Abstract
Calcium (Ca2+) signals are generated across a broad time range. Kinetic considerations impact how information is processed to encode and decode Ca2+ signals, the choreography of responses that ensure specific and efficient signaling and the overall temporal gearing such that ephemeral Ca2+ signals have lasting physiological value. The reciprocal importance of timing for Ca2+ signaling, and Ca2+ signaling for timing is exemplified by the altered kinetic profiles of Ca2+ signals in certain diseases and the likely role of basal Ca2+ fluctuations in the perception of time itself.
Introduction
Crudely, the business of Ca2+ signaling is one of information delivery. How do biological systems interpret environmental cues to choreograph the generation of Ca2+ signals and thereby execute appropriate physiological responses? Understanding how inputs are processed and relayed via changes in cytoplasmic Ca2+ concentration (‘encoding’) to impact the activity of only a desired subset of Ca2+-sensitive targets (‘decoding’) has proved to be a durable and mechanistically intriguing field of research, as well as one of increasing pathological pertinence.
Ca2+ signals display great spatiotemporal malleability. They are generated across wide spatial and temporal ranges (nanometer to centimeter, microsecond to hour). This broad scope disguises additional flexibility in the size, source, spread, persistence and rhythm of cytoplasmic Ca2+ changes coordinated through the specific organization and properties (the ‘functional architecture’) of Ca2+ channels, pumps, buffers and exchangers in any given cell type. Therefore, as spatial and temporal controls are inseparable orchestrators of Ca2+ signals, our focus here on issues of ‘timing’ is somewhat contrived. Consequently, we direct readers to broader reviews [1-4] and discuss here solely principles of timing in Ca2+ signaling and examples that showcase their application.
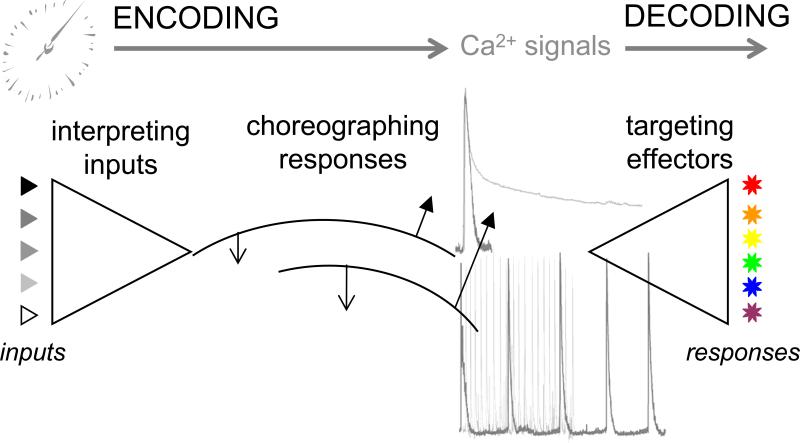
Timing pervades all aspects of Ca2+ signaling, affecting how environmental information is compiled, encrypted and deciphered (overview in Figure 1). Temporal considerations govern: (1) how incoming information is both processed at the cell surface and resolved by effectors (‘interpreting inputs’), (2) how reactions are set into motion with appropriate sequentiality and interdependence, cueing processes in an order determined by feedback from and dialog with other signaling pathways (‘choreographing responses’) and (3) how Ca2+-dependent effectors are differentially activated (‘targeting effectors’) by temporal aspects of cytoplasmic Ca2+ signals (notably their frequency or duration). These topics are discussed below.
Figure 1. Kinetic orchestration of Ca2+ signals.
Schematic overview of the impact of timing in cellular Ca2+ signaling. Aspects of timing impact how extracellular signals are interpreted and choreographed into specific profiles of cytoplasmic [Ca2+] signals (‘encoding’). Different Ca2+ signals result depending on how cells interpret dynamic environmental cues (‘integrating inputs’, triangles) to contextualize and temporally order specific signaling processes (‘choreographing responses’). Temporal aspects of the resultant cytoplasmic Ca2+ signal – notably, variability in duration (top) and periodicity (lower) – are sensed by subsets of Ca2+-sensitive effectors via Ca2+-binding sites of varied affinity, kinetics and interdependence, resulting in their selective activation (‘decoding: targeting effectors’) to yield discrete responses (stars).
Interpreting Inputs
Single Inputs: Context
A first example of time-dependent sensitivity in Ca2+ signaling is ‘context’: scenarios where responsiveness to a signal is state-dependent (Figure 2a). At one end of the spectrum, this represents gain-of-function scenarios where a signal is ineffective at evoking a response at one point in time, but not at another. More subtly, different outcomes may be associated with the same input when presented at different times (e.g different antigen-evoked Ca2+ signals in naïve and primed lymphocytes [4,5]). If time is ‘that great gift of nature which keeps everything from happening at once’, context is the timekeeper that paces change via external or autonomous cues. In short, such contextual cues distill specificity from pervasive signals.
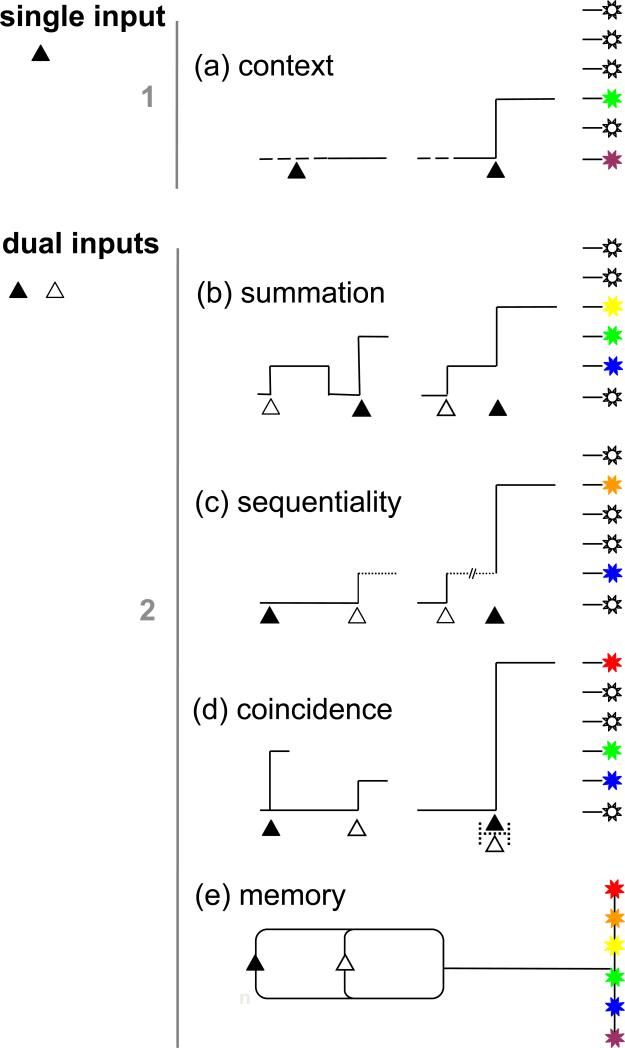
Figure 2. Temporal integration empowers diverse outcomes from limited inputs.
Principles of timing impacting signal interpretation for single and multiple (dual) inputs. (a) ‘Context’ determines whether a single (‘1’) signal will be effective at evoking a response (▲ → no response/response). Bottom, Two inputs associated with specific individual responses can produce several different responses (right) when (b) the duration of their effects compound (‘summation’), (c) the order of their presentation matters (‘sequentiality’; ▲△→response ‘x’, △▲→ response ‘y’), (d) the relative timing, but not necessarily order, of the arrival of each input is critical (‘coincidence detection’, ▲...△/△...▲→ response ‘x’, △▲ or ▲△→ response ‘y’) or (e) an example of ‘associative’ memory where specific combinations of presented signals (e.g. one of two signals ▲ and △ presented ‘n’ times) can trigger a number of unique (≤2n) responses. Strategies for temporal ‘memory’ of Ca2+ signals (e.g. priming & persistence, are discussed under ‘duration’).
