Agonists for seven TLRs induce similar responses when tested in murine iris/ciliary body cultures and have more disparate responses when tested in eyes in vivo.
Keywords: PAMPs, cytokines, iritis, leukocyte chemotaxis
Abstract
TLRs are critical for host defense and innate immunity. Emerging evidence also supports a role for TLRs in many chronic inflammatory diseases, including inflammatory eye disease, known as uveitis. The activation of TLR4 by endotoxin induces a standard model of murine uveitis. How activation of additional TLRs influences the onset and/or severity of anterior uveitis has not been examined. We sought to elucidate the potential of TLRs (TLR1/2, TLR2/6, TLR3, TLR4, TLR5, TLR7/8, and TLR9) to trigger uveitis in mice. Directly stimulated iris/ciliary body explants demonstrated a marked increase in production of inflammatory cytokines TNF-α, IL-6, IP-10/CXCL10, MCP-1, and KC with relatively little production of IFN-γ, IL-10, IL-12p40, IL-12p70, IL-17, IL-1β, IL-4, or RANTES. The cytokine-response profiles were comparable amongst the TLR agonists, albeit some differences were noted, such as greater IP-10 production following TLR3 activation. Intra-ocular injection of TLR agonists increased leukocyte interactions with the endothelium of the iris vasculature and resulted in chemotaxis into the iris tissue. Assessment of leukocytic responses by ivt videomicroscopy and histology revealed quantitative differences amongst responses to the TLR agonists with respect to the timing and numbers of rolling, adhering, iris-infiltrating, and aqueous humor-infiltrating cells, along with cytokine levels in vivo. Our data demonstrate the eye's responsiveness to a diverse array of microbial products, which activates TLRs, and reveal differences in relative cellular response among the various TLR agonists in vivo.
Introduction
TLRs form a large family of well-characterized PRRs with at least 10 members in humans and 12 in mice [1]. TLRs 1–9 are highly conserved across the two species. TLRs have evolved to recognize a wide variety of microbial components or PAMPs, and their activation is critical for innate immune responses, as well as shaping adaptive immune responses. Some of the TLRs, such as TLR1/2, -2/6, -4, and -5, preferentially respond to bacterial proteins and lipids, whereas others, such as TLR3, -7, -8, and -9, mainly respond to bacterial and viral nucleic acids. More recently, TLRs have also been implicated in potentially recognizing alarmins, which are endogenous or host-derived agonists, in situations of chronic inflammation.
Although TLRs are clearly critical to host defense through initiation of the earliest immune responses, their dysregulation could potentially be involved in chronic inflammatory diseases. TLRs have been linked to predisposition to diseases, such as rheumatoid arthritis, inflammatory bowel disease, Crohn's disease, psoriasis, asthma, myocarditis, hepatitis, systemic lupus erythematosus, multiple sclerosis, and ankylosing spondylitis [2–7]. Exactly how TLRs are involved in inflammatory disorders is not entirely known, although they could potentially contribute through various capacities, such as providing the initial trigger in an infectious setting or propagation of chronic inflammation through the recognition of endogenous ligands and/or breakdown of immune tolerance. The necessity of TLRs in directing an autoreactive immune response is indicated by the need for an adjuvant in many experimental models of disease, including murine models of arthritis, experimental autoimmune encephalomyelitis, and EAU [2]. The role for aberrant host responses against infection and the participation of gut microflora in shaping immunity are underscored by studies of arthritis in the HLA-B27 transgenic rats, the IL-1R antagonist KO mice, and the SKG mice, each of which develops peripheral joint disease in standard but not in germ-free housing conditions [7–9].
Uveitis, or intraocular inflammatory disease, is one of the leading causes of blindness and human visual disability [10]. Uveitis encompasses a heterogeneous group of diseases, wherein the uvea (the middle vascular coat of the eye) is consistently afflicted. Uveitis can arise from microbial or viral infection or can be associated with various “noninfectious” systemic diseases, many of which were mentioned above (including inflammatory bowel disease, Crohn's disease, multiple sclerosis, sarcoidosis, ankylosing spondylitis, Behçet's disease, and many others). Despite numerous studies that investigated the involvement of TLRs in the pathogenesis of inflammatory disorders described above, the contribution of TLRs to uveitis is poorly understood. Recent research has documented the mRNA expression of all known TLRs in eye tissue [11, 12]. With the exception of TLR4, functional studies to delineate the potential of individual TLRs to trigger uveitis in vivo are lacking.
In this study, we focused on the anterior uveal tract, in particular, the iris, which is the tissue consistently affected in anterior uveitis—the most frequently diagnosed type of uveitis. The inflammatory potentials of the TLRs conserved across humans and mice (TLR1/2, TLR2/6, TLR3, TLR4, TLR5, TLR7/8, and TLR9) were assessed in murine iris/ciliary body cultures, as well as in mouse eyes. Using intravital microscopy, we were able to assess ongoing leukocytic responses within the iris tissue in vivo following local administration of different TLR agonists. This technique allowed us to enumerate specific cellular responses, such as leukocytic rolling and adherence on the vascular endothelium, as well as the subsequent extravasation and chemotaxis into the iris stroma. Since the discovery of TLRs, important advances have been made in our understanding of immunity. This study was a step toward understanding the inflammatory potential of individual TLRs in the setting of murine uveitis.
