not known how these two markers are expressed during postnatal development of the immune system.
The trafficking of lymphocytes depends on interactions between tissue-specific chemokines and endothelial adhesion molecules and the corresponding receptors on the lymphocyte subsets. In humans, naïve CD45RA+ T cells express the homing receptors CD62L and CCR7 and in infants, also α4β7 [19–21]. These homing receptors allow the naïve T cell to enter the secondary lymphoid tissue to be activated [20–22]. In contrast, memory CD45RO+ T cells display a homing receptor phenotype depending on the site of activation [19, 23]. Thus, T cells activated in LNs draining the skin will express the homing receptor CCR4 [24], whereas T cells activated in gut-associated lymphoid tissue will express the homing receptors α4β7 and CCR9 [25]. The homing phenotype may be used as a differentiation marker in CD4+ T cells, as we showed recently that CD4+CD25+ T cells undergo homing receptor switch, i.e., from being α4β7-positive to be CCR4-positive, which coincides with their differentiation from a naïve to a memory phenotype [26]. Yet, it remains to be determined whether FOXP3+ or CTLA-4+ Tregs early in life are associated with naïve or memory CD4+ T cells later in life.
To address these issues, we have examined blood lymphocytes from a prospectively followed newborn-infant cohort of 65 infants regarding the proportion of the CD4+CD25+ T cell subsets that expresses intracellular FOXP3 or CTLA-4 and the expression of naïve and memory markers (CD45RA and CD45RO) and homing receptors (α4β7, CD62L, and CCR4) on CD4+ T cells. For the first time, we show that high percentages of FOXP3+ or CTLA-4+ Tregs within the CD4+CD25+ T cell population in infants are negatively associated with the expression of the memory markers CD45RO and CCR4 on circulating CD4+ T cells later in childhood using multivariate factor analysis. Thus, our results indicate that a high proportion of FOXP3+ and CTLA-4+ Tregs early in infancy may modulate the proportion of memory CD4+ T cells later in life.
MATERIALS AND METHODS
Subjects and collection of blood samples
Blood samples were collected from a prospective newborn-infant cohort, aiming at investigating the maturation of the immune system. The study included 65 healthy infants (33 boys and 32 girls), born at term (≥38 gestational weeks) in rural areas of Southwest Sweden. Cord blood samples were obtained at birth from 48 infants, and peripheral blood samples were obtained at 3–5 days (n=59), 1 month (n=59), 4 months (n=58), 18 months (n=63), and 36 months (n=50) of age. For simultaneous staining for FOXP3 and CTLA-4, cord blood was obtained from healthy newborn infants born at term at the Sahlgrenska University Hospital/Mölndal (Gothenburg, Sweden). Peripheral blood was also obtained from 20 healthy adult volunteers aged 25–55 years. All blood samples were collected in preservative-free heparin tubes. Informed consent was obtained from the parents and volunteers, and the study was approved by the Human Research Ethics Committee of the Medical Faculty, University of Gothenburg (Sweden).
Flow cytometry
Phenotypic analysis of T lymphocytes was performed by flow cytometry within 72 h of venepuncture. Initial experiments showed that proportions and numbers of different lymphocyte populations were unaffected by 72-h storage in room temperature compared with freshly isolated samples (data not shown). Whole blood (50 μl/tube for surface staining or 100 μl/tube for surface and intracellular staining) was incubated for 20 min at 4°C with the following antihuman mAb: allophycocyanin-conjugated anti-CD25 (clone 2A3; BD Biosciences, Erembodegem, Belgium); FITC-conjugated anti-CD45RA (clone L48; BD Biosciences), anti-CD62L (clone Dreg-56; BD Biosciences), and anti-CD49d (clone 44H6; Serotec, UK); PerCP-conjugated anti-CD4 (clone SK3; BD Biosciences); and PE-conjugated anti-CD45RO (clone UCHL-1; BD Biosciences), anti-β7-integrin (clone FIB504; BD Biosciences), and anti-CCR4 (clone IG1; BD Biosciences). RBCs were then lysed (FACS lysing solution, BD Biosciences), and the remaining cells were washed twice with FACS buffer. After completed cell-surface staining, the cells were intracellularly stained for FOXP3 or CTLA-4. Fixation, permeabilization, and staining for FOXP3 were performed using the kit PE-antihuman FOXP3 staining set (eBioscience, San Diego, CA, USA), whereas for CTLA-4, fixation and permeabilization were performed using the Cytofix/Cytoperm kit (BD Biosciences), followed by staining with biotin-conjugated CTLA-4 (clone BNI3; BD PharMingen, San Diego, CA, USA), followed by PE-conjugated streptavidin (BD Biosciences), according to the manufacturer's instructions. Moreover, simultaneous staining for intracellular FOXP3 and CTLA-4 in the CD4+CD25 T cell population was performed on cells from newborn children, 36-month-old children, and adults. Peripheral mononuclear cells were isolated by density gradient centrifugation using Lymphoprep (Nycomed, Oslo, Norway). The cells were stained with Horizon V450-conjugated anti-CD4 (clone RPA-T4; BD Biosciences) and allophycocyanin-conjugated anti-CD25 for 20 min at 4°C in the dark. Thereafter, permeabilization and staining were performed using the kit PE-antihuman FOXP3 staining set, followed by staining with PE-conjugated FOXP3 (clone PCH101; eBioscience) and biotin-conjugated CTLA-4 (clone BNI3; BD PharMingen), followed by PE-Cy7-conjugated streptavidin (BD Biosciences). All isotype controls were purchased from BD Biosciences, except for the isotype control for FOXP3, which was purchased from eBioscience. A FACSCanto II (BD Biosciences), equipped with FACSDiva software, was used to analyze the simultaneous staining for CTLA-4 and FOXP3. All other stainings were analyzed on a FACSCalibur (BD Biosciences) equipped with CellQuestPro software. All flow cytometry data were analyzed using Flow Jo software (Tree Star, Ashland, OR, USA). FOXP3+ Tregs and CTLA-4+ T cells, within the CD4+CD25+ and CD4+CD25high T cell populations, were defined by the procedure shown in Supplemental Fig. 1: based on the expression CD25 on CD4+ T cells, three gates were set to identify CD25−, CD25+, and CD25high T cells (2% of the CD4+ T cells that expressed high CD25). The gate for FOXP3 and CTLA-4 were set by comparing the expression of FOXP3 or CTLA-4 within the CD25−, CD25+, or CD25high populations with each other and their isotype controls. The expression of FOXP3, CTLA-4, CD45RA, and CD45RO was analyzed at birth, 3–5 days, and 1, 4, 18, and 36 months, whereas the expression of HLA-DR, α4β7, CD62L, and CCR4 was analyzed at birth and 4, 18, and 36 months of age.
