Abstract
Purpose
To determine the prognostic value of FOXO1, GATA3 and Annexin-1 expression in breast cancer.
Methods
Tissue microarray and individual paraffin tissue slides from 131 patients were used for the study. The association of FOXO1, GATA3 and Annexin-1 expression with clinicopathological features of breast cancer and disease outcome was examined in retrospective samples. Kaplan-Meier survival curves and Cox regression with multivariate analysis were used for assessing the relative risk (RR) and disease-free survival (DFS). The expression of FOXO1, GATA3 and Annexin-1 were determined by immunohistochemistry and the association among the three proteins was analyzed by Logistic regression analysis.
Results
The nuclear expression of FOXO1 was observed in most of the normal breast tissues and 51.3% of the malignant breast tissues. GATA3 and Annexin-1 were expressed at 73% and 24.6% respectively in breast cancer tissues. The expression of FOXO1, GATA3 and Annexin-1 were all inversely correlated with lymph node-positive tumors. Both FOXO1 and Annexin-1 expression were also inversely associated with HER2-overexpressing tumors. FOXO1 expression was significantly associated with both GATA3 and Annexin-1 expression. In addition, Multivariate analyses confirm that only FOXO1 levels independently predict DFS.
Conclusion
FOXO1 expression in breast cancer is regulated by the PI3K/Akt pathway. The expression of FOXO1 is also associated with GATA3 and/or Annexin-1. Restoring or targeting FOXO1 to the cell nucleus in breast cancer tissues may improve response to therapy and disease outcome. Further clinical studies are warranted to test this hypothesis.
Keywords: FOXO1, GATA3, annexin-1, survival, breast cancer
Introduction
Breast cancer survival has improved significantly over the last 30 years [1]; however, it still ranks second among cancer deaths in women. Therapeutic failure and distant metastasis has been a major challenge in the treatment of breast cancer. The expression of receptors status in tumor tissue at the time of diagnosis affects prognosis and treatment options. About 60% to 70% of breast cancers are ER and/or PR positive and those tumor cells grow in response to the estrogen. Additionally, 20% to 25% of breast cancers overexpress HER2 (HER2/neu or ErbB2) receptors and these breast cancers tend to be much more aggressive and fast-growing [2, 3]. For decades, treatment protocols for breast cancer have been reliant on expression of those receptors. Patients with ER and/or PR expressing tumors will receive hormonal therapy, such as tamoxifen, and tumors with HER2 overexpressing will be treated with trastuzumab, in addition to chemotherapy. However, the responsiveness for both trastuzumab and tamoxifen has not been satisfactory. Approximately 30% of ER positive tumors do not respond to the treatment [4] and greater than 70% of patients with HER2 overexpressing tumors show poor response to trastuzumab [5, 6]. In these patients, the overall survival (OS) and the time to relapse are significantly shorter. Furthermore, 10% to 20% of breast cancers testing negative for both ER and PR and HER2 are classified as triple-negative tumors. This type of breast cancer tends to be more aggressive than other types of breast cancer and lacks targets for treatment. These patients are more likely to have poor disease outcome [7, 8]. Therefore, exploring more markers to predict responsiveness of treatment, tumor progression and potential target therapies is becoming more and more important.
Forkhead box protein O1 (FOXO1) is a member of the subfamily of mammalian FOXO (forkhead box O; forkhead members of the O subclass) transcription factors. In mammals this family consists of FOXO1, FOXO3a, FOXO4 and FOXO6 and regulates a variety of biological processes [9]. FOXO is known to be a direct phosphorylation target of the protein kinase Akt [10, 11]. The activation of Akt with subsequent functional loss of FOXO family of transcription factors has been observed to promote tumorigenesis and cancer progression in different cancers; therefore it has become a major target in preventing tumorigenesis [12, 13]. Recently, we reported that increased activity of the PI3K/Akt pathway in HER2 overexpressing breast cancer cells results in down-regulation of FOXO1 and inhibition of trastuzumab induced cell apoptosis [14]. Furthermore, blocking active Akt signaling significantly increased FOXO1 expression and rendered the cells more responsive to trastuzumab induced growth inhibition. Breast cancer patients with HER2 overexpressing tumors are more likely to increase phosphorylation of Akt (pAkt) in their tumor tissue and have poor disease outcome [15]. It is possible that those patients who had increased pAkt in tumors may have deregulated FOXO1.
FOXO1 phosphorylation and inactivation has been reported to be inversely correlated with OS or DFS in patients with prostate cancer [16], ovarian cancer [17] and bladder cancer [18]. The association of FOXO1 expression and disease outcome in breast cancer patients still remains unknown. We have hypothesized that the increased pAkt in breast tumor tissues will result in inactive FOXO1 and that, patients with increased pAkt and decreased FOXO1 in their tumor tissue cells would become resistant to treatment and will have decreased DFS.
Since breast cancer occurs in the luminal epithelium, it is well known that the maintenance of the luminal epithelium cell differentiation requires GATA3, a zinc-finger transcription factor [19]. Recent studies have showed that GA-TA3 is an important luminal marker and intertwined in the ER pathway [20, 21]. GATA3 has been found to be inversely associated with histological grade and HER2 expression in breast cancer [22]. Loss of expression of GATA3 in breast cancer has been associated with aggressive tumors and poor survival rates [22]. GATA3 may also functionally link to activation of Akt [23]. This evidence implies that GATA3 may associate with FOXO1 in predicting response to treatment and survival in breast cancer.
Gene array analysis recently performed in our laboratory showed that Annexin-1, from the Annexin family of Ca2+-dependent phospholipid-binding proteins, was decreased more than 2 fold in HER2 overexpressing trastuzumab resistant cells compared to trastuzumab responsive cells. Differential expression of Annexin-1 has been reported in different human cancers. Annexin-1 has been reported to be decreased in prostate, head and neck as well as esophageal cancers [24-26]; however, it is increased in pancreatic cancer [27]. Reduced expression of Annexin-1 was also seen in both ductal and invasive breast carcinoma [28]. In vitro Annexin-1 has been shown to be involved in proliferation, differentiation and apoptosis in cancer cells [29, 30].
