Abstract
Mucosal surfaces of the reproductive tract as well as their secretions have important roles in preventing sexual transmission of HIV-1. In the current study, the majority of the intrinsic anti-HIV-1 activity of human seminal plasma (SP) was determined to reside in the cationic polypeptide fraction. Antiviral assays utilizing luciferase reporter cells and lymphocytic cells revealed the ability of whole SP to prevent HIV-1 infection, even when SP was diluted 3200-fold. Subsequent fractionation by continuous flow acid-urea (AU)-PAGE and antiviral testing revealed that cationic polypeptides within SP were responsible for the majority of anti-HIV-1 activity. A proteomic approach was utilized to resolve and identify 52 individual cationic polypeptides that contribute to the aggregate anti-HIV-1 activity of SP. One peptide fragment of semenogelin I, termed SG-1, was purified from SP by a multistep chromatographic approach, protein sequenced, and determined to exhibit anti-HIV-1 activity against HIV-1. Anti-HIV-1 activity was transient, as whole SP incubated for prolonged time intervals exhibited a proportional decrease in anti-HIV-1 activity that was directly attributed to the degradation of semenogelin I peptides. Collectively, these results indicate that the cationic polypeptide fraction of SP is active against HIV-1, and that semenogelin-derived peptides contribute to the intrinsic anti-HIV-1 activity of SP.—Martellini, J. A., Cole, A. C., Venkataraman, N., Quinn, G. A., Svoboda, P., Gangrade, B. K., Pohl, J., Sørensen, O. E., Cole, A. M. Cationic polypeptides contribute to the anti-HIV-1 activity of human seminal plasma.
Keywords: antigens, epitopes, AIDS, reproductive immunology, viral, antimicrobial
HIV has become a pandemic over the past few decades, newly infecting an estimated 2.7 million people in 2007 alone. Due to the rapid mutation rate of HIV-1, and unforeseen problems in the clinic, vaccine development has gained insignificant headway over viral spread. Even in the absence of preventive measures, natural mucosal host defenses are thought to play a role in preventing or suppressing HIV-1 transmission. Principal molecular effectors of mucosal host defense include antimicrobial polypeptides, which exhibit a broad range of actions against gram-negative and gram-positive bacteria, fungi, and viruses (1,2,3). Mucosal tissues throughout the human body elaborate a number of antimicrobial polypeptides that, once released into the overlying fluid, quickly and effectively eradicate invading pathogens. While biological evidence for antibacterial and antifungal host defense has been established (4), only a comparably few studies have focused on the antiviral activities and mechanisms of these polypeptides (5).
The main antimicrobial peptides and proteins found on mucosal surfaces are cationic in nature and include lysozyme, secretory leukocyte protease inhibitor (SLPI), lactoferrin, defensins, and the cathelicidin LL-37. Alone they exhibit only modest antiviral activity against HIV-1, yet in concert with other peptides (e.g., example in human vaginal fluid), they can exert significant anti-HIV-1 activity (6). While human seminal plasma (SP) contains several antimicrobial polypeptides, including hCAP18, the precursor protein of LL-37, and defensins (7, 8), a number of other cationic polypeptides and their roles in antiviral host defense have not yet been studied. The most abundant proteins in SP are semenogelin I and semenogelin II, secreted from the seminal vesicles. These proteins immediately form a noncovalently linked coagulum, which is subsequently liquefied by proteases that hydrolyze the gel proteins into soluble fragments (7, 9,10,11). Studies have reported antibacterial activity of semenogelin-derived peptides (12, 13), which our group has recently confirmed and extended (14).
While studies have determined the antibacterial effects of individual cationic polypeptides found in SP, the antiviral activity of the cationic component of SP has not been investigated. Moreover, histidine-rich peptides have not been reported to play a major role in anti-HIV-1 host defense. In the current study, the anti-HIV-1 activity of SP was found to be contained in the cationic polypeptide fraction, revealing a significant host defense function against HIV-1. Further, electrophoretic and chromatographic fractionation followed by proteomic analyses identified 52 separate cationic polypeptides, many of which contributed to the aggregate anti-HIV-1 activity of SP. Notably, a recent study reported semen-mediated enhancement of HIV transmission, due to prostatic acid phosphatase (PAP)-derived amyloid fibrils (15). While certain methods differ between this report and our current study, taken together, it is clear that both proviral and antiviral factors are present within SP. Our results provide biological evidence that SP is naturally antiviral against HIV-1, suggesting an innate defense mechanism that prevents HIV-1 transmission.
MATERIALS AND METHODS
Collection and processing of human SP
Samples of human semen were collected by the Center for Reproductive Medicine (Orlando, FL, USA) for routine seminal analyses, and the discarded deidentified SP component was used for this study. Patients were asked to refrain from ejaculation for 2–3 d, but no more than 5 d, prior to collection, and semen was obtained using dry masturbation into a sterile polypropylene cup. All samples were allowed to liquefy for 30 min at room temperature and centrifuged for 30 min at 1500 g in order to remove any cellular debris. A total of 103 SP samples were collected, with volumes ranging from 0.5 to 6 ml, and equal volumes of all donor samples were pooled, portioned into aliquots, and stored at −80°C until needed. The average protein concentration of SP was acquired using a Micro BCA Protein Assay (Pierce, Rockford, IL, USA) using diluted bovine serum albumin (BSA) as a standard. The assay was repeated 3 times in triplicate, revealing an average protein concentration of 161.3 mg/ml. Average pH of whole undiluted SP was determined with colorpHast pH indicator strips (EMD Chemicals Inc., Gibbstown, NJ, USA) and observed at a pH of 7.75 (n=3). Whole SP utilized for antiviral cell culture assays was not manipulated further.
Cell lines and viruses
The TZM-bl and PM1 cell lines were obtained from the National Institutes of Health (NIH) AIDS Research and Reference Reagent Program (Germantown, MD, USA). TZM-bl cells are a stable line of HeLa-derived epithelia. They contain Tat-regulated reporter genes, and neutralize virus after single-round infections. When used with Env-pseudotyped laboratory strains of HIV, TZM-bl cells can undergo a luciferase (Luc) reporter gene assay that performs a sensitive and rapid report of the extent of viral infection. TZM-bl cells were grown in D10 medium: high-glucose DMEM (Mediatech, Manassas, VA, USA) supplemented with 100 U/ml penicillin, 100 μg/ml streptomycin, and 10% (v/v) fetal bovine serum. PM1 cell cultures were maintained at a density of 0.4–0.8 × 106 cells/ml in R20 medium: RPMI 1640 supplemented with 100 U/ml penicillin, 100 μg/ml streptomycin, 100 mM HEPES, and 20% (v/v) fetal bovine serum (Gemini Bio-Products, West Sacramento, CA, USA). The HIV-1 strain BaL (R5) was obtained from the NIH AIDS Research and Reference Reagent Program. HIV-1 BaL was propagated in PM1 cells over 16 d, and virus-containing supernatant was collected every other day starting on d 5 of infection. Supernatant was filtered through a 0.45-μm pore-size filter and stored in 0.5- or 1-ml aliquots at −80°C until needed. Viral quantification was achieved via a sensitive commercial ELISA for p24gag (PerkinElmer, Waltham, MA, USA).