Statistical analysis
The relationship among the different T cell subsets at different ages was analyzed using the multivariate factor analyses: PCA and OPLS (SIMCA-P+ software version 12, Umetrics, Umeå, Sweden). PCA, including data for each individual, was first performed, as it generates hypotheses by providing an overview of groupings and trends and makes it possible to detect possible outliers regarding the CD4+ T cell naïve and memory phenotype, homing receptor expression, and the proportions of circulating FOXP3+ or CTLA-4+Tregs. The quality of the PCA was assessed based on the parameters R2 and Q2, i.e., the percentages of the variation of the dataset explained (R2) and predicted (Q2) by the model, respectively. OPLS is a regression development of PCA, which was implemented to correlate the X and Y data matrices, where X represents expression of memory markers and homing receptors for each individual, and Y represents the fraction of FOXP3+ or CTLA-4+Tregs for each subject. The generated OPLS model correlates the X matrices with Y using a linear multivariate model. The importance of each X variable to Y is represented by column bars. Jack-knifing was used to calculate standard errors displayed as error bars on each column (representing the 95% confidence interval). Univariate correlation tests were only performed between the proportions of FOXP3+ or CTLA-4+Tregs and the X variables (naïve, memory, and homing markers), which contributed most to Y, i.e., variables that displayed large bars with error bars not crossing the x-axis in the OPLS analysis. Univariate data analysis, including Spearman's rank correlation test, Kruskal-Wallis test followed by Dunn's multiple comparison test or Mann-Whitney U test (GraphPad Prism, GraphPad Software, San Diego, CA, USA), was used as described in the figure legends. P ≤ 0.05 was considered as significant (*P≤0.05; **P≤0.01; and ***P≤0.001).
RESULTS
Proportions of FOXP3+ Tregs and CTLA-4+ T cells during infancy and in adults
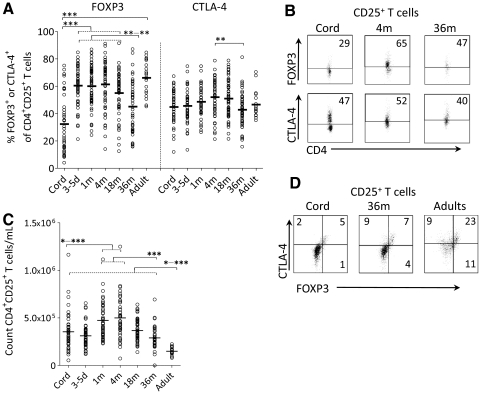
We analyzed the proportion of FOXP3+ Tregs and CTLA-4+ T cells within the CD4+CD25+ (CD25+) T cell population at birth, 3–5 days, and 1, 4, 18, and 36 months of age and compared them with adults. As shown in Fig. 1A and B, the proportion of FOXP3+ Tregs displayed a striking rise between birth and 3–5 days of age, but during the following 18 months, no further increase occurred. The proportion of CTLA-4, on the other hand, remained constant during the first 18 months of life. However, at 36 months of age, the fractions of FOXP3+ Tregs and CTLA-4+ T cells were significantly lower than that observed at 4 months of age (Fig. 1A and B; regarding FOXP3, all data at birth, 3–5 days, 1 and 4 months, as well as part of the 18-month data have been published previously by Grindebacke et al. [26]). The increase of the proportion of FOXP3+ Tregs between birth and 3–5 days of age was not a result of an increment in the total CD25+ T cell count, as the total numbers of CD25+ T cells did not rise during the first week of life, as demonstrated in Fig. 1C.
Figure 1. The proportion of circulating FOXP3+ Tregs and CTLA-4+ T cells during the first 36 months of life.