Based on the connections among FOXO1, GATA3, Annexin-1, HER2 and ER/PR, as well as their functional complex in breast cancer biology, we decided not only to examine FOXO1, but also to examine GATA3 and Annexin-1 expression in this study. We used tissue microarray and an immunohistochemical (IHC) approach to determine the clinical significance of FOXO1, GATA3 and Annexin-1 and their association in breast cancer. The cohort of patients used for the current study was the same as the patients used in our previous study to examine the clinical association of pAkt and HER2 [15]. We found that decreasing FOXO1 expression predicts shorter DFS independently in breast cancer. Unlike FOXO1, GATA3 and Annexin-1 do not significantly predicted DFS in this cohort of patients. Interestingly, both GATA3 and Annexin-1 expression were significantly associated with FOXO1 expression.
Materials and methods
Patients
Approval from the institution review board was obtained before initiating the study. As mentioned above, this is the same cohort of patients that had been examined by us for pAkt expression [15]. Briefly, a series of 131 patients (53% African American and 47% Latina) was included in this study. At the time of diagnosis with breast cancer, 48.1% of our subjects were under 50 years of age, and the remaining 51.9% were above 50 years of age. The mean age was 50.7 years old. The patients had undergone breast surgery and were treated with chemotherapy or adjuvant chemotherapy at King/Drew Medical Center from 1999 to 2005. Tumor pathology, histology, treatment protocols and disease outcomes were compiled for patients from our retrospective database.
The ER/PR status and HER2 status reported in patient pathology reports were determined by IHC and provided by Impath, the Cancer Information Company (Los Angeles, CA, USA) [15]. The IHC for HER2 was performed with the DAKO HERCEPTest as described previously [15, 31]. We excluded those cases for which breast cancer was not the primary cancer, had no detailed information on their tumor pathology, or had not completed their treatment protocols for non-clinical reasons. To compare the expression of FOXO1, GATA3 and Annexin-1 in breast cancer tissue and non-cancer tissue, uninvolved tumor tissue from 48 breast cancer patients were also included in the study.
Tissue microarray (TMA) construction and IHC
The breast TMA was constructed similarly to the array previously described [32]. Histology of each specimen on haematoxylin and eosin (H&E) stained sections was carefully reviewed and marked on corresponding individual paraffin block by clinical pathologist. Three 0.6 mm tissue cores were taken from each selected specimen and placed in one receiver arrayed paraffin block. Non-neoplastic breast tissue cores were also included in each tissue microarray block.
IHC was performed on both tissue microarray and paraffin tissue section from individual samples by using antibodies for the detection of FOXO1 (ab39659; Abcam), GATA3 (clone HG3-31, sc-268; Santa Cruze), Annexin-1 (610066; BD Transduction Laboratories). The positive staining was detected using diaminobenzidene (DAB) (Vector Lab, CA) according to the manufacturer's instructions.
Evaluation of IHC results
The scoring method used for FOXO1, GATA3 and Annexin-1 expression was based on semi-quantitative score system [33]. The intensity and percentage of cells with positive staining were both scored. Light microscopy and digital computer software (DigiPro, Labomed, Inc., Culver City, CA, USA) were used to identify the protein staining intensity level and quantify the proportion of positive cells. Each tissue section was evaluated and scored by two clinical pathologists who were blinded to the origin of the tissue. In only 3% to 5% cases did the pathologists disagreed. For those cases, we discussed them with the pathologists and then objectively re-scored them by the two pathologists.
The intensity of positive staining in tissue samples were scored using three pluses (+++) for high intensity staining, two pluses (++) for moderate staining, one plus (+) for low intensity and negative (no staining). The percentage of staining was categorized as: 0=negative; 1=1% to 10% positive tumor cells; 2=11% to 20%; and so on. The maximum score was 10=91% to 100% positive tumor cells. The final quantification of IHC results for both variables (the intensity of the staining and the percentage cells with positive staining) were considered (score=intensity × positive). The range of score was from 0 (negative staining) to 30. The cutoff value for each marker was according to its expression level in non-cancer sections. The expression level for the marker was considered reduced or negative if its score in tumor cells was less than 50 quartiles below that in non-cancer sections.
In this cohort of patients the FOXO1 expression remained mainly in the nucleus and fewer sections showed cytoplasm positive in non tumor cells. However, FOXO1 expression was observed in both the nuclear and cytoplasmic sections of tumor cells. The score system for FOXO1, therefore, has been separated as nuclear positive only and/or nuclear/cytoplasm positive (total FOXO1 expression score). Based on the scoring method mentioned above, the nuclear staining score more than 25 was defined as nuclear FOXO1 positive; and total expression score more than 12 was characterized as FOXO1 positive in nuclear and cytoplasmic regions. GATA3 positive was defined as nuclear score more than 7; and Annexin-1 positive was defined as the score more than 14.
Statistical analysis
All of the analysis was performed with a statistical package, SPSS (SPSS Inc., Chicago, IL, USA). DFS was defined as the time of tumor removal to the development of either local recurrence or distant metastases. Kaplan-Meier survival curves with log-rank testing were used to assess the DFS. The RR of shorter DFS was determined by Cox proportional hazard regression with multivariate analysis. The association of FOXO1, GATA3, Annexin-1 and pAkt was determined by Logistic regression. The personal chi-square test was used to examine the statistically significant differences between FOXO1, GATA3, Annexin-1 expression and other known predictive markers (tumor size, node involvement, staging, ER/PR status and HER2 status). A p-value of less than 0.05 was considered statistically significant. If the frequency in any category was less than 5, Fisher's exact test was compared; 2-side exact p-value less than 0.05 was considerate statistically significant.