Experimental determination of anti-HIV-1 activity and cytotoxic effects
For antiviral experiments with whole SP, fractionated SP, and synthetic and purified cationic peptide fragments of semenogelin I, which we termed SG-1, Tzm-bl cells were trypsinized, counted via hemocytometer, and seeded to 6 × 104 cells/ml (6000 cells/well) in a 96-well microtiter plate. After 24 h, cells were treated in triplicate with 50 μl of D10 containing treatment sample. Immediately following treatment, 50 μl of virus diluted to a constant final concentration (5 ng p24/ml) in D10 was added to each well and incubated at 37°C in 5% CO2. After a 24-h infection period, cells were lysed using a Bright Glo luciferase system (Promega, Madison, WI, USA), and luciferase was quantified with a Spectramax luminometer (Molecular Devices Corp., Sunnyvale, CA, USA), with an integration time of 5 s/well (16). Ability to prevent HIV-1 infection was measured as a percentage reduction in luciferase (relative light units) compared to the positive viral control (medium and virus only), while the negative control (medium only, no virus) acted as the baseline comparison. Metabolic activity and cytotoxicity of the cells were verified by a tetrazolium-based [3-(4,5-dimethyl-2-thizolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT)] assay according to the manufacturer’s instructions (R&D Systems, Minneapolis, MN, USA) and a standard trypan blue dye-exclusion assay (17), performed in parallel with the HIV-treated cells.
PM1 cells (1.5×105/0.1 ml) were treated with whole SP diluted in R20, infected with HIV-1 BaL (2 ng p24/ml), and incubated for 3 h at 37°C in 5% CO2 with agitation of the cell suspension every 30 min. The cell suspension was then diluted with 2 ml of R20; pelleted; resuspended in 0.5 ml of diluted whole SP in R20 medium, or vehicle only; and incubated at 37°C in 5% CO2. At 3 d postinfection, the supernatants were collected and stored at −20°C, and the remaining cells in culture were resuspended in 0.5 ml of diluted whole SP or R20 alone. On d 5, supernatants were collected and stored, and a trypan blue dye assay was performed on the cells, as described previously. To quantify the viral inhibition of SP, d 5 supernatants underwent an ELISA for p24gag (PerkinElmer) and were compared to vehicle-only control.
Resolution of SP via 2-dimensional polyacrylamide gel electrophoresis (2-D PAGE)
Whole SP was electrophoresed on a 2-D acid-urea (AU)/tricine-SDS-PAGE as described previously (6). Briefly, SP was prepared in a 1:1 ratio with 0.2% cetyltrimethyl ammonium bromide (CETAB) and then admixed 1:1 with 3× AU loading dye (9 M urea, 5% acetic acid, and methyl green). A 12.5% native AU-PAGE was used as the first dimension to separate peptides based on their overall cationic charge density. The gel was electrophoresed at 65 V for 20–22 h and stained with 10% (v/v) amido black, and each lane was excised and prepared for the second dimension by soaking in equilibrium buffer containing 10 mg/ml DTT. Gel strips were inserted horizontally and electrophoresed on a 16% tricine-SDS-PAGE (second dimension) at 35 mA, for 27–29 h. Individual protein spots were visualized using Sypro Ruby gel stain (Bio-Rad, Hercules, CA, USA), excised from the gel, and stored in 0.01% acetic acid at −20°C until analyzed by mass spectrometry.
Identification of individual cationic components in human SP
Excised 2-D PAGE spots underwent trypsin digestion and mass spectrometric (MALDI-TOF/TOF MS/MS) analyses as described previously (6). The resulting digested peptide fragments were identified utilizing GPS Explorer 2.0 software (Applied Biosystems, Foster City, CA, USA) and a Mascot (http://www.matrixscience.com) search engine. The NCBInr database (National Center for Biotechnology Information, Bethesda, MD, USA) and Homo sapiens taxonomy were used for the searches.
Fractionation of SP based on charge density
Whole SP was prepared for fractionation by adding 3× AU loading dye (1:2 with 70 μl SP-1). The Miniprep Cell Fractionation and Continuous-Elution Electrophoresis System (Bio-Rad) with a 12.5% AU-PAGE column gel was utilized to collect larger amounts of cationic polypeptides. Elution buffer was 5% acetic acid using an isocratic gradient collecting at 0.1 ml/min. The column gel was electrophoresed at 70 V overnight, and fractions were collected every 15 min (1.5 ml/fraction). Fractions (40) were collected for each run and either processed for purification immediately or stored at 4°C overnight.
Processing of fractionated SP
Fractions collected from the AU-PAGE column gel were dried under vacuum (Speedvac; ThermoSavant, Holbrook, NY, USA) with no heat. Fractions were then resuspended in high-pressure liquid chromatography (HPLC)-grade H2O and electrophoresed in order of elution (fractions 1–40) on mini-16% tricine-SDS gels (4 μl sample + 5 μl Laemli buffer + 3 μl SDS-loading buffer). These single-dimension electropherograms were compared with the 2-D PAGE gels to identify bands.
Fractions 1–40 then underwent solid-phase extraction to desalt and concentrate each sample in preparation for cell culture. Sep-Pak C18 polypropylene syringe-barrel cartridges (1 cc, 50 mg; Waters Corp., Milford, MA, USA) were used according to the manufacturer’s instructions. Cartridges were preconditioned with ddH2O/0.1% trifluoroacetic acid (TFA) and eluted at a rate of ∼0.25 ml/min. To ensure maximum binding, the flow-through was reapplied to the column bed 3 times, washed with ddH2O/0.1% TFA, and eluted with 60% acetonitrile (ACN) in HPLC-grade H2O, followed by 100% methanol. All elutions were collected, dried under vacuum, and resuspended in HPLC-grade H2O. Mini-16% tricine-SDS gels were used to confirm that the protein banding patterns were identical to those of predesalted fractions.
Purification of SG-1 utilizing reverse-phase (RP)-HPLC
Fractions 5–9 (of 40) containing the cationic peptide fragment of semenogelin I that we termed SG-1 were pooled and subjected to RP-HPLC. For RP-HPLC, a Waters 2795 separation module and dual-wavelength absorbance detector with a Waters XTerra analytical column (silica, C18, 4.6×250 mm, 5.0-μm particle size) were used. The aqueous mobile phase used was HPLC-grade H2O with 0.1% TFA, against a 1-ml/min gradient from 0–60% ACN/0.08%TFA. Peak fractions (280-nm absorbance) were vacuum-dried and resuspended in 0.1% TFA and HPLC-grade H2O. All fractions containing the semipurified peptide were pooled together and were subjected to microbore RP-HPLC (silica C18, 1.0×150 mm, 3.5-μm bead size, XTerra column; Waters Corp.) under a similar ACN gradient. Eluted fractions corresponding to the specific SG-1 peak on the chromatogram were vacuum-dried and resuspended in HPLC-grade H2O. A Micro BCA Protein Assay (Pierce), using diluted BSA as a standard, was performed to quantify the concentration of purified SG-1 in the pooled sample. Purified SG-1 was analyzed by MALDI-TOF-MS and N-terminal Edman degradation to determine the exact mass of the polypeptide sequence.