MATERIALS AND METHODS
TLR agonists and other reagents
The following synthetic TLR agonists were used in these studies: Pam3CSK4, FSL-1, poly (I:C), synthetic monophosphoryl lipid A, flagellin, resiquimod (R848), and stimulatory unmethylated CpG-containing synthetic ODNs (InvivoGen, San Diego, CA, USA). All ligands were dissolved in endotoxin-free, sterile saline prior to use. All agonists [except poly (I:C), which tested below 0.05 EU/mg sample] were endotoxin-free, as measured by the Limulus amoebocyte lysate assay. As additional controls, control ODN (InvivoGen) and BSA (Sigma-Aldrich, St. Louis, MO, USA) were used to test for the lack of any nonspecific responses to ODNs or protein (data not shown).
Mice
Female BALB/c mice (8–10 weeks) were purchased from The Jackson Laboratory (Bar Harbor, ME, USA). Mice were housed in a facility approved by the Association for Assessment and Accreditation of Laboratory Animal Care International. Procedures were conducted in accordance with National Institutes of Health and the Association for Research in Vision and Ophthalmology guidelines and Oregon Health and Science University Institutional Animal Care and Use Committee (Portland, OR, USA) policies.
Intra-ocular injections
ivt injections were performed by a method described previously [13]. Briefly, mice were anesthetized by 1.7% isoflurane in oxygen, and injections were performed with 30-gauge Hamilton syringes under direct visualization through a surgical microscope. Mice received a 2-μl injection of the specified TLR agonists in one eye and a corresponding volume of saline, BSA, or control ODNs in the contralateral eye. Pilot studies were performed to optimize the dose of each TLR agonist that achieved consistently measurable responses. In some cases, the maximum possible dose of a given TLR agonist was administered as a consequence of its weak inflammatory potential and the limited quantity that we were able to inject into the vitreous.
Intra-ocular videomicroscopy
Leukocytic responses within the iris vasculature and extravascular tissue were measured using a method of intravital microscopy described previously [13, 14]. Briefly, at the time of videomicroscopy, mice were i.p.-injected with 35 mg/kg rhodamine 6G (Sigma-Aldrich) to label all intravascular leukocytes. Mice were anesthetized with 1.7% isoflurane in oxygen, and 10-s digital video images of the iris vasculature and tissue were recorded using a black-and-white video camera (Kappa Scientific, Gleichen, Germany) on an epifluorescence ivt microscope (modified Orthoplan; Leica, Wetzlar, Germany) in three independent regions of each eye. The numbers of each phenotype of each leukocyte (i.e., rolling, adhering, or infiltrating) were counted. Vessel diameters and lengths and areas of iris tissue were quantified using Image J public-domain analysis software.
Histopathological examination
Eyes were enucleated and prepared for histological assessment as described previously [14]. Briefly, eyes were fixed overnight in 10% neutral-buffered formalin, followed by 80% ethanol, and then embedded in paraffin. Sections (7 μm) were cut through the middle region near the papillary-optic nerve plane and stained with H&E. The numbers of leukocytes within the aqueous humor in four sections were counted by a masked observer and averaged for each eye.
Explant cultures and multiplex ELISA Luminex assays
Organotypic retinal and iris/ciliary body cultures were prepared from naïve BALB/c mouse tissues and maintained at 37°C in 5% CO2 for the duration of the experiment. Briefly, the neuroretinal and iris/ciliary body tissues were carefully excised from enucleated eyes. Tissues were cut into one-thirds; cultured in Falcon Microtest 96-well polystyrene plates (Becton Dickinson and Co., Franklin Lakes, NJ, USA), containing 70 μl minimal essential medium, containing 2 mM L-glutamine and Earle's balanced salts; and supplemented with 5% heat-inactivated FBS (FBS-HI, Hyclone, Logan, UT, USA). Dissected tissues were incubated for 24 h in media, with or without the indicated TLR agonists at 10 μg/ml, and then the culture supernatants were collected for analysis. The cytokines IFN-γ, IL-1β, IL-4, IL-6, IL-10, IL-12(p40), IL-12(p70), IL-17, IP-10, KC, MCP-1, RANTES, and TNF-α were measured with a custom Milliplex mouse cytokine/chemokine ELISA (Millipore, Billerica, MA, USA) using Model L100 IS (Luminex, Austin, TX, USA). The same multiplex ELISA cytokine panel was used for assessment of cytokine levels in protein isolated from whole eye-tissue homogenates as described previously [15]. Protein was quantified by BCA assay (Bio-Rad, Hercules, CA, USA), and equal amounts of protein were used to perform the Luminex assays. Data were analyzed with BeadView multiplex analysis software (version 1.0, Upstate Cell Signaling Solutions, Lake Placid, NY, USA).
Statistical analysis
Data are represented as mean ± sem. Mean differences between treatments were analyzed using a one-way analysis of variation with t test post hoc analyses. Differences were considered statistically significant when P < 0.05.