Results
FOXO1, GATA3 and Annexin-1 expression in breast cancer and non-cancer tissues
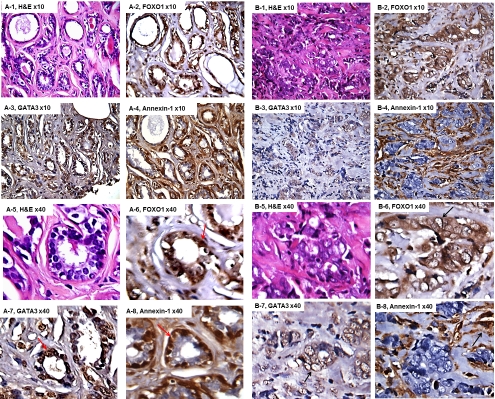
From the total 131 cases, we selected those tissue sections with clear and integer nuclear structure after staining for IHC scoring only. As shown in Table 1, positive nuclear expression of FOXO1 protein was detected in ∼ 91% normal breast tissue and ∼ 51% breast cancer tissues. We observed that about 80% FOXO1 nuclear positive breast cancer tissues also had positive cytoplasm expression. GATA3 was 100% positive in all normal breast tissues and 73.7% positive in breast cancer tissues (Table 1). Our data showed that 83% normal breast tissue myoepithelila cells were positive for Annexin-1; while Annexin-1 was lost in more than 75% breast cancer tissues (Table 1). Figure 1A-1 and 1A-5 showed H&E staining of normal breast tissue from a non-cancer subject and Figure 1A-2 to 1A-4 and Figure 1A-6 to 1A-8 demonstrated an expressing pattern of FOXO1, GATA3 and Annexin-1 in the normal tissue. Both FOXO1 (Figure 1A-2 and 1A-6) and GATA3 (Figure 1A-3 and 1A-7) were expressed in the nuclear regions of epithelial cells and Annexin-1 (Figure 1A-4 and 1A-8) was expressed primarily in the myoepithelila cells. Figure 1B-1 and 1B-5 showed H&E staining of an invasive ductal carcinoma from a breast cancer patient with ER/ PR negative and HER2 positive tumor. The nuclear expression of FOXO1 and GATA3 were significantly reduced and the myoepithelial expression of Annexin-1 was also lost in the cancer tissue (Figure 1B-2 to 1B-4 and Figure 1B-6 to 1B-8).
Table 1.
FOXO1, GATA3 and Annexin-1 expression in cancer and non-cancer tissue
| Cancer N% | Non-Cancer N% | P | |
|---|---|---|---|
| FOXO1 | |||
| Nuclei+ | 60(51.3) | 30(90.9) | |
| Nuclei- | 57(48.7) | 3(9.1) | 0.01 |
| GATA3 | |||
| Positive | 73(73.3) | 30(100) | |
| Negative | 26(26.3) | 0 | 0.001 |
| Annexin-1 | |||
| Positive | 15(24.6) | 10(83.3) | |
| Negative | 46(75.4) | 2(16.7) | <0.001 |
Figure 1.
FOXO1, GATA3 and Annexin-1 expression in non-cancer and cancer tissues. A-1 and A-8 were normal breast tissue from a non-cancer subject and B-1 and B-8 were invasive ductal carcinoma from a breast cancer patient with ER/PR negative and HER2 positive tumor. A-1 to A-4 and B-1 to B4 were low-power and A-5 to A-8 and B-5 to B-8 were high-power. The red arrows indicated positive expression of FOXO1 and GATA3 in nuclear of epithelial cells and Annexin-1 in the myoepithelila cells. The black arrows indicated membrane and cytoplasm expression of FOXO1 and GATA3 and the storm tissue expression of Annexin-1.
Association of FOXO1, GATA3 and Annexin-1 expression with clinicopathological features of breast cancer
The correlations between the expression of FOXO1, GATA3, Annexin-1 and the clinicopathological features of breast cancer are summarized in Table 2. An inverse correlation between the expression of FOXO1 and positive lymph node was observed (p=0.038). There was no difference in FOXO1 expression between different tumor size, stage and ER status in this cohort's samples. At the same time, the lower nuclear expression of FOXO1 was observed in PR-negative tumors (p=0.031) and HER2 positive tumors (p=0.01). As shown in Figure 1B-2 and 1B-6, FOXO1 expression was more likely to be in the cytoplasm in the ER/PR negative and HER2 overexpressing tumors.
Table 2.
Association of FOXO1, GATA3 and Annexin-1 expression with clinicopathological features of breast cancer
| Variables | FOXO1 | GATA3 | Annexin-1 | |||
|---|---|---|---|---|---|---|
| Positive | Negative | Positive | Negative | Positive | Negative | |
| N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | |
| Tumor size | ||||||
| ≤5cm | 38(53.5) | 33(46.5) | 41(78.8) | 11(21.2) | 10(32.3) | 21(67.7) |
| > 5cm | 16(41.0) | 23(59.0) | 27(65.9) | 14(34.1) | 5(16.7) | 25(83.3) |
| N = 110, p=0.093 | N=93, p=0.163 | N = 61, p=0.161 | ||||
| Lymph nodes | ||||||
| Negative | 30(63.8) | 17(36.2) | 35(87.5) | 5(12.5) | 11(44.0) | 14(56.0) |
| Positive | 30(42.9) | 40(57.1) | 33(62.3) | 20(37.7) | 4(11.1) | 32(88.9) |
| N = 117, p=0.038 | N=93,p=0.007 | N = 61, p=0.004 | ||||
| Stage | ||||||
| I/II | 43(57.3) | 32(42.7) | 44(80.0) | 11(20.0) | 10(29.4) | 24(70.6) |
| III/IV | 17(40.5) | 25(59.5) | 24(63.2) | 14(36.8) | 5(18.5) | 22(81.5) |
| N = 117, p=0.087 | N=93,p=0.073 | N = 61, p=0.330 | ||||
| ER | ||||||
| Positive | 23(57.5) | 17 (42.5) | 33(84.6) | 6(15.4) | 1(3.7) | 26(96.3) |
| Negative | 25(41.7) | 35 (58.3) | 40(66.7) | 20(33.3) | 14(41.2) | 20(58.8) |
| N = 100, p=0.122 | N=99,p=0.049 | N = 61, p=0.001 | ||||
| PR | ||||||
| Positive | 23(62.2) | 14 (37.8) | 27(79.4) | 7(20.6) | 0 | 22 (100) |
| Negative | 25(39.7) | 38 (60.3) | 46(70.8) | 19(29.2) | 15 (38.5) | 24(61.5) |
| N = 100, p=0.031 | N=99,p=0.356 | N = 61, p=0.001 | ||||
| HER2 | ||||||
| Positive | 12(33.3) | 24 (66.7) | 13(68.4) | 6(31.6) | 0 | 11(100) |
| Negative | 48(59.3) | 33 (40.7) | 60(75.0) | 20(25.0) | 11(22.0) | 39(78.0) |
| N = 117, p=0.01 | N=99,p=0.560 | N = 61, p=0.051 | ||||
HER2 positive: 3+ positive tested by immunohistochemistry, ER: estrogen receptor, PR: progesterone receptor
The GATA3 expression also showed an inverse association with positive lymph node (p=0.007). There was no difference in GATA3 expression with tumor size, staging and HER2 status. In contrast to FOXO1, the GATA3 expression was more positive in ER-positive tumors than ER-negative tumors (p=0.049), whereas there was no difference between PR-positive and PR-negative tumors.
