Abstract
Intestinal resection and malformations in adult and pediatric patients result in devastating consequences. Unfortunately, allogeneic transplantation of intestinal tissue into patients has not been met with the same measure of success as the transplantation of other organs. Attempts to engineer intestinal tissue in vitro include disaggregation of adult rat intestine into subunits called organoids, harvesting native adult stem cells from mouse intestine and spontaneous generation of intestinal tissue from embryoid bodies. Recently, by utilizing principles gained from the study of developmental biology, human pluripotent stem cells have been demonstrated to be capable of directed differentiation into intestinal tissue in vitro. Pluripotent stem cells offer a unique and promising means to generate intestinal tissue for the purposes of modeling intestinal disease, understanding embryonic development and providing a source of material for therapeutic transplantation.
Keywords: development, differentiation, embryoid bodies, human, intestine, organoids, pluripotent, regeneration, stem cell, tissue engineering, transplantation
Tissue engineering is a broad and emerging field that includes a range of research areas from developmental biology and disease to regeneration and generation of tissues and organs for transplantation. The underlying principle of tissue engineering involves manipulating cells in combination with biologic or synthetic materials in order to cultivate new tissues and organs [1]. This can be done fully in vitro to derive engineered tissue, or scaffolds can be transplanted in vivo and then seeded by endogenous cell types. Recent emphasis has been placed on personalized medicine, where a patient's own cells are used to generate autologous tissue, which eliminates the need for immunosuppressive therapy following transplantation in order to prevent tissue rejection. Human clinical trials using tissue engineering include the transplantation of skin, cartilage, bone, blood vessels, corneas, urinary structures and lung bronchi [2–15]. As of 2007, the commercial market for tissue engineered products comprised 50 firms or businesses employing 3000 people and generating revenues of US$1.3 billion annual [16].
Since the emergence of tissue engineering biology in the 1980s, initially for the growth and development of liver tissue in vitro [17–22], various strategies have been employed including ex vivo manipulation of organs or organoid units, fabrication of organs by seeding cells on extracellular scaffolds, isolation and manipulation of adult stem cells in vivo and in vitro, and, more recently, the directed differentiation of pluripotent stem cells (PSCs) into organ cell types and tissues [23–25]. There are two types of human PSC (hPSC): human embryonic stem cells (hESCs), derived from the inner cell mass of the blastocyst of an embryo, and human induced pluripotent stem (hiPS) cells [26–28]. hiPS cells are generated by nuclear reprogramming of somatic cell types into hESC-like cells through introduction of molecular factors that control pluripotency [29–31]. In the last several years, hPSCs have been used in vitro in unprecedented studies of human developmental biology to identify molecular pathways that regulate the differentiation of progenitor cell types into organ-specific cells [32–36]. In this article, we will discuss how basic studies of intestinal developmental biology and adult intestinal stem cells have led to recent progress in engineering intestinal tissue.
Intestine structure & function
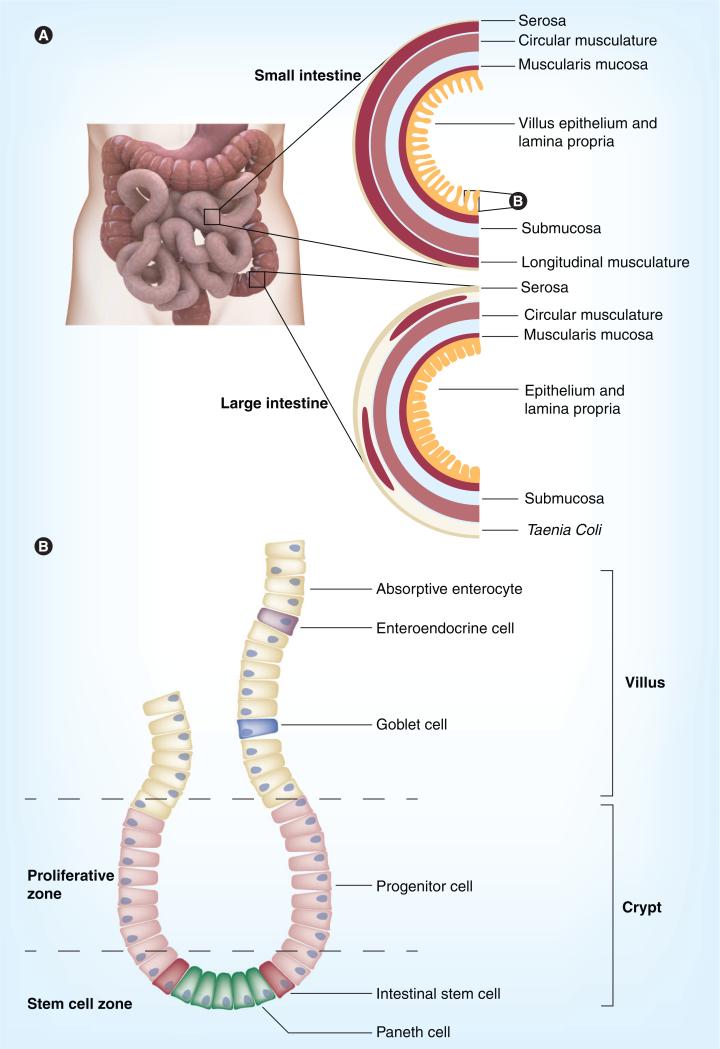
One major challenge of tissue engineering is generating tissues that have the full complement of organ function in vitro. An example is the intestine, which is comprised of two hierarchal structures: the small and large bowel (Figure 1A). The bowel's chief functions are to process and absorb nutrients from food and eliminate enteral waste from the body. Along the length of the intestine, distinct regionalization exists, and defined functions are performed in each segment. For example, the small bowel contains the subdivisions of duodenum (most proximal), jejunum and ileum (most distal) and participates in the neutralization of stomach acid during digestion, enzymatic processing of food products within the lumen, and both passive and active absorption of nutrients [37,38]. The large bowel is composed of cecum (most proximal); ascending, transverse, descending and sigmoid colon; and rectum (most distal) [39]. The main purpose of the colon is to resorb water and sodium while concentrating fecal matter for eventual excretion from the body. In cross-section, the small and large intestine are radially symmetrical and both are composed of outer layers of serosa and musculature which participate in peristalsis, middle layers of submucosa and muscularis mucosa, and innermost layers of lamina propria and a simple columnar epithelium, which lines the luminal surface (Figure 1A) [37,40]. In the colon, large longitudinal muscular bundles, called taenia coli, are present to aid in peristalsis. During digestion, a coordinated interplay occurs between the intestinal layers, which is mediated by hormone signals, an enteric nervous system and vasculature to allow for the controlled movement and efficient processing of food, nutrient absorption and endocrine-mediated communication with other systems to control numerous processes, including glucose homeostasis and satiety. Within the lumen, bacterial symbiosis also allows for breakdown of molecules that the body cannot handle [41].
Figure 1. Histology of intestinal layers and crypts.
(A) In cross-section, both small and large intestine contain outer layers of serosa and both longitudinal and circular musculature. The large intestine has large muscular ribbons, called taenia coli, to aid in contraction and peristalsis. Middle layers include submucosa and muscularis mucosa. The innermost layers are the lamina propria and epithelium. In the small intestine, villi project into the intestinal lumen, which expands the surface area available for absorption. Villi are absent in the large intestine. (B) Intestinal crypts contain resident stem cells capable of generating all cell types of the mature epithelium (small intestinal crypt and villus). In the small intestine, Paneth cells provide a crucial microenvironmental niche for stem cells. Paneth cells are largely absent in the large intestine. Following asymmetrical cell division, stem cells give rise to progenitor cells that differentiate into enterocytes (capable of absorption of nutrients and water) hormone secreting enteroendocrine cells, mucin secreting goblet cells and the aforementioned Paneth cells.
The intestinal epithelium is a complex environment composed of active cell proliferation and differentiation of many cell types (Figure 1B). In the small intestine, villus and microvillus projections into the lumen exponentially increase the surface area available for nutrient absorption, whereas these projections are absent in the colon. Intestinal stem cells reside near the bottom of epithelial crypts and give rise to progenitor cells, which rapidly divide and differentiate into subtypes of cells that comprise the intestinal epithelium (Figure 1B) [37]. The most common intestinal epithelial cells are enterocytes which are mainly responsible for absorption of nutrients and water via active and passive transport. Goblet cells, which are more abundant in the colon than the small intestine, secrete mucins and other proteins that are used for lubrication and as a barrier defense against pathogens, and Paneth cells secrete lysozyme to prevent bacterial infection. Paneth cells also play a key role in providing a niche for the stem cells in the crypts (described in more detail later) [42,43]. Enteroendocrine cells are the rarest of gut epithelial cells (comprising 1% of the epithelium) and include approximately ten different subtypes, which secrete a variety of hormones that participate in glucose homeostasis (glucose insulinotropic peptide and glucagon-like peptide 1), satiety (ghrelin), pH balance (secretin), gall bladder contraction (cholecystokinin), neuromuscular contraction and gut motility (neurotensin and motilin) and the regulation of pancreatic and pituitary hormone secretion (somatostatin) [44–47]. Subtypes of enteroendocrine cells are often confined to specific segments of the gut. Given that different proximal–distal segments of the gut have different cellular compositions and functions, the gut represents an unusual challenge for tissue engineering efforts.