Semenogelin-specific Western blot analysis
Samples were electrophoresed in either 16% mini-tricine-SDS gels or 12.5% mini-AU gels, as described previously (18, 19). Gels were either stained with Sypro Ruby gel stain (Bio-Rad) or transferred onto an Immobilon-P membrane (Millipore, Billerica, MA, USA) activated with methanol and Tris-buffered saline (TBS) at 180 mA for 45 min using the Mighty Small Transphor vertical electrophoresis system (Amersham Biosciences, Piscataway, NJ, USA). Membranes were fixed with 0.05% glutaraldehyde in TBS for 20 min, and blocked for 30 min at 37°C with Superblock (Pierce). Membranes were incubated overnight with a mouse polyclonal anti-semenogelin I (SGI) antibody (Novus Biologicals, Littleton, CO, USA), immunoreactive against the full-length human protein, diluted 1:1000 in antibody buffer (Superblock in TBS+0.05% Tween-20). Once washed, blocked at 37°C for 15 min, and incubated with peroxidase-conjugated anti-mouse immunoglobulin-G for 2 h, the membranes were again washed and developed using the Immun-Star HRP (Bio-Rad). Membranes were imaged and analyzed using ChemiDoc XRS with Quantity One software (Bio-Rad).
Statistical analyses
The antiviral and cytotoxicity assays were performed in triplicate for each treatment of each experimental condition. For the TZM-bl antiviral assays, luciferase was measured as relative light units (RLU), and the infected vehicle-only controls were set as 100% infection. For the PM1 antiviral assays, p24 ELISA quantification established the infected, vehicle-only control as 100% infection. Metabolic and cytotoxicity assays compared results to the untreated, vehicle-only control, calculating variations as a percentage of the baseline. Individual treatments were analyzed by either 1-way ANOVA with Tukey’s multiple posttest comparisons, or 2-tailed unpaired t test. Mass spectrometric analysis of excised spots from the 2-D PAGE were performed, and identified proteins with a confidence index >85% and ion score >40 were considered positive.
RESULTS
Human SP exhibits significant anti-HIV-1 activity
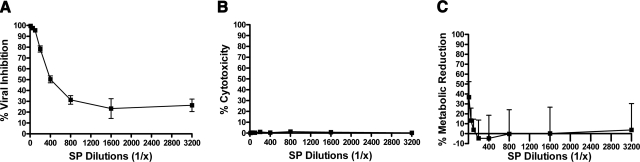
To determine the intrinsic anti-HIV-1 activity of SP, we assayed whole SP against the R5 strain HIV-1 BaL in TZM-bl cells. An R5 phenotype was chosen since it is primarily responsible for sexually transmitted HIV-1. After 24 h of infection, the cells were lysed, and luciferase activity was measured (Fig. 1A; P<0.001; n=3). Whole SP revealed significant antiviral activity: at 1% concentration (v/v, 1:100), viral inhibition remained >95%, and SP-1 dilutions >1:100 reduced the rates of infection in a dose-dependent manner in the absence of cytotoxicity (Fig. 1B; n=3) or adverse effects on cellular metabolism (Fig. 1C; n=3). Because SP diluted up to 1:3200 retained anti-HIV-1 activity, these data suggest that the SP component of semen is potently active against HIV-1.
Figure 1.
Whole SP inhibits HIV-1 infection. A) TZM-bl cell cultures were treated with serial dilutions of SP or a PBS vehicle control, and infected with the BaL laboratory strain of HIV-1 (5 ng/ml p24) for 24 h. Inhibition of viral infection was measured as percentage reduction in luciferase activity (RLU) vs. an infected, vehicle-only control. All dilutions vs. control were significantly different; P < 0.0001. B) Trypan blue assays were performed at 24 h to measure the cytotoxicity of SP and were expressed as percentage of nonviable cells over total number of cells. C) An MTT assay was also performed in the absence of infection and measured at 24 h posttreatment. Reduction in cellular metabolic rate was measured as percentage of decreased tetrazolium production vs. vehicle-only control. Only the 1:10 dilution statistically reduced metabolic activity vs. control; P < 0.0001; n = 3. Error bars = sem.
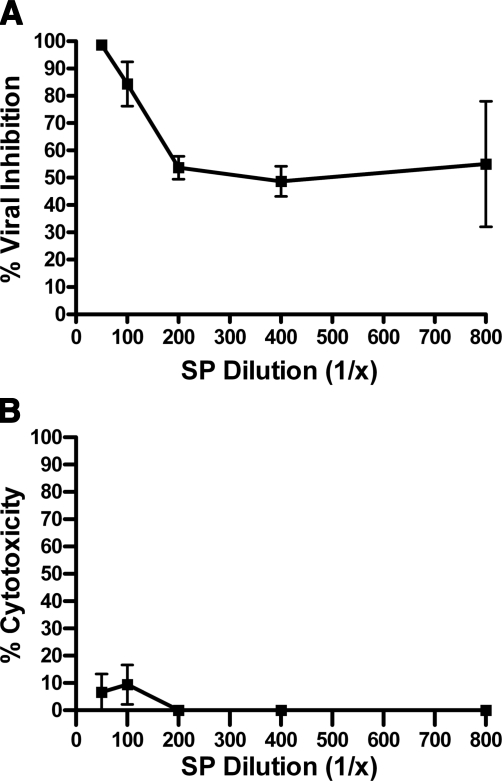
To further validate the anti-HIV activity of SP, the antiviral activity was confirmed by infecting PM1 cells with HIV-1 BaL and measuring viral p24 release. Virus in the presence or absence of vehicle or serial dilutions of SP was incubated with PM1 cells for 5 d. As compared to a vehicle-only control, the lowest dilution (1:50) of SP exhibited complete viral inhibition, while serial dilutions displayed dose-dependent antiviral activity (Fig. 2A; P<0.0001; n=3), in the absence of appreciable cytotoxicity (Fig. 2B). Taken together, these results confirm the anti-HIV-1 activity of SP.
Figure 2.
Whole SP inhibits HIV-1 release. A) PM1 cells were infected with HIV-1 BaL (2 ng/ml p24) in the presence or absence of serially diluted SP or vehicle-only control. Five days postinfection, supernatant was collected to quantify p24 release by ELISA. Data are expressed as percentage inhibition of infection vs. infected vehicle-only control; P < 0.0001 for all dilutions. B) PM1 cells were subjected to trypan blue assay; cytotoxicity was expressed as percentage of cells vs. total number of cells in the infected vehicle-only control. Only the 1:50 dilution was cytotoxic; P < 0.05; n = 3–6. Error bars = sem.
