Abstract
Tumor Necrosis Factor-Related Apoptosis Inducing Ligand (TRAIL/TNFSF10) has been reported to specifically induce malignant cell death being relatively nontoxic to normal cells. Since its identification 15 years ago, the antitumor activity and therapeutic value of TRAIL have been extensively studied. Five receptors quickly emerged, two of them being able to induce programmed cell death in tumor cells. This review takes a comprehensive look at this ligand and its receptors, and its potential role in primary bone tumors (osteosarcoma and Ewing's sarcoma) therapy. The main limit of clinical use of TRAIL being the innate or acquired resistance mechanisms, different possibilities to sensitize resistant cells are discussed in this review, together with the impact of bone microenvironment in the regulation of TRAIL activity.
Keywords: Tumor Necrosis Factor-Related Apoptosis Inducing Ligand, TRAIL/TNFSF10, receptor, signalingbone tumor, microenvironment
TRAIL: structure and functions
Structure
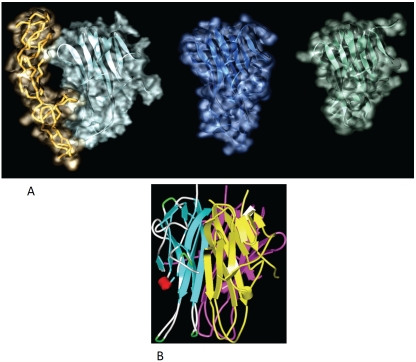
Tumor Necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL/Apo2L/TNFSF10) is a member of the TNF superfamily identified independently by 2 teams which both reported a sequence homology with the extracellular domain of CD95 ligand (28%) (Fas-L) and of TNF (23%) [1, 2]. Located on chromosome 3, the gene that encodes the 20 kDa TRAIL protein is composed of five exons and four introns. Unlike other members of the TNF superfamily whose expression is often inducible and detected transiently in activated cells, TRAIL mRNA was identified constitutively in most human tissues with an expression significantly greater in the spleen and prostate [2]. TRAIL, like other members of the TNF superfamily is expressed as a type II transmembrane protein composed of 281 amino acids (aa) in the human form. Cleavage of its C-terminus part corresponding to the extracellular domain, releases a soluble form of the protein [3]. The crystal structure of the monomeric form of TRAIL also presents a strong homology with the TNF and CD40 Ligand structures (Figure 1A) [4]. TRAIL binds to its receptors as a homotrimer form, this trimeric form being biologically much more active than the monomeric one (Figure 1B) [2].
Figure 1.
structure of TRAIL. A: Surface representation of TRAIL (cyan) bounded to its cognate receptor DR5 (tube representation, light orange), TNF-alpha (blue), and CD40L (green). TNF-alpha and CD40L were superposed using the conserved anti-parallel beta-sheets scaffold to present the same orientation as DR5 (this scaffold is represented in ribbon representation). The variations observed in the surface rendered highlight the differences in loops shapes and size between ligands. Crystallographic coordinates for TRAIL, TNF-alpha and CD40L were taken from 1DU3, 1TNF and 1ALY respectively [136-138]. The superposition was made using Discovery Studio 2.5.5 (Accelrys Inc., San Diego, USA) and the final rendering using POV-Ray (http://www.povray.org) and The GIMP (http://www.gimp.org). B: Ribbon structure of the TRAIL homotrimer (blue, yellow, pink).
Functions
The ability of TRAIL to induce apoptosis of transformed cells has been widely demonstrated but its physiological role is not well defined. Mice deficient for the receptor or the murine form of TRAIL (mTRAIL) develop normally, suggesting a lack of role of this signaling pathway in embryonic development [5, 6]. TRAIL is specifically expressed on the surface of natural killer cells (NK), cytotoxic T lymphocytes, macrophages and dendritic cells. The transcritpion of its mRNA can be induced by interferon type I [7]. The selective expression of TRAIL on the surface of effector cells of the immune system suggests a role of TRAIL in the formation of the immune repertoire and in the regulation of the immune response. Studies in humans and in animal models suggest that TRAIL plays a role in regulating the immune response in order to avoid excessive reactions against infectious agents such as cytomegalovirus or Listeria [5, 8] and self-antigens involved in autoimmune diseases such as arthritis, diabetes and multiple sclerosis [9-11]. Other studies suggest that TRAIL would have a role as tumor suppressor involved in immune surveillance against the development of primary tumors or metastases. Thus, in mice, inhibiting the activity of TRAIL by gene knockout, by blocking antibodies or by inhibiting the mouse form of one of its death receptors has confirmed the role of tumor suppression in spontaneous or induced models of metastases from cutaneous lymphoma or carcinoma [12-14]. Moreover, a recent study of 368 human breast tumor samples showed a correlation between a subregulation of TRAIL in these tumors and the presence of brain metastases [15]. If the role of TRAIL in inhibiting metastasis development has been well demonstrated, its role in the suppression of primary tumors is still debated. Thus, TRAIL-deficient mice do not develop more spontaneous tumors than wild type mice and the development of skin tumors in response to chemical carcinogens is not augmented [14].
TRAIL receptors
TRAIL is able to interact with five receptors (Figure 2): 2 death receptors and 3 decoy receptors. It is able to bind to DR4/TRAIL-R1/TNFRSF10A and DR5/TRAIL-R2/KILLER/TNFRSF10B death receptors (Figure 1A) which can transduce a death signal, and to decoy receptors DcR1/TRAIL-R3/TNFRSF10C and DcR2/TRAIL -R4/TNFRSF10D which cannot [16-18]. It has been shown that TRAIL is able of binding to its death receptor with a higher affinity than to the decoys DcR1 and DcR2 [19]. In addition, TRAIL is able to bind with low affinity to a third soluble decoy receptor, osteoprotegerin (OPG/TNFRSF11B) [20]. In mice, however, mTRAIL can bind to only one death receptor mDR5 (mTRAILR2/mKILLER) that shares the same degree of homology with DR4 and DR5 [21].
Figure 2.
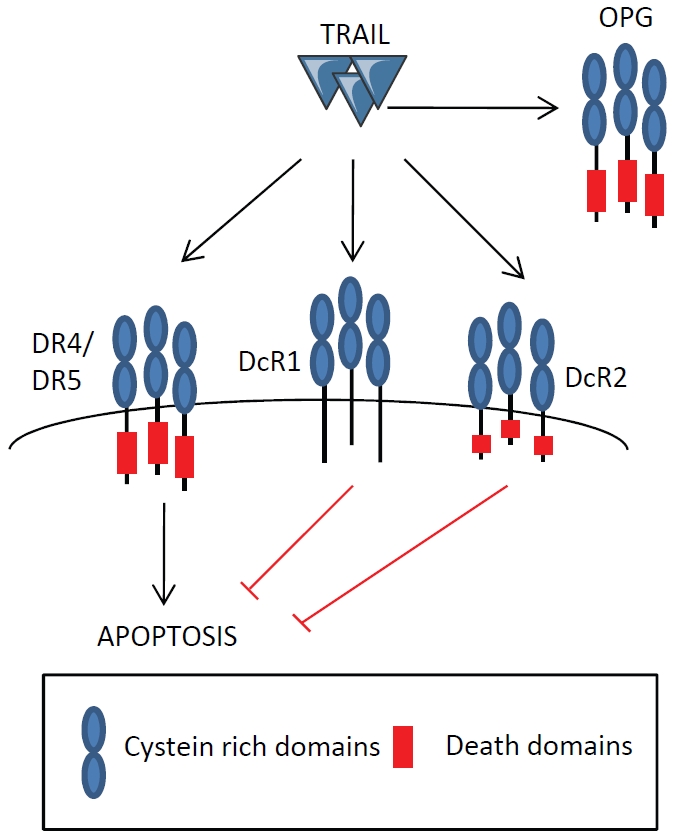
TRAIL is able to interact with five receptors: 2 transmembrane death receptors which can transduce a death signal (DR4 and DR5) and 3 decoy receptors DcR1, DcR2 and the soluble osteoprotegerin (OPG) which cannot.
DR4 and DR5 death receptors
DR4 and DR5 receptors are type I transmembrane proteins containing in their cytoplasmic domain a death domain (DD) capable of binding to other death domains. It was long believed that death receptors are present at the membrane in their monomeric form and trimerized only when interacting with their trimeric ligand. Now, several data indicate that these receptors are oligomeric already before the fixation of their ligand [22, 23]. The DR4 and DR5 expression may be regulated by p53 [24]. Indeed, the DR5 gene promoter has a responsive element to p53 and is overexpressed in response to γ-irradiation through p53 [25, 26]. Post-translational regulation of DR4 and DR5 death receptors such as glycosylation and palmitoylation were also shown to be important regulators of TRAIL induced signaling. Thus, there is a correlation between the expression of the enzyme initiating the GALNT14 glycosylation and sensitivity to TRAIL in pancreatic carcinoma cells, lung cancer and malignant melanoma [27]. However, these O-glycosylations do not appear to alter the expression of DR4 and DR5 to the membrane or their affinity for TRAIL. They seem, however, to affect the receptor oligomerization in response to TRAIL binding, this oligomerization being necessary for the formation of the Death-Inducing Signaling Complex (DISC) and further caspase 8 activation (Figure 3). Palmitoylation is necessary to localize DR4 in lipid rafts allowing DR4 to trimerize in the absence of ligand, but the effect of its inhibition on the tumor cell sensitivity to TRAIL has not yet been demonstrated [28]. The ligand binding to its receptors often induces endocytosis of the ligand-receptor complex and the role of this internalization has recently been studied in the case of TRAIL signaling pathway. After binding TRAIL, DR4 and DR5 are both internalized by a mechanism dependent on dynamin [29, 30]. The biological role of this endocytosis is currently poorly understood and its inhibition does not appear to influence the TRAIL induced apoptosis [29, 30].
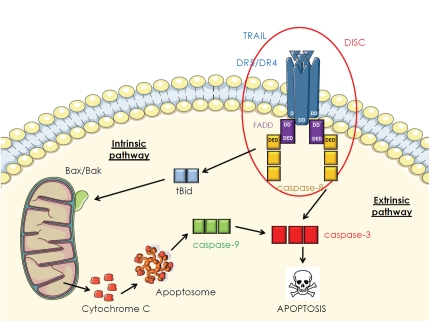
Figure 3.
TRAIL-induced apoptosis signaling pathway via the death receptors DR4 and DR5. The incoming trimeric ligand TRAIL recruits three receptors into a complex. The induced juxtaposition of the intracellular domains triggers recruitment of the intracellular signaling components forming the Death-Inducing Signaling Complex (DISC) and leading to the caspase cascade and cell death. DD: Death Domain; DED: Death Effector Domains; FADD: Fas-Associated protein with Death Domain; c-FLIP: cellular FLICE-inhibitory protein; BAK: B cell-CELL/Lymphoma 2 (Bcl-2) homologous Antagonist Killer; BAX: Bcl-2-Associated X; Bid: BH3-interacting domain death agonist.
DcR1 and DcR2 decoy receptors
The expression of these receptors has been shown to prevent the induction of apoptosis in different human tumor cell lines derived from lymphoma, renal, breast and prostate carcinoma [31-33] but their involvement in TRAIL resistance remains controversial [34]. About their modes of action, DcR1 is a receptor with a glycophosphatidylinositol (GPI) anchor but with no intracellular domains able to sequester TRAIL in lipid rafts. DcR2 has a truncated death domain and interacts with DR5 to form a heterotrimeric complex in the DISC to inhibit cas-pase-8 activation [35], this interaction might even be upstream of the interaction with TRAIL [19].
Osteoprotegerin (OPG)
OPG was first described as a secreted member of the TNF receptor family (TNFRSF11B) that regulates bone resorption [36]. Unlike other members of the TNF receptor family OPG does not possess a transmembrane domain. Then a first report has provided evidence that OPG is a receptor for TRAIL, binding the ligand with an affinity of 3.0 nM and inhibiting TRAIL-mediated apoptosis of sensitive Jurkat cells [20].