Intestinal disorders & transplantation
Worldwide, only approximately 200 allogeneic intestinal transplants occur each year due to both donor shortage and the technical challenges inherent in the procedure [48]. Among adults, the most common reasons for transplant include ischemic bowel, inflammatory bowel disease (such as Crohn's disease or ulcerative colitis) and traumatic injury complicated by bowel resection [48]. In the pediatric population, especially neonates, the primary reasons for a bowel transplant are congenital malformations, such as Hirschsprung's disease or intestinal atresias, acquired dysfunction or short gut syndrome as a result of intestinal resection due to necrotizing enterocolitis, volvulus or gastroschisis. Bowel transplantation as part of a multivisceral procedure involving the liver, intestine, stomach and pancreas occurs in approximately a quarter of all pediatric transplant cases [48]. Transplants are also required in some cases of severe gastroparesis and in complications of resection caused by meconium ileus and cystic fibrosis, among others. In children aged 1–18 years, 5-year survival rates from intestinal transplants are significantly lower (46–76%) when compared with renal (95–96%), liver (77–86%) and heart (72–77%) transplants [49,50]. This higher morbidity and mortality is believed to be caused by a greater need for post-transplant immunosuppression, since a large part of the immune system resides in the intestine, rendering it highly immunogenic [51]. Undersuppression can lead to graft rejection and elimination of intestinal barrier function, while oversuppression can yield local or systemic infection in the recipient. It is the hope of physicians, scientists and patients that intestinal engineering efforts will improve the outcome of intestinal transplants through generating autologous tissue or by devising methods for stimulating tissue regeneration in situ.
Ex vivo manipulation of intestinal organoids for tissue engineering
In the 1980s, studies demonstrated that a rat duodenal cell line (IEC-17), when cultured long term, formed organized structures composed of closed central lumens and stratified layers of polarized tissue [52]. These observations raised the possibility of ex vivo manipulation of intestinal tissue as a means to engineer intestine. Subsequent studies demonstrated that disaggregated small intestinal tissue from rat harvested by enzymatic digestion formed self-contained intestinal units when transplanted subcutaneously into donor rats [53]. Grafts contained tissue with a circumferential epithelium surrounding a central lumen and exhibited expression of markers of absorptive enterocytes, goblet cells, Paneth cells and enteroendocrine cells. Work in fetal intestine clarified the necessity of mesenchymal–epithelial interactions for the survival and ultimate differentiation potential of ex vivo intestinal tissue [54,55]. The Vacanti laboratory demonstrated that disaggregated fetal intestinal units, which they termed organoids, could be seeded onto sheets of nonwoven polyglycolic acid and incubated for 7 days in vitro prior to transplantation into the peritoneal cavity of recipient Lewis rats [56,57]. After harvest, these implants, also called tissue engineered neointestine (TENI), showed crypt structures and mature intestinal histology.
Other laboratories attempted to dissociate the small intestine of adult rats into organoids for culture and transplantation into recipients with only modest recovery of mature intestinal tissue [1,58,59]. As the polyester scaffolds commonly used to grow intestine in vitro and in vivo have become more refined, addition of collagen and poly-l-lactic acid to scaffolds of polyglycolic acid improved engraftment rate of organoids in rats, and increased the size and number of villus structures present in the resulting tissue [60,61]. Further work demonstrated that anastomosis of TENI to native bowel of rats significantly improved weight loss and malabsorption commonly associated with massive bowel resection [62–65].
Attempts to isolate organoid units from regions of the gut other than the small intestine have also met with success. Grikscheit et al. derived tissue engineered colon by disaggregation of rat colon similar to previous methods used to isolate small intestine organoids [66,67]. When cultured with polymer scaffolds and transplanted into Lewis rats with ileal anastomoses, they contributed to neocolon tissue in vivo, and treated rats had decreased stool transit time, lower stool moisture content and higher total serum bile acids compared with rats with end-ileostomies alone, suggesting some functional competency of the transplanted colon. The same group had similar results following dissociation of esophagus and stomach organoid units and showed functional contribution of these tissues in respective, transplant recipient rats [68,69]. Interestingly, the functional capacity of region-specific intestinal organoid segments from adult animals seems to be preserved after harvesting, ex vivo expansion and transplantation into alternate intestinal sites. For example, organoids derived from rat ileum retained the ability to express ileal-specific bile acid transporter protein when transplanted into rat jejunum [70]. This would suggest that adult intestinal tissue and perhaps the stem cells therein are limited in their capacity to adopt a new regional cell fate.
Neovasculaturization, lymphangiogenesis and immune cell contribution have all been observed in engrafted organoid units that contribute to the intestine [71–73]. Recent work has demonstrated that anal sphincter musculature can be harvested from humans and engineered ex vivo into an innervated construct by coculture with fetal enteric neurons [74,75]. Subcutaneous implantation of this tissue in immunocompromised recipient mice yielded grafts with functional properties of anal sphincter musculature including the ability to respond to neurotransmitters that relaxed or contracted the tissue appropriately. More recently, TENI derived from mouse intestine has been isolated and transplanted into recipients with contribution to functional small intestine, which now allows for the use of mouse genetics in future TENI studies [76]. In addition, disaggregated small intestinal and stomach organoids have also been derived from large animals, including pigs, and autologous transplantation has been performed following generation of TENI [77].
Isolation & culture of intestinal stem cells
Intestinal stem cells are known to exist in the base of crypts, where they are maintained in a unique microenvironmental niche capable of preserving multipotency and allowing differentiation of progenitor cells into all epithelial cell lineages. Several trophic factors are provided by adjacent cell types, including mesenchyme and Paneth cells, to promote active WNT signaling in the stem cell [78–80]. WNT antagonist studies have demonstrated disrupted epithelial architecture in the small intestine and reduced proliferation of epithelial subtypes, suggesting that WNT signaling supports the stem cell niche and allows for proliferation of stem cells [81–85]. Studies have demonstrated that BMP is expressed in villi, where it opposes cell proliferation and promotes cell differentiation [86,87]. Use of BrdU, LacZ and Cre-lox reporter systems have demonstrated that the lineage of all the cells of several adjacent villi can be traced to stem cells within crypts [88,89].
Ootani et al. studied intestinal stem cells within disaggregated organoid units derived from rat small intestine in long-term ex vivo cultures and found that growth was slowed in the presence of the WNT inhibitor DKK1 and stimulated by the presence of Rspondin-1, a WNT agonist [90]. Treatment with the γ-secretase inhibitor, dibenzapine, as well as overexpression of neurogenin-3, a transcription factor essential for enteroendocrine cell differentiation, induced goblet and enteroendocrine cell formation within organoid cultures. These results showed that ex vivo manipulated intestinal tissue has the ability to respond to normal stem cell signaling to direct proliferation and differentiation of intestinal epithelium. However, to date, no comprehensive study has been undertaken to characterize similarities and differences in the functional properties and differentiation potential of intestinal stem cells located in different region-specific segments of the intestine.
Multiple intestinal stem cell markers have been identified in mice, including Lgr-5, Bmi-1, Msi-1, Ephb-2 and Dcamkl-1 [91–95]. Lgr-5, a G-protein coupled receptor closely related to the thyroid-stimulating hormone receptor, is regulated by the WNT/APC/β-catenin pathway and is perhaps the most well-studied marker [88,96,97]. It is expressed in a limited number of columnar epithelial cells located at the crypt base suggesting that its location is restricted to the stem cell compartment. Sato et al. demonstrated that Lgr-5+ cells can be isolated from the crypts of postnatal mice, purified by FACS, and cultured as single cells in the presence of EGF, Noggin and R-spondin-1 to give rise to spherically shaped organoid units with an inner luminal structure, villus domains and with outward projecting crypt-like structures containing Lgr-5+ stem cells [98]. Further ana lysis showed the presence of goblet, Paneth and enteroendocrine cell subtypes within each organoid. The fact that each organoid structure was derived from single Lgr-5+ cells demonstrates the extraordinary capability of these cells to contribute to all known intestinal epithelial cells.
Sato et al. also demonstrated that coculture of Lgr-5+ cells with FACS sorted CD24+ Paneth cells significantly improved in vitro organoid formation, and genetic removal of Paneth cells in vivo is associated with loss of Lgr-5+ cells [99]. This work suggests that the Paneth cells are part of the intestinal stem cell niche and provide important microenvironmental cues. Along with WNT signaling, Notch, a cell surface receptor that interacts with its ligand (Delta) via cell-to-cell contact, is known to play a role in intestinal stem cell homeostasis, which is probably facilitated by intimate interaction between cells within the intestinal crypt [100]. Inhibition of Notch signaling provides instruction to stem cells to undergo differentiation into secretory cell lineages; although the timing, duration and coordination of these signals are not well studied [101,102].