RESULTS
TLR stimulation of iris/ciliary body tissue results in cytokine production
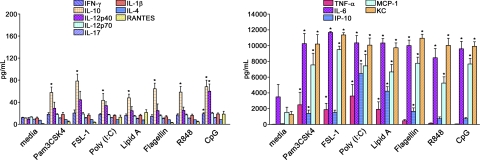
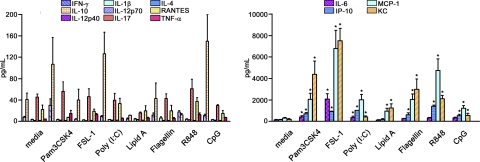
To examine the potential of TLRs within anterior eye tissue, specifically the iris/ciliary body, to promote inflammation, cytokines were measured in the supernatants of organotypic cultures that had been treated with the indicated synthetic TLR agonists. We found that all TLR agonists were capable of eliciting greater production of TNF-α, IL-6, IP-10, MCP-1, and KC (Fig. 1, right) than the other cytokines measured (Fig. 1, left). At the doses and timing tested here, negligible levels of the cytokines IL-12p70, IL-17, IL-1β, and IL-4 were observed in response to all TLR agonists. Some differences amongst the TLR responses were noted. For example, TLR9 stimulation by CpG-ODN increased IL-12p40 more than all other TLRs. Stimulation of TLR5, TLR7/8, or TLR9 resulted in relatively little TNF-α compared with the other TLR agonists, and although IP-10 production was detected in response to all TLR agonists, it was strongest in response to TLR3 and TLR4 stimulation, possibly indicating TRIF/TRAM-dependent pathway activation. We performed the same experiments with retina explants, and few significant cytokine changes were observed. Increased IP-10, IL-6, MCP-1, and KC levels were observed in a similar profile to that of iris/ciliary body tissue, albeit stimulated retina explants achieved much lower levels: an approximate twofold increase over media controls was discovered (data not shown). We find it interesting that overall, the iris/ciliary body tissue appears to demonstrate a greater sensitivity to TLR stimulation than retinal tissue. These results indicate that the iris/ciliary body is responsive to agonists for all TLRs and is capable of promoting inflammatory cytokine production to a much greater extent than the retinal tissue.
Figure 1. TLR stimulation of the iris/ciliary body ex vivo results in unique profiles of cytokine production.
Organotypic iris/ciliary body cultures were stimulated with 10 μg/ml of the indicated TLR agonists. Cytokine levels in the culture supernatants were determined by multiplex ELISA (Luminex) at 24 h poststimulation. Data are shown as mean ± sem of combined values from three independent experiments with three mice each. *P < 0.05 comparison between media control and TLR agonist-stimulated samples.
Activation of TLR4 by lipid A in vivo
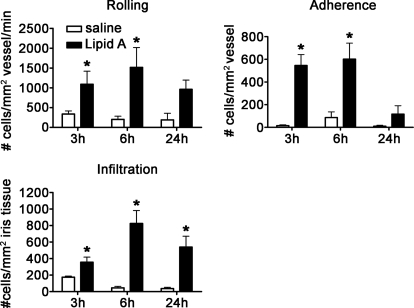
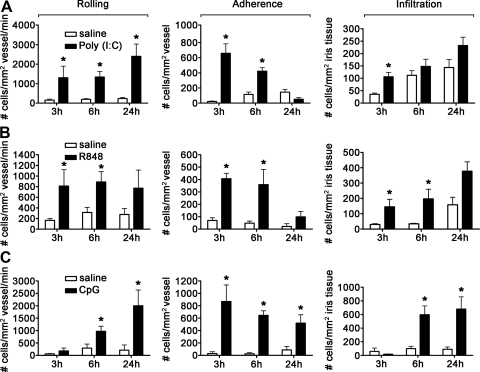
Based on our observations that the iris/ciliary body tissue is responsive to stimulation by agonists for each TLR, we sought to elucidate the functional activity of each TLR within the eye. For this study, we used the technique of intravital microscopy, which allows us to assess ongoing leukocytic changes quantitatively within the iris in vivo. We are able to identify differences in cellular responses based on behavior, such as rolling and adhesion on the iris vasculature and infiltration into the iris stroma. We first assessed TLR4 activation, as historically, its activation mediates a standardized model, EIU. We delivered synthetic lipid A, which is the moiety of the LPS chemical structure considered to be responsible for the biological activities induced by LPS, directly into the eye via an ivt injection. As shown in Fig. 2, lipid A resulted in a significant increase in the numbers of rolling, adhering, and infiltrating leukocytes within the iris as early as 3 h postinjection. This effect was sustained for at least 24 h. These data support the potential of TLR4 to trigger uveitis, which is consistent with the murine EIU models and a prior report in rats comparing LPS versus lipid A [16].
Figure 2. Activation of TLR4 by lipid A.
The numbers of rolling, adhering, and infiltrating cells within the iris vasculature and tissue as a function of time in response to 250 ng lipid A were enumerated with ivt videomicroscopy. Data are shown as mean ± sem of combined values from two independent experiments. *P < 0.05 comparison between lipid A-injected eyes and saline-injected control eyes (n=9–15 mice/treatment/time-point).
Activation of TLR2/1 and TLR2/6 heterodimers by Pam3CSK4 and FSL-1
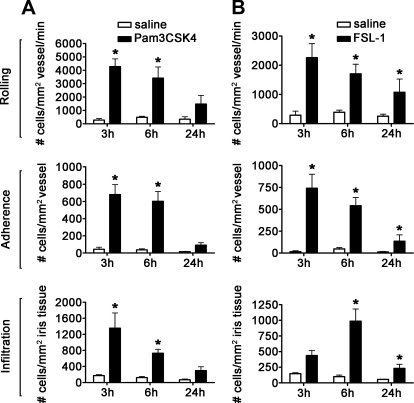
TLR2, in association with TLR1 or TLR6, plays an important role in recognizing bacterial lipoproteins and lipopeptides. TLR2 associated with TLR1 responds to triacyl lipopeptides, whereas TLR6 association with TLR2 changes its specificity to recognize diacyl lipopeptides [17, 18]. We tested the functions of the two possible TLR2 heterodimers in mice by injecting Pam3CKS4 to activate TLR2/1 or FSL-1 to activate TLR2/6. As shown in Fig. 3A, a significant increase in rolling, adherence, and infiltration was observed within 3 h, with near-complete resolution by 24 h postinjection, in response to Pam3CSK4. FSL-1 also demonstrated a similar inflammatory capacity to increase rolling, adherence, and infiltration (Fig. 3B); albeit, a somewhat different kinetics with respect to cellular infiltration within the iris tissue was noted.
