Abstract
Discussion on exudate neutrophils as a more accurate model of the “working” functional in vivo neutrophil than their circulating progenitors.
Keywords: inflammation, exudate, apoptosis, diapedesis
Almost all of the current understanding of neutrophil biology has come from the study of circulating neutrophils isolated from blood. These purified cells, transformed as they are by shear and gravitational forces as well as ionic and temperature shocks, are widely considered as ″resting″ neutrophils. Furthermore, whereas the resulting population of cells is often ≥90% neutrophils, the other cells present (e.g., eosinophils, lymphocytes, and monocytes) have unique functions and can alter neutrophil functions significantly. For example, small numbers of monocytes, similar to those contaminating most commonly used neutrophil-preparative methods, can alter neutrophil responses (including apoptosis) to LPS [1]. One must ask: are these cells derived from the circulation really a true model of the working neutrophil that has adhered to the endothelium, traversed it, and migrated through the tissue to an infectious/inflammatory focus?
In this issue of the Journal of Leukocyte Biology, Christenson et al. [2] add to the growing and oft-ignored list of functional differences between circulating and exudate neutrophils, i.e., resistance to antiapoptotic agents that prolong peripheral neutrophil survival. The report of Christenson et al. [2] similar to the other rather sparse reports, comparing circulating neutrophils with exudate neutrophils, alludes to the Janus-like character of the neutrophil.
How the physiologic processes involved in their departure from the circulation alter the human neutrophil is only barely understood and has been inferred mostly from animal studies. Whereas inflammatory exudates and pus can be obtained from patients with various infectious and inflammatory diseases, only a few experimentally tractable models exist for the study of such exudates in human subjects. The earliest method, the so-called Rebuck skin window, involves abrasion of the superficial layers of the skin and placement of a coverslip to allow adherence and recovery of these cells [3]. Another model involves inflammatory exudates forming within blisters or after the epithelium over the blister (the roof) has been removed and the wound bathed with various buffers [4]. Both chemical agents, such as cantharidin (Spanish fly) [5] and suction [4], have been used to generate such blisters. The method used by Christenson et al. [2] was reported by Hellum and Solberg in 1977 [6] and is widely used (see the excellent review by Follin [7]). Briefly, inflammatory exudates are formed after the dermis and epidermis have been separated by suction and the latter removed to allow the wound to be bathed, usually with autologous serum, to induce exudation of neutrophils. One advantage of this model is the final purity of the neutrophil preparation. Depending on the time-point, relatively few, if any, cells are present in the exudate other than neutrophils. The decreased abundance of monocytes in exudate neutrophil preparations (relative to their abundance in purified, circulating neutrophil preparations) may account for the failure of exudate neutrophils to respond robustly to LPS [8], a point that requires further investigation in the report by Christenson et al. [2].
Whether any of these models accurately reflect natural inflammation in vivo remains to be shown; however, limited comparisons have demonstrated similarities between blister exudate neutrophils and those isolated from pus [9]. Exudates formed by the Rebuck skin window and suction blister methods have been compared [10], indicating that monocytes are a significant, early component of inflammation in the skin window (≥20% of cells within 6 h), whereas in blisters, they do not appear until much later (∼5% of cells at 20 h). Clearly, the cellular makeup of the inflammatory exudate will have a major impact on the function of these cells and necessitates some precise knowledge of the cellular composition when determining relative biological functions.
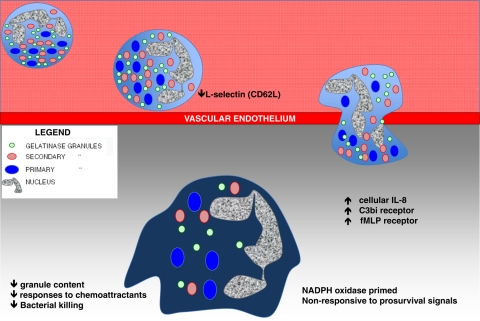
What are the environmental signals that a neutrophil may encounter? For all of these techniques, it can be assumed that the exudate cells are exposed to microbial products in the form of skin-resident microflora, whether viable or dead. Aside from these host-extrinsic factors (e.g., microbial toxins, patterns, products), it must be the case that the spectrum of host-inflammatory mediators plays a central role in determining the functional abilities of neutrophils that interact with them. In addition to the diverse stimuli that induce neutrophil exudation (e.g., C5a, IL-8, formyl peptides, etc.), the complex inflammatory milieu containing cytokines and lipid mediators of inflammation must determine the function of neutrophils encountering these signals. Whether a setting is normoxic or hypoxic, acidic or basic, or warm or cool will also impact neutrophil function. These caveats aside, as shown in Fig. 1, several differences are known that distinguish an exudate neutrophil from a circulating neutrophil. For example, during exudation in vivo, neutrophils undergo exocytosis of specific granules, alteration of surface marker expression [11], and an increase in intracellular IL-8 [12]. The mobilization of distinct neutrophil granule subsets during exudation recapitulates what has been observed in vitro [13]. Given that different granule types contain distinct spectra of receptors and other molecules [14], the regulated release of granules during extravasation dynamically alters neutrophil functional attributes.
Figure 1. Transformation of a circulating neutrophil to an exudate neutrophil.
Adhesion of circulating neutrophils to the vascular endothelium results in down-regulation of L-selectin (CD62L). The process of diapedesis or transendothelial migration, either by traversing the endothelium at the juncture of cells or by passing through the endothelial cell body, is not well understood, but is likely to be a major signal to the neutrophil. Once out of the circulation, exudate neutrophils acquire increased intracellular IL-8 and increased surface expression of the complement C3bi receptor and formyl peptide receptor that is attributed to partial degranulation. The reduction in granule content of exudate neutrophils has been associated with decreased bacterial killing but increased NADPH oxidase priming. Non-responsiveness to pro-survival signals and chemoattractants limits neutrophil mobility and lifespan.
Ideally, studies of neutrophil function should examine both faces of the neutrophil: the inflammatory face of the exudate neutrophil, as well as the relatively quiescent face of the circulating neutrophil. Human exudate experiments are fraught with many potential pitfalls, not the least of which is that usually, only ∼107 exudate neutrophils can be recovered per donor using the suction blister model. The tremendous advances made recently in the miniaturization of biologic analysis should reinvigorate research of these cells. For example, microarray expression analysis predicted an antiapoptotic phenotype of exudate neutrophil compared with circulating neutrophil [15]. The study by Christenson et al. [2] suggests that one of the major biologic processes that control inflammation in vivo (i.e, apoptosis) is different in transmigrated versus circulating neutrophils. Specifically, once in the tissues, the lifespan of the neutrophil is no longer extended by inflammatory stimuli, as it is in the circulating neutrophil. It may make good biologic sense for inflammatory signals to extend the lifespan of a circulating neutrophil in transit to a site of infection. In contrast, once the neutrophil has reached its destination, extending its life further may have a detrimental impact, promoting excessive inflammation and/or tissue damage. Whether the phenomenon described by Christenson et al. [2] contributes to the balance between beneficial and detrimental neutrophil functions in tissues remains to be seen. However, their use of in vivo-generated exudate neutrophils to contrast ″working″ neutrophils from their circulating progenitors is an important step in understanding neutrophil biology. Despite the difficulty of performing such studies, exudate neutrophils provide a more accurate model of the working neutrophil than their circulating progenitors and provide clearer insight into the many functions of the neutrophil in vivo.
ACKNOWLEDGMENTS
This project has been funded in whole or in part with federal funds from the National Cancer Institute, National lnstitutes of Health, under contract no. HHSN261200800001E. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
Footnotes
SEE CORRESPONDING ARTICLE ON PAGE 1055
REFERENCES
- 1. Sabroe I., Prince L. R., Dower S. K., Walmsley S. R., Chilvers E. R., Whyte M. K. (2004) What can we learn from highly purified neutrophils? Biochem. Soc. Trans. 32, 468–469 [DOI] [PubMed] [Google Scholar]
- 2. Christenson K., Björkman L., Karlsson J., Sundqvist M., Movitz C., Speert D. P., Dahlgren C., Bylund J. (2011) In vivo-transmigrated human neutrophils are resistant to antiapoptotic stimulation. J. Leuk. Biol. 90, 1055–1063 [DOI] [PubMed] [Google Scholar]
- 3. Rebuck J. W., Crowley J. H. (1955) A method of studying leukocytic functions in vivo. Ann. N. Y. Acad. Sci. 59, 757–805 [DOI] [PubMed] [Google Scholar]
- 4. Kiistala U., Mustakallio K. K. (1964) In-vivo separation of epidermis by production of suction blisters. Lancet 1, 1444–1445 [DOI] [PubMed] [Google Scholar]
- 5. Boggs D. R., Athens J. W., Haab O. P., Raab S. O., Cartwright G. E., Wintrobe M. M. (1964) Induced inflammatory exudates in normal man. A method designed to study the qualitative and quantitative cellular response to a pyogenic stimulus. Am. J. Pathol. 44, 61–71 [PMC free article] [PubMed] [Google Scholar]
- 6. Hellum K. B., Solberg C. O. (1977) Human leucocyte migration: studies with an improved skin chamber technique. Acta Pathol. Microbiol. Scand. [C] 85C, 413–423 [DOI] [PubMed] [Google Scholar]
- 7. Follin P. (1999) Skin chamber technique for study of in vivo exudated human neutrophils. J. Immunol. Methods 232, 55–65 [DOI] [PubMed] [Google Scholar]
- 8. Marmon S., Hinchey J., Oh P., Cammer M., de Almeida C. J., Gunther L., Raine C. S., Lisanti M. P. (2009) Caveolin-1 expression determines the route of neutrophil extravasation through skin microvasculature. Am. J. Pathol. 174, 684–692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kuhns D. B., Long Priel D. A., Gallin J. I. (1995) Loss of L-selectin (CD62L) on human neutrophils following exudation in vivo. Cell. Immunol. 164, 306–310 [DOI] [PubMed] [Google Scholar]
- 10. Zimmerli W., Gallin J. I. (1987) Monocytes accumulate on Rebuck skin window coverslips but not in skin chamber fluid. A comparative evaluation of two in vivo migration models. J. Immunol. Methods 96, 11–17 [DOI] [PubMed] [Google Scholar]
- 11. Zimmerli W., Seligmann B., Gallin J. I. (1986) Exudation primes human and guinea pig neutrophils for subsequent responsiveness to the chemotactic peptide N-formylmethionylleucylphenylalanine and increases complement component C3bi receptor expression. J. Clin. Invest. 77, 925–933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kuhns D. B., Gallin J. I. (1995) Increased cell-associated IL-8 in human exudative and A23187-treated peripheral blood neutrophils. J. Immunol. 154, 6556–6562 [PubMed] [Google Scholar]
- 13. Sengeløv H., Follin P., Kjeldsen L., Lollike K., Dahlgren C., Borregaard N. (1995) Mobilization of granules and secretory vesicles during in vivo exudation of human neutrophils. J. Immunol. 154, 4157–4165 [PubMed] [Google Scholar]
- 14. Borregaard N., Sorensen O. E., Theilgaard-Monch K. (2007) Neutrophil granules: a library of innate immunity proteins. Trends Immunol. 28, 340–345 [DOI] [PubMed] [Google Scholar]
- 15. Theilgaard-Mönch K., Knudsen S., Follin P., Borregaard N. (2004) The transcriptional activation program of human neutrophils in skin lesions supports their important role in wound healing. J. Immunol. 172, 7684–7693 [DOI] [PubMed] [Google Scholar]