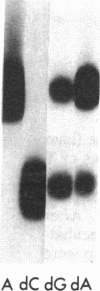
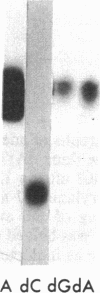
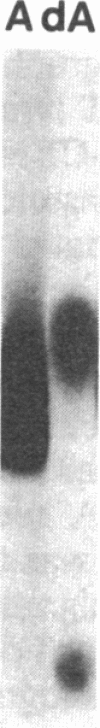
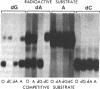
Abstract
Nucleoside kinases catalyze the initial step leading to the accumulation of deoxypurine nucleotides that occurs in patients with inherited deficiencies of adenosine deaminase (adenosine aminohydrolase, EC 3.5.4.4) and purine-nucleoside phosphorylase (purine-nucleoside:orthophosphate ribosyltransferase, EC 2.4.2.1). This accumulation is thought to interfere with DNA synthesis in lymphocytes and, thus, to cause the immune defects associated with these enzymopathies. However, there is controversy about the identity of the nucleoside kinases that are responsible for intracellular phosphorylation of deoxyadenosine in adenosine deaminase deficiency and deoxyguanosine in purine nucleoside phosphorylase deficiency. To distinguish the nucleoside kinases present in T and B lymphoblastoid cells, we have coupled discontinuous PAGE with autoradiography. This procedure showed that deoxycytidine kinase (NTP:deoxycytidine 5'-phototransferase, EC 2.7.1.74), deoxyadenosine kinase (ATP:deoxyadenosine 5'-phosphotransferase, EC 2.7.1.76), and adenosine kinase (ATP:adenosine 5'-phosphotransferase, EC 2.7.1.20) are all present in both T and B lymphoblasts. While adenosine kinase is expressed at nearly equal levels in B and T cells, the deoxynucleoside kinases are expressed at much lower levels in B cells than in T cells. The autoradiographic data agreed with assays of the nucleoside kinase activities. Molecular weights were determined by using 5-10% polyacrylamide gels. Mr values were 29,000 for adenosine kinase, 41,000 for deoxyadenosine kinase, and 53,000 for deoxycytidine kinase and its isozyme. The reduced expression of deoxycytidine and deoxyadenosine kinases in B lymphoblasts may account for the lower accumulation of deoxypurine nucleotides in B cells as compared with T cells.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barker J., Lewis R. A. Deoxyguanosine kinase of neonatal mouse skin tissue. Biochim Biophys Acta. 1981 Mar 13;658(1):111–123. doi: 10.1016/0005-2744(81)90254-0. [DOI] [PubMed] [Google Scholar]
- Bryan J. K. Molecular weights of protein multimers from polyacrylamide gel electrophoresis. Anal Biochem. 1977 Apr;78(2):513–519. doi: 10.1016/0003-2697(77)90111-7. [DOI] [PubMed] [Google Scholar]
- Carson D. A., Kaye J., Seegmiller J. E. Differential sensitivity of human leukemic T cell lines and B cell lines to growth inhibition by deoxyadenosine. J Immunol. 1978 Nov;121(5):1726–1731. [PubMed] [Google Scholar]
- Carson D. A., Kaye J., Seegmiller J. E. Lymphospecific toxicity in adenosine deaminase deficiency and purine nucleoside phosphorylase deficiency: possible role of nucleoside kinase(s). Proc Natl Acad Sci U S A. 1977 Dec;74(12):5677–5681. doi: 10.1073/pnas.74.12.5677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FERGUSON K. A. STARCH-GEL ELECTROPHORESIS--APPLICATION TO THE CLASSIFICATION OF PITUITARY PROTEINS AND POLYPEPTIDES. Metabolism. 1964 Oct;13:SUPPL–SUPPL1002. doi: 10.1016/s0026-0495(64)80018-4. [DOI] [PubMed] [Google Scholar]
- Fabianowska-Majewska K., Greger J., Gorzkiewicz B. Purification and properties of deoxyadenosine kinase from rat liver mitochondria. I. Purification and physical properties. Enzyme. 1984;31(1):27–32. [PubMed] [Google Scholar]
- Giblett E. R., Ammann A. J., Wara D. W., Sandman R., Diamond L. K. Nucleoside-phosphorylase deficiency in a child with severely defective T-cell immunity and normal B-cell immunity. Lancet. 1975 May 3;1(7914):1010–1013. doi: 10.1016/s0140-6736(75)91950-9. [DOI] [PubMed] [Google Scholar]
- Giblett E. R., Anderson J. E., Cohen F., Pollara B., Meuwissen H. J. Adenosine-deaminase deficiency in two patients with severely impaired cellular immunity. Lancet. 1972 Nov 18;2(7786):1067–1069. doi: 10.1016/s0140-6736(72)92345-8. [DOI] [PubMed] [Google Scholar]
- Gower W. R., Jr, Carr M. C., Ives D. H. Deoxyguanosine kinase. Distinct molecular forms in mitochondria and cytosol. J Biol Chem. 1979 Apr 10;254(7):2180–2183. [PubMed] [Google Scholar]
- Hershfield M. S., Fetter J. E., Small W. C., Bagnara A. S., Williams S. R., Ullman B., Martin D. W., Jr, Wasson D. B., Carson D. A. Effects of mutational loss of adenosine kinase and deoxycytidine kinase on deoxyATP accumulation and deoxyadenosine toxicity in cultured CEM human T-lymphoblastoid cells. J Biol Chem. 1982 Jun 10;257(11):6380–6386. [PubMed] [Google Scholar]
- Hurley M. C., Palella T. D., Fox I. H. Human placental deoxyadenosine and deoxyguanosine phosphorylating activity. J Biol Chem. 1983 Dec 25;258(24):15021–15027. [PubMed] [Google Scholar]