Figure 3. Activation of TLR2/1 and TLR2/6 heterodimers by Pam3CSK4 and FSL-1.
The numbers of rolling, adhering, and infiltrating cells within the iris vasculature and tissue as a function of time in response to 1 μg Pam3CSK4 (A) or 0.4 μg FSL-1(B) were enumerated with ivt videomicroscopy. Data are shown as mean ± sem of combined values from two independently performed experiments. *P < 0.05 comparison between Pam3CSK4- or FSL-1-injected eyes and saline-injected control eyes (n=9–20 mice/treatment/time-point).
Activation of TLR5 by flagellin
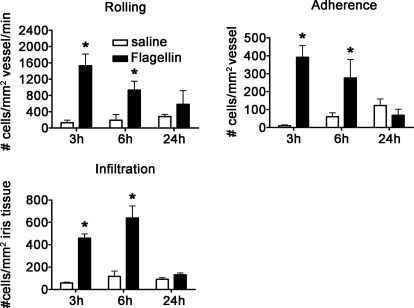
TLR5 is the primary sensor for flagellin, which is the major component of bacterial flagellar filaments derived from Gram-positive and Gram-negative bacteria. A recent report implicates the NOD-like receptor protein, Ipaf, as a secondary and downstream effector of caspase-1-dependent responses to flagellin, thereby implicating a combinatorial role for the two receptors [19, 20]. Both receptors might contribute to the results presented here. Nonetheless, no prior study has investigated the inflammatory effects of flagellin-injected ivt in mice. We therefore tested whether flagellin is capable of triggering uveitis. As shown in Fig. 4, flagellin significantly increased the number of rolling, adhering, and infiltrating leukocytes at 3 and 6 h postinjection. These responses were resolved by 24 h. Control studies, wherein mice were injected with comparable amounts of an irrelevant protein (BSA), demonstrated a lack of any significant response compared with ivt saline injection (data not shown) and thereby, substantiated the specificity of the response to flagellin.
Figure 4. Activation of TLR5 by flagellin.
The numbers of rolling, adhering, and infiltrating cells within the iris vasculature and tissue as a function of time in response to 0.4 μg flagellin were enumerated with ivt videomicroscopy. Data are shown as mean ± sem of combined values from two independently performed experiments. *P < 0.05 comparison between poly (I:C)-injected eyes and saline-injected control eyes (n=9–15 mice/treatment/time-point).
Activation of TLR3, TLR7/8, and TLR9 by nucleic acids
TLR3, -7, -8, and -9 mainly respond to bacterial and viral nucleic acids. In Fig. 5, we show how activation of these receptors may influence uveitis in mice. TLR3 mediates responses to dsRNA and is considered important in the setting of viral infections. Notably, TLR3 is known to elicit inflammation in a MyD88-independent pathway involving TRIF/TRAM, which is in contrast to all other TLRs tested here requiring MyD88. To examine the ocular inflammatory function of TLR3 in mice, we administered the synthetic RNA poly (I:C). As shown in Fig. 5A, we find that injection of poly (I:C) had its greatest impact on leukocyte rolling and adherence within the iris vasculature but had little influence on leukocyte infiltration within the iris tissue itself. A significant difference relative to saline was noted only at 3 h postinjection, indicating that poly (I:C) is not an especially potent trigger of uveitis. Indeed, this is the maximum dose of poly (I:C), with which we are able to inject ivt into mice, further attesting to its relatively minor inflammatory potential in the development of uveitis.
Figure 5. Uveitic response to nucleic acids that trigger TLR3, -7, -8, or -9.
The numbers of rolling, adhering, and infiltrating cells within the iris vasculature and tissue as a function of time in response to 4 μg poly (I:C) (A), 4 μg R848 (B), or 6.5 μg CpG (C) were enumerated by ivt videomicroscopy. Data are shown as mean ± sem of combined values from two independently performed experiments. *P < 0.05 comparison between TLR agonist-injected eyes and saline-injected control eyes (n=9–15 mice/treatment/time-point).
R848 (resiquimod) is a synthetic mimetic of guanosine/uridine-rich, short ssRNA, which is known to activate cells expressing TLR7 and -8, both of which are involved in antiviral responses. Although mice do express TLR7 and TLR8, only TLR7 is believed to be functional in mice [21, 22] and presumably mediates any responses we observe here. We find that mice respond to locally administered R848, as a significant increase in the number of rolling, adhering, and infiltrating leukocytes was observed (Fig. 5B). Infiltration was observed within the first 6 h of injection, although this degree of infiltration is relatively low compared with some of the other TLR responses reported here.
Unmethylated, CpG-ODNs account for the stimulatory effect of bacterial and viral DNA. The most active immune-stimulatory sequences in mice contain an unmethylated CpG-dinucleotide flanked at the 5′-end by two purines and at the 3′-end by two pyrimidines. As shown in Fig. 5C, activation of TLR9 by synthetic CpG-ODNs resulted in a progressive increase in the numbers of rolling and adherent cells with corresponding infiltration at 6 h and 24 h postinjection. Control studies, wherein eyes were injected with comparable amounts of irrelevant ODN, demonstrated a lack of inflammation (data not shown), indicating a CpG-ODN/TLR9-specific response. We observed a sustained and significant increase in the numbers of rolling, adhering, and infiltrating cells out to 48 h following injection with CpG-ODN, and this was resolved by 7 days (data not shown).