Besides these 5 cognate receptors, transcriptional modifications have been identified such as alternative splicing in the TRAIL-R2/DR5 and TRAIL-R4 transcripts [37, 38]. First, two iso-forms of DR5/TRAIL-R2, i.e. TRICK2A and TRICK2B which differ in the presence of a 23 aa extension between the transmembrane region and the cysteine-rich domain has been described by Screaton and coll [37]. Then Krieg and coll. identified a novel alternative splice variant of DcR2/TRAIL-R4/TRUNDD, designated TRAIL-R4-β [38]. The lack of exon 3 of the DcR2/TRAIL-R4 gene results in truncation of the first complete cysteine rich domain 1 which seems to be essential for TRAIL-binding.
TRAIL proapoptotic signaling pathways
The binding of TRAIL to its death receptors DR4 and DR5 induces a conformational change of their death domains which, along with their oligomerization, is the functional activation of the receptor. This activation will induce the recruitment of Fas-Associated protein with Death Domain (FADD) on the death receptors by interaction of their respective death domains (Figure 3). Acting as an adapter protein, FADD will allow the recruitment of procaspase-8/-10 by interaction of their respective Death Effector Domains (DED) [39]. The multiprotein complex resulting from these associations is called DISC and induces self-activation of initiator caspases [40]. In response to their activation, caspases-8 and 10 will induce the proteolytic cleavage of the effector caspase-3 which, itself, will cleave other caspases and other regulators and structural proteins [41]. This will result in a sprouting of the plasma membrane, nucleus condensation and cleavage of the chromatin leading to cell apoptosis known as the extrinsic pathway (death receptor pathway) [42]. A bridge between extrinsic and intrinsic (mitochondrial) apoptosis pathways exists through the protein-BH3 interacting domain death agonist (Bid), a member of the B cell-CELL/Lymphoma 2 (Bcl-2) family, a substrate for caspase 8. Depending on the cell type, the cleavage of Bid (p22) into tBid (p15) will be linked or not to the primary mechanism of apoptosis induction in response to TRAIL [43]. The presence or absence of X-linked inhibitor of apoptosis protein (XIAP) and the negative regulation of caspase 3 activation might also be involved in this categorization of cells. The cleaved form of the Bid protein tBid, initiates the intrinsic pathway of apoptosis by binding to Bcl-2-Associated X (BAX) and Bcl-2 homologous Antagonist Killer (BAK). This will result in their oligomerization and translocation to the outer membrane of mitochondria that initiates the permabilization of this membrane [44]. Pro-apoptotic proteins such as cytochrome c and Second mitochondria-derived activator of caspase/Direct Inhibitor of Apoptosis-Binding protein with Low pI (Smac/DIABLO), normally located in the membrane inter-space of the mitochondria membrane will be released in the cytosol [45]. Cytochrome c associates with ATP and Apoptotic Peptidase Activating Factor-I (APAF-1) to form a structure called the apopto-some, which will induce caspase-9 activation. In turn, caspase-9 will activate caspase-3, -6 and -7 leading to cell apoptosis called the intrinsic pathway [46].
TRAIL induced non-apoptotic signaling pathways
Many studies have shown that TRAIL is not only involved in the induction of apoptosis through its death receptors but is also able to induce survival signals and cell proliferation. The nuclear factor kappa B (NF-kB) would be activated via DR4, DR5 and DcR2 receptors by the formation of a secondary signaling complex generated after the DISC formation [47, 48] (Figure 4). This complex involves FADD, activated caspase-8, the Receptor Interacting Protein 1 (RIP1), TNF-Receptor Associated Protein (TRAF-2), TNF Receptor-Associated Death Domain (TRADD) and Inhibitor of kappa B kinase (IKKγ/NEMO) [49] (Figure 4). Thus, after recruitment, RIP will interact with IKKγ leading to the recruitment of IKKα and β, which will phosphorylate the Inhibitor of kappa B (IκB) inducing its proteolytic degradation and the further activation of NF-kB. This activation of NF-kB is able to induce cell survival through overexpression of various anti-apoptotic proteins such as c-FLIP, BCL-extra large (BCL-Xl), Myeloid cell leukemia sequence 1 (MCL1) and cIAPS [50, 51]. In addition, this complex is able to induce survival signals involving phosphoinositide 3-kinase (PI3K)-Akt and mitogen-activated protein kinase (MAPK: Jun-terminal kinase (JNK), extracellular signal-regulated kinase 2 (Erk2) and p38) [52, 53]. It seems that in resistant cells, the main signal induced by TRAIL is the one involving NF-kB. A study of a resistant form of cholangiocarcinoma to apoptosis has shown that the activation of NF -kB may promote invasion and metastasis [54]. Similarly, it has been shown in a preclinical model of adenocarcinoma resistant to apoptosis induced in Severe Combined ImmunoDeficiency (SCID) mice that a treatment with TRAIL increases the formation of metastases [50].
Figure 4.
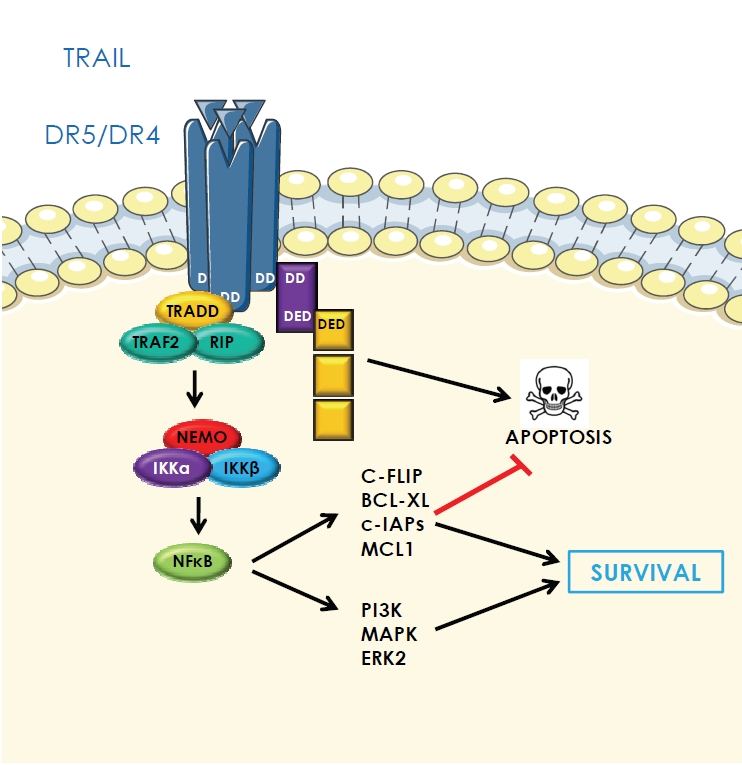
TRAIL-induced non apoptotic signaling pathways. The trimeric TRAIL can also induce recruitment of intracel-lular components leading to activation of NF-kB. RIP1: Receptor Interacting Protein 1, TRAF2: TNF-Receptor Associated Protein, TRADD: TNF-Receptor Associated Death Domain, IKKγ/ NEMO: Inhibitor of ?B Kinase, BCL-Xl: BCL-extra large, MCL1: Myeloid cell leukemia sequence 1, cIAPs: cellular inhibitors of apoptosis, PI3K: Phos-phoInositide 3-Kinase-Akt, MAPK: mito-gen-activated protein kinase, Erk2: extracellular signal-regulated kinase 2.
Using TRAIL in cancer therapy
Preclinical studies
Over the past 10 years, TRAIL has become the most promising members of the TNF super-family to be used in cancer therapy. This interest arises from the ability of TRAIL to induce apoptosis specifically in cell lines derived from solid and hematological tumors in combination with various chemotherapy agents or with radiation in vitro but also in vivo without significant effects on normal cells [1, 2, 55-61]. The results of the specific antitumor activity of TRAIL have suggested that it might represent a better candidate for clinical use than CD95L or TNF, which have deleterious side effects in vivo. However, the use of TRAIL as a therapeutic agent in clinical trials has been delayed due to in vitro studies showing apoptosis induction in hepatocyte cultures treated with a recombinant form of TRAIL (rhTRAIL) [62-64]. However, all the recombinant forms of TRAIL used in these studies had an exogenous polyhistidine tag or flag and it was subsequently shown that these tagged forms have an aberrant tertiary structure inducing an over-aggregation of receptors, responsible for side effects observed in hepatocytes [63]. It has been shown since that a non-tagged recombinant form of TRAIL specifically induces apoptosis of tumor cells without side effects on hepatocytes [65]. Agonist antibodies to TRAIL death receptors have also been tested in several experimental models. For example, the ability of 2 human monoclonal antibodies (mAbs) directed against DR4 and DR5 have been evaluated to kill human myeloma cells by the cleavage of Myeloid cell leukemia (Mcl)-1L, a major molecule for myeloma survival [66].
Clinical trials (Table 1)
Table 1.
Clinical trials using recombinant human TRAIL (rhTRAIL) or agonist antibodies (Mapatumumab, Lexatumumab and Apomab) of death receptors DR4 or DR5. CRC: colorectal cancer; O: ovarian; L: lung; M: myeloma; NHL: non-Hodgkin lymphoma; NSCLC: nonsmall cell lung cancer.
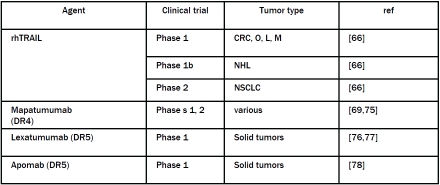 |
Clinical trials were conducted to evaluate the antitumor potential of TRAIL for solid and hematologic tumors. A phase 1 clinical trial was conducted on 51 adult patients including 36 with colorectal, ovarian, lung and melanoma cancer to study the pharmacokinetics and safety of rhTRAIL [67]. During this test, TRAIL showed no toxicity and no antibody directed against rhTRAIL was detected. Preclinical studies having shown a potentiation of TRAIL effect in combination with chemotherapeutic agents [68, 69], a phase 1b clinical trial was conducted with a combination of rituximab (anti-CD20) with rhTRAIL on patients with non Hodgkin's low grade lymphoma [67]. Of 7 patients, 2 had a complete response, 1 a partial response and 2 a stabilization of the disease. This study showed that a combination of rituximab with rhTRAIL had no side effects and showed a positive activity in these patients. Another clinical trial was conducted in patients with advanced lung cancer with a combination of rhTRAIL and paclitaxel, carboplatin and bevacizumab (anti-vascular endothelial growth factor A). Results of this trial confirmed the safety of the combination of rhTRAIL with chemotherapy agents as well as a promising anti-tumor efficacy. The results of this clinical trial show that of 18 patients, 10 experienced a confirmed response (9 partial and 1 complete) with a response rate of 56%. Given these promising results, a phase 2 clinical trial is currently underway on the same pathology.
Because rhTRAIL hepatotoxicity, monoclonal antibodies agonists of its death receptors have been developed and used in clinical trials (Table 1). Several phase 1 and 2 clinical trials are being or have been conducted with the antibody agonist of DR4, the mapatumumab on advanced solid tumors or non-Hodgkin lymphoma (recurrent or resistant) with or without chemotherapy agents such as paclitaxel plus carboplatin or gemcitabine plus cisplatin [70-76]. The mapatumumab is well tolerated showing a half-life of about 18 days and patients have shown clinical benefits with partial responses or stable disease. However, elevated liver markers were measured in some patients, probably due to the administration of mapatumumab.
Clinical phase 1 trials were also conducted with DR5 agonist antibody, the lexatumumab on patients with advanced solid tumors in combination or not with a cocktail of chemotherapeutic agents consisting of gemcitabime, premetrexeb, doxorubicin and FOLFIRI (leuvovorin, fluorouracil and irinotecan) [77, 78]. This anti-body is well tolerated and has a half-life of approximately 16 days. Again some patients had stable disease and partial responses. The apomab [79], another DR5 agonist antibody is currently tested in a Phase 1 clinical trial in advanced solid tumors resistant to treatment. Half -life was found to be 15-20 days and it is also well tolerated. No objective response was observed but 2 patients appeared to have clinical benefits.
Primary malignant bone tumors
Osteosarcoma is the most frequent malignant tumor of the bone in the pediatric age group, with an incidence of 8.7 per million in children and adolescents under the age of 20 years [80]. Its incidence peaks during the second decade of life coinciding with the adolescent growth spurt. It is more commonly seen in the metaphyseal region of long bones: the disteal femur, proximal tibia and proximal humerus being the most common sites and corresponding to areas of active growth in the body (Figure 5A). Overall survival rates in nonmetastatic osteosarcoma were a dismal 10% prior to the 1970s when surgery was the only treatment option. Since the 1980s, the use of multidrug chemotherapy and refined surgical techniques has dramatically increased to 65% the overall survival rates in nonmetastatic osteosarcoma [81]. The outlook for metastatic osteosarcoma continues to be grim with reported survival rates of 20% [82].
Figure 5.
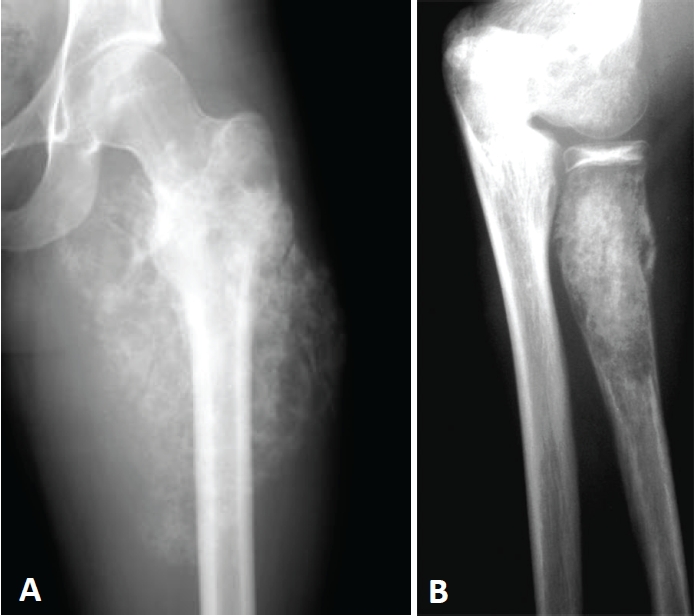
Radiographies of osteosarcoma (A) and Ewing's sarcoma (B). Ill defined osteolytic lesion involving metaphysis (osteosarcoma) or diaphysis (Ewing's sarcoma) of long bone is the most common feature. The underlying cortex is commonly partially destroyed, and periosteal new bone is commonly present at the periphery of the tumor. Characteristic permeative bone destruction is often associated with “onion-skin” or “sunburst” periosteal reaction. In Ewing's sarcoma, a large ill-defined soft tissue mass is frequently seen. In addition, expansile bone destruction with soap-bubble appearance might be seen.
Ewing's sarcomas are the second most frequent malignant bone tumors arising predominantly in the bones of children and young adults, the median age at diagnosis being 14 years with most patients presenting between the ages of 5 and 20 years [83]. Tumors may arise in bone or soft tissue, and symptoms generally include localized pain and a visible or palpable mass (Figure 5B). The femur is the most common long bone involved, and bones of the pelvis are the next most commonly involved site. Extraosseous Ewing's sarcomas comprise 15% of cases and may arise anywhere else in the body, including skin and visceral organs. Patients with Ewing's sarcoma who present with localized disease have a better prognosis than those with metastatic disease. The most common sites of metastasis are the lungs, bone and bone marrow. The Ewing's Sarcoma family of tumors (ESFT) comprises morphologically heterogeneous tumors that are characterized by nonrandom chromosomal translocations involving the EWS gene and one of the several members of the ETS family of transcription factor genes [84]. The mainstay of therapy for Ewing's sarcoma includes a combination of systemic chemotherapy and local control with surgery and/or radiation therapy [85].
Chondrosarcomas are the second most common primary bone sarcoma in adults after osteosarcoma. Conventional chondrosarcomas are characterized by the synthesis of pure hyaline cartilage by malignant cells [86]. Unlike osteosarcoma, chondrosarcoma is extremely unusual in the first two decades of life and tend to affect axial skeleton.
Altogether, the primary bone cancers, Ewing's sarcoma, osteosarcoma and chondrosarcoma account for 4/5 primary bone sarcomas [87]. These malignancies require not only adequate local control measures (i.e. surgery or radiotherapy) but also systemic therapy to reduce the high probability of lung and/or bone metastases (Figure 6). The improvement in survival observed in the mid 1980's has been attributed to the use of intensive multidrug chemotherapy given in combination with advanced surgery. However, since then no substantial further improvement of survival is observed [88, 89]. Despite survival about 65-75% in non metastatic Ewing's Sarcoma and Osteosarcoma, there remains much room for improvement in therapy. This would include regimens with fewer short-and long-term side effects and better results for difficult locations and patients with recurrent disease.
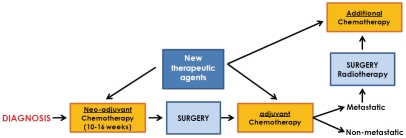
Figure 6.
Current osteosarcoma and Ewing's sarcoma local and systemic treatment. Approximately 10-16 weeks of preadjuvant therapy is given to patients before local control measures (surgery and/or radiation for Ewing's sarcoma) in an effort to control micrometastases. In osteosarcoma, the choice of adjuvant chemotherapy is influenced by the chemotherapy response. At the end of the standard treatment therapy, high-risk subsets with lung metastases get local control.
Because Ewing's sarcoma and osteosarcoma tend to occur during periods of rapid bone growth, i.e. adolescents and young adults, and because of pleiotropic effects of insulin-like Growth Factor (IGF)-1R signaling on cancer cell proliferation and survival pathways, these targeted agents have been of great interest in Ewing's sarcoma and osteosarcoma [90]. The status of other investigational agents for OS includes liposomal muramyltripeptide phos-phatidylethanolamine (L-MTP-PE), aerosol therapies including granulocyte-macrophage colony stimulating factor (GM-CSF), cisplatin and gemcitabine, and bone-specific approaches. Indeed, recent favorable experience in a benign bone tumor (giant cell tumor of bone) with the anti-RANKL (receptor activator of NF-kB Ligand) antibody denosumab® shows that agents that modify bone physiology may provide potential benefit for patients with bone tumors [91].
Therefore, considering the lack of improvement in overall survival rates for both Ewing's sarcoma and osteosarcoma since three decades and the potential therapeutic impact of TRAIL in several cancers, the use of this agent or the agonist antibodies to its death receptors may provide promising therapeutic value in such bone tumors.
TRAIL and bone tumors
Many experimental studies of the application of TRAIL to osteosarcoma cells have been reported [92, 93]. However, treatment of osteosarcoma cells with TRAIL did not lead to satisfactory results, since sensitivity of osteosarcoma cells to TRAIL-induced apoptosis is lower than that of other types of tumor cells. Indeed, cell sensitivity to TRAIL is different in various cells and the lack of sensitivity in osteosarcoma cells remain unclear [94]. Therefore, additional agents to sensitize osteosarcoma cells to TRAIL-induced apoptosis have been proposed but the problem of side-effects still remains [95, 96].
In contrast, TRAIL can induce caspase-dependent apoptosis in the majority of Ewing's sarcoma cell lines studied thus showing the importance of this therapeutic approach [97-99]. Ewing's sarcoma cell lines have been identified as highly sensitive to TRAIL in vitro, especially in cells that express TRAIL receptors [97-100]. In patient biopsies, a study showed that 80% of Ewing's tumor samples express DR4 and that all of them express DR5 [100]. Another study performed with 32 samples of Ewing's sarcoma has shown that 72% express both DR4 and DR5 receptors, 25% one of them, and 3% of these samples express none of the two death receptors [98]. These results suggest a high probability of sensitivity of Ewing's sarcoma to TRAIL-induced apoptosis and justify the use of such treatment by activating this apoptosis pathway.
In vivo, few groups studied the therapeutic effect of TRAIL and DR5 agonist in xenograft models of Ewing's sarcoma [101, 102]. The results were very encouraging in ES models induced by sensitive cell lines, showing a 87% inhibition of primary bone tumor incidence and growth, and the prevention of tumor-induced osteolysis, leading to a significant 2-fold increase in animal survival 40 days after tumor induction. Furthermore, TRAIL inhibited tumor nodule dissemination in lungs and increased survival in an osteosarcoma model [101].
Currently, no clinical trial using TRAIL or DR4/DR5 agonist antibodies is underway in primary bone tumors. However, the major limitation of clinical use of TRAIL or DR4/DR5 agonists is the appearance of resistance phenomena. However, some cell lines resistant to conventional chemotherapy agents such as adriamycin or etoposide, are sensitive to TRAIL, demonstrating the interest of TRAIL for an alternative treatment for these patients whose survival rate at 5 years is very low. These chemotherapy agents were even able to sensitize tumor cells to TRAIL-induced apoptosis in lung carcinoma cell lines [103].
Combination TRAIL-chemotherapy
Chemotherapeutic agents such as doxorubicin (Dox) administered in combination with TRAIL have a synergistic effect in some cancers such as colorectal cancer even in a cell line rendered resistant to TRAIL [104]. TRAIL activates primarily the extrinsic pathway of apoptosis and Dox the intrinsic pathway which may explain a synergistic effect of these two molecules [105]. Furthermore, Dox can increase the pro-apoptotic potential of TRAIL in a preclinical model of prostate cancer by inhibiting the expression of c-FLIP, the key inhibitor of the extrinsic apoptosis pathway [106]. Concerning Ewing's sarcoma, TRAIL may also have a synergistic effect in vitro with bortezomib, an inhibitor of the proteasome used in multiple myeloma and lymphoma [107]. Concerning bone tumors, a study investigated the efficacy of TRAIL as a single agent and in combination with clinically relevant chemotherapeutic drugs in fresh isolates of primary malignant cells obtained from biopsy material [93]. The data demonstrated that chemotherapeutic agents were moderately effective alone and TRAIL alone had little or no effect in cells in culture. However, the combination of both produced a significant increase in tumor cell death, with doxorubicin and TRAIL providing the most effective combination [93]. These results also demonstrate the benefit of using TRAIL in cancer therapy in combination with chemotherapy agents, which can result in a synergistic effect or counteract the resistance of tumor cells to TRAIL, a major problem for its clinical use.
Limits of using TRAIL in therapy: resistance mechanisms to TRAIL
Despite the great interest of TRAIL for its ability to specifically induce apoptosis of tumor cells, many malignancies are resistant. Many resistance mechanisms developed by cancer cells have already been described in the signaling pathway of TRAIL (Figure 7) and understanding these mechanisms is necessary to consider a therapeutic use of TRAIL or its death receptor agonists. Thus, if TRAIL is to be used as part of the therapeutic arsenal for Ewing's sarcoma and osteosarcoma, there is a need to identify means of sensitizing these tumors to the effects of TRAIL to address local and metastatic disease.
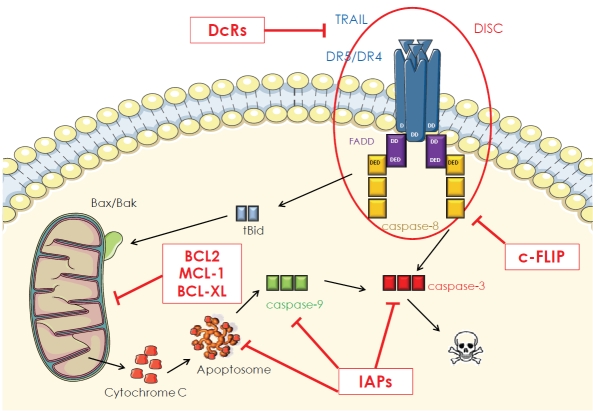
Figure 7.
Resistance mechanisms in the TRAIL signaling pathway. Numerous resistance mechanisms developed by cancer cells have been described at different levels of the intrinsic and extrinsic TRAIL signaling pathways. DcRs: Decoy Receptors, DD: Death Domain, DED: Death Effector Domains, FADD: Fas-Associated protein with Death Domain, c-FLIP: cellular FLICE-inhibitory Protein, Bid: BH3-interacting domain death agonist, BCL-Xl: BCL-extra large, MCL1: Myeloid cell leukemia sequence 1, IAPs: inhibitors of apoptosis.
The first control point is located at the membrane with TRAIL receptors, the interaction with death receptors being required for signal transduction leading to apoptosis of tumor cells. Thus, a lack of DR4 at the membrane was correlated with the resistance phenomena in ovarian cancer and a decreased location at the membrane due to a lack of transport has been shown to amplify the resistance in colon cancer [108, 109]. However, the activating receptors are not the only ones to play a role in these resistance phenomena. Thus, some tumors such as meningioma and medulloblastoma have a high rate of membrane DR4 and DR5 but are resistant to TRAIL [110].
Concerning Ewing's sarcoma, sensitization of death receptor-induced apoptosis and abrogation of TRAIL resistance in vitro lead to increased tumor cell sensitivity to TRAIL and the concomitant inhibition of Ewing's sarcoma growth [111]. According to previous studies, resistance to TRAIL in Ewing's sarcoma may not be due to the expression of activating or decoy receptors, the rate of DR4 and DR5 being relatively constant between sensitive and resistant cell strains, and the decoy receptors hardly detectable in these lines (sensitive or resistant). For example, TRAIL as a single agent induces apoptosis in 7 out of 9 Ewing's sarcoma cell lines [100]. No difference in the surface TRAIL-receptors was observed between sensitive and resistant cell lines. Pre-incubation with interferon-gamma (IFN-γ) rendered the 2 resistant cell lines sensitive by inducing caspase-8 activation [100]. Other studies from the same group confirmed the relevance of using IFN-γ to enhance caspase-8 activation, thereby sensitizing resistant cell lines to TRAIL [99, 112]. The use of fenretinide in Ewing's sarcomas was shown to upregulate cell surface expression of TRAIL death receptors in an ASK1- and p38-dependent manner [113]. The correlation between death receptor expression and TRAIL sensitivity is controversial depending on the studies. For example, a study from Mitsiades and coll describes that 9 of 10 Ewing's sarcoma family tumors underwent apoptosis with TRAIL through activation of caspase-10, caspase-8 (FLICE), caspase-3 and caspase-9 [98]. These 9 cell lines express both DR4 and DR5 while the resistant line expressed only DR4. The resistance of the cell line was overcome by restoration of DR5 levels by transfection. In addition, levels of DcR1, DcR2 or levels of FLICE-inhibitory protein (FLIP) did not correlate with TRAIL resistance. However, in our recent study a correlation between DR4 expression and TRAIL sensitivity was observed in 7 human Ewing's sarcoma cell lines [101]. These discrepancies may rely to the cell lines studied, their culture conditions or TRAIL concentrations used.
Several other therapeutic combinations targeting different steps of the TRAIL induced proapoptotic pathway have been proposed to sensitize resistant cells to TRAIL. Concerning osteosarcoma, a recent study determines the effects of bisphosphonates (BPs) which are very effective drugs in the treatment of bone diseases, on TRAIL-resistant MG-63 human osteosarcoma cells [114]. These cells showed no response to TRAIL alone; however, pre-treatment with BPs significantly increased TRAIL -mediated apoptosis and cellular activation of caspase-3, by inducing DR5 mRNA expression and protein. Moreover, it has been previously demonstrated that MG-63 osteosarcoma cells are heterozygous for a dominant-negative mutation in the death domain of DR4 which can confer TRAIL resistance [115]. Another study from Takeda and coll. demonstrated that LKB1, a serine/threonine kinase expressed in bone and soft tissue sarcoma cells, associates with Death Associated Protein 3 (DAP3), which plays a critical role in TRAIL-mediated apoptosis through activation of pro-caspase 8 [116]. These authors showed that co-expression of LKB1 with DAP3 strongly induced apoptosis in osteosarcoma cells.
More data are available from studies performed in Ewing's Sarcoma Family of Tumors (ESFT). Several studies revealed that TRAIL resistance can be also overcome by proteasome inhibitors [97, 107]. In a first study, the cytotoxic responsiveness of 40 cell lines derived from representatives of the ESFT including 26 Ewing's sarcoma cell lines was investigated [97]. Cell death was induced by TRAIL incubation in more than 60% of these cell lines. Inhibitors of macromolecule synthesis (actinomycin D, cycloheximide) augmented susceptibility to TRAIL in TRAIL-responsive cell lines, while these agents did not render TRAIL-resistant cell lines susceptible to TRAIL. However, the proteasome inhibitor MG132 sensitized these cell lines to TRAIL. Another more recent study confirm these results showing that bortezomib, a novel proteasome inhibitor, exhibited synergistic activity against two Ewing's sarcoma cell lines when combined with TRAIL [107]. Another therapeutic combination to overcome TRAIL resistance was demonstrated by Wang and coll. who have shown that Platelet-Derived Growth Factor (PDGF)R-β blockade by using Gleevec combined with TRAIL resulted in antihuman Ewing's sarcoma activity in vitro and in vivo, suggesting the possibility that combining these treatments will improve anti-Ewing's sarcoma therapy [117]. The effects of histone deacetylase inhibitors (HDI) have been also studied alone or in combination with TRAIL in EFST [118]. The authors showed that HDIs cooperated with TRAIL in inducing cell death in Ewing's Sarcoma cell lines, but HDI-induced cytotoxicity does not depend on the death receptor pathway.
OPG and TRAIL interplay in tumor cell biology
OPG, a soluble member of the TNF receptor superfamily not only plays a role in bone metabolism as an inhibitor of osteoclastogenesis by its interaction to the cytokine RANKL, but also in tumor cell survival as a ligand for TRAIL.
OPG transgenic mice were born with high bone mass associated with a marked decrease in the number and activity of osteoclasts [36]. OPG comprises 401 aa of which 21 form a signal peptide that is cleaved generating mature form of 380 aa. At the N terminus, there are four domains which have cysteine-rich TNF receptor homologous motifs and are necessary and sufficient for binding to its major target RANKL, therefore inhibiting osteoclastic differentiation and activity both in vitro and in vivo [119]. OPG, by efficiently binding RANKL and TRAIL, prevents their association with their transmembrane receptors and therefore counteracts both the RANKL-mediated osteoclastogenesis as well as the pro-apoptotic activity of TRAIL [120]. Therefore, OPG may be involved in survival of a number of tumor cell types via this mechanism [121-123]. This could be of particular importance regarding the ability of tumor cells to evade cell death, since host immune cells present in the tumor microenvironment produce TRAIL [7, 124, 125]. As such, release of OPG by tumor cells is a potential mechanism of resistance by these cells to TRAIL-induced apoptosis [121]. The biological importance of OPG-TRAIL interactions is underscored by recent findings that at physiological conditions, OPG can bind TRAIL with an affinity similar to that of RANKL [126]. In terms of tumor cell survival, in vitro expression studies have demonstrated both breast and prostate cancer cell lines to produce sufficient OPG to protect them against TRAIL-induced apoptosis [121, 127]. Taking this into account, additional studies have been performed using bone marrow stromal cells (BMSCs) derived from either breast or prostate cancer patients. In both cancer types, BMSCs were found to produce sufficient levels of OPG to protect tumor cells from TRAIL-induced apoptosis. This suggests that bone-derived OPG may be involved in promoting survival of these tumor cell types within the bone microenvironment [122, 128]. Similarly, both BMSCs and MG-63 osteosarcoma cells have been demonstrated to produce OPG that can inhibit apoptosis of human myeloma cells [123]. The observation that OPG can inhibit TRAIL-induced apoptosis of multiple myeloma cells suggests there is a fine balance involved between the beneficial effects of OPG in cancer-induced bone disease and potential detrimental effects of inhibiting TRAIL-mediated tumor cell apoptosis. Taking this into account, an OPG peptidomimetic OP3-4 has been developed [129]. Treatment of murine blood mononuclear cells with OP3-4 inhibited osteoclast formation by these cells and, unlike OPG, did not inhibit TRAIL-induced apoptosis of multiple myeloma cells [129]. In addition, it has been shown that multiple myeloma cells inhibit OPG release by stromal cells, thereby promoting osteoclast activation and lytic bone disease (by enhancing RANKL availability) while at the same time exposing themselves to higher levels of ambient TRAIL [130]. Thus, as a recurring theme, the relative levels of pro- versus anti-apoptotic molecules that act in a cell autonomous manner or in the milieu of the bone marrow microenvironment determine the outcome of potentially lethal signals.
The majority of in vivo models indicate that OPG can decrease bone lysis associated with cancer-induced bone disease, leading indirectly to a reduction in tumor growth due to space limitations and possibly inhibition of bone-derived growth factor release. Concerning osteosarcoma, Lamoureux and coll. have previously demonstrated that OPG administered by gene transfer was effective in preventing the formation of osteolytic lesions associated with tumor development in a preclinical model of osteosarcoma, in reducing tumor incidence and the local tumor growth, leading to a 4-fold augmentation of mice survival 28 days postimplantation [131]. Because OPG has no direct activity on osteosarcoma cells in vitro (cell binding, cell proliferation, apoptosis, or cell cycle distribution), the authors suggest that OPG exerts indirect inhibitory effect on tumor progression through the inhibition of RANKL whose production is enhanced in bone tumor environment. In addition, no pro-tumoral effect through TRAIL inhibition could be observed in these conditions. However, employing a new assay system for the TRAIL-OPG complex, Mogi and Kondo recently demonstrated that this complex is constitutively present in the human osteosarcoma cell lines HOS and MG-63 [132].
The role of OPG in vivo seems therefore to be dependent upon the relative concentrations, timing and location of OPG, TRAIL and their ligands, particularly in the local bone marrow environment. The extent of crosstalk between tumor cells and the surrounding stroma will also influence the expression levels of the molecules involved in the OPG/RANKL system, both in the primary tumor and in bone metastases [133]. As OPG is abundantly released by cells from the tumor and normal microenvironment including MSC, fibroblasts and endothelial cells, a recent study investigated the effect of TRAIL on OPG release [134]. Unexpectedly, recombinant TRAIL decreased the spontaneous OPG release in all cell types examined through decreased of the phosphorylation levels of p38/MAPK, suggesting that this pathway is involved in the stabilization of OPG mRNA.
This interplay is even more complex when considering the study of Nicolin and Narducci [135]. The authors have shown that the breast cancer cell line MDA-MB-231 produces a sufficient amount of OPG to bind TRAIL, resulting in an upregulation of RANKL mRNA expression. The authors conclude that the presence of OPG as secreted by this cell line and acting as a paracrine factor could affect breast cancer RANKL production inducing an enhancement of osteolysis and the perpetuation of a vicious cycle.
Conclusion
Several years of intensive research have proved that TRAIL could play a major role in cancer therapy, as a single agent or combined with chemotherapy by overcoming the drug resistance in specific cancers. However, the story of TRAIL and its receptors is very complex with conflicting results, reflecting the interplay between several receptors, possible redundancy and differing expression and sensitivity between tissues. For example, TRAIL resistance remains a crucial limit for its clinical use, especially in osteosarcoma which are poorly sensitive to TRAIL. The better knowledge of TRAIL receptors and signaling pathways has allowed a better management of TRAIL resistance, by its combination with several drugs able to resensitize cancer cells to TRAIL. Concerning bone tumors, another level of regulation is added by the presence and production by bone cells and MSC of OPG which is able to interact with TRAIL. Therefore, complementary studies are needed to better understand the complex tumor cell-host cell interactions in the bone microenvironment and of autocrine and paracrine effects of the secreted (from tumor cells) and released (from bone matrix) factors which may facilitate the development of effective TRAIL strategies to inhibit disease progression.
References
- 1.Pitti RM, Marsters SA, Ruppert S, Donahue CJ, Moore A, Ashkenazi A. Induction of apoptosis by Apo-2 ligand, a new member of the tumor necrosis factor cytokine family. J Biol Chem. 1996;271:12687–12690. doi: 10.1074/jbc.271.22.12687. [DOI] [PubMed] [Google Scholar]
- 2.Wiley SR, Schooley K, Smolak PJ, Din WS, Huang CP, Nicholl JK, Sutherland GR, Smith TD, Rauch C, Smith CA, Goodwin RG. Identification and characterization of a new member of the TNF family that induces apoptosis. Immunity. 1995;3:673–682. doi: 10.1016/1074-7613(95)90057-8. [DOI] [PubMed] [Google Scholar]
- 3.Kimberley FC, Screaton GR. Following a TRAIL: update on a ligand and its five receptors. Cell Res. 2004;14:359–372. doi: 10.1038/sj.cr.7290236. [DOI] [PubMed] [Google Scholar]
- 4.Cha SS, Kim MS, Choi YH, Sung BJ, Shin NK, Shin HC, Sung YC, Oh BH. 2.8 A resolution crystal structure of human TRAIL, a cytokine with selective antitumor activity. Immunity. 1999;11:253–261. doi: 10.1016/s1074-7613(00)80100-4. [DOI] [PubMed] [Google Scholar]
- 5.Diehl GE, Yue HH, Hsieh K, Kuang AA, Ho M, Morici LA, Lenz LL, Cado D, Riley LW, Winoto A. TRAIL-R as a negative regulator of innate immune cell responses. Immunity. 2004;21:877–889. doi: 10.1016/j.immuni.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 6.Cretney E, Takeda K, Yagita H, Glaccum M, Peschon JJ, Smyth MJ. Increased susceptibility to tumor initiation and metastasis in TNF-related apoptosis-inducing ligand-deficient mice. J Immunol. 2002;168:1356–1361. doi: 10.4049/jimmunol.168.3.1356. [DOI] [PubMed] [Google Scholar]
- 7.Almasan A, Ashkenazi A. Apo2L/TRAIL: apoptosis signaling, biology, and potential for cancer therapy. Cytokine Growth Factor Rev. 2003;14:337–348. doi: 10.1016/s1359-6101(03)00029-7. [DOI] [PubMed] [Google Scholar]
- 8.Zheng SJ, Jiang J, Shen H, Chen YH. Reduced apoptosis and ameliorated listeriosis in TRAIL-null mice. J Immunol. 2004;173:5652–5658. doi: 10.4049/jimmunol.173.9.5652. [DOI] [PubMed] [Google Scholar]
- 9.Wandinger KP, Lünemann JD, Wengert O, Bellmann Strobl J, Aktas O, Weber A, Grundström E, Ehrlich S, Wernecke KD, Volk HD, Zipp F. TNF-related apoptosis inducing ligand (TRAIL) as a potential response marker for interferon-beta treatment in multiple sclerosis. Lancet. 2003;361:2036–2043. doi: 10.1016/S0140-6736(03)13641-0. [DOI] [PubMed] [Google Scholar]
- 10.Lub de Hooge MN, de Vries EGE, de Jong S, Bijl M. Soluble TRAIL concentrations are raised in patients with systemic lupus erythematosus. Ann Rheum Dis. 2005;64:854–858. doi: 10.1136/ard.2004.029058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lamhamedi Cherradi SE, Zheng SJ, Maguschak KA, Peschon J, Chen YH. Defective thymocyte apoptosis and accelerated autoimmune diseases in TRAIL-/- mice. Nat Immunol. 2003;4:255–260. doi: 10.1038/ni894. [DOI] [PubMed] [Google Scholar]
- 12.Takeda K, Hayakawa Y, Smyth MJ, Kayagaki N, Yamaguchi N, Kakuta S, Iwakura Y, Yagita H, Okumura K. Involvement of tumor necrosis factor-related apoptosis-inducing ligand in surveillance of tumor metastasis by liver natural killer cells. Nat Med. 2001;7:94–100. doi: 10.1038/83416. [DOI] [PubMed] [Google Scholar]
- 13.Finnberg N, Klein Szanto AJP, El Deiry WS. TRAIL-R deficiency in mice promotes susceptibility to chronic inflammation and tumorigenesis. J Clin Invest. 2008;118:111–123. doi: 10.1172/JCI29900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grosse Wilde A, Voloshanenko O, Bailey SL, Longton GM, Schaefer U, Csernok AI, Schütz G, Greiner EF, Kemp CJ, Walczak H. TRAIL-R deficiency in mice enhances lymph node me tastasis without affecting primary tumor devel opment. J Clin Invest. 2008;118:100–110. doi: 10.1172/JCI33061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bos PD, Zhang XHF, Nadal C, Shu W, Gomis RR, Nguyen DX, Minn AJ, van de Vijver MJ, Gerald WL, Foekens JA, Massagué J. Genes that mediate breast cancer metastasis to the brain. Nature. 2009;459:1005–1009. doi: 10.1038/nature08021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pan G, O'Rourke K, Chinnaiyan AM, Gentz R, Ebner R, Ni J, Dixit VM. The receptor for the cytotoxic ligand TRAIL. Science. 1997;276:111–113. doi: 10.1126/science.276.5309.111. [DOI] [PubMed] [Google Scholar]
- 17.Pan G, Ni J, Wei YF, Yu G, Gentz R, Dixit VM. An antagonist decoy receptor and a death domain-containing receptor for TRAIL. Science. 1997;277:815–818. doi: 10.1126/science.277.5327.815. [DOI] [PubMed] [Google Scholar]
- 18.Sheridan JP, Marsters SA, Pitti RM, Gurney A, Skubatch M, Baldwin D, Ramakrishnan L, Gray CL, Baker K, Wood WI, Goddard AD, Godowski P, Ashkenazi A. Control of TRAIL-induced apoptosis by a family of signaling and decoy receptors. Science. 1997;277:818–821. doi: 10.1126/science.277.5327.818. [DOI] [PubMed] [Google Scholar]
- 19.Clancy L, Mruk K, Archer K, Woelfel M, Mongkolsapaya J, Screaton G, Lenardo MJ, Chan FK. Preligand assembly domain-mediated ligand-independent association between TRAIL receptor 4 (TR4) and TR2 regulates TRAIL-induced apoptosis. Proc Natl Acad Sci USA. 2005;102:18099–18104. doi: 10.1073/pnas.0507329102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Emery JG, McDonnell P, Burke MB, Deen KC, Lyn S, Silverman C, Dul E, Appelbaum ER, Eichman C, DiPrinzio R, Dodds RA, James IE, Rosenberg M, Lee JC, Young PR. Osteopro-tegerin is a receptor for the cytotoxic ligand TRAIL. J Biol Chem. 1998;273:14363–14367. doi: 10.1074/jbc.273.23.14363. [DOI] [PubMed] [Google Scholar]
- 21.Wu GS, Burns TF, Zhan Y, Alnemri ES, El Deiry WS. Molecular cloning and functional analysis of the mouse homologue of the KILLER/DR5 tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) death receptor. Cancer Res. 1999;59:2770–2775. [PubMed] [Google Scholar]
- 22.Chan FKM. Three is better than one: preligand receptor assembly in the regulation of TNF receptor signaling. Cytokine. 2007;37:101–107. doi: 10.1016/j.cyto.2007.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wagner KW, Punnoose EA, Januario T, Lawrence DA, Pitti RM, Lancaster K, Lee D, von Goetz M, Yee SF, Totpal K, Huw L, Katta V, Cavet G, Hymowitz SG, Amler L, Ashkenazi A. Death-receptor O-glycosylation controls tumor-cell sensitivity to the proapoptotic ligand Apo2L/TRAIL. Nat Med. 2007;13:1070–1077. doi: 10.1038/nm1627. [DOI] [PubMed] [Google Scholar]
- 24.Nimmanapalli R, Perkins CL, Orlando M, O'Bryan E, Nguyen D, Bhalla KN. Pretreatment with paclitaxel enhances apo-2 ligand/tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis of prostate cancer cells by inducing death receptors 4 and 5 protein levels. Cancer Res. 2001;61:759–763. [PubMed] [Google Scholar]
- 25.Takimoto R, El Deiry WS. Wild-type p53 trans-activates the KILLER/DR5 gene through an intronic sequence-specific DNA-binding site. Oncogene. 2000;19:1735–1743. doi: 10.1038/sj.onc.1203489. [DOI] [PubMed] [Google Scholar]
- 26.Burns TF, Bernhard EJ, El Deiry WS. Tissue specific expression of p53 target genes suggests a key role for KILLER/DR5 in p53-dependent apoptosis in vivo. Oncogene. 2001;20:4601–4612. doi: 10.1038/sj.onc.1204484. [DOI] [PubMed] [Google Scholar]
- 27.Gonzalvez F, Ashkenazi A. New insights into apoptosis signaling by Apo2L/TRAIL. Oncogene. 2010;29:4752–4765. doi: 10.1038/onc.2010.221. [DOI] [PubMed] [Google Scholar]
- 28.Rossin A, Derouet M, Abdel Sater F, Hueber AO. Palmitoylation of the TRAIL receptor DR4 confers an efficient TRAIL-induced cell death signalling. Biochem J. 2009;419:185–192. doi: 10.1042/BJ20081212. 2 p following 192. [DOI] [PubMed] [Google Scholar]
- 29.Austin CD, Lawrence DA, Peden AA, Varfolomeev EE, Totpal K, De Mazière AM, Klumperman J, Arnott D, Pham V, Scheller RH, Ashkenazi A. Death-receptor activation halts clathrin-dependent endocytosis. Proc Natl Acad Sci USA. 2006;103:10283–10288. doi: 10.1073/pnas.0604044103. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 30.Kohlhaas SL, Craxton A, Sun XM, Pinkoski MJ, Cohen GM. Receptor-mediated endocytosis is not required for tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-induced apoptosis. J Biol Chem. 2007;282:12831–12841. doi: 10.1074/jbc.M700438200. [DOI] [PubMed] [Google Scholar]
- 31.Sanlioglu AD, Karacay B, Koksal IT, Griffith TS, Sanlioglu S. DcR2 (TRAIL-R4) siRNA and ade- novirus delivery of TRAIL (Ad5hTRAIL) break down in vitro tumorigenic potential of prostate carcinoma cells. Cancer Gene Ther. 2007;14:976–984. doi: 10.1038/sj.cgt.7701087. [DOI] [PubMed] [Google Scholar]
- 32.Sanlioglu AD, Korcum AF, Pestereli E, Erdogan G, Karaveli S, Savas B, Griffith TS, Sanlioglu S. TRAIL death receptor-4 expression positively correlates with the tumor grade in breast cancer patients with invasive ductal carcinoma. Int J Radiat Oncol Biol Phys. 2007;69:716–723. doi: 10.1016/j.ijrobp.2007.03.057. [DOI] [PubMed] [Google Scholar]
- 33.Riccioni R, Pasquini L, Mariani G, Saulle E, Rossini A, Diverio D, Pelosi E, Vitale A, Chierichini A, Cedrone M, Foà R, Lo Coco F, Peschle C, Testa U. TRAIL decoy receptors mediate resistance of acute myeloid leukemia cells to TRAIL. Haematologica. 2005;90:612–624. [PubMed] [Google Scholar]
- 34.Griffith TS, Chin WA, Jackson GC, Lynch DH, Kubin MZ. Intracellular regulation of TRAIL- induced apoptosis in human melanoma cells. J Immunol. 1998;161:2833–2840. [PubMed] [Google Scholar]
- 35.Mérino D, Lalaoui N, Morizot A, Schneider P, Solary E, Micheau O. Differential inhibition of TRAIL-mediated DR5-DISC formation by decoy receptors 1 and 2. Mol Cell Biol. 2006;26:7046–7055. doi: 10.1128/MCB.00520-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Simonet WS, Lacey DL, Dunstan CR, Kelley M, Chang MS, Luthy R, Nguyen HQ, Wooden S, Bennett L, Boone T, Shimamoto G, DeRose M, Elliott R, Colombero A, Tan HL, Trail G, Sullivan J, Davy E, Bucay N, Renshaw Gegg L, Hughes TM, Hill D, Pattison W, Campbell P, Sander S, Van G, Tarpley J, Derby P, Lee R, Program AE, Boyle WJ. Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell. 1997;89:309–319. doi: 10.1016/s0092-8674(00)80209-3. [DOI] [PubMed] [Google Scholar]
- 37.Screaton GR, Mongkolsapaya J, Xu XN, Cowper AE, McMichael AJ, Bell JI. TRICK2, a new alternatively spliced receptor that transduces the cytotoxic signal from TRAIL. Curr Biol. 1997;7:693–696. doi: 10.1016/s0960-9822(06)00297-1. [DOI] [PubMed] [Google Scholar]
- 38.Krieg A, Schulte am Esch J, 2nd, Ramp U, Hosch SB, Knoefel WT, Gabber HE, Mahotka C. TRAIL-R4-β: a new splice variant of TRAIL-receptor 4 lacking the cysteine rich domain 1. Biochem Biophys Res Commun. 2006;349:115–121. doi: 10.1016/j.bbrc.2006.08.031. [DOI] [PubMed] [Google Scholar]
- 39.Medema JP, Scaffidi C, Kischkel FC, Shevchenko A, Mann M, Krammer PH, Peter ME. FLICE is activated by association with the CD95 death-inducing signaling complex (DISC) EMBO J. 1997;16:2794–2804. doi: 10.1093/emboj/16.10.2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kischkel FC, Lawrence DA, Chuntharapai A, Schow P, Kim KJ, Ashkenazi A. Apo2L/TRAIL-dependent recruitment of endogenous FADD and caspase-8 to death receptors 4 and 5. Immunity. 2000;12:611–620. doi: 10.1016/s1074-7613(00)80212-5. [DOI] [PubMed] [Google Scholar]
- 41.Danial NN, Korsmeyer SJ. Cell death: critical control points. Cell. 2004;116:205–219. doi: 10.1016/s0092-8674(04)00046-7. [DOI] [PubMed] [Google Scholar]
- 42.Galluzzi L, Maiuri MC, Vitale I, Zischka H, Castedo M, Zitvogel L, Kroemer G. Cell death modalities: classification and pathophysiological implications. Cell Death Differ. 2007;14:1237–1243. doi: 10.1038/sj.cdd.4402148. [DOI] [PubMed] [Google Scholar]
- 43.Scaffidi C, Fulda S, Srinivasan A, Friesen C, Li F, Tomaselli KJ, Debatin KM, Krammer PH, Peter ME. Two CD95 (APO-1/Fas) signaling pathways. EMBO J. 1998;17:1675–1687. doi: 10.1093/emboj/17.6.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li H, Zhu H, Xu CJ, Yuan J. Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell. 1998;94:491–501. doi: 10.1016/s0092-8674(00)81590-1. [DOI] [PubMed] [Google Scholar]
- 45.Luo X, Budihardjo I, Zou H, Slaughter C, Wang X. Bid, a Bcl2 interacting protein, mediates cytochrome c release from mitochondria in response to activation of cell surface death receptors. Cell. 1998;94:481–490. doi: 10.1016/s0092-8674(00)81589-5. [DOI] [PubMed] [Google Scholar]
- 46.Zou H, Henzel WJ, Liu X, Lutschg A, Wang X. Apaf-1, a human protein homologous to C. elegans CED-4, participates in cytochrome c-dependent activation of caspase-3. Cell. 1997;90:405–413. doi: 10.1016/s0092-8674(00)80501-2. [DOI] [PubMed] [Google Scholar]
- 47.MacFarlane M. TRAIL-induced signalling and apoptosis. Toxicol Lett. 2003;139:89–97. doi: 10.1016/s0378-4274(02)00422-8. [DOI] [PubMed] [Google Scholar]
- 48.Degli Esposti MA, Dougall WC, Smolak PJ, Waugh JY, Smith CA, Goodwin RG. The novel receptor TRAIL-R4 induces NF-kappaB and protects against TRAIL-mediated apoptosis, yet retains an incomplete death domain. Immunity. 1997;7:813–820. doi: 10.1016/s1074-7613(00)80399-4. [DOI] [PubMed] [Google Scholar]
- 49.Li H, Lin X. Positive and negative signaling components involved in TNFalpha-induced NF-kappaB activation. Cytokine. 2008;41:1–8. doi: 10.1016/j.cyto.2007.09.016. [DOI] [PubMed] [Google Scholar]
- 50.Trauzold A, Siegmund D, Schniewind B, Sipos B, Egberts J, Zorenkov D, Emme D, Röder C, Kalthoff H, Wajant H. TRAIL promotes metastasis of human pancreatic ductal adenocarcinoma. Oncogene. 2006;25:7434–7439. doi: 10.1038/sj.onc.1209719. [DOI] [PubMed] [Google Scholar]
- 51.Ricci MS, Kim SH, Ogi K, Plastaras JP, Ling J, Wang W, Jin Z, Liu YY, Dicker DT, Chiao PJ, Flaherty KT, Smith CD, El Deiry WS. Reduction of TRAIL-induced Mcl-1 and cIAP2 by c-Myc or sorafenib sensitizes resistant human cancer cells to TRAIL-induced death. Cancer Cell. 2007;12:66–80. doi: 10.1016/j.ccr.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 52.Secchiero P, Gonelli A, Carnevale E, Milani D, Pandolfi A, Zella D, Zauli G. TRAIL promotes the survival and proliferation of primary human vascular endothelial cells by activating the Akt and ERK pathways. Circulation. 2003;107:2250–2256. doi: 10.1161/01.CIR.0000062702.60708.C4. [DOI] [PubMed] [Google Scholar]
- 53.Belyanskaya LL, Ziogas A, Hopkins Donaldson S, Kurtz S, Simon HU, Stahel R, Zangemeister Wittke U. TRAIL-induced survival and proliferation of SCLC cells is mediated by ERK and dependent on TRAIL-R2/DR5 expression in the absence of caspase-8. Lung Cancer. 2008;60:355–365. doi: 10.1016/j.lungcan.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 54.Ishimura N, Isomoto H, Bronk SF, Gores GJ. Trail induces cell migration and invasion in apoptosis-resistant cholangiocarcinoma cells. Am J Physiol Gastrointest Liver Physiol. 2006;290:G129–136. doi: 10.1152/ajpgi.00242.2005. [DOI] [PubMed] [Google Scholar]
- 55.Gazitt Y. TRAIL is a potent inducer of apoptosis in myeloma cells derived from multiple myeloma patients and is not cytotoxic to hematopoietic stem cells. Leukemia. 1999;13:1817–1824. doi: 10.1038/sj.leu.2401501. [DOI] [PubMed] [Google Scholar]
- 56.Walczak H, Miller RE, Ariail K, Gliniak B, Griffith TS, Kubin M, Chin W, Jones J, Woodward A, Le T, Smith C, Smolak P, Goodwin RG, Rauch CT, Schuh JC, Lynch DH. Tumoricidal activity of tumor necrosis factor-related apoptosis-inducing ligand in vivo. Nat Med. 1999;5:157–163. doi: 10.1038/5517. [DOI] [PubMed] [Google Scholar]
- 57.Ashkenazi A, Pai RC, Fong S, Leung S, Lawrence DA, Marsters SA, Blackie C, Chang L, McMurtrey AE, Hebert A, DeForge L, Koumenis IL, Lewis D, Harris L, Bussiere J, Koeppen H, Shahrokh Z, Schwall RH. Safety and antitumor activity of recombinant soluble Apo2 ligand. J Clin Invest. 1999;104:155–162. doi: 10.1172/JCI6926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kelley SK, Harris LA, Xie D, Deforge L, Totpal K, Bussiere J, Fox JA. Preclinical studies to predict the disposition of Apo2L/tumor necro sis factor-related apoptosis-inducing ligand in humans: characterization of in vivo efficacy, pharmacokinetics, and safety. J Pharmacol Exp Ther. 2001;299:31–38. [PubMed] [Google Scholar]
- 59.Pollack IF, Erff M, Ashkenazi A. Direct stimulation of apoptotic signaling by soluble Apo2l/tumor necrosis factor-related apoptosis- inducing ligand leads to selective killing of glioma cells. Clin Cancer Res. 2001;7:1362–1369. [PubMed] [Google Scholar]
- 60.Marini P, Schmid A, Jendrossek V, Faltin H, Daniel PT, Budach W, Belka C. Irradiation specifically sensitises solid tumour cell lines to TRAIL mediated apoptosis. BMC Cancer. 2005;5(5) doi: 10.1186/1471-2407-5-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Song K, Benhaga N, Anderson RL, Khosravi Far R. Transduction of tumor necrosis factorrelated apoptosis-inducing ligand into hematopoietic cells leads to inhibition of syngeneic tumor growth in vivo. Cancer Res. 2006;66:6304–6311. doi: 10.1158/0008-5472.CAN-05-3501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jo M, Kim TH, Seol DW, Esplen JE, Dorko K, Billiar TR, Strom SC. Apoptosis induced in normal human hepatocytes by tumor necrosis factor-related apoptosis-inducing ligand. Nat Med. 2000;6:564–567. doi: 10.1038/75045. [DOI] [PubMed] [Google Scholar]
- 63.Lawrence D, Shahrokh Z, Marsters S, Achilles K, Shih D, Mounho B, Hillan K, Totpal K, De-Forge L, Schow P, Hooley J, Sherwood S, Pai R, Leung S, Khan L, Gliniak B, Bussiere J, Smith CA, Strom SS, Kelley S, Fox JA, Thomas D, Ashkenazi A. Differential hepatocyte toxicity of recombinant Apo2L/TRAIL versions. Nat Med. 2001;7:383–385. doi: 10.1038/86397. [DOI] [PubMed] [Google Scholar]
- 64.Ganten TM, Koschny R, Sykora J, Schulze Bergkamen H, Büchler P, Haas TL, Schader MB, Untergasser A, Stremmel W, Walczak H. Preclinical differentiation between apparently safe and potentially hepatotoxic applications of TRAIL either alone or in combination with chemotherapeutic drugs. Clin Cancer Res. 2006;12:2640–2646. doi: 10.1158/1078-0432.CCR-05-2635. [DOI] [PubMed] [Google Scholar]
- 65.Hao C, Song JH, Hsi B, Lewis J, Song DK, Petruk KC, Tyrrell DL, Kneteman NM. TRAIL inhibits tumor growth but is nontoxic to human hepatocytes in chimeric mice. Cancer Res. 2004;64:8502–8506. doi: 10.1158/0008-5472.CAN-04-2599. [DOI] [PubMed] [Google Scholar]
- 66.Menoret E, Gomez Bougie P, Geffroy Luseau A, Daniels S, Moreau P, Le Gouill S, Harousseau JL, bataille R, Amiot M, Pellat-Deceunynck C. Mcl-1L cleavage is involved in TRAIL-R1- and TRAIL-R2-mediated apoptosis induced by HGS-ETR1 and HGS-ETR2 human mAbs in myeloma cells. Blood. 2006;108:1346–1352. doi: 10.1182/blood-2005-12-007971. [DOI] [PubMed] [Google Scholar]
- 67.Wu GS. TRAIL as a target in anti-cancer therapy. Cancer Lett. 2009;285:1–5. doi: 10.1016/j.canlet.2009.02.029. [DOI] [PubMed] [Google Scholar]
- 68.Nagane M, Pan G, Weddle JJ, Dixit VM, Cavenee WK, Huang HJ. Increased death receptor 5 expression by chemotherapeutic agents in human gliomas causes synergistic cytotoxicity with tumor necrosis factor-related apoptosis-inducing ligand in vitro and in vivo. Cancer Res. 2000;60:847–853. [PubMed] [Google Scholar]
- 69.Cuello M, Ettenberg SA, Nau MM, Lipkowitz S. Synergistic induction of apoptosis by the combination of trail and chemotherapy in chemo-resistant ovarian cancer cells. Gyneco Oncol. 2001;81:380–390. doi: 10.1006/gyno.2001.6194. [DOI] [PubMed] [Google Scholar]
- 70.Tolcher AW, Mita M, Meropol NJ, von Mehren M, Patnaik A, Padavic K, Hill M, Mays T, McCoy T, Fox NL, Halpern W, Corey A, Cohen RB. Phase I pharmacokinetic and biologic correlative study of mapatumumab, a fully human monoclonal antibody with agonist activity to tumor necrosis factor-related apoptosis-inducing ligand receptor-1. J Clin Oncol. 2007;25:1390–1395. doi: 10.1200/JCO.2006.08.8898. [DOI] [PubMed] [Google Scholar]
- 71.Mom CH, Verweij J, Oldenhuis CN, Gietema JA, Fox NL, Miceli R, Eskens FA, Loos WJ, de Vries EG, Sleijfer S. Mapatumumab, a fully human agonistic monoclonal antibody that targets TRAIL-R1, in combination with gemcitabine and cisplatin: a phase I study. Clin Cancer Res. 2009;15:5584–5590. doi: 10.1158/1078-0432.CCR-09-0996. [DOI] [PubMed] [Google Scholar]
- 72.Younes A, Vose JM, Zelenetz AD, Smith MR, Burris HA, Ansell SM, Klein J, Halpern W, Miceli R, Kumm E, Fox NL, Czuczman MS. A Phase 1b/2 trial of mapatumumab in patients with relapsed/refractory non-Hodgkin's lymphoma. Br J Cancer. 2010;103:1783–1787. doi: 10.1038/sj.bjc.6605987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hotte SJ, Hirte HW, Chen EX, Siu LL, Le LH, Corey A, Iacobucci A, MacLean M, Lo L, Fox NL, Oza AM. A phase 1 study of mapatumumab (fully human monoclonal antibody to TRAIL-R1) in patients with advanced solid malignancies. Clin Cancer Res. 2008;14:3450–3455. doi: 10.1158/1078-0432.CCR-07-1416. [DOI] [PubMed] [Google Scholar]
- 74.Greco FA, Bonomi P, Crawford J, Kelly K, Oh Y, Halpern W, Lo L, Gallant G, Klein J. Phase 2 study of mapatumumab, a fully human agonistic monoclonal antibody which targets and activates the TRAIL receptor-1, in patients with advanced non-small cell lung cancer. Lung Cancer. 2008;61:82–90. doi: 10.1016/j.lungcan.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 75.Trarbach T, Moehler M, Heinemann V, Köhne CH, Przyborek M, Schulz C, Sneller V, Gallant G, Kanzler S. Phase II trial of mapatumumab, a fully human agonistic monoclonal antibody that targets and activates the tumour necrosis factor apoptosis-inducing ligand receptor-1 (TRAIL-R1), in patients with refractory colorectal cancer. Br J Cancer. 2010;102:506–512. doi: 10.1038/sj.bjc.6605507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Leong S, Cohen RB, Gustafson DL, Langer CJ, Camidge DR, Padavic K, Gore L, Smith M, Chow LQ, von Mehren M, O'Bryant C, Hariharan S, Diab S, Fox NL, Miceli R, Eckhardt SG. Mapatumumab, an antibody targeting TRAIL-R1, in combination with paclitaxel and carboplatin in patients with advanced solid malignancies: results of a phase I and pharmacokinetic study. J Clin Oncol. 2009;27:4413–4421. doi: 10.1200/JCO.2008.21.7422. [DOI] [PubMed] [Google Scholar]
- 77.Plummer R, Attard G, Pacey S, Li L, Razak A, Perrett R, Barrett M, Judson I, Kaye S, Fox NL, Halpern W, Corey A, Calvert H, de Bono J. Phase 1 and pharmacokinetic study of lexatumumab in patients with advanced cancers. Clin Cancer Res. 2007;13:6187–6194. doi: 10.1158/1078-0432.CCR-07-0950. [DOI] [PubMed] [Google Scholar]
- 78.Wakelee HA, Patnaik A, Sikic BI, Mita M, Fox NL, Miceli R, Ullrich SJ, Fisher GA, Tolcher AW. Phase I and pharmacokinetic study of lexatumumab (HGS-ETR2) given every 2 weeks in patients with advanced solid tumors. Ann Oncol. 2010;21:376–381. doi: 10.1093/annonc/mdp292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Adams C, Totpal K, Lawrence D, Marsters S, Pitti R, Yee S, Ross S, Deforge L, Koeppen H, Sagolla M, Compaan D, Lowman H, Hymowitz S, Ashkenazi A. Structural and functional analysis of the interaction between the agonistic monoclonal antibody Apomab and the proapoptotic receptor DR5. Cell Death Differ. 2008;15:751–761. doi: 10.1038/sj.cdd.4402306. [DOI] [PubMed] [Google Scholar]
- 80.Mirabello L, Troisi RJ, Savage SA. International osteosarcoma incidence patterns in children and adolescents, middle age and elderly persons. Int J Cancer. 2009;125:229–234. doi: 10.1002/ijc.24320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Link MP, Gebhardt MC, Meyers PA. Osteosarcoma. In: Pizzo PA, Poplack DG, editors. principles and practices of pediatric oncology (5th edition) PA, USA: Lippincott Williams & Wilkins; 2006. pp. 1074–1115. [Google Scholar]
- 82.Wu PK, Chen WM, Chen CF, Lee OK, Haung CK, Chen TH. Primary osteogenic sarcoma with pulmonary metastasis, clinical results and prognostic factors in 91 patients. Jpn J Clin Oncol. 2009;39:514–522. doi: 10.1093/jjco/hyp057. [DOI] [PubMed] [Google Scholar]
- 83.Wilkins RM, Pritchard DJ, Burgert EO, Jr, Unni KK. Ewing's sarcoma of bone. Experience with 140 patients. Cancer. 1986;58:2551–2555. doi: 10.1002/1097-0142(19861201)58:11<2551::aid-cncr2820581132>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 84.Kovar H, Aryee D, Zoubek A. The Ewing family of tumors and the search for the Achilles’ heel. Curr Opin Oncol. 1999;11:275–284. doi: 10.1097/00001622-199907000-00007. [DOI] [PubMed] [Google Scholar]
- 85.Rodriguez Galindo C. Pharmacological management of Ewing sarcoma family of tumours. Expert Opin Pharmacother. 2004;5:1257–1270. doi: 10.1517/14656566.5.6.1257. [DOI] [PubMed] [Google Scholar]
- 86.Barnes R, Catto M. Chondrosarcoma of bone. J Bone Joint Surg Br. 1966;48:729–764. [PubMed] [Google Scholar]
- 87.Linet MS, Ries LA, Smith MA, Tarone RE, Devesa SS. Cancer surveillance series: recent trends in childhood cancer incidence and mortality in the United States. J Natl Cancer Inst. 1999;91:1051–1058. doi: 10.1093/jnci/91.12.1051. [DOI] [PubMed] [Google Scholar]
- 88.Mirabello L, Troisi RJ, Savage SA. Osteosarcoma incidence and survival rates from 1973 to 2004: data from the Surveillance, Epidemiology, and End Results Program. Cancer. 2009;115:1531–1543. doi: 10.1002/cncr.24121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gatta G, Capocaccia R, Stiller C, Kaatsch P, Berrino F, Terenziani M, EUROCARE Working Group Childhood cancer survival trends in Europe: a EUROCARE Working group study. J Clin Oncol. 2005;23:3742–3751. doi: 10.1200/JCO.2005.00.554. [DOI] [PubMed] [Google Scholar]
- 90.Kolb EA, Gorlick R, Houghton PJ, Morton CL, Lock R, Carol H, Reynolds CP, Maris JM, Keir ST, Billups CA, Smith MA. Initial testing (phase 1) of a monoclonal antibody (SCH 717454) against the IGF-1 receptor by the pediatric preclinical testing program. Pediatr Blood Cancer. 2008;50:1190–1197. doi: 10.1002/pbc.21450. [DOI] [PubMed] [Google Scholar]
- 91.Thomas D, Henshaw R, Skubitz K, Chawla S, Staddon A, Blay JY, Roudier M, Smith J, Ye Z, Sohn W, Dansey R, Jun S. Denosumab in patients with giant-cell tumour of bone: an open-label, phase 2 study. Lancet Oncol. 2010;11:275–80. doi: 10.1016/S1470-2045(10)70010-3. [DOI] [PubMed] [Google Scholar]
- 92.Evdokiou A, Bouralexis S, Atkins GJ, Chai F, Hay S, Clayer M, Findlay DM. Chemotherapeutic agents sensitize osteogenic sarcoma cells, but not normal human bone cells, to Apo2L/TRAIL-induced apoptosis. Int J Cancer. 2002;99:491–504. doi: 10.1002/ijc.10376. [DOI] [PubMed] [Google Scholar]
- 93.Bouralexis S, Clayer M, Atkins GJ, Labridinis A, Hay S, Graves S, Findlay DM, Evdokiou A. Sensitivity of fresh isolates of soft tissue sarcoma, osteosarcoma and giant cell tumour cells to Apo2L/TRAIL and doxorubicin. Int J Oncol. 2004;24:1263–1270. doi: 10.3892/ijo.24.5.1263. [DOI] [PubMed] [Google Scholar]
- 94.Bouralexis S, Findlay DM, Atkins GJ, Labrinidis A, Hay S, Evdokiou A. Progressive resistance of BTK-143 osteosarcoma cells to Apo2L/TRAIL-induced apoptosis is mediated by acquisition of DcR2/TRAIL-R4 expression: resensitisation with chemotherapy. Br J Cancer. 2003;89:206–214. doi: 10.1038/sj.bjc.6601021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hotta T, Suzuki H, Nagai S, Yamamoto K, Imakiire A, Takada E, Itoh M, Mizuguchi J. Chemotherapeutic agents sensitize sarcoma cell lines to tumor necrosis factor-related apoptosis-inducing ligand-induced caspase-8 activation, apoptosis and loss of mitochondrial membrane potential. J Orthop Res. 2003;21:949–957. doi: 10.1016/S0736-0266(03)00062-7. [DOI] [PubMed] [Google Scholar]
- 96.Cenni V, Maraldi NM, Ruggeri A, Secchiero P, Del Coco R, De Pol A, Cocco L, Marmiroli S. Sensitization of multidrug resistant human ostesarcoma cells to Apo2 Ligand/TRAIL- induced apoptosis by inhibition of the Akt/PKB kinase. Int J Oncol. 2004;25:1599–1608. [PubMed] [Google Scholar]
- 97.Van Valen F, Fulda S, Truckenbrod B, Eckervogt V, Sonnemann J, Hillmann A, Rödl R, Hoffmann C, Winkelmann W, Schäfer L, Dockhorn Dworniczak B, Wessel T, Boos J, Debatin KM, Jürgens H. Apoptotic responsiveness of the Ewing's sarcoma family of tumours to tumour necrosis factor-related apoptosis-inducing ligand (TRAIL) Int J Cancer. 2000;88:252–259. doi: 10.1002/1097-0215(20001015)88:2<252::aid-ijc17>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 98.Mitsiades N, Poulaki V, Mitsiades C, Tsokos M. Ewing's sarcoma family tumors are sensitive to tumor necrosis factor-related apoptosis-inducing ligand and express death receptor 4 and death receptor 5. Cancer Res. 2001;61:2704–2712. [PubMed] [Google Scholar]
- 99.Kumar A, Jasmin A, Eby MT, Chaudhary PM. Cytotoxicity of Tumor necrosis factor related apoptosis-inducing ligand towards Ewing's sarcoma cell lines. Oncogene. 2001;20:1010–1014. doi: 10.1038/sj.onc.1204154. [DOI] [PubMed] [Google Scholar]
- 100.Kontny HU, Hämmerler K, Klein R, Shayan P, Mackall CL, Niemeyer CM. Sensitivity of Ewing's sarcoma to TRAIL-induced apoptosis. Cell Death Differ. 2001;8:506–514. doi: 10.1038/sj.cdd.4400836. [DOI] [PubMed] [Google Scholar]
- 101.Picarda G, Lamoureux F, Geffroy L, Delepine P, Montier T, Laud K, Tirode F, Delattre O, Heymann D, Rédini F. Preclinical evidence that use of TRAIL in Ewing's sarcoma and osteosarcoma therapy inhibits tumor growth, prevents osteolysis, and increases animal survival. Clin Cancer Res. 2010;16:2363–2374. doi: 10.1158/1078-0432.CCR-09-1779. [DOI] [PubMed] [Google Scholar]
- 102.Merchant MS, Yang X, Melchionda F, Romero M, Klein R, Thiele CJ, Tsokos M, Kontny HU, Mackall CL. Interferon gamma enhances the effectiveness of tumor necrosis factor-related apoptosis-inducing ligand receptor agonists in a xenograft model of Ewing's sarcoma. Cancer Res. 2004;64:8349–8356. doi: 10.1158/0008-5472.CAN-04-1705. [DOI] [PubMed] [Google Scholar]
- 103.Vaculova A, Kaminskyy V, Jalalvand E, Surova O, Zhivotovsky B. Doxorubicin and etoposide sensitize small cell lung carcinoma cells expressing caspase-8 to TRAIL. Mol. Cancer. 2010;9:87. doi: 10.1186/1476-4598-9-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lee ALZ, Dhillon SHK, Wang Y, Pervaiz S, Fan W, Yang YY. Synergistic anti-cancer effects via co-delivery of TNF-related apoptosis-inducing ligand (TRAIL/Apo2L) and doxorubicin using micellar nanoparticles. Mol Biosyst. 2011;7:1512–1522. doi: 10.1039/c0mb00266f. [DOI] [PubMed] [Google Scholar]