The proportion of circulating CD4+CD25+ T cells that expresses (A) FOXP3 (FOXP3+ Tregs) or CTLA-4 (CTLA-4+ T cells) at birth (Cord), 3–5 days, and 1, 4, 18, and 36 months and in adults. Regarding FOXP3+ Tregs, all data at birth, 3–5 days, and 1 and 4 months of age and in adults, as well as part of the 18-month data, have been published previously by Grindebacke et al. [26]. (B) Representative dot plots showing FOXP3+ Tregs and CTLA-4+ T cells at birth, 4 months, and 36 months of age. (C) Absolute count of CD4+ CD25+ T cells at birth, 3–5 days, and 1, 4, 18, and 36 months and in adults. Each symbol represents one individual, and horizontal bars indicate the median value. (D) Representative dot plots showing the coexpression of FOXP3 and CTLA-4 in CD4+CD25+ T cells at birth and 36 months of age and in adults. Numbers represent the percentage of cells within the gate. *P ≤ 0.05; **P ≤ 0.01; and ***P ≤ 0.001 (Kruskal-Wallis test followed by Dunn's multiple comparison test).
To examine whether FOXP3 and CTLA-4 were coexpressed on the same CD25+ T cells, we simultaneously stained the cells with these two markers (Fig. 1D). However, intracellular double-staining for FOXP3 and CTLA-4 quenched each other, making it difficult to gate properly. Accordingly, the data from double-stained samples were not consistent with the data from single-stained samples. Therefore, single-stained FOXP3+ or CTLA-4+ T cells within the CD25+ T cell population were used to study the relationship between Tregs and naïve or memory T cells.
Relationship between FOXP3+ or CTLA-4+ Tregs and the expression of memory markers and homing receptors on circulating CD4+ T cells during infancy
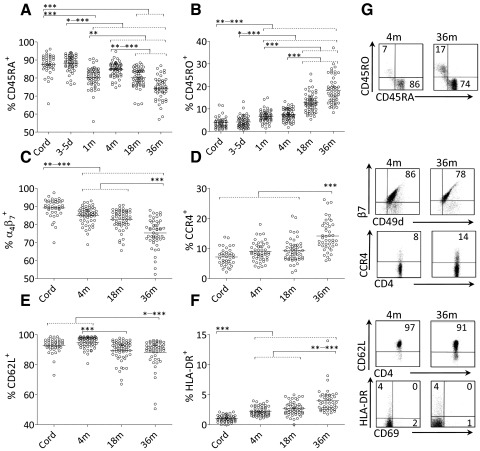
We first examined the proportions of CD4+ T cells that expressed CD45RA (naïve T cells), CD45RO (memory T cells), HLA-DR, α4β7, CCR4, or CD62L in blood samples obtained at birth, 3–5 days, and 1, 4, 18, and 36 months of age using flow cytometry. As shown in Fig. 2A–G, the proportions of naïve CD45RA+, α4β7+, or CD62L+ T cells decreased significantly with age, whereas the proportions of memory or activated CD4+ T cells, which expressed CD45RO, HLA-DR, or CCR4, increased with age (Kruskal-Wallis test followed by Dunn's multiple comparison test). We found a large individual variation in the proportions of CD4+ T cells that expressed CD45RA, CD45RO, α4β7, CD62L, or CCR4 (Fig. 2A–E and G).
Figure 2. Percentages of circulating CD4+ T cells that express CD45RA, CD45RO, HLA-DR, α4β7, CCR4, or CD62L during the first 36 months of life.
The percentage of circulating CD4+ that express (A) CD45RA, (B) CD45RO, (C) α4β7, (D) CCR4, (E) CD62L, or (F) HLA-DR at different time-points during the first 36 months of life. Each symbol represents one individual, and horizontal bars indicate the median value. (G) Representative dot plots showing CD45RA+, CD45RO+, α4β7+ (CD49d+β7+), CCR4+, CD62L+, and HLA-DR+ cells within the CD4+ T cell population at 4 and 36 months of age. Numbers represent the percentage of cells within the gate. *P ≤ 0.05; **P ≤ 0.01; and ***P ≤ 0.001 (Kruskal-Wallis test followed by Dunn's multiple comparison test).
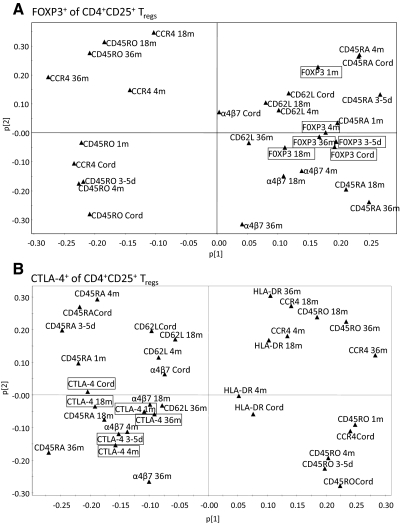
Next, we examined whether FOXP3+ or CTLA-4+ Tregs within the CD25+ T cell population were related to CD4+ T cells that expressed CD45RA, CD45RO, HLA-DR, α4β7, CCR4, or CD62L using multivariate factor analysis. To examine these relationships, we used the proportions of the different T cell subtypes, as absolute cell counts were dependent on the absolute number of the CD4+ T cells (data not shown). PCA was first performed to obtain an overview of how the different variables related to one another [27]. As depicted in the score scatter plots, we found that the proportions of CD4+ T cells that expressed CD45RA, α4β7, or CD62L and the proportion of FOXP3+ or CTLA-4+ Tregs within the CD25+ T cell population were all projected on one side of the x-axis at all ages (upper- and lower-right quadrants in Fig. 3A and upper- and lower-left quadrants in Fig. 3B), which demonstrates a positive association between these T cell markers. In contrast, the proportions of memory CD45RO- or CCR4-expressing T cells were projected on the opposite side of the x-axis (upper- and lower-left quadrants and upper- and lower-right quadrants in Fig. 3A and B, respectively), demonstrating an inverse relationship between the proportion of FOXP3+ or CTLA-4+ Tregs and the proportion of CD45RO- or CCR4-expressing CD4+ T cells. We observed the same relationships when investigating the association between FOXP3+ or CTLA-4+ Tregs within the CD25high T cell population and the naïve or memory CD4+ T cells (Supplemental Fig. 2). Taken together, a high fraction of FOXP3+ or CTLA-4+ Tregs, within the CD4+CD25+ or CD4+CD25high T cell populations, was associated with a high proportion of naïve CD4+ T cells in the circulation during the first 36 months of life.