Human SP contains numerous cationic polypeptides
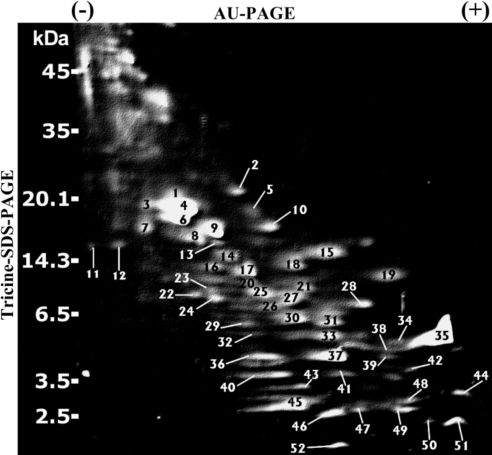
We have reported previously that the majority of cationic polypeptides within human vaginal fluid collectively inhibit HIV-1 infection (6). Herein, we sought to determine whether human SP contains cationic polypeptides, whether these polypeptides are similar to those of human vaginal fluid, and whether the polypeptides exert activity against HIV-1. A unique 2-D AU/tricine-SDS-PAGE approach was utilized to resolve the cationic polypeptide fraction of SP. Figure 3 reveals a Sypro Ruby-stained 2-D electropherogram of whole SP (6.5 μl) electrophoresed in an AU-PAGE gel in the first dimension (horizontal), followed by tricine-SDS-PAGE in the second dimension (vertical), with each spot representing an individual polypeptide. Spots were excised from the gel and digested with trypsin, and 52 individual polypeptides were identified by MALDI-TOF/TOF MS/MS. The identity of each of the 52 polypeptides in Fig. 3 is provided in Table 1. Interestingly, 33 of the spots were found to represent fragments of 8 parental proteins commonly found in SP, several of which have putative roles in innate host defense: semenogelin I, semenogelin II, prolactin-induced protein (PIP), PAP, lactoferrin, prostatic-specific antigen, β-microseminoprotein, and cystatin C.
Figure 3.
Resolution and identification of 52 individual cationic polypeptides in human SP. Whole SP (6.5 μl SP-1) was electrophoresed by AU-PAGE in the first dimension, followed by a tricine-SDS-PAGE in the second dimension, and stained with Sypro Ruby. Note that polypeptides with the highest cationic charge density migrated toward the bottom right side of the gel. Protein spots were excised and identified by MALDI-TOF/TOF MS/MS analysis. All labeled spots (spots 1–52) correspond to proteins described in Table 1.
TABLE 1.
Identified cationic polypeptides from 2-D PAGE and MALDI-TOF/TOF MS/MS analysis
| Spot | Spot ID | Accession | Spot mass (kDa) | Ion score | CI (%) | Protein mass (kDa) |
|---|---|---|---|---|---|---|
| 1 | Prolactin-induced protein | gi 4505821 | ∼20 | 312 | 100 | 16.56 |
| 2 | Semenogelin I | gi 37731930 | ∼20 | 18 | 99.46 | 10.38 |
| 3 | Prolactin-induced protein | gi 4505821 | ∼19 | 148 | 100 | 16.56 |
| 4 | Prolactin-induced protein | gi 4505821 | ∼19 | 0 | 99.35 | 16.56 |
| 5 | Ig heavy-chain V region | gi 346162 | ∼18.5 | 64 | 99.69 | 13.15 |
| 6 | Prolactin-induced protein/PSA | gi 4505821/gi 14250150 | ∼18 | 277/42 | 100/99.8 | 16.56/44.48 |
| 7 | Keratin 1, type II, cytoskeleton | gi 7428712 | ∼17 | 320 | 100 | 65.45 |
| 8 | PAP/prolactin induced protein | gi 27574174/gi 4505821 | ∼16.5 | 154/86 | 100/100 | 40.97/16.56 |
| 9 | Semenogelin II | gi 47683071 | ∼17 | 0 | 99.94 | 65.43 |
| 10 | Semenogelin I preproprotein | gi 33392699 | ∼17 | 25 | 99.55 | 45.26 |
| 11 | Lactoferrin | gi 386855 | ∼15 | 53 | 99.58 | 30.56 |
| 12 | Lactoferrin | gi 386855 | ∼15 | 67 | 99.99 | 30.56 |
| 13 | Cystatin C precursor/semenogelin II | gi 15341822/gi 32699322 | ∼16 | 135/62 | 100/99.9 | 15.78/65.43 |
| 14 | Keratin 1, type II, cytoskeleton | gi 7331218 | ∼14.3 | 279 | 100 | 65.97 |
| 15 | Microseminoprotein β | gi 225159 | ∼14.5 | 91 | 100 | 10.64 |
| 16 | Prolactin-induced protein | gi 4505821 | ∼14 | 196 | 100 | 16.56 |
| 17 | Semenogelin II | gi 4506885 | ∼13 | 62 | 99.979 | 65.4 |
| 18 | Bullous pemphigoid antigen | gi 27923959 | ∼14 | 70 | 99.92 | 371.9 |
| 19 | Protein product of HMFN1661 | gi 51555816 | ∼12 | 46 | 82.09 | 19.68 |
| 20 | Keratin 10 | gi 623409 | ∼12 | 56 | 99.72 | 57.21 |
| 21 | Semenogelin II precursor | gi 47683071 | ∼10 | 76 | 100 | 65.43 |
| 22 | Prostatic acid phosphatase | gi 6137673 | ∼9 | 58 | 99.66 | 39.7 |
| 23 | Hypothetical protein | gi 21732890 | ∼10 | 61 | 99.37 | 196.9 |
| 24 | Prostatic acid phosphatase | gi 16740983 | ∼8.5 | 42 | 98.037 | 44.51 |
| 25 | Semenogelin I preproprotein | gi 38049014 | ∼9 | 207 | 100 | 45.27 |
| 26 | Semenogelin I | gi 32450803 | ∼8 | 30 | 99.836 | 45.29 |
| 27 | Semenogelin I preproprotein | gi 32450803 | ∼8.5 | 117 | 100 | 45.29 |
| 28 | ATP synthase, H+ transporting, mitochondrial | gi 12654679 | ∼8 | 55 | 97.97 | 12.58 |
| 29 | Prostatic acid phosphatase | gi 515997 | ∼7 | 74 | 99.99 | 44.53 |
| 30 | Semenogelin I preproprotein | gi 38049014 | ∼7.5 | 115 | 100 | 45.27 |
| 31 | Semenogelin I | gi 37728854 | ∼7 | 85 | 100 | 23.79 |
| 32 | Semenogelin I | gi 33392699 | ∼6 | 47 | 99.65 | 45.26 |
| 33 | Semenogelin I preproprotein | gi 32450803 | ∼6 | 58 | 99.99 | 45.29 |
| 34 | DAP-2 | gi 89028478 | ∼5 | 40 | 26.15 | 9.169 |
| 35 | Semenogelin I | gi 37728828 | ∼5–8 | 12 | 99.49 | 19.92 |
| 36 | Clusterin | gi 42716297 | ∼5 | 108 | 100 | 57.79 |
| 37 | Semenogelin I | gi 32450803 | ∼5 | 80 | 100 | 45.29 |
| 38 | FLJ00150 protein | gi 18676506 | ∼4.75 | 49 | 90.04 | 207.2 |
| 39 | Microtubule-actin crosslinking factor | gi 55665463 | ∼4.5 | 44 | 73.18 | 505.3 |
| 40 | Semenogelin I | gi 37728854 | ∼4 | 41 | 81.02 | 23.79 |
| 41 | SHQ1 homolog | gi 34193077 | ∼4 | 51 | 93.85 | 65.11 |
| 42 | Intercellular adhesion molecule | gi 85068578 | ∼4 | 48 | 89.45 | 29.63 |
| 43 | Semenogelin II | gi 4506885 | ∼3.5 | 44 | 97.82 | 65.4 |
| 44 | NPD003 | gi 5531825 | ∼3.5 | 41 | 41.34 | 33.31 |
| 45 | Semenogelin I preproprotein | gi 37728854 | ∼3 | 54 | 99.28 | 23.79 |
| 46 | Semenogelin I preproprotein | gi 38049014 | ∼2.8 | 80 | 100 | 45.27 |
| 47 | Semenogelin I | gi 37728854 | ∼3 | 74 | 99.99 | 23.79 |
| 48 | RQCD1 protein | gi 13937977 | ∼3 | 45 | 77.7 | 29.11 |
| 49 | Semenogelin II | gi 32699322 | ∼2.8 | 67 | 100 | 65.43 |
| 50 | HSPC337 | gi 6841324 | ∼2.8 | 53 | 96.12 | 20.39 |
| 51 | Microtubule-actin crosslinking factor | gi 55665463 | ∼2.75 | 49 | 90.48 | 505.3 |
| 52 | Growth differentiation factor 2 | gi 7705308 | ∼2 | 40 | 17.14 | 47.29 |
Table identifies spots shown in Fig. 3. CI, confidence index.