- Ives D. H., Durham J. P., Tucker V. S. Rapid determination of nucleoside kinase and nucleotidase activities with tritium-labeled substrates. Anal Biochem. 1969 Apr 4;28(1):192–205. doi: 10.1016/0003-2697(69)90170-5. [DOI] [PubMed] [Google Scholar]
- Krenitsky T. A., Tuttle J. V., Koszalka G. W., Chen I. S., Beacham L. M., 3rd, Rideout J. L., Elion G. B. Deoxycytidine kinase from calf thymus. Substrate and inhibitor specificity. J Biol Chem. 1976 Jul 10;251(13):4055–4061. [PubMed] [Google Scholar]
- Krygier V., Momparler R. L. Mammalian deoxynucleoside kinases. II. Deoxyadenosine kinase: purification and properties. J Biol Chem. 1971 May 10;246(9):2745–2751. [PubMed] [Google Scholar]
- Miller R. L., Adamczyk D. L., Miller W. H. Adenosine kinase from rabbit liver. I. Purification by affinity chromatography and properties. J Biol Chem. 1979 Apr 10;254(7):2339–2345. [PubMed] [Google Scholar]
- Miller R. L., Adamczyk D. L., Miller W. H., Koszalka G. W., Rideout J. L., Beacham L. M., 3rd, Chao E. Y., Haggerty J. J., Krenitsky T. A., Elion G. B. Adenosine kinase from rabbit liver. II. Substrate and inhibitor specificity. J Biol Chem. 1979 Apr 10;254(7):2346–2352. [PubMed] [Google Scholar]
- Mitchell B. S., Mejias E., Daddona P. E., Kelley W. N. Purinogenic immunodeficiency diseases: selective toxicity of deoxyribonucleosides for T cells. Proc Natl Acad Sci U S A. 1978 Oct;75(10):5011–5014. doi: 10.1073/pnas.75.10.5011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochs U. H., Chen S. H., Ochs H. D., Osborne W. R., Scott C. R. Purine nucleoside phosphorylase deficiency: a molecular model for selective loss of T cell function. J Immunol. 1979 Jun;122(6):2424–2429. [PubMed] [Google Scholar]
- Osborne W. R. Human red cell purine nucleoside phosphorylase. Purification by biospecific affinity chromatography and physical properties. J Biol Chem. 1980 Aug 10;255(15):7089–7092. [PubMed] [Google Scholar]
- Osborne W. R., Scott C. R. The metabolism of deoxyguanosine and guanosine in human B and T lymphoblasts. A role for deoxyguanosine kinase activity in the selective T-cell defect associated with purine nucleoside phosphorylase deficiency. Biochem J. 1983 Sep 15;214(3):711–718. doi: 10.1042/bj2140711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne W. R., Sullivan J. L., Scott C. R. Formycin B, purine nucleoside phosphorylase and lymphocyte function. Immunol Commun. 1980;9(3):257–267. doi: 10.3109/08820138009065998. [DOI] [PubMed] [Google Scholar]
- Schnebli H. P., Hill D. L., Bennett L. L., Jr Purification and properties of adenosine kinase from human tumor cells of type H. Ep. No. 2. J Biol Chem. 1967 May 10;242(9):1997–2004. [PubMed] [Google Scholar]
- Tischfield J. A., Bernhard H. P., Ruddle F. H. A new electrophoretic-autoradiographic method for the visual detection of phosphotransferases. Anal Biochem. 1973 Jun;53(2):545–554. doi: 10.1016/0003-2697(73)90105-x. [DOI] [PubMed] [Google Scholar]
- Ullman B., Gudas L. J., Cohen A., Martin D. W., Jr Deoxyadenosine metabolism and cytotoxicity in cultured mouse T lymphoma cells: a model for immunodeficiency disease. Cell. 1978 Jun;14(2):365–375. doi: 10.1016/0092-8674(78)90122-8. [DOI] [PubMed] [Google Scholar]
- Ullman B., Levinson B. B., Hershfield M. S., Martin D. W., Jr A biochemical genetic study of the role of specific nucleoside kinases in deoxyadenosine phosphorylation by cultured human cells. J Biol Chem. 1981 Jan 25;256(2):848–852. [PubMed] [Google Scholar]
- Verhoef V., Sarup J., Fridland A. Identification of the mechanism of activation of 9-beta-D-arabinofuranosyladenine in human lymphoid cells using mutants deficient in nucleoside kinases. Cancer Res. 1981 Nov;41(11 Pt 1):4478–4483. [PubMed] [Google Scholar]
- Willis R. C., Carson D. A., Seegmiller J. E. Adenosine kinase initiates the major route of ribavirin activation in a cultured human cell line. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3042–3044. doi: 10.1073/pnas.75.7.3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wortmann R. L., Mitchell B. S., Edwards N. L., Fox I. H. Biochemical basis for differential deoxyadenosine toxicity to T and B lymphoblasts: role for 5'-nucleotidase. Proc Natl Acad Sci U S A. 1979 May;76(5):2434–2437. doi: 10.1073/pnas.76.5.2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada Y., Goto H., Ogasawara N. Purine nucleoside kinases in human T- and B-lymphoblasts. Biochim Biophys Acta. 1983 Nov 22;761(1):34–40. doi: 10.1016/0304-4165(83)90359-8. [DOI] [PubMed] [Google Scholar]
- Yamada Y., Goto H., Ogasawara N. T-lymphoblast-specific nucleoside kinase: characterization and comparison with deoxycytidine kinase. Int J Biochem. 1985;17(3):425–428. doi: 10.1016/0020-711x(85)90221-6. [DOI] [PubMed] [Google Scholar]