Figure 3. Relationship between FOXP3+ or CTLA-4+ Tregs and expression of memory markers and homing receptors on CD4+ T cells in blood during infancy.
PCA plot showing an overview of the data, including percentage of FOXP3+ (A) and CTLA-4+ Tregs (B) within the CD4+CD25+ T cells and the fraction of CD4+ T cells expressing CD45RA and CD45RO at birth, 3–5 days, and 1, 4, 18, and 36 months and HLA-DR, α4β7, CD62L, or CCR4 at birth and 4, 18, and 36 months of age.
High proportions of FOXP3+ or CTLA-4+ Tregs early in life are associated with a low proportion of CD4+ T cells with a memory phenotype later in infancy
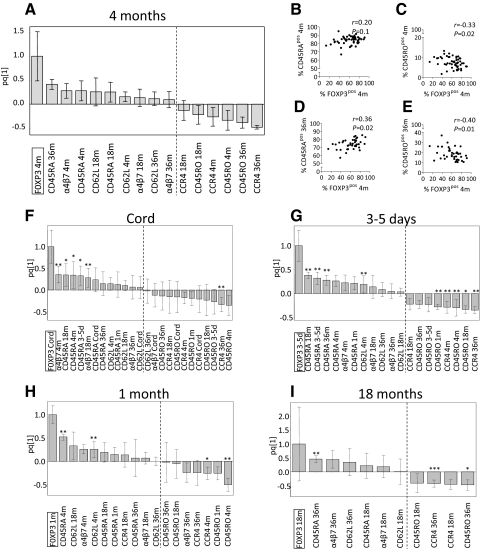
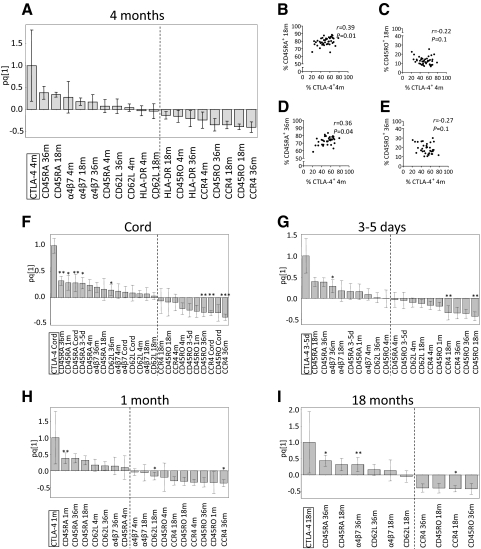
As the proportions of FOXP3+ or CTLA-4+ Tregs were positively associated with naïve T cells and inversely correlated with memory T cells within the CD4+ T cell fraction, we performed OPLS to examine the X variables that displayed the strongest association (positive or negative) with a high proportion of FOXP3+ or CTLA-4+ Tregs at the different ages. As shown in the representative OPLS loading column plots (Figs. 4A and 5A), X variables lying in the same direction as FOXP3+ or CTLA-4+ Tregs within the CD25+ T cell population at 4 months of age (located to the far left) were positively associated (CD45RA, α4β7, and CD62L at 4, 18, and 36 months of age), whereas variables in the opposite direction were inversely correlated (CD45RO, HLA-DR, and CCR4 at 4, 18, and 36 months of age). The larger the bar and smaller the error bar in the OPLS loading column plot, the larger and more certain is the contribution to the model. Similar associations were observed for FOXP3+ or CTLA-4+ Tregs and the X variables in the OPLS analyses at birth, 3–5 days, and 1 and 18 months of age (Figs. 4F–I and 5F–I, respectively).
Figure 4. Correlations between FOXP3+ Tregs and expression of homing receptors and memory markers during infancy.
OPLS was used to correlate the data matrices, X (the expression of CD45RA, CD45RO, α4β7, CD62L, or CCR4 on CD4+ T cells at 4, 18, and 36 months of age) and Y (FOXP3+ of CD4+CD25+ T cells), to each other by a linear multivariate model. X parameters lying in the same direction as FOXP3+ Tregs in the OPLS plots are positively associated, whereas parameters in the opposite direction are inversely correlated. Each column displays uncertainty bars with 95% confidence interval. (A) OPLS plot of the population of FOXP3+ Tregs at 4 months of age and the X variables. (B–E) Univariate correlations between the fraction of FOXP3+ Tregs at 4 months and the percentage of CD45RA+ (B and D) and CD45RO+ (C and E) on CD4+ T cells at 4 months (B and C) or 36 months (D and E) of age. (F–I) OPLS plots of the proportions of FOXP3+ Tregs at birth (F), 3–5 days (G), 1 month (H), and 18 months (I) of age and the X variables. (B–E) P ≤ 0.05 is considered significant (Spearman′s rank correlation test). (F–I) *P < 0.05, **P < 0.01, and ***P < 0.001, according to univariate analyses (Spearman′s rank correlation test) of the correlation between the proportion of FOXP3+ Tregs and the percentages of the X variables.