Cationic polypeptides are responsible for the anti-HIV-1 activity of SP
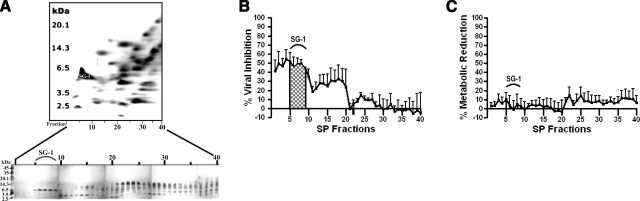
We utilized a continuous-elution semipreparative AU-PAGE column gel to isolate each cationic polypeptide fraction from whole SP for downstream analysis of anti-HIV-1 activity and cytotoxicity. Forty SP fractions, which corresponded to the majority of cationic polypeptides present in Fig. 3, were collected and individually subjected to tricine-SDS-PAGE. Note that the combination of using a fractionated AU column gel and tricine-SDS gels is equivalent to the first and second dimensions, respectively, of the analytical 2-D AU/tricine-SDS-PAGE. Referencing the protein bands in the tricine-SDS gels (Fig. 4A) to the corresponding spots of a 2-D PAGE electropherogram of SP (Figs. 3 and 4A) afforded the identification of each protein band.
Figure 4.
Cationic polypeptide fractions of SP exhibit significant anti-HIV-1 activity. A) After column-gel AU-fractionation of whole SP, 40 fractions were collected and electrophoresed on mini-tricine-SDS gels. Silver staining revealed peptide bands retained in each individual fraction. To reference fractions to the corresponding spots previously identified by 2-D PAGE, the 2-D Sypro Ruby-stained gel image was inverted and displayed as a negative digital effect, and flipped in the horizontal axis as compared with Fig. 1. In this orientation, the most cationic polypeptides are to the left of the gel. B) Cells were treated in a TZM-bl antiviral assay with the fractionated SP (fractions 1–40) at a dilution of 1:10. Fractions 5–9 were most active and contained the identified semenogelin I peptide termed SG-1 (Fig. 1, spot 35). C) An MTT assay conducted in parallel to the antiviral assay measured metabolic reduction as percentage reduction of the medium-only negative control; n = 4. Error bars = sem.
Each of the 40 SP fractions was subsequently prepared for antiviral assays by undergoing lyophilization to remove volatile acetic acid, followed by solid-phase extraction to desalt the samples. Samples were diluted 1:10 in ddH2O and subjected to antiviral assays against HIV-1 BaL as described above. Fractions 1–10, which contained polypeptides with the highest cationic charge density, exhibited the greatest activity against HIV-1 (Fig. 4B; n=4) as well as negligible cytotoxicity and reduction of cellular metabolic activity (Fig. 4C; n=4).
SG-1, a 52-residue semenogelin I-derived peptide, is active against HIV-1
Next, we assessed whether individual cationic polypeptides in SP were directly active against HIV-1. The most abundant, highly cationic protein on Sypro Ruby-stained 2-D PAGE gels (Fig. 3, spot 35) was chosen since the corresponding fractions, eluted from an AU-PAGE column gel, exhibited appreciable activity against HIV-1 (Fig. 4B). Fractions 5–9 from an AU-PAGE column gel (Fig. 4A) were pooled and subjected to a 2-step RP-HPLC purification (Fig. 5). The resulting single-protein band was visualized (Fig. 6) and sequenced by a combination of N-terminal Edman peptide sequencing and MALDI-TOF MS (Fig. 7A). The peptide, termed SG-1, was identified as a 5750.638-Da peptide whose mass and N-terminal sequence (HNKQEGRDHDKSKGH-) corresponded to the theoretical mass of 5750.837 Da from the 52-aa sequence NH2-HNKQEGRDHDKSKGHFHRVVIHHKGGKAHRGT QNPSQDQGNSPSGKGISSQY-COOH, which is a naturally cleaved fragment of the mature 462-aa semenogelin I polypeptide (9). The SG-1 peptide had been identified previously as one of several basic proteins in liquefied SP (20).
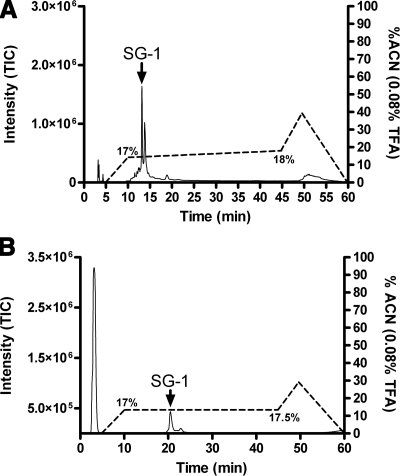
Figure 5.
Purification of SG-1 by analytical and microbore RP-HPLC. SG-1 was purified by a 2-step RP-HPLC, with fractionation using the organic solvent ACN and 0.08% TFA as an ion-pairing agent. Solvent gradient is shown as a percentage (dotted line); eluted peptides are represented as intensity of the total ion current (TIC; solid line). A) Analytical column was loaded with the fractionated SP-1 pool (Fig. 4, fractions 5–9). B) Semipure peak fraction representing SG-1 was loaded onto a microbore column, and SG-1 was eluted at 17.25% of the ACN solvent.
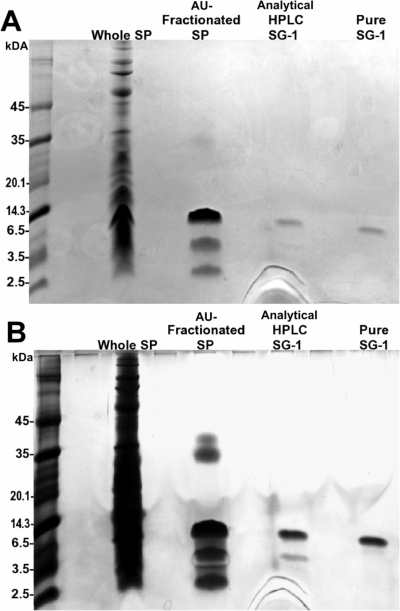
Figure 6.