Intestinal embryonic development
One approach to tissue engineering intestine is to differentiate PSCs into intestinal cell types and tissues. Historically, differentiation of embryonic and induced pluripotent stem cells into specific organ cell types such as liver and pancreas has required an intimate knowledge of the molecular pathways that regulate embryonic development of that organ. Similar to the liver and pancreas, the intestines are derived from the endoderm primary germ layer that arises following gastrulation of the developing embryo (Figure 2) [103–106]. Nodal, a member of the TGF-β superfamily, is required for specification of endoderm tissue in all vertebrate species [107,108]. A complex series of morphogenetic events occurs to transform a simple sheet of definitive endoderm (DE) into a 3D gut tube, starting with initial closure of the tube at the anterior and posterior intestinal portals, which then proceeds toward the middle of the embryo resulting in a primitive gut tube that spans the length of the embryo (Figure 2). During its formation, the gut tube epithelium undergoes elongation and patterning along the anterior–posterior axis to form specific subdomains: the foregut, midgut and hindgut. The foregut gives rise to the thyroid, esophagus, lungs, stomach, pancreas, liver, gall bladder and duodenum; and the mid and hindgut give rise to the small and large intestine, respectively. Although relatively little is known about the mechanisms that control the early stages of gut tube morphogenesis [109], patterning is known to involve WNT, FGF and BMP signaling Figure 2) [110–117]. At this developmental stage, WNT and FGF signaling each repress foregut fate and promote development of mid and hindgut lineages [109,117,118]. Refining the regional identity of the gut tube into duodenum, jejunum, ileum and colon is then established through interplay between region-specific factors in the mesenchym and epithelium. This involves signaling molecules such as BMP, hedgehog, WNT, PDGF and EGF [119–123] and transcription factors including CDX2 and various HOX proteins [119,120,124–128] that function in regionalization and formation of villus structures, which proceeds in an anterior to posterior wave along the entire length of the intestine. Final maturation of the intestinal epithelium occurs during the prenatal and neonatal period and involves the formation of crypts, the establishment of flora and the activation of digestive programs in response to feeding.
Figure 2. Embryonic development of vertebrate endoderm and intestine.
Following gastrulation of the blastocyst and migration of cells through the primitive streak, the formation of definitive endoderm is induced by exposure to the TGF-b superfamily member Nodal. The endoderm layer eventually forms a primitive gut tube along the anterior–posterior axis that defines proximal and distal gut structures. During gut tube morphogenesis, the influence of various factors establishes patterning of tissue along the anterior–posterior axis such that different domains are able to undergo region-specific organogenesis. During this stage, WNT, FGF and BMP signaling induces hindgut specification and represses the development of foregut tissue. Initial closure of the gut tube occurs at the anterior and posterior ends and proceeds toward the middle until complete. Foregut develops into anterior structures including thyroid, lung, esophagus, stomach, liver, gall bladder and pancreas. Midgut domains develop into small intestine and the proximal large intestine, while hindgut develops into the remainder of the colon.
Differentiation of PSCs into intestinal tissue
Mouse intestinal differentiation
The molecular pathways that control embryonic intestinal development have proven central to recent successes in the directed differentiation of PSCs into intestinal cell types and tissues. Mouse embryonic stem cells (mESCs) are derived from the inner cell mass of the murine blastocyst and have the ability differentiate into all cell types of the developing embryo [129–131]. Efforts to generate specific cell and tissue types in vitro from mESCs have broadly taken two approaches. The first involves the formation of spherical, multicellular aggregates known as embryoid bodies (EBs), which favors cell–cell interactions, but is stochastic in nature and results in less control over differentiation. The second approach is to direct the differentiation of monolayer cultures with addition and removal of soluble factors to mimic embryonic intestinal development. This approach allows for better directed differentiation into specific cell types, but loses the advantage of the normal 3D environment of the embryo and the resulting cell–cell contact.
Spontaneous differentiation of murine EBs has resulted in formation of gut-like tissue in vitro [132–135]. These reports described both physiologic and molecular evidence for the formation of intestinal tissue, including rhythmic beating reminiscent of intestinal peristalsis and cells that histologically resembled intestinal cell types such as goblet cells. Expression of molecular markers that are present during embryonic intestinal development, including Foxa2/HNF3b, Sox17, Id2 and Gata4 were detected in in vitro gut-like structures by PCR and in situ hybridization. Formation of gut-like structures from spontaneous differentiation of EBs was also observed with murine induced pluripotent stem (miPS) cells. These reports all benefited from the strength of 3D EB cultures, but also suffered from the primary weakness of EB-based approaches: that spontaneous differentiation results in the formation of many organ tissues, the result being relatively inefficient formation of intestinal tissue.
Engraftment and subsequent growth of EBs in vivo resulted in more differentiated intestinal tissues [136,137]. Markers of hindgut formation, including Cdx2 and 5-hydroxytryptamine (serotonin) were present, but the stomach-specific marker H+/K+ ATPase was absent. The intestinal stem cell marker, Bmi-1, was also present in transplanted tissues. Host organisms contributed blood vessels and neuronal innervation. In another functional study of mESC contribution to intestinal differentiation in vivo, Kudo et al. directly injected male mESCs into irradiated intestinal mucosa of female recipients and demonstrated that Y-chromosome-positive cells were present in the mouse intestine at 14 days [138].
A recent report improved upon the low efficiency of spontaneous differentiation of EBs by incorporating growth factor manipulations that were designed to direct EB differentiation into intestine [139]. Mouse EBs were generated and then first exposed to activin A, a Nodal analogue, for 6 days to enrich for the formation of DE. EBs were then exposed to Wnt3a and cell-conditioned media, which elevated the expression of intestinal markers including Cdx2, Fabp2 and Id2. Within the EB-derived tissues, there were regions of epithelium and some of these expressed markers including Ephb-2 and Lgr-5. Impressively, injection of DE derived from green fluorescent protein-expressing mESCs into the colonic mucosa of mice resulted in contribution of green fluorescent protein-positive cells to endogenous intestinal tissue. As to whether or not these cells acquired molecular and functional properties of intestine in vivo will undoubtedly be the focus of future studies.
Human intestinal differentiation
As in mice, hPSCs come from embryos (hESC [140]) or can come from nuclear reprogramming of somatic cells (hiPS cells [30,31]). Both have the capacity for differentiation into organ cell types derived from the three primary germ layers. One unique advantage of hiPS cells is that they can be derived from specific patients and, therefore, tissue derived from these hiPS cells can be transplanted autologously. hiPS cells are derived from adult somatic cells, such as fibroblasts or keratinocytes, and are induced toward a pluripo-tent state by several different methods including expression of transcription factors including Nanog, Oct4, Sox2, Klf4 and c-Myc [29,30,141], or more recently through expression of siRNAs [142]. hiPS cell lines can be maintained indefinitely in culture in the same manner as hESCs; however molecular comparison of hESCs and hiPS cells demonstrate some subtle differences, and some evidence suggests that hiPS cells retain a certain degree of epigenetic memory of the tissue from which they are derived [143]. In addition, recent data suggest that pluripotent miPS cells are unable to efficiently form teratomas when transplanted into genetically identical recipient mice and induce a T-cell infiltration at the site of transplantation, suggesting that these cells are rejected by the host immune system [144]. The molecular basis of this rejection is not known, and it could be caused by the method or efficiency of reprogramming. In addition, it is unclear whether differentiated tissues derived from induced pluripotent stem cells will be similarly immunogenic. Since tissue derivatives from hiPS cells have only been tested in immunocompromised animal models, it remains to be seen if they are capable of generating an immune response in autologous transplants as well. Nevertheless, both hESCs and hiPS cells represent powerful tools for the in vitro generation of human tissue types and for the study of developmental biology and organo-genesis and the potential use of PSC derivatives in animal models of disease will undoubtedly be an area of intense study in the future.
A recent approach has been described to efficiently direct hESCs and hiPS cells into intestinal tissue in vitro that utilized both 3D growth Figure 3) and directed differentiation, based on the signaling pathways that control the different stages of vertebrate embryonic intestinal development [145]. In this protocol, monolayers of hPSCs were first directed into the DE lineage using a highly efficient protocol involving low serum and activin A exposure for 3 days, resulting in cultures of 90% DE. Subsequent exposure to WNT3A and FGF4 efficiently directed the formation of posteriorized, CDX2-expressing hindgut endoderm. Specification of hindgut endoderm resulted in the spontaneous induction of 3D, gut tube-like morphogenesis and the formation gut tube spheroids. When grown in 3D conditions that are known to favor intestinal growth Figure 3A) spheroids undergo intestinal morphogenesis and eventually yield intestinal organoids that contain an intestinal epithelium that is almost entirely CDX2+, from which LGR-5+ crypt-like domains, intervillus regions, villus-like projections and a microvillus brush border develop. Intestinal organoids also contain a laminated intestinal mesenchyme with layers of smooth muscle actin expressing cells and intestinal subepithelial myofibroblasts.
Figure 3. Human pluripotent cells are capable of directed differentiation into mature human intestine.
(A) Intestinal organoids can be generated by directed differentiation of human pluripotent cells first into definitive endoderm by low serum and exposure to activin A in vitro. Following culture in WNT3a and FGF4, floating intestinal progenitor spheroids form. When collected and plated at day 0 in the semisolid matrix (Matrigel™; BD Biosciences, Franklin Lakes, NJ, USA), they grow in size and complexity by 28 days to form epithelium with crypt and villus-like structures (arrowheads). Day 28 organoid epithelium contains all known mature cell types including (B) MUC2-expressing goblet cells, (C) LYZ-expressing Paneth cells and (D) CHGA-expressing enteroendocrine cells.
CHGA: Chromogranin A; LYZ: Lysozyme; MUC: Mucin.