Similar to FOXO1 and GATA3, the Annexin-1 was also inversely associated with positive lymph node (p=0.004) and there was no difference between tumor size and staging. Annexin-1 also showed an inverse association with HER2 positive tumors (p=0.051). As shown in Figure 1B-4 and 1B-8, Annexin-1 was more likely to be expressed in stromal tissue than tumor cells. However, the positive expression of Annexin-1 was more associated with both ER and PR negative tumors in this cohort of patients (p=0.001 for both).
We further evaluated the association of FOXO1 GATA3, and Annexin-1 expression and their expression in relation with pAkt status in tumors. Increased pAkt in breast cancer was significantly associated with reduced nuclear and cytoplasmic expression of FOXO1 (Table 3). GATA3 and Annexin-1 expression were significantly associated with FOXO1 expression (Table 3). In particular, the decrease in expression of GATA3 or Annexin-1 was more related to decrease nuclear expression of FOXO1. There was no association in expression of GATA3 and Annexin-1 (Table 3). The GATA3 and Annexin-1 expression were also not associated with pAkt status in this cohort of patients (Table 3).
Table 3.
Association of reducing FOXO1, GATA3, Annexin-1 and pAkt expression
| FOXO1 | GATA3 | Annexin-1 | ||||
|---|---|---|---|---|---|---|
| OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | |
| pAkt | ||||||
| Positive vs. Negative | 10.7(4.2-27) | <0.001 | 1.8 (0.4-9.7) | 0.47 | 2.1(0.3-17.6) | 0.49 |
| GATA3 | ||||||
| Negative vs.Positive | 2.8(1.1-7.3) | 0.033 | - | 0.7 (0.2-2.1) | 0.524 | |
| Annexin-1 | ||||||
| Negative vs. positive | 5.8(2.0-16.8) | 0.001 | 0.7 (0.2-2.1) | 0.524 | - | |
| FOXO1 | ||||||
| Negative vs. positive | - | 2.8 (1.1-7.3) | 0.033 | 5.8 (2.0-16.8) | 0.001 | |
Expression of FOXO1, GATA3 and Annexin-1 for predicting DFS
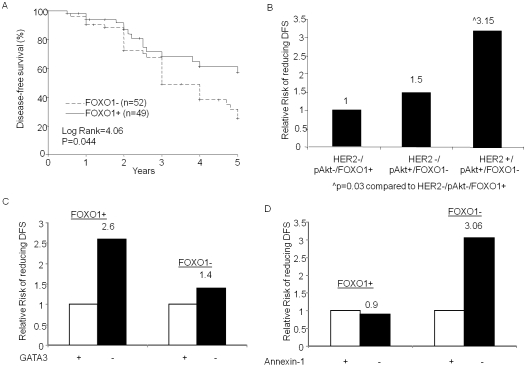
We examined the expression of FOXO1, GATA3 and Annexin-1 in relation to DFS in all breast cancer patients. Kaplan-Meier survival analysis showed that patients with positive expression of FOXO1 had longer DFS (p=0.044, Figure 2). Interestingly, when we stratified according to the ER/PR and HER2 status of their tumors, the more significant difference towards longer DFS was observed in those patients with PR-negative or HER2 overexpressing tumors (data not shown).
Figure 2.
DFS and FOXO1 expression. (A) Kaplan-Meier survival curves were used to compare the 5-year DFS between the FOXO1 positive and negative tumors. The differences between the curves were estimated by log-rank test and p<0.05 was considered statistically significant. (B to D), The RR of shorter DFS was determined by univariate Cox regression analysis. (B) The patients were grouped as (I) HER2-/pAkt+/FOXO1-, (II) HER2+/pAkt+/FOXO1- and (III) HER2-/pAkt-/FOXO1+ tumors. The RR was compared to (III) HER2-/pAkt-/FOXO1+ group. (C and D), The patients were first grouped as FOXO1-positive and FOXO1-negative and then RR of shorter DFS in GATA3-negative tumor was compared with that in GATA3-positive tumor in each group (C); or Annexin-1-negative tumor was compared with Annexin-1 -positive tumor (D) in each group. p<0.05 was considered statistically significant only.
In accordance with the results shown by Kaplan-Meier analysis the univariate Cox proportional hazard analysis also demonstrated that FOXO1 was a significant predictor for DFS (Table 4).
Table 4.