Figure 5. Correlations between CTLA-4+ Tregs and expression of homing receptors and memory markers during infancy.
OPLS was used to correlate the data matrices, X (the expression of CD45RA, CD45RO, α4β7, CD62L, or CCR4 on CD4+ T cells at 4, 18, and 36 months of age) and Y (CTLA-4+ of CD4+CD25+ T cells), to each other by a linear multivariate model. X parameters lying in the same direction as CTLA-4+ Tregs in the OPLS plots are positively associated, whereas parameters in the opposite direction are inversely correlated. Each column displays uncertainty bars with 95% confidence interval. (A) OPLS plot of the population of CTLA-4+ Tregs at 4 months of age and the X variables. (B–E) Univariate correlations between the fraction of CTLA-4+ Tregs at 4 months and the percentage of CD45RA+ (B and D) and CD45RO+ (C and E) on CD4+ T cells at 18 months (B and C) or 36 months (D and E) of age. (F–I) OPLS plots of the proportions of CTLA-4+ Tregs at birth (F), 3–5 days (G), 1 month (H), and 18 months (I) of age and the X variables. (B–E) P ≤ 0.05 is considered significant (Spearman′s rank correlation test). (F–I) *P < 0.05, **P < 0.01, and ***P < 0.001, according to univariate analyses (Spearman′s rank correlation test) of the correlation between the proportion of CTLA-4+ Tregs and the percentages of the X variables.
Univariate correlation analyses were performed for the proportions of FOXP3+ or CTLA-4+ Tregs and the memory and homing markers that displayed large bars with error bars not crossing the x-axis in the OPLS analyses (Figs. 4A and F–I and 5A and F–I). In particular, the fraction of FOXP3+ Tregs at 4 months correlated positively to the percentage of CD4+ T cells expressing CD45RA and negatively to CD45RO at 36 months of age (Fig. 4D and E). The percentage of CTLA-4+ Tregs at 4 months was positively correlated to the proportions of CD4+ T cells expressing CD45RA at 18 and 36 months of age (Fig. 5B and D). However, CTLA-4+ Tregs at 4 months of age did not correlate significantly with the fraction of CD4+ T cells that expressed CD45RO at 18 and 36 months of age (Fig. 5C and E). Moreover, a high fraction of FOXP3+ or CTLA-4+ Tregs at birth, 3–5 days, and 1 and 18 months also correlated positively or negatively with the proportions of either or both CD45RA and CD45RO at 4, 18, and 36 months of age (Figs. 4F–I and Fig. 5F–I). In summary, infants with a high proportion of FOXP3+ or CTLA-4+ Tregs early in life had a low proportion of memory T cells later in life, which indicates that FOXP3+ or CTLA-4+ Tregs may modulate the proportion of CD4+ memory T cells later in infancy.
We recently showed that CD25+ T cells undergo homing receptor switch, i.e., from being α4β7-positive to being CCR4-positive, during their differentiation to a memory CD45RO phenotype [26]. Accordingly, the proportion of FOXP3+ Tregs at 4 months was inversely correlated to CCR4+ T cells at 4 and 36 months of age (r=–0.28, P=0.04, and r=–0.44, P=0.006, respectively). The α4β7 expression at 4 and 18 months correlated positively (r=0.50, P=0.001, and r=0.40, P=0.01, respectively) with FOXP3+ Tregs at birth. Furthermore, the proportion of CTLA-4+ Tregs at 4 months was inversely correlated to the expression of CCR4 on CD4+ T cells at 4, 18, and 36 months of age (r=–0.36, P=0.01; r=–0.32, P=0.04; and r=–0.35, P=0.05, respectively). Taken together, within the circulating CD25+ T cell population, a high proportion of FOXP3+ or CTLA-4+ Tregs might modulate homing receptor switch on CD4+ T cells later in infancy.
DISCUSSION
FOXP3+ Tregs and CTLA-4+ T cells are important immune regulators in mice and in humans [12–14]. In adults, T cells expressing these markers suppress in vitro proliferation [12, 13], and it was shown recently that CTLA-4+ T cells also possess the ability to prevent autoantigen-specific T cell migration into target tissues in mice [14, 17]. These observations led us to investigate the relationships between the proportions of FOXP3+ or CTLA-4+ Tregs of CD25+ T cells and naïve, memory, and homing receptors expressed on CD4+ T cells during early childhood using a prospective cohort study design. We show here for the first time that a high proportion of FOXP3+ or CTLA-4+ Tregs in early infancy is associated with a low fraction of memory T cells expressing CD45RO later in childhood using multivariate factor analysis. Moreover, a high proportion of FOXP3+ or CTLA-4+ Tregs was also found to be associated with a low proportion of CD4+ T cells that expresses the skin homing receptor CCR4. Accordingly, it has been demonstrated that upon TCR stimulation, the newly activated T cells up-regulate CCR4 in adults [28], and we recently showed that CCR4 expression is linked to the T cell memory phenotype in infants [26].