- 105.Aroui S, Mili D, Brahim S, De Waard M, Kenani A. Doxorubicin coupled to penetratin promotes apoptosis in CHO cells by a mechanism involving c-Jun NH2-terminal kinase. Biochem Biophys Res Commun. 2010;396:908–914. doi: 10.1016/j.bbrc.2010.05.020. [DOI] [PubMed] [Google Scholar]
- 106.El Zawahry A, McKillop J, Voelkel Johnson C. Doxorubicin increases the effectiveness of Apo2L/TRAIL for tumor growth inhibition of prostate cancer xenografts. BMC Cancer. 2005;5:2. doi: 10.1186/1471-2407-5-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lu G, Punj V, Chaudhary PM. Proteasome inhibitor Bortezomib induces cell cycle arrest and apoptosis in cell lines derived from Ewing's sarcoma family of tumors and synergizes with TRAIL. Cancer Biol Ther. 2008;7:603–608. doi: 10.4161/cbt.7.4.5564. [DOI] [PubMed] [Google Scholar]
- 108.Horak P, Pils D, Haller G, et al. Contribution of epigenetic silencing of tumor necrosis factor-related apoptosis inducing ligand receptor 1 (DR4) to TRAIL resistance and ovarian cancer. Mol Cancer Res. 2005;3:335–343. doi: 10.1158/1541-7786.MCR-04-0136. [DOI] [PubMed] [Google Scholar]
- 109.Jin Z, McDonald ER, Dicker DT, El Deiry WS. Deficient tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) death receptor transport to the cell surface in human colon cancer cells selected for resistance to TRAIL-induced apoptosis. J Biol Chem. 2004;279:35829–35839. doi: 10.1074/jbc.M405538200. [DOI] [PubMed] [Google Scholar]
- 110.Dyer MJS, MacFarlane M, Cohen GM. Barriers to effective TRAIL-targeted therapy of malignancy. J Clin Oncol. 2007;25:4505–4506. doi: 10.1200/JCO.2007.13.1011. [DOI] [PubMed] [Google Scholar]
- 111.Fulda S, Küfer MU, Meyer E, van Valen F, Dockhorn Dworniczak B, Debatin KM. Sensitization for death receptor- or drug-induced apoptosis by re-expression of caspase-8 through demethylation or gene transfer. Oncogene. 2001;20:5865–5877. doi: 10.1038/sj.onc.1204750. [DOI] [PubMed] [Google Scholar]
- 112.issat A, Vraetz T, Tsokos M, Klein R, Braun M, Koutelia N, Fisch P, Romero ME, Long L, Noellke P, Mackall CL, Niemeyer CM, Kontny U. Interferon-gamma sensitizes resistant Ewing's sarcoma cells to tumor necrosis factor related apoptosis inducing ligand-induced apoptosis by up-regulation of caspase-8 without altering chemosensitivity. Am J Pathol. 2007;170:1917–1930. doi: 10.2353/ajpath.2007.060993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.White DE, Burchill SA. Fenretinide-dependent upregulation of death receptors through ASK1 and p38D enhances death receptor ligand-induced cell death in Ewing's sarcoma family of tumours. Br J Cancer. 2010;103:1380–1390. doi: 10.1038/sj.bjc.6605896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Moon MH, Jeong JK, Seo JS, Lee YJ, Park SY. Bisphosphonate enhances TRAIL sensitivity to human osteosarcoma cells via DR-5 upregulation. Exp Mol Med. 2011;43:138–145. doi: 10.3858/emm.2011.43.3.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Locklin RM, Federici E, Espina B, Hulley PA, Russell RG, Edwards CM. Selective targeting of death receptor 5 circumvents resistance of MG-63 osteosarcoma cells to TRAIL-induced apoptosis. Mol Cancer Ther. 2007;6:3219–3228. doi: 10.1158/1535-7163.MCT-07-0275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Takeda S, Iwai A, Nakashima M, Fujikura D, Chiba S, Li HM, Uehara J, Kawaguchi S, Kaya M, Nagoya S, Wada T, Yuan J, Rayter S, Ashworth A, Reed JC, Yamashita T, Uede T, Miyazaki T. LKB1 is crucial for TRAIL-mediated apoptosis induction in osteosarcoma. Anticancer Res. 2007;27:761–768. [PubMed] [Google Scholar]
- 117.Wang Y, Mandal D, Wang S, Kleinerman ES, Pollock RE, Lev D, Hayes Jordan A. Platelet- derived growth factor b inhibition increases tumor necrosis factor-related apoptosis- inducing ligand (TRAIL) sensitivity. Cancer. 2010;116:3892–3902. doi: 10.1002/cncr.25107. [DOI] [PubMed] [Google Scholar]
- 118.Sonnemann J, Dreyer L, Hartwig M, Palani CD, Hong le TT, Klier U, Bröker B, Völker U, Beck JF. Histone deacetylase inhibitors induce cell death and enhance the apoptosis-inducing activity of TRAIL in Ewing's sarcoma cells. J Cancer Res Clin Oncol. 2007;133:847–858. doi: 10.1007/s00432-007-0227-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Theoleyre S, Wittrant Y, Tat SK, Fortun Y, Redini F, Heymann D. The molecular triad OPG/RANK/RANKL: involvement in the orchestration of pathophysiological bone remodeling. Cyt Gr Fact Rev. 2004;15:457–475. doi: 10.1016/j.cytogfr.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 120.Lamoureux F, Moriceau G, Picarda G, Rousseau J, Trichet V, Rédini F. Regulation of os-teoprotegerin pro- or anti-tumoral activity by bone tumor microenvironment. Biophys Biochim Acta Rev on Cancer. 2010;1805:17–24. doi: 10.1016/j.bbcan.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 121.Holen I, Croucher PI, Hamdy FC, Eaton CL. Osteoprotegerin (OPG) is a survival factor for human prostate cancer cells. Cancer Res. 2002;62:1619–1623. [PubMed] [Google Scholar]
- 122.Neville Webb HL, Cross NA, Eaton CL, Nyambo R, Evans CA, Coleman RE, Holen I. Osteopro tegerin (OPG) produced by bone marrow stromal cells protects breast cancer cells from TRAIL-induced apoptosis. Breast Cancer Res Treat. 2004;86:269–279. doi: 10.1023/b:brea.0000036900.48763.b3. [DOI] [PubMed] [Google Scholar]
- 123.Shipman CM, Croucher PI. Osteoprotegerin is a soluble decoy receptor for tumor necrosis factor-related apoptosis-inducing ligand/Apo2 ligand and can function as a paracrine survival factor for human myeloma cells. Cancer Res. 2003;63:912–916. [PubMed] [Google Scholar]
- 124.Griffith TS, Wiley SR, Kubin MZ, Sedger LM, Maliszewski CR, Fanger NA. Monocyte-mediated tumoricidal activity via the tumor necrosis factor-related cytokine, TRAIL. J Exp Med. 1999;189:1343–1354. doi: 10.1084/jem.189.8.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Takeda K, Smyth MJ, Cretney E, Hayakawa Y, Kayagaki N, Yagita H, Okumura K. Critical role for tumour necrosis factor-related apoptosis- inducing ligand in immune surveillance against tumour development. J Exp Med. 2002;195:161–169. doi: 10.1084/jem.20011171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Vitovski JS, Phillips JR, Sayers, Croucher PI. Investigating the interaction between osteoprotegerin and RANKL or TRAIL: evidence for a pivotal role for osteoprotegerin in regulating two distinct pathways. J Biol Chem. 2007;282:31601–31609. doi: 10.1074/jbc.M706078200. [DOI] [PubMed] [Google Scholar]
- 127.Holen I, Cross SS, Neville Webb H, Cross NA, Balasubramanian SP, Croucher PI, Evans CA, Lippitt JM, Coleman RE, Eaton CL. Osteoprotegerin (OPG) expression by breast cancer cells in vitro and breast tumours in vivo: a role in tumour cell survival? Breast Cancer Res Treat. 2005;92:207–215. doi: 10.1007/s10549-005-2419-8. [DOI] [PubMed] [Google Scholar]
- 128.Nyambo R, Cross NA, Lippitt JM, Holen I, Bryden G, Hamdy FC, Eaton CL. Human bone marrow stromal cells protect prostate cancer cells from TRAIL-induced apoptosis. J Bone Min Res. 2004;10:269–279. doi: 10.1359/JBMR.040703. [DOI] [PubMed] [Google Scholar]
- 129.Heath DJ, Vanderkerken K, Cheng X, Gallagher O, Prideaux M, Murali R, Croucher PI. An osteoprotegerin-like peptidomimetic inhibits osteoclastic bone resorption and osteolytic bone disease in myeloma. Cancer Res. 2007;67:202–208. doi: 10.1158/0008-5472.CAN-06-1287. [DOI] [PubMed] [Google Scholar]
- 130.Barillé Nion S, Barlogie B, Bataille R, Bergsagel PL, Epstein J, Fenton RG, Jacobson J, Kuehl WM, Shaughnessy J, Tricot G. Advances in biology and therapy of multiple myeloma. Hematology Am Soc Hematol Educ. program;2003:248–278. [PubMed] [Google Scholar]
- 131.Lamoureux F, Richard P, Wittrant Y, Battaglia S, Pilet P, Trichet V, Blanchard F, Gouin F, Pitard B, Heymann D, Redini F. Therapeutic relevance of osteoprotegerin gene therapy in osteosarcoma: blockade of the vicious cycle between tumor cell proliferation and bone resorption. Cancer Res. 2007;67:7308–7318. doi: 10.1158/0008-5472.CAN-06-4130. [DOI] [PubMed] [Google Scholar]
- 132.Mogi M, Kondo A. The presence of TRAIL-OPG complex in human osteosarcoma and human salivary gland adenocarcinoma. J Immunoassay Immunochem. 2011;32:70–78. doi: 10.1080/15321819.2010.538110. [DOI] [PubMed] [Google Scholar]
- 133.Cross NA, Papageorgiou M, Eaton CL. Bone marrow stromal cells promote growth and survival of prostate cancer cells. Biochem Soc Trans. 2007;35:698–700. doi: 10.1042/BST0350698. [DOI] [PubMed] [Google Scholar]
- 134.Corallini F, Celeghini C, Rimondi E, di Lasio MG, Gonelli A, Secchiero P, Zauli G. TRAIL down-regulates the release of osteoprotegerin (OPG) by primary stromal cells. J Cell Physiol. 2010 doi: 10.1002/jcp.22564. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 135.Nicolin V, Narducci P. Soluble TRAIL could enhance bone destruction acting on RANK-Ligand in oestrogen-independent human breast cancer cell line MDA-MB-231. Acta Histochem. 2008;110:388–396. doi: 10.1016/j.acthis.2008.09.011. [DOI] [PubMed] [Google Scholar]
- 136.Cha SS, Sung BJ, Kim YA, Song YL, Kim HJ, Kim S, Lee MS, Oh BH. Crystal structure of TRAIL-DR5 complex identifies a critical role of the unique frame insertion in conferring recognition specificity. J Biol Chem. 2000;275:31171–31177. doi: 10.1074/jbc.M004414200. [DOI] [PubMed] [Google Scholar]
- 137.Eck MJ, Sprang SR. The structure of tumor necrosis factor-alpha at 2.6 A resolution. Implications for receptor binding. J Biol Chem. 1989;264:17595–17605. doi: 10.2210/pdb1tnf/pdb. [DOI] [PubMed] [Google Scholar]
- 138.Karpusas M, Hsu YM, Wang JH, Thompson J, Lederman S, Chess L, Thomas D. 2 A crystal structure of an extracellular fragment of human CD40 ligand. Structure. 1995;3:1031–1039. doi: 10.1016/s0969-2126(01)00239-8. [DOI] [PubMed] [Google Scholar]