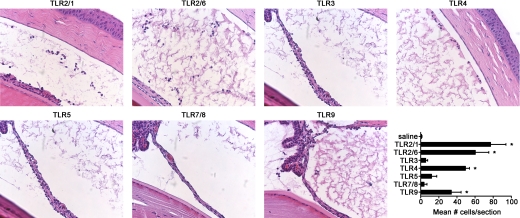
Histological assessment of the impact of TLR activation on inflammation within the anterior segment
To further assess the influence of TLR activation on development of anterior uveitis, we performed histology on eyes at 6 h post-ivt injection of each indicated TLR agonist and counted the number of cells that had migrated into the aqueous humor of the anterior segment. As shown in Fig. 6, in most cases, the quantification of leukocytic responses within the iris by ivt microscopy is corroborated histologically. Representative photos of histopathology observed for each TLR agonist are shown, and the mean numbers of infiltrating cells within the aqueous humor are depicted in the graph. Significant responses with varying degrees were observed for all TLR agonists, except for that of poly (I:C), R848, or flagellin. For all four TLR agonists, Pam3CSK4, FSL-1, lipid A, and CpG-ODN (which triggered a significant cellular infiltration into the aqueous humor), neutrophils represent the predominating cell type (>80% of cells within the aqueous humor). This observation is consistent with EIU and the role for neutrophils as early responder cells during innate immune activation.
Figure 6. Histological assessment of the impact of TLR activation on inflammation within the anterior segment.
Representative photos (original magnifications, 400×) of tissue sections of the eyes from mice injected with each of the TLR agonists and assessed 6 h postinjection by H&E staining. The numbers of cells within the aqueous humor of the anterior segments are graphically depicted (lower right panel). Data are shown as mean ± sem. *P < 0.05 comparison between TLR agonist-injected eyes and saline-injected control eyes (n=6 mice/treatment).
TLR stimulation results in cytokine production in vivo
To examine the potential of TLRs to promote inflammation in vivo, cytokines were measured in whole eye-tissue homogenates from mice previously administered ivt injections of each of the indicated agonists. Overall, we observed a similar cytokine profile to that of iris explants in vitro, wherein the cytokines IFN-γ, IL-10, IL-12, IL-1β, IL-17, IL-4, RANTES, and TNF-α (Fig. 7, left) were produced at minimal levels compared with IL-6, IP-10, MCP-1, and KC (Fig. 7, right). We find it interesting that KC, IP-10, and MCP-1 are consistently the most abundant, which relates to the increased presence of neutrophils and monocytes in vivo. However, these results would also suggest that additional unmeasured factors are produced, which act to shape the different cellular responses observed in vivo to TLR activation.
Figure 7. Evaluation of cytokine responses to TLR agonists in vivo.
Whole eye-tissue homogenates were evaluated for the indicated levels of cytokines by multiplex ELISA at 6 h postinjection. Data are shown as mean ± sem (n=8 mice/treatment). *P < 0.05 comparison between ivt saline controls and TLR agonist-injected mice.
DISCUSSION
The present study elucidates the inflammatory potential of TLRs to trigger murine uveitis in vivo. We demonstrate that functionally, all TLRs tested are capable of directly promoting cytokine production in iris/ciliary body cultures. Somewhat surprisingly, we find that although activation of the various TLRs induced comparable cytokine production ex vivo, the leukocytic changes within the iris tissue in vivo differed.
Despite the extensive advances that have been made in our understanding of TLRs in immunity, the participation of TLRs in uveitis is poorly understood. A role for TLRs in influencing adaptive immune responses has been reported in EAU, a retinal autoimmunity model, wherein mice, conventionally immunized with the retinal antigen interphotoreceptor retinoid-binding protein, along with adjuvant consisting of CFA with Mycobacterium tuberculosis and Bordetella pertussis toxin, develop EAU. In that study, MyD88 KO mice, but not mice lacking TLR2, TLR4, or TLR9, had impaired EAU development [23]. These findings may reflect the redundancy of the TLRs tested in this system. A more recent report by the same group reiterated the redundancy of TLR2, -3, -4, and -9 in the adjuvant effect required to induce EAU [24]. Mice lacking two TLRs (TLR2 and -4, TLR2 and -9, or TLR4 and -9) developed EAU to the same extent as congenic controls with the standard immunization protocol, and agonists of single TLRs [Pam3CSK4, poly (I:C), or CpG to activate TLR2/1, TLR3, or TLR9, respectively] exacerbated EAU in conjunction with a suboptimal immunization protocol. Consistent with these findings, the TLR agonists peptidoglycan, lipoteichoic acid, poly (I:C), LPS, flagellin, and CpG-ODN triggered autoimmune uveitis in mice by activating naïve T cells specific to HEL to subsequently induce ocular inflammation in mice. In this model, HEL is expressed specifically in the lens under control of the αA-crystallin promoter [25]. Collectively, these studies indicate a role for TLRs in pathogenic autoimmunity involving the eye.
The reports mentioned above focused on autoimmune models involving T cell responses and also focused on the pathology of the posterior segment of the eye, primarily the retina. As the most frequently diagnosed type of uveitis is anterior and involves the iris, we chose to focus on acute, inflammatory responses within the iris and further examined innate responses triggered by TLR agonists, rather than their adjuvant potential for adaptive responses. Most of the studies about the potential of TLRs to elicit uveitis in vivo have focused on activation of TLR4 by LPS to produce EIU. This model has been used for over 30 years [26] as a tool to investigate the initiating events in acute inflammatory uveitis. Other suggestions that TLRs can trigger uveitis come from studies actually performed prior to the discovery of TLRs. Injection of bacterial preparations, which we now know contained agonists for TLR2, TLR4, and another PRR, NOD2, resulted in uveitis in rabbits and rats [27, 28]. We have characterized more recently the potential of NOD1 and NOD2 agonists to induce ocular inflammation [14, 29]. Mutations in NOD2 are known to induce a rare, autosomal-dominant form of uveitis in patients [30], and this observation suggests that other PRRs, such as the TLRs, could be important in inducing uveitis. We, therefore, considered it critical to learn whether agonists of additional TLRs trigger uveitis. With the recent characterization of the molecular structure of TLR agonists and the availability of synthesized reagents specific for each TLR, we are now able to adopt a reductionist approach to examine individual TLRs systematically. To our knowledge, this is the first report that comprehensively assessed TLRs in the onset of murine uveitis in vivo.
TLR activation of signaling pathways originates from their cytoplasmic TIR domains and ultimately leads to activation of transcription factors such as NF-κB, AP-1, or IFN regulatory factor 3, thereby enhancing production of various inflammatory mediators, including cytokines, chemokines, and adhesion molecules. Two downstream signaling pathways activated by TLRs have been an area of intense research, and their signal transduction can be distinguished by the initiating adaptors MyD88/TIR domain-containing adaptor protein (a.k.a., Mal) and TRIF/TRAM [1]. We demonstrated that all TLRs tested are functional in iris/ciliary body tissue, as stimulation with agonists for each TLR resulted in cytokine production. We observed the production of cytokines from the MyD88-dependent pathway, e.g., IL-6, as well as the MyD88-independent pathway, e.g., IP-10, indicating that both arms of TLR signaling were intact. One caveat in interpreting the results at 24 h poststimulation is that cytokines expressed early might have induced others by this point of time. In general, the pattern of cytokine production was comparable amongst the TLRs, although some differences were noted, such as greater amounts of IP-10 in response to poly (I:C). The extent to which the MyD88-dependent and independent pathways influence uveitis is not known. Our own work recently demonstrated [31] an essential role for MyD88 in the development of uveitis triggered by TLR2/1 or TLR2/6. We also recently reported [15] that the MyD88 pathway plays a greater role in EIU than does the TRIF pathway. Future studies that delineate downstream signaling pathways used by TLRs should prove informative.
How the anterior uvea, which includes the iris and ciliary body, responds to TLR agonists in vivo is especially pertinent, given that this tissue is consistently afflicted in anterior uveitis. Our observations indicate that activated TLRs vary in their ability to trigger uveitis, despite their comparable cytokine response profiles ex vivo and in vivo. Several factors likely contribute to the discordance between our findings in vivo and ex vivo. The presence of many immunosuppressive factors, such as TGF-β, might impact the nature of the responses to TLR agonists in vivo. We did not observe, however, evidence for a uniform, global suppression of TLR responses.
Differential induction of inflammatory mediators, such as adhesion molecules, chemokines, and cytokines, which shape the leukocyte response, is suggested by the differences in behavior that we observed in vivo across the spectrum of TLR agonists. For example, activation of most TLRs resulted in coordinated leukocyte rolling, adherence, and infiltration, but activation of TLR3 by poly (I:C) resulted in increased leukocyte rolling and adherence, which did not correlate with the extent of cell infiltration into the iris tissue or aqueous humor. Following TLR3 activation, there appears to be a relative insufficiency in the appropriate chemotactic signals needed for this latter step or alternatively, stimulation of an inhibitory signal, such as type I IFN. The relatively low inflammatory potential of poly (I:C) in vivo might indicate that the MyD88/Mal pathway exerts a greater ability to elicit inflammation than the TRIF/TRAM pathway, and this might be a result of a difference in the responding cell type or might reflect a difference in the pharmacokinetics of poly (I:C) compared with the other agonists tested. Another notable difference is that following activation of TLR5 and -7/8, leukocytes infiltrated the iris tissue but did not emigrate into the aqueous humor. Intravital microscopy is an especially valuable tool for quantification of cellular responses, such as leukocyte rolling, which would not otherwise be evident histologically. Moreover, this technique provides insight into the types of chemotactic signals elicited based on differentiation of leukocyte behavior (rolling, adherence, infiltration), which are mediated by distinct molecular processes. A limitation, however, is that we were unable to distinguish responder cell types. Our histological studies, however, indicated that neutrophils were the predominant leukocyte that extravasated into the anterior uveal tract. Of note, KC, a potent neutrophil chemoattractant, is consistently produced by activation of TLR agonists in vitro and in vivo. Further studies will be needed to test possible explanations for these results, again including responding cell types and pharmacokinetics.
The eye is considered an immune-privileged site as a consequence of various mechanisms that are in place to prevent vision-threatening side-effects of excessive inflammation within the eye. The presence of immunosuppressive factors and the morphological features of eyes likely influence responsiveness to TLR agonists in vivo. Consequently, studies of cell and organotypic cultures may provide useful information about the potential of TLR responses, but they don't necessarily reflect what occurs in vivo. Studying a different species, such as rat, or a different route of injection, such as i.v., might have produced different outcomes; the results that we present here illustrate the discordance between in vivo and ex vivo models. Functional analysis of individual TLRs in vivo is clearly necessary to understand how they shape the ocular inflammatory responses involved in uveitis. Nonetheless, contrasting the responses in vivo and ex vivo provides clues to possible areas of regulation worthy of further study.
ACKNOWLEDGMENTS
This work was made possible by the support from National Eye Institute at NIH [grants EY019020 (H.L.R.), EY019604 (J.T.R.), and EY010572 (departmental core grant)]. Additional support was provided by the Stan and Madelle Rosenfeld Family Trust, the William and Mary Bauman Foundation, the William C. Kuzell Foundation, and Research to Prevent Blindness. H.L.R. also receives career development support from the Research to Prevent Blindness and the American College of Rheumatology. The authors thank C. Kintzley and M. Hannah for their assistance in the quantification of intravital microscopic images.
Footnotes
- EAU
- experimental autoimmune uveitis
- EIU
- endotoxin-induced uveitis
- FSL-1
- fibroblast-stimulating lipopeptide 1
- HEL
- hen egg lysozyme
- IP-10
- IFN-inducible protein 10
- ivt
- intravitreal
- KC
- keratinocyte-derived chemokine CXCL1
- KO
- knockout
- Mal
- MyD88 adapter-like
- NOD
- nucleotide-binding oligomerization
- ODN
- oligodeoxynucleotide
- Pam3CSK4
- palmitoyl-3-cysteine-serine-lysine-4
- poly (I:C)
- polyinosinic:polycytidylic acid
- TIR
- Toll/IL-1R
- TRAM
- Toll/IL-1R-domain-containing adaptor-inducing IFN-β-related adaptor molecule
- TRIF
- Toll/IL-1R-domain-containing adaptor-inducing IFN-β
AUTHORSHIP
J.J.A. and H.L.R. conceived of and performed the experiments, analyzed data, and prepared the manuscript. J.T.R. and S.R.P. participated in interpretation of data and manuscript preparation.
REFERENCES
- 1. Kawai T., Akira S. (2010) The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat. Immunol. 11, 373–384 [DOI] [PubMed] [Google Scholar]
- 2. Li M., Zhou Y., Feng G., Su S. B. (2009) The critical role of Toll-like receptor signaling pathways in the induction and progression of autoimmune diseases. Curr. Mol. Med. 9, 365–374 [DOI] [PubMed] [Google Scholar]
- 3. Takeda K., Akira S. (2005) Toll-like receptors in innate immunity. Int. Immunol. 17, 1–14 [DOI] [PubMed] [Google Scholar]
- 4. Assassi S., Reveille J. D., Arnett F. C., Weisman M. H., Ward M. M., Agarwal S. K., Gourh P., Bhula J., Sharif R., Sampat K., Mayes M. D., Tan F. K. (2011) Whole-blood gene expression profiling in ankylosing spondylitis shows upregulation of Toll-like receptor 4 and 5. J. Rheumatol. 38, 87–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Corr S. C., O'Neill L. A. (2009) Genetic variation in Toll-like receptor signalling and the risk of inflammatory and immune diseases. J. Innate Immun. 1, 350–357 [DOI] [PubMed] [Google Scholar]
- 6. McCormack W. J., Parker A. E., O'Neill L. A. (2009) Toll-like receptors and NOD-like receptors in rheumatic diseases. Arthritis Res. Ther. 11, 243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yoshitomi H., Sakagushi N., Kobayashi K., Brown G. D., Tagami T., Sakihama T., Hirota K., Tanaka S., Nomura T., Miki I., Gordon S., Akira S., Nakamura T., Sakagushi S. (2005) A role for fungal {β}-glucans and their receptor Dectin-1 in the induction of autoimmune arthritis in genetically susceptible mice. J. Exp. Med. 201, 949–960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Taurog J. D., Richardson J. A., Croft J. T., Simmons W. A., Zhou M., Fernandez-Sueiro J. L., Balish E., Hammer R. E. (1994) The germfree state prevents development of gut and joint inflammatory disease in HLA-B27 transgenic rats. J. Exp. Med. 180, 2359–2364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Abdollahi-Roodsaz S., Joosten L. A., Koenders M. I., Devesa I., Roelofs M. F., Radstake T. R., Heuvelmans-Jacobs M., Akira S., Nicklin M. J., Ribeiro-Dias F., van den Berg W. B. (2008) Stimulation of TLR2 and TLR4 differentially skews the balance of T cells in a mouse model of arthritis. J. Clin. Invest. 118, 205–216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chang J. H., Wakefield D. (2002) Uveitis: a global perspective. Ocul. Immunol. Inflamm. 10, 263–279 [DOI] [PubMed] [Google Scholar]
- 11. Rodríguez-Martínez S., Cancino-Diaz M. E., Jimenez-Zamudio L., Garcia-Latorre E., Cancino-Diaz J. C. (2005) TLRs and NODs mRNA expression pattern in healthy mouse eye. Br. J. Ophthalmol. 89, 904–910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chang J. H., McCluskey P. J., Wakefield D. (2006) Toll-like receptors in ocular immunity and the immunopathogenesis of inflammatory eye disease. Br. J. Ophthalmol. 90, 103–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Becker M. D., Nobiling R., Planck S. R., Rosenbaum J. T. (2000) Digital video-imaging of leukocyte migration in the iris: intravital microscopy in a physiological model during the onset of endotoxin-induced uveitis. J. Immunol. Methods 240, 23–37 [DOI] [PubMed] [Google Scholar]
- 14. Rosenzweig H. L., Martin T. M., Jann M. M., Planck S. R., Davey M. P., Kobayashi K., Flavell R. A., Rosenbaum J. T. (2008) NOD2, the gene responsible for familial granulomatous uveitis, is essential in a mouse model of muramyl dipeptide-induced uveitis. Invest. Ophthalmol. Vis. Sci. 49, 1518–1524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kezic J., Taylor S., Gupta S., Planck S.R., Rosenzweig H.L., Rosenbaum J.T. (2011) Endotoxin-induced uveitis is primarily dependent on radiation resistant cells and on MyD88 but not TRIF. J. Leukoc. Biol. 90, 305–311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hanashiro R. K., Fujino Y., Gugunfu, Samura M., Takahashi T., Masuda K. (1997) Synthetic lipid A-induced uveitis and endotoxin-induced uveitis—a comparative study. Jpn. J. Ophthalmol. 41, 355–361 [DOI] [PubMed] [Google Scholar]
- 17. Jin M. S., Kim S. E., Heo J. Y., Lee M. E., Kim H. M., Paik S. G., Lee H., Lee J. O. (2007) Crystal structure of the TLR1-TLR2 heterodimer induced by binding of a tri-acylated lipopeptide. Cell 130, 1071–1082 [DOI] [PubMed] [Google Scholar]
- 18. Kang J. Y., Nan X., Jin M. S., Youn S. J., Ryu Y. H., Mah S., Han S. H., Lee H., Paik S. G., Lee J. O. (2009) Recognition of lipopeptide patterns by Toll-like receptor 2-Toll-like receptor 6 heterodimer. Immunity 31, 873–884 [DOI] [PubMed] [Google Scholar]
- 19. Amer A., Franchi L., Kanneganti T. D., Body-Malapel M., Ozoren N., Brady G., Meshinchi S., Jagirdar R., Gewirtz A., Akira S., Núñez G. (2006) Regulation of Legionella phagosome maturation and infection through flagellin and host Ipaf. J. Biol. Chem. 281, 35217–35223 [DOI] [PubMed] [Google Scholar]
- 20. Vijay-Kumar M., Carvalho F. A., Aitken J. D., Fifadara N. H., Gerwirtz A. T. (2010) TLR5 or NLRC4 is necessary and sufficient for promotion of humoral immunity by flagellin. Eur. J. Immunol. 40, 3528–3534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Akira S., Uematsu S., Takeuchi O. (2006) Pathogen recognition and innate immunity. Cell 124, 783–801 [DOI] [PubMed] [Google Scholar]
- 22. Kawai T., Akira S. (2006) Innate immune recognition of viral infection. Nat. Immunol. 7, 131–137 [DOI] [PubMed] [Google Scholar]
- 23. Su S. B., Silver P. B., Grajewski R. S., Agarwal R. K., Tang J., Chan C. C., Caspi R. R. (2005) Essential role of the MyD88 pathway, but nonessential roles of TLRs 2, 4, and 9, in the adjuvant effect promoting Th1-mediated autoimmunity. J. Immunol. 175, 6303–6310 [DOI] [PubMed] [Google Scholar]
- 24. Fang J., Fang D., Silver P. B., Wen F., Li B., Ren X., Lin Q., Caspi R. R., Su S. B. (2010) The role of TLR2, TRL3, TRL4, and TRL9 signaling in the pathogenesis of autoimmune disease in a retinal autoimmunity model. Invest. Ophthalmol. Vis. Sci. 51, 3092–3099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fujimoto C., Shi G., Gery I. (2008) Microbial products trigger autoimmune ocular inflammation. Ophthalmic Res. 40, 193–199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rosenbaum J. T., McDevitt H. O., Guss R. B., Egbert P. R. (1980) Endotoxin-induced uveitis in rats as a model for human disease. Nature 286, 611–613 [DOI] [PubMed] [Google Scholar]
- 27. Fox A., Hammer M. E., Lill P., Burch T. G., Burrish G. (1984) Experimental uveitis elicited by peptidoglycan-polysaccharide complexes, lipopolysaccharide, and muramyl dipeptide. Arch. Ophthalmol. 102, 1063–1067 [DOI] [PubMed] [Google Scholar]
- 28. Kufoy E. A., Fox K., Fox A., Parks C., Pakalnis V. A. (1990) Modulation of the blood-aqueous barrier by gram positive and gram negative bacterial cell wall components in the rat and rabbit. Exp. Eye Res. 50, 189–195 [DOI] [PubMed] [Google Scholar]
- 29. Rosenzweig H. L., Galster K., Planck S., Rosenbaum J. (2009) NOD1 expression in the eye and functional contribution to IL-1{β} dependent ocular inflammation in mice. Invest. Ophthalmol. Vis. Sci. 50, 1746–1753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Miceli-Richard C., Lesage S., Rybojad M., Prieur A. M., Manouvrier-Hanu S., Hafner R., Chamaillard M., Zouali H., Thomas G., Hugot J. P. (2001) CARD15 mutations in Blau syndrome. Nat. Genet. 29, 19–20 [DOI] [PubMed] [Google Scholar]
- 31. Rosenzweig H. L., Galster K., Vance E. E., Ensign-Lewis J., Nunez G., Davey M. P., Rosenbaum J. T. (2011) NOD2 deficiency results in increased susceptibility to peptidoglycan-induced uveitis in mice. Invest. Ophthalmol. Vis. Sci. 52, 4106–4112 [DOI] [PMC free article] [PubMed] [Google Scholar]