Human intestinal organoids contained all known intestinal epithelial cell types including absorptive enterocytes, mucin-secreting goblet cells, lysozyme secreting Paneth cells and chromogranin A-positive enteroendocrine cells Figure 3B) and had both absorptive and secretory function. The utility of this culture system for molecular studies of human development and disease were demonstrated using the transcription factor neurogenin-3, in which mutations in humans cause loss of enteroendocrine cells and enteric anendocrinosis. In intestinal organoids, endogenous neurogenin-3 function was knocked down by introduction of shRNA and resulted in the loss of enteroendocrine cells. By contrast, overexpression of neurogenin-3 resulted in a five-fold increase in the number of enteroendocrine cells [145]. Together, these data suggest that the in vitro derived intestinal organoid system should allow both basic studies of human intestinal development and disease.
Future perspective
With increasing frequency, stem cells are being utilized in the study of human disease and basic developmental biology. With the advent of induced pluripotent stem cells and their demonstrated capacity for directed tissue differentiation, it is now theoretically possible to harvest cells from patients for the purpose of reprogramming them into hiPS cells, which can be used to generate intestine for autologous transplantation. Furthermore, when harvested from patients with genetic syndromes or mutations, hiPS cells are a powerful tool with which to study the developmental origin and pathophysiology of the disease. The immunogenicity of pluripotent miPS cells has recently been described in genetically identical recipient mice [144]; however, no detailed evidence of the immunogenic potential of differentiated tissue derived from induced pluripotent stem cells exist, although this will no doubt be the subject of further evaluation. Nevertheless, careful evaluation of the safety of tissues derived from human pluripotent sources is needed prior to initiation of transplantation into allogeneic or autologous recipients.
Directed differentiation of PSCs into human intestine represents a leap forward in human developmental biology, since the direct investigation and observation of human embryonic development is limited. However, critical gaps in our understanding of intestinal development remain, which limit the utility of PSC-derived intestinal tissue. For example, it is not well understood how regional segments of the intestine are established during embryonic development and this information would facilitate generation of region-specific segments of intestine from PSCs. In vitro derivation of specific segments of human intestinal tissue would facilitate pharmacological study of drug effects and toxins, reveal the impact of specific nutrients on enteroendocrine cell behavior and allow for studies of regional stem cell properties. In addition, testing of medical devices, surgical techniques, tissue injury and wound healing, and studies of nutrient and environmental exposure could potentially be conducted on human intestinal tissue that is grown outside the body. Studies of the functional contribution of in vitro hPSC-derived intestinal organoids to the native intestines of injured animal transplant recipients are ongoing. Future advances are needed, such as the use of bioengineered tissue scaffolding, which could allow for the massive expansion of human intestinal organoids from current sizes (a few millimeters in diameter) to that which would be adequate for both in vitro studies and for transplantation into human patients.
Executive summary.
Need for intestinal tissue for transplantation
■ Intestinal disorders and resection caused by congenital or acquired anomalies result in the need for intestinal tissue for transplantation in both adults and children.
■ Data indicate that allogeneic intestinal transplantation has a poor 5-year graft survival and patient mortality compared with transplantation of other solid organs including kidney, liver and heart.
■ Tissue engineering offers a promising means to generate nascent intestinal tissue in vitro utilizing stem cells and developmental biology principles to provide tissue capable of autologous transplantation.
Intestinal disaggregation & use of polyester scaffolding to generate in vitro intestinal tissue
■ Several research laboratories have demonstrated that disaggregated intestinal tissue from rats is able to generate sustainable units of intestinal tissue, termed organoids, when cultured in vitro.
■ Transplantation of organoids into recipient animals generates donor-derived intestinal tissue capable of functional properties of the region in which they are transplanted.
■ Polyester scaffolding, comprised of polyglycolic acid or poly-l-lactic acid, has been demonstrated to increase the size of intestinal grafts and has been successfully used in intestinal transplantation into recipient animals.
Intestinal stem cell identification & characterization
■ Recent advances have demonstrated that single LGR-5+ cells harvested from adult mouse intestine are capable of regenerating all cell types of the intestinal epithelium ex vivo.
■ The intestinal stem cell is supported in intestinal crypts by Paneth cells and is maintained in a microenvironmental niche through signaling via WNT and Notch.
■ The signaling events that instruct the differentiation of intestinal stem and progenitor cells into the various mature epithelial cell types are not well understood.
Spontaneous generation of intestinal tissue with embryonic stem cells
■ Embryoid bodies derived from mouse embryonic stem cells have been demonstrated to spontaneously give rise to cells expressing intestinal-specific markers.
■ Transplantation of embryoid body-derived intestinal cells can contribute to formation of new intestinal tissue following surgical injury or radiation.
■ Few published attempts have been made to direct the fate of embryonic stem cells or embryoid bodies into intestinal cells with specific culture conditions and soluble factors utilizing developmental biology principles.
Directed differentiation of human pluripotent stem cells into intestinal tissue
■ A recent study has demonstrated the ability of human pluripotent stem cells to undergo directed differentiation into intestinal tissue through stages that recapitulate embryonic development.
■ Definitive endoderm derived in vitro is capable of intestinal organoid formation by exposure to high levels of WNT and FGF.
■ 3D culture conditions support the growth of organoids into mature intestinal tissue containing a laminated mesenchyme and an epithelium containing all known intestinal cell types and capable of functional peptide transport.
■ Use of induced pluripotent stem cells to generate human intestinal tissue offers the possibility to model disease in the intestine, test pharmaceuticals in an in vitro environment and provide material for autologous transplantation of intestine.
Acknowledgements
The authors wish to thank Jason R Spence for providing the confocal microscopy images of human intestinal organoids in Figure 3B.
Footnotes
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
Bibliography
Papers of special note have been highlighted as:
■ of interest
■■ of considerable interest
- 1.Penkala RA, Kim SS. Gastrointestinal tissue engineering. Exp. Rev. Med. Devices. 2007;4(1):65–72. doi: 10.1586/17434440.4.1.65. [DOI] [PubMed] [Google Scholar]
- 2.Vacanti J. Tissue engineering and regenerative medicine: from first principles to state of the art. J. Pediatr. Surg. 2010;45(2):291–294. doi: 10.1016/j.jpedsurg.2009.10.063. [DOI] [PubMed] [Google Scholar]
- 3.Chistiakov DA. Endogenous and exogenous stem cells: a role in lung repair and use in airway tissue engineering and transplantation. J. Biomed. Sci. 2010;17:92. doi: 10.1186/1423-0127-17-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kusuma S, Gerecht S. Engineering blood vessels using stem cells: innovative approaches to treat vascular disorders. Exp. Rev. Cardiovasc. Ther. 2010;8(10):1433–1445. doi: 10.1586/erc.10.121. [DOI] [PubMed] [Google Scholar]