Cox proportional hazard analysis for disease-free survival
| Variables | Univariate analysis | *Multivariate analysis | ||
|---|---|---|---|---|
| RR 95% CI | p-value | RR 95% CI | p-value | |
| FOXO1 | ||||
| Positive | 1 | 1 | ||
| Negative | 1.8(1.0-3.1) | 0.05 | 1.9 (1.0-3.5) | 0.039 |
| GATA3 | ||||
| Positive | 1 | 1 | ||
| Negative | 1.2(0.5-3.1) | 0.714 | 1.1 (0.4-3.1) | 0.796 |
| Annexin-1 | ||||
| Positive | 1 | 1 | ||
| Negative | 1.35(0.3-5.4) | 0.670 | 0.989 (0.5-5.5) | 0.990 |
Adjusted for ER, PR, HER2, Tumor size and node stage
Next, we compared the RR of shorter DFS among patients expressing different levels of pAkt and FOXO1 in both HER2-positive and negative tumors. Figure 2B shows that in the HER2 negative group the RR of shorter DFS increased by 1.5 fold in patients with elevated pAkt and reduced FOXO1 compared to those with normal pAkt and positive expression of FOXO1. The RR was further increased to 3.15 fold (p=0.03) in patients whose tumors had HER2 overexpression, increased pAkt and decreased FOXO1 (Figure 2B). There were no significant differences in DFS based on different GATA3 and Annexin-1 in this cohort of patients (data not shown). However, patients who had reduced GATA3 expression in the tumor tissue tend to have increased risk to reduce DFS (Figure 2C). As shown in Figure 2C, a greater increase in RR (RR=2.6) by reducing GATA3 was observed in patients with FOXO1 positive tumors. An increased RR of shorter DFS was also shown in patients with reduced Annexin-1 expression in their tumor tissues. In contrast to GATA3, the increased RR by decreased Annexin-1 was seen in those with FOXO1 negative tumors only (RR=3.06, Figure 2D). This data suggested that the both GATA3 and Annexin-1 may interact with FOXO1 to influence the DFS.
As previous data from the same cohort of patients showed, the tumor size, lymph node, ER/ PR and HER2 status were all significantly affecting DFS [15]. We next performed multivariate analysis adjusted for those factors and found that patients with reduced FOXO1 expression in either nuclear or cytoplasm had significantly increased risk to reduce DFS (RR=1.9, p=0.029; Table 4). The data presented here confirm that FOXO1 is an independent predictor for DFS in breast cancer.
GATA3 and Annexin-1 alone were not shown to be significant in both univariate and multivariate analysis.
Discussion
Activation of PI3K/Akt pathway and cytoplasmic localization are necessary for inhibiting the tumor suppressor properties of FOXOs, including FOXO1 ubiquitination and subsequent deregulation [9, 12, 34]. The deregulation of FOXO1 has been shown to promote cell proliferation and tumorgenesis in prostate, breast and endo-metrial cancer cells [35-37]. Regression of FOXO1 function in prostate cancer cells has been shown in both androgen receptor dependent and independent pathways [35, 38, 39]. The phosphorylation and nuclear exclusion of FOXO1 in breast cancer cells have been reported to be stimulated by growth factors including ErbB family receptors [36]. The altered FOXO1 regulation has been closely linked to drug resistance in both prostate and breast cancer cells [38, 40, 41]. We have recently demonstrated that HER2 overexpressing breast cancer cells, SKBR3 transfected with myr-Akt1 (activated Akt) have decreased nuclear expression of FOXO1 and the cells were less responsive to trastuzumab [14].
The nuclear FOXO1 expression is significantly decreased in primary prostate cancer compared to normal prostate tissue [17]. An increased phosphorylated FOXO1 (pFOXO1) in cytoplasm was associated with shorter DFS and OS in soft tissue sarcoma [42]. The pFOXO1 was reported to be lower in metastatic colon cancer than in primary tumors [43]. The clinical significance of FOXO1 expression and localization in breast cancer has not been established yet.
In this study we showed that FOXO1 expression in normal breast tissue mainly remained in the nucleus, while in breast cancer tissue the FOXO1 was expressed in both nucleus and cytoplasm. Breast cancer patients who had increased pAkt had lower expression of FOXO1. In correlation with pAkt levels [15], the expression of FOXO1 in both nucleus and cytoplasm was significantly reduced in Lymph node positive and HER2 overexpressing tumors. In contrast only nuclear expression of FOXO1 was associated with PR status. A low expression of nuclear FOXO1 was observed in PR negative tumors. The two nuclear proteins, FOXO1 and PR regulate each other's transactivation function in endometrial cells [44]. The correlation of FOXO1 and PR in the same cellular localization in breast cancer tissue from our current study provided further evidence supporting the data from endometrial cells. It will require more functional studies to understand that if the same localization leads to the interaction between FOXO1 and PR in different cancer cells.
Overall, reduced FOXO1 expression in both nucleus and cytoplasm predicted poor DFS in this cohort of patients (p=0.05), especially in HER2 overexpressing tumors. In HER2 overexpressing tumors with elevated pAkt and decreased FOXO1 expression significantly increased RR of shorter DFS. These data suggest that the FOXO1 mediated by Akt pathway plays a significant role in disease outcome of HER2 positive tumors. The multivariate analysis further confirmed the independent predicting value of FOXO1.
In the present study we also examined clinical significance of GATA3 and Annexin-1 protein expression. We observed the positive staining of GATA3 in both nucleus and cytoplasm. Consistent with its function, the clinical significance of GATA3 was related only with nuclear expression in our study. The nuclear expression of GATA3 has been considered to be a prognostic indicator of breast cancer [22]. The low expression of nuclear GATA3 was more associated with larger tumor size, positive lymph node, ER/PR negative and HER2 positive tumors [22]. Our data from the current study partially supported those observations and showed that the expression of GATA3 was inversely correlated with lymph node stage significantly. High expression of GATA3 was more associated with ER-positive status (p=0.049). We observed only slightly more GATA3 positive expression in PR-positive, small size and early stage tumors, although the differences were not significant. It could become more significant if the sample size was larger.
