Review of the mechanisms by which external factors, such as alcohol, cigarette smoke, air pollution, and others impact macrophage phagocytosis.
Keywords: cigarette smoke, alcohol, diesel exhaust, ozone
Abstract
The ability of a pathogen to evade host immunity successfully, in contrast to the host's capacity to defend itself against a foreign invader, is a complex struggle, in which eradication of infection is dictated by a robust immunologic response. Often, there are external factors that can alter the outcome by tipping the scale to benefit pathogen establishment rather than resolution by the host's defense system. These external sources, such a cigarettes, alcohol, or environmental pollutants, can negatively influence the effectiveness of the immune system's response to a pathogen. The observed suppression of immune function can be attributed to dysregulated cytokine and chemokine production, the loss of migratory potential, or the inability to phagocytose pathogens by immune cells. This review will focus on the mechanisms involved during the toxin-induced suppression of phagocytosis. The accumulated data support the importance of studying the mechanisms of phagocytosis following exposure to these factors, in that this effect alone cannot only leave the host susceptible to infection but also promote alterations in many other macrophage functions necessary for pathogen clearance and restoration of homeostasis.
Introduction
The importance in understanding macrophage function is underscored when considering the role of this multifaceted cell type in initiating an inflammatory response against a pathogen, including ingesting pathogens, presenting of antigen, stimulating other immune cells, and at the conclusion of an inflammatory response, clearing immune cells and debris. One component central to these functions is phagocytosis. Initiation of phagocytosis has been shown to induce cytokine release, promoting an inflammatory response to a pathogen, and allows antigen presentation to coordinate the adaptive immune response. Additionally, terminating the inflammatory response by removing other cells, such as phagocytosis of apoptotic neutrophils, is critical in prevention of a hyperinflammatory response in tissues, such as the lungs, which can be susceptible to damage from prolonged or uncontrolled inflammation. Although macrophage phagocytosis is a complex process, many studies have elucidated the importance of receptor express, actin cytoskeleton, small GTPases, PI3K, and others described in this review as central mechanisms in controlling this function [1–3].
Currently, there is a growing concern about the effects of environmental pollutants on health and their contribution to disease. Toxicity from alcohol consumption and cigarette smoking can be prevented, although both are common practice for hundreds of millions of people on a daily basis. Other factors, such as air pollution, including diesel exhaust and O3, and second-hand smoke, can be controlled to a lesser extent. Both sets of factors can trigger or exacerbate the health state of children, elderly, and otherwise healthy individuals.
In this review, we will be describing the impact of a range of environmental factors shown to negatively influence mechanisms controlling phagocytosis in human, animal, and cell line experimental paradigms. Specifically, the toxic effects of alcohol consumption, cigarette smoke, air pollution (PM), diesel exhaust, O3, and others will be discussed. As efficient phagocytosis and clearance of foreign material are critical roles of macrophages, we will focus on understanding the mechanisms by which these toxic factors can suppress macrophage phagocytosis.
THE PROCESS OF PHAGOCYTOSIS
The process of phagocytosis is the cellular uptake of pathogens, cellular debris, and apoptotic cells (efferocytosis). Although the basic concepts of phagocytosis were described in the late 1800s and early 1900s by Nobel Laureate Elie Metchnikoff, its definition and the mechanisms used during this process have been described more recently in much greater detail [4]. Phagocytosis is distinguished from other forms of cellular engulfment, such as endocytosis or pinocytosis, by three major characteristics: 1) phagocytosis involves the ingestion of particles at least 0.5 μm in diameter; 2) the process is initiated by the interaction of molecules with PAMPs or opsonized particles binding to specific cellular receptors located on the phagocyte; and 3) ligand-receptor complex triggers the local reorganization of the actin cytoskeleton that provides a driving force for the engulfment of the particle [5–7]. Phagocytosis can be differentiated further from endocytosis of particles, as activation of endocytosis does not require receptor stimulation, and endocytosis can use clathrin rather than actin to engulf the product [8–10]. Phagocytosis is initiated by the activation of receptors and examples include: the fragment, crystallizable FcR, which binds to the Fc portion of Ig; CR (consisting of CD11b and CD18 or the integrins aM/β2, respectively), which binds to complement-opsonized particles; the mannose and SRs binding to mannose moieties and modified LDL, respectively; and CD14/TLR4, which binds to LPS [11–15]. Binding and activation of these receptors induce the formation of a phagosome, a complex organelle that links internalization of a pathogen or apoptotic bodies with immune responses, cytoskeletal elements, Src kinases, and many other cellular networks, some of which will be described in this review [16, 17].
Actin filaments allow the connection of the extracellular pathogen with the intracellular machinery of the phagocyte via adhesion complexes. Polymerized actin has been shown to assemble underneath Ig-opsonized particles and pathogens such as Pseudomonas aeruginosa and Borrelia burgdorferi [1, 2, 18, 19]. The importance of actin polymerization during phagocytosis is confirmed in studies that show that blocking F-actin formation with latrunculin A or cytochalasin inhibits phagocytosis [20, 21]. Activation of small GTPases is involved in actin polymeration, which in turn plays a role in the formation of the lamellipodial extensions and which a phagocyte extends toward a pathogen. Additionally, regulation of actin polymerization by small GTPases mediates binding of actin to the cellular anchoring components and actinomyosin, which contributes to mechanical properties or forces required for pathogen ingestion.
Although there are conflicting reports about the type of receptors that initiate receptor-mediated phagocytosis and the small GTPase used during the process, Rac and Cdc42 are typically associated as the signaling molecules downstream of the FcRs, whereas Rho is associated with CR-mediated phagocytosis [1–3]. FcγR-mediated phagocytosis by macrophage cell lines, RAW264.7 and J774.A1, or FcγRIIA-transfected COS cells are inhibited by the use of the dominant-negative Rac1 or Cdc42, plasmid Rac1N17 or Cdc42 N17, respectively [1, 2]. This effect was also observed using IgE-opsonized particles and measuring RBL-2H3 mast cell phagocytosis after inhibition of Rac or Cdc42 [22]. Studies have also revealed that clustering of constitutively active Cdc42 or Rac1 leads to actin rearrangement, membrane ruffling, and particle engulfment [23, 24]. Moreover, FcγR-mediated phagocytosis has been shown to activate Rac in J774A.1 macrophages, Cdc42 in RAW264.7 macrophages, and Rac and Cdc42 in FcγR-transfected COS cells [25–27]. Downstream of this receptor, these small GTPases are activated independently of each other via the Rac guanine nucleotide exchange factor Vav and an unknown Cdc42 exchange factor 8, respectively [25]. Although Rho does not appear to be required for FcγR-mediated phagocytosis [1, 3, 5], some studies do support differing roles for Rho in FcγR-mediated phagocytosis [28]. One study noted that microinjection of the Rho inhibitor C3 into J774 macrophages ablated FcγR-mediated Ca2+ signaling and phagocytosis, as well as actin polymerization to the phagosome [28]. Contrary to these observations, another group reported that exposure of bone marrow-derived macrophages with the Rho inhibitor reduced FcγR-mediated phagocytosis without altering actin polymerization to the phagosome [29]. The involvement of small GTPases in phagocytosis represents a commonality between both studies and a mechanism that is conserved among multiple species (for a review, see ref. [30]).
The control of CR-mediated phagocytosis is less clear than FcγR-mediated phagocytosis. In contrast to the small GTPases Rac and Cdc42, Rho has been shown to be required for CR-mediated phagocytosis [1]. The importance of Rho in controlling mechanisms downstream of CR-mediated phagocytosis was revealed with the use of the same Rho inhibitor, C3. J774.A1 macrophages treated with C3 prior to phagocytosis of CR3-opsonized beads had a loss of actin and Arp2/3 recruitment to the phagosome, whereas transfection of dominant-negative Rac and Cdc42 into macrophages did not show a change in actin or Arp2/3 during the process [3]. The variant morphology of actin assembly at CR3-mediated phagosomes compared with FcγR-mediated phagocytosis may partially explain the differences in the use of the small GTPases [1]. As expected, bone marrow-derived macrophages that are deficient for Rac1/2 have suppressed FcγR-mediated phagocytosis; however, these cells also exhibited ablated C3bi-opsonized RBC phagocytosis [29]. These authors also reported a C3-mediated suppression of FcγR-mediated phagocytosis, which is contradictory to most published literature about FcγR-mediated phagocytosis. To further complicate this issue, bone marrow-derived macrophages, which are deficient for Vav1/3, have diminished C3bi-mediated phagocytosis. This defect can be rescued by transfecting a constitutively active Rac vector [29]. Although there is little doubt that the Rho family of small GTPases plays a major role in phagocytosis, there are conflicting data in describing which small GTPase is the dominant player in the various types of phagocytosis. In general, the consensus suggests that FcγR-mediated phagocytosis is controlled predominately by Rac/Cdc42, and CR-mediated phagocytosis is controlled primarily by Rho.
Phagocytosis and the processes occurring in conjunction with phagocytosis, such as ROS production and the delivery of membranes to the phagosome, have been shown to be controlled partially by PI3K [31]. PI(3,4,5)P3 transiently localizes to the forming phagosome and rapidly dissociates upon closure in macrophages and other cells activating the FcγRs (except the inhibitory FcγRIIb, which attenuates PI3K activation) [31–33]. Additionally, the use of Wortmannin and/or Ly294002 (potent PI3K inhibitors) blocked IgG-dependent phagocytosis in neutrophils, macrophages, and monocytes [31, 34]. Early in the phagocytic process, pseudopodial extension, mediated by actin remodeling at the phagocytic cup, is dependent on PI3K activity and formation of PI(3,4,5)P3. However, PI3K does not seem to be necessary for the initial actin polymerization at this site [31, 35, 36]. PI3K activity appears to be mediated by associating with tyrosine-phosphorylated substrates of the protein kinase Syk. As the majority of the research to date uses FcRs, there are a few studies linking the importance of PI3K and reorganization to the phagosome in CR-mediated phagocytosis [37, 38].
The differences in observed macrophage phagocytosis following exposure to environmental factors may also be explained by the activation state of this innate immune cell. Depending on the cytokine profile to which macrophages are exposed, they can be in an M1 phenotype (induced by IFN-γ, release of TNF-α, IL-6, IL-12, and a state in which macrophages are primed to phagocytose and kill pathogen) or M2 phenotype (induced by IL-4, IL-13, etc., and a state in which macrophages up-regulate SRs often during the “clean-up” process following the clearance of an infection) state. Many publications have supported the suppressive effects of ethanol, for example, on proinflammatory cytokine production, suggesting a shift from an M1 to an M2 phenotype, alterations in immune-modulating signals (e.g., corticosterone), or a loss in a specific pathway that would normally induce cytokine production (ERK1/2, for example) [39–41]. That being said, downstream of receptor activation (which can be different depending on the activation state of the macrophage), a number of the universal mechanisms involved during phagocytosis are commonly activated (adhesions, actin cytoskeleton, etc.), independent of the receptor activated. Unfortunately, the majority of studies examining the effects of an external factor on phagocytosis do not comment on the activation state of these macrophages; hence, it is not possible to elaborate on this at the present time.
ETHANOL AND PHAGOCYTOSIS IN MACROPHAGES
Alcohol (ethanol) consumption is a global health problem, and the average person consumes 6.2 L/year worldwide. Although one-half of the world's total population abstains from alcohol, it is estimated that 3.8% of all global deaths and 4.6% of global disease, including the reduction of healthy living years, is attributed to alcohol consumption [42]. NIH defines binge drinking (acute ethanol exposure) as an amount of alcohol sufficient to increase blood alcohol levels above 80 mg/dl (the legal driving limit in most states) in the blood and chronic alcohol abuse as a prolonged and repetitive exposure to these levels of alcohol (http://www.niaaa.nih.gov/). This level of blood alcohol concentration is typically the equivalent of about five drinks for males and four drinks for females in one 2-h sitting, a quantity that can vary depending on the weight of an individual and food intake. Alcohol consumption is known to have a suppressive effect on immune function [43]. Regardless of duration of alcohol exposure (acute or chronic), alcohol increases complications after infections, demonstrated by the decreased ability to clear opportunistic pathogens.
Clinical and laboratory evidence has shown that exposure to ethanol exhibits a variety of effects on immune cell function, including decreasing lymphocyte responsiveness to mitogens, suppressing chemotactic and phagocytic functions, and altering the production of cytokines by lymphocytes and macrophages [44–47]. In some organs, such as the lungs, the effect of ethanol on inflammation is independent of the length of ethanol exposure (for a review, see ref. [43]). In other organs, such as the spleen, there is a biphasic effect of ethanol on the inflammatory response, depending on the duration of ethanol exposure [48]. In general, chronic ethanol exposure is said to exacerbate inflammatory processes, such as atherosclerosis, multiple sclerosis, Alzheimer's disease, liver disease, and rheumatoid arthritis [49–52]. This is in contrast with acute ethanol exposure, which suppresses inflammatory responses [39, 53–56]. A commonality observed following acute or chronic ethanol exposure is the suppression of phagocytosis.