- 5.Petersen TH, Calle EA, Zhao L, et al. Tissue-engineered lungs for in vivo implantation. Science. 2010;329(5991):538–541. doi: 10.1126/science.1189345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Proulx S, Fradette J, Gauvin R, Larouche D, Germain L. Stem cells of the skin and cornea: their clinical applications in regenerative medicine. Curr. Opin. Organ Transplant. 2011;16(1):87–89. doi: 10.1097/MOT.0b013e32834254f1. [DOI] [PubMed] [Google Scholar]
- 7.Tare RS, Kanczler J, Aarvold A, Jones AM, Dunlop DG, Oreffo RO. Skeletal stem cells and bone regeneration: translational strategies from bench to clinic. Proc. Inst. Mech. Eng. H. 2010;224(12):1455–1470. doi: 10.1243/09544119JEIM750. [DOI] [PubMed] [Google Scholar]
- 8.Toh WS, Lee EH, Cao T. Potential of human embryonic stem cells in cartilage tissue engineering and regenerative medicine. Stem Cell Rev. 2011;7(3):544–559. doi: 10.1007/s12015-010-9222-6. [DOI] [PubMed] [Google Scholar]
- 9.Calle EA, Petersen TH, Niklason LE. Procedure for lung engineering. J. Vis. Exp. 2011;49:e2651. doi: 10.3791/2651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heiligenstein S, Cucchiarini M, Laschke MW, et al. In vitro and in vivo characterization of non-biomedical and biomedical grade alginates for articular chondrocyte transplantation. Tissue Eng. Part C Methods. 2011 doi: 10.1089/ten.tec.2010.0681. doi: 10.1089/ten. TEA.2010.0681. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 11.Marazzi M, Crovato F, Bucco M, et al. GMP-compliant culture of human hair follicle cells for encapsulation and transplantation. Cell Transplant. 2011 doi: 10.3727/096368911X565010. doi: 10.3727/096368911X565010. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 12.Orlando G, Baptista P, Birchall M, et al. Regenerative medicine as applied to solid organ transplantation: current status and future challenges. Transpl. Int. 2011;24(3):223–232. doi: 10.1111/j.1432-2277.2010.01182.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ouellet G, Dube J, Gauvin R, Laterreur V, Bouhout S, Bolduc S. Production of an optimized tissue-engineered pig connective tissue for the reconstruction of the urinary tract. Tissue Eng. Part A. 2011;17(11–12):1625–1633. doi: 10.1089/ten.TEA.2010.0324. [DOI] [PubMed] [Google Scholar]
- 14.Raya-Rivera A, Esquiliano DR, Yoo JJ, Lopez-Bayghen E, Soker S, Atala A. Tissue-engineered autologous urethras for patients who need reconstruction: an observational study. Lancet. 2011;377(9772):1175–1182. doi: 10.1016/S0140-6736(10)62354-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang X, Yang J, Li Y, et al. Functional neovascularization in tissue engineering with porcine acellular dermal matrix and human umbilical vein endothelial cells. Tissue Eng. Part C Methods. 2011;17(4):423–433. doi: 10.1089/ten.TEC.2010.0466. [DOI] [PubMed] [Google Scholar]
- 16.Lysaght MJ, Jaklenec A, Deweerd E. Great expectations: private sector activity in tissue engineering, regenerative medicine, and stem cell therapeutics. Tissue Eng. Part A. 2008;14(2):305–315. doi: 10.1089/tea.2007.0267. [DOI] [PubMed] [Google Scholar]
- 17.Saito S, Sakagami K, Orita K. A new hybrid artificial liver using a combination of hepatocytes and biomatrix. ASAIO Trans. 1987;33(3):459–462. [PubMed] [Google Scholar]
- 18.Cai ZH, Shi ZQ, O'Shea GM, Sun AM. Microencapsulated hepatocytes for bioartificial liver support. Artif. Organs. 1988;12(5):388–393. doi: 10.1111/j.1525-1594.1988.tb02793.x. [DOI] [PubMed] [Google Scholar]
- 19.Uchino J, Tsuburaya T, Kumagai F, et al. A hybrid bioartificial liver composed of multiplated hepatocyte monolayers. ASAIO Trans. 1988;34(4):972–977. [PubMed] [Google Scholar]
- 20.Wong H, Chang TM. The viability and regeneration of artificial cell microencapsulated rat hepatocyte xenograft transplants in mice. Biomater. Artif. Cells Artif. Organs. 1988;16(4):731–739. doi: 10.3109/10731198809117565. [DOI] [PubMed] [Google Scholar]
- 21.Cai ZH, Shi ZQ, Sherman M, Sun AM. Development and evaluation of a system of microencapsulation of primary rat hepatocytes. Hepatology. 1989;10(5):855–860. doi: 10.1002/hep.1840100518. [DOI] [PubMed] [Google Scholar]
- 22.Nose Y. Artificial liver for a bridge to liver transplantation. Artif. Organs. 1989;13(5):415–416. doi: 10.1111/j.1525-1594.1989.tb01549.x. [DOI] [PubMed] [Google Scholar]
- 23.Vacanti JP, Morse MA, Saltzman WM, Domb AJ, Perez-Atayde A, Langer R. Selective cell transplantation using bioabsorbable artificial polymers as matrices. J. Pediatr. Surg. 1988;23(1 Pt 2):3–9. doi: 10.1016/s0022-3468(88)80529-3. [DOI] [PubMed] [Google Scholar]
- 24.Langer R, Vacanti JP. Tissue engineering. Science. 1993;260(5110):920–926. doi: 10.1126/science.8493529. [DOI] [PubMed] [Google Scholar]
- 25.Vacanti CA. The history of tissue engineering. J. Cell. Mol. Med. 2006;10(3):569–576. doi: 10.1111/j.1582-4934.2006.tb00421.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Deng W. Exploiting pluripotency for therapeutic gain. Panminerva Med. 2010;52(2):167–173. [PubMed] [Google Scholar]
- 27.Stadtfeld M, Hochedlinger K. Induced pluripotency: history, mechanisms, and applications. Genes Dev. 2010;24(20):2239–2263. doi: 10.1101/gad.1963910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sullivan GJ, Bai Y, Fletcher J, Wilmut I. Induced pluripotent stem cells: epigenetic memories and practical implications. Mol. Hum. Reprod. 2010;16(12):880–885. doi: 10.1093/molehr/gaq091. [DOI] [PubMed] [Google Scholar]
- 29■■.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663–676. doi: 10.1016/j.cell.2006.07.024. [First description of the ability to generate induced pluripotent stem cells by the reprogramming of mouse fibroblasts with defined factors.] [DOI] [PubMed] [Google Scholar]
- 30■■.Yu J, Vodyanik MA, Smuga-Otto K, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318(5858):1917–1920. doi: 10.1126/science.1151526. [First description of the ability to generate human induced pluripotent stem cells by reprogramming human somatic cells with defined factors.] [DOI] [PubMed] [Google Scholar]
- 31.Nakagawa M, Koyanagi M, Tanabe K, et al. Generation of induced pluripotent stem cells without Myc from mouse and human fibroblasts. Nat. Biotechnol. 2008;26(1):101–106. doi: 10.1038/nbt1374. [DOI] [PubMed] [Google Scholar]
- 32.Jiang M, Lv L, Ji H, et al. Induction of pluripotent stem cells transplantation therapy for ischemic stroke. Mol. Cell. Biochem. 2011;354(1–2):67–75. doi: 10.1007/s11010-011-0806-5. [DOI] [PubMed] [Google Scholar]
- 33.Liu H, Kim Y, Sharkis S, Marchionni L, Jang YY. In vivo liver regeneration potential of human induced pluripotent stem cells from diverse origins. Sci. Transl. Med. 2011;3(82):82ra39. doi: 10.1126/scitranslmed.3002376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mauritz C, Martens A, Rojas SV, et al. Induced pluripotent stem cell (iPSC)-derived Flk-1 progenitor cells engraft, differentiate, and improve heart function in a mouse model of acute myocardial infarction. Eur. Heart J. 2011 doi: 10.1093/eurheartj/ehr166. doi: 10.1093/eurheartj/ehr166. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 35.Rhee YH, Ko JY, Chang MY, et al. Protein-based human iPS cells efficiently generate functional dopamine neurons and can treat a rat model of Parkinson disease. J. Clin. Invest. 2011;121(6):2326–2335. doi: 10.1172/JCI45794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang A, Tang Z, Park IH, et al. Induced pluripotent stem cells for neural tissue engineering. Biomaterials. 2011;32(22):5023–5032. doi: 10.1016/j.biomaterials.2011.03.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cheng LK, O'Grady G, Du P, Egbuji JU, Windsor JA, Pullan AJ. Gastrointestinal system. Wiley Interdiscip. Rev. Syst. Biol. Med. 2010;2(1):65–79. doi: 10.1002/wsbm.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Venkatasubramanian J, Ao M, Rao MC. Ion transport in the small intestine. Curr. Opin. Gastroenterol. 2010;26(2):123–128. doi: 10.1097/MOG.0b013e3283358a45. [DOI] [PubMed] [Google Scholar]
- 39.Bharucha AE. Update of tests of colon and rectal structure and function. J. Clin. Gastroenterol. 2006;40(2):96–103. doi: 10.1097/01.mcg.0000196190.42296.a9. [DOI] [PubMed] [Google Scholar]
- 40.Pabst R, Rothkotter HJ. Structure and function of the gut mucosal immune system. Adv. Exp. Med. Biol. 2006;579:1–14. doi: 10.1007/0-387-33778-4_1. [DOI] [PubMed] [Google Scholar]