Clearcut examples of state-dependent responsiveness are found during the natural temporal progression of development and cellular differentiation, and during acute refractory states in excitable tissue. Sperm-evoked Ca2+ signaling is ineffective at fertilizing oocytes, until maturation (minutes to days) renders a competent egg. Depolarizing stimuli are ineffective at stimulating Ca2+ entry until the developmental timepoint when voltage-gated Ca2+ channels are expressed [6,7]. Defined periods of ligand sensitivity are seen with several Ca2+-modulating agonists [7,8]. Windows of ‘competency’ to inductive signaling during embryogenesis exist transiently and execute irreversibly on receiving appropriate inputs. Since the activity of many inducing factors modulate, and/or are modulated by local Ca2+ concentration [9-12], it is unsurprising that embryonic Ca2+ transients/gradients are implicated in patterning and specification [13-16]. Potential mechanisms delimiting these periods include temporal regulation of receptor transcription/translation, and restricted spatial expression of Ca2+ channels [17,18]. Alternatively, targets may be present but inhibited, and time must pass before attenuatory regulation is relieved [19,20]. Finally, it is important to point out that the functional readout of Ca2+ signaling networks may change over developmental time [13]: early non-canonical Wnt (Wnt-Ca2+) signaling is associated with specification events (ventral patterning, [15,16]) but later with morphogenesis (e.g. convergent extension, [21]); Ca2+ oscillations can either initiate development or terminate cellular viability, depending on the age of the egg when fertilized [22].
Ca2+ signals observed during cellular differentiation [6,23,24], reinforce differentiative events and possibly impact their intrinsic timing. One example is the ordered production of first neurons, then glia from multipotent neuronal stem cells in the developing vertebrate cortex. This timing mechanism is contextual: environmental cues bias outcomes, even though the intrinsic timing mechanism is hard-wired into isolated clones [25]. Spontaneous Ca2+ entry signals observed during neuronal progenitor differentiation [6,23,24] may impact neuro/gliogenic switching through global epigenetic control. Methyl-CpG-binding protein 2, a transcriptional repressor with affinity for glial specific promoters, is subject to a Ca2+ influx dependent phosphorylation that relieves transcriptional repression [12,25,26].
Multiple Inputs: Summation, Sequentiality, Coincidence & Memory
Timing provides further flexibility in information processing where multiple inputs are involved (Figure 2). Kinetic considerations ensure that two inputs do not invariantly produce only two outputs. Changes in the duration, sequentiality and temporal convergence of incoming signals ensure a multiplicity of biological outcomes to a pair of stimuli. Here, we have broadly corralled examples under the headings of ‘summation’ (the effects of two Ca2+ signals compound to ensure a discrete outcome, Figure 2b), ‘sequentiality’ (the order of presentation of two signals dictates different outcomes, Figure 2c), ‘coincidence detection’ (the arrival of two different signals within a set timeframe ensures a discrete outcome, Figure 2d) and ‘memory’ (certain combinations/sequences of repetitive signals associate with specific responses, Figure 2e). Although this categorization is fluid (for example, many coincidence detectors are also sensitive to the order inputs are presented), they provide a simple framework for discussion.
Summation is frequently the basis of ‘amplitude modulation’ [4] in Ca2+ signaling (Figure 2b). When different inputs converge to the same signaling currency (Ca2+) and their effects overlap, the resulting cytosolic Ca2+ increases compound to yield unique responses. Summation of Ca2+ signals is not only important for activating targets of progressively lower Ca2+ affinity, but also for triggering cellular Ca2+ signals. In the majority of cell types, initiation of a Ca2+ wave is regulated by local summation of short lasting Ca2+ fluxes that drive the ambient cytoplasmic Ca2+ concentration ([Ca2+]cyt) toward a threshold at which regenerative Ca2+ release occurs [2,27,28]. As Ca2+ channels integrate diverse regulatory inputs, there can be considerable variability in the occurrence and duration of unitary Ca2+ release events that impact their summation. The threshold local [Ca2+]cyt needed to evoke regenerative Ca2+ release is also variable, related to the global behaviour of the cytoplasm as an ‘excitable medium’, an integrative gauge of second messenger levels and the repleteness of cellular Ca2+ stores at any point in time. Therefore, temporal summation shapes both the generation of Ca2+ signals and the ensuing responses.
In paradigms of ‘sequentiality’ (Figure 2c), the order of presentation of inputs is important. Certain input combinations render one response, other combinations another or no response at all. A classic paradigm involving sequentiality (and co-incidence) is spike-timing dependent plasticity (STDP), where the same presynaptic and postsynaptic action potentials can strengthen or weaken synaptic efficiency depending on their order [29,30]. At the molecular level, sequentiality is encoded by mechanisms that endow interdependence to binding/regulatory sites including overlap [31], conformational masking [32-35], de novo generation of binding sites/functionality by intermolecular assembly [36,37] or spatial translocation [33,38]. An elegant example of a scenario where one signal is ineffective unless another has been presented first is the sequential activation of conventional PKC isoforms. DAG analogs do not cause translocation of full length PKC unless Ca2+ is elevated [33]. This is because the diacylglycerol (DAG) binding sites (C12 domain) of the kinase are rendered inaccessible by a pseudosubstrate clamp until Ca2+ binds (C2 domain) to effect plasma membrane translocation and a series of stabilizing interactions (including DAG binding) that must be maintained to relieve kinase inhibition. Therefore overlapping Ca2+ (first step, translocation) and DAG signals (second step, activity), but neither signal independently, produces maximal kinase activity.
Many processes involved in Ca2+ signaling integrate different signals by detecting ‘coincident’ inputs (Figure 2d). In some cases, simple overlap of different signals is sufficient to evoke a supralinear response. In more stringent examples, the arrival of an input may define a set time window in which the coincident input must be received, or the ordering of co-incident inputs is important. Most notably in STDP, the type and extent of synaptic modification is dependent on both the respective timing (millisecond discrimination, [29]), and ordering of pre- and post-synaptic action potentials [29,30,39]. Examples of molecular coincidence detectors include Ca2+ channels [30,34,39-41]), Ca2+-regulated enzymes [42,43] and transcription regulators [44], all of which sense pairings of extracellular signals (agonists, depolarization), G proteins or second messengers (IP3, Ca2+, cAMP, DAG).
Finally, the involvement of Ca2+ signaling in ‘memory’ (Figure 2e) spans work on speculative mechanisms of memory deposition in individual Ca2+ sensors [38], through to intensely researched changes in local synaptic plasticity/connectivity that likely underpin neuronal ‘clique’ and network firing patterns involved in the deposition and consolidation of memories [30,45,46]. Ca2+ entry and Ca2+ release signals regulate short term (<hrs) activity-dependent changes in synaptic efficiency (of pre-existing synaptic components), as well as longer term changes (>days) in synaptic function and architecture [30,47-49]. Short term working memory (seconds), spatial memory (hours) and the reactivation of consolidated memories embedded via transcription/translational events harbor a common dependency on Ca2+ fluxes [48-50]. The increasing technical ability to monitor, and manipulate, the firing patterns of many neurons during mnemonic episodes [46,51] demonstrates how temporal precision (neuronal discharge frequency, latencies) is paramount for coupling clique dynamics and for preserving the fidelity of signals traversing neuronal networks.
Choreography
Signal transduction is an engagement of interlinked modules of reactions that adjust cellular behavior to environmental cues. Efficient signaling necessitates ordered temporal execution of these modules and their constituent reactions, each of which may regulate preceding or subsequent events. Kinetic considerations define the speed by which information is relayed between effectors and thereby the timeframe of the overall response, the oscillations in activity of individual components, as well as the impact of positive and negative feedback in determining the sensitivity, range and stability of output from the overall module.
