Abstract
MicroRNAs (miRNAs) are small non-coding RNAs that regulate gene expression by binding to complementary sequences in mRNAs encoding downstream target genes. A large variety of cellular processes, including differentiation, development, apoptosis and cell cycle progression, are dependent on miRNA-mediated suppression of gene expression for their regulation. As such, it is unsurprising that these small RNA molecules are associated with signaling networks that are often altered in various diseases, including many blood cancers. One such network is the nuclear factor-κB (NF-κB) pathways that universally stimulate transcription of proteins which generally promote cell survival, inhibit apoptosis, allow cellular growth, induce angiogenesis and generate many pro-inflammatory responses. The NF-κB signalling pathway is often constitutively activated in blood cell cancers including myelodysplastic syndrome (MDS), acute myeloid leukaemia (AML), acute lymphocytic leukaemia (ALL), chronic myeloid leukaemia (CML), chronic lymphocytic leukaemia (CLL), lymphomas and in multiple myeloma (MM). This review focuses on the function of miRNAs that directly target NF-κB signaling cascade. Recent findings that connect this pathway through various miRNA families to human blood cancers are reviewed, and support for using miRNA-based therapy as a novel method to counteract this tumour-promoting signalling event is discussed.
Keywords: Leukemia, NF-κB, microRNA, gene regulation, apoptosis
Introduction
miRNAs are small non-coding RNA molecules that are about 20-25 nucleotides in length and are transcriptional regulators [1]. miRNA can control the expression of genes involved in processes such as development, differentiation, proliferation and apoptosis and play an important role in cancer. Moreover, miRNA can regulate the expression of more than 30% of protein -coding genes and interestingly, more than 50% of miRNA genes are located in cancer-associated genomic regions, suggesting they possess a role in the pathogenesis of human cancers [2, 3].
The first miRNA (lin-4) was discovered in 1993 [4] and since then, hundreds if not thousands of miRNAs have been identified and added to various databases including (microRNA.org and MirBASE.org). Understanding the function of miRNA stems from appreciating their production, which begins with the transcription of primary transcripts called pri-miRNAs by RNA polymerase II [5]. Pri-miRNAs range from hundreds to thousands of nucleotides in length and so must undergo three cleavage steps before their activation to regulate genes [6]. Processing begins in the nucleus using ribonuclease the Drosha and DGCR8 (DiGeorge syndrome critical region 8) complex to form an intermediate hairpin called a pre-miRNA of about 70-100 nucleotides long [7]. The pre-miRNA is transported out of the nucleus by exportin-5 and taken to the cytoplasm to be converted to an 18-25 nucleotide [8], then mature double-stranded miRNA is processed using a ribonuclease named Dicer. Subsequently, the miRNA double-strand is separated to form a mature, active miRNA using an effector complex called RNA-induced silencing complex (RISC)[9]. Following the formation of this mature miRNA, the specific complementary region of the 3’ untranslated region (UTR) of a messenger RNA (mRNA) can be targeted [10]. Upon binding of the mRNA and miRNA, the RISC complex induces degradation of the double-stranded mRNA [10]. Another possible mechanism that miRNAs use is the blocking of protein translation processes, therefore resulting in the elimination of that specific protein that is being expressed [11].
Examination of tumour-specific miRNA expression profiles has revealed that miRNAs could be master regulators of many aspects of tumour biology [1]. Many studies have shown that miRNA themselves can function as tumour suppressor genes or oncogenes, where gene repression or overexpression can have a diagnostic and prognostic significance [1, 12]. The tumour suppressor miR-98, for example, was identified by Johnson et al [13] to be reduced in some tumours resulting in amplified Ras oncogene activation and thereby aberrantly increasing the proliferation of cells [13]. In contrast, upregulation of oncogenic miR-17 by cMyc was found to affect cell cycle control mechanisms and to disrupt the apoptotic regulator E2F1 [14]. Following these key discoveries in 2005, many correlations between miRNA expression, with Weinberg's six hallmarks of cancer having been established [15].
Despite the surge in epigenetic research in the last decade, the roles of miRNA in many human cancers such as leukaemia and lymphoma have not been clearly defined. Manipulation of miRNA regulation could be a novel approach to achieving an understanding for the regulatory mechanisms of miRNA in cancer. Changes in the expression of a number of transcription factors have been associated with various blood cancers [16, 17], with increasing evidence supporting a role for the regulation of transcription factors by miRNAs [10].
This review reports data published about the miRNAs which directly target the NF-κB pathway in human cancer with particular emphasis on leukaemia, myeloma and lymphoma. It also describes the molecular mechanisms underlying miRNA and NF-κB dysregulation in these haematological malignancies.