- 41.Possemiers S, Grootaert C, Vermeiren J, et al. The intestinal environment in health and disease – recent insights on the potential of intestinal bacteria to influence human health. Curr. Pharm. Des. 2009;15(18):2051–2065. doi: 10.2174/138161209788489159. [DOI] [PubMed] [Google Scholar]
- 42.Dharmani P, Srivastava V, Kissoon-Singh V, Chadee K. Role of intestinal mucins in innate host defense mechanisms against pathogens. J. Innate Immun. 2009;1(2):123–135. doi: 10.1159/000163037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Defoneska A, Kaunitz JD. Gastroduodenal mucosal defense. Curr. Opin. Gastroenterol. 2010;26(6):604–610. doi: 10.1097/MOG.0b013e32833f1222. [DOI] [PubMed] [Google Scholar]
- 44.Efendic S, Portwood N. Overview of incretin hormones. Horm. Metab. Res. 2004;36(11–12):742–746. doi: 10.1055/s-2004-826157. [DOI] [PubMed] [Google Scholar]
- 45.Lee CS, Kaestner KH. Clinical endocrinology and metabolism. Development of gut endocrine cells. Best Pract. Res. Clin. Endocrinol. Metab. 2004;18(4):453–462. doi: 10.1016/j.beem.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 46.Rindi G, Leiter AB, Kopin AS, Bordi C, Solcia E. The ‘normal’ endocrine cell of the gut: changing concepts and new evidences. Ann. NY Acad. Sci. 2004;1014:1–12. doi: 10.1196/annals.1294.001. [DOI] [PubMed] [Google Scholar]
- 47.Vilsboll T, Holst JJ. Incretins, insulin secretion and Type 2 diabetes mellitus. Diabetologia. 2004;47(3):357–366. doi: 10.1007/s00125-004-1342-6. [DOI] [PubMed] [Google Scholar]
- 48.Ueno T, Fukuzawa M. Current status of intestinal transplantation. Surg. Today. 2010;40(12):1112–1122. doi: 10.1007/s00595-010-4324-y. [DOI] [PubMed] [Google Scholar]
- 49.Larosa C, Glah C, Baluarte HJ, Meyers KE. Solid-organ transplantation in childhood: transitioning to adult health care. Pediatrics. 2011;127(4):742–753. doi: 10.1542/peds.2010-1232. [DOI] [PubMed] [Google Scholar]
- 50.Larosa C, Baluarte HJ, Meyers KE. Outcomes in pediatric solid-organ transplantation. Pediatr. Transplant. 2011;15(2):128–141. doi: 10.1111/j.1399-3046.2010.01434.x. [DOI] [PubMed] [Google Scholar]
- 51.Garg M, Jones RM, Vaughan RB, Testro AG. Intestinal transplantation: current status and future directions. J. Gastroenterol. Hepatol. 2011;26(8):1221–1228. doi: 10.1111/j.1440-1746.2011.06783.x. [DOI] [PubMed] [Google Scholar]
- 52.Sambuy Y, De Angelis I. Formation of organoid structures and extracellular matrix production in an intestinal epithelial cell line during long-term in vitro culture. Cell. Differ. 1986;19(2):139–147. doi: 10.1016/0045-6039(86)90071-0. [DOI] [PubMed] [Google Scholar]
- 53.Tait IS, Flint N, Campbell FC, Evans GS. Generation of neomucosa in vivo by transplantation of dissociated rat postnatal small intestinal epithelium. Differentiation. 1994;56(1–2):91–100. doi: 10.1046/j.1432-0436.1994.56120091.x. [DOI] [PubMed] [Google Scholar]
- 54.Kedinger M, Simon-Assmann P, Haffen K. Growth and differentiation of intestinal endodermal cells in a coculture system. Gut. 1987;28(Suppl.):237–241. doi: 10.1136/gut.28.suppl.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Haffen K, Kedinger M, Simon-Assmann P. Mesenchyme-dependent differentiation of epithelial progenitor cells in the gut. J. Pediatr. Gastroenterol. Nutr. 1987;6(1):14–23. doi: 10.1097/00005176-198701000-00005. [DOI] [PubMed] [Google Scholar]
- 56.Evans GS, Flint N, Somers AS, Eyden B, Potten CS. The development of a method for the preparation of rat intestinal epithelial cell primary cultures. J. Cell. Sci. 1992;101(Pt 1):219–231. doi: 10.1242/jcs.101.1.219. [DOI] [PubMed] [Google Scholar]
- 57.Choi RS, Vacanti JP. Preliminary studies of tissue-engineered intestine using isolated epithelial organoid units on tubular synthetic biodegradable scaffolds. Transplant. Proc. 1997;29(1–2):848–851. doi: 10.1016/s0041-1345(96)00164-9. [DOI] [PubMed] [Google Scholar]
- 58.Organ GM, Mooney DJ, Hansen LK, Schloo B, Vacanti JP. Enterocyte transplantation using cell-polymer devices to create intestinal epithelial-lined tubes. Transplant. Proc. 1993;25(1 Pt 2):998–1001. [PubMed] [Google Scholar]
- 59.Gupta A, Dixit A, Sales KM, Winslet MC, Seifalian AM. Tissue engineering of small intestine – current status. Biomacromolecules. 2006;7(10):2701–2709. doi: 10.1021/bm060383e. [DOI] [PubMed] [Google Scholar]
- 60.Sosnowski S, Wozniak P, Lewandowska Szumiel M. Polyester scaffolds with bimodal pore size distribution for tissue engineering. Macromol. Biosci. 2006;6(6):425–434. doi: 10.1002/mabi.200600003. [DOI] [PubMed] [Google Scholar]
- 61.Choi RS, Riegler M, Pothoulakis C, et al. Studies of brush border enzymes, basement membrane components, and electrophysiology of tissue-engineered neointestine. J. Pediatr. Surg. 1998;33(7):991–996. doi: 10.1016/s0022-3468(98)90520-6. discussion 996–997. [DOI] [PubMed] [Google Scholar]
- 62.Grikscheit TC, Siddique A, Ochoa ER, et al. Tissue-engineered small intestine improves recovery after massive small bowel resection. Ann. Surg. 2004;240(5):748–754. doi: 10.1097/01.sla.0000143246.07277.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kaihara S, Kim S, Benvenuto M, et al. End-to-end anastomosis between tissue-engineered intestine and native small bowel. Tissue Eng. 1999;5(4):339–346. doi: 10.1089/ten.1999.5.339. [DOI] [PubMed] [Google Scholar]
- 64.Kim SS, Kaihara S, Benvenuto M, et al. Regenerative signals for tissue-engineered small intestine. Transplant. Proc. 1999;31(1–2):657–660. doi: 10.1016/s0041-1345(98)01737-0. [DOI] [PubMed] [Google Scholar]
- 65.Kim SS, Kaihara S, Benvenuto MS, et al. Regenerative signals for intestinal epithelial organoid units transplanted on biodegradable polymer scaffolds for tissue engineering of small intestine. Transplantation. 1999;67(2):227–233. doi: 10.1097/00007890-199901270-00007. [DOI] [PubMed] [Google Scholar]
- 66.Grikscheit TC, Ogilvie JB, Ochoa ER, Alsberg E, Mooney D, Vacanti JP. Tissue-engineered colon exhibits function in vivo. Surgery. 2002;132(2):200–204. doi: 10.1067/msy.2002.125310. [DOI] [PubMed] [Google Scholar]
- 67.Grikscheit TC, Ochoa ER, Ramsanahie A, et al. Tissue-engineered large intestine resembles native colon with appropriate in vitro physiology and architecture. Ann. Surg. 2003;238(1):35–41. doi: 10.1097/01.SLA.0000074964.77367.4a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Grikscheit T, Ochoa ER, Srinivasan A, Gaissert H, Vacanti JP. Tissue-engineered esophagus: experimental substitution by onlay patch or interposition. J. Thorac. Cardiovasc. Surg. 2003;126(2):537–544. doi: 10.1016/s0022-5223(03)00032-1. [DOI] [PubMed] [Google Scholar]
- 69.Grikscheit T, Srinivasan A, Vacanti JP. Tissue-engineered stomach: a preliminary report of a versatile in vivo model with therapeutic potential. J. Pediatr. Surg. 2003;38(9):1305–1309. doi: 10.1016/s0022-3468(03)00386-5. [DOI] [PubMed] [Google Scholar]
- 70.Avansino JR, Chen DC, Hoagland VD, Woolman JD, Stelzner M. Orthotopic transplantation of intestinal mucosal organoids in rodents. Surgery. 2006;140(3):423–434. doi: 10.1016/j.surg.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 71.Perez A, Grikscheit TC, Blumberg RS, Ashley SW, Vacanti JP, Whang EE. Tissue-engineered small intestine: ontogeny of the immune system. Transplantation. 2002;74(5):619–623. doi: 10.1097/00007890-200209150-00006. [DOI] [PubMed] [Google Scholar]
- 72.Gardner-Thorpe J, Grikscheit TC, Ito H, et al. Angiogenesis in tissue-engineered small intestine. Tissue Eng. 2003;9(6):1255–1261. doi: 10.1089/10763270360728161. [DOI] [PubMed] [Google Scholar]
- 73.Duxbury MS, Grikscheit TC, Gardner Thorpe J, et al. Lymphangiogenesis in tissue-engineered small intestine. Transplantation. 2004;77(8):1162–1166. doi: 10.1097/01.tp.0000121506.34924.3c. [DOI] [PubMed] [Google Scholar]
- 74.Raghavan S, Gilmont RR, Miyasaka EA, et al. Successful implantation of bioengineered, intrinsically innervated, human internal anal sphincter. Gastroenterology. 2011;141(1):310–319. doi: 10.1053/j.gastro.2011.03.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Miyasaka EA, Raghavan S, Gilmont RR, et al. In vivo growth of a bioengineered internal anal sphincter: comparison of growth factors for optimization of growth and survival. Pediatr. Surg. Int. 2011;27(2):137–143. doi: 10.1007/s00383-010-2786-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sala FG, Matthews JA, Speer AL, Torashima Y, Barthel ER, Grikscheit TC. A multicellular approach forms a significant amount of tissue-engineered small intestine in the mouse. Tissue Eng. Part A. 2011;17(13–14):1841–1850. doi: 10.1089/ten.tea.2010.0564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sala FG, Kunisaki SM, Ochoa ER, Vacanti J, Grikscheit TC. Tissue-engineered small intestine and stomach form from autologous tissue in a preclinical large animal model. J. Surg. Res. 2009;156(2):205–212. doi: 10.1016/j.jss.2009.03.062. [DOI] [PubMed] [Google Scholar]