Stepwise purification of the SG-1 peptide. Purification of SG-1 from whole SP to isolated pure peptide was represented by electrophoresing samples from each step of purification on a mini-tricine-SDS gel. A 1:100 dilution of SP, a sample of fractionated SP (pooled fractions 5–9), a sample of the semipurified SG-1 from analytical RP-HPLC, and the purified SG-1 from the microbore RP-HPLC column (4 μl each) were visualized with Coomassie blue (A) and silver staining (B). Native SG-1 was utilized in the remaining assays.
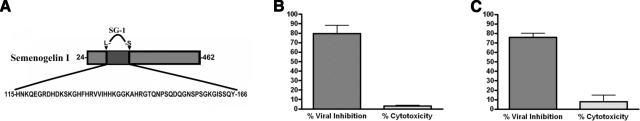
Figure 7.
SG-1 peptide is naturally active against HIV-1. SG-1 was sequenced and identified by Edman degradation and mass spectrometric analysis. A) Mature semenogelin I, lacking the 23-residue signal peptide sequence; the 52-aa SG-1 sequence is identified. B) A TZM-bl antiviral assay against HIV-1 BaL (5 ng/ml p24) was performed with the natural SG-1 peptide (5 μg/ml). Inhibition of viral infection was measured as percentage reduction in luciferase activity vs. vehicle-only control. A trypan blue assay was performed in parallel to measure cytotoxicity, expressed as percentage of nonviable cells vs. total cells. C) PM1 cells were infected with HIV-1 BaL (2 ng/ml p24) in the presence or absence of natural SG-1 peptide (15 μg/ml) or vehicle-only control. Supernatant was collected 5 d postinfection to quantify p24 release by ELISA. Data are expressed as percentage inhibition of infection vs. infected vehicle-only control. On d 5, postinfection PM1 cells were subjected to trypan blue assay; cytotoxicity was expressed as percentage of cells vs. total cells in the infected vehicle-only control; n = 3. Error bars = sem.
To determine whether SG-1 exhibited antiviral activity, the purified peptide was subjected to anti-HIV-1 assays. SG-1 maintained anti-HIV-1 activity in both a TZM-bl cell assay (Fig. 7B; n=3), and a PM1 cell antiviral assay (Fig. 7C; n=3), and displayed negligible cytotoxicity (Fig. 7B, C). As the concentration of SG-1 active against HIV-1 in vitro is far less than the estimated physiological concentration (∼80 μg SG-1/ml whole SP, data not shown), it is likely that the SG-1 peptide contributes significantly to the antiviral host defense of SP.
Prolonged incubation of whole SP reduces anti-HIV-1 activity and degrades the majority of semenogelin I peptides
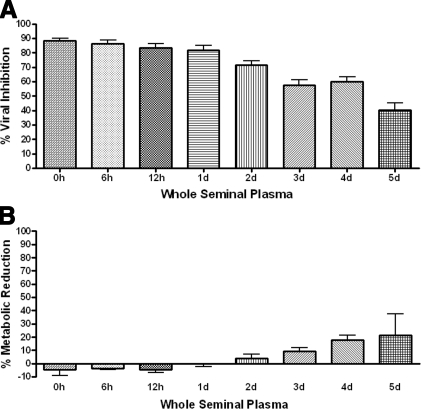
Since semenogelin-derived peptides continue to degrade over time, we wanted to determine whether proteolytic cleavage affects the anti-HIV-1 activity of human SP. The SP samples were incubated at timed intervals at 37°C with constant mild agitation (250 rpm). Incubated SP was tested for antiviral activity in comparison to whole SP without incubation in a TZM-bl cell assay (Fig. 8A; n=3). SP incubated ≤24 h retained significant anti-HIV-1 activity in the absence of cytotoxicity (Fig. 8B), while incubation intervals >24 h exhibited a reduction in antiviral activity. These data suggest that the anti-HIV-1 activity of incubated SP decreases proportionally with increased incubation time.
Figure 8.
Incubation of whole SP affects anti-HIV-1 activity. A) TZM-bl cells were treated with 1:100 dilutions of whole SP incubated for timed intervals or a PBS vehicle control (0, 6, and 12 h, and 1, 2, 3, 4, and 5 d at 37°C, with gentle agitation; 250 rpm). Cells were infected with HIV-1 BaL (5 ng/ml p24) for 24 h. Inhibition of viral infection was measured as percentage reduction in luciferase activity vs. infected vehicle-only control. All SP samples were significantly higher in activity vs. control; P < 0.0001. B) MTT assays were also performed, in the absence of infection, and measured at 24 h posttreatment. Reduction in cellular metabolic activity was measured as percentage of decreased tetrazolium production vs. vehicle-only control. No dilution statistically reduced metabolic activity vs. control; n = 3. Error bars = sem.
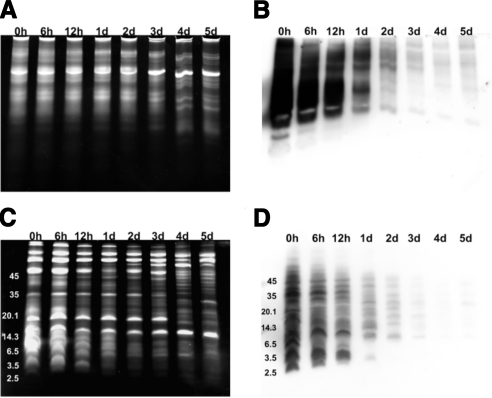
Postejaculation, the SP coagulum liquefies due to proteolytic processing; however, this proteolytic cleavage does not cease once the coagulum is liquefied (21). To determine if the effects of prolonged SP incubation included degradation of cationic polypeptides, the samples were subjected to AU-PAGE (Fig. 9A), and tricine-SDS-PAGE (Fig. 9C). Protein-band patterns visibly changed at 6 h of incubation and progressively dispersed into protein bands with higher cationic charges and smaller molecular mass. To track the effects of prolonged incubation on the antimicrobial semenogelin I proteins, samples were subjected to Western blot analysis (Fig. 9B, D) with an antisemenogelin I polyclonal antibody. It has been shown that semenogelins are degraded selectively over time (14). The effects of incubation on semenogelin I proteins were visible after 6 h, exhibiting an increasingly degraded protein-band pattern in samples incubated for 24 h or longer. These data suggest that the decreased anti-HIV-1 activity of incubated SP is correlated directly with the increased degradation of semenogelin I proteins. Due to the abundance of semenogelin-derived peptides in comparison to other identified peptides observed in our study (Table 1), and the activity exhibited by semenogelin-derived peptides, this study confirms the importance of semenogelins to the intrinsic antiviral activity of SP.
Figure 9.
Prolonged incubation of SP results in increasingly degraded semenogelin I. Incubated SP samples (1 μl/lane) were electrophoresed on minipolyacrylamide gels. Samples were electrophoresed on 12.5% mini-AU gels and stained with Sypro Ruby protein stain (A) or Western blotting with a polyclonal anti-semenogelin I antibody(B). Degradation of cationic semenogelin I peptides was evident with prolonged incubation time. SP samples were also electrophoresed on 16% mini-tricine-SDS gel and stained with Sypro Ruby protein stain (C) or Western blotting with the anti-semenogelin I antibody (D). A similar pattern of peptide degradation was shown with prolonged incubation.