NF-κB and human cancer
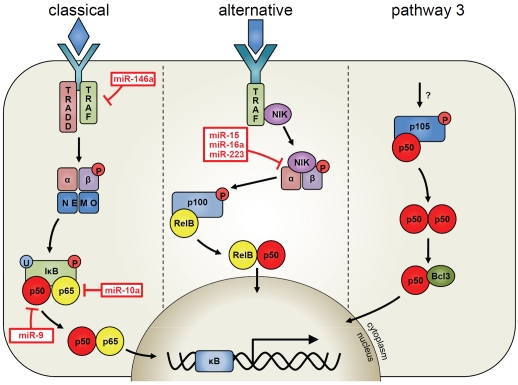
NF-κB proteins are dimeric transcription factors that induce the expression of genes involved in cell survival, proliferation and in immune responses [18]. NF-κB is activated by many stimuli, including pro-inflammatory cytokines such as tumour necrosis factor-α (TNF), interleukin-1β (IL-1β) and epidermal growth factor (EGF) [19, 20]. There are two main pathways involved in NF-κB signalling, namely the classical pathway (which is mostly involved in the activation of innate immunity) and the alternative pathway (required for the development of lymphoid tissue)[18-21]. NF-κB is composed of five sub-units named p65 (RelA), RelB, cRel, p50 and p52 (Figure 1). In unstimulated cells, NF-κB is located in the cytoplasm whereby its nuclear localisation sequences are masked, enabling NF-κB to be retained in an inactive state [18]. The classical NF-κB pathway is initiated by various stimuli including TNF which through its receptors can activate TNF receptor-associated factor (TRAF) adaptors. Following this, activation of the I-κB kinase (IKK) kinase complex causes phosphorylation of IκB proteins and triggers their proteosomal degradation [22]. In turn, the NF-κB subunits translocate from the cytoplasm to the nucleus and bind to the κB complex as dimers, including p50/p65 and p65/cRel. This enables transcription of a plethora of genes that drive inflammation as well as regulating apoptosis (eg TNF, survivin and FLIP) [23, 24]. The classical NF-κB pathway is constitutively active in many blood cancers, including myelodysplastic syndrome (MDS), acute myeloid leukaemia (AML), acute lymphocytic leukaemia (ALL), chronic myeloid leukaemia (CML), chronic lymphocytic leukaemia (CLL), lymphomas and in multiple myeloma (MM)[16, 25, 26]. Constitutively active NF-κB results in the deregulated expression of NF-κB controlled genes and can have advantageous survival effects for the cancer cell. For example, overexpression of antiapoptotic genes such as surviving, FLIP and IAPs can enhance the cells ability to resist cytotoxic chemotherapy [17, 20, 27].
Figure 1.
Schematic representation of the NF-κB pathways that are regulated by miRNA in human blood cancer.
The overexpression of NF-κB and its antiapoptotic cytoprotective effect suggests that it might be a useful therapeutic target for the treatment of haematologic malignancies. Several drugs effective for the treatment of MM, including proteasome inhibitors, thalidomide, lenalidomide and arsenic trioxide, block NF-κB activation [28-32]. New agents with NF-κB inhibitory activity enhance the anti-MM effects of conventional chemotherapeutic agents and reduce different side-effects. Triptolide (diterpenoid triepoxyde), a purified component of a traditional Chinese medicine, extracted from a shrub-like vine named Trypterygium wilfordii Hook F (TWHF) inhibits transcriptional activation of NF-κB and downregulates the expression of various NF-κB-regulated genes [33]. Triptolide induces apoptosis of MM cells and effectively inhibits cell growth of MM cells. NF-κB activation can be also inhibited by IKKβ-selective inhibitors, PS-1145 dihydrochloride, MLN120B (Millennium Pharmaceuticals)[30, 34] and BMS-345541 (Bristol-Myers Squibb)[35]. LC-1 the dimethylamino-parthenolide derivative demonstrated significant cytotoxicity to AML blasts targeting NF-κB [36]. Taken together these data suggest a multidrug approach to target NF-κB in human cancers, however with the knowledge that we need a more complete understanding of how miRNA targets NF-κB, we are still a long way from effectively manipulating NF-κB pathways in human disease.
miRNA and human blood cancers
In 2004 Chen and colleagues first established the connection between miRNAs and haematopoiesis regulation, determining that individual haematopoietic cell types differentially expressed miRNAs, given that the same miRNAs were not always expressed in all lineages [37]. Their findings suggested that the specific miRNAs are induced during lineage differentiation and could influence haematopoietic lineage differentiation in mice, although differences in the expression pattern of the same miRNAs in humans have been reported [38-40]. Moreover, mature miRNAs were found to regulate haematopoietic differentiation-associated mRNA on CD34+ cells, and especially miR-155 represented an inhibitor of haematopoietic stem progenitor differentiation [41]. It has also been reported that members of the miR-30 family by targeting the transcription factor PRDM1, regulate the differentiation of lymphocytes to plasma cells [42, 43]. More than the deregulation of miRNAs, haematopoietic deletion of AGO2 or of Dicer resulted in disruption of erythropoiesis, with severe anemia, splenomegaly, and maturation arrest of erythroid precursors. Aberrant miRNA expression profiles have been reported in almost all types of haematological malignancies, including various types of NHL, Hodgkin lymphoma, CLL, AML, APL, ALL and MM [44-48].
The potential use of miRNAs as prognostic markers in clinical practice has already been demonstrated, as expression levels of miRNAs could predict the time to first treatment in CLL patients, were associated with mutations of established molecular prognostic factors [49], and were also associated with overall survival in patients with hepatocellular carcinoma, pancreatic cancer, colon adenocarcinoma, lung cancer, esophageal cancer, and melanoma. Moreover, miRNAs have been evaluated in the context of chemosensitivity to assess the individual chemoresponse in both in vitro and in vivo models [43, 50]. The measurement of miRNAs levels in plasma or serum has rendered them useful in the diagnosis of solid malignancies such colorectal cancer, lung, prostate, and kidney cancer. However, it has not been elucidated whether miRNA circulating levels are tumour-created or represent a systemic response, and it is not clear yet which is the best specimen among serum, plasma, or peripheral blood mononuclear cells, used for the miRNA signature detection. The important functions of miRNAs in cancer make them attractive therapeutic targets, therefore efforts should be made to identify which miRNAs could be used to achieve clinical benefits against cancer [51].
There is convincing evidence for a major role of miRNAs in cancer. This connection was first suggested in 2002 by Calin et al [52], with the discovery that miR-15 and miR-16 were located on chromosome 13q14, a region frequently deleted in CLL. Upon examining the expression levels of these miRNAs, miR-15 and miR-16 were reduced or eliminated in 68% of all CLL cases tested [52]. They also noted that the 13q14 deletion was frequently the only genetic abnormality in patients and thus the deletion of miR-15/16 may be a direct cause of CLL. Upon examination of genomic locations of miRNAs, they reported that many miRNA-coding regions are located in fragile regions of the genome that are frequently amplified or deleted in many cancers, arguing that gain or loss of miRNAs were selected for in cancerous cells and underlie important tumourigenic steps [53].