Currently the expression of Annexin-1 in breast cancer is still under investigation. An earlier study reported an increased Annexin-1 expression in various types of breast cancers, including noninvasive ductal carcinoma in situ and invasive and metastatic breast tumors, although the sample size (n=33) was relatively small [45]. A recent report on tissue microarray analyses showed that decreased Annexin-1 expression in myoepithelial cells in breast ductal carcinoma and the decreased expression of Annexin-1 in breast cancer tissue could be correlated with breast cancer progression [28]. Our data was in agreement with the finding from microarray analysis that Annexin-1 was highly expressed in cytoplasm and nuclei of normal myoepithelial and ductal breast cells, but decreased significantly in malignant cells. In our study, the reduced Annexin-1 expression in malignant cells was most likely due to the absence of the myoepithelial cells. In addition, we found that Annexin-1 expression was decreased in lymph node positive and HER2 overexpressing breast cancer. In HER2 overexpressing tumors, Annexin-1 was more frequently expressed in stromal tissues. We also observed more positive Annexin-1 expression in ER/PR negative tumors. These results need to be further validated by a larger sample size. In this cohort of patients, even though the expression of GATA3 and Annexin-1 did not predict DFS in multivariate analysis, both GATA3 and Annexin-1 expression were significantly associated with nuclear expression of FOXO1. The reduced GATA3 expression increased RR of shorter DFS, especially in the FOXO1 positive group, and the reduced Annexin-1 were able to increase RR of shorter DFS in the FOXO1 negative group. Although the increased RR was not shown to be statistically significant, the evidence suggests that there are correlated roles between FOXO1 and GATA3 and/or FOXO1 and Annexin-1 in regulation of DFS. We postulate that a larger sample size may increase RR and reach statistical significance.
We did not find differences in FOXO1, GATA3 and Annexin-1 levels between African-American and Latina women in this cohort of patient.
The uniqueness of this study is that we used the same cohort of patients that had been examined for pAkt and further investigated the clinical significance of FOXO1, GATA3 and Annexin-1. The information from this study elicited the Akt pathway in relation to tumor pathology and in predicting breast cancer progression. The findings from this study confirmed our previous study in vitro that expression and localization of FOXO1 is strongly associated with disease outcome of HER2 overexpressing tumors. Using tissue microarray approach, our study also reported for the first time that FOXO1 expression and localization is related to GATA3 and Annexin -1 in breast cancer. The association of nuclear expression of FOXO1 and GATA3 or FOXO1 and Annexin-1 seem likely to have an important role in predicting disease outcome for breast cancer.
The limitation of this study was that this cohort of patients was not prospective and had a limited sample size. Therefore, it was not able to evaluate the value of FOXO1 in response to trastuzumab or other specific chemo/hormonal therapy directly. Nonetheless, based on the independent role of FOXO1 in predicting DFS, especially in HER2 overexpressing tumors, we can consider expression of FOXO1 may be important to the trastuzumab response phenotype of breast cancer.
Conclusion
Overall data from this study in addition to current knowledge suggest that the overexpression of HER2 receptor in breast cancer could deregulate PI3K/Akt pathway and then inhibit or deregulate FOXO1 expression. Assessment of FOXO1 expression in clinical samples can predict the recurrence and metastatic behavior of breast cancer, especially for HER2 overexpressing tumors. Although the expression of GATA3 or Annexin-1 alone did not independently predict DFS, the expression and localization of FOXO1 was strongly associated with GATA3 and Annexin-1 expression. The intrinsic high correlation of FOXO1 with GATA3 and Annexin-1 may provide a new option for designing target therapy.
Acknowledgments
This work was supported in part by grants from NIH/National Cancer Institute 1U54CA14393-01; U56 CA101599-01; CA15083-25S3; R25DK067015-01; and Department of Defense Breast Cancer Research Program grant BC043180 to JV Vadgama.
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Wu Y, Khan H, Chillar R, Vadgama JV. Prognostic value of plasma HER-2/neu in African American and Hispanic women with breast cancer. Int J Oncol. 1999;14:1021–1037. doi: 10.3892/ijo.14.6.1021. [DOI] [PubMed] [Google Scholar]
- 3.Hynes NE, Stern DF. The biology of erbB-2/ neu/HER-2 and its role in cancer. Biochim Biophys Acta. 1994;1198:165–184. doi: 10.1016/0304-419x(94)90012-4. [DOI] [PubMed] [Google Scholar]
- 4.Milla Santos A, Milla L, Portella J, Rallo L, Pons M, Rodes E, Casanovas J, Puig Gali M, Anastrozole V. Tamoxifen as First-Line Therapy in Postmenopausal Patients With Hormone-Dependent Advanced Breast Cancer: A Prospective, Randomized, Phase III Study. American Journal of Clinical Oncology. 2003;26:317–322. doi: 10.1097/01.COC.0000047126.10522.F9. [DOI] [PubMed] [Google Scholar]
- 5.Cobleigh MA, Vogel CL, Tripathy D, Robert NJ, Scholl S, Fehrenbacher L. Multinational study of the efficacy and safety of humanized anti-HER2 monoclonal antibody in women who have HER-2 over-expressing metastatic breast cancer that has progressed after chemotherapy for metastatic disease. J Clin Oncol. 1999;17:2639–2648. doi: 10.1200/JCO.1999.17.9.2639. [DOI] [PubMed] [Google Scholar]