Obviously, different signaling modules execute over different timeframes in different cells: synaptic [Ca2+] changes within microseconds, compared to latencies of up to tens of seconds with agonist-evoked Ca2+ signaling in non-excitable cells. The same module can however be customized to work over a malleable timeframe, as exemplified by considering the ubiquitous phospholipase C (PLCβ) Ca2+ signaling cascade. At one extreme, where this module underpins Drosophila visual transduction, responses are executed with minimal latency (<25ms), an obvious temporal adaptation to the demands of flight. A major contributing factor is the spatial compartmentalization and precoupling of signaling components by the scaffolding protein InaD in the rhabdomere, minimizing the need for amplification steps upstream of PLCβ [52]. The tight spatial coupling of this module permits the high temporal fidelity of phototransduction events. Indeed, the latency of the single photon response (time between activation of a rhodopsin molecule and ‘quantum bump’ depolarization), is increased ~6-fold in mutants lacking such spatial organization through InaD [52]. Scaffolding proteins impact signaling kinetics both generally - by increasing reaction speed and efficiency via compartmentalizing proteins within restricted surfaces that favor productive and privileged interactions - but also specifically, as is increasingly recognized (e.g. InaD [53], Homer-2 [54]) by regulating individual kinetic interactions within Ca2+ signaling modules. In contrast, the coupling of remotely synthesized hormones to phosphoinositide-coupled Ca2+ oscillations in the intact liver occurs over a timeframe >10,000-fold longer and more compatible with metabolic cycling [55]. Here, each protein component is separated by signal relay steps impacted by issues of messenger durability, such as agonist range, GTPase activity (e.g. RGS proteins [56]), IP3 diffusion/metabolism and regionalized Ca2+ buffering.
Positive and negative feedback regulation of proteins in these modules is critical in shaping the periodicity and kinetics of cytoplasmic Ca2+ signals. Feedback regulation ensures that all the relay steps (G proteins, IP3, Ca2+ [57]) as well as interlinked signaling outputs (e.g. phosphorylation, cAMP [43,58]) can display oscillatory, phase-locked changes in activity. The differential kinetic timecourse of individual feedback steps, even on the same component, is important in ordering reactions, sensing spatial distance and for switching reliably and stably between activity states [59]. For example, many intracellular Ca2+ channels exhibit time-dependent regulation by agonists, where the same ligand concentration may increase or decrease Ca2+ release depending on the duration of exposure [40,60]. The activity of distinct Ca2+ signaling modules is further choreographed by steps that interlink their ordered temporal execution, establishing complex regulatory circuits and crosstalk with other signaling pathways that orchestrate short term responses while maintaining longer term homeostatic balance.
Temporal decoding of Ca2+ signals
Cytoplasmic Ca2+ signals relay information to Ca2+-sensitive effectors. Issues of timing – notably the periodicity and duration of Ca2+ transients – are crucial in determining how information is decoded into appropriate cellular responses. These parameters are discussed separately below, prefaced with a reminder about their in vivo interdependence: the efficiency of frequency encoding of Ca2+ signals is impacted by the specific profile of Ca2+ spike duration and amplitude, as well as baseline level of Ca2+ over which they occur [33,61-65].
Frequency
Repetitive fluctuations in cytosolic Ca2+ occur over an exceptionally broad time range (Figure 3). These fluctuations encompass rhythmic changes in baseline Ca2+ observed in fungi, plants and animals [66-69], upon which are superimposed stimulation-evoked Ca2+ transients that occur episodically as bursts of oscillations or repetitive Ca2+ spikes with regulable intermittency [3,70] and cell-/agonist- specific profiles [71]. The duration of trains of Ca2+ spikes (which can be evoked for hours in experiments) is physiologically significant [72]. The ~107-fold range in periodicity (likely constrained by our inability to resolve exceptionally slow, or fast signals without summation) is exemplified at the extremes by the rapid bursts of Ca2+ spikes generated within sound-producing muscles (e.g. ≥10ms spike duration in toadfish swimbladder muscle [73]) and the protracted and persisent (e.g. ≤24hr) oscillations in basal [Ca2+]cyt associated with circadian rhythms [67-69]. In this latter case, Ca2+ signaling messengers may actually orchestrate the perception of time via entrainment and pacing of the oscillator itself [74]. Therefore the variable periodicity of Ca2+ signals coordinates responses from the cellular to the systems level.
Figure 3. Physiological harmonics: frequency encoding of Ca2+ signals.
The ~10,000,000-fold range in the periodicity (Hz, left) of repetitive Ca2+ changes observed in different cells/tissues, using data from non-excitable cells (orange, [112,113]), muscle (purple, [73,114,115]), embryos (green, [14,116]) and neurons (blue [67,117]). Numbers represent citations to examples delimiting each range. Right (red), estimates of optimal Ca2+ spiking frequencies for half-maximal activation of the indicated proteins from: Ras [75], NF-kB [64], NFAT [64], CamKII [63], PKC [33]. These obviously represent single point estimates from a broader in vivo range.
Irrespective of the absolute frequency of Ca2+ oscillations, the same key issues hold: what is the mechanistic basis of the oscillator and how is this oscillator read? This latter question may simply entail understanding ‘target selection’ (i.e. how the affinity, kinetics and localization of Ca2+ binding sites delimit which effectors respond) for outputs that directly track Ca2+ signals. More elaborately, many effectors have the ability to transduce different frequencies of Ca2+ transients into graded levels of activation (‘frequency-modulation’). Over the ~30 years since repetitive Ca2+ oscillations were resolved, considerable effort has been spent addressing these questions [2,4,57]. Several molecules (and many more processes) have been identified that are optimally regulated by specific Ca2+ spiking frequencies, including Ca2+-dependent transcription factors (NF-AT [64,65], NF-kB [64], Oct/OAP [64]), Ras [75], CamKII [61,63] and PKC [33]). Collectively these data show that different Ca2+ sensors are regulated, over distinct ranges (Figure 3) and between distinct limits, by the periodicity of Ca2+ transients [58,61,63,64,75].
Considerably less is known, however, about the molecular basis of frequency decoding. Non-linear activation mechanisms in most models are underpinned by elements of co-incidence and reinforcement (e.g. interdependent, cooperative or temporally geared reactions where active intermediates persist beyond the transient Ca2+ spike) to render integrative behavior. Seemingly subtle kinetic delays are critical in allowing activities to be summated at high Ca2+ spiking frequencies, as shown, for example, by the short delays (seconds) in PKCγ and DAG association and dissociation as Ca2+ rises and falls [33]. The strongest structural insight into frequency decoding is probably provided by CaMKII, and experimental observations that different CaMKII isoforms [63], and notably different splice variants of the same isoform (βCaMKII [61]), display unique in vitro sensitivities (~10-fold range) to the frequency of Ca2+ oscillations. Specific structural changes impacting initial autophosphorylation rates [61], or residues determining the dramatic decrease in calmodulin dissociation rate from the autophosphorylated holoenzyme are likely crucial [76]. Coupling such biochemical insight with crystallographic solutions [77] will ultimately reveal the conformational mechanics of frequency decoding.
It suffices to conclude that the diversity of responses regulated by Ca2+ oscillation frequency emphasizes the utility of frequency encoding as a strategy for relaying information [78]. Ca2+ spiking decreases the threshold [Ca2+]cyt for activating responses, preserves signal fidelity and specificity while minimizing metabolic cost, effector desensitization and cytotoxic risk associated with sustained increases in [Ca2+]cyt.
Duration
The duration of Ca2+ transients varies considerably: Ca2+ signals can be rapid (and likely localized), or more protracted (and likely pervasive). Their individualized duration is determined by the kinetic interplay of Ca2+ fluxes that increase, buffer and remove Ca2+ ions from the cytosol, the balance between which is regulated physiologically and disturbed pathologically through changes in the functional architecture of Ca2+ signaling molecules. The duration of a Ca2+ signal therefore depends on the precise molecular complement of Ca2+ transporters engaged during a response. Involvement of the endoplasmic reticulum (ER) is particularly crucial: Ca2+ signals that evoke regenerative Ca2+-induced Ca2+ release via IP3Rs or RyRs trigger propagating Ca2+ waves that extend the duration and spatial reach of cytoplasmic Ca2+ changes. The consequent ER Ca2+ depletion stimulates a homeostatic ‘store-operated’ Ca2+ entry, which in conjunction with other ‘receptor-operated’ Ca2+ influx pathways, refill the ER with Ca2+ via delayed but enduring Ca2+ entry currents [79]. Therefore, the involvement of different families of intracellular Ca2+ channels in amplifying, and triggering the amplification of, Ca2+ signals is a key determinant of Ca2+ signal duration.
The timecourse of a Ca2+ signal is important as early and late phases of Ca2+ signals are often associated with different responses - sustained Ca2+ elevations will reach more targets, and occupy sites of appropriate affinity for longer. For example, transient Ca2+ signals are often insufficient to activate transcriptional responses [80] and the duration of more sustained Ca2+ signals is important in specifying which transcription factors are most efficiently activated [70,81]. Further examples of Ca2+ signals of different durations directing unique cellular responses are as diverse as stomatal opening/closing [62], exocrine function [82], the integrated initiation of Ca2+-dependent events during egg activation [83], positive and negative thymocyte selection [84] and the selective modulation of plasticity in neurons (LTD vs LTP, [29,30]).
In terms of decoding, one would underestimate the versatility of Ca2+ as messenger by assuming the effective duration of a Ca2+ signal was set only by the occupancy of Ca2+ binding sites on Ca2+ sensors with singular affinities. Ca2+-dependent effectors exhibit a variety of ‘temporal gearing’ strategies to ensure the consequences of transient Ca2+ signals persist beyond the duration of the Ca2+ signal itself. While cellular Ca2+ binding proteins exhibit a wide range of intrinsic Ca2+ binding affinities, even within specific subfamilies of Ca2+ sensors [85-87], these ‘basal’ affinities can change dramatically on regulatory modification (>25000-fold [76]), through cooperativity with other Ca2+ binding modules in the same proteins, and on forming interactions with substrate or additional partners. Such mechanisms ensure that ephemeral Ca2+ signals have lasting physiological value.
Many Ca2+-triggered interactions are enabled by Ca2+-dependent translocations to cellular membranes [88], such as those mediated by C2 domains (e.g. PKC [33,58], Ras regulators [89]), Ca2+/myristoyl switches [86], annexin repeats [87] or via exposure of binding sites for specific target proteins (e.g. CaMKII [90]). After these initial Ca2+-driven translocation, activities may become entirely Ca2+-independent (‘autonomous’) thereby extending the temporal reach of Ca2+ signals, exemplified by the importance of CaMK autonomy for transcription [91] and plasticity [92]. Localized autonomous CaMKII activity is maintained by autophosphorylation (e.g. on cardiac Ca2+ channels [93] and certain PSD targets [94]), or simply by immobilization on the target itself (e.g. for NR2B, [95] and certain K+ channels [96]). These varied routes to autonomy define whether temporal gearing is controlled by local phosphatase activity, target dissociation, or both. Therefore the extent of temporal amplification through translocation is likely unique for different subcellular compartments, as well as different targets.
Mistiming
When timing goes awry, pathological outcomes ensue. There are many examples of how the temporal profile of cytosolic Ca2+ signals is adversely remodeled by disease and specifically by mutations that impact the expression, activity and spatial organization of individual Ca2+ homeostatic genes (see reviews in [97]). In the heart, for example, abnormal Ca2+ cycling predisposes to arrhythmias [98] via decreased expression/functionality of Ca2+ buffers [99] or mutational dysfunction of voltage-operated Ca2+ channels [100], intracellular Ca2+ channels (e.g. ≤70 mutations in human RyR2) or proteins that compartmentalize Ca2+ channels and transporters [101]. Protracted Ca2+ transients diagnostic of diastolic dysfunction can result from decreased sarcoplasmic reticulum Ca2+ ATPase (SERCA) expression or mutational dysfunction of its regulators thereby impairing recovery from Ca2+ loads [102,103]. Timing of mutations is also critical for disease progression, by impairing [104] or persistently potentiating [105,106] Ca2+ signaling events at key points in the pathogenic timeline. For example, PKD1 is a gene mutated in a majority of patients with autosomal dominant polycystic kidney disease. It encodes polycystin-1 (TRPP1/polycystin-1), a cell surface protein which functions as part of a Ca2+-dependent mechanosensitive complex with PKD2 during renal development. Loss of polycystin-1 function results in differential pathological outcomes depending on when functionality is impaired. If deleted shortly after birth, kidney cysts form rapidly and mice die in a few weeks, whereas loss of function in adults is associated with a much milder phenotype [104].
Beyond the many ‘loss-of-function’ mutations that impact global Ca2+ cycling, rarer ‘gain-of-function’ mutations increase the duration of cellular Ca2+ fluxes. This is exemplified by mutants in several Ca2+ channelopathies where normal channel inactivation kinetics are delayed [107-109]. A dramatic example is the de novo Ca2+ channel mutation associated with Timothy syndrome (G406R, Cav1.2) where a near complete failure of voltage-dependent channel inactivation precipitates cardiac, neuronal and developmental defects [100]. Many kinetic changes are however much subtler, yet still cause disease. For instance, certain mutations (3 from 50+) identified in ATP2A2, which encodes the housekeeping SERCA2b isoform, confer relatively normal Ca2+ transport and ATP hydrolytic activities (>65% of wild type [110]). These mutants are none-the-less associated with the Darier disease pedigrees [111]. Modulation of SERCA2 affinities in the heart – either to increase or decrease the net Ca2+ affinity of SR Ca2+ sequestration – can both result in cardiomyopathies [103]. An important principle from such examples is that although different cell types show incredible versatility in their individualized ‘Ca2+ signatures’, there is considerably smaller tolerance for temporal deviation from differentiated Ca2+ profiles without pathological consequences.
Conclusions
The diversity of examples discussed above underscore the many ways in which timing impacts how information is relayed from external stimuli via Ca2+ signals into specific physiological outcomes. The broad community of Ca2+ handling proteins that increase, decrease and buffer cytoplasmic Ca2+ provide kinetic diversity in assembling signaling modules customized to cellular function at any point in time to generate Ca2+ signals across a remarkable range of durations and periodicities. The malleable role of Ca2+ as a messenger depends on precise temporal control of the activity of these individual molecules, as well as controlled regulation of the extent of feedback, diversification and gearing between them that rapidly set up and dissipate spatial gradients of activity within cells and tissues. Consequently, quantifying the tolerance of this kinetic architecture to pathological cues is key for delimiting how the temporal dynamics of specific modules within proteins contribute to Ca2+-related pathologies at a systems-level.
Acknowledgements
Work supported by NIH (NS046783) and a NSF CAREER Fellowship (JSM, 0237946). Space considerations restricted discussion of many examples and we apologize for omission of pertinent work.
References
- 1.Clapham DE. Calcium signaling. Cell. 2007;131:1047–1058. doi: 10.1016/j.cell.2007.11.028. [DOI] [PubMed] [Google Scholar]
- 2.Berridge MJ, Bootman MD, Roderick HL. Calcium signaling: dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol. 2003;4:517–529. doi: 10.1038/nrm1155. [DOI] [PubMed] [Google Scholar]
- 3.Berridge MJ, Lipp P, Bootman MD. The versatility and universality of calcium signaling. Nat Rev Mol Cell Biol. 2000;1:11–21. doi: 10.1038/35036035. [DOI] [PubMed] [Google Scholar]
- 4.Berridge MJ. The AM and FM of calcium signalling. Nature. 1997;386:759–760. doi: 10.1038/386759a0. [DOI] [PubMed] [Google Scholar]
- 5.Lioudyno MI, Kozak JA, Penna A, Safrina O, Zhang SL, Send D, Roos J, Stauderman KA, Cahalan MD. Orai1 and STIM1 move to the immunological synapse and are up-regulated during T cell activation. PNAS. 2008;105:2011–2016. doi: 10.1073/pnas.0706122105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Spitzer NC. Electrical activity in early neuronal development. Nature. 2006;444:707–712. doi: 10.1038/nature05300. [DOI] [PubMed] [Google Scholar]
- 7.Spitzer NC. A developmental handshake: neuronal control of ionic currents and their control of neuronal differentiation. J Neurobiol. 1991;22:659–673. doi: 10.1002/neu.480220702. [DOI] [PubMed] [Google Scholar]
- 8.Emerson M, Travis AR, Bathgate R, Stojanov T, Cook DI, Harding E, Lu DP, O'Neill C. Characterization and functional significance of calcium transients in the 2-cell mouse embryo induced by an autocrine growth factor. J Biol Chem. 2000;275:21905–21913. doi: 10.1074/jbc.M001719200. [DOI] [PubMed] [Google Scholar]
- 9.Bottcher RT, Niehrs C. Fibroblast Growth Factor signaling during early vertebrate development. Endocr Rev. 2004;26:63–77. doi: 10.1210/er.2003-0040. [DOI] [PubMed] [Google Scholar]
- 10.Kohn AD, Moon RT. Wnt and calcium signaling: β-catenin-independent pathways. Cell Calcium. 2005;38:439–446. doi: 10.1016/j.ceca.2005.06.022. [DOI] [PubMed] [Google Scholar]
- 11.Raya A, Kawakami Y, Rodriguez-Esteban C, Ibanes M, Rasskin-Gutman D, Rodriguez-Leon J, Busher D, Feijo JA, Izpisua Belmonte JC. Notch activity acts as a sensor for extracellular calcium during vertebrate left-right determination. Nature. 2004;427:121–129. doi: 10.1038/nature02190. [DOI] [PubMed] [Google Scholar]
- 12.Zhou Z, Hong EJ, Cohen S, Zhao W-N, Ho H-YH, Schmidt L, Chen WG, Lin Y, Savner E, Griffith EC, et al. Brain-specific phosphorylation of MeCP2 regulates activity-dependent BDNF transcription, dendritic growth, and spine maturation. Neuron. 2006;52:255–269. doi: 10.1016/j.neuron.2006.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Whitaker M. Calcium at fertilization and in early development. Physiol Rev. 2006;86:25–88. doi: 10.1152/physrev.00023.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gilland E, Miller AL, Karplus E, Baker R, Webb SE. Imaging of multicellular large-scale rhythmic calcium waves during zebrafish gastrulation. PNAS. 2000;96:157–161. doi: 10.1073/pnas.96.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Slusarski DC, Pelegri F. Calcium signaling in vertebrate embryonic patterning and morphogenesis. Dev Biol. 2007;307:1–13. doi: 10.1016/j.ydbio.2007.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saneyoshi T, Kume S, Amasaki Y, Mikoshiba K. The Wnt/calcium pathway activates NF/AT and promotes ventral cell fate in Xenopus embryos. Nature. 2002;417:295–299. doi: 10.1038/417295a. [DOI] [PubMed] [Google Scholar]
- 17.Dale B, Yazaki I, Tosti E. Polarized distribution of L-type calcium channels in early sea urchin embryos. Am J Physiol. 1997;273:822–825. doi: 10.1152/ajpcell.1997.273.3.C822. [DOI] [PubMed] [Google Scholar]
- 18.Albrieux M, Villaz M. Bilateral asymmetry of the inositol trisphosphate-mediated calcium signaling in two-cell ascidian embryos. Biol Cell. 2000;92:277–284. doi: 10.1016/s0248-4900(00)01066-2. [DOI] [PubMed] [Google Scholar]
- 19.Okagaki R, Izumi H, Okada T, Nagahora H, Nakajo K, Okamura Y. The maternal transcript for truncated voltage-dependent Ca2+ channels in the ascidian embryo: a potential suppressive role in Ca2+ channel expression. Dev Biol. 2001;230:258–277. doi: 10.1006/dbio.2000.0119. [DOI] [PubMed] [Google Scholar]
- 20.Boulware MJ, Marchant JS. IP3 receptor activity is differentially regulated in endoplasmic reticulum subdomains during oocyte maturation. Curr Biol. 2005;15:765–770. doi: 10.1016/j.cub.2005.02.065. [DOI] [PubMed] [Google Scholar]
- 21.Wallingford JB, Fraser SE, Harland RM. Convergent extension: The molecular control of polarized cell movement during embryonic development. Dev Cell. 2002;2:695–706. doi: 10.1016/s1534-5807(02)00197-1. [DOI] [PubMed] [Google Scholar]
- 22.Gordo AC, Rodrigues P, Kurokawa M, Jellerette T, Exley GE, Warner C, Fissore R. Intracellular Ca2+ oscillations signal apoptosis rather than activation in in vitro aged mouse eggs. Biol Reprod. 2002;66:1828–1837. doi: 10.1095/biolreprod66.6.1828. [DOI] [PubMed] [Google Scholar]
- 23.Ciccolini F, Collins TJ, Sudhoelter J, Lipp P, Berridge MJ, Bootman MD. Local and global spontaneous calcium events regulate neurite outgrowth and onset of GABAergic phenotype during neural precursor differentiation. J Neurosci. 2003;23:103–111. doi: 10.1523/JNEUROSCI.23-01-00103.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gu X, Spitzer NC. Distinct aspects of neuronal differentiation encoded by frequency of spontaneous Ca2+ transients. Nature. 1995;375:784–787. doi: 10.1038/375784a0. [DOI] [PubMed] [Google Scholar]
- 25.Miller FD, Gauthier AS. Timing is everything: Making neurons versus glia in the developing cortex. Neuron. 2007;54:357–369. doi: 10.1016/j.neuron.2007.04.019. [DOI] [PubMed] [Google Scholar]
- 26.Chen WG, Chanq Q, Lin Y, Meissner A, West AE, Griffith EC, Jaenisch R, Greenberg ME. Depression of BDNF transcription involves calcium-dependent phosphorylation of MeCP2. Science. 2003;302:885–889. doi: 10.1126/science.1086446. [DOI] [PubMed] [Google Scholar]
- 27.Bootman MD, Berridge MJ, Lipp P. Cooking with calcium; the recipes for composing global signals from elementary events. Cell. 1997;91:367–373. doi: 10.1016/s0092-8674(00)80420-1. [DOI] [PubMed] [Google Scholar]
- 28.Marchant JS, Callamaras N, Parker I. Initiation of IP3 mediated Ca2+ waves in Xenopus oocytes. EMBO J. 1999;18:5285–5299. doi: 10.1093/emboj/18.19.5285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abbott LF, Nelson SB. Synaptic plasticity: taming the beast. Nat Neurosci. 2000;3:1178–1183. doi: 10.1038/81453. [DOI] [PubMed] [Google Scholar]
- 30.Citri A, Malenka RC. Synaptic Plasticity: Multiple forms, functions and mechanisms. Neuropsychopharmacology. 2008;33:18–41. doi: 10.1038/sj.npp.1301559. [DOI] [PubMed] [Google Scholar]
- 31.Kwon Y, Hofmann T, Montell C. Integration of phosphoinositide- and calmodulin- mediated regulation of TRPC6. Mol Cell. 2007;25:491–503. doi: 10.1016/j.molcel.2007.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sweatt JD, Atkins CM, Johnson J, English JD, Roberson ED, Chen SJ, Newton A, Klann E. Protected-site phosphorylation of protein kinase C in hippocampal long-term potentiation. J Neurochem. 1998;71:1075–1085. doi: 10.1046/j.1471-4159.1998.71031075.x. [DOI] [PubMed] [Google Scholar]
- 33.Oancea E, Meyer T. Protein kinase C as a molecular machine for decoding calcium and diacylglycerol signals. Cell. 1998;95:307–318. doi: 10.1016/s0092-8674(00)81763-8. [DOI] [PubMed] [Google Scholar]
- 34.Marchant JS, Taylor CW. Cooperative activation of inositol trisphosphate receptors by sequential binding of IP3 and Ca2+ safeguards against spontaneous activity. Curr Biol. 1997;7:510–518. doi: 10.1016/s0960-9822(06)00222-3. [DOI] [PubMed] [Google Scholar]
- 35.Griffith LC. Regulation of calcium/calmodulin-dependent protein kinase II activation by intramolecular and intermolecular interactions. J Neurosci. 2004;24:8394–8398. doi: 10.1523/JNEUROSCI.3604-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Van Rossum DB, Patterson RL, Sharma S, Barrow RK, Kornberg M, Gill DL, Snyder SH. Phospholipase Cγ1 controls surface expression of TRPC3 through an intermolecular PH domain. Nature. 2005;434:99–104. doi: 10.1038/nature03340. [DOI] [PubMed] [Google Scholar]
- 37.Liou J, Fivaz M, Inoue T, Meyer T. Live-cell imaging reveals sequential oligomerization and local plasma membrane targeting of stromal interaction molecule 1 after Ca2+ store depletion. PNAS. 2007;104:9301–9306. doi: 10.1073/pnas.0702866104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu Q, Walker SA, Gao D, Taylor JA, Dai Y, Arkell RS, Bootman MD, Roderick HL, Cullen PJ, Lockyer PJ. CAPRI and RASAL impose different modes of information processing on Ras due to contrasting temporal filtering of Ca2+. J Cell Biol. 2005;170:183–190. doi: 10.1083/jcb.200504167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sarkisov DV, Wang SS-H. Order-dependent coincidence detection in cerebllar Purkinje neurons at the inositol trisphosphate receptor. J Neurosci. 2008;28:133–142. doi: 10.1523/JNEUROSCI.1729-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Foskett JK, White C, Cheung KH, Mak DO. Inositol trisphosphate receptor Ca2+ release channels. Physiol Rev. 2007;87:593–658. doi: 10.1152/physrev.00035.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chuang HH, Neuhausser WM, Julius D. The super-cooling agent icilin reveals a mechanism of coincidence detection by a temperature-sensitive TRP channel. Neuron. 2004;43:859–869. doi: 10.1016/j.neuron.2004.08.038. [DOI] [PubMed] [Google Scholar]
- 42.Hashimotodani Y, Ohno-Shosaku T, Tsubokawa H, Ogata H, Emoto K, Maejima T, Araishi K, Shin HS, Kano M. Phospholipase Cβ serves as a coincidence detector through its Ca2+ dependency for triggering retrograde endocannabinoid signal. Neuron. 2005;45:257–268. doi: 10.1016/j.neuron.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 43.Willoughby D, Cooper DMF. Ca2+ stimulation of adenylyl cyclase generates dynamic oscillations in cyclic AMP. J Cell Sci. 2005;119:828–836. doi: 10.1242/jcs.02812. [DOI] [PubMed] [Google Scholar]
- 44.Kovacs KA, Steullet P, Steinmann M, Do KQ, Magistretti PJ, Halfon O, Cardinaux JR. TORC1 is a calcium- and cAMP-sensitive coincidence detector involved in hippocampal long-term synaptic plasticity. PNAS. 2007;104:4700–4705. doi: 10.1073/pnas.0607524104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Neves G, Cooke SF, Bliss TV. Synaptic plasticity, memory and the hippocampus: a neural network approach to causality. Nature Reviews Neuroscience. 2008;9:65–75. doi: 10.1038/nrn2303. [DOI] [PubMed] [Google Scholar]
- 46.Lin L, Osan R, Tsien JZ. Organizing principles of real-time memory encoding: neural clique assemblies and universal neural codes. Trends Neurosci. 2006;29:48–57. doi: 10.1016/j.tins.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 47.Bardo S, Cavazzini MG, Emptage N. The role of the endoplasmic reticulum Ca2+ store in the plasticity of central neurons. Trends Pharmacol Sci. 2006;27:78–84. doi: 10.1016/j.tips.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 48.Monqillo G, Barak O, Tsodyks M. Synaptic theory of working memory. Science. 2008;319:1543–6. doi: 10.1126/science.1150769. [DOI] [PubMed] [Google Scholar]
- 49.Mamou CB, Gamach K, Nader K. NMDA receptors are critical for unleashing consolidated auditory fear memories. Nat Neurosci. 2006;9:1237–1239. doi: 10.1038/nn1778. [DOI] [PubMed] [Google Scholar]
- 50.Pastalkova E, Serrano P, Pinkhasova D, Wallace E, Fenton AA, Sacktor TC. Storage of spatial information by the maintenance mechanism of LTP. Science. 2006;313:1141–1144. doi: 10.1126/science.1128657. [DOI] [PubMed] [Google Scholar]
- 51.Nikolenko V, Poskanzer KE, Yuste R. Two-photon photostimulation and imaging of neuronal circuits. Nature Methods. 2007;4:943–950. doi: 10.1038/nmeth1105. [DOI] [PubMed] [Google Scholar]
- 52.Scott K, Zuker CS. Assembly of the Drosophila phototransduction cascade shapes elementary responses. Nature. 1998;395:805–808. doi: 10.1038/27448. [DOI] [PubMed] [Google Scholar]
- 53.Mishra P, Socolich M, Wall M, Graves J, Wang Z, Ranganathan R. Dynamic scaffolding in a G protein-coupled signaling system. Cell. 2007;131:80–92. doi: 10.1016/j.cell.2007.07.037. [DOI] [PubMed] [Google Scholar]
- 54.Shin DM, Dehoff M, Luo X, Kang SH, Tu J, Nayak SK, Ross EM, Worley PF, Muallem S. Homer 2 tunes G-protein-coupled receptors stimulus intensity by regulating RGS proteins and PLCβ GAP activities. J Cell Biol. 2003;162:293–303. doi: 10.1083/jcb.200210109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Robb-Gaspers LD, Thomas AP. Coordination of Ca2+ signalling by intercellular propagation of Ca2+ waves in the intact liver. J Biol Chem. 1995;270:8102–8107. doi: 10.1074/jbc.270.14.8102. [DOI] [PubMed] [Google Scholar]
- 56.Luo X, Popov S, Kanti Bera A, Wilkie TM, Muallem S. RGS proteins provide biochemical control of agonist-evoked [Ca2+]i oscillations. Mol Cell. 2001;7:651–660. doi: 10.1016/s1097-2765(01)00211-8. [DOI] [PubMed] [Google Scholar]
- 57.Taylor CW, Thorn P. Calcium signalling: IP3 rises again...and again. Curr Biol. 2001;11:R352–R355. doi: 10.1016/s0960-9822(01)00192-0. [DOI] [PubMed] [Google Scholar]
- 58.Violin JD, Zhang J, Tsien RY, Newton AC. A genetically encoded fluorescent reporter reveals oscillatory phosphorylation by protein kinase C. J Cell Biol. 2003;161:899–909. doi: 10.1083/jcb.200302125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Brandman O, Ferell JE, Li R, Meyer T. Interlinked fast and slow positive feedback loops drive reliable cell decisions. Science. 2005;310:496–498. doi: 10.1126/science.1113834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Churamani D, Dickinson GD, Ziegler M, Patel S. Time sensing by NAADP receptors. Biochem J. 2006;397:313–320. doi: 10.1042/BJ20060179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bayer KU, De Knoninck P, Schulman H. Alternative splicing modulates the frequency-dependent response of CamKII to Ca 2+ oscillations. EMBO J. 2002;21:3590–3597. doi: 10.1093/emboj/cdf360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Allen GJ, Chu SP, Harrington CL, Schumacher K, Hoffmann T, Tang YY, Greill E, Schroeder JI. A defined range of guard cell calcium oscillation parameters encodes stomatal movements. Nature. 2001;411:1053–1057. doi: 10.1038/35082575. [DOI] [PubMed] [Google Scholar]
- 63.De Koninck P, Schulman H. Sensitivity of CaM Kinase II to the frequency of Ca2+ oscillations. Science. 1998;279:227–230. doi: 10.1126/science.279.5348.227. [DOI] [PubMed] [Google Scholar]
- 64.Dolmetsch RE, Xu K, Lewis R. Calcium oscillations increase the efficiency and specificity of gene expression. Nature. 1998;392:933–936. doi: 10.1038/31960. [DOI] [PubMed] [Google Scholar]
- 65.Li WH, Llopis J, Whitney M, Zlokarnik G, Tsien RY. Cell-permeant caged InsP3 ester shows that Ca2+ spike frequency can optimize gene expression. Nature. 1998;392:936–941. doi: 10.1038/31965. [DOI] [PubMed] [Google Scholar]
- 66.Kippert F. Cellular signalling and the complexity of biological timing: insights from the ultradian clock of Schizosaccharomyces pombe. Phil Trans R Soc Lond B. 2001;356:1725–1733. doi: 10.1098/rstb.2001.0935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ikeda M, Sugiyama T, Wallace CS, Gompf HS, Yoshioka T, Miyawaki A, Allen CN. Circadian dynamics of cytosolic and nuclear Ca2+ in single suprachiasmatic nucleus neurons. Neuron. 2003;38:253–263. doi: 10.1016/s0896-6273(03)00164-8. [DOI] [PubMed] [Google Scholar]
- 68.Johnson CH, Knight MR, Kondo T, Masson P, Sedbrook J, Haley A, Trewavas A. Circadian oscillations of cytosolic and chloroplastic free calcium in plants. Science. 1995;269:1863–1865. doi: 10.1126/science.7569925. [DOI] [PubMed] [Google Scholar]
- 69.Tang R-H, Han S, Zheng H, Cook CW, Choi CS, Woerner TE, Jackson RB, Pei Z-M. Coupling diurnal cytosolic Ca2+ oscillations to the CAS-IP3 pathway in Arabidopsis. Science. 2007;315:1423–1436. doi: 10.1126/science.1134457. [DOI] [PubMed] [Google Scholar]
- 70.Dolmetsch RE, Lewis RS, Goodnow CC, Healy JI. Differential activation of transcription factors induced by Ca2+ response amplitude and duration. Nature. 1997;386:855–858. doi: 10.1038/386855a0. [DOI] [PubMed] [Google Scholar]
- 71.Kiselyov K, Shin DM, Muallem S. Signalling specificity in GPCR-dependent Ca2+ signaling. Cell Signal. 2003;15:243–253. doi: 10.1016/s0898-6568(02)00074-8. [DOI] [PubMed] [Google Scholar]
- 72.Ozil J-P, Banrezes B, Toth S, Pan H, Schultz RM. Ca2+ oscillatory pattern in fertilized mouse eggs affects gene expression and development to term. Dev Biol. 2006;300:534–544. doi: 10.1016/j.ydbio.2006.08.041. [DOI] [PubMed] [Google Scholar]
- 73.Rome LC. Design and function of superfast muscles: new insights into the physiology of skeletal muscle. Annu Rev Physiol. 2006;68:193–221. doi: 10.1146/annurev.physiol.68.040104.105418. [DOI] [PubMed] [Google Scholar]
- 74.Dodd AN, Gardner MJ, Hotta CT, Hubbard KE, Dalchau N, Love J, Assie J-M, Robertson FC, Jakobsen MK, Goncalves J, et al. The Arabidopsis circadian clock incorporates a cADPR-based feedback loop. Science. 2007;318:1789–1792. doi: 10.1126/science.1146757. [DOI] [PubMed] [Google Scholar]
- 75.Kupzig S, Walker SA, Cullen PJ. The frequencies of calcium oscillations are optimized for efficient calcium-mediated activation of Ras and the ERK/MAPK cascade. PNAS. 2005;102:7577–82. doi: 10.1073/pnas.0409611102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Singla SI, Hudmon A, Goldberg JM, Smith JL, Schulman H. Molecular characterization of calmodulin trapping by calcium/calmodulin-dependent protein kinase II. J Biol Chem. 2001;276:29353–29360. doi: 10.1074/jbc.M101744200. [DOI] [PubMed] [Google Scholar]
- 77.Rosenberg OS, Deindl S, Sung R-J, Nairn AC, Kuriyan Structure of the autoinhibited kinase domain of CaMKII and SAXS analysis of the holoenzyme. Cell. 2005;123:849–860. doi: 10.1016/j.cell.2005.10.029. [DOI] [PubMed] [Google Scholar]
- 78.Rapp PE, Mees AI, Sparrow CT. Frequency encoded biochemical regulation is more accurate than amplitude dependent control. J Theor Biol. 1981;90:531–544. doi: 10.1016/0022-5193(81)90304-0. [DOI] [PubMed] [Google Scholar]
- 79.Venkatachalam K, van Rossum DB, Patterson RL, Ma H-T, Gill DJ. The cellular and molecular basis of store-operated calcium entry. Nat Cell Biol. 2002;4:E263–E272. doi: 10.1038/ncb1102-e263. [DOI] [PubMed] [Google Scholar]
- 80.Gallo EM, Cante-Barett K, Crabtree GR. Lymphocyte calcium signaling from membrane to nucleus. Nature Immunology. 2006;7:25–32. doi: 10.1038/ni1295. [DOI] [PubMed] [Google Scholar]
- 81.Chawla S, Bading H. CREB/CBP and SRE-interacting transcriptional regulators are fast on-off switches: duration of calcium transients specifies the magnitude of transcriptional responses. J Neurochem. 2001;79:849–858. doi: 10.1046/j.1471-4159.2001.00645.x. [DOI] [PubMed] [Google Scholar]
- 82.Petersen OH, Tepikin AV. Polarized calcium signaling in exocrine gland cells. Ann Rev Physiol. 2008;70:273–279. doi: 10.1146/annurev.physiol.70.113006.100618. [DOI] [PubMed] [Google Scholar]
- 83.Ozil J-P, Markoulaki S, Toth S, Matson S, Banrezes B, Knott JG, Schultz RM, Huneau D, Ducibella T. Egg activation events are regulated by the duration of a sustained [Ca2+]cyt signal in the mouse. Dev Biol. 2005;282:39–54. doi: 10.1016/j.ydbio.2005.02.035. [DOI] [PubMed] [Google Scholar]
- 84.Werlen G, Hausmann B, Naeher D, Palmer E. Signaling life and death in the thymus: timing is everything. Science. 2003;299:1859–1862. doi: 10.1126/science.1067833. [DOI] [PubMed] [Google Scholar]
- 85.Xu J, Mashimo T, Sudhof TC. Synaptotagmin -1, -2, and -9: Ca2+ sensors for fast release that specify distinct presynaptic properties in subsets of neurons. Neuron. 2007;54:567–581. doi: 10.1016/j.neuron.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 86.Burgoyne RD. Neuronal calcium sensor proteins: generating diversity in neuronal Ca2+ signalling. Nat Rev Neurosci. 2007;8:182–193. doi: 10.1038/nrn2093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Monastyrskaya K, Babiychuk EB, Hostettler A, Rescher U, Draeger A. Annexins as intracellular calcium sensors. Cell Calcium. 2007;47:207–219. doi: 10.1016/j.ceca.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 88.Fivaz M, Meyer T. Specific localization and Timing in Neuronal Signal Transduction mediated by protein-lipid interactions. Neuron. 2003;40:319–330. doi: 10.1016/s0896-6273(03)00634-2. [DOI] [PubMed] [Google Scholar]
- 89.Liu Q, Walker SA, Gao D, Taylor JA, Dai Y, Arkell RS, Bootman MD, Roderick HL, Cullen PJ, Lockyer PJ. CAPRI and RASAL impose different modes of information processing on Ras due to contrasting temporal filtering of Ca2+. J Cell Biol. 2005;170:183–190. doi: 10.1083/jcb.200504167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Merrill MA, Chen Y, Strack S, Hell JW. Activity-driven postsynaptic translocation of CaMKII. Trends Pharmacol Sci. 2005;26:645–653. doi: 10.1016/j.tips.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 91.Chow FA, Anderson KA, Noeldner PK, Means AR. The autonomous activity of calcium/calmodulin-dependent protein kinase IV is required for its role in transcription. J Biol Chem. 2005;280:20530–20538. doi: 10.1074/jbc.M500067200. [DOI] [PubMed] [Google Scholar]
- 92.Giese KP, Fedorov NB, Filipkowski RK, Silva AJ. Autophosphorylation at Thr286 of the α calcium-calmodulin kinase II in LTP and learning. Science. 1998;279:870–873. doi: 10.1126/science.279.5352.870. [DOI] [PubMed] [Google Scholar]
- 93.Hudmon A, Schulman H, Kim J, Maltez JM, Tsien RW, Pitt GS. CamKII tethers to L-type Ca2+ channels, establishing a local and dedicated integrator of Ca2+ signals for facilitation. J Cell Biol. 2005;171:537–547. doi: 10.1083/jcb.200505155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Shen K, Teruel MN, Connor JH, Shenolikar S, Meyer T. Molecular memory by reversible translocation of calcium/calmodulin-dependent protein kinase II. Nat Neurosci. 2000;3:881–886. doi: 10.1038/78783. [DOI] [PubMed] [Google Scholar]
- 95.Bayer K-U, De Koninick P, Leonard AS, Hell JW, Schulman H. Interaction with the NMDA receptor locks CaMKII in an active conformation. Nature. 2001;411:801–804. doi: 10.1038/35081080. [DOI] [PubMed] [Google Scholar]
- 96.Sun XX, Hodge JJ, Zhou Y, Nguyen M, Griffith LC. The eag potassium channel binds and locally activates calcium/calmodulin-dependent protein kinase II. J Biol Chem. 2004;279:10206–10214. doi: 10.1074/jbc.M310728200. [DOI] [PubMed] [Google Scholar]
- 97.Carafoli E, Brini M. Subcellular Biochemistry. Springer Publishing Company; New York: 2007. 45. [Google Scholar]
- 98.Ter Keurs HEDJ, Boyden PA. Calcium and Arrhythmogenesis. Physiol Rev. 2007;87:457–506. doi: 10.1152/physrev.00011.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.di Barletta MR, Viatchenko-Karpinski S, Nori A, Memmi M, Terentyev D, Turcato F, Valle G, Rizzi N, Napolitano C, Gyorke S, et al. Clinical phenotype and functional characterization of CASQ2 mutations associated with catecholaminergic polymorphic ventricular tachycardia. Circulation. 2006;114:1012–1019. doi: 10.1161/CIRCULATIONAHA.106.623793. [DOI] [PubMed] [Google Scholar]
- 100.Splawski I, Timothy KW, Sharpe LM, Decher N, Kumar P, Bloise R, Napolitano C, Schwartz PJ, Joseph RM, Condouris K, et al. Cav1.2 calcium channel dysfuntion causes a multisystem disorder including arrhythmia and autism. Cell. 2004;119:19–31. doi: 10.1016/j.cell.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 101.Mohler PJ, Schott JJ, Gramolini AO, Dilly KW, Guatimosim S, duBell WH, Song LS, Hautogne K, Kyndt F, Ali ME, et al. Ankyrin-B mutation causes type 4 long-QT cardiac arrhythmia and sudden cardica death. Nature. 2003;421:634–639. doi: 10.1038/nature01335. [DOI] [PubMed] [Google Scholar]
- 102.Schmitt JP, Kamisago M, Asahi M, Li GH, Ahmad F, Mende U, Kranias EG, MacLennan DH, Seidman JG, Seidman CE. Dilated cardiomyopathy and heart failure caused by a mutation in phospholamban. Science. 2003;299:1410–1413. doi: 10.1126/science.1081578. [DOI] [PubMed] [Google Scholar]
- 103.Vangheluwe P, Sipido KR, Raeymaekers L, Wuytack F. New perspectives on the role of SERCA2's Ca2+ affinity in cardiac function. Biochim Biophys Acta. 2006;1763:1216–1228. doi: 10.1016/j.bbamcr.2006.08.025. [DOI] [PubMed] [Google Scholar]
- 104.Piontek K, Menezes LF, Garcia-Gonzalez MA, Huso DL, Germino GG. A critical developmental switch defines the kinetics of kidney cyst formation after loss of Pkd1. Nat Med. 2007;13:1490–1495. doi: 10.1038/nm1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Habib T, Park H, Tsang M, Moreno de Alboran I, Nicks A, Wilson L, Knoepfler PS, Andrews S, Rawlings DJ, Eisenman RN, et al. Myc stimulates B lymphocyte differentiation and amplifies calcium signaling. J Cell Biol. 2007;179:717–731. doi: 10.1083/jcb.200704173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Prevarskaya N, Zhang L, Barritt G. TRP channels in cancer. Biochim Biophys Acta. 2007;1772:937–946. doi: 10.1016/j.bbadis.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 107.Tottene A, Pivotto F, Fellin T, Cesetti T, van den Maagdenberg AMJM, Pietrobon D. Specific kinetic alterations of human CaV2.1 calcium channels produced by mutation S218L causing familial hemiplegic migraine and delayed cerebral edema and coma after minor head trauma. J Biol Chem. 2005;280:17678–17686. doi: 10.1074/jbc.M501110200. [DOI] [PubMed] [Google Scholar]
- 108.Peloquin JB, Rehak R, Doering CJ, McRory JE. Functional analysis of congenital stationary night blindness type-2 CACNA1F mutations F742C, G1007R, and R1049W. Neuroscience. 2007;150:335–45. doi: 10.1016/j.neuroscience.2007.09.021. [DOI] [PubMed] [Google Scholar]
- 109.Vitko I, Chen Y, Arias JM, Shen Y, Wu X-R, Perez-Reyes E. Functional characterization and neuronal modeling of the effects of childhood absence epilepsy variants of CACNA1H, a T-Type calcium channel. J Neurosci. 2005;25:4844–4855. doi: 10.1523/JNEUROSCI.0847-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Miyauchi Y, Daiho T, Yamasaki K, Takahashi H, Ishida-Yamamoto A, Danko S, Suzuki H, Iizuka H. Comprehensive analysis of expression and function of 51 sarco(endo)plasmic reticulum Ca2+-ATPase mutants associated with Darier disease. J Biol Chem. 2006;281:22882–22895. doi: 10.1074/jbc.M601966200. [DOI] [PubMed] [Google Scholar]
- 111.Sato K, Yamasaki K, Daiho T, Miyauchi Y, Takahashi H, Ishida-Yamamoto A, Nakamura S, Iizuka H, Suzuki H. Distinct types of abnormality in kinetic properties of three Darier disease-causing sarco(endo)plasmic reticulum Ca2+-ATPase mutants that exhibit normal expression and high Ca2+ transport activity. J Biol Chem. 2004;279:35595–603. doi: 10.1074/jbc.M404887200. [DOI] [PubMed] [Google Scholar]
- 112.Hellman B, Gylfe E, Grapengiesser E, Lund P-E, Berts A. Cytoplasmic Ca2+ oscillations in pancreatic β-cells. Biochim Biophys Acta. 1992;1113:295–305. doi: 10.1016/0304-4157(92)90003-s. [DOI] [PubMed] [Google Scholar]
- 113.Woods NM, Cuthbertson KSR, Cobbold PH. Repetitive transient rises in cytoplasmic free calcium in hormone-stimulated hepatocytes. Nature. 1986;319:600–602. doi: 10.1038/319600a0. [DOI] [PubMed] [Google Scholar]
- 114.Haddock RE, Hill CE. Differential activation of ion channels by inositol 1,4,5-trisphosphate (IP3)- and ryanodine-sensitive calcium stores in rat basilar artery vasomotion. J Physiol. 2002;545:615–627. doi: 10.1113/jphysiol.2002.027904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.McCloskey KD, Toland HM, Hollywood MA, Thornbury KD, McHale NG. Hyperpolarisation-activated inward current in isolated sheep mesenteric lymphatic smooth muscle. J Physiol. 1999;521:201–211. doi: 10.1111/j.1469-7793.1999.00201.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kubota HY, Yoshimoto Y, Hiramoto Y. Oscillation of intracellular free calcium in cleaving and cleavage-arrested embryos of Xenopus laevis. Dev Biol. 1993;160:512–518. doi: 10.1006/dbio.1993.1325. [DOI] [PubMed] [Google Scholar]
- 117.Koester HJ, Sakmann B. Calcium dynamics associated with action potentials in single nerve terminals of pyramidal cells in layer 2/3 of the young rat neocortex. J Physiol. 2000;529:625–646. doi: 10.1111/j.1469-7793.2000.00625.x. [DOI] [PMC free article] [PubMed] [Google Scholar]