Global expression profiling revealed alterations of miRNA expression patterns first in CLL [2] and then in other malignancies [54]. These studies reviewed by Munker et al [54] showed that the expression of several miRNAs (miR-17-5p, miR-20a, miR-21, miR-92, miR-106a and miR-155) was increased in the majority of tumour types, arguing that these may be common oncogenic miRNAs. It was also noted in these studies that miRNA expression patterns could distinguish tumours and tissue types, indicating that miRNA expression levels may be useful biomarkers for cancer. Such miRNA expression patterns were then found to be associated with poor prognosis of CLL and lung cancer, offering further demonstration of such potential. Subsequent mechanistic studies demonstrated that alteration of specific miRNAs could affect cell proliferation, apoptosis, tumour growth and angiogenesis in mouse models. Altogether, the evidence is convincing that alterations of miRNAs occur during and contribute towards leukaemogenesis.
miRNA that modulate NF-κB signalling
The role of miRNA in regulating the NF-κB pathway is not fully appreciated especially in blood cancers, particularly since a large proportion of blood cancers have constitutively active NF-κB. This section will hopefully allow the reader to digest the influence of miRNA on the NF-κB pathway. Figure 1 shows the miRNAs that directly target specific elements in one of the three NF-κB pathways (from published data only). Table 1 lists and references the miRNA involved in regulating NF-κB pathways in human blood cancers. Moreover, we also describe the influence of NF-κB on miRNA expression.
Table 1.
Key miRNA in regulating NF-κB pathways in human blood cancer.
| miR-9 | NF-κB1 | Ovarian cancer | Down-regulated in human ovarian cancer correlating with high levels of NF-κB1 [62]. |
|---|---|---|---|
| miR-10a | MAP3K7 βTRC | AML | Over-expressed in AML and down-regulated in CML [59] [60]. |
| CML | Targets both MAP3K7 and βTRC, key regulators of IκBα [61]. | ||
| miR-15a/-16 | Bcl2 IKKα | CLL | Decreased during monocyte-macrophage differentiation, correlating with an increase in IKKα, p52 and a stabilisation of NIK [45]. |
| Deleted in 68% of CLL cases [52]. | |||
| miR-146a | TRAF6 | Myeloid sarcoma Lymphoma | Expression is NF-κB dependent and feeds back to inhibit NF-κB by targeting an adaptor protein TRAF6 [55]. |
| Adult T-cell leukaemia | Loss drives development of myeloid malignancies [56]. | ||
| Over-expression enhances growth of T-cells [58]. | |||
| miR-155 | TP53INP1 | CLL | Can induce hyperproliferation of B cells [39]. |
| AML | Over-expression correlates with repression of pro-apoptotic tumour p53-induced nuclear protein 1[40]. | ||
| CML | |||
| miR-223 | NFI-A | Ovarian cancer | Co-regulated with miR-9 in ovarian cancer [72]. |
| IKKα | Decreased during monocyte-macrophage differentiation, correlating with an increase in IKKα [45]. | ||
To begin this section we will start with probably the most well known miRNA, miR-146a. Mir-146a is a member of the miR-146 miRNA family, consisting of two evolutionary conserved miRNA genes: miR-146a and miR-146b. In people, these loci are located on separate chromosomes, in quite unrelated sequence contexts, but differ in their mature sequence only by 2 nucleotides at the 3’ end. Initially, it was shown that both genes respond to lipopolysaccharide in human monocytes, but only miR-146a is processed to a mature form, with induction of expression of miR-146a an NF-κB dependent process [55]. Based on further work miR-146a was shown to act as a negative feedback regulator of the NF-κB by targeting two adapter protein, TRAF6 (TNF receptor-associated factor 6), which is crucial for NF-κB signalling [55]. Also, Zhao et al have recently shown that knock-out of miR-146a gene in mice leads to histologically and immunophenotypically defined myeloid sarcomas and some lymphomas [56]. In adult T-cell leukaemia (which is an aggressive and fatal CD4+ T-cell malignancy) the human T-cell leukaemia virus type 1 (HTLV-1) which is the causative agent of this disease, up regulates miR-146a [57]. In another study, HTLV-1 induced Tax protein to up-regulate miR-146a expression in a NF-κB-dependent manner which results in the inhibition of miR-146a target genes [58]. Inhibition of miR-146a function by an anti-miRNA inhibitor reduced the proliferation of HTLV-1-infected T-cell lines but not that of uninfected T-cell lines. Moreover, overexpression of miR-146a enhanced the growth of an HTLV-1-infected T-cell line. These findings suggest that miR-146a is a potentially suitable therapeutic target of adult T-cell leukaemia.
miR-155 is another oncogenic miRNA [124] that is regulated in a NF-κB dependent manner and associated with a number of different blood cancers including CLL [39], AML and CML [40, 44]. Increased miR-155 expression can also be found in the bone marrow of leukaemic patients and overexpression of miR-155 in mouse models causes hyperproliferation of B-cells, a common hallmark of leukaemia and lymphoma [102]. Over-expression of miR-155 also causes the repression of tumour p53-induced nuclear protein 1, which is a pro-apoptotic gene downstream of p53 signalling [129]. The suppression of tumour p53-induced nuclear protein 1 is a likely mechanism for pro-tumourigenic functions of miR-155 and a possible mediator of inflammation-induced carcinogenesis.
Another very interesting miRNA is miR-10a, which has been found to be overexpressed in association with NPM1 mutations and MDM4 downregulation in intermediate-risk AML [44, 59]. Moreover, mir-10a is also down-regulated in 71% of newly diagnosed CML patients [60]. In a separate study by Fang et al [61], it was shown that phosphorylation of IκBα (a prerequisite for IκBα proteolysis and NF-κB activation) was significantly up-regulated in miR-10a knock -down cells and was accompanied by increased nuclear expression of NF-κB p65. Conversely, knock-in of miR-10a (a conservative 25-fold increase) inhibited the basal expression of VCAM-1 and E-selectin. Two key regulators of IκBα degradation mitogen-activated kinase kinase kinase 7 (MAP3K7; TAK1) and beta-transducin repeat-containing gene (betaTRC) contain a highly conserved miR-10a binding site in the 3’ UTR. Both molecules were up-regulated by miR-10a knock-down and suppressed by miR-10a knock-in, and evidence of direct miR-10a binding to the 3’ UTR was demonstrated by luciferase assay. Taken together these experiments demonstrate a direct link between mir-10a and NF-κB regulation which could be influential in leukaemogenesis [61].
miR-9 has emerged as a regulator of NF-κB in another study that has shown that miR-9 is downregulated in human ovarian cancer relative to normal ovary, and overexpression of miR-9 suppresses cell growth in vitro. Furthermore, the 3'-UTR of p50 NF-κB1 mRNA is found to be regulated directly by miR-9, demonstrating that NF-κB1 is a functionally important target of miR-9 in ovarian cancer cells. When miR-9 is over-expressed in ovarian cancer cells, the mRNA and protein levels of NF-κB1 are both suppressed, whereas inhibition of miR-9 results in an increase in NF-κB1 expression levels. Ovarian cancer tissues display significantly lower expression of miR-9 and a higher level of NF-κB1 compared with normal tissues, indicating that regulation of NF-κB1 by miR-9 is an important mechanism for miR-9 to inhibit ovarian cancer cell proliferative processes.
Dergulation of miR-15a and miR-16 in human CLL have been shown in the majority of patients as highlighted previously [39]. In another study by Hanlon et al [45] miR-15a and miR-16 were decreased considerably during human monocyte-macrophage differentiation, and expression of the miRNAs miR-223, miR-15a and miR-16, which led to higher expression of the serinethreonine kinase IKKα in macrophages [45]. In macrophages, higher IKKα expression in conjunction with stabilization of the NF-κB-inducing kinase, NIK, induced larger amounts of p52 NF-κB2. Moreover, with the knowledge that p52 and bcl-3 genes being involved in chromosomal translocations described in chronic and myeloid leukaemias [62], this is a major finding that links both NF-κB regulation and miRNA in the tumourigenic nature of these types of leukaemia.
The final part of this section examines the capacity for virus-induced miRNA manipulation of the NF-κB pathway. In this section we have previously touched on the role of HTLV-1 in upregulating miR-146a and its role in causing adult T-cell leukaemia. Another example of viruses regulating NF-κB is published by Lei et al [63] which showed that in Kaposi's sarcoma-associated herpesvirus (KSHV or HHV8), deletion of a miRNA cluster from the KSHV genome causes reduced NF-κB activity [63]. Down-regulation of NF-κB activity was due to binding of the viral miRNA (miR-K1) to the 3'UTR of the IκBα inhibitor, therefore silencing its expression. Moreover with the knowledge that HSHV is known to be associated with different types of lymphoproliferative disorders [64], it is high likely that miR-K1 could be the cause of high nuclear NF-κB levels in the majority of infected KSHV-associated blood cancers. The role of Epstein-Barr virus (EBV) in regulating miRNA which targets or regulates NF-κB should also be mentioned here. It has been shown over the past few years that EBV-encoded latent membrane protein 1 (LMP1) is a functional homologue of the TNF receptor family and contributes substantially to the oncogenic potential of EBV by inducing a number of miRNAs through its capacity to activate the NF-KB pathway including miR-155 and miR-146a [65, 66]. Taken together with the plethora of publications regarding the involvement of EBV in pathogenesis of human blood cancers, it is inevitable that this virus can induce miRNAs which regulate the role of NF-κB, ultimately leading to the oncogenic and chemoresistant nature of many of those associated cancers.
miRNA-targeted therapy
The discovery of small noncoding RNA molecules that repress translation has provided the research community with an opportunity to control the expression of specific genes. Small-molecule inhibitors and monoclonal antibodies have led to many successful therapies in cancer [67], however many cancer targets are difficult to inhibit and some inhibitors have many off-target side-effects and as such, possess undesirable toxicity [68]. It is possible that future cancer therapeutics could use small complementary miRNA sequences known as anti-miRs or antagomiRs to inhibit oncogenic miRNA activity, while miRNA mimics themselves could enable tumour suppressor gene function to be restored in a tumour cell [69]. The potential use of miRNA mimics and anti-miRs have also been associated with many treatment advances in diverse fields of disease including cardiac disease and allergic airway diseases [70, 71]. The most promising approach proposed is to employ miRNA mimics, which can restore miRNA function that is lost within a disease. This approach uses synthetic oligonucleotides which are identical in sequence to endogenous miRNAs, and replicate their response. The second approach is to employ anti-miRs which repress rather than mimic responses of endogenous miRNAs; towards which means several approaches have been employed including: antisense oligonucleotides; miR-sponges and sponge constructs; synthetic oligonucleotide miR-masks; as well as potential small molecule inhibitors [70, 71]. The limiting factor in the use of either anti-miRs or miRNA mimics is the delivery of these agents into cells. The limiting factor in their use presently is the delivery of these agents into cells. However, their future pharmacological use is enormous and their potential to treat a variety of human cancers (not just blood cancers) should be planned.
Concluding remarks
During the past decade, much has been uncovered regarding the role of NF-κB in supporting tumourigenesis in various cancers. It is now also known that miRNAs target many genes that affect the activity of the NF-κB pathways, however, after reviewing the role of miRNA in regulating specific NF-κB subunits or kinases, we have shown that less is known about this process (Figure 1). This suggests that there is still a huge amount to discover regarding the impact of miRNA on NF-κB signalling and the system's usefulness in battling cancer.
Acknowledgments
The authors thank the Association for International Cancer Research, Leukaemia and Lymphoma Research, The Big C and the University of East Anglia for support.
Conflict of interest
The authors declare they have no conflicts of interest.
References
- 1.Drakaki A, Iliopoulos D. MicroRNA Gene Networks in Oncogenesis. Curr Genomics. 2009;10:35–41. doi: 10.2174/138920209787581299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Calin GA, Sevignani C, Dumitru CD, Hyslop T, Noch E, Yendamuri S, Shimizu M, Rattan S, Bullrich F, Negrini M, Croce CM. Human microRNA genes are frequently located at fragile sites and genomic regions involved in cancers. Proc Natl Acad Sci USA. 2004;101:2999–3004. doi: 10.1073/pnas.0307323101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carthew RW. Gene regulation by microRNAs. Curr Opin Genet Dev. 2006;16:203–8. doi: 10.1016/j.gde.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 4.Lee RC, Feinbaum RL, Ambros V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell. 1993;75:843–54. doi: 10.1016/0092-8674(93)90529-y. [DOI] [PubMed] [Google Scholar]
- 5.Rodriguez A, Griffiths-Jones S, Ashurst JL, Bradley A. Identification of mammalian microRNA host genes and transcription units. Genome Res. 2004;14:1902–10. doi: 10.1101/gr.2722704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rushworth SA. Targeting the oncogenic role of miRNA in human cancer using naturally occurring compounds. Br J Pharmacol. 2011;162:346–8. doi: 10.1111/j.1476-5381.2010.01075.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Landthaler M, Yalcin A, Tuschl T. The human DiGeorge syndrome critical region gene 8 and Its D. melanogaster homolog are required for miRNA biogenesis. Curr Biol. 2004;14:2162–7. doi: 10.1016/j.cub.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 8.Bohnsack MT, Czaplinski K, Gorlich D. Exportin 5 is a RanGTP-dependent dsRNA-binding protein that mediates nuclear export of pre-miRNAs. RNA. 2004;10:185–91. doi: 10.1261/rna.5167604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hutvagner G, Zamore PD. A microRNA in a multiple-turnover RNAi enzyme complex. Science. 2002;297:2056–60. doi: 10.1126/science.1073827. [DOI] [PubMed] [Google Scholar]
- 10.Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215–33. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sangokoya C, Telen MJ, Chi J-T. MicroRNA miR -144 modulates oxidative stress tolerance and associates with anemia severity in sickle cell disease. Blood. 2009;116:4338–48. doi: 10.1182/blood-2009-04-214817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dalmay T, Edwards DR. MicroRNAs and the hallmarks of cancer. Oncogene. 2006;25:6170–5. doi: 10.1038/sj.onc.1209911. [DOI] [PubMed] [Google Scholar]
- 13.Johnson CD, Esquela-Kerscher A, Stefani G, Byrom M, Kelnar K, Ovcharenko D, Wilson M, Wang X, Shelton J, Shingara J, Chin L, Brown D, Slack FJ. The let-7 microRNA represses cell proliferation pathways in human cells. Cancer Res. 2007;67:7713–22. doi: 10.1158/0008-5472.CAN-07-1083. [DOI] [PubMed] [Google Scholar]
- 14.O'Donnell KA, Wentzel EA, Zeller KI, Dang CV, Mendell JT. c-Myc-regulated microRNAs modulate E2F1 expression. Nature. 2005;435:839–43. doi: 10.1038/nature03677. [DOI] [PubMed] [Google Scholar]
- 15.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 16.Frelin C, Imbert V, Griessinger E, Peyron AC, Rochet N, Philip P, Dageville C, Sirvent A, Hummelsberger M, Bérard E, Dreano M, Sirvent N, Peyron JF. Targeting NF-κB activation via pharmacologic inhibition of IKK2- induced apoptosis of human acute myeloid leukemia cells. Blood. 2005;105:804–11. doi: 10.1182/blood-2004-04-1463. [DOI] [PubMed] [Google Scholar]
- 17.Kamihira S, Yamada Y, Hirakata Y, Tomonaga M, Sugahara K, Hayashi T, Dateki N, Harasawa H, Nakayama K. Aberrant Expression of Caspase Cascade Regulatory Genes in Adult T-Cell Leukaemia: Survivin Is an Important Determinant for Prognosis. Brit J Haematol. 2001;114:63–9. doi: 10.1046/j.1365-2141.2001.02902.x. [DOI] [PubMed] [Google Scholar]
- 18.Bonizzi G, Karin M. The two NF-κB activation pathways and their role in innate and adaptive immunity. Trends Immunol. 2004;25:280–8. doi: 10.1016/j.it.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 19.Baud V, Karin M. Signal Transduction by Tumor Necrosis Factor and Its Relatives. Trends in Cell Biology. 2001;11:372–7. doi: 10.1016/s0962-8924(01)02064-5. [DOI] [PubMed] [Google Scholar]
- 20.Rae C, Langa S, Tucker SJ, MacEwan DJ. Elevated NF-κB responses and FLIP levels in leukemic but not normal lymphocytes: reduction by salicylate allows TNF-induced apoptosis. Proc Natl Acad Sci USA. 2007;104:12790–5. doi: 10.1073/pnas.0701437104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kreuz S, Siegmund D, Rumpf JJ, Samel D, Leverkus M, Janssen O, Häcker G, Dittrich-Breiholz O, Kracht M, Scheurich P, Wajant H. NF-κB activation by Fas is mediated through FADD, caspase-8, and RIP and is inhibited by FLIP. J Cell Biol. 2004;166:369–80. doi: 10.1083/jcb.200401036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Skaug B, Jiang X, Chen ZJ. The role of ubiquitin in NF-κB regulatory pathways. Annu Rev Biochem. 2009;78:769–96. doi: 10.1146/annurev.biochem.78.070907.102750. [DOI] [PubMed] [Google Scholar]
- 23.Rushworth SA, Taylor A, Langa S, MacEwan DJ. TNF signaling gets FLIPped off - TNF-induced regulation of FLIP. Cell Cycle. 2008;7:194–9. doi: 10.4161/cc.7.2.5159. [DOI] [PubMed] [Google Scholar]
- 24.Zaitseva L, Rushworth SA, MacEwan DJ. Silencing FLIPL modifies TNF-induced apoptotic protein expression. Cell Cycle. 2011;10:1067–72. doi: 10.4161/cc.10.7.15247. [DOI] [PubMed] [Google Scholar]
- 25.Guzman ML, Neering SJ, Upchurch D, Grimes B, Howard DS, Rizzieri DA, Luger SM, Jordan CT. Nuclear factor-κB is constitutively activated in primitive human acute myelogenous leukemia cells. Blood. 2001;98:2301–7. doi: 10.1182/blood.v98.8.2301. [DOI] [PubMed] [Google Scholar]
- 26.Rushworth SA, Bowles KM, Raninga P, MacEwan DJ. NF-κB-inhibited acute myeloid leukemia cells are rescued from apoptosis by heme oxygenase-1 induction. Cancer Res. 2010;70:2973–83. doi: 10.1158/0008-5472.CAN-09-3407. [DOI] [PubMed] [Google Scholar]
- 27.Dai Y, Chen S, Wang L, Pei XY, Kramer LB, Dent P, Grant S. Bortezomib interacts synergistically with belinostat in human acute myeloid leukaemia and acute lymphoblastic leukaemia cells in association with perturbation in NF-κB and Bim. Br J Haematol. 2011;153:222–235. doi: 10.1111/j.1365-2141.2011.08591.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rushworth SA, Bowles KM, MacEwan DJ. High basal nuclear levels of Nrf2 in acute myeloid leukemia reduces sensitivity to proteasome inhibitors. Cancer Res. 2011;71:1999–2009. doi: 10.1158/0008-5472.CAN-10-3018. [DOI] [PubMed] [Google Scholar]
- 29.Jackson G, Einsele H, Moreau P, Miguel JS. Bortezomib, a novel proteasome inhibitor, in the treatment of hematologic malignancies. Cancer Treatment Rev. 2005;31:591–602. doi: 10.1016/j.ctrv.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 30.Hideshima T, Chauhan D, Richardson P, Mitsiades C, Mitsiades N, Hayashi T, Munshi N, Dang L, Castro A, Palombella V, Adams J, Anderson KC. NF-κB as a therapeutic target in multiple myeloma. J Biol Chem. 2002;277:16639–47. doi: 10.1074/jbc.M200360200. [DOI] [PubMed] [Google Scholar]
- 31.Baer MR, Gojo I. Novel agents for the treatment of acute myeloid leukemia in the older patient. J Natl Compr Cancer Netw. 2011;9:331–5. doi: 10.6004/jnccn.2011.0029. [DOI] [PubMed] [Google Scholar]
- 32.Estrov Z, Manna SK, Harris D, Van Q, Estey EH, Kantarjian HM, Talpaz M, Aggarwal BB. Phenylarsine Oxide Blocks Interleukin-1 Beta-Induced Activation of the Nuclear Transcription Factor NF-κB, Inhibits Proliferation, and Induces Apoptosis of Acute Myelogenous Leukemia Cells. Blood. 1999;94:2844–53. [PubMed] [Google Scholar]
- 33.Xu B, Guo X, Mathew S, Armesilla AL, Cassidy J, Darling JL, Wang W. Triptolide simultaneously induces reactive oxygen species, inhibits NF-κB activity and sensitizes 5-fluorouracil in colorectal cancer cell lines. Cancer Lett. 2010;291:200–8. doi: 10.1016/j.canlet.2009.10.013. [DOI] [PubMed] [Google Scholar]
- 34.Hideshima T, Ikeda H, Chauhan D, Okawa Y, Raje N, Podar K, Mitsiades C, Munshi NC, Richardson PG, Carrasco RD, Anderson KC. Bortezomib induces canonical nuclear factor-κB activation in multiple myeloma cells. Blood. 2009;114:1046–52. doi: 10.1182/blood-2009-01-199604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jani TS, DeVecchio J, Mazumdar T, Agyeman A, Houghton JA. Inhibition of NF-κB signaling by quinacrine is cytotoxic to human colon carcinoma cell lines and is synergistic in combination with tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) or oxaliplatin. J Biol Chem. 2010;285:19162–72. doi: 10.1074/jbc.M109.091645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jenkins C, Hewamana S, Gilkes A, Neelakantan S, Crooks P, Mills K, Pepper C, Burnett A. Nuclear factor-κB as a potential therapeutic target for the novel cytotoxic agent LC-1 in acute myeloid leukaemia. Br J Haematol. 2008;143:661–71. doi: 10.1111/j.1365-2141.2008.07392.x. [DOI] [PubMed] [Google Scholar]
- 37.Chen CZ, Li L, Lodish HF, Bartel DP. MicroRNAs modulate hematopoietic lineage differentiation. Science. 2004;303:83–6. doi: 10.1126/science.1091903. [DOI] [PubMed] [Google Scholar]
- 38.Marcucci G, Mrózek K, Radmacher MD, Garzon R, Bloomfield CD. The prognostic and functional role of microRNAs in acute myeloid leukemia. Blood. 2011;117:1121–9. doi: 10.1182/blood-2010-09-191312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li S, Moffett HF, Lu J, Werner L, Zhang H, Ritz J, Neuberg D, Wucherpfennig KW, Brown JR, Novina CD. MicroRNA expression profiling identifies activated B cell status in chronic lymphocytic leukemia cells. PLoS One. 2011;6:e16956. doi: 10.1371/journal.pone.0016956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Machova Polakova K, Lopotova T, Klamova H, Burda P, Trněný M, Stopka T, Moravcová J. Expression patterns of microRNAs associated with CML phases and their disease related targets. Mol Cancer. 2011;10:41. doi: 10.1186/1476-4598-10-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Donahue RE, Jin P, Bonifacino AC, Metzger ME, Ren J, Wang E, Stroncek DF. Plerixafor (AMD3100) and granulocyte colonystimulating factor (G-CSF) mobilize different CD34+ cell populations based on global gene and microRNA expression signatures. Blood. 2009;114:2530–41. doi: 10.1182/blood-2009-04-214403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lin J, Lwin T, Zhao JJ, Tam W, Choi YS, Moscinski LC, Dalton WS, Sotomayor EM, Wright KL, Tao J. Follicular dendritic cell-induced microRNA-mediated upregulation of PRDM1 and downregulation of BCL-6 in non-Hodgkin's B-cell lymphomas. Leukemia. 2011;25:145–52. doi: 10.1038/leu.2010.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang P, Zou F, Zhang X, Li H, Dulak A, Tomko RJ, Jr, Lazo JS, Wang Z, Zhang L, Yu J. microRNA-21 negatively regulates Cdc25A and cell cycle progression in colon cancer cells. Cancer Res. 2009;69:8157–65. doi: 10.1158/0008-5472.CAN-09-1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Garzon R, Garofalo M, Martelli MP, Briesewitz R, Wang L, Fernandez-Cymering C, Volinia S, Liu CG, Schnittger S, Haferlach T, Liso A, Diverio D, Mancini M, Meloni G, Foa R, Martelli MF, Mecucci C, Croce CM, Falini B. Distinctive microRNA signature of acute myeloid leukemia bearing cytoplasmic mutated nucleophosmin. Proc Natl Acad Sci USA. 2008;105:3945–50. doi: 10.1073/pnas.0800135105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hanlon K, Rudin CE, Harries LW. Investigating the targets of MIR-15a and MIR-16-1 in patients with chronic lymphocytic leukemia (CLL) PLoS One. 2009;4:e7169. doi: 10.1371/journal.pone.0007169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mavrakis KJ, Wolfe AL, Oricchio E, Palomero T, de Keersmaecker K, McJunkin K, Zuber J, James T, Khan AA, Leslie CS, Parker JS, Paddison PJ, Tam W, Ferrando A, Wendel HG. Genome-wide RNA-mediated interference screen identifies miR-19 targets in Notch-induced T-cell acute lymphoblastic leukaemia. Nat Cell Biol. 2010;12:372–9. doi: 10.1038/ncb2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nagel S, Venturini L, Przybylski GK, Grabarczyk P, Schmidt CA, Meyer C, Drexler HG, Macleod RA, Scherr M. Activation of miR-17-92 by NK-like homeodomain proteins suppresses apoptosis via reduction of E2F1 in T-cell acute lymphoblastic leukemia. Leuk Lymphoma. 2009;50:101–8. doi: 10.1080/10428190802626632. [DOI] [PubMed] [Google Scholar]
- 48.Schotte D, De Menezes RX, Akbari Moqadam F, Khankahdani LM, Lange-Turenhout E, Chen C, Pieters R, Den Boer ML. MicroRNAs characterize genetic diversity and drug resistance in pediatric acute lymphoblastic leukemia. Haematologica. 2011 doi: 10.3324/haematol.2010.026138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nana-Sinkam SP, Croce CM. MicroRNA in chronic lymphocytic leukemia: transitioning from laboratory-based investigation to clinical application. Cancer Genet Cytogenet. 2010;203:127–33. doi: 10.1016/j.cancergencyto.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 50.Nakajima G, Hayashi K, Xi Y, Kudo K, Uchida K, Takasaki K, Yamamoto M, Ju J. Non-coding MicroRNAs hsa-let-7g and hsa-miR-181b are Associated with Chemoresponse to S-1 in Colon Cancer. Cancer Genomics Proteomics. 2006;3:317–24. [PMC free article] [PubMed] [Google Scholar]
- 51.Kota J, Chivukula RR, O'Donnell KA, Wentzel EA, Montgomery CL, Hwang HW, Chang TC, Vivekanandan P, Torbenson M, Clark KR, Mendell JR, Mendell JT. Therapeutic microRNA delivery suppresses tumorigenesis in a murine liver cancer model. Cell. 2009;137:1005–17. doi: 10.1016/j.cell.2009.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Calin GA, Dumitru CD, Shimizu M, Bichi R, Zupo S, Noch E, Aldler H, Rattan S, Keating M, Rai K, Rassenti L, Kipps T, Negrini M, Bullrich F, Croce CM. Frequent deletions and down-regulation of micro- RNA genes miR15 and miR16 at 13q14 in chronic lymphocytic leukemia. Proc Natl Acad Sci USA. 2002;99:15524–9. doi: 10.1073/pnas.242606799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Starczynowski DT, Morin R, McPherson A, Lam J, Chari R, Wegrzyn J, Kuchenbauer F, Hirst M, Tohyama K, Humphries RK, Lam WL, Marra M, Karsan A. Genome-wide identification of human microRNAs located in leukemia-associated genomic alterations. Blood. 2011;117:595–607. doi: 10.1182/blood-2010-03-277012. [DOI] [PubMed] [Google Scholar]
- 54.Munker R, Calin GA. MicroRNA profiling in cancer. Clin Sci. 2011;121:141–58. doi: 10.1042/CS20110005. [DOI] [PubMed] [Google Scholar]