Several studies have demonstrated that CCR4-expressing Th2 cells are implicated in the development of allergic asthma and dermatitis [29–31], and blocking the CCR4 pathway results in hindered trafficking of activated T cells to their target tissue, leading to attenuated allergic inflammation [29]. Homing receptors involved in human autoimmune disorders remain to be elucidated, but in mice, CTLA-4-expressing T cells prevent tissue-specific T cell migration via yet-unclear immunological mechanisms [14, 17]. Thus, our results that indicate that Tregs modulate T cell activation, including homing receptor switch, could be of importance for explaining what factors mediate protection from inflammatory disease later in life. Indeed, lack of FOXP3+ Tregs or CTLA-4+ T cells is linked to various allergy- and autoimmune-related inflammatory conditions [7, 8, 15, 16].
The immunological mechanisms by which FOXP3+ or CTLA-4+ Tregs may regulate T cell maturation in vivo in infants remain speculative. However, APCs, i.e., DCs, have been implicated as a target for Treg immune regulation in mice and humans [32, 33]. FOXP3+ Tregs express the adhesion molecule LFA-1, which permits aggregation with DCs to a higher degree than naïve T cells and may thus out-compete these cells from interacting with the APCs [33]. Regarding CTLA-4, it has been shown that its interaction with the costimulatory molecules CD80 and/or CD86 on DCs inhibits the accumulation of the primary T cell growth factor IL-2 [34]. Furthermore, CTLA-4 per se, independently of in which cell type it is expressed, down-regulates CD80 and/or CD86 on DCs [12, 33, 35] by binding and removing these molecules via transendocytosis, which will inhibit T cell activation and differentiation [18]. Moreover, blocking the interaction between CTLA-4 and CD80 and/or CD86 has been shown to expand the activated T cell population [36]. This is in accordance with our results in infants, showing that a high proportion of not only FOXP3+ Tregs but also CTLA-4+ Tregs is associated to a low fraction of memory T cells.
In our hands, it was not possible to elucidate whether FOXP3 and CTLA-4 are coexpressed on the same cells using flow cytometry. We found that intracellular double-staining for FOXP3 and CTLA-4 leads to suboptimal gating strategies, as the two intracellular markers influence each other. Accordingly, it was demonstrated recently that staining for more than one intracellular marker will quench other fluorochromes used [37]. Thus, in present study, data obtained from double-stained samples were not comparable with those obtained from samples stained with FOXP3 or CTLA-4 alone. Staining for CD25 and CD127 to identify Tregs [38] and study their expression of CTLA-4 in infants could have been a better approach. Unfortunately, the importance of CD127 to human Treg identification was not discovered when initiating the present prospective cohort study.
In conclusion, a high proportion of FOXP3+ or CTLA-4+ Tregs in early infancy is associated with a low fraction of memory T cells expressing CD45RO and CCR4 later in childhood. We suggest that FOXP3+ and CTLA-4+ Tregs possess immunoregulatory properties in vivo in infants, which may reduce activation and tissue trafficking of T cells.
Supplementary Material
ACKNOWLEDGMENTS
This work was funded by the Swedish Council for Working Life and Social Research, by the Swedish Research Council (grant K2009-57X-14455-08-3), by the Vårdal Foundation, by the Region Västra Götaland (agreement concerning research and education of doctors; ALF), and by Torsten and Ragnar Söderberg's Foundation. We thank study nurses Anders Nordberg and Helen Andersson at Skaraborgs Hospital, Skövde and Lidköping, respectively. The skillful technical assistance of Inger Nordström along with the staff at the Clinical Immunology Laboratory of the Sahlgrenska University Hospital is highly appreciated. Finally, we thank all of the families who took part in the study.
The online version of this paper, found at www.jleukbio.org, includes supplemental information.
- CD62L
- CD62 ligand
- FOXP3
- Forkhead box P3
- OPLS
- orthogonal projection to latent structures by means of partial least squares
- PCA
- principal component analysis
- Tregs
- regulatory T cells
AUTHORSHIP
H.R. analyzed and interpreted all data, designed all figures, and wrote the paper. A-C.L. was involved in analysis and interpretation of the data and wrote the paper. K.A. was involved in collecting samples and in data analysis. I.A. and A.E.W. were involved in the birth-cohort study design and final approval of the manuscript to be submitted. A.R. was involved in the birth-cohort study design and continuously supervised all aspects of the work.
DISCLOSURE
The authors have no financial conflict of interest.