The recognition, internalization, and degradation of a pathogen are necessary for normal clearance of an infection. Dysregulation in these processes, such as seen after ethanol exposure, can be detrimental to the host defense (Table 1). Studies from the early 1960s and 1970s have shown immunomodulatory effects of ethanol on bacterial clearance [71, 72]. Mörland's groups [63, 73] reported that at 6 h and 24 h after in vitro ethanol exposure, human monocytes exhibited a decreased ability to phagocytose IgG-opsonized erythrocytes compared with monocytes that were not exposed to ethanol. The lowest dose that yielded modest but significant suppression after ethanol exposure was 28 mM, comparable with a blood alcohol content of 130 mg/dl. Their study included a range of doses of ethanol from 14 mM (65 mg/dl), a dose that did not suppress monocyte phagocytosis of IgG-coated erythrocytes, to 220 mM (1000 mg/dl), which suppressed FcγR-mediated phagocytosis by 65%. Rimland and Hand [57] reported that rabbit alveolar macrophages exposed to ethanol are not only less capable of phagocytosing latex beads and radiolabeled Staphylococcus aureus but are also less efficient in destroying the bacteria once internalized. In the 1990s, Zuiable's group [64] showed that human blood monocytes exposed to 100, 200, or 300 mg/dl ethanol in vitro for as little as 1 h and as long as 24 h triggered a reduction in phagocytosis and killing of nonopsonized Candida albicans. Additionally, treating peripheral blood monocytes with 100 mg/dl ethanol initially resulted in elevated phagocytosis of C. albicans by Day 7 compared with monocytes not exposed to ethanol. Interestingly, replenishing the media every day with fresh ethanol, up to Day 7, suppressed C. albicans phagocytosis by the monocytes. These data suggest that there may be a compensatory mechanism following ethanol exposure that allows an increase in phagocytosis at later time-points and support the view that ethanol effects on immunomodulation are time-dependent. In parallel to measuring the effect of ethanol on yeast phagocytosis, Zuiable's group [64] also examined human monocyte phagocytosis of IgG-opsonized erythrocytes. In contrast to the suppressed phagocytosis that occurs with yeast after 1 h and 1 day, phagocytosis of IgG-coated erythrocytes was only suppressed after 1 h of 100, 200, or 300 mg/dl ethanol. There was no effect of ethanol on FcγR-mediated phagocytosis after 1 or 7 days of ethanol exposure, suggesting a receptor-specific sensitivity to ethanol manipulation. In another report, mice receiving 4 consecutive days of a higher 4.5-g/kg level of ethanol, via intragastric administration, resulted in impaired ex vivo peritoneal macrophage phagocytosis of FITC-expressing Leishmania promastigotes, 24 h after the last exposure [62]. More recently, studies from our laboratory revealed that 3 h of ethanol exposure in vivo (3 g/kg) or in vitro (50 mM) suppressed phagocytosis of EGFP-P. aeruginosa by alveolar macrophages and the macrophage cell line, RAW264.7 [40]. This effect was not observed at earlier than 90 min or later than 24 h, suggesting a temporal effect of ethanol. A short, 1-h exposure to ethanol, given in vivo (3 g/kg given i.p.) or in vitro (25–100 mM), suppressed alveolar macrophage efferocytosis or phagocytosis of apoptotic cells [74]. These data are of critical importance when considering the importance of inflammation resolution following lung infection.
Table 1. Acute and Chronic Ethanol Exposure Affects Phagocytosis, Molecules That Facilitate Phagocytosis, and Subsequent Functions Downstream of Phagocytosis in Macrophages.
| Acute exposure to ethanol | Chronic exposure to ethanol | |
|---|---|---|
| Phagocytosis | ↓ Alveolar macrophages [57] | ↓ Kupffer cells [58–60] |
| ↓ Splenic macrophages | ↓ Alveolar macrophages [53, 61] | |
| ↓ Peritoneal macrophages [59] | ↑ Splenic macrophages [62] | |
| ↓ Monocytes [63, 64] | ↓ Microglia [65] | |
| ↑ Kupffer cells (LPS-stimulated) [66] | ||
| Phagocytic receptor expression | No macrophage data | ↓ β2 integrin in Kupffer cells [67] |
| Lysosomal vesicle conformation | ↓ H2O2 in alveolar macrophages [68] | ↑ H2O2 [58] |
| ↓ Superoxide in Kupffer cells [69] | ↓ GSH in alveolar macrophages [54] | |
| ↑ Superoxide in blood PMN [69] | ↑ Superoxide in alveolar macrophages [67] | |
| Allostimulatory potential | No macrophage data | ↑ CD80 and CD86 in CD11b+ splenocytes [70] |
Similar to the suppressive effects on phagocytosis seen after acute ethanol exposure, chronic ethanol exposure also attenuates macrophage phagocytosis. Escherichia coli phagocytosis, as well as suppression of chemotaxis and fMLP-induced superoxide anion production, was observed in Kupffer cells isolated from rats exposed to chronic ethanol ingestion [58]. Furthermore, studies in the late 1980s showed that in vivo phagocytosis of latex beads by Kupffer cells was decreased in rats chronically fed ethanol compared with animals not exposed to ethanol in their diet [59]. In contrast, these same studies revealed an increase in splenic macrophage phagocytosis of injected latex beads after chronic ethanol exposure when compared with splenic macrophages of saline-exposed rats, an effect that may be compensatory or receptor- or tissue-specific. Other organs, such as the lungs, have shown sensitivity to chronic ethanol exposure, with a decrease in alveolar macrophage phagocytosis of microorganisms and a subsequent increased risk of pneumonia in rats chronically exposed to ethanol [61]. The addition of glutathione precursors to the diet restored alveolar macrophage function and decreased sensitivity to endotoxemia-induced acute lung injury. This may be a result of glutathione's reduced state being able to donate a reducing equivalent to an ethanol-induced oxidized environment. Other studies showed reduced membrane expression of the GM-CSFR as a possible cause for decreased alveolar macrophage phagocytosis [53]. In parallel, ethanol ingestion reduced cellular expression and nuclear binding of PU.1, the master transcription factor that activates GM-CSF-dependent macrophage functions [53]. Ultimately, treatment of ethanol-fed rats with rGM-CSF restored GM-CSFR expression at the surface of alveolar macrophages, as well as PU.1 protein expression and nuclear binding in these cells. In the context of the CNS, ethanol-exposed microglia had decreased phagocytosis of opsonized E. coli. Additionally, ethanol treatment limited PMA-stimulated superoxide anion production, implying not only a decreased ability to uptake a pathogen in ethanol-exposed cells but also an inability to destroy the pathogen once it is taken up into the cell [65]. Suppressed phagocytosis of apoptotic neutrophils was also observed following chronic ethanol exposure. In C57Bl/6 mice chronically exposed to ethanol, LPS-induced lung injury resulted in increased neutrophil accumulation as a result of the decreased efferocytosis by alveolar macrophages [74]. This process was suggested not to be controlled by the small GTPase Rho, as the suppression of Rho (using C3 transferase) or the inhibition of Rho kinase (via Y27632) did not alter alveolar macrophage efferocytosis in control or ethanol-exposed cells. Additionally, the diminished efferocytosis may occur as a result of ethanol-induced receptor modifications. Uptake of apoptotic cells, via the asialoglycoprotein receptor, was impaired in cells obtained from ethanol-fed animals, indicating that ethanol also reduces the clearance of apoptotic cells in the liver [75]. Chronic ingestion of ethanol was shown to be associated with a decrease in complement-mediated clearance of opsonized erythrocytes [60]. In support of a receptor-specific effect of ethanol, chronic ingestion of ethanol was shown to be associated with a decrease in complement-mediated clearance of opsonized erythrocytes, an effect that was not observed in Fc- or nonopsonized erythrocytes [60].
Taken together, these reports conclusively demonstrate that phagocytosis is impaired in macrophages acutely exposed to ethanol to a similar extent as the same cell type isolated from chronic ethanol-treated animal models [48]. It is interesting to note that although ethanol attenuates macrophage phagocytosis, regardless of the duration of exposure, acute and chronic ethanol studies demonstrate a profound dichotomy with respect to other aspects of the inflammatory response. As the focus of this review is to discuss factors that impact macrophage phagocytosis, the diverging impact of ethanol of other host-immune functions will not be discussed.
To further elucidate the mechanisms by which exposure to ethanol suppresses phagocytosis, specifically targeting the actin cytoskeleton, it is important to understand how suppression occurs. The ability of ethanol to modify actin polymerization has been observed by many laboratories. Following stimulation with LPS, RAW264.7 cells cluster TLR4 and CD14 at the level of the cell membrane, which was shown to be controlled by actin polymerization at these foci (Fig. 1) [76]. Pretreating these cells with 0.4% w/v (∼300 mg/dl) ethanol for 10 min prior to LPS stimulation reduced actin-mediated TLR4 and CD14 aggregation to the same extent as cytochalasin D, an actin depolymerizer. In support of this work, unpublished data from our laboratory revealed that alveolar macrophages and RAW264.7 cells exposed to 2.2 g/kg in vivo or 50 mM in vitro ethanol, respectively, have reduced actin polymerization around the phagosome following activation via the FcγR. Other cell types, including PMNs, have altered actin regulation following exposure to ethanol [77, 78]. Total polymerized actin, or F-actin, was increased following fMLP stimulation of PMNs isolated from rats receiving ethanol infusion for 3 h (peak 180 mg/dl for males; 200 mg/dl for females) compared with cells isolated from control rats [78]. In this study, F-actin was measured by staining the polymerized actin and quantifying the fluorescent intensity by flow cytometry. However, the authors failed to consider the total actin level or determine the effect of ethanol on actin distribution. As adhesion molecules (discussed above) and small GTPases (below) function as binding partners and regulators of actin, it is plausible that ethanol-mediated alterations in these mediators may contribute to aberrant actin polymerization.
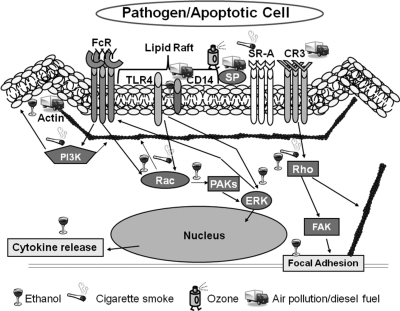
Figure 1. Known effects of external toxin exposure on pathways downstream of phagocytic receptors.
The mechanistic effects of ethanol, cigarette smoke, O3, and air pollution/diesel exposure on phagocytic pathways. Small GTPase regulation, actin reorganization, SPs, receptor expression, and MAPKs are known to be affecting proper phagocytosis following exposure to these environmental factors. PAK, p21-Activated protein; FAK, focal adhesion kinase.
As mentioned above, in spite of the extensive documentation of the control of phagocytosis by small GTPases, published data are conflicting concerning their overall contribution to phagocytosis modulation. In contrast, the number of reports describing the effects of acute exposure to ethanol on small GTPases are not as abundant but less contradictory. Work from our laboratory supports the published data that propose that Rac is the dominant GTPase during FcγR-mediated phagocytosis, and Rho does not seem to affect actin polymerization following FcγR activation [1, 2]. Moreover, this research revealed that acute ethanol exposure suppresses basal Rac activity, as well as Rac activation, during FcγR-mediated phagocytosis (unpublished results). This is not surprising, as this small GTPase is necessary for extension of lamellipodia and migration, and studies have previously shown that ethanol blunts chemotaxis toward a stimulus. Additionally, actin polymerization at the site of the phagosome is known to be controlled by Rac [24]. A decrease in Rac activity may partially explain the limited actin polymerization observed after FcγR stimulation in ethanol-exposed cells. Rac has also been associated with phagosomal adhesion formation, which may explain why vinculin phosphorylation after FcγR-mediated phagocytosis is lowered (unpublished results). Other studies showed that in astrocytes exposed to 10 mM ethanol for 10 min, there may be indirect effects, such as suppressed Rac activity by inducing RhoE activation, a known inhibitor of Rac [79]. The defect in Rac activity, which we and others observed, can explain the inhibition in actin polymerization to the phagosome and the decreased bacterial phagocytosis following ethanol exposure. In contrast to these studies, Boé et al. [74] showed that 60 min in vivo exposure of alveolar macrophages resulted in a reduction in Rho activity compared with macrophages isolated from mice receiving saline; however, a link between a lower level of Rho activity and altered efferocytosis by alveolar macrophages could not be established. This may be, in part, a result of the clearance of apoptotic cells through the FcRs, which is under the control of the small GTPase Rac.
Ethanol, through its ability to modify the plasma membrane, can suppress phagocytosis indirectly. Following acute exposure, ethanol is incorporated into the plasma membrane, directly affecting membrane fluidity and subsequent lipid raft stability, in addition to physically hindering receptor aggregation. Szabo's groups [80, 81] showed that ethanol can inhibit TLR4 engagement with CD14 in lipid rafts, the region where these molecules are purported to colocalize following LPS stimulation. The authors attribute these affects to a reduction in actin polymerization and loss of actin recruitment to clusters of TLR4. In addition to prohibiting aggregation between TLR4 and CD14, acute exposure to ethanol has been associated with increased membrane fluidity in rat liver hepatocytes, a phenomenon that is maintained following chronic ethanol exposure in these cells [82–84]. Studies have also documented the importance of TLR signaling during phagocytosis, suggesting that the involvement of the MyD88 pathway results in the ethanol-mediated reduction in phagocytosis [85]. Presumably, the increase in membrane fluidity would destabilize lipid rafts, further compromising TLR activation and phagocytosis. The increase in membrane fluidity and receptor hindrance caused by ethanol incorporation into the plasma membrane, in conjunction with downstream ethanol-induced signaling defects, may be additional contributing factors to ethanol-mediated suppression of macrophage phagocytosis.
Although a low daily intake (5–10 g/day) of alcohol is suggested to improve outcomes of certain pathologic conditions, such as ischemic cardiovascular complications, as well as diabetes, evidence suggests that the detrimental effects on disease and injury outweigh the benefits [86, 87]. It is important to highlight that the health-related benefits of alcohol is relevant only for populations with a higher risk of heart disease, such as men and postmenopausal women over 45 years of age. With this in mind, ethanol may be promoting cardiovascular health through inhibition of leukocyte activation and phagocytosis. The development of foam cells is induced by the phagocytosis of LDL particles by monocytes [88]. Preventing phagocytosis through the consumption of alcohol, as described above in other models of ethanol exposure, may assist in limiting foam-cell production and cardiovascular plaque formation. Additionally, HDL cholesterol has been shown to protect against atherosclerosis by influencing its production [89]. HDL cholesterol inhibits LDL cholesterol oxidation, thus preventing uptake by macrophages and foam-cell formation. With this in mind, studies have proposed that moderate alcohol consumption results in increased HDL, although conflicting data show decreased foam-cell accumulation without evidence of an ethanol-induced increase in HDL levels [90, 91].
The increased morbidity and mortality of patients admitted to the hospital, who have been previously exposed to ethanol, are extensive [92]. Patients presenting with pneumonia or septic shock and a history of alcohol abuse have a higher probability of becoming infected with P. aeruginosa [93]. Moreover, alcohol abusers suffer a three- to sevenfold greater mortality rate from bacterial pneumonia compared with subjects who have pneumonia without prior alcohol exposure [94]. Upon admission to the hospital, patients with alcohol in their system have longer lengths of stay and increased Intensive Care Unit stay, resulting in greater health care costs [95]. These data reinforce the importance of studying the immunosuppressive effects of ethanol from all perspectives.
CIGARETTE SMOKING AND PHAGOCYTOSIS
The CDC reported that 20.6% of all adults (23.5% of men; 17.9% of women) were smokers in 2009, numbers that coincide with more than 46 million U.S. adults [96]. Based on data collected from 1995 to 1999, the CDC estimated that adult male smokers lost an average of 13.2 years of life, and female smokers lost 14.5 years of life. Smoking cigarettes is the primary risk factor for the development of COPD, lung (and many other types of) cancer, and respiratory infections [97]. Exposure to cigarette smoke is known to compromise lung immunity by disruption of the mucociliary function, mucus hypersecretion, and disturbance of the mucosal integrity [98]. Additionally, the oxidative stress caused by cigarette smoke triggers local lung inflammation by activating epithelial cells, alveolar macrophages, neutrophils, and lymphocytes [99]. As a result of the fragile alveolar architecture of the lungs, the release of inflammatory cytokines, proteases, and ROS by immune cells can result in irreversible damage to the airway, thereby allowing increased exposure to incoming pathogens [100–104].
The impact of cigarette smoking on macrophage phagocytosis has been described for over one-half of a century, but more recently, smoking has been specifically linked to impaired alveolar macrophage phagocytosis in smokers or patients with COPD (Table 2) [105–107]. Effective efferocytosis is critical in maintenance of the host environment, and reduced efferocytosis results in a necrotic and proinflammatory environment, which promotes COPD pathogenesis. Multiple signaling pathways are involved in the process of efferocytosis, including the balance of the small GTPases Rac-1 and RhoA, as well as PI3K (Fig. 1) [131–133]. Interestingly, efferocytosis relies on Rac activity to induce membrane ruffling during phagocytosis [134]. In contrast, RhoA and its downstream effector Rho kinase negatively regulate the process by inducing stress fiber, focal adhesion formation, and cell spreading [131, 133]. With this in mind, alveolar macrophages from mice exposed to cigarette smoke (25 and 100 mg/m3; total PM) for 5 h had depressed efferocytosis, which lasted for 2 days [108]. The higher smoke exposure levels (100 mg/m3) given for 5 h/day for 5 days resulted in prolonged impairment of macrophage function, for up to 1 week. Chronic exposure with even higher doses (250 mg/m3; 5 h/day for 22 weeks) yielded a reduction in macrophage efferocytosis for 20 weeks. This group suggested that the inhibitory effect of cigarette smoke on efferocytosis was a result of oxidant-dependent activation of the RhoA-Rho kinase pathway, as seen in studies using extracellular superoxide dismutase knockout mice showing smoke-induced suppression of efferocytosis. Additionally, pretreatment of alveolar macrophages with the antioxidants N-acetylcysteine or manganese (III) tetrakis (4-benzoic acid) porphyrin returned efferocytosis to levels seen under control conditions. Moreover, inhibition of the RhoA-Rho kinase pathway with C3 transferase or Y27632 prevented the long-term inhibition (1 and 2 days postsmoke inhalation) of alveolar macrophage efferocytosis in mice exposed to 100 mg/m3 [108]. MBL also regulates efferocytosis through the activation of small GTPases [135]. A loss in MBL was observed in the BALF of COPD patients and smokers [136], which correlated with the cigarette smoke-induced decrease in efferocytosis by alveolar macrophages. Supplementing with aerosolized MBL resulted in a partial restoration of normal efferocytosis by alveolar macrophages from smoke-exposed mice through the induction Rac1/2/3 levels in these cells. One study specifically targets ceramide as the major contributor to the poor efferocytosis observed following exposure to cigarette smoke [109]. Exposure of human and rat alveolar macrophages to ceramide, a product of cigarette smoke exposure, for 4 h resulted in decreased efferocytosis comparable with treatment with cigarette smoke extract. Ceramide exposure was also associated with a decrease in Rac recruitment to the plasma membrane during efferocytosis. Additionally, rat alveolar macrophages overexpressing Rac showed a resistance to ceramide-induced reduction of efferocytosis [109].
Table 2. Cigarette Smoke, Air Pollution/Diesel Exhaust, and O3 Exposure Affect Phagocytosis, Molecules That Facilitate Phagocytosis, and Subsequent Functions Downstream of Phagocytosis in Macrophages and Blood Monocytes.
| Cigarette smoking | Air pollution/diesel exhaust | O3 | |
|---|---|---|---|
| Phagocytosis | ↓ Alveolar macrophages [108–110] | ↓ Alveolar macrophages [111–113] | ↓ Alveolar macrophages [114–117] |
| Phagocytic receptor expression | ↓ SR-A in PMs [118] | ↓ CD11b and CD29 in alveolar macrophages and blood monocytes [119] | ↑ TLR4 in alveolar macrophages [120] |
| ↓ CD31, CD91, and CD44 in alveolar macrophages [106] | ↓ CD14 in blood monocytes [119] | ↑ MARCO in alveolar macrophages [121] | |
| ↓ CD11c in alveolar macrophages [119] | ↑ CD11b/c in alveolar macrophages [122] | ||
| Lysosomal vesicle conformation | ↑ Superoxide and H2O2 in alveolar macrophages [123] | ↓ Superoxide by alveolar macrophages [124] | ↓ Oxidative burst in alveolar macrophages and blood monocyt es [119] |
| ↑ Superoxide in alveolar macrophages following MAP stimulation [125] | ↑ ROS in unstimulated alveolar macrophages [126, 127] | ↓ Superoxide in alveolar macrophages [128] | |
| ↓ Superoxide in alveolar macrophages [129] | ↓ ROS in PMA-stimulated alveolar macrophages [126] | ||
| ↓↓ Superoxide when O3 and cigarette smoke are combined in alveolar macrophages [129] | ↓ Superoxide by alveolar macrophages at 2 h and ↑ after 24 h [130] | ||
| Allostimulatory potential | No macrophage data | No macrophage data | ↑ CD80 and CD86 in alveolar macrophages [122] |
MARCO, Macrophage receptor with collagenous structure.
Phagocytosis of invading pathogens is also attenuated following exposure to cigarette smoke. Alveolar macrophage clearance of Haemophilus influenza is decreased in the presence of cigarette smoke extract. For example, the alveolar macrophage cell line MH-S, exposed to 10% cigarette smoke extract for as little as 3 h, resulted in decreased H. influenza binding to the macrophages and reduced internalization of the bacteria [110]. The loss in phagocytosis was suggested to be a result of a loss in PI3K activity, because of the cigarette smoke-induced decrease in phosphorylated AKT, a downstream effector of PI3K. Additionally, H. influenza, normally an activator of AKT, was incapable of inducing phosphorylation of AKT following pretreatment of the macrophages with LY294002, a PI3K inhibitor. Pretreatment with LY294002 also inhibited H. influenza uptake, suggesting the importance of PI3K during phagocytosis, as well as implicating a mechanism by which cigarette smoke is mediating its effects. Phagocytosis of other pathogens, such as P. aeruginosa, has also been shown to thrive in the lungs of human and rodent subjects exposed to cigarette smoke, although the mechanisms by which phagocytosis is impaired are not described thoroughly [137, 138].
As a result of the complex composition of cigarette smoke, inhibition of receptor binding to its complementary ligand and expression of receptors should also be considered when determining possible mechanisms by which suppression of phagocytosis occurs. For example, cigarette smoke includes aldehydes acrolein and 4-hydroxynoneal, which have been shown to adhere to macrophage SR-A, CD36, and the hyaluronan receptor (CD44) [118, 139, 140]. Alveolar macrophage receptor expression of smokers with COPD and smokers with no diagnosed health conditions, compared with healthy patients, was decreased as well. A study by Hodge et al. [106] reported a reduction in the expression of PECAM (CD31), LDLR-related protein (CD91), and CD44 on alveolar macrophages from smokers. These receptors have been shown to be used as recognition molecules involved in the phagocytosis of apoptotic cells [141–143]. In this 2007 study [106], the authors credited the decreased receptor expression to higher levels of cAMP after incubation of human alveolar macrophages in medium containing 2.5% or 10% cigarette smoke extract (equivalent to one-half to two packs of cigarettes/day). Interestingly, like alcohol exposure, the decrease in alveolar macrophage phagocytosis in patients with COPD was attributed to a reduction in GSH levels in BALF and provided a means of correcting the attenuated efferocytosis by supplementing with procysteine [107]. Not only did chronic exposure to ethanol reduce GSH levels in the lungs of mice [54], but they also showed that supplementing with the GSH precursor procysteine reversed the ethanol-induced suppression of macrophage phagocytosis.
The study of smoking or consuming alcohol alone can be considered incomplete without investigating the combined effects of cigarette smoke and ethanol on macrophage phagocytosis. To support the importance of these studies, evidence suggests that adolescents are five times and adults are twice as likely to smoke if they participated in binge drinking in the previous 30 days [144]. Although studies by Vander Top et al. [145] have shown a decrease in PMN killing of Streptococcus pneumonia following smoke and ethanol exposure, there are no current published studies measuring these effects on macrophage phagocytosis.
Extensive evidence reveals that cigarette smoking is associated with increased morbidities, including COPD, asthma, cancer, infections, and many others. Because of the extensiveness of the serious health issues, $97 billion is spent annually on public and private health care resulting from cigarette smoke. Currently, most efforts are put into the treatment of the morbidities of smoking cigarettes once they arise. As described above, one mechanism by which cigarette smoking inhibited phagocytosis was by decreasing MBL, and clinical studies are under way to examine the safety of rMBL to improve immune function in these subjects [146].
O3 AND PHAGOCYTOSIS
Air pollutants can typically be classified as primary or secondary. Primary pollutants are emitted directly from a process, such as ash from a volcanic eruption, fuel exhaust from a motor vehicle exhaust, or sulfur dioxide released from factories. Secondary pollutants are not emitted directly but rather, are formed in the air when primary pollutants react or interact with surrounding components. A commonly encountered secondary air pollutant is ground-level O3, which is a major component of smog and is produced by interaction of volatile organic and NO compounds brought about by the combustion of fossil fuels. Studies have attributed O3-related morbidities in the general human population and have emphasized that children and aged individuals (over 65 years) are particularly vulnerable to low levels of inhaled O3 [147–154]. Above a certain concentration, inhalation of O3 can be fatal. For each 25-ppb-measured increase in the level of ambient O3, there is a 4% rise in mortality [155]. Furthermore, recent estimates showed that for each 10-ppb elevation in a 1-h daily maximum of O3, there is a 0.39–0.87% increase in mortality [156, 157]. Even at 15 ppb, which are levels of ambient O3 and well below the current U.S. Environmental Protection Agency standard, an association of heightened mortality persists. Increased levels of O3 can exacerbate severity of respiratory illness, although some debate exists as to whether this is secondary to changes in airway mechanics or a result of dysregulation of immune function [158]. Below, we will focus on the immunological effects of O3, specifically targeting phagocytosis and the mechanisms by which phagocytosis may be decreased.
O3 exposure has been associated with the inability to properly phagocytose and kill pathogenic organisms, including Streptococcus zooepidemicus and pyogenes, S. aureus, Klebsiella pneumonia, Mycobacterium tuberculosis, Lysteria monocytogenes, herpes simplex virus, P. aeruginosa, and others [114–117, 159–162]. Although some suggest O3 susceptibility seems, in part, to be dependent on the genetic disposition in humans and mice, the mechanisms that suppress phagocytosis directly have not been researched extensively [163, 164]. Phagocytic activity, but not superoxide production, by alveolar macrophages isolated from 3-h O3-exposed (0.1, 0.3, or 0.6 ppm) rabbits was reduced when compared with macrophages isolated from control animals [165]. In contrast, a study by Ryer-Powder et al. [128] showed that alveolar macrophages from mice that inhaled 0.11 ppm O3 for 3 h had decreased superoxide production but no changes in phagocytic ability of latex beads or sheep RBCs. Additionally, alveolar macrophage cytochrome b558 levels were lower after exposure to 3 ppm O3 for 3 h, and the authors suggest this as a mechanism by which superoxide levels may be suppressed. However, 3 ppm O3 exposure is higher than the levels used by other groups, and as a result of the lack of cytochrome b558 changes at the lower levels of O3, alternative mechanisms of superoxide suppression may exist.
Furthermore, a study looking at acute and chronic O3 exposure revealed that 2-h exposure of 0.1 ppm O3 for 13 days induced alveolar macrophage (and neutrophil) recruitment to the lungs of rabbits on Days 7 and 14 [166]. This exposure to O3 also limited the number of alveolar macrophages able to phagocytose on Days 3 and 7. The recruitment of immune cells may be a response to the induction of inflammatory cytokines induced by O3 [167]. Pulmonary surfactants, which traditionally have been thought to reduce surface tension of the alveoli, have also played a role in phagocytosis. A recent study by Mikerov et al. [117] demonstrated that O3-induced oxidation of SP-A negatively affects alveolar macrophage phagocytosis of P. aeruginosa and S. aureus (Fig. 1). These results were observed following in vivo or in vitro oxidation of SP-A1 and SP-A2 and suggest that one has to consider the lungs redox status, not only as a functional difference of SPs but also when considering the immune response to pulmonary infection. Finally, this group went on to show that SP-A−/− knockout mice, given a K. pneumonia infection, had decreased survival when compared with WT mice exposed to filtered air. O3-exposed WT mice had attenuated survival compared with a filtered air-exposed mouse, and there was a synergistic mortality in mice exposed to O3 with a SP-A−/− phenotype [168]. Similar to studies described above, O3 increased infiltration of PMNs to the lung and SP-A oxidation. This would again support a genetic control of O3-mediated immune suppression observed by others.
AIR POLLUTION/DIESEL FUEL EXHAUST AND PHAGOCYTOSIS
According to the World Health Organization, air pollution is the cause of ∼1.2 million deaths annually [169]. Environmental (air) pollutants have been shown to drive the processes that are involved in pulmonary and nasal allergic disease and more serious morbidities, including COPD and asthma [170–179]. Not surprisingly, there is a correlation between the increase in the diagnosis of these diseases and the increase in fossil-fuel combustion and emission of particulate pollutants [178]. Specifically, PM10 (describing a mass metric that measures particles in the air with a 50% efficiency for particles with an aerodynamic diameter of 10 μm) [180] or less can exacerbate pulmonary disorders, including COPD and asthma, as well as increase the intensity of allergic inflammation [172, 173, 177, 178, 181, 182]. As expected, PM10 pollutants are comprised of an amalgamate of various chemicals, PM, and/or biological materials. A major component of PM10 pollutants includes DEP, consisting of 40% of total PM10 levels in areas such as the Los Angeles basin, resulting in a daily exposure rate as high as 300 mg DEP/day [172]. Clinical and laboratory studies have shown that DEP increase IgE production, Th2 cytokine production, and mucosal inflammation in humans and animals challenged by allergens [183, 184]. Additionally, there is a strong body of literature showing a severe depression in phagocytosis in macrophages exposed to DEP, which will be described.
Air pollution is a significant problem of public health for the general population in urban areas and for road workers. As a result of its heterogeneous composition of carbon particulates, DEP, and other PM of various sizes, studies focus on a specific, aforementioned particle or as a general PM of a certain size. Many studies have shown that fine particulate atmospheric pollution was associated with increased respiratory and cardiovascular morbidity and mortality [185–187]. Additionally, there have been numerous investigations showing how carbon or DEP or PM suppress phagocytosis of bacteria, fungi, and inert particles [111–113, 180, 188]. Focusing specifically on how these particles affect macrophage phagocytosis, our first example complements studies done with O3 exposure. Diesel exhaust and carbon-loaded alveolar macrophages induced lipid peroxidation of a surfactant during a 5-h exposure to these particles [111]. This is attributed to the decrease in S. pneumonia killing and phagocytosis, as described by others [189]. Cytoskeletal elements have also been shown to be affected by carbon and DEP in dog alveolar macrophages and the macrophage cell line J774A.1 [190]. Both particle types triggered a relaxation, coupled by an increase in the stiffening of the cytoskeleton, which in turn, resulted in a loss in phagosome motion inside the cell. Although there are currently no studies that have measured the effects of these particles on small GTPases, it is likely that the actin cytoskeletal manipulations observed are being controlled by small GTPases. Receptor expression on alveolar macrophages and blood monocytes is decreased following exposure to air pollution particles (PM10) [119]. Both cell types displayed lower levels of expression of CD11b and CD29 after 18 h exposure to PM10 (Fig. 1). CD11c was also reduced in alveolar macrophages, although no change was observed in the blood monocytes. In contrast, CD14 levels were attenuated in only the blood monocytes and not altered in the alveolar macrophages following PM10 exposure. A reduction in the surface levels of CD11b, a heterodimer in the CR3, would impair the ability of phagocytes to properly bind to an opsonized pathogen. Additionally, CD14, through its interactions with TLR4, allows a cell to properly respond to LPS. As studies have shown that TLR4 signaling is critical during phagocytosis, the loss of the costimulatory molecule CD14 could result in insufficient signaling through this pathway. Furthermore, CD14 was shown to be required for monocyte phagocytosis of gram-negative bacteria [191]. DEP may be suppressing phagocytosis by indirect means as well. IFN-γ, a cytokine that enhances phagocytosis, is produced at lower levels by lymphocytes exposed to 5–50 μg/ml DEP and L. monocytogenes [192]. More recently, another study by Yin et al. [193] revealed that rat alveolar macrophages given DEP had a dose-dependent reduction in proinflammatory mediators, TNF-α, IL-1β, and IL-12, and augmented secretion of the anti-inflammatory cytokine IL-10 following stimulation with L. monocytogenes. Although most would not consider breathing in air as a potential threat to our immune system, ample data have supported the importance of considering a cleaner environment, especially in urban regions.
Clean air laws, prohibiting the amount of pollutants that factories and automobiles can release, have been implemented to reduce the environmental and health effects of these toxins. Although not as easily assessed, air pollution has been linked to increased daily clinic visits as well as hospitalizations among people over the age of 65 [194, 195]. Additionally, environmental factors should be taken into consideration when people exercise, especially in highly polluted areas [196].
OTHER EXTERNAL FACTORS AND PHAGOCYTOSIS
This review covered the most commonly studied external factors that alter phagocytosis; this list is not exhaustive. Many additional factors have been shown to inhibit phagocytosis and include lead, marijuana smoke, trichloroethylene, chloroform, and others [197–200]. Unfortunately, the complete mechanisms that control the various types of phagocytosis and the subsequent internalization have yet to be fully elucidated following exposure to these substances. Taking into consideration the known, recurring mechanisms of phagocytosis that are suppressed following exposure to environmental factors, we can assume important directions lie in the small GTPases, the cytoskeleton, and ROS production.
CONCLUSION
Environmental pollutants, alcohol, and cigarette smoke alter a variety of physiologic functions, including regulation of the immune response and promoting increased susceptibility to infection. In general, these factors suppress the inflammatory response and immune cell phagocytosis, compromising a critical line of defense against pathogens, tissue injury, and malignancy. This disruption of the “normal” immune response can increase vulnerability to infectious challenge and impair proper clearance of infectious organisms, as well as altering the course of the postinflammation return to homeostasis. Considering the significance of the cytoskeleton and small GTPases in many cellular functions and the multitude and complex sociological issues associated with external toxins, there is a clear need to continue the investigation of these important biological observations initiated by the research reported here. Gaining a better understanding of the mechanisms responsible for toxin-induced suppression of phagocytosis, as well as the inflammatory and immune responses, would permit the development of treatments that could offset the detrimental consequences of ethanol ingestion, cigarette smoking, and living in polluted areas.
ACKNOWLEDGMENTS
This work was supported by NIH R01 AA12034 (E.J.K.), NIH F31 AA017027 (J.K.), NIH T32 AA013527, NIH R01 AA018859 (E.J.K.), and Ralph and Marian C. Falk Research Trust (E.J.K.). We thank Aleah Brubaker and Jessica Remus for critical evaluation of the manuscript.
Footnotes
- Arp2/3
- actin-related protein 2/3
- BALF
- BAL fluid
- CDC
- Centers for Disease Control and Prevention
- COPD
- chronic obstructive pulmonary disease
- CR
- complement receptor
- DEP
- diesel exhaust particles
- MBL
- mannose-binding lectin
- O3
- ozone
- PI(3,4,5)P3
- phosphatidylinositol (3,4,5)-trisphosphate
- PM
- particulate matter
- PM10
- particulate pollutants of 10 μm
- PMN
- polymorphonuclear leukocyte
- SP
- surfactant protein
- SR
- scavenger receptor
AUTHORSHIP
J.K. designed and performed the study, interpreted the results, and wrote the manuscript. E.J.K supervised the work, interpreted the results, and wrote the manuscript.
REFERENCES
- 1. Caron E., Hall A. (1998) Identification of two distinct mechanisms of phagocytosis controlled by different Rho GTPases. Science 282, 1717–1721 [DOI] [PubMed] [Google Scholar]
- 2. Cox D., Chang P., Zhang Q., Reddy P. G., Bokoch G. M., Greenberg S. (1997) Requirements for both Rac1 and Cdc42 in membrane ruffling and phagocytosis in leukocytes. J. Exp. Med. 186, 1487–1494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. May R. C., Caron E., Hall A., Machesky L. M. (2000) Involvement of the Arp2/3 complex in phagocytosis mediated by FcγR or CR3. Nat. Cell Biol. 2, 246–248 [DOI] [PubMed] [Google Scholar]
- 4. Metchnikoff E. (1968) Lectures on the comparative pathology of inflammation, delivered at the Pasteur Institute in 1891. Dover Publications, New York, USA [Google Scholar]
- 5. May R. C., Machesky L. M. (2001) Phagocytosis and the actin cytoskeleton. J. Cell Sci. 114, 1061–1077 [DOI] [PubMed] [Google Scholar]
- 6. Aderem A., Underhill D. M. (1999) Mechanisms of phagocytosis in macrophages. Annu. Rev. Immunol. 17, 593–623 [DOI] [PubMed] [Google Scholar]
- 7. Kwiatkowska K., Sobota A. (1999) Signaling pathways in phagocytosis. Bioessays 21, 422–431 [DOI] [PubMed] [Google Scholar]
- 8. Steinman R. M., Mellman I. S., Muller W. A., Cohn Z. A. (1983) Endocytosis and the recycling of plasma membrane. J. Cell Biol. 96, 1–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Conner S. D., Schmid S. L. (2003) Regulated portals of entry into the cell. Nature 422, 37–44 [DOI] [PubMed] [Google Scholar]
- 10. Grant B. D., Donaldson J. G. (2009) Pathways and mechanisms of endocytic recycling. Nat. Rev. Mol. Cell Biol. 10, 597–608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sánchez-Mejorada G., Rosales C. (1998) Signal transduction by immunoglobulin Fc receptors. J. Leukoc. Biol. 63, 521–533 [DOI] [PubMed] [Google Scholar]
- 12. Brown E. J. (1991) Complement receptors and phagocytosis. Curr. Opin. Immunol. 3, 76–82 [DOI] [PubMed] [Google Scholar]
- 13. Stahl P. D., Ezekowitz R. A. (1998) The mannose receptor is a pattern recognition receptor involved in host defense. Curr. Opin. Immunol. 10, 50–55 [DOI] [PubMed] [Google Scholar]
- 14. Devitt A., Moffatt O. D., Raykundalia C., Capra J. D., Simmons D. L., Gregory C. D. (1998) Human CD14 mediates recognition and phagocytosis of apoptotic cells. Nature 392, 505–509 [DOI] [PubMed] [Google Scholar]
- 15. Platt N., da Silva R. P., Gordon S. (1998) Recognizing death: the phagocytosis of apoptotic cells. Trends Cell Biol. 8, 365–372 [DOI] [PubMed] [Google Scholar]
- 16. Garin J., Diez R., Kieffer S., Dermine J. F., Duclos S., Gagnon E., Sadoul R., Rondeau C., Desjardins M. (2001) The phagosome proteome: insight into phagosome functions. J. Cell Biol. 152, 165–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Trost M., English L., Lemieux S., Courcelles M., Desjardins M., Thibault P. (2009) The phagosomal proteome in interferon-γ-activated macrophages. Immunity 30, 143–154 [DOI] [PubMed] [Google Scholar]
- 18. Linder S., Heimerl C., Fingerle V., Aepfelbacher M., Wilske B. (2001) Coiling phagocytosis of Borrelia burgdorferi by primary human macrophages is controlled by CDC42Hs and Rac1 and involves recruitment of Wiskott-Aldrich syndrome protein and Arp2/3 complex. Infect. Immun. 69, 1739–1746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lee D. J., Cox D., Li J., Greenberg S. (2000) Rac1 and Cdc42 are required for phagocytosis, but not NF-κB-dependent gene expression, in macrophages challenged with Pseudomonas aeruginosa. J. Biol. Chem. 275, 141–146 [DOI] [PubMed] [Google Scholar]
- 20. de Oliveira C. A., Mantovani B. (1988) Latrunculin A is a potent inhibitor of phagocytosis by macrophages. Life Sci. 43, 1825–1830 [DOI] [PubMed] [Google Scholar]
- 21. Malawista S. E., Gee J. B., Bensch K. G. (1971) Cytochalasin B reversibly inhibits phagocytosis: functional, metabolic, and ultrastructural effects in human blood leukocytes and rabbit alveolar macrophages. Yale J. Biol. Med. 44, 286–300 [PMC free article] [PubMed] [Google Scholar]
- 22. Massol P., Montcourrier P., Guillemot J. C., Chavrier P. (1998) Fc receptor-mediated phagocytosis requires CDC42 and Rac1. EMBO J. 17, 6219–6229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Castellano F., Montcourrier P., Guillemot J. C., Gouin E., Machesky L., Cossart P., Chavrier P. (1999) Inducible recruitment of Cdc42 or WASP to a cell-surface receptor triggers actin polymerization and filopodium formation. Curr. Biol. 9, 351–360 [DOI] [PubMed] [Google Scholar]
- 24. Castellano F., Montcourrier P., Chavrier P. (2000) Membrane recruitment of Rac1 triggers phagocytosis. J. Cell Sci. 113, 2955–2961 [DOI] [PubMed] [Google Scholar]
- 25. Patel J. C., Hall A., Caron E. (2002) Vav regulates activation of Rac but not Cdc42 during FcγR-mediated phagocytosis. Mol. Biol. Cell 13, 1215–1226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kant A. M., De P., Peng X., Yi T., Rawlings D. J., Kim J. S., Durden D. L. (2002) SHP-1 regulates Fcγ receptor-mediated phagocytosis and the activation of RAC. Blood 100, 1852–1859 [PubMed] [Google Scholar]
- 27. Niedergang F., Colucci-Guyon E., Dubois T., Raposo G., Chavrier P. (2003) ADP ribosylation factor 6 is activated and controls membrane delivery during phagocytosis in macrophages. J. Cell Biol. 161, 1143–1150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hackam D. J., Rotstein O. D., Schreiber A., Zhang W., Grinstein S. (1997) Rho is required for the initiation of calcium signaling and phagocytosis by Fcγ receptors in macrophages. J. Exp. Med. 186, 955–966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hall A. B., Gakidis M. A., Glogauer M., Wilsbacher J. L., Gao S., Swat W., Brugge J. S. (2006) Requirements for Vav guanine nucleotide exchange factors and Rho GTPases in FcγR- and complement-mediated phagocytosis. Immunity 24, 305–316 [DOI] [PubMed] [Google Scholar]
- 30. Niedergang F., Chavrier P. (2005) Regulation of phagocytosis by Rho GTPases. Curr. Top. Microbiol. Immunol. 291, 43–60 [DOI] [PubMed] [Google Scholar]
- 31. Cox D., Tseng C. C., Bjekic G., Greenberg S. (1999) A requirement for phosphatidylinositol 3-kinase in pseudopod extension. J. Biol. Chem. 274, 1240–1247 [DOI] [PubMed] [Google Scholar]
- 32. Kanakaraj P., Duckworth B., Azzoni L., Kamoun M., Cantley L. C., Perussia B. (1994) Phosphatidylinositol-3 kinase activation induced upon Fc γ RIIIA-ligand interaction. J. Exp. Med. 179, 551–558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gratacap M. P., Payrastre B., Viala C., Mauco G., Plantavid M., Chap H. (1998) Phosphatidylinositol 3,4,5-trisphosphate-dependent stimulation of phospholipase C-γ2 is an early key event in FcγRIIA-mediated activation of human platelets. J. Biol. Chem. 273, 24314–24321 [DOI] [PubMed] [Google Scholar]
- 34. Ninomiya N., Hazeki K., Fukui Y., Seya T., Okada T., Hazeki O., Ui M. (1994) Involvement of phosphatidylinositol 3-kinase in Fc γ receptor signaling. J. Biol. Chem. 269, 22732–22737 [PubMed] [Google Scholar]
- 35. Marshall J. G., Booth J. W., Stambolic V., Mak T., Balla T., Schreiber A. D., Meyer T., Grinstein S. (2001) Restricted accumulation of phosphatidylinositol 3-kinase products in a plasmalemmal subdomain during Fc γ receptor-mediated phagocytosis. J. Cell Biol. 153, 1369–1380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Araki N., Johnson M. T., Swanson J. A. (1996) A role for phosphoinositide 3-kinase in the completion of macropinocytosis and phagocytosis by macrophages. J. Cell Biol. 135, 1249–1260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bohdanowicz M., Cosio G., Backer J. M., Grinstein S. (2010) Class I and class III phosphoinositide 3-kinases are required for actin polymerization that propels phagosomes. J. Cell Biol. 191, 999–1012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sendide K., Reiner N. E., Lee J. S., Bourgoin S., Talal A., Hmama Z. (2005) Cross-talk between CD14 and complement receptor 3 promotes phagocytosis of mycobacteria: regulation by phosphatidylinositol 3-kinase and cytohesin-1. J. Immunol. 174, 4210–4219 [DOI] [PubMed] [Google Scholar]
- 39. Goral J., Choudhry M. A., Kovacs E. J. (2004) Acute ethanol exposure inhibits macrophage IL-6 production: role of p38 and ERK1/2 MAPK. J. Leukoc. Biol. 75, 553–559 [DOI] [PubMed] [Google Scholar]
- 40. Karavitis J., Murdoch E. L., Gomez C. R., Ramirez L., Kovacs E. J. (2008) Acute ethanol exposure attenuates pattern recognition receptor activated macrophage functions. J. Interferon Cytokine Res. 28, 413–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Glover M., Pruett S. B. (2006) Role of corticosterone in immunosuppressive effects of acute ethanol exposure on Toll-like receptor mediated cytokine production. J. Neuroimmune Pharmacol. 1, 435–442 [DOI] [PubMed] [Google Scholar]
- 42. Rehm J., Mathers C., Popova S., Thavorncharoensap M., Teerawattananon Y., Patra J. (2009) Global burden of disease and injury and economic cost attributable to alcohol use and alcohol-use disorders. Lancet 373, 2223–2233 [DOI] [PubMed] [Google Scholar]
- 43. Happel K. I., Nelson S. (2005) Alcohol, immunosuppression, and the lung. Proc. Am. Thorac. Soc. 2, 428–432 [DOI] [PubMed] [Google Scholar]
- 44. Napolitano L. M., Koruda M. J., Zimmerman K., McCowan K., Chang J., Meyer A. A. (1995) Chronic ethanol intake and burn injury: evidence for synergistic alteration in gut and immune integrity. J. Trauma 38, 198–207 [DOI] [PubMed] [Google Scholar]
- 45. Messingham K. A., Faunce D. E., Kovacs E. J. (2002) Alcohol, injury, and cellular immunity. Alcohol 28, 137–149 [DOI] [PubMed] [Google Scholar]
- 46. Cook R. T. (1998) Alcohol abuse, alcoholism, and damage to the immune system—a review. Alcohol. Clin. Exp. Res. 22, 1927–1942 [PubMed] [Google Scholar]
- 47. Szabo G. (1998) Monocytes, alcohol use, and altered immunity. Alcohol. Clin. Exp. Res. 22, 216S–219S [DOI] [PubMed] [Google Scholar]
- 48. Goral J., Karavitis J., Kovacs E. J. (2008) Exposure-dependent effects of ethanol on the innate immune system. Alcohol 42, 237–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Cunningham C., Wilcockson D. C., Campion S., Lunnon K., Perry V. H. (2005) Central and systemic endotoxin challenges exacerbate the local inflammatory response and increase neuronal death during chronic neurodegeneration. J. Neurosci. 25, 9275–9284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Feldmann M., Brennan F. M., Maini R. N. (1996) Role of cytokines in rheumatoid arthritis. Annu. Rev. Immunol. 14, 397–440 [DOI] [PubMed] [Google Scholar]
- 51. Libby P. (2002) Inflammation in atherosclerosis. Nature 420, 868–874 [DOI] [PubMed] [Google Scholar]
- 52. Perry V. H., Cunningham C., Holmes C. (2007) Systemic infections and inflammation affect chronic neurodegeneration. Nat. Rev. Immunol. 7, 161–167 [DOI] [PubMed] [Google Scholar]
- 53. Joshi P. C., Applewhite L., Ritzenthaler J. D., Roman J., Fernandez A. L., Eaton D. C., Brown L. A., Guidot D. M. (2005) Chronic ethanol ingestion in rats decreases granulocyte-macrophage colony-stimulating factor receptor expression and downstream signaling in the alveolar macrophage. J. Immunol. 175, 6837–6845 [DOI] [PubMed] [Google Scholar]
- 54. Brown L. A., Ping X. D., Harris F. L., Gauthier T. W. (2007) Glutathione availability modulates alveolar macrophage function in the chronic ethanol-fed rat. Am. J. Physiol. Lung Cell. Mol. Physiol. 292, L824–L832 [DOI] [PubMed] [Google Scholar]
- 55. Libon C., Forestier F., Cotte-Laffitte J., Labarre C., Quero A. M. (1993) Effect of acute oral administration of alcohol on superoxide anion production from mouse alveolar macrophages. J. Leukoc. Biol. 53, 93–98 [DOI] [PubMed] [Google Scholar]
- 56. Goral J., Kovacs E. J. (2005) In vivo ethanol exposure down-regulates TLR2-, TLR4-, and TLR9-mediated macrophage inflammatory response by limiting p38 and ERK1/2 activation. J. Immunol. 174, 456–463 [DOI] [PubMed] [Google Scholar]
- 57. Rimland D., Hand W. L. (1980) The effect of ethanol on adherence and phagocytosis by rabbit alveolar macrophages. J. Lab. Clin. Med. 95, 918–926 [PubMed] [Google Scholar]
- 58. Bautista A. P. (2002) Chronic alcohol intoxication primes Kupffer cells and endothelial cells for enhanced CC-chemokine production and concomitantly suppresses phagocytosis and chemotaxis. Front. Biosci. 7, a117–a125 [DOI] [PubMed] [Google Scholar]
- 59. Shiratori Y., Jin'nai H., Teraoka H., Matano S., Matsumoto K., Kamii K., Tanaka M., Okano K. (1989) Phagocytic properties of hepatic endothelial cells and splenic macrophages compensating for a decreased phagocytic function of Kupffer cells in the chronically ethanol-fed rats. Exp. Cell Biol. 57, 300–309 [DOI] [PubMed] [Google Scholar]
- 60. Messner R. P., Meryhew N. L., DeMaster E. G. (1993) Effect of ethanol on immune clearance in mice: biphasic alteration of complement-mediated clearance with chronic ethanol ingestion. J. Lab. Clin. Med. 122, 506–517 [PubMed] [Google Scholar]
- 61. Brown L. A., Harris F. L., Ping X. D., Gauthier T. W. (2004) Chronic ethanol ingestion and the risk of acute lung injury: a role for glutathione availability? Alcohol 33, 191–197 [DOI] [PubMed] [Google Scholar]
- 62. Andrade M. C., Albernaz M. J., Araujo M. S., Santos B. P., Teixeira-Carvalho A., Faria A. M., Martins-Filho O. A. (2009) Short-term administration of ethanol in mice deviates antigen presentation activity towards B cells. Scand. J. Immunol. 70, 226–237 [DOI] [PubMed] [Google Scholar]
- 63. Mørland B., Mørland J. (1984) Reduced Fc-receptor function in human monocytes exposed to ethanol in vitro. Alcohol Alcohol. 19, 211–217 [PubMed] [Google Scholar]
- 64. Zuiable A., Wiener E., Wickramasinghe S. N. (1992) In vitro effects of ethanol on the phagocytic and microbial killing activities of normal human monocytes and monocyte-derived macrophages. Clin. Lab. Haematol. 14, 137–147 [DOI] [PubMed] [Google Scholar]
- 65. Aroor A. R., Baker R. C. (1998) Ethanol inhibition of phagocytosis and superoxide anion production by microglia. Alcohol 15, 277–280 [DOI] [PubMed] [Google Scholar]
- 66. Spitzer J. A., Zhang P. (1996) Gender differences in phagocytic responses in the blood and liver, and the generation of cytokine-induced neutrophil chemoattractant in the liver of acutely ethanol-intoxicated rats. Alcohol. Clin. Exp. Res. 20, 914–920 [DOI] [PubMed] [Google Scholar]
- 67. Morio L. A., Chiu H., Sprowles K. A., Laskin D. L. (2000) Functional heterogeneity of rat hepatic and alveolar macrophages: effects of chronic ethanol administration. J. Leukoc. Biol. 68, 614–620 [PubMed] [Google Scholar]
- 68. Zhang P., Nelson S., Summer W. R., Spitzer J. A. (1997) Acute ethanol intoxication suppresses the pulmonary inflammatory response in rats challenged with intrapulmonary endotoxin. Alcohol. Clin. Exp. Res. 21, 773–778 [PubMed] [Google Scholar]
- 69. Bautista A. P., Elliott K. E. (1994) Acute ethanol intoxication regulates f-Met-Leu-Phe-induced chemotaxis and superoxide release by neutrophils and Kupffer cells through modulation of the formyl peptide receptor in the rat. Life Sci. 54, 721–730 [DOI] [PubMed] [Google Scholar]
- 70. Zhu X., Coleman R. A., Alber C., Ballas Z. K., Waldschmidt T. J., Ray N. B., Krieg A. M., Cook R. T. (2004) Chronic ethanol ingestion by mice increases expression of CD80 and CD86 by activated macrophages. Alcohol 32, 91–100 [DOI] [PubMed] [Google Scholar]
- 71. Auerbach-Rubin F., Ottolenghi-Nightingale E. (1971) Effect of ethanol on the clearance of airborne pneumococci and the rate of pneumococcal transformations in the lung. Infect. Immun. 3, 688–693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Laurenzi G. A., Guarneri J. J., Endriga R. B., Carey J. P. (1963) Clearance of bacteria by the lower respiratory tract. Science 142, 1572–1573 [DOI] [PubMed] [Google Scholar]
- 73. Preus H., Tollefsen T., Mörland B. (1987) Effects of tetracycline on human monocyte phagocytosis and lymphocyte proliferation. Acta Odontol. Scand. 45, 297–302 [DOI] [PubMed] [Google Scholar]
- 74. Boé D. M., Richens T. R., Horstmann S. A., Burnham E. L., Janssen W. J., Henson P. M., Moss M., Vandivier R. W. (2010) Acute and chronic alcohol exposure impair the phagocytosis of apoptotic cells and enhance the pulmonary inflammatory response. Alcohol. Clin. Exp. Res. 34, 1723–1732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. McVicker B. L., Tuma D. J., Kubik J. A., Hindemith A. M., Baldwin C. R., Casey C. A. (2002) The effect of ethanol on asialoglycoprotein receptor-mediated phagocytosis of apoptotic cells by rat hepatocytes. Hepatology 36, 1478–1487 [DOI] [PubMed] [Google Scholar]
- 76. Dai Q., Pruett S. B. (2006) Ethanol suppresses LPS-induced Toll-like receptor 4 clustering, reorganization of the actin cytoskeleton, and associated TNF-α production. Alcohol. Clin. Exp. Res. 30, 1436–1444 [DOI] [PubMed] [Google Scholar]
- 77. Allansson L., Khatibi S., Olsson T., Hansson E. (2001) Acute ethanol exposure induces [Ca2+]i transients, cell swelling and transformation of actin cytoskeleton in astroglial primary cultures. J. Neurochem. 76, 472–479 [DOI] [PubMed] [Google Scholar]
- 78. Zhang P., Spitzer J. A. (1997) Acute ethanol administration modulates leukocyte actin polymerization in endotoxic rats. Alcohol. Clin. Exp. Res. 21, 779–783 [PubMed] [Google Scholar]
- 79. Guasch R. M., Blanco A. M., Perez-Arago A., Minambres R., Talens-Visconti R., Peris B., Guerri C. (2007) RhoE participates in the stimulation of the inflammatory response induced by ethanol in astrocytes. Exp. Cell Res. 313, 3779–3788 [DOI] [PubMed] [Google Scholar]
- 80. Szabo G., Dolganiuc A., Dai Q., Pruett S. B. (2007) TLR4, ethanol, and lipid rafts: a new mechanism of ethanol action with implications for other receptor-mediated effects. J. Immunol. 178, 1243–1249 [DOI] [PubMed] [Google Scholar]
- 81. Dolganiuc A., Bakis G., Kodys K., Mandrekar P., Szabo G. (2006) Acute ethanol treatment modulates Toll-like receptor-4 association with lipid rafts. Alcohol. Clin. Exp. Res. 30, 76–85 [DOI] [PubMed] [Google Scholar]
- 82. Benedetti A., Tangorra A., Svegliati Baroni G., Ferretti G., Marucci L., Jezequel M., Orlandi F. (1994) Plasma membrane order parameter in periportal and perivenular hepatocytes isolated from ethanol-treated rats. Am. J. Physiol. 266, G282–G291 [DOI] [PubMed] [Google Scholar]
- 83. Yamada S., Lieber C. S. (1984) Decrease in microviscosity and cholesterol content of rat liver plasma membranes after chronic ethanol feeding. J. Clin. Invest. 74, 2285–2289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Polokoff M. A., Simon T. J., Harris R. A., Simon F. R., Iwahashi M. (1985) Chronic ethanol increases liver plasma membrane fluidity. Biochemistry 24, 3114–3120 [DOI] [PubMed] [Google Scholar]
- 85. Shen Y., Kawamura I., Nomura T., Tsuchiya K., Hara H., Dewamitta S. R., Sakai S., Qu H., Daim S., Yamamoto T., Mitsuyama M. (2010) Toll-like receptor 2- and MyD88-dependent phosphatidylinositol 3-kinase and Rac1 activation facilitates the phagocytosis of Listeria monocytogenes by murine macrophages. Infect. Immun. 78, 2857–2867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Rehm J., Room R., Graham K., Monteiro M., Gmel G., Sempos C. T. (2003) The relationship of average volume of alcohol consumption and patterns of drinking to burden of disease: an overview. Addiction 98, 1209–1228 [DOI] [PubMed] [Google Scholar]
- 87. Puddey I. B., Rakic V., Dimmitt S. B., Beilin L. J. (1999) Influence of pattern of drinking on cardiovascular disease and cardiovascular risk factors—a review. Addiction 94, 649–663 [DOI] [PubMed] [Google Scholar]
- 88. Witztum J. L., Steinberg D. (1991) Role of oxidized low density lipoprotein in atherogenesis. J. Clin. Invest. 88, 1785–1792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. De Oliveira E Silva E. R., Foster D., McGee Harper M., Seidman C. E., Smith J. D., Breslow J. L., Brinton E. A. (2000) Alcohol consumption raises HDL cholesterol levels by increasing the transport rate of apolipoproteins A-I and A-II. Circulation 102, 2347–2352 [DOI] [PubMed] [Google Scholar]
- 90. Linn S., Carroll M., Johnson C., Fulwood R., Kalsbeek W., Briefel R. (1993) High-density lipoprotein cholesterol and alcohol consumption in US white and black adults: data from NHANES II. Am. J. Public Health 83, 811–816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Merritt R., Guruge B. L., Miller D. D., Chaitman B. R., Bora P. S. (1997) Moderate alcohol feeding attenuates postinjury vascular cell proliferation in rabbit angioplasty model. J. Cardiovasc. Pharmacol. 30, 19–25 [DOI] [PubMed] [Google Scholar]
- 92. Moss M., Burnham E. L. (2003) Chronic alcohol abuse, acute respiratory distress syndrome, and multiple organ dysfunction. Crit. Care Med. 31, S207–S212 [DOI] [PubMed] [Google Scholar]
- 93. Marik P. E. (2000) The clinical features of severe community-acquired pneumonia presenting as septic shock. Norasept II Study Investigators. J. Crit. Care 15, 85–90 [DOI] [PubMed] [Google Scholar]
- 94. Schmidt W., De Lint J. (1972) Causes of death of alcoholics. Q. J. Stud. Alcohol 33, 171–185 [PubMed] [Google Scholar]
- 95. Saitz R., Ghali W. A., Moskowitz M. A. (1997) The impact of alcohol-related diagnoses on pneumonia outcomes. Arch. Intern. Med. 157, 1446–1452 [PubMed] [Google Scholar]
- 96. Dube S. R., McClave A., James C., Caraballo R., Kaufmann R., Pechacek T. (2010) Vital signs: current cigarette smoking among adults aged ≥18 years—United States, 2009. Morbidity and Mortality Weekly Report (MMWR), vol. 59, 1135–1140 [PubMed] [Google Scholar]
- 97. Ruiz M., Ewig S., Marcos M. A., Martinez J. A., Arancibia F., Mensa J., Torres A. (1999) Etiology of community-acquired pneumonia: impact of age, comorbidity, and severity. Am. J. Respir. Crit. Care Med. 160, 397–405 [DOI] [PubMed] [Google Scholar]
- 98. Sopori M. (2002) Effects of cigarette smoke on the immune system. Nat. Rev. Immunol. 2, 372–377 [DOI] [PubMed] [Google Scholar]
- 99. Barnes P. J. (2008) Immunology of asthma and chronic obstructive pulmonary disease. Nat. Rev. Immunol. 8, 183–192 [DOI] [PubMed] [Google Scholar]
- 100. Sethi S. (2000) Bacterial infection and the pathogenesis of COPD. Chest 117, 286S–291S [DOI] [PubMed] [Google Scholar]
- 101. Sethi S., Murphy T. F. (2001) Bacterial infection in chronic obstructive pulmonary disease in 2000: a state-of-the-art review. Clin. Microbiol. Rev. 14, 336–363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Sethi S., Murphy T. F. (2008) Infection in the pathogenesis and course of chronic obstructive pulmonary disease. N. Engl. J. Med. 359, 2355–2365 [DOI] [PubMed] [Google Scholar]
- 103. Wang H., Liu X., Umino T., Skold C. M., Zhu Y., Kohyama T., Spurzem J. R., Romberger D. J., Rennard S. I. (2001) Cigarette smoke inhibits human bronchial epithelial cell repair processes. Am. J. Respir. Cell Mol. Biol. 25, 772–779 [DOI] [PubMed] [Google Scholar]
- 104. Wickenden J. A., Clarke M. C., Rossi A. G., Rahman I., Faux S. P., Donaldson K., MacNee W. (2003) Cigarette smoke prevents apoptosis through inhibition of caspase activation and induces necrosis. Am. J. Respir. Cell Mol. Biol. 29, 562–570 [DOI] [PubMed] [Google Scholar]
- 105. Shephard R. J. (1978) Cigarette smoking and reactions to air pollutants. Can. Med. Assoc. J. 118, 379–381, 383, 392 [PMC free article] [PubMed] [Google Scholar]
- 106. Hodge S., Hodge G., Ahern J., Jersmann H., Holmes M., Reynolds P. N. (2007) Smoking alters alveolar macrophage recognition and phagocytic ability: implications in chronic obstructive pulmonary disease. Am. J. Respir. Cell Mol. Biol. 37, 748–755 [DOI] [PubMed] [Google Scholar]
- 107. Hodge S., Matthews G., Mukaro V., Ahern J., Shivam A., Hodge G., Holmes M., Jersmann H., Reynolds P. N. (2011) Cigarette smoke-induced changes to alveolar macrophage phenotype and function is improved by treatment with procysteine. Am. J. Respir. Cell Mol. Biol. 44, 673–681 [DOI] [PubMed] [Google Scholar]
- 108. Richens T. R., Linderman D. J., Horstmann S. A., Lambert C., Xiao Y. Q., Keith R. L., Boe D. M., Morimoto K., Bowler R. P., Day B. J., Janssen W. J., Henson P. M., Vandivier R. W. (2009) Cigarette smoke impairs clearance of apoptotic cells through oxidant-dependent activation of RhoA. Am. J. Respir. Crit. Care Med. 179, 1011–1021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Petrusca D. N., Gu Y., Adamowicz J. J., Rush N. I., Hubbard W. C., Smith P. A., Berdyshev E. V., Birukov K. G., Lee C. H., Tuder R. M., Twig H. L., III, Vandivier R. W., Petrache I. (2010) Sphingolipid-mediated inhibition of apoptotic cell clearance by alveolar macrophages. J. Biol. Chem. 285, 40322–40332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Martí-Lliteras P., Regueiro V., Morey P., Hood D. W., Saus C., Sauleda J., Agusti A. G., Bengoechea J. A., Garmendia J. (2009) Nontypeable haemophilus influenzae clearance by alveolar macrophages is impaired by exposure to cigarette smoke. Infect. Immun. 77, 4232–4242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Lundborg M., Dahlen S. E., Johard U., Gerde P., Jarstrand C., Camner P., Lastbom L. (2006) Aggregates of ultrafine particles impair phagocytosis of microorganisms by human alveolar macrophages. Environ. Res. 100, 197–204 [DOI] [PubMed] [Google Scholar]
- 112. Jakab G. J., Risby T. H., Sehnert S. S., Hmieleski R. R., Farrington J. E. (1990) Suppression of alveolar macrophage membrane receptor-mediated phagocytosis by model and actual particle-adsorbate complexes. Initial contact with the alveolar macrophage membrane. Environ. Health Perspect. 86, 337–344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Zhou H., Kobzik L. (2007) Effect of concentrated ambient particles on macrophage phagocytosis and killing of Streptococcus pneumoniae. Am. J. Respir. Cell Mol. Biol. 36, 460–465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Gilmour M. I., Park P., Doerfler D., Selgrade M. K. (1993) Factors that influence the suppression of pulmonary antibacterial defenses in mice exposed to ozone. Exp. Lung Res. 19, 299–314 [DOI] [PubMed] [Google Scholar]
- 115. Van Loveren H., Rombout P. J., Wagenaar S. S., Walvoort H. C., Vos J. G. (1988) Effects of ozone on the defense to a respiratory Listeria monocytogenes infection in the rat. Suppression of macrophage function and cellular immunity and aggravation of histopathology in lung and liver during infection. Toxicol. Appl. Pharmacol. 94, 374–393 [DOI] [PubMed] [Google Scholar]
- 116. Oosting R. S., Van Iwaarden J. F., Van Bree L., Verhoef J., Van Golde L. M., Haagsman H. P. (1992) Exposure of surfactant protein A to ozone in vitro and in vivo impairs its interactions with alveolar cells. Am. J. Physiol. 262, L63–L68 [DOI] [PubMed] [Google Scholar]
- 117. Mikerov A. N., Umstead T. M., Gan X., Huang W., Guo X., Wang G., Phelps D. S., Floros J. (2008) Impact of ozone exposure on the phagocytic activity of human surfactant protein A (SP-A) and SP-A variants. Am. J. Physiol. Lung Cell. Mol. Physiol. 294, L121–L130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Kirkham P. A., Spooner G., Ffoulkes-Jones C., Calvez R. (2003) Cigarette smoke triggers macrophage adhesion and activation: role of lipid peroxidation products and scavenger receptor. Free Radic. Biol. Med. 35, 697–710 [DOI] [PubMed] [Google Scholar]
- 119. Becker S., Soukup J. M. (1998) Decreased CD11b expression, phagocytosis, and oxidative burst in urban particulate pollution-exposed human monocytes and alveolar macrophages. J. Toxicol. Environ. Health A 55, 455–477 [DOI] [PubMed] [Google Scholar]
- 120. Hollingsworth J. W., Maruoka S., Li Z., Potts E. N., Brass D. M., Garantziotis S., Fong A., Foster W. M., Schwartz D. A. (2007) Ambient ozone primes pulmonary innate immunity in mice. J. Immunol. 179, 4367–4375 [DOI] [PubMed] [Google Scholar]
- 121. Dahl M., Bauer A. K., Arredouani M., Soininen R., Tryggvason K., Kleeberger S. R., Kobzik L. (2007) Protection against inhaled oxidants through scavenging of oxidized lipids by macrophage receptors MARCO and SR-AI/II. J. Clin. Invest. 117, 757–764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Koike E., Kobayashi T., Shimojo N. (2001) Ozone exposure enhances expression of cell-surface molecules associated with antigen-presenting activity on bronchoalveolar lavage cells in rats. Toxicol. Sci. 63, 115–124 [DOI] [PubMed] [Google Scholar]
- 123. Ishida T., Pinkerton K. E., Takeuchi M. (2009) Alveolar macrophage from cigarette smoke-exposed mice inhibits B lymphocyte proliferation stimulated with LPS. Respiration 77, 91–95 [DOI] [PubMed] [Google Scholar]
- 124. Sawyer K., Mundandhara S., Ghio A. J., Madden M. C. (2010) The effects of ambient particulate matter on human alveolar macrophage oxidative and inflammatory responses. J. Toxicol. Environ. Health A 73, 41–57 [DOI] [PubMed] [Google Scholar]
- 125. Gunella G., Bardelli C., Amoruso A., Viano I., Balbo P., Brunelleschi S. (2006) Macrophage-stimulating protein differently affects human alveolar macrophages from smoker and non-smoker patients: evaluation of respiratory burst, cytokine release and NF-κB pathway. Br. J. Pharmacol. 148, 478–489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Aam B. B., Fonnum F. (2007) ROS scavenging effects of organic extract of diesel exhaust particles on human neutrophil granulocytes and rat alveolar macrophages. Toxicology 230, 207–218 [DOI] [PubMed] [Google Scholar]
- 127. Yin X. J., Ma J. Y., Antonini J. M., Castranova V., Ma J. K. (2004) Roles of reactive oxygen species and heme oxygenase-1 in modulation of alveolar macrophage-mediated pulmonary immune responses to Listeria monocytogenes by diesel exhaust particles. Toxicol. Sci. 82, 143–153 [DOI] [PubMed] [Google Scholar]
- 128. Ryer-Powder J. E., Amoruso M. A., Czerniecki B., Witz G., Goldstein B. D. (1988) Inhalation of ozone produces a decrease in superoxide anion radical production in mouse alveolar macrophages. Am. Rev. Respir. Dis. 138, 1129–1133 [DOI] [PubMed] [Google Scholar]
- 129. March T. H., Barr E. B., Finch G. L., Nikula K. J., Seagrave J. C. (2002) Effects of concurrent ozone exposure on the pathogenesis of cigarette smoke-induced emphysema in B6C3F1 mice. Inhal. Toxicol. 14, 1187–1213 [DOI] [PubMed] [Google Scholar]
- 130. Wang S., Lantz R. C., Vermeulen M. W., Chen G. J., Breceda V., Robledo R. F., Hays A. M., Young S., Witten M. L. (1999) Functional alterations of alveolar macrophages subjected to smoke exposure and antioxidant lazaroids. Toxicol. Ind. Health 15, 464–469 [DOI] [PubMed] [Google Scholar]
- 131. Tosello-Trampont A. C., Nakada-Tsukui K., Ravichandran K. S. (2003) Engulfment of apoptotic cells is negatively regulated by Rho-mediated signaling. J. Biol. Chem. 278, 49911–49919 [DOI] [PubMed] [Google Scholar]
- 132. Vandivier R. W., Henson P. M., Douglas I. S. (2006) Burying the dead: the impact of failed apoptotic cell removal (efferocytosis) on chronic inflammatory lung disease. Chest 129, 1673–1682 [DOI] [PubMed] [Google Scholar]
- 133. Leverrier Y., Ridley A. J. (2001) Requirement for Rho GTPases and PI 3-kinases during apoptotic cell phagocytosis by macrophages. Curr. Biol. 11, 195–199 [DOI] [PubMed] [Google Scholar]
- 134. Park D., Tosello-Trampont A. C., Elliott M. R., Lu M., Haney L. B., Ma Z., Klibanov A. L., Mandell J. W., Ravichandran K. S. (2007) BAI1 is an engulfment receptor for apoptotic cells upstream of the ELMO/Dock180/Rac module. Nature 450, 430–434 [DOI] [PubMed] [Google Scholar]
- 135. Ogden C. A., deCathelineau A., Hoffmann P. R., Bratton D., Ghebrehiwet B., Fadok V. A., Henson P. M. (2001) C1q and mannose binding lectin engagement of cell surface calreticulin and CD91 initiates macropinocytosis and uptake of apoptotic cells. J. Exp. Med. 194, 781–795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Hodge S., Matthews G., Dean M. M., Ahern J., Djukic M., Hodge G., Jersmann H., Holmes M., Reynolds P. N. (2010) Therapeutic role for mannose-binding lectin in cigarette smoke-induced lung inflammation? Evidence from a murine model. Am. J. Respir. Cell Mol. Biol. 42, 235–242 [DOI] [PubMed] [Google Scholar]
- 137. Drannik A. G., Pouladi M. A., Robbins C. S., Goncharova S. I., Kianpour S., Stampfli M. R. (2004) Impact of cigarette smoke on clearance and inflammation after Pseudomonas aeruginosa infection. Am. J. Respir. Crit. Care Med. 170, 1164–1171 [DOI] [PubMed] [Google Scholar]
- 138. Thomas W. R., Holt P. G., Keast D. (1978) Cigarette smoke and phagocyte function: effect of chronic exposure in vivo and acute exposure in vitro. Infect. Immun. 20, 468–475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Esterbauer H., Schaur R. J., Zollner H. (1991) Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic. Biol. Med. 11, 81–128 [DOI] [PubMed] [Google Scholar]
- 140. Kirkham P. A., Spooner G., Rahman I., Rossi A. G. (2004) Macrophage phagocytosis of apoptotic neutrophils is compromised by matrix proteins modified by cigarette smoke and lipid peroxidation products. Biochem. Biophys. Res. Commun. 318, 32–37 [DOI] [PubMed] [Google Scholar]
- 141. Brown S., Heinisch I., Ross E., Shaw K., Buckley C. D., Savill J. (2002) Apoptosis disables CD31-mediated cell detachment from phagocytes promoting binding and engulfment. Nature 418, 200–203 [DOI] [PubMed] [Google Scholar]
- 142. Gardai S. J., Xiao Y. Q., Dickinson M., Nick J. A., Voelker D. R., Greene K. E., Henson P. M. (2003) By binding SIRPα or calreticulin/CD91, lung collectins act as dual function surveillance molecules to suppress or enhance inflammation. Cell 115, 13–23 [DOI] [PubMed] [Google Scholar]
- 143. Hart S. P., Dougherty G. J., Haslett C., Dransfield I. (1997) CD44 regulates phagocytosis of apoptotic neutrophil granulocytes, but not apoptotic lymphocytes, by human macrophages. J. Immunol. 159, 919–925 [PubMed] [Google Scholar]
- 144. Bobo J. K., Husten C. (2000) Sociocultural influences on smoking and drinking. Alcohol Res. Health 24, 225–232 [PMC free article] [PubMed] [Google Scholar]
- 145. Vander Top E. A., Perry G. A., Snitily M. U., Gentry-Nielsen M. J. (2006) Smoke exposure and ethanol ingestion modulate intrapulmonary polymorphonuclear leukocyte killing, but not recruitment or phagocytosis. Alcohol. Clin. Exp. Res. 30, 1599–1607 [DOI] [PubMed] [Google Scholar]
- 146. Valdimarsson H., Vikingsdottir T., Bang P., Saevarsdottir S., Gudjonsson J. E., Oskarsson O., Christiansen M., Blou L., Laursen I., Koch C. (2004) Human plasma-derived mannose-binding lectin: a phase I safety and pharmacokinetic study. Scand. J. Immunol. 59, 97–102 [DOI] [PubMed] [Google Scholar]
- 147. Dockery D. W., Pope C. A., III, Xu X., Spengler J. D., Ware J. H., Fay M. E., Ferris B. G., Jr., Speizer F. E. (1993) An association between air pollution and mortality in six U.S. cities. N. Engl. J. Med. 329, 1753–1759 [DOI] [PubMed] [Google Scholar]
- 148. Bell M. L., McDermott A., Zeger S. L., Samet J. M., Dominici F. (2004) Ozone and short-term mortality in 95 US urban communities, 1987–2000. JAMA 292, 2372–2378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Gryparis A., Forsberg B., Katsouyanni K., Analitis A., Touloumi G., Schwartz J., Samoli E., Medina S., Anderson H. R., Niciu E. M., Wichmann H. E., Kriz B., Kosnik M., Skorkovsky J., Vonk J. M., Dörtbudak Z. (2004) Acute effects of ozone on mortality from the “air pollution and health: a European approach” project. Am. J. Respir. Crit. Care Med. 170, 1080–1087 [DOI] [PubMed] [Google Scholar]
- 150. Katsouyanni K., Zmirou D., Spix C., Sunyer J., Schouten J. P., Ponka A., Anderson H. R., Le Moullec Y., Wojtyniak B., Vigotti M. A., Bacharova L., Schwartz J. (1995) Short-term effects of air pollution on health: a European approach using epidemiological time-series data. The APHEA project: background, objectives, design. Eur. Respir. J. 8, 1030–1038 [PubMed] [Google Scholar]
- 151. Hubbell B. J., Hallberg A., McCubbin D. R., Post E. (2005) Health-related benefits of attaining the 8-hr ozone standard. Environ. Health Perspect. 113, 73–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Gent J. F., Triche E. W., Holford T. R., Belanger K., Bracken M. B., Beckett W. S., Leaderer B. P. (2003) Association of low-level ozone and fine particles with respiratory symptoms in children with asthma. JAMA 290, 1859–1867 [DOI] [PubMed] [Google Scholar]
- 153. Burnett R. T., Smith-Doiron M., Stieb D., Raizenne M. E., Brook J. R., Dales R. E., Leech J. A., Cakmak S., Krewski D. (2001) Association between ozone and hospitalization for acute respiratory diseases in children less than 2 years of age. Am. J. Epidemiol. 153, 444–452 [DOI] [PubMed] [Google Scholar]
- 154. Schwartz J. (1995) Short term fluctuations in air pollution and hospital admissions of the elderly for respiratory disease. Thorax 50, 531–538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Parodi S., Vercelli M., Garrone E., Fontana V., Izzotti A. (2005) Ozone air pollution and daily mortality in Genoa, Italy between 1993 and 1996. Public Health 119, 844–850 [DOI] [PubMed] [Google Scholar]
- 156. Ito K., De Leon S. F., Lippmann M. (2005) Associations between ozone and daily mortality: analysis and meta-analysis. Epidemiology 16, 446–457 [DOI] [PubMed] [Google Scholar]
- 157. Levy J. I., Chemerynski S. M., Sarnat J. A. (2005) Ozone exposure and mortality: an empiric bayes metaregression analysis. Epidemiology 16, 458–468 [DOI] [PubMed] [Google Scholar]
- 158. Medina-Ramón M., Zanobetti A., Schwartz J. (2006) The effect of ozone and PM10 on hospital admissions for pneumonia and chronic obstructive pulmonary disease: a national multicity study. Am. J. Epidemiol. 163, 579–588 [DOI] [PubMed] [Google Scholar]
- 159. Aranyi C., Vana S. C., Thomas P. T., Bradof J. N., Fenters J. D., Graham J. A., Miller F. J. (1983) Effects of subchronic exposure to a mixture of O3, SO2, and (NH4)2SO4 on host defenses of mice. J. Toxicol. Environ. Health 12, 55–71 [DOI] [PubMed] [Google Scholar]
- 160. Goldstein E., Tyler W. S., Hoeprich P. D., Eagle C. (1971) Ozone and the antibacterial defense mechanisms of the murine lung. Arch. Intern. Med. 127, 1099–1102 [PubMed] [Google Scholar]
- 161. Miller S., Ehrlich R. (1958) Susceptibility to respiratory infections of animals exposed to ozone. I. Susceptibility to Klebsiella pneumoniae. J. Infect. Dis. 103, 145–149 [DOI] [PubMed] [Google Scholar]
- 162. Thomas G. B., Fenters J. D., Ehrlich R., Gardner D. E. (1981) Effects of exposure to ozone on susceptibility to experimental tuberculosis. Toxicol. Lett. 9, 11–17 [DOI] [PubMed] [Google Scholar]
- 163. Weinmann G. G., Bowes S. M., Gerbase M. W., Kimball A. W., Frank R. (1995) Response to acute ozone exposure in healthy men. Results of a screening procedure. Am. J. Respir. Crit. Care Med. 151, 33–40 [DOI] [PubMed] [Google Scholar]
- 164. Prows D. R., Shertzer H. G., Daly M. J., Sidman C. L., Leikauf G. D. (1997) Genetic analysis of ozone-induced acute lung injury in sensitive and resistant strains of mice. Nat. Genet. 17, 471–474 [DOI] [PubMed] [Google Scholar]
- 165. Schlesinger R. B., Zelikoff J. T., Chen L. C., Kinney P. L. (1992) Assessment of toxicologic interactions resulting from acute inhalation exposure to sulfuric acid and ozone mixtures. Toxicol. Appl. Pharmacol. 115, 183–190 [DOI] [PubMed] [Google Scholar]
- 166. Driscoll K. E., Vollmuth T. A., Schlesinger R. B. (1987) Acute and subchronic ozone inhalation in the rabbit: response of alveolar macrophages. J. Toxicol. Environ. Health 21, 27–43 [DOI] [PubMed] [Google Scholar]
- 167. Becker S., Madden M. C., Newman S. L., Devlin R. B., Koren H. S. (1991) Modulation of human alveolar macrophage properties by ozone exposure in vitro. Toxicol. Appl. Pharmacol. 110, 403–415 [DOI] [PubMed] [Google Scholar]
- 168. Mikerov A. N., Haque R., Gan X., Guo X., Phelps D. S., Floros J. (2008) Ablation of SP-A has a negative impact on the susceptibility of mice to Klebsiella pneumoniae infection after ozone exposure: sex differences. Respir. Res. 9, 77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Kenworthy J., Laube F. (2002) Urban transport patterns in a global sample of cities and their linkages to transport infrastructures, land use, economics and environment. World Transport Policy and Practice, vol. 8, Eco-Logica, Lancaster, UK, 5–19 [Google Scholar]
- 170. Aberg N. (1989) Asthma and allergic rhinitis in Swedish conscripts. Clin. Exp. Allergy 19, 59–63 [DOI] [PubMed] [Google Scholar]
- 171. Burney P. (1988) Asthma deaths in England and Wales 1931–85: evidence for a true increase in asthma mortality. J. Epidemiol. Community Health 42, 316–320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Diaz-Sanchez D. (1997) The role of diesel exhaust particles and their associated polyaromatic hydrocarbons in the induction of allergic airway disease. Allergy 52, 52–56, discussion 57–58 [DOI] [PubMed] [Google Scholar]
- 173. Diaz-Sanchez D., Dotson A. R., Takenaka H., Saxon A. (1994) Diesel exhaust particles induce local IgE production in vivo and alter the pattern of IgE messenger RNA isoforms. J. Clin. Invest. 94, 1417–1425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Haahtela T., Lindholm H., Bjorksten F., Koskenvuo K., Laitinen L. A. (1990) Prevalence of asthma in Finnish young men. BMJ 301, 266–268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Li X. Y., Gilmour P. S., Donaldson K., MacNee W. (1996) Free radical activity and pro-inflammatory effects of particulate air pollution (PM10) in vivo and in vitro. Thorax 51, 1216–1222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Miyamoto T. (1997) Epidemiology of pollution-induced airway disease in Japan. Allergy 52, 30–34, discussion 35–36 [DOI] [PubMed] [Google Scholar]
- 177. Nel A. E., Diaz-Sanchez D., Ng D., Hiura T., Saxon A. (1998) Enhancement of allergic inflammation by the interaction between diesel exhaust particles and the immune system. J. Allergy Clin. Immunol. 102, 539–554 [DOI] [PubMed] [Google Scholar]
- 178. Peterson B., Saxon A. (1996) Global increases in allergic respiratory disease: the possible role of diesel exhaust particles. Ann. Allergy Asthma Immunol. 77, 263–268, quiz 269–270 [DOI] [PubMed] [Google Scholar]
- 179. Yunginger J. W., Reed C. E., O'Connell E. J., Melton L. J., III, O'Fallon W. M., Silverstein M. D. (1992) A community-based study of the epidemiology of asthma. Incidence rates, 1964–1983. Am. Rev. Respir. Dis. 146, 888–894 [DOI] [PubMed] [Google Scholar]
- 180. Barlow P. G., Brown D. M., Donaldson K., MacCallum J., Stone V. (2008) Reduced alveolar macrophage migration induced by acute ambient particle (PM10) exposure. Cell Biol. Toxicol. 24, 243–252 [DOI] [PubMed] [Google Scholar]
- 181. Diaz-Sanchez D., Tsien A., Fleming J., Saxon A. (1997) Combined diesel exhaust particulate and ragweed allergen challenge markedly enhances human in vivo nasal ragweed-specific IgE and skews cytokine production to a T helper cell 2-type pattern. J. Immunol. 158, 2406–2413 [PubMed] [Google Scholar]
- 182. Miyabara Y., Takano H., Ichinose T., Lim H. B., Sagai M. (1998) Diesel exhaust enhances allergic airway inflammation and hyperresponsiveness in mice. Am. J. Respir. Crit. Care Med. 157, 1138–1144 [DOI] [PubMed] [Google Scholar]
- 183. Takano H., Yoshikawa T., Ichinose T., Miyabara Y., Imaoka K., Sagai M. (1997) Diesel exhaust particles enhance antigen-induced airway inflammation and local cytokine expression in mice. Am. J. Respir. Crit. Care Med. 156, 36–42 [DOI] [PubMed] [Google Scholar]
- 184. Fujimaki H., Saneyoshi K., Shiraishi F., Imai T., Endo T. (1997) Inhalation of diesel exhaust enhances antigen-specific IgE antibody production in mice. Toxicology 116, 227–233 [DOI] [PubMed] [Google Scholar]
- 185. Künzli N., Kaiser R., Medina S., Studnicka M., Chanel O., Filliger P., Herry M., Horak F., Jr., Puybonnieux-Texier V., Quénel P., Schneider J., Seethaler R., Vergnaud J. C., Sommer H. (2000) Public-health impact of outdoor and traffic-related air pollution: a European assessment. Lancet 356, 795–801 [DOI] [PubMed] [Google Scholar]
- 186. Pope C. A., III, Burnett R. T., Thun M. J., Calle E. E., Krewski D., Ito K., Thurston G. D. (2002) Lung cancer, cardiopulmonary mortality, and long-term exposure to fine particulate air pollution. JAMA 287, 1132–1141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Dockery D. W., Schwartz J., Spengler J. D. (1992) Air pollution and daily mortality: associations with particulates and acid aerosols. Environ. Res. 59, 362–373 [DOI] [PubMed] [Google Scholar]
- 188. Rudell B., Blomberg A., Helleday R., Ledin M. C., Lundback B., Stjernberg N., Horstedt P., Sandstrom T. (1999) Bronchoalveolar inflammation after exposure to diesel exhaust: comparison between unfiltered and particle trap filtered exhaust. Occup. Environ. Med. 56, 527–534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Bouhafs R. K., Jarstrand C., Robertson B. (2004) Lipid peroxidation of lung surfactant in experimental neonatal group B streptococcal pneumonia. Lung 182, 61–72 [DOI] [PubMed] [Google Scholar]
- 190. Möller W., Hofer T., Ziesenis A., Karg E., Heyder J. (2002) Ultrafine particles cause cytoskeletal dysfunctions in macrophages. Toxicol. Appl. Pharmacol. 182, 197–207 [DOI] [PubMed] [Google Scholar]
- 191. Grunwald U., Fan X., Jack R. S., Workalemahu G., Kallies A., Stelter F., Schutt C. (1996) Monocytes can phagocytose Gram-negative bacteria by a CD14-dependent mechanism. J. Immunol. 157, 4119–4125 [PubMed] [Google Scholar]
- 192. Yin X. J., Schafer R., Ma J. Y., Antonini J. M., Roberts J. R., Weissman D. N., Siegel P. D., Ma J. K. (2003) Alteration of pulmonary immunity to Listeria monocytogenes by diesel exhaust particles (DEPs). II. Effects of DEPs on T-cell-mediated immune responses in rats. Environ. Health Perspect. 111, 524–530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Yin X. J., Dong C. C., Ma J. Y., Roberts J. R., Antonini J. M., Ma J. K. (2007) Suppression of phagocytic and bactericidal functions of rat alveolar macrophages by the organic component of diesel exhaust particles. J. Toxicol. Environ. Health A 70, 820–828 [DOI] [PubMed] [Google Scholar]
- 194. Hwang J. S., Chan C. C. (2002) Effects of air pollution on daily clinic visits for lower respiratory tract illness. Am. J. Epidemiol. 155, 1–10 [DOI] [PubMed] [Google Scholar]
- 195. Fung K. Y., Luginaah I. N., Gorey K. M. (2007) Impact of air pollution on hospital admissions in Southwestern Ontario, Canada: generating hypotheses in sentinel high-exposure places. Environ. Health 6, 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Zebrowska A., Mankowski R. (2010) Effects of long-term exposure to air pollution on respiratory function and physical efficiency of pre-adolescent children. Eur. J. Med. Res. 15 (Suppl. 2), 224–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Zelikoff J. T., Parsons E., Schlesinger R. B. (1993) Inhalation of particulate lead oxide disrupts pulmonary macrophage-mediated functions important for host defense and tumor surveillance in the lung. Environ. Res. 62, 207–222 [DOI] [PubMed] [Google Scholar]
- 198. Selgrade M. K., Gilmour M. I. (2010) Suppression of pulmonary host defenses and enhanced susceptibility to respiratory bacterial infection in mice following inhalation exposure to trichloroethylene and chloroform. J. Immunotoxicol. 7, 350–356 [DOI] [PubMed] [Google Scholar]
- 199. Singh V. K., Mishra K. P., Rani R., Yadav V. S., Awasthi S. K., Garg S. K. (2003) Immunomodulation by lead. Immunol. Res. 28, 151–166 [DOI] [PubMed] [Google Scholar]
- 200. Tashkin D. P. (2005) Smoked marijuana as a cause of lung injury. Monaldi Arch. Chest Dis. 63, 93–100 [DOI] [PubMed] [Google Scholar]