- 55.Taganov KD, Boldin MP, Chang KJ, Baltimore D. NF-κB-dependent induction of microRNA miR-146, an inhibitor targeted to signaling proteins of innate immune responses. Proc Natl Acad Sci USA. 2006;103:12481–6. doi: 10.1073/pnas.0605298103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhao JL, Rao DS, Boldin MP, Taganov KD, O'Connell RM, Baltimore D. NF-κB dysregulation in microRNA-146a-deficient mice drives the development of myeloid malignancies. Proc Natl Acad Sci USA. 2011;108:9184–9. doi: 10.1073/pnas.1105398108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bellon M, Lepelletier Y, Hermine O, Nicot C. Deregulation of microRNA involved in hematopoiesis and the immune response in HTLV-I adult T-cell leukemia. Blood. 2009;113:4914–7. doi: 10.1182/blood-2008-11-189845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tomita M, Tanaka Y, Mori N. MicroRNA miR-146a is induced by HTLV-1 tax and increases the growth of HTLV-1-infected T-cells. Int J Cancer. 2011 doi: 10.1002/ijc.25115. in press. [DOI] [PubMed] [Google Scholar]
- 59.Ovcharenko D, Stolzel F, Poitz D, Fierro F, Schaich M, Neubauer A, Kelnar K, Davison T, Müller-Tidow C, Thiede C, Bornhäuser M, Ehninger G, Brown D, Illmer T. miR-10a overexpression is associated with NPM1 mutations and MDM4 downregulation in intermediaterisk acute myeloid leukemia. Exp Hematol. 2011;39:1030–1042. doi: 10.1016/j.exphem.2011.07.008. [DOI] [PubMed] [Google Scholar]
- 60.Agirre X, Jimenez-Velasco A, San José-Enériz E, Garate L, Bandrés E, Cordeu L, Aparicio O, Saez B, Navarro G, Vilas-Zornoza A, Pérez-Roger I, García-Foncillas J, Torres A, Heiniger A, Calasanz MJ, Fortes P, Román-Gómez J, Prósper F. Down-regulation of hsa-miR-10a in chronic myeloid leukemia CD34+ cells increases USF2-mediated cell growth. Mol Cancer Res. 2008;6:1830–40. doi: 10.1158/1541-7786.MCR-08-0167. [DOI] [PubMed] [Google Scholar]
- 61.Fang Y, Shi C, Manduchi E, Civelek M, Davies PF. MicroRNA-10a regulation of proinflammatory phenotype in athero-susceptible endothelium in vivo and in vitro. Proc Natl Acad Sci USA. 2010;107:13450–5. doi: 10.1073/pnas.1002120107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Munzert G, Kreitmeier S, Bergmann L. Normal structure of NFKB2, C-REL and BCL-3 gene loci in lymphoproliferative and myeloproliferative disorders. Leukemia Lymphoma. 2000;38:395–400. doi: 10.3109/10428190009087031. [DOI] [PubMed] [Google Scholar]
- 63.Lei X, Bai Z, Ye F, Xie J, Kim CG, Huang Y, Gao SJ. Regulation of NF-κB inhibitor IκBa and viral replication by a KSHV microRNA. Nat Cell Biol. 2010;12:193–9. doi: 10.1038/ncb2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Deloose ST, Smit LA, Pals FT, Kersten MJ, van Noesel CJ, Pals ST. High incidence of Kaposi sarcoma-associated herpesvirus infection in HIV-related solid immunoblastic/plasmablastic diffuse large B-cell lymphoma. Leukemia. 2005;19:851–5. doi: 10.1038/sj.leu.2403709. [DOI] [PubMed] [Google Scholar]
- 65.Gatto G, Rossi A, Rossi D, Kroening S, Bonatti S, Mallardo M. Epstein-Barr virus latent membrane protein 1 trans-activates miR-155 transcription through the NF-κB pathway. Nucleic Acids Res. 2008;36:6608–19. doi: 10.1093/nar/gkn666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cameron JE, Yin Q, Fewell C, Lacey M, McBride J, Wang X, Lin Z, Schaefer BC, Flemington EK. Epstein-Barr virus latent membrane protein 1 induces cellular MicroRNA miR-146a, a modulator of lymphocyte signaling pathways. J Virol. 2008;82:1946–58. doi: 10.1128/JVI.02136-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hochhaus A, Druker B, Sawyers C, Guilhot F, Schiffer CA, Cortes J, Niederwieser DW, Gambacorti-Passerini C, Stone RM, Goldman J, Fischer T, O'Brien SG, Reiffers JJ, Mone M, Krahnke T, Talpaz M, Kantarjian HM. Favorable long-term follow-up results over 6 years for response, survival, and safety with imatinib mesylate therapy in chronic-phase chronic myeloid leukemia after failure of interferonalpha treatment. Blood. 2008;111:1039–43. doi: 10.1182/blood-2007-07-103523. [DOI] [PubMed] [Google Scholar]
- 68.Pecot CV, Calin GA, Coleman RL, Lopez-Berestein G, Sood AK. RNA interference in the clinic: challenges and future directions. Nat Rev Cancer. 2011;11:59–67. doi: 10.1038/nrc2966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Garzon R, Marcucci G, Croce CM. Targeting microRNAs in cancer: rationale, strategies and challenges. Nat Rev Drug Discov. 2010;9:775–89. doi: 10.1038/nrd3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dorn GW., 2nd Therapeutic potential of microRNAs in heart failure. Curr Cardiol Rep. 2010;12:209–15. doi: 10.1007/s11886-010-0096-7. [DOI] [PubMed] [Google Scholar]
- 71.Maes T, Tournoy KG, Joos GF. Gene therapy for allergic airway diseases. Curr Allergy Asthma Rep. 2011;11:163–72. doi: 10.1007/s11882-011-0177-8. [DOI] [PubMed] [Google Scholar]
- 72.Laios A, O'Toole S, Flavin R, Martin C, Kelly L, Ring M, et al. Potential role of miR-9 and miR-223 in recurrent ovarian cancer. Mol Cancer. 2008;7:35. doi: 10.1186/1476-4598-7-35. [DOI] [PMC free article] [PubMed] [Google Scholar]