- 78.Kapoor A, Li HJ, Leiter AB. Intestinal development: the many faces of Wnt signaling. Gastroenterology. 2007;133(2):710–712. doi: 10.1053/j.gastro.2007.06.029. [DOI] [PubMed] [Google Scholar]
- 79.Garcia MI, Ghiani M, Lefort A, Libert F, Strollo S, Vassart G. LGR-5 deficiency deregulates Wnt signaling and leads to precocious Paneth cell differentiation in the fetal intestine. Dev. Biol. 2009;331(1):58–67. doi: 10.1016/j.ydbio.2009.04.020. [DOI] [PubMed] [Google Scholar]
- 80.Gregorieff A, Stange DE, Kujala P, et al. The Ets-domain transcription factor Spdef promotes maturation of goblet and Paneth cells in the intestinal epithelium. Gastroenterology. 2009;137(4):1333–1345. e1–3. doi: 10.1053/j.gastro.2009.06.044. [DOI] [PubMed] [Google Scholar]
- 81.Tian Q, He XC, Hood L, Li L. Bridging the BMP and Wnt pathways by PI3 kinase/Akt and 14-13-3ζ. Cell Cycle. 2005;4(2):215–216. [PubMed] [Google Scholar]
- 82.Yi F, Brubaker PL, Jin T. TCF-4 mediates cell type-specific regulation of proglucagon gene expression by b-catenin and glycogen synthase kinase-3b. J. Biol. Chem. 2005;280(2):1457–1464. doi: 10.1074/jbc.M411487200. [DOI] [PubMed] [Google Scholar]
- 83.Aguilera O, Fraga MF, Ballestar E, et al. Epigenetic inactivation of the Wnt antagonist DICKKOPF-1 (DKK-1) gene in human colorectal cancer. Oncogene. 2006;25(29):4116–4121. doi: 10.1038/sj.onc.1209439. [DOI] [PubMed] [Google Scholar]
- 84.Boman B, Kopelovich L, Siracusa LD, et al. A Tcf4-GFP reporter mouse model for monitoring effects of Apc mutations during intestinal tumorigenesis. Mol. Carcinog. 2009;48(9):821–831. doi: 10.1002/mc.20526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hughes KR, Sablitzky F, Mahida YR. Expression profiling of Wnt family of genes in normal and inflammatory bowel disease primary human intestinal myofibroblasts and normal human colonic crypt epithelial cells. Inflamm. Bowel Dis. 2011;17(1):213–220. doi: 10.1002/ibd.21353. [DOI] [PubMed] [Google Scholar]
- 86.Haramis AP, Begthel H, Van Den Born M, et al. De novo crypt formation and juvenile polyposis on BMP inhibition in mouse intestine. Science. 2004;303(5664):1684–1686. doi: 10.1126/science.1093587. [DOI] [PubMed] [Google Scholar]
- 87.Batts LE, Polk DB, Dubois RN, Kulessa H. Bmp signaling is required for intestinal growth and morphogenesis. Dev. Dyn. 2006;235(6):1563–1570. doi: 10.1002/dvdy.20741. [DOI] [PubMed] [Google Scholar]
- 88.Barker N, Van Es JH, Jaks V, et al. Very long-term self-renewal of small intestine, colon, and hair follicles from cycling Lgr-5+ve stem cells. Cold Spring Harb. Symp. Quant. Biol. 2008;73:351–356. doi: 10.1101/sqb.2008.72.003. [DOI] [PubMed] [Google Scholar]
- 89.Barker N, Clevers H. Lineage tracing in the intestinal epithelium. Curr. Protoc. Stem Cell Biol. 2010 doi: 10.1002/9780470151808.sc05a04s13. Chapter 5, Unit 5A.4. [DOI] [PubMed] [Google Scholar]
- 90.Ootani A, Li X, Sangiorgi E, et al. Sustained in vitro intestinal epithelial culture within a Wnt-dependent stem cell niche. Nat. Med. 2009;15(6):701–706. doi: 10.1038/nm.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Asai R, Okano H, Yasugi S. Correlation between Musashi-1 and c-hairy-1 expression and cell proliferation activity in the developing intestine and stomach of both chicken and mouse. Dev. Growth Differ. 2005;47(8):501–510. doi: 10.1111/j.1440-169X.2005.00825.x. [DOI] [PubMed] [Google Scholar]
- 92.Holmberg J, Genander M, Halford MM, et al. EphB receptors coordinate migration and proliferation in the intestinal stem cell niche. Cell. 2006;125(6):1151–1163. doi: 10.1016/j.cell.2006.04.030. [DOI] [PubMed] [Google Scholar]
- 93.Yuqi L, Chengtang W, Ying W, Shangtong L, Kangxiong L. The expression of Msi-1 and its significance in small intestinal mucosa severely damaged by high-dose 5-FU. Dig. Dis. Sci. 2008;53(9):2436–2442. doi: 10.1007/s10620-007-0155-0. [DOI] [PubMed] [Google Scholar]
- 94.May R, Sureban SM, Hoang N, et al. Doublecortin and CaM kinase-like-1 and leucine-rich-repeat-containing G-protein-coupled receptor mark quiescent and cycling intestinal stem cells, respectively. Stem Cells. 2009;27(10):2571–2579. doi: 10.1002/stem.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sangiorgi E, Capecchi MR. Bmi-1 is expressed in vivo in intestinal stem cells. Nat. Genet. 2008;40(7):915–920. doi: 10.1038/ng.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Barker N, Van Es JH, Kuipers J, et al. Identification of stem cells in small intestine and colon by marker gene Lgr-5. Nature. 2007;449(7165):1003–1007. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- 97.Haegebarth A, Clevers H. Wnt signaling, Lgr-5, and stem cells in the intestine and skin. Am. J. Pathol. 2009;174(3):715–721. doi: 10.2353/ajpath.2009.080758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98■■.Sato T, Vries RG, Snippert HJ, et al. Single Lgr-5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature. 2009;459(7244):262–265. doi: 10.1038/nature07935. [Isolation of single adult intestinal stem cells and expansion in vitro into intestinal epithelial structures demonstrated clonality of the stem cell compartment defined by the presence of surface Lgr-5 espression.] [DOI] [PubMed] [Google Scholar]
- 99.Sato T, Van Es JH, Snippert HJ, et al. Paneth cells constitute the niche for Lgr-5 stem cells in intestinal crypts. Nature. 2011;469(7330):415–418. doi: 10.1038/nature09637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Scoville DH, Sato T, He XC, Li L. Current view: intestinal stem cells and signaling. Gastroenterology. 2008;134(3):849–864. doi: 10.1053/j.gastro.2008.01.079. [DOI] [PubMed] [Google Scholar]
- 101.Van Es JH, Van Gijn ME, Riccio O, et al. Notch/γ-secretase inhibition turns proliferative cells in intestinal crypts and adenomas into goblet cells. Nature. 2005;435(7044):959–963. doi: 10.1038/nature03659. [DOI] [PubMed] [Google Scholar]
- 102.Fre S, Huyghe M, Mourikis P, Robine S, Louvard D, Artavanis-Tsakonas S. Notch signals control the fate of immature progenitor cells in the intestine. Nature. 2005;435(7044):964–968. doi: 10.1038/nature03589. [DOI] [PubMed] [Google Scholar]
- 103.Wells JM, Melton DA. Vertebrate endoderm development. Ann. Rev. Cell. Dev. Biol. 1999;15:393–410. doi: 10.1146/annurev.cellbio.15.1.393. [DOI] [PubMed] [Google Scholar]
- 104.Piotrowska K, Zernicka-Goetz M. Early patterning of the mouse embryo – contributions of sperm and egg. Development. 2002;129(24):5803–5813. doi: 10.1242/dev.00170. [DOI] [PubMed] [Google Scholar]
- 105.Tam PP, Kanai-Azuma M, Kanai Y. Early endoderm development in vertebrates: lineage differentiation and morphogenetic function. Curr. Opin. Genet. Dev. 2003;13(4):393–400. doi: 10.1016/s0959-437x(03)00085-6. [DOI] [PubMed] [Google Scholar]
- 106.Lewis SL, Tam PP. Definitive endoderm of the mouse embryo: formation, cell fates, and morphogenetic function. Dev. Dyn. 2006;235(9):2315–2329. doi: 10.1002/dvdy.20846. [DOI] [PubMed] [Google Scholar]
- 107.Hagos EG, Dougan ST. Time-dependent patterning of the mesoderm and endoderm by nodal signals in zebrafish. BMC Dev. Biol. 2007;7:22. doi: 10.1186/1471-213X-7-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Clements D, Friday RV, Woodland HR. Mode of action of VegT in mesoderm and endoderm formation. Development. 1999;126(21):4903–4911. doi: 10.1242/dev.126.21.4903. [DOI] [PubMed] [Google Scholar]
- 109.Spence JR, Lauf R, Shroyer NF. Vertebrate intestinal endoderm development. Dev. Dyn. 2011;240(3):501–520. doi: 10.1002/dvdy.22540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hardwick JC, Van Den Brink GR, Bleuming SA, et al. Bone morphogenetic protein 2 is expressed by, and acts upon, mature epithelial cells in the colon. Gastroenterology. 2004;126(1):111–121. doi: 10.1053/j.gastro.2003.10.067. [DOI] [PubMed] [Google Scholar]
- 111.Wells JM, Melton DA. Early mouse endoderm is patterned by soluble factors from adjacent germ layers. Development. 2000;127(8):1563–1572. doi: 10.1242/dev.127.8.1563. [DOI] [PubMed] [Google Scholar]