REFERENCES
- 1. Sakaguchi S., Sakaguchi N., Asano M., Itoh M., Toda M. (1995) Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor α-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J. Immunol. 155, 1151–1164 [PubMed] [Google Scholar]
- 2. Sakaguchi S., Yamaguchi T., Nomura T., Ono M. (2008) Regulatory T cells and immune tolerance. Cell 133, 775–787 [DOI] [PubMed] [Google Scholar]
- 3. Baecher-Allan C., Brown J. A., Freeman G. J., Hafler D. A. (2001) CD4+CD25high regulatory cells in human peripheral blood. J. Immunol. 167, 1245–1253 [DOI] [PubMed] [Google Scholar]
- 4. Grindebacke H., Wing K., Andersson A. C., Suri-Payer E., Rak S., Rudin A. (2004) Defective suppression of Th2 cytokines by CD4CD25 regulatory T cells in birch allergics during birch pollen season. Clin. Exp. Allergy 34, 1364–1372 [DOI] [PubMed] [Google Scholar]
- 5. Wing K., Larsson P., Sandstrom K., Lundin S. B., Suri-Payer E., Rudin A. (2005) CD4+ CD25+ FOXP3+ regulatory T cells from human thymus and cord blood suppress antigen-specific T cell responses. Immunology 115, 516–525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hori S., Nomura T., Sakaguchi S. (2003) Control of regulatory T cell development by the transcription factor Foxp3. Science 299, 1057–1061 [DOI] [PubMed] [Google Scholar]
- 7. Wildin R. S., Smyk-Pearson S., Filipovich A. H. (2002) Clinical and molecular features of the immunodysregulation, polyendocrinopathy, enteropathy, X linked (IPEX) syndrome. J. Med. Genet. 39, 537–545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chatila T. A., Blaeser F., Ho N., Lederman H. M., Voulgaropoulos C., Helms C., Bowcock A. M. (2000) JM2, encoding a fork head-related protein, is mutated in X-linked autoimmunity-allergic disregulation syndrome. J. Clin. Invest. 106, R75–R81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Miyara M., Yoshioka Y., Kitoh A., Shima T., Wing K., Niwa A., Parizot C., Taflin C., Heike T., Valeyre D., Mathian A., Nakahata T., Yamaguchi T., Nomura T., Ono M., Amoura Z., Gorochov G., Sakaguchi S. (2009) Functional delineation and differentiation dynamics of human CD4+ T cells expressing the FoxP3 transcription factor. Immunity 30, 899–911 [DOI] [PubMed] [Google Scholar]
- 10. Takahashi T., Tagami T., Yamazaki S., Uede T., Shimizu J., Sakaguchi N., Mak T. W., Sakaguchi S. (2000) Immunologic self-tolerance maintained by CD25(+)CD4(+) regulatory T cells constitutively expressing cytotoxic T lymphocyte-associated antigen 4. J. Exp. Med. 192, 303–310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Friedline R. H., Brown D. S., Nguyen H., Kornfeld H., Lee J., Zhang Y., Appleby M., Der S. D., Kang J., Chambers C. A. (2009) CD4+ regulatory T cells require CTLA-4 for the maintenance of systemic tolerance. J. Exp. Med. 206, 421–434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wing K., Onishi Y., Prieto-Martin P., Yamaguchi T., Miyara M., Fehervari Z., Nomura T., Sakaguchi S. (2008) CTLA-4 control over Foxp3+ regulatory T cell function. Science 322, 271–275 [DOI] [PubMed] [Google Scholar]
- 13. Zheng Y., Manzotti C. N., Burke F., Dussably L., Qureshi O., Walker L. S., Sansom D. M. (2008) Acquisition of suppressive function by activated human CD4+ CD25– T cells is associated with the expression of CTLA-4 not FoxP3. J. Immunol. 181, 1683–1691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ise W., Kohyama M., Nutsch K. M., Lee H. M., Suri A., Unanue E. R., Murphy T. L., Murphy K. M. (2010) CTLA-4 suppresses the pathogenicity of self antigen-specific T cells by cell-intrinsic and cell-extrinsic mechanisms. Nat. Immunol. 11, 129–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tivol E. A., Borriello F., Schweitzer A. N., Lynch W. P., Bluestone J. A., Sharpe A. H. (1995) Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity 3, 541–547 [DOI] [PubMed] [Google Scholar]
- 16. Waterhouse P., Penninger J. M., Timms E., Wakeham A., Shahinian A., Lee K. P., Thompson C. B., Griesser H., Mak T. W. (1995) Lymphoproliferative disorders with early lethality in mice deficient in CTLA-4. Science 270, 985–988 [DOI] [PubMed] [Google Scholar]
- 17. Jain N., Nguyen H., Chambers C., Kang J. (2010) Dual function of CTLA-4 in regulatory T cells and conventional T cells to prevent multiorgan autoimmunity. Proc. Natl. Acad. Sci. USA 107, 1524–1528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Qureshi O. S., Zheng Y., Nakamura K., Attridge K., Manzotti C., Schmidt E. M., Baker J., Jeffery L. E., Kaur S., Briggs Z., Hou T. Z., Futter C. E., Anderson G., Walker L. S., Sansom D. M. (2011) Trans-endocytosis of CD80 and CD86: a molecular basis for the cell-extrinsic function of CTLA-4. Science 332, 600–603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Campbell D. J., Butcher E. C. (2002) Rapid acquisition of tissue-specific homing phenotypes by CD4(+) T cells activated in cutaneous or mucosal lymphoid tissues. J. Exp. Med. 195, 135–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sallusto F., Lenig D., Forster R., Lipp M., Lanzavecchia A. (1999) Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature 401, 708–712 [DOI] [PubMed] [Google Scholar]