- 6.Vogel CL, Cobleigh MA, Tripathy D, Gutheil JC, Harris LN, Fehrenbacher L. Efficacy and safety of trastuzumab as a single agent in first-line treatment of HER2-over-expressing metastatic breast cancer. J Clin Oncol. 2002;2:719–726. doi: 10.1200/JCO.2002.20.3.719. [DOI] [PubMed] [Google Scholar]
- 7.Carey LA, Perou CM, Livasy CA, Dressler LG, Cowan D, Conway K, Karaca G, Troester MA, Tse CK, Edmiston S, Deming SL, Geradts J, Cheang MC, Nielsen TO, Moorman PG, Earp HS, Millikan RC. Race, breast cancer subtypes and survival in the Carolina Breast Cancer Study. JAMA. 2006;295:2492–2502. doi: 10.1001/jama.295.21.2492. [DOI] [PubMed] [Google Scholar]
- 8.Ihemelandu CU, Leffall LD, Jr, Dewitty RL, Naab TJ, Mezghebe HM, Makambi KH, Adams Campbell L, Frederick WA. Molecular breast cancer subtypes in premenopausal and postmenopausal African-American women: age-specific prevalence and survival. J Surg Res. 2007;143:109–118. doi: 10.1016/j.jss.2007.03.085. [DOI] [PubMed] [Google Scholar]
- 9.Greer EL, Brunet A. FOXO transcription factors at the interface between longevity and tumor suppression. Oncogene. 2005;24:7410–7425. doi: 10.1038/sj.onc.1209086. [DOI] [PubMed] [Google Scholar]
- 10.Myatt SS, Lam EW. The emerging roles of forkhead box (Fox) proteins in cancer. Nat Rev Cancer. 2007;7:847–859. doi: 10.1038/nrc2223. [DOI] [PubMed] [Google Scholar]
- 11.Gomes AR, Brosens JJ, Lam EW. Resist or die: FOXO transcription factors determine the cellular response to chemotherapy. Cell Cycle. 2008;7:3133–3136. doi: 10.4161/cc.7.20.6920. [DOI] [PubMed] [Google Scholar]
- 12.Arden KC. Multiple roles of FOXO transcription factors in mammalian cells point to multiple roles in cancer. Experimental Gerontology. 2006;41:709–717. doi: 10.1016/j.exger.2006.05.015. [DOI] [PubMed] [Google Scholar]
- 13.Yang JY, Hung MC. A new fork for clinical application: targeting forkhead transcription factors in cancer. Clin Cancer Res. 2009;15:752–757. doi: 10.1158/1078-0432.CCR-08-0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu Y, Shang X, Sarkissyan M, Slamon D, Vadgama JV. FOXO1A is a target for HER2-Overexpressing breast tumors. Cancer Res. 2010;70:5475–5485. doi: 10.1158/0008-5472.CAN-10-0176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu Y, Mohammed H, Chillar R, Clayton S, Slamon D, Vadgama JV. Clinical significance of Akt and HER2/neu overexpression in African American and Latina women with breast cancer. Breast Cancer Res. 2008;10:R3. doi: 10.1186/bcr1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li R, Erdamar S, Dai H, Wheeler TM, Frolov A, Scardino PT. Forkhead protein FKHR and its phosphorylated from p-FKHR in human prostate cancer. Hum Pathol. 2007;38:1501–1507. doi: 10.1016/j.humpath.2007.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goto T, Takano M, Hirata J, Tsuda H. The involvement of FOXO1 in cytotoxic stress and drug-resistance induced by paclitaxel in ovarian cancers. Br J Cancer. 2008;98:1068–1075. doi: 10.1038/sj.bjc.6604279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim TH, Jo SW, Lee YS, Kim YJ, Lee SC, Kim WJ, Yun SJ. Forkhead box O-class 1 and Forkhead box G1 as prognostic markers for bladder cancer. J Korean Med Sci. 2009;24:468–473. doi: 10.3346/jkms.2009.24.3.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kouros Mehr H, Slorach EM, Sternlicht MD, Werb Z. GATA-3 maintains the differentiation of luminal cell fate in the mammary gland. Cell. 2006;127:1041–1055. doi: 10.1016/j.cell.2006.09.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wilson BJ, Giguere V. Meta-analysis of human cancer microarrays reveals GATA3 is integral to the estrogen receptor alpha pathway. Molecular Cancer. 2008;7:49. doi: 10.1186/1476-4598-7-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eeckhoute J, Keeton EK, Lupien M, Krum SA, Carroll JS, Brown M. Positive cross-regulatory loop ties GATA-3 to estrogen receptor α expression in breast cancer. Cancer Res. 2007;67:6477–6483. doi: 10.1158/0008-5472.CAN-07-0746. [DOI] [PubMed] [Google Scholar]
- 22.Mehra R, Varambally S, Ding L, Shen R, Sabel M, Ghosh D, Chinnaiyan AM, Kleer CG. Identification of GATA3 as breast cancer prognostic marker by global gene expression meta-analysis. Cancer Res. 2005;65:11259–11264. doi: 10.1158/0008-5472.CAN-05-2495. [DOI] [PubMed] [Google Scholar]
- 23.McCune K, Bhat Nakshatri P, Thorat MA, Nephew KP, Badve S, Nakshatri H. Prognosis of hormone-dependent breast cancers: implications of the presence of dysfunctional transcriptional networks activated by insulin via the immune transcription factor T-bet. Cancer Res. 2010;70:685–696. doi: 10.1158/0008-5472.CAN-09-1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xin W, Rhodes DR, Ingold C, Chinnaiyan AM, Rubin MA. Dysregulation of the Annexin family protein family is associated with prostate cancer progression. Am J Pathol. 2003;162:255–261. doi: 10.1016/S0002-9440(10)63816-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Garcia Pedreo JM, Fernandez MP, Morgan RO, Herrero Zapatero A, Gonzalez MV, Suarez Nieto C. Annexin A1 down-regulation in head and neck cancer is associated with epithelial differentiation status. Am J Pathol. 2004;164:73–79. doi: 10.1016/S0002-9440(10)63098-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hu N, Flaig MJ, Su H, Shou JZ, Roth MJ, Li WJ. Comprehensive characterization of Annexin I alterations in esophageal squamous cell carcinoma. Clin Cancer Res. 2004;10:6013–6022. doi: 10.1158/1078-0432.CCR-04-0317. [DOI] [PubMed] [Google Scholar]