DISCUSSION
Mucosal secretions are thought to play a fundamental role in host defense, due in large part to intrinsic antimicrobial peptides and proteins elaborated in the fluid. In the current study, we revealed that SP exhibited significant anti-HIV-1 activity and that the activity resided mainly in the cationic polypeptide fraction. Furthermore, polypeptide fractions with greater cationic charge density demonstrated increased anti-HIV-1 activity as compared to less cationic polypeptide fractions, suggesting that charge was an essential determinant of antiviral activity. On prolonged incubation of SP, antiviral activity decreased concomitant with the degradation of semenogelin I peptides, further emphasizing the role of cationic antimicrobial polypeptides in anti-HIV-1 host defense. While our study is the first to associate the majority of cationic polypeptides found in human SP with the fluid’s intrinsic anti-HIV-1 activity, this finding was not altogether surprising: The vast majority of polypeptides with reported activity against microbes and viruses are cationic in nature (22).
The 2-D AU/tricine-SDS-PAGE system was chosen for its unique ability to separate cationic polypeptides. Indeed, we have utilized this technique to identify cationic polypeptides that contribute to the antimicrobial and antiviral host defenses of human nasal fluid and vaginal fluids (6, 23). In the current study, 33 of the identified polypeptides were relevant to host defense. Many polypeptides were cleavage products of semenogelin I and semenogelin II, the most abundant proteins in SP (10, 24, 25), both of which have been reported to exhibit antibacterial activity (13, 14). In addition, the protease PAP is involved in the processing and degradation of semenogelins and likely plays an important role during postcoital coagulum processing to liberate active anti-HIV-1 peptides (26). The seminal vesicle-derived PIP, also known as gp17 glycoprotein, is a high-affinity CD4 ligand that has been shown to inhibit HIV-1 gp120-mediated syncytium formation (27). Lactoferrin, a broad-spectrum antimicrobial protein, can prevent the early stages of HIV-1 infection by preventing the virus from entering host cells (28,29,30,31). β-Microseminoprotein is one of the predominant proteins secreted from the prostate, and while no definitive biological functions are ascribed to this protein, it has been shown to decrease tumor growth in the prostate and has purported roles in host defense (32, 33). The cysteine proteinase inhibitor cystatin C reportedly prevents viral replication of HSV and coronaviruses (34,35,36). Collectively, the identity and function of these polypeptides strongly suggest a function for SP in antiviral host defense. As most of these polypeptides are not components of vaginal fluid, it remains to be determined whether the antiviral polypeptides from SP work in concert with polypeptides from vaginal fluid to prevent sexual transmission of HIV-1.
SP is a complex biological fluid, containing both antiviral and proviral factors (37). Notably, a recent study revealed that naturally occurring PAP fragments in semen form amyloid fibrils that enhance HIV-1 infection by capturing virions and promoting attachment to target cells (15). Several differences between their approach and our current study might explain these contrasting findings. Methodological differences in our study include the initial liquefaction of SP, the use of FBS in HIV-1 infection assays, and extended incubation of target cells with SP. While their study defends the washing of cell monolayers after 3 h due to concerns of cytotoxicity, our study suggests that at the low concentrations of SP needed to observe antiviral activity (<2% v/v of SP) cellular metabolism was normal and insignificant cytotoxicity was observed. Our 2-D analysis displayed PAP fragments at ∼16.5 kDa, but we did not observe any at the designated 4- to 4.5-kDa range previously published as HIV enhancers. While these differences prevent a truly paralleled comparison, both studies should be taken together as they suggest that proviral and antiviral forces may be in direct competition in SP in vivo. In fact, we have also shown that vaginal fluid elaborates components (e.g., calgranulin A) that are permissive for viral infection (6). Whether the relative concentrations of proviral and antiviral components in an individual’s seminal or vaginal fluid are predictive of susceptibility to HIV-1 infection is a subject that warrants further investigation.
Our study demonstrated that human SP exhibits significant intrinsic anti-HIV-1 activity and characterized a cationic polypeptide, SG-1, as an anti-HIV-1 component of SP. Future studies, which we are actively pursuing, will further analyze the mode of viral inhibition SG-1 uses. While previously established as antibacterial, no other studies have determined that semenogelins exhibit significant antiviral activity. These findings set the stage for future studies to determine the host defense role of the myriad other cationic polypeptides we identified in SP. The presence of such an anti-HIV-1 mechanism in SP may be one reason why the chance of infection is so low (∼3 in 1000 coital acts) in healthy populations (38).
Acknowledgments
We thank the laboratory staff at the Center for Reproductive Medicine (Orlando, FL, USA) for their expert technical assistance.
References
- Lehrer R. I., Ganz T. Cathelicidins: a family of endogenous antimicrobial peptides. Curr Opin Hematol. 2002;9:18–22. doi: 10.1097/00062752-200201000-00004. [DOI] [PubMed] [Google Scholar]
- Ganz T., Weiss J. Antimicrobial peptides of phagocytes and epithelia. Semin Hematol. 1997;34:343–354. [PubMed] [Google Scholar]
- Klotman M. E., Chang T. L. Defensins in innate antiviral immunity. Nat Rev Immunol. 2006;6:447–456. doi: 10.1038/nri1860. [DOI] [PubMed] [Google Scholar]
- Ganz T., Selsted M. E., Szklarek D., Harwig S. S., Daher K., Bainton D. F., Lehrer R. I. Defensins Natural peptide antibiotics of human neutrophils. J Clin Invest. 1985;76:1427–1435. doi: 10.1172/JCI112120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole A. M., Lehrer R. I. Minidefensins: antimicrobial peptides with activity against HIV-1. Curr Pharm Des. 2003;9:1463–1473. doi: 10.2174/1381612033454667. [DOI] [PubMed] [Google Scholar]
- Venkataraman N., Cole A. L., Svoboda P., Pohl J., Cole A. M. Cationic polypeptides are required for anti-HIV-1 activity of human vaginal fluid. J Immunol. 2005;175:7560–7567. doi: 10.4049/jimmunol.175.11.7560. [DOI] [PubMed] [Google Scholar]