- 112.Dessimoz J, Opoka R, Kordich JJ, Grapin Botton A, Wells JM. FGF signaling is necessary for establishing gut tube domains along the anterior–posterior axis in vivo. Mech. Dev. 2006;123(1):42–55. doi: 10.1016/j.mod.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 113.Li Y, Litingtung Y, Ten Dijke P, Chiang C. Aberrant Bmp signaling and notochord delamination in the pathogenesis of esophageal atresia. Dev. Dyn. 2007;236(3):746–754. doi: 10.1002/dvdy.21075. [DOI] [PubMed] [Google Scholar]
- 114.Wells JM, Esni F, Boivin GP, et al. Wnt/ β-catenin signaling is required for development of the exocrine pancreas. BMC Dev. Biol. 2007;7:e4. doi: 10.1186/1471-213X-7-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zorn AM, Wells JM. Molecular basis of vertebrate endoderm development. Int. Rev. Cytol. 2007;259:49–111. doi: 10.1016/S0074-7696(06)59002-3. [DOI] [PubMed] [Google Scholar]
- 116.Danesh SM, Villasenor A, Chong D, Soukup C, Cleaver O. BMP and BMP receptor expression during murine organogenesis. Gene Expr. Patterns. 2009;9(5):255–265. doi: 10.1016/j.gep.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zorn AM, Wells JM. Vertebrate endoderm development and organ formation. Ann. Rev. Cell. Dev. Biol. 2009;25:221–251. doi: 10.1146/annurev.cellbio.042308.113344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Que J, Choi M, Ziel JW, Klingensmith J, Hogan BL. Morphogenesis of the trachea and esophagus: current players and new roles for noggin and Bmps. Differentiation. 2006;74(7):422–437. doi: 10.1111/j.1432-0436.2006.00096.x. [DOI] [PubMed] [Google Scholar]
- 119.Gao N, White P, Kaestner KH. Establishment of intestinal identity and epithelial–mesenchymal signaling by Cdx2. Dev. Cell. 2009;16(4):588–599. doi: 10.1016/j.devcel.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Grainger S, Savory JG, Lohnes D. Cdx2 regulates patterning of the intestinal epithelium. Dev. Biol. 2010;339(1):155–165. doi: 10.1016/j.ydbio.2009.12.025. [DOI] [PubMed] [Google Scholar]
- 121.Van Den Brink GR, Bleuming SA, Hardwick JC, et al. Indian hedgehog is an antagonist of Wnt signaling in colonic epithelial cell differentiation. Nat. Genet. 2004;36(3):277–282. doi: 10.1038/ng1304. [DOI] [PubMed] [Google Scholar]
- 122.Mandhan P, Quan QB, Beasley S, Sullivan M. Sonic hedgehog, BMP4, and Hox genes in the development of anorectal malformations in Ethylenethiourea-exposed fetal rats. J. Pediatr. Surg. 2006;41(12):2041–2045. doi: 10.1016/j.jpedsurg.2006.08.035. [DOI] [PubMed] [Google Scholar]
- 123.Mandhan P, Beasley S, Hale T, Ellmers L, Roake J, Sullivan M. Sonic hedgehog expression in the development of hindgut in ETU-exposed fetal rats. Pediatr. Surg. Int. 2006;22(1):31–36. doi: 10.1007/s00383-005-1575-6. [DOI] [PubMed] [Google Scholar]
- 124.Aubin J, Dery U, Lemieux M, Chailler P, Jeannotte L. Stomach regional specification requires Hoxa5-driven mesenchymal–epithelial signaling. Development. 2002;129(17):4075–4087. doi: 10.1242/dev.129.17.4075. [DOI] [PubMed] [Google Scholar]
- 125.Dan Z, Bo ZZ, Tao Z, et al. Hoxd-13 expression in the development of hindgut in ethylenethiourea-exposed fetal rats. J. Pediatr. Surg. 2010;45(4):755–761. doi: 10.1016/j.jpedsurg.2009.11.011. [DOI] [PubMed] [Google Scholar]
- 126.Kawazoe Y, Sekimoto T, Araki M, Takagi K, Araki K, Yamamura K. Region-specific gastrointestinal Hox code during murine embryonal gut development. Dev. Growth Differ. 2002;44(1):77–84. doi: 10.1046/j.1440-169x.2002.00623.x. [DOI] [PubMed] [Google Scholar]
- 127.Pitera JE, Smith VV, Thorogood P, Milla PJ. Coordinated expression of 3′ hox genes during murine embryonal gut development: an enteric Hox code. Gastroenterology. 1999;117(6):1339–1351. doi: 10.1016/s0016-5085(99)70284-2. [DOI] [PubMed] [Google Scholar]
- 128.Zacchetti G, Duboule D, Zakany J. Hox gene function in vertebrate gut morphogenesis: the case of the caecum. Development. 2007;134(22):3967–3973. doi: 10.1242/dev.010991. [DOI] [PubMed] [Google Scholar]
- 129.Martin GR. Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc. Natl Acad. Sci. USA. 1981;78(12):7634–7638. doi: 10.1073/pnas.78.12.7634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Gossler A, Doetschman T, Korn R, Serfling E, Kemler R. Transgenesis by means of blastocyst-derived embryonic stem cell lines. Proc. Natl Acad. Sci. USA. 1986;83(23):9065–9069. doi: 10.1073/pnas.83.23.9065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Suda Y, Suzuki M, Ikawa Y, Aizawa S. Mouse embryonic stem cells exhibit indefinite proliferative potential. J. Cell. Physiol. 1987;133(1):197–201. doi: 10.1002/jcp.1041330127. [DOI] [PubMed] [Google Scholar]
- 132.Yamada T, Yoshikawa M, Takaki M, et al. In vitro functional gut-like organ formation from mouse embryonic stem cells. Stem Cells. 2002;20(1):41–49. doi: 10.1634/stemcells.20-1-41. [DOI] [PubMed] [Google Scholar]
- 133.Kuwahara M, Ogaeri T, Matsuura R, Kogo H, Fujimoto T, Torihashi S. In vitro organogenesis of gut-like structures from mouse embryonic stem cells. Neurogastroenterol. Motil. 2004;16(Suppl. 1):14–18. doi: 10.1111/j.1743-3150.2004.00468.x. [DOI] [PubMed] [Google Scholar]
- 134.Matsuura R, Kogo H, Ogaeri T, et al. Crucial transcription factors in endoderm and embryonic gut development are expressed in gut-like structures from mouse ES cells. Stem Cells. 2006;24(3):624–630. doi: 10.1634/stemcells.2005-0344. [DOI] [PubMed] [Google Scholar]
- 135.Ueda T, Yamada T, Hokuto D, et al. Generation of functional gut-like organ from mouse induced pluripotent stem cells. Biochem. Biophys. Res. Comm. 2010;391(1):38–42. doi: 10.1016/j.bbrc.2009.10.157. [DOI] [PubMed] [Google Scholar]
- 136.Torihashi S, Kuwahara M, Ogaeri T, Zhu P, Kurahashi M, Fujimoto T. Gut-like structures from mouse embryonic stem cells as an in vitro model for gut organogenesis preserving developmental potential after transplantation. Stem Cells. 2006;24(12):2618–2626. doi: 10.1634/stemcells.2006-0148. [DOI] [PubMed] [Google Scholar]
- 137.Konuma N, Wakabayashi K, Matsumoto T, et al. Mouse embryonic stem cells give rise to gut-like morphogenesis, including intestinal stem cells, in the embryoid body model. Stem Cells Dev. 2009;18(1):113–126. doi: 10.1089/scd.2008.0045. [DOI] [PubMed] [Google Scholar]
- 138.Kudo K, Abe Y, Hu DL, Kijima H, Nakane A. Colonization and differentiation of transplanted embryonic stem cells in the irradiated intestine of mice. Tohoku J. Exp. Med. 2007;212(2):143–150. doi: 10.1620/tjem.212.143. [DOI] [PubMed] [Google Scholar]
- 139■.Cao L, Gibson JD, Miyamoto S, et al. Intestinal lineage commitment of embryonic stem cells. Differentiation. 2011;81(1):1–10. doi: 10.1016/j.diff.2010.09.182. [Use of exogenous growth factors to efficiently direct the differentiation of intestinal tissue from mouse pluripotent cells.] [DOI] [PubMed] [Google Scholar]
- 140.Thomson JA, Itskovitz-Eldor J, Shapiro SS, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282(5391):1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 141.Voelkel C, Galla M, Maetzig T, et al. Protein transduction from retroviral Gag precursors. Proc. Natl Acad. Sci. USA. 2010;107(17):7805–7810. doi: 10.1073/pnas.0914517107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Anokye-Danso F, Trivedi CM, Juhr D, et al. Highly efficient miRNA-mediated reprogramming of mouse and human somatic cells to pluripotency. Cell Stem Cell. 2011;8(4):376–388. doi: 10.1016/j.stem.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Kim K, Doi A, Wen B, et al. Epigenetic memory in induced pluripotent stem cells. Nature. 2010;467(7313):285–290. doi: 10.1038/nature09342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Zhao T, Zhang ZN, Rong Z, Xu Y. Immunogenicity of induced pluripotent stem cells. Nature. 2011;474(7350):212–215. doi: 10.1038/nature10135. [DOI] [PubMed] [Google Scholar]
- 145■■.Spence JR, Mayhew CN, Rankin SA, et al. Directed differentiation of human pluripotent stem cells into intestinal tissue in vitro. Nature. 2011;470(7332):105–109. doi: 10.1038/nature09691. [First study to demonstrate that intestinal tissue can be derived in vitro by robust, efficient differentiation of human pluripotent cells by the manipulation of exogenous growth factors.] [DOI] [PMC free article] [PubMed] [Google Scholar]