- 27.Bai XF, Ni XG, Zhao P, Liu SM, Wang HX, Guo B. Overexpression of Annexin 1 in pancreatic cancer and its clinical significance. Word J Gastroenterol. 2004;10:1466–1470. doi: 10.3748/wjg.v10.i10.1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shen D, Nooraie F, Elshimali Y, Lonsberry V, He J, Bose S, Chia D, Seligson D, Chang HR, Goodglick L. Decreased expression of annexin A1 is correlated with breast cancer development and progression as determined by a tissue microarray analysis. Human Pathology. 2006;37:1583–1591. doi: 10.1016/j.humpath.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 29.Ang EZF, Nguyen HT, Sim HK, Putti TC, Lim LHK. Annexin-1 regulates growth arrest induced by high levels of estrogen in MCF-7 breast cancer cells. Mol Cancer Res. 2009;7:266–274. doi: 10.1158/1541-7786.MCR-08-0147. [DOI] [PubMed] [Google Scholar]
- 30.Khau T, Langenbach SY, Schuliga M, Harris T, Johnstone CN, anderson RL, Stewart AG. Annexin-1 signals mitogen-stimulated breast tumor cell proliferation by activation of the formyl peptide receptors (FPRs) 1 and 2. FASEB J. 2011;25:483–496. doi: 10.1096/fj.09-154096. [DOI] [PubMed] [Google Scholar]
- 31.Press MF, Slamon DJ, Flom KJ, Park J, Zhou JY, Bernstein L. Evaluation of HER-2/neu gene amplification and overexpression: comparison of frequently used assay methods in a molecularly characterized cohort of breast cancer specimens. J Clin Oncol. 2002;20:3095–3105. doi: 10.1200/JCO.2002.09.094. [DOI] [PubMed] [Google Scholar]
- 32.Seligson DB, Horvath S, Shi T, Yu H, Tze S, Grunstein M, Kurdistani SK. Global histone modification patterns predict risk of prostate cancer recurrence. Nature. 2005;435:1262–1266. doi: 10.1038/nature03672. [DOI] [PubMed] [Google Scholar]
- 33.Albergaria A, Paredes J, Sousa B, Milanezi F, Carneiro V, Bastos J, Costa S, Vieira D, Lopes N, Lam EW, Lunet N, Schmitt F. Expression of FOXA1 and GATA3 in breast cancer: the prognostic significance in hormone receptor-negative tumors. Breast Cancer Research. 2009;11:R40. doi: 10.1186/bcr2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reagan Shaw S, Ahmad N. The role of Forkhead-box class O (FoxO) transcription factors in cancer: A target for management of cancer. Toxicology and Applied Pharmacology. 2007;224:360–368. doi: 10.1016/j.taap.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 35.Huang H, Muddiman DC, Tindall DJ. Androgens negative regulate forkhead transctiption factor FKHR (FOXO1) through a proteolytic mechanism in prostate cancer cells. J Biol Chem. 2004;279:13866–13877. doi: 10.1074/jbc.M314143200. [DOI] [PubMed] [Google Scholar]
- 36.Jackson JG, Kreisberg JI, Koterba AP, Yee D, Brattain MG. Phosphorylation and nuclear exclusion of forkhead transcription factor FKHR after epidermal growth factor treatment in human breast cancer cells. Oncogene. 2000;19:4574–4581. doi: 10.1038/sj.onc.1203825. [DOI] [PubMed] [Google Scholar]
- 37.Goto T, Takano M, Albergaria A, Briese J, Pomeranz KM, Cloke B, Fusi L, Feroze Zaidi F, Maywald N, Sajin M, Dina RE, Ishihara O, Takeda S, Lam EWF, Bamberger AM, Ghaem Magharm S, Brosens JJ. Mechanism and function consequences of loss of FOXO1 expression in endometrioid endometrial cancer cells. Oncogene. 2008;27:9–19. doi: 10.1038/sj.onc.1210626. [DOI] [PubMed] [Google Scholar]
- 38.Gan L, Chen S, Wang Y, Watahiki A, Bohrer L, Sun Z, Wang YH, Huang H. Inhibition of androgen receptor as a novel mechanism of Taxol chemotherapy in prostate cancer. Cancer Res. 2009;69:8386–8394. doi: 10.1158/0008-5472.CAN-09-1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang H, Fang J, Yao D, Wu Y, Ip C, Dong Y. Activation of FOXO1 is critical for the antican-cer effect of methylseleninic acid in prostate cancer cells. Prostate. 2010;70:1265–1273. doi: 10.1002/pros.21162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Han CY, Cho KB, Choi HS, Han HK, Kang KW. Role of FoxO1 activation in MDR1 expression in adriamycin-resistant breast cancer cells. Carcinogenesis. 2008;29:1837–1844. doi: 10.1093/carcin/bgn092. [DOI] [PubMed] [Google Scholar]
- 41.Silva J, Cavazos DA, Donzis E, Friedrichs WE, Marciniak R, deGraffenried LA. Aktinduced Tamoxifen resistance is associated with altered FKHR regulation. Cancer Investigation. 2007;25:569–573. doi: 10.1080/07357900701513538. [DOI] [PubMed] [Google Scholar]
- 42.Zhang B, Tomita Y, Ch'ng E, Qiu Y, He J, Jin YF, Tomoeda M, Hamada KI, Ueda T, Aozasa K. Prognostic significance of phosphorylated FOXO1 expression in soft tissue sarcoma. Ann Surg Oncol. 2009 doi: 10.1245/s10434-009-0481-x. DOI 10.1245/s10434-009-0481. [DOI] [PubMed] [Google Scholar]
- 43.Bravou V, Klironomos G, Papadaki E, Taravias S, Varakis J. ILK over-expression in human colon cancer progression correlates with activation of β-catenin, down-regulation of E-cadherin and activation of the Akt-FKHR pathway. J Pathol. 2006;208:91–99. doi: 10.1002/path.1860. [DOI] [PubMed] [Google Scholar]
- 44.Kim JJ, Buzzio OL, Li S, Lu Z. Role of FOXO1A in the regulation of insulin-like growth factor binding protein-1 in human endometrial cells: interaction with progesterone receptor. Biol Reprod. 2005;73:833–839. doi: 10.1095/biolreprod.105.043182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ahn SH, Sawada H, Ro JY, Nicolson GL. Differential expression of annexin-1 in human mammary ductal epithelial cells in normal and benign and malignant breast tissues. Clin Exp Metastasis. 1997;15:151–156. doi: 10.1023/a:1018452810915. [DOI] [PubMed] [Google Scholar]