- Malm J., Sorensen O., Persson T., Frohm-Nilsson M., Johansson B., Bjartell A., Lilja H., Stahle-Backdahl M., Borregaard N., Egesten A. The human cationic antimicrobial protein (hCAP-18) is expressed in the epithelium of human epididymis, is present in seminal plasma at high concentrations, and is attached to spermatozoa. Infect Immun. 2000;68:4297–4302. doi: 10.1128/iai.68.7.4297-4302.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Com E., Bourgeon F., Evrard B., Ganz T., Colleu D., Jegou B., Pineau C. Expression of antimicrobial defensins in the male reproductive tract of rats, mice, and humans. Biol Reprod. 2003;68:95–104. doi: 10.1095/biolreprod.102.005389. [DOI] [PubMed] [Google Scholar]
- Lilja H. Structure, function, and regulation of the enzyme activity of prostate-specific antigen. World J Urol. 1993;11:188–191. doi: 10.1007/BF00185066. [DOI] [PubMed] [Google Scholar]
- Robert M., Gagnon C. Semenogelin I: a coagulum forming, multifunctional seminal vesicle protein. Cell Mol Life Sci. 1999;55:944–960. doi: 10.1007/s000180050346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert M., Gagnon C. Sperm motility inhibitor from human seminal plasma: association with semen coagulum. Hum Reprod. 1995;10:2192–2197. doi: 10.1093/oxfordjournals.humrep.a136267. [DOI] [PubMed] [Google Scholar]
- Bourgeon F., Evrard B., Brillard-Bourdet M., Colleu D., Jegou B., Pineau C. Involvement of semenogelin-derived peptides in the antibacterial activity of human seminal plasma. Biol Reprod. 2004;70:768–774. doi: 10.1095/biolreprod.103.022533. [DOI] [PubMed] [Google Scholar]
- Zhao H., Lee W. H., Shen J. H., Li H., Zhang Y. Identification of novel semenogelin I-derived antimicrobial peptide from liquefied human seminal plasma. Peptides. 2008;29:505–511. doi: 10.1016/j.peptides.2008.01.009. [DOI] [PubMed] [Google Scholar]
- Edstrom A. M., Malm J., Frohm B., Martellini J. A., Giwercman A., Morgelin M., Cole A. M., Sorensen O. E. The major bactericidal activity of human seminal plasma is zinc-dependent and derived from fragmentation of the semenogelins. J Immunol. 2008;181:3413–3421. doi: 10.4049/jimmunol.181.5.3413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munch J., Rucker E., Standker L., Adermann K., Goffinet C., Schindler M., Wildum S., Chinnadurai R., Rajan D., Specht A., Gimenez-Gallego G., Sanchez P. C., Fowler D. M., Koulov A., Kelly J. W., Mothes W., Grivel J. C., Margolis L., Keppler O. T., Forssmann W. G., Kirchhoff F. Semen-derived amyloid fibrils drastically enhance HIV infection. Cell. 2007;131:1059–1071. doi: 10.1016/j.cell.2007.10.014. [DOI] [PubMed] [Google Scholar]
- Cole A. L., Yang O. O., Warren A. D., Waring A. J., Lehrer R. I., Cole A. M. HIV-1 adapts to a retrocyclin with cationic amino acid substitutions that reduce fusion efficiency of gp41. J Immunol. 2006;176:6900–6905. doi: 10.4049/jimmunol.176.11.6900. [DOI] [PubMed] [Google Scholar]
- Da Costa A. O., de Assis M. C., Marques Ede A., Plotkowski M. C. Comparative analysis of three methods to assess viability of mammalian cells in culture. Biocell. 1999;23:65–72. [PubMed] [Google Scholar]
- Harwig S. S., Chen N. P., Park A. S., Lehrer R. I. Purification of cysteine-rich bioactive peptides from leukocytes by continuous acid-urea-polyacrylamide gel electrophoresis. Anal Biochem. 1993;208:382–386. doi: 10.1006/abio.1993.1065. [DOI] [PubMed] [Google Scholar]
- Schagger H., von Jagow G. tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem. 1987;166:368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- Lilja H., Jeppsson J. O. Amino acid sequence of the predominant basic protein in human seminal plasma. FEBS Lett. 1985;182:181–184. doi: 10.1016/0014-5793(85)81179-0. [DOI] [PubMed] [Google Scholar]
- Robert M., Gibbs B. F., Jacobson E., Gagnon C. Characterization of prostate-specific antigen proteolytic activity on its major physiological substrate, the sperm motility inhibitor precursor/semenogelin I. Biochemistry. 1997;36:3811–3819. doi: 10.1021/bi9626158. [DOI] [PubMed] [Google Scholar]
- Ganz T. Antimicrobial polypeptides. J Leukoc Biol. 2004;75:34–38. doi: 10.1189/jlb.0403150. [DOI] [PubMed] [Google Scholar]
- Cole A. M., Liao H. I., Stuchlik O., Tilan J., Pohl J., Ganz T. Cationic polypeptides are required for antibacterial activity of human airway fluid. J Immunol. 2002;169:6985–6991. doi: 10.4049/jimmunol.169.12.6985. [DOI] [PubMed] [Google Scholar]
- Jonsson M., Lundwall A., Malm J. The semenogelins: proteins with functions beyond reproduction? Cell Mol Life Sci. 2006;63:2886–2888. doi: 10.1007/s00018-006-6287-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilja H. Cell biology of semenogelin. Andrologia. 1990;22(Suppl. 1):132–141. doi: 10.1111/j.1439-0272.1990.tb02079.x. [DOI] [PubMed] [Google Scholar]
- Brillard-Bourdet M., Rehault S., Juliano L., Ferrer M., Moreau T., Gauthier F. Amidolytic activity of prostatic acid phosphatase on human semenogelins and semenogelin-derived synthetic substrates. Eur J Biochem. 2002;269:390–395. doi: 10.1046/j.0014-2956.2001.02667.x. [DOI] [PubMed] [Google Scholar]
- Autiero M., Gaubin M., Mani J. C., Castejon C., Martin M., el Marhomy S., Guardiola J., Piatier-Tonneau D. Surface plasmon resonance analysis of gp17, a natural CD4 ligand from human seminal plasma inhibiting human immunodeficiency virus type-1 gp120-mediated syncytium formation. Eur J Biochem. 1997;245:208–213. doi: 10.1111/j.1432-1033.1997.00208.x. [DOI] [PubMed] [Google Scholar]
- Berkhout B., Floris R., Recio I., Visser S. The antiviral activity of the milk protein lactoferrin against the human immunodeficiency virus type 1. Biometals. 2004;17:291–294. doi: 10.1023/b:biom.0000027707.82911.be. [DOI] [PubMed] [Google Scholar]
- Groot F., Geijtenbeek T. B., Sanders R. W., Baldwin C. E., Sanchez-Hernandez M., Floris R., van Kooyk Y., de Jong E. C., Berkhout B. Lactoferrin prevents dendritic cell-mediated human immunodeficiency virus type 1 transmission by blocking the DC-SIGN–gp120 interaction. J Virol. 2005;79:3009–3015. doi: 10.1128/JVI.79.5.3009-3015.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swart P. J., Kuipers E. M., Smit C., Van Der Strate B. W., Harmsen M. C., Meijer D. K. Lactoferrin Antiviral activity of lactoferrin. Adv Exp Med Biol. 1998;443:205–213. [PubMed] [Google Scholar]
- Van der Strate B. W., Beljaars L., Molema G., Harmsen M. C., Meijer D. K. Antiviral activities of lactoferrin. Antiviral Res. 2001;52:225–239. doi: 10.1016/s0166-3542(01)00195-4. [DOI] [PubMed] [Google Scholar]
- Shukeir N., Arakelian A., Kadhim S., Garde S., Rabbani S. A. Prostate secretory protein PSP-94 decreases tumor growth and hypercalcemia of malignancy in a syngenic in vivo model of prostate cancer. Cancer Res. 2003;63:2072–2078. [PubMed] [Google Scholar]
- Udby L., Lundwall A., Johnsen A. H., Fernlund P., Valtonen-Andre C., Blom A. M., Lilja H., Borregaard N., Kjeldsen L., Bjartell A. beta-Microseminoprotein binds CRