IRW Bone marrow progenitors express fewer IL-5α receptors and elevated levels of soluble non-signaling receptor which may contribute to diminished eosinophil differentiation.
Keywords: cytokine, allergy, mast cells, bone marrow
Abstract
We examine the proliferation and differentiation of bone marrow (BM) progenitors from inbred Rocky Mountain White (IRW) mice, a strain used primarily for retrovirus infection studies. In contrast to findings with BALB/c and C57BL/6 strains, IRW BM cells cannot proliferate or generate pure eosinophil cultures ex vivo in response to a defined cytokine regimen. Analysis of IRW BM at baseline was unremarkable, including 0.08 ± 0.03% Lin–Sca-1+c-kit+ (LSK) hematopoietic stem cells and 5.2 ± 0.3% eosinophils; the percentage of eosinophil progenitors (EoPs; Lin–Sca-1–c-kit+CD34+IL-5Rα+) was similar in all three mouse strains. Transcripts encoding GM-CSFRα and the IL-3/IL-5/GM-CSF common β chain were detected at equivalent levels in IRW and BALB/c BM, whereas expression of transcripts encoding IL-5Rα, IL-3Rα, and GATA-2 was diminished in IRW BM compared with BALB/c. Expression of membrane-bound IL-5Rα and intracellular STAT5 proteins was also diminished in IRW BM cells. Diminished expression of transcripts encoding IL-5Rα and GATA-2 and immunoreactive STAT5 in IRW BM persisted after 4 days in culture, along with diminished expression of GATA-1. Western blot revealed that cells from IRW BM overexpress nonsignaling soluble IL-5Rα protein. Interestingly, OVA sensitization and challenge resulted in BM and airway eosinophilia in IRW mice; however, the responses were significantly blunted. These results suggest that IRW mice have diminished capacity to generate eosinophils in culture and in vivo, likely as a result of diminished signaling via IL-5Rα.
Introduction
We have recently described a method via which mouse eosinophils are generated ex vivo from unselected BM progenitors [1–3]. We have successfully generated eosinophils using this method (bmEos) from BALB/c and C57BL/6, as well as gene-deleted mice, on these backgrounds. As part of this approach, BM cells are cultured for 4 days in SCF and FLT3L and then IL-5 alone thereafter. FLT3L has been characterized as an agent that promotes the growth of primitive HSCs [4], and SCF, likewise, supports progenitor growth [5]. IL-5 is a Th2 cytokine, which is widely recognized for its ability to promote eosinophilia, complete with proliferation, migration, and activation. IL-5 exerts its influence via a heterodimeric receptor consisting of a unique IL-5Rα subunit and a common βc, which is shared with the IL-3 and GM-CSF heterodimeric receptors [6]. Interestingly, alternative splicing of the IL-5Rα mRNA [7–9] leads to the production of an intact transmembrane-bound receptor (60 kDa), which transduces signal via the JAK2-STAT5 pathway [10], and a smaller, soluble decoy receptor (40 kDa), which does not signal and therefore, effectively reduces the bioavailability of IL-5 [11]. The bmEos, produced from unselected BM progenitors, have polymorphic nuclei and cytoplasmic granules; express immunoreactive major basic protein, Siglec F, and IL-5Rα; can undergo chemotaxis in response to eotaxin-1 [12]; and degranulate in response to platelet-activating factor and related phospholipid mediators [12].
As part of an extensive exploration of the responses of mouse bmEos from TLR gene-deleted mice, we examined TLR7 gene-deleted mice on the IRW background [13], together with an IRW WT control. IRW mice first appeared in the literature in 1983 as an original line generated by Chesebro and colleagues [14]. Although there are few additional details regarding the characterization of these mice, they have been maintained as an inbred colony at RML, NIAID (Hamilton, MT, USA), and have been used extensively by researchers at this site for studies focused mainly on retrovirus infection and pathogenesis [14–24].
We report here our findings on the unusual nature and unique hematopoietic responses of the BM progenitors from IRW mice.
MATERIALS AND METHODS
Mice
Six- to 8-week-old WT BALB/c and C57BL/6 mice were purchased from Taconic Farms (Rockville, MD, USA). Breeding pairs and single male mice of the IRW strain were obtained from RML, NIAID, and were maintained on-site. This study was reviewed, and all protocols were carried out in accordance with the Institute's Animal Care and Use Committee Guidelines (Animal Study Protocol LAD 7E).
Ex vivo culture of eosinophils and mast cells
The method for eosinophil culture from unselected BM progenitors was as described previously [1]. Briefly, the femur and tibia were flushed with RPMI 1640 (Invitrogen Life Technologies, Carlsbad, CA, USA), the RBCs were lysed, and the remaining cells were suspended RPMI 1640 (Invitrogen Life Technologies). BM progenitors were used immediately (freshly isolated BM) or seeded at 106/mL in 10–15 mL media containing RPMI 1640 with 20% FBS (Cambrex, East Rutherford, NJ, USA); 100 IU/mL penicillin and 10 μg/mL streptomycin (Cellgro Mediatech, Manassas, VA, USA); 2 mM glutamine (Invitrogen Life Technologies); 25 mM HEPES, 1× nonessential amino acids, and 1 mM sodium pyruvate (Invitrogen Life Technologies); and 50 μM β-ME (Sigma-Aldrich, St. Louis, MO, USA), supplemented with 100 ng/mL SCF and 100 ng/mL FLT3L (PeproTech, Rocky Hill, NJ, USA); cells were maintained in this medium from Day 0 to Day 4. On Day 4, the medium containing SCF and FLT3L was replaced with fresh medium containing 10 ng/mL rmIL-5 (R&D Systems, Minneapolis, MN, USA). One-half of the media was replaced with fresh cytokine-containing media every other day, and the total cell number was determined by staining with trypan blue and counting on a hemocytometer.
Mouse BMMCs were obtained by culturing the cells for 4–6 weeks in RPMI 1640, supplemented with 10% FBS, glutamine (4 mM), sodium pyruvate (1 mM), penicillin (100 units/mL), streptomycin (100 μg/mL), and nonessential amino acids (Sigma-Aldrich) and HEPES (25 mM), β-ME (50 μM), and rmIL-3 (30 ng/mL; PeproTech), with or without SCF (100 ng/mL), according to previously established protocols [25]. Total cell number was determined each week by counting on a hemocytometer.
OVA sensitization and challenge
Mice were immunized with OVA (Sigma-Aldrich), according to the protocol of Hansen et al. [26], with minor modifications. Briefly, mice were injected i.p. with OVA (50 μg) in aluminum potassium sulfate (Imject Alum, Pierce, Rockford, IL, USA) on Days 1 and 14 and challenged intranasally (100 μg OVA) in sterile PBS on Days 21, 22, and 23. Blood samples were collected prior to start of protocol and again on Day 24. The mice were killed, and BM and BALF were collected on Day 25 as follows: mice were killed by cervical dislocation while under anesthesia and then subjected to BAL with 2× 800 μL 1% BSA/PBS, yielding a total of ∼1 mL/mouse. The RBCs were lysed, the total cell number was obtained from each sample, and slides were prepared as described.
Enumeration of eosinophils
Manual counting was accomplished by generating slides from BM, blood, and BALF samples. Briefly, RBCs were lysed, the cells were counted, and 50,000 cells in 100 μL 0.1% BSA/PBS were placed in a cytofunnel (Thermo Scientific, Waltham, MA, USA) and centrifuged at 500 g for 5 min. The slides were stained with Diff-Quik (Siemens, Newark, DE, USA), as follows, to maximize detection of eosinophils: 5 min in fixative and then 2 min air dry; 3 min in solution 1 (xanthene dye/eosin), followed by three washes in dH2O, and then 2 min air dry; 10 s in solution 2 (counterstain), followed by three washes in dH2O, and then air or blot dry and coverslip with cytoseal-60 (Richard-Allan Scientific, Kalamazoo, MI, USA). Five hundred cells from each slide were counted under high-powered objective, and eosinophils are reported as percentage of total.
Flow cytometry
BM cells were suspended in HBSS (Lonza, Walkersville, MD, USA) at 106/mL and stained with violet live-dead (Invitrogen Life Technologies) for 30 min at 4°C in the dark. After washing in 1% BSA/PBS, IL-5Rα (CD125) expression was examined via detection with the pan-leukocyte marker anti-CD45-Alexa Fluor 700 (clone 30-F11; BD Biosciences, Franklin Lakes, NJ, USA) and anti-IL-5Rα/CD125-Alexa Fluor 488 (clone T21; BD Biosciences) antibodies together with isotype controls. Expression of CD11b (clone M1/70 Alexa Fluor 647 conjugate; BD Biosciences) and Siglec F (clone E50-2440 PE conjugate; BD Biosciences) was examined in a separate experiments. Each antibody was evaluated in the presence of 0.5 μg blocking antibody, anti-CD16/CD32 (clone 2.4G2; BD PharMingen, San Diego, CA, USA), and incubated for 1 h at 4°C in the dark. After washing in BSA/PBS, the cells were fixed overnight in 4% formaldehyde in PBS (Thermo Scientific). One hundred thousand events were collected on an LSRII flow cytometer (BD Biosciences), and the data were analyzed in FlowJo 7.5 (Tree Star, Ashland, OR, USA). Compensation was performed in FlowJo postcollection. All analysis was performed on live cells only, and the data are reported as percentage of live cells.
Analysis of LSK and EoP cells
BM was isolated, erythrocytes were lysed, and the lineage-positive cells were removed using a lineage cell depletion kit (Miltenyi Biotec, Auburn, CA, USA). The Lin– cells (50,000–194,000/107 BM cells) were subjected to staining with anti-CD117/c-kit (Miltenyi Biotec), anti-Sca-1 (Miltenyi Biotec), anti-CD34 (Ram34, BD PharMingen), and anti-CD125/IL-5Rα (T21, BD PharMingen) antibodies. Single- and double-stained cells were run on an LSRII flow cytometer (BD Biosciences), and the data were analyzed in FlowJo 7.5 (Tree Star). Compensation was performed in FlowJo postcollection. Data are reported as percentage of Lin– cells and percentage of total cells evaluated.
RNA isolation and qRT-PCR
RNA samples were isolated from BM using the SuperArray RT2 qPCR-grade RNA isolation kit, per the manufacturer's directions; RNA samples were treated with DNase I as part of the isolation procedure. RNA (2 μg), prepared as described, was reverse-transcribed using a First Strand cDNA synthesis kit for RT-PCR (AMV; Roche Diagnostics, Indianapolis, IN, USA). cDNA (1 μL) was subjected to Taqman qPCR using Fam-labeled probe and primers specific to each indicated mouse transcript. All primer probe sets were purchased from ABI (Carlsbad, CA, USA) and are as follows: IL-5Rα, Mm00434283_m1; IL-3Rα, Mm00434273_m1; GM-CSF-Rα, Mm00438331_g1; common βc, Mm00655745_m1; GATA-1, Mm00484678_m1; GATA-2, Mm00492300_m1. All experiments included no RT, and no template controls and mouse GAPDH (ABI #4308313) were used as the endogenous control. Each transcript at each time-point is normalized to GAPDH to account for variations in initial template and then reported relative to a single, randomly selected BALB/c sample unless indicated.
Whole cell lysates for Western blotting
Freshly isolated BM cells were isolated by centrifugation and incubated in tissue-culture media without rmIL-5 for 6 h prior to stimulation with rmIL-5 (10 ng/mL) for 15 min. Cells were then isolated by centrifugation and resuspended at 3 × 107/mL in RIPA buffer (150 mM NaCl, 0.1% SDS, 1% Nonidet P-40, 0.5% Na-deoxycholate, 5 mM MgCl2, and 40 mM Tris-HCl) with 2 mM EDTA, 250 μM cathepsin G inhibitor I (Calbiochem EMD Chemicals, Gibbstown, NJ, USA), and additional protease and phosphatase inhibitors (Roche Diagnostics; catalog #4693159 and #4906837, respectively). Cell lysates were incubated on ice for 30 min with occasional vortexing. At the end of the incubation period, an equal volume of 2× SDS protein gel-loading solution (Quality Biological, Gaithersburg, MD, USA), containing a 1:20 dilution of β-ME, was added to the cell lysates, and the entire solution heated at 100°C for 10 min. Samples were then stored at –20°C or used directly in experiments.
Western blotting
Samples were run on Tris-glycine gels from 4% to 20% (Invitrogen Life Technologies). After separation by electrophoresis, protein was transferred to nitrocellulose membranes using the iBlot dry-blotting system (Invitrogen Life Technologies). Blots were blocked for 1 h in 5% BSA/TBST and then incubated overnight with specified primary antibody. Antibodies used are as follows: anti-STAT5 (Santa Cruz Biotechnology, Santa Cruz, CA, USA; #835) at 1:1000 dilution; anti-IL-5Rα (R&D Systems; #AF553) at 1:1000 dilution; anti-β-actin (Cell Signaling Technology, Beverly, MA, USA; #4968) at 1:1000 dilution. Blots were also probed with anti-IL-3Rα/CD123 from Santa Cruz Biotechnology (cat. 681), but no signal was detected. Blots were washed 5× with TBST and transferred to the appropriate secondary antibody for 1 h at 4°C (goat anti-rabbit from Cell Signaling Technology used at 1:2000). Blots were washed 5× with TBST and then developed using Western Lightning Plus-ECL (PerkinElmer, Waltham, MA, USA) and BioMax maximum resolution film (Eastman Kodak, Rochester, NY, USA). Densitometry was carried out using NIH Image software [27].
Statistics
Data are reported as representative experiments or mean ± sem. All analysis was performed in GraphPad Prism 5 (GraphPad Software, La Jolla, CA, USA). Statistical significance was determined as noted in each figure legend.
RESULTS
Proliferation and differentiation of IRW BM progenitors are not supported by cytokines ex vivo
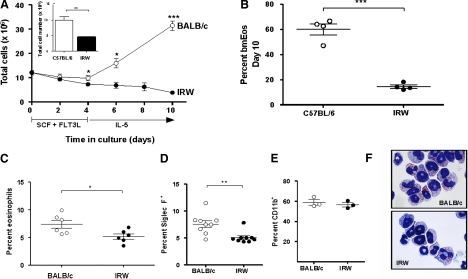
As demonstrated in our earlier publications [1–3, 28], BM cells from inbred BALB/c mice proliferate ex vivo and differentiate into eosinophils when cultured for 4 days in SCF and FLT3L, followed by IL-5 alone thereafter. Typically, BALB/c cultures seeded with 107 unselected BM progenitors will yield ∼3 × 107 phenotypically and functionally mature eosinophils at Day 12 [1]. C57BL/6 cultures seeded with 107 unselected progenitors will yield ∼107 mature eosinophils [3]. bmEos generated from BALB/c or C57BL/6 progenitors can be maintained as viable in culture with IL-5 through Day 21. Fig. 1A illustrates the typical proliferation pattern for BALB/c mice, which includes stable cell numbers through Day 4, followed by rapid expansion after introduction of the eosinophilopoietic cytokine, IL-5. These BALB/c cultures expanded approximately threefold to 31.6 ± 1.9 × 106 cells by Day 10. In contrast, cell numbers in the IRW cultures initially paralleled those of the BALB/c but deviated downward upon the introduction of IL-5. Only 3.9 ± 0.3 × 106 cells were detected at Day 10, notably fewer than the number of cells used to initiate the culture. A similar, diminished growth rate was noted when IRW progenitor cultures were compared with those from C57BL/6 mice (Fig. 1A, inset). Furthermore, Day 10 cultures from C57BL/6 progenitors included 60 ± 4% eosinophils, whereas only 14 ± 1% eosinophils were present in IRW cultures, documenting a minimal response to IL-5 (Fig. 1B). Fewer eosinophils were detected in freshly isolated BM from IRW mice than were detected in BM from BALB/c mice (5.2±0.3% vs. 7.4±0.5%, respectively; P<0.05; Fig. 1C); this fraction is similar to what has been observed in IL-5 gene-deleted and IL-5R gene-deleted mouse strains [29, 30]. Eosinophils were detected in BALB/c and IRW BM, respectively, using anti-Siglec F, a marker that has been used for specific enumeration of eosinophils in this tissue [31] (Fig. 1D). In contrast, no differential detection of cells bearing the granulocyte/monocyte marker, CD11b, was observed (Fig. 1E). Eosinophils from IRW BM are normal in appearance (Fig. 1F), as are the few eosinophils that develop in response to cytokines in culture.
Figure 1. Proliferation and eosinophil differentiation of BM progenitors derived from IRW mice is not supported by cytokines ex vivo.
(A) BALB/c-derived BM cells (○) proliferate ex vivo in response to SCF, FLT3L, and IL-5; IRW-derived BM cells (●) do not. Shown are pooled data from six experiments. (Inset) Total cells at Day 10 from C57BL/6- and IRW-derived BM cells; n = 4; *P < 0.05; **P < 0.01; ***P < 0.001, unpaired t test. (B) Percent eosinophils at Day 10 in cultures from BM isolated from IRW and C57BL/6 mice. Each point represents an individual mouse. (C) Percent eosinophils in freshly isolated BM from IRW and BALB/c mice determined by visual inspection of stained cells; each point represents an individual mouse. (D) Percent Siglec F+ cells in freshly isolated BM; each point represents an individual mouse. (E) As in D; percent CD11b+ cells. (F) Cultured bmEos (Day 10) from progenitors from BALB/c (upper panel) and IRW mice (lower panel); original magnification, 100×.
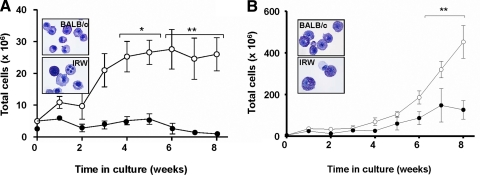
IRW BM cells do not proliferate in response to IL-3, culture conditions that are used to generate BMMCs [25] (Fig. 2A). Although markedly less responsive than BALB/c BM, IRW progenitors can proliferate in culture response to IL-3 and SCF (Fig. 2B). The mast cells generated from the IRW cultures were clearly granulated and stained normally with toluidine blue. Tissue mast cells were also detected in toluidine blue-stained skin sections from IRW mice (data not shown).
Figure 2. Mast cell proliferation ex vivo is diminished in BM progenitor cultures derived from IRW mice.
BM cells from IRW mice (●; A) do not proliferate in response to rmIL-3 (30 ng/mL) alone and (B) respond, but less effectively than BM cells isolated from C57BL/6 mice to rmIL-3 and SCF (100 ng/mL) (○). (Insets) Phenotypically mature mast cells stained with toluidine blue; shown are cells from Week 4 cultures generated with rmIL-3 alone or rmIL-3 and SCF. Data pooled from three independent experiments; *P < 0.05; **P < 0.01.
Evaluation of the HSCs in IRW mice
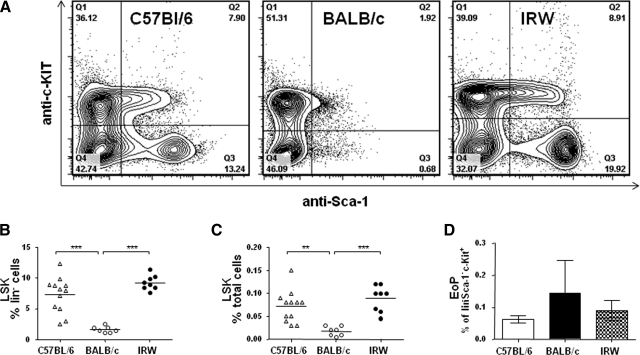
Isolated Lin– cells were evaluated for the expression of the progenitor markers Sca-1 and c-kit [32, 33]. In IRW mice, the HSCs (LSK cells) represented 0.09 ± 0.01% of the total cells in the BM, a range indistinguishable from that determined for HSCs from C57BL/6 mice (0.07±0.01%). The HSC population represents a significantly smaller fraction in BALB/c BM (0.02±0.004), primarily as a result of the diminished Lin–Sca-1+ population (Fig. 3A–C). BM cells were evaluated for EoPs [34] (Lin–Sca-1–ckit+CD34+IL-5Rα+); no quantitative differences were detected among the three strains evaluated (Fig. 3D).
Figure 3. Analysis of BM LSK HSCs.
(A) Representative contour plots of Lin– cells isolated from BM of C57BL/6, BALB/c, and IRW mice and probed with anti-Sca-1 and anti-c-kit antibodies. HSCs (LSK) identified as (B) percent of Lin– cells and (C) percent of total BM. Each point represents an individual mouse. **P < 0.01; ***P < 0.001 by ANOVA with Bonferroni post-test. (D) EoPs identified as a percent of Lin–Sca-1–c-kit+ cells (Q1, panel A); n = 3–4 mice/strain.
Expression of cytokine receptors, transcription factors, and signaling molecules that promote eosinophil proliferation and differentiation
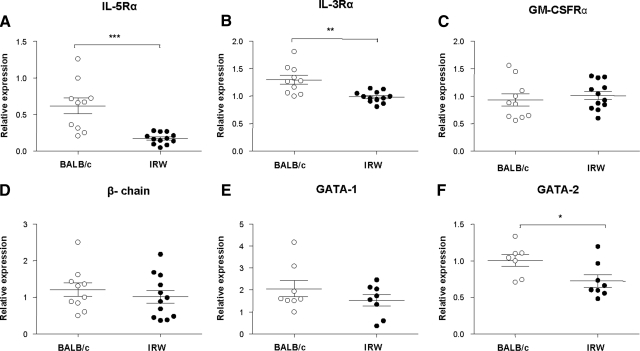
Transcripts encoding eosinophil-related transcription factors GATA-1 and GATA-2 [35, 36] and cytokine receptor subunits, IL-3Rα, IL-5Rα, GM-CSFRα, and the common βc, were detected by qRT-PCR in RNA prepared from BM from BALB/c and IRW mice (Fig. 4). Whereas transcripts encoding the GM-CSFRα, the common βc, and GATA-1 were expressed at levels that were indistinguishable from one another, expression levels of GATA-2, IL-5Rα, and IL-3Rα were diminished in IRW BM cells compared with BALB/c. After 4 days of culture with SCF and FLT3L, diminished expression of transcripts encoding IL-5Rα and GATA-2 persists; we likewise observed differential expression GATA-1 at this time (Supplemental Fig. 1).
Figure 4. Relative expression of cytokine receptor subunits and transcription factors in BM progenitor cells of BALB/c and IRW mice.
(A) IL-5Rα; (B) IL-3Rα; (C) GM-CSFRα; (D) common βc; and (E) GATA-1 and (F) GATA-2 transcription factors. Each point represents results for an individual mouse; *P < 0.05; **P < 0.01; ***P < 0.001, unpaired t test.
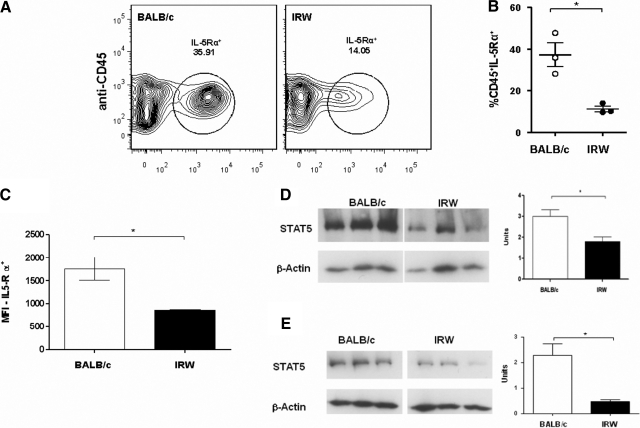
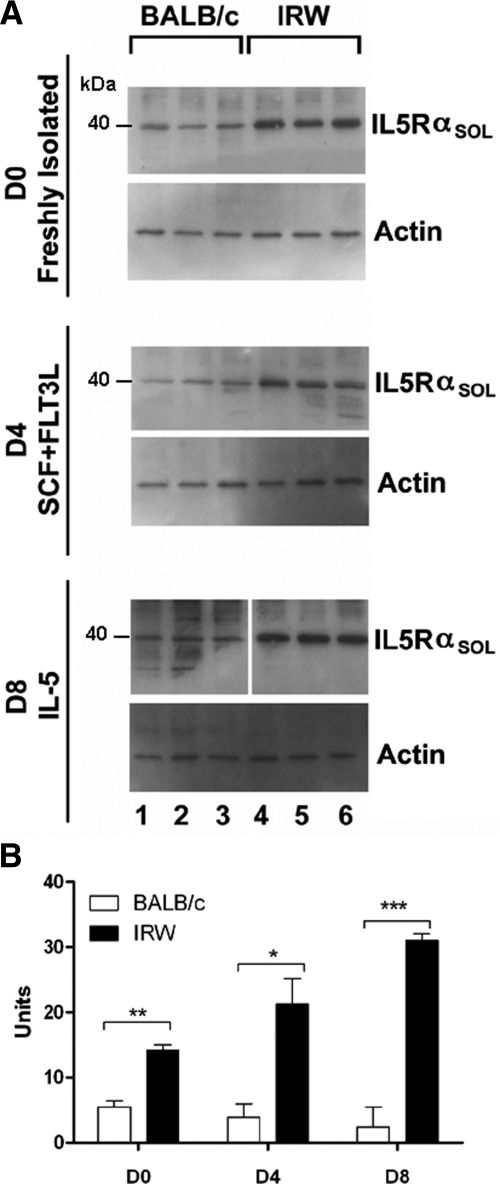
Surface expression of IL-5Rα was diminished in freshly isolated BM cells from IRW mice when compared with those from BALB/c. Not only were there fewer IL-5Rα+ cells in IRW BM (Fig. 5A and B), but also, the number of IL-5Rα+ surface receptors per cell was diminished, as inferred from geometric MFI measurements (Fig. 5C). Similarly, expression of the intracellular signaling molecule STAT5 was reduced in IRW progenitors (Fig. 5D), and diminished expression of STAT5 persists after 4 days in culture (Fig. 5E). Expression of the 40-kDa soluble form of IL-5Rα, a nonsignaling receptor variant, is elevated in BM precursors isolated from IRW mice and remains so through Day 8 in culture (Fig. 6), suggesting a mechanism to explain why these progenitors are and remain unresponsive to IL-5.
Figure 5. Expression of immunoreactive IL-5Rα and STAT5 in BM cells from IRW and BALB/c mice.
Surface expression of IL-5Rα. (A) Representative contour plots of BALB/c and IRW BM cells probed with anti-CD45 and anti-IL-5Rα. (B) Percent CD45+IL-5Rα+ cells in BM of IRW and BALB/c mice; *P < 0.05, unpaired t test. (C) Relative MFI determined for BM cells isolated from BALB/c and IRW mice; n = 3 mice/sample. (D) Western analysis of lysates from freshly isolated BM cells from BALB/c and IRW mice; each lane contains protein from an individual mouse. Lanes shown are from a single nitrocellulose membrane with intervening lanes removed. On the right, densitometry; units on y-axis refer to signal from anti-STAT5 antibody divided by signal from anti-β-actin antibody; n = 3 mice. (E) As in D; analysis of lysates from BM cells in culture, Day 4.
Figure 6. Detection of soluble IL-5Rα in BM cells.
(A) Protein was extracted from freshly isolated BM of BALB/c mice (lanes 1–3) and IRW mice (lanes 4–6) and from BM cells that have been cultured for 4 or 8 days (D4 or D8, respectively) in the cytokines indicated. Western blots were probed with anti-IL-5Rα (IL5Rαsol) and antiactin antibodies as indicated. (B) Results of densitometry of Westerns are shown in the graph. Each set of samples was run on the same membrane with intervening marker lanes excised as indicated. *P < 0.05; **P < 0.01; ***P < 0.001.
OVA sensitization and challenge in IRW mice
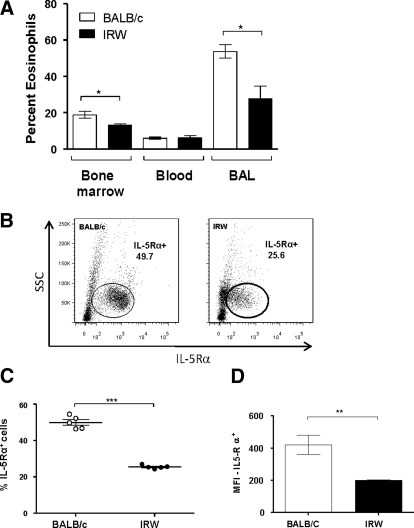
The responses of IRW and BALB/c mice to OVA sensitization and challenge were determined by quantitative assessment of eosinophilia in BM, blood, and BALF. Although IRW BM progenitors do not proliferate and differentiate into eosinophils in response to cytokines in culture, IRW mice are capable of generating BM and tissue eosinophilia in vivo. BALB/c and IRW mice respond to OVA sensitization and challenge with an approximate threefold expansion of the BM eosinophil pool, each from its own initial baseline (data not shown). Eosinophils are recruited to the airways, although significantly fewer are detected in BM and BALF of IRW mice (Fig. 7A). Differential expression of IL-5Rα on BM progenitors from IRW and BALB/c mice persists during this heightened Th2 state (Fig. 7B and C), likewise, with reduced expression of IL-5Rα per cell, as inferred from MFI (Fig. 7D).
Figure 7. OVA sensitization and challenge elicits eosinophilia in IRW and BALB/c mice.
(A) Percent eosinophils in tissue from OVA-sensitized and -challenged mice, as determined by Diff-Quik staining and visual inspection. There was no difference in the total cells isolated from the BALF of BALB/c and IRW mice following OVA sensitization and challenge; *P < 0.05, unpaired t test. (B) Surface expression of IL-5Rα in BM of OVA-sensitized and -challenged BALB/c and IRW mice; representative contour plots probed anti-IL-5Rα. SSC, Side-scatter. (C) Percent IL-5Rα+ cells isolated from the BM of OVA-sensitized and -challenged BALB/c (○) and IRW (●) mice; n = 5; ***P < 0.001, unpaired t test. (D) MFI for IL-5Rα+ cells isolated from BM from OVA-sensitized and -challenged BALB/c and IRW mice; **P < 0.01; unpaired t test.
DISCUSSION
Tissue-culture models are crucial to our understanding of the hematopoietic process [37]. Discrepancies between events occurring in vivo and those occurring in tissue culture can yield intriguing insights into mechanisms of biological control. One example of this is our study of cultured BM progenitors from ΔdblGATA mice, which have undergone ablation of a palindromic enhancer in the hematopoietic promoter of the gene encoding GATA-1 and are serendipitously devoid of eosinophils in vivo [38]. We found that BM progenitors in culture with SCF, FLT3L followed by GM-CSF, IL-3, and IL-5 (a protocol that differs from the one used here) were capable of generating eosinophils (6–7% of total cells) by using a proximal promoter, one whose activity is apparently suppressed in vivo [39].
In the study reported here, we use a cytokine-induced growth protocol that is designed to augment progenitor proliferation and to promote eosinophil commitment and differentiation. Using unselected BM cells from BALB/c mice, we typically observe a threefold expansion and 100% eosinophils at Days 12–14 in culture [1]. Although proliferation is not as robust, BM cells from C57BL/6 mice likewise undergo differentiation, and the culture yields 100% eosinophils by Days 12–14 in response to this cytokine regimen. In contrast, BM cells from IRW mice are unable to proliferate or differentiate under these defined conditions. We have determined that BM cells from IRW mice have diminished numbers of IL-5Rα-positive cells, express fewer IL-5Rs per cell, and overexpress soluble IL-5Rα, a nonsignaling splice variant, which may function as a decoy receptor. Although IL-5 can bind to the soluble receptor [11, 40], and this receptor has been detected in vivo in association with human disease [41–43], its physiologic role as a modulator of IL-5 bioavailability remains unproven. Nonetheless, one or the combination of these mechanisms may serve to reduce the impact of exogenous IL-5 and prevent the signals necessary to promote differentiation into the eosinophilic phenotype in response to these cytokines.
In this study, we examined the populations of HSCs or LSK cells (defined by the cell-surface profile LSK) and committed EoPs (Lin–Sca-1–ckit+CD34+IL-5Rα+) at baseline in BM progenitors of IRW, BALB/c, and C57BL/6 mice. Interestingly, we detected fewer LSK cells among the BM progenitors in the BALB/c strain, demonstrating that the BALB/c, not the IRW strain, is effectively the “outlier” (Fig. 3A). The progenitor populations in the IRW mice are quantitatively indistinguishable from those in the C57BL/6 mice; the latter strain responds effectively to the defined cytokine regimen and generates eosinophils in culture [2, 3]. Although the analysis of LSK cells and EoPs in the IRW BM did not provide any specific clues regarding eosinophil hematopoiesis in this mouse strain, it will be intriguing to explore the contributions of these progenitor populations to cytokine-driven eosinophil hematopoiesis in our routine protocols.
Interestingly, despite the differential expression of IL-5Rα subunits, overexpression of the soluble IL-5Rα decoy receptor in BM progenitors at baseline, and diminished capacity to respond to IL-5 ex vivo, IRW progenitors can clearly proliferate and generate eosinophils in vivo; it remains intriguing and unclear exactly what the nature of the in vivo compensation mechanism might be. Our results with the mast cell cultures suggest that response to SCF may be one contributing factor. IRW BM progenitors include a typical percentage of c-kit+ LSK HSCs, comparable with C57BL/6 mice, and are clearly capable of responding to SCF; for example, IRW BM progenitors do not proliferate in response IL-3 alone but do so in response to IL-3 in the presence of SCF. Interestingly, Metcalf and colleagues [5] implicate SCF as an eosinophilopoietic cytokine in vivo, with actions that are independent of the IL-3/IL-5/GM-CSF/common βc type I cytokine receptors. Klein and colleagues [44, 45] and Finotto and colleagues [46] have documented the production of SCF in lung tissue in response to OVA sensitization and challenge, as well as a role in eosinophil kinetics. The presence of SCF, IL-5, and IL-3, coincidentally, during allergen challenge in vivo, may compensate for the limited capacity of IRW progenitors to respond to IL-5 alone.
In summary, the BM progenitors from IRW mice have several unique properties. They are unable to proliferate or differentiate appropriately in response to conditions defined for eosinophil culture and do not proliferate effectively under conditions defined for the generation of BMMCs. Although eosinophils are present in IRW BM, we observe diminished expression of IL-5Rα, fewer receptors per cell, and overexpression of the soluble IL-5Rα decoy receptor, which overall, may result in a global reduction in the ability to respond effectively to the eosinophilopoietic cytokine, IL-5. Interestingly, despite the inability of these progenitors to proliferate in response to IL-5 ex vivo, OVA sensitization and challenge resulted in eosinophilia in IRW mice. These results suggest that there are compensation mechanisms in vivo, the nature and specificity of which are worthy of further exploration.
Supplementary Material
ACKNOWLEDGMENTS
This work is supported by NIAID, Division of Intramural Research Funding, #AI000941 and #AI000965. The authors are grateful for the assistance of Mr. Ricardo Dreyfuss of Medical Arts, who prepared the photomicrographs for publication.
The online version of this paper, found at www.jleukbio.org, includes supplemental information.
- βc
- β-chain
- ABI
- Applied Biosystems
- BALF
- BAL fluid
- BM
- bone marrow
- bmEos
- method via which mouse eosinophils are generated ex vivo from unselected bone marrow progenitors
- BSA/PBS
- BSA in PBS
- dH2O
- distilled water
- EoP
- eosinophil progenitor
- FLT3L
- fetal liver tyrosine kinase-3 ligand
- HSC
- hematopoietic stem cell
- IRW
- inbred Rocky Mountain White
- Lin–
- lineage-negative
- LSK
- Lin–Sca-1+c-kit+
- MFI
- mean fluorescence intensity
- qRT-PCR
- quantitative RT-PCR
- rm
- recombinant mouse
- RML, NIAID
- Rocky Mountain Laboratories, National Institute of Allergy and Infectious Diseases
- Sca-1
- stem cell antigen-1
- SCF
- stem cell factor
AUTHORSHIP
K.D.D. conceived of and performed the BM eosinophil differentiation experiments and prepared an initial draft of the paper. K.E.G-C. performed many of the qPCR experiments and provided editorial advice on the final draft of the paper. C.M.P. provided technical assistance on many aspects of the research and provided editorial advice on the final draft of the paper. A.B.B. provided technical assistance with the Western analysis and provided editorial advice on the final draft of the paper. T.I. performed the BMMC differentiation experiments and provided editorial advice on the final draft of the paper. K.E.P. provided IRW mice and insight into their use, as well as providing editorial advice on the final draft of the paper. A.M.G. provided assistance with the BMMC differentiation experiments and provided editorial advice on the final draft of the paper. H.F.R. provided oversight on the design and execution of the project and preparation of the manuscript.
REFERENCES
- 1. Dyer K. D., Moser J. M., Czapiga M., Siegel S. J., Percopo C. M., Rosenberg H. F. (2008) Functionally competent eosinophils differentiated ex vivo in high purity from normal mouse bone marrow. J. Immunol. 181, 4004–4009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dyer K. D., Percopo C. M., Fischer E. R., Gabryszewski S. J., Rosenberg H. F. (2009) Pneumoviruses infect eosinophils and elicit MyD88-dependent release of chemoattractant cytokines and interleukin-6. Blood 114, 2649–2656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dyer K. D., Percopo C. M., Rosenberg H. F. (2009) Generation of eosinophils from unselected bone marrow progenitors: wild-type, TLR- and eosinophil-deficient mice. Open Immunol. J. 2, 163–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hudak S., Hunte B., Culpepper J., Menon S., Hannum C., Thompson-Snipes L., Rennick D. (1995) FLT3/FLK2 ligand promotes the growth of murine stem cells and the expansion of colony-forming cells and spleen colony-forming units. Blood 85, 2747–2755 [PubMed] [Google Scholar]
- 5. Metcalf D., Mifsud S., Di Rago L. (2002) Stem cell factor can stimulate the formation of eosinophils by two types of murine eosinophil progenitor cells. Stem Cells 20, 460–469 [DOI] [PubMed] [Google Scholar]
- 6. Miyajima A., Mui A. L., Ogorochi T., Sakamaki K. (1993) Receptors for granulocyte-macrophage colony-stimulating factor, interleukin-3, and interleukin-5. Blood 82, 1960–1974 [PubMed] [Google Scholar]
- 7. Takaki S., Tominaga A., Hitoshi Y., Mita S., Sonoda E., Yamaguchi N., Takatsu K. (1990) Molecular cloning and expression of the murine interleukin-5 receptor. EMBO J. 9, 4367–4374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Murata Y., Takaki S., Migita M., Kikuchi Y., Tominaga A., Takatsu K. (1992) Molecular cloning and expression of the human interleukin 5 receptor. J. Exp. Med. 175, 341–351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Imamura F., Takaki S., Akagi K., Ando M., Yamamura K., Takatsu K., Tominaga A. (1994) The murine interleukin-5 receptor α-subunit gene: characterization of the gene structure and chromosome mapping. DNA Cell Biol. 13, 283–292 [DOI] [PubMed] [Google Scholar]
- 10. Mui A. L., Wakao H., Harada N., O'Farrell A. M., Miyajima A. (1995) Interleukin-3, granulocyte-macrophage colony-stimulating factor, and interleukin-5 transduce signals through two forms of STAT5. J. Leukoc. Biol. 57, 799–803 [DOI] [PubMed] [Google Scholar]
- 11. Monahan J., Siegel N., Keith R., Caparon M., Christine L., Compton R., Cusik S., Hirsch J., Huynh M., Devine C., Polazzi J., Rangwala S., Tsai B., Portanova J. (1997) Attenuation of IL-5-mediated signal transduction, eosinophil survival, and inflammatory mediator release by a soluble human IL-5 receptor. J. Immunol. 159, 4024–4034 [PubMed] [Google Scholar]
- 12. Dyer K. D., Percopo C. M., Xie Z., Yang Z., Kim J. D., Davoine F., Lacy P., Druey K. M., Moqbel R., Rosenberg H. F. (2010) Mouse and human eosinophils degranulate in response to platelet-activating factor (PAF) and lysoPAF via a PAF-receptor-independent mechanism: evidence for a novel receptor. J. Immunol. 184, 6327–6334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Butchi N. B., Pourciau S., Du M., Morgan T. W., Peterson K. E. (2008) Analysis of the neuroinflammatory response to TLR7 stimulation in the brain: comparison of multiple TLR7 and/or TLR8 agonists. J. Immunol. 180, 7604–7612 [DOI] [PubMed] [Google Scholar]
- 14. Chesebro B., Portis J. L., Wehrly K., Nishio J. (1983) Effect of murine host genotype on MCF virus expression, latency, and leukemia cell type of leukemias induced by Friend murine leukemia helper virus. Virology 128, 221–233 [DOI] [PubMed] [Google Scholar]
- 15. Bessen R. A., Lynch W. P., Portis J. L. (1995) Inhibition of murine retrovirus-induced neurodegeneration in the spinal cord by explant culture. J. Virol. 69, 7300–7303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Buller R. S., Ahmed A., Portis J. L. (1987) Identification of two forms of an endogenous murine retroviral env gene linked to the Rmcf locus. J. Virol. 61, 29–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Buller R. S., Sitbon M., Portis J. L. (1988) The endogenous mink cell focus-forming (MCF) gp70 linked to the Rmcf gene restricts MCF virus replication in vivo and provides partial resistance to erythroleukemia induced by Friend murine leukemia virus. J. Exp. Med. 167, 1535–1546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Buller R. S., Van Zant G., Eldridge P. W., Portis J. L. (1989) A population of murine hematopoietic progenitors expresses an endogenous retroviral gp70 linked to the Rmcf gene and associated with resistance to erythroleukemia. J. Exp. Med. 169, 865–880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Buller R. S., Wehrly K., Portis J. L., Chesebro B. (1990) Host genes conferring resistance to a central nervous system disease induced by a polytropic recombinant Friend murine retrovirus. J. Virol. 64, 493–498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Du M., Butchi N. B., Woods T., Morgan T. W., Peterson K. E. (2010) Neuropeptide Y has a protective role during murine retrovirus-induced neurological disease. J. Virol. 84, 11076–11088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Peterson K. E., Evans L. H., Wehrly K., Chesebro B. (2006) Increased proinflammatory cytokine and chemokine responses and microglial infection following inoculation with neural stem cells infected with polytropic murine retroviruses. Virology 354, 143–153 [DOI] [PubMed] [Google Scholar]
- 22. Portis J. L., Czub S., Garon C. F., McAtee F. J. (1990) Neurodegenerative disease induced by the wild mouse ecotropic retrovirus is markedly accelerated by long terminal repeat and gag-pol sequences from nondefective Friend murine leukemia virus. J. Virol. 64, 1648–1656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Portis J. L., McAtee F. J., Hayes S. F. (1987) Horizontal transmission of murine retroviruses. J. Virol. 61, 1037–1044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sitbon M., Sola B., Evans L., Nishio J., Hayes S. F., Nathanson K., Garon C. F., Chesebro B. (1986) Hemolytic anemia and erythroleukemia, two distinct pathogenic effects of Friend MuLV: mapping of the effects to different regions of the viral genome. Cell 47, 851–859 [DOI] [PubMed] [Google Scholar]
- 25. Jensen B. M., Swindle E. J., Iwaki S., Gilfillan A. M. (2006) Generation, isolation, and maintenance of rodent mast cells and mast cell lines. Curr. Protoc. Immunol. Sept., Chapter 3, Unit 3.23 [DOI] [PubMed] [Google Scholar]
- 26. Hansen G., Berry G., DeKruyff R. H., Umetsu D. T. (1999) Allergen-specific Th1 cells fail to counterbalance Th2 cell-induced airway hyperreactivity but cause severe airway inflammation. J. Clin. Invest. 103, 175–183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Abramoff M. D., Magalhaes P. J., Ram S. J. (2004) Image processing with ImageJ. Biophotonics International 11, 36–42 [Google Scholar]
- 28. Dyer K. D., Percopo C. M., Xie Z., Yang Z., Kim J. D., Davoine F., Lacy P., Druey K. M., Moqbel R., Rosenberg H. F. (2010) Mouse and human eosinophils degranulate in response to platelet-activating factor (PAF) and lysoPAF via a PAF-receptor-independent mechanism: evidence for a novel receptor. J. Immunol. 184, 6327–6334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kopf M., Brombacher F., Hodgkin P. D., Ramsay A. J., Milbourne E. A., Dai W. J., Ovington K. S., Behm C. A., Kohler G., Young I. G., Matthaei K. I. (1996) IL-5-deficient mice have a developmental defect in CD5+ B-1 cells and lack eosinophilia but have normal antibody and cytotoxic T cell responses. Immunity 4, 15–24 [DOI] [PubMed] [Google Scholar]
- 30. Yoshida T., Ikuta K., Sugaya H., Maki K., Takagi M., Kanazawa H., Sunaga S., Kinashi T., Yoshimura K., Miyazaki J., Takaki S., Takatsu K. (1996) Defective B-1 cell development and impaired immunity against Angiostrongylus cantonensis in IL-5R α-deficient mice. Immunity 4, 483–494 [DOI] [PubMed] [Google Scholar]
- 31. Dyer K. D., Garcia-Crespo K. E., Killoran K. E., Rosenberg H. F. (2011) Antigen profiles for the quantitative assessment of eosinophils in mouse tissues by flow cytometry. J. Immunol. Methods 369, 91–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cavazzana-Calvo M., Fischer A., Bushman F. D., Payen E., Hacein-Bey-Abina S., Leboulch P. (2011) Is normal hematopoiesis maintained solely by long-term multipotent stem cells? Blood 117, 4420–4424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wilson A., Laurenti E., Trumpp A. (2009) Balancing dormant and self-renewing hematopoietic stem cells. Curr. Opin. Genet. Dev. 19, 461–468 [DOI] [PubMed] [Google Scholar]
- 34. Iwasaki H., Mizuno S., Mayfield R., Shigematsu H., Arinobu Y., Seed B., Gurish M. F., Takatsu K., Akashi K. (2005) Identification of eosinophil lineage-committed progenitors in the murine bone marrow. J. Exp. Med. 201, 1891–1897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hirasawa R., Shimizu R., Takahashi S., Osawa M., Takayanagi S., Kato Y., Onodera M., Minegishi N., Yamamoto M., Fukao K., Taniguchi H., Nakauchi H., Iwama A. (2002) Essential and instructive roles of GATA factors in eosinophil development. J. Exp. Med. 195, 1379–1386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Iwasaki H., Mizuno S., Arinobu Y., Ozawa H., Mori Y., Shigematsu H., Takatsu K., Tenen D. G., Akashi K. (2006) The order of expression of transcription factors directs hierarchical specification of hematopoietic lineages. Genes Dev. 20, 3010–3021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gauvreau G. M., Ellis A. K., Denburg J. A. (2009) Haemopoietic processes in allergic disease: eosinophil/basophil development. Clin. Exp. Allergy 39, 1297–1306 [DOI] [PubMed] [Google Scholar]
- 38. Yu C., Cantor A. B., Yang H., Browne C., Wells R. A., Fujiwara Y., Orkin S. H. (2002) Targeted deletion of a high-affinity GATA-binding site in the GATA-1 promoter leads to selective loss of the eosinophil lineage in vivo. J. Exp. Med. 195, 1387–1395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Dyer K. D., Czapiga M., Foster B., Foster P. S., Kang E. M., Lappas C. M., Moser J. M., Naumann N., Percopo C. M., Siegel S. J., Swartz J. M., Ting-De Ravin S., Rosenberg H. F. (2007) Eosinophils from lineage-ablated Δ dblGATA bone marrow progenitors: the dblGATA enhancer in the promoter of GATA-1 is not essential for differentiation ex vivo. J. Immunol. 179, 1693–1699 [DOI] [PubMed] [Google Scholar]
- 40. Devos R., Guisez Y., Cornelis S., Verhee A., Van der Heyden J., Manneberg M., Lahm H. W., Fiers W., Tavernier J., Plaetinck G. (1993) Recombinant soluble human interleukin-5 (hIL-5) receptor molecules. Cross-linking and stoichiometry of binding to IL-5. J. Biol. Chem. 268, 6581–6587 [PubMed] [Google Scholar]
- 41. Gevaert P., Bachert C., Holtappels G., Novo C. P., Van der Heyden J., Fransen L., Depraetere S., Walter H., van Cauwenberge P., Tavernier J. (2003) Enhanced soluble interleukin-5 receptor α expression in nasal polyposis. Allergy 58, 371–379 [DOI] [PubMed] [Google Scholar]
- 42. Liu L. Y., Sedgwick J. B., Bates M. E., Vrtis R. F., Gern J. E., Kita H., Jarjour N. N., Busse W. W., Kelly E. A. (2002) Decreased expression of membrane IL-5 receptor α on human eosinophils: I. Loss of membrane IL-5 receptor α on airway eosinophils and increased soluble IL-5 receptor α in the airway after allergen challenge. J. Immunol. 169, 6452–6458 [DOI] [PubMed] [Google Scholar]
- 43. Rohde G., Gevaert P., Holtappels G., Fransen L., Borg I., Wiethege A., Arinir U., Tavernier J., Schultze-Werninghaus G., Bachert C. (2004) Soluble interleukin-5 receptor α is increased in acute exacerbation of chronic obstructive pulmonary disease. Int. Arch. Allergy Immunol. 135, 54–61 [DOI] [PubMed] [Google Scholar]
- 44. Klein A., Talvani A., Cara D. C., Gomes K. L., Lukacs N. W., Teixeira M. M. (2000) Stem cell factor plays a major role in the recruitment of eosinophils in allergic pleurisy in mice via the production of leukotriene B4. J. Immunol. 164, 4271–4276 [DOI] [PubMed] [Google Scholar]
- 45. Klein A., Talvani A., Silva P. M., Martins M. A., Wells T. N., Proudfoot A., Luckacs N. W., Teixeira M. M. (2001) Stem cell factor-induced leukotriene B4 production cooperates with eotaxin to mediate the recruitment of eosinophils during allergic pleurisy in mice. J. Immunol. 167, 524–531 [DOI] [PubMed] [Google Scholar]
- 46. Finotto S., Buerke M., Lingnau K., Schmitt E., Galle P. R., Neurath M. F. (2001) Local administration of antisense phosphorothioate oligonucleotides to the c-kit ligand, stem cell factor, suppresses airway inflammation and IL-4 production in a murine model of asthma. J. Allergy Clin. Immunol. 107, 279–286 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.