Abstract
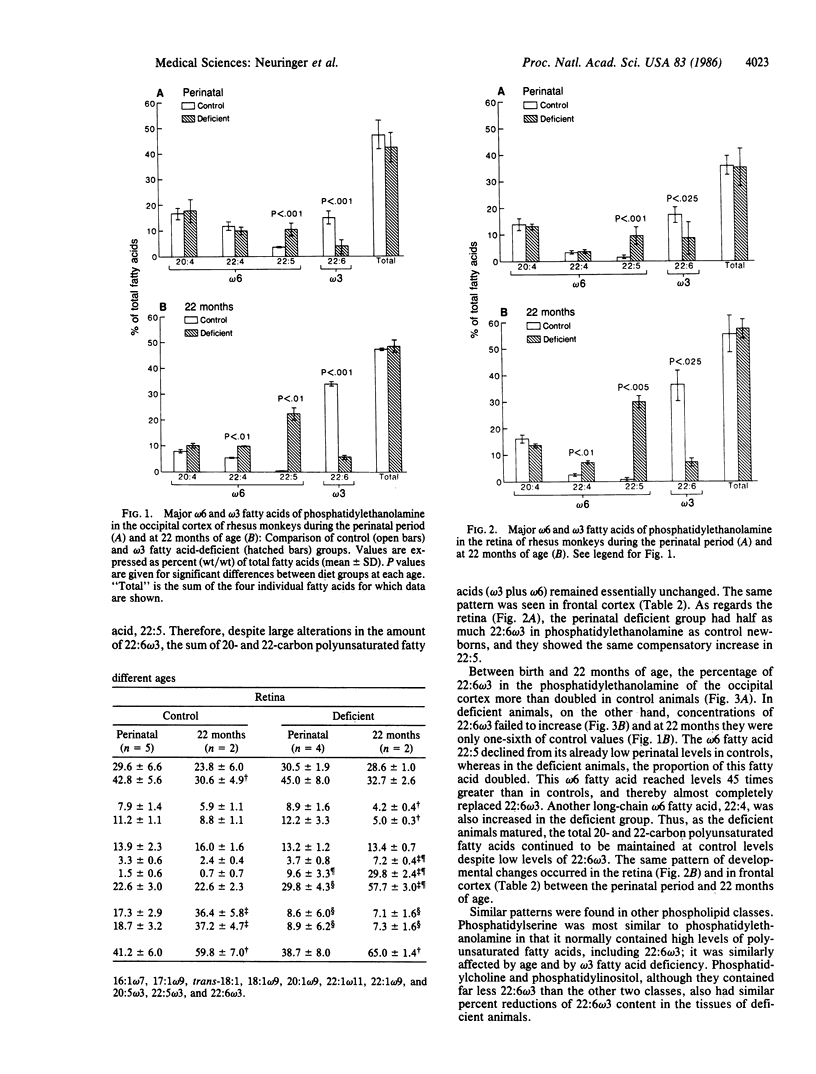
Docosahexaenoic acid [22:6 omega 3; 22:6-(4,7,10,13,16,19)] is the major polyunsaturated fatty acid in the photoreceptor membranes of the retina and in cerebral gray matter. It must be obtained either from the diet or by synthesis from other omega 3 fatty acids, chiefly alpha-linolenic acid (18:3 omega 3). We tested the effect of dietary omega 3 fatty acid deprivation during gestation and postnatal development upon the fatty acid composition of the retina and cerebral cortex and upon visual function. Rhesus monkeys (Macaca mulatta) were fed semipurified diets very low in 18:3 omega 3 throughout pregnancy, and their infants received a similar diet from birth. A control group of females and their infants received a semipurified diet supplying ample 18:3 omega 3. In near-term fetuses and newborn infants of the deficient group, the 22:6 omega 3 content of phosphatidylethanolamine was one-half of control values in the retina and one-fourth in cerebral cortex. By 22 months of age, the content of 22:6 omega 3 in these tissues approximately doubled in control monkeys, but it failed to increase in the deficient group. Low levels of 22:6 omega 3 in the deficient animals' tissues were accompanied by a compensatory increase in longer-chain omega 6 fatty acids, particularly 22:5 omega 6. Functionally, the deficient animals had subnormal visual acuity at 4-12 weeks of age and prolonged recovery time of the dark-adapted electroretinogram after a saturating flash. Abnormally low levels of 22:6 omega 3 may produce alterations in the biophysical properties of photoreceptor and neural membranes that may underlie these functional impairments. The results of this study suggest that dietary omega 3 fatty acids are retina and brain.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Gilfillan A. M., Chu A. J., Smart D. A., Rooney S. A. Single plate separation of lung phospholipids including disaturated phosphatidylcholine. J Lipid Res. 1983 Dec;24(12):1651–1656. [PubMed] [Google Scholar]
- Holman R. T., Johnson S. B., Hatch T. F. A case of human linolenic acid deficiency involving neurological abnormalities. Am J Clin Nutr. 1982 Mar;35(3):617–623. doi: 10.1093/ajcn/35.3.617. [DOI] [PubMed] [Google Scholar]
- Lamptey M. S., Walker B. L. A possible essential role for dietary linolenic acid in the development of the young rat. J Nutr. 1976 Jan;106(1):86–93. doi: 10.1093/jn/106.1.86. [DOI] [PubMed] [Google Scholar]
- Neuringer M., Connor W. E., Van Petten C., Barstad L. Dietary omega-3 fatty acid deficiency and visual loss in infant rhesus monkeys. J Clin Invest. 1984 Jan;73(1):272–276. doi: 10.1172/JCI111202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portman O. W., Alexander M., Illingworth D. R. Changes in brain and sciatic nerve composition with development of the rhesus monkey (Macaca mulatta). Brain Res. 1972 Aug 11;43(1):197–213. doi: 10.1016/0006-8993(72)90284-3. [DOI] [PubMed] [Google Scholar]
- Rapp J. H., Connor W. E., Lin D. S., Inahara T., Porter J. M. Lipids of human atherosclerotic plaques and xanthomas: clues to the mechanism of plaque progression. J Lipid Res. 1983 Oct;24(10):1329–1335. [PubMed] [Google Scholar]
- Sinnhuber R. O., Castell J. D., Lee D. J. Essential fatty acid requirement of the rainbow trout, Salmo gairdneri. Fed Proc. 1972 Sep-Oct;31(5):1436–1441. [PubMed] [Google Scholar]
- Svennerholm L. Distribution and fatty acid composition of phosphoglycerides in normal human brain. J Lipid Res. 1968 Sep;9(5):570–579. [PubMed] [Google Scholar]
- Tinoco J., Williams M. A., Hincenbergs I., Lyman R. L. Evidence for nonessentiality of linolenic acid in the diet of the rat. J Nutr. 1971 Jul;101(7):937–945. doi: 10.1093/jn/101.7.937. [DOI] [PubMed] [Google Scholar]
- Vitiello F., Zanetta J. P. Thin-layer chromatography of phospholipids. J Chromatogr. 1978 Dec 11;166(2):637–640. doi: 10.1016/s0021-9673(00)95654-1. [DOI] [PubMed] [Google Scholar]
- Wheeler T. G., Benolken R. M., Anderson R. E. Visual membranes: specificity of fatty acid precursors for the electrical response to illumination. Science. 1975 Jun 27;188(4195):1312–1314. doi: 10.1126/science.1145197. [DOI] [PubMed] [Google Scholar]