- 21. Salmi M., Alanen K., Grenman S., Briskin M., Butcher E. C., Jalkanen S. (2001) Immune cell trafficking in uterus and early life is dominated by the mucosal addressin MAdCAM-1 in humans. Gastroenterology 121, 853–864 [DOI] [PubMed] [Google Scholar]
- 22. Campbell J. J., Murphy K. E., Kunkel E. J., Brightling C. E., Soler D., Shen Z., Boisvert J., Greenberg H. B., Vierra M. A., Goodman S. B., Genovese M. C., Wardlaw A. J., Butcher E. C., Wu L. (2001) CCR7 expression and memory T cell diversity in humans. J. Immunol. 166, 877–884 [DOI] [PubMed] [Google Scholar]
- 23. Kantele A., Zivny J., Hakkinen M., Elson C. O., Mestecky J. (1999) Differential homing commitments of antigen-specific T cells after oral or parenteral immunization in humans. J. Immunol. 162, 5173–5177 [PubMed] [Google Scholar]
- 24. Campbell J. J., Haraldsen G., Pan J., Rottman J., Qin S., Ponath P., Andrew D. P., Warnke R., Ruffing N., Kassam N., Wu L., Butcher E. C. (1999) The chemokine receptor CCR4 in vascular recognition by cutaneous but not intestinal memory T cells. Nature 400, 776–780 [DOI] [PubMed] [Google Scholar]
- 25. Kunkel E. J., Campbell J. J., Haraldsen G., Pan J., Boisvert J., Roberts A. I., Ebert E. C., Vierra M. A., Goodman S. B., Genovese M. C., Wardlaw A. J., Greenberg H. B., Parker C. M., Butcher E. C., Andrew D. P., Agace W. W. (2000) Lymphocyte CC chemokine receptor 9 and epithelial thymus-expressed chemokine (TECK) expression distinguish the small intestinal immune compartment: epithelial expression of tissue-specific chemokines as an organizing principle in regional immunity. J. Exp. Med. 192, 761–768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Grindebacke H., Stenstad H., Quiding-Jarbrink M., Waldenstrom J., Adlerberth I., Wold A. E., Rudin A. (2009) Dynamic development of homing receptor expression and memory cell differentiation of infant CD4+CD25high regulatory T cells. J. Immunol. 183, 4360–4370 [DOI] [PubMed] [Google Scholar]
- 27. Wiklund S., Johansson E., Sjostrom L., Mellerowicz E. J., Edlund U., Shockcor J. P., Gottfries J., Moritz T., Trygg J. (2008) Visualization of GC/TOF-MS-based metabolomics data for identification of biochemically interesting compounds using OPLS class models. Anal. Chem. 80, 115–122 [DOI] [PubMed] [Google Scholar]
- 28. Sallusto F., Kremmer E., Palermo B., Hoy A., Ponath P., Qin S., Forster R., Lipp M., Lanzavecchia A. (1999) Switch in chemokine receptor expression upon TCR stimulation reveals novel homing potential for recently activated T cells. Eur. J. Immunol. 29, 2037–2045 [DOI] [PubMed] [Google Scholar]
- 29. Perros F., Hoogsteden H. C., Coyle A. J., Lambrecht B. N., Hammad H. (2009) Blockade of CCR4 in a humanized model of asthma reveals a critical role for DC-derived CCL17 and CCL22 in attracting Th2 cells and inducing airway inflammation. Allergy 64, 995–1002 [DOI] [PubMed] [Google Scholar]
- 30. Tian L., Li W., Wang J., Zhang Y., Zheng Y., Qi H., Guo X., Zhang Y., Ma D., Shen H., Wang Y. (2011) The CKLF1–C19 peptide attenuates allergic lung inflammation by inhibiting CCR3- and CCR4-mediated chemotaxis in a mouse model of asthma. Allergy 66, 287–297 [DOI] [PubMed] [Google Scholar]
- 31. Vestergaard C., Deleuran M., Gesser B., Gronhoj Larsen C. (2003) Expression of the T-helper 2-specific chemokine receptor CCR4 on CCR10-positive lymphocytes in atopic dermatitis skin but not in psoriasis skin. Br. J. Dermatol. 149, 457–463 [DOI] [PubMed] [Google Scholar]
- 32. Misra N., Bayry J., Lacroix-Desmazes S., Kazatchkine M. D., Kaveri S. V. (2004) Cutting edge: human CD4+CD25+ T cells restrain the maturation and antigen-presenting function of dendritic cells. J. Immunol. 172, 4676–4680 [DOI] [PubMed] [Google Scholar]
- 33. Onishi Y., Fehervari Z., Yamaguchi T., Sakaguchi S. (2008) Foxp3+ natural regulatory T cells preferentially form aggregates on dendritic cells in vitro and actively inhibit their maturation. Proc. Natl. Acad. Sci. USA 105, 10113–10118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Krummel M. F., Allison J. P. (1996) CTLA-4 engagement inhibits IL-2 accumulation and cell cycle progression upon activation of resting T cells. J. Exp. Med. 183, 2533–2540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ying H., Yang L., Qiao G., Li Z., Zhang L., Yin F., Xie D., Zhang J. (2010) Cutting edge: CTLA-4–B7 interaction suppresses Th17 cell differentiation. J. Immunol. 185, 1375–1378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kavanagh B., O'Brien S., Lee D., Hou Y., Weinberg V., Rini B., Allison J. P., Small E. J., Fong L. (2008) CTLA4 blockade expands FoxP3+ regulatory and activated effector CD4+ T cells in a dose-dependent fashion. Blood 112, 1175–1183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Fazekas de St Groth B., Zhu E., Asad S., Lee L. (2011) Flow cytometric detection of human regulatory T cells. Methods Mol. Biol. 707, 263–279 [DOI] [PubMed] [Google Scholar]
- 38. Seddiki N., Santner-Nanan B., Martinson J., Zaunders J., Sasson S., Landay A., Solomon M., Selby W., Alexander S. I., Nanan R., Kelleher A., Fazekas de St Groth B. (2006) Expression of interleukin (IL)-2 and IL-7 receptors discriminates between human regulatory and activated T cells. J. Exp. Med. 203, 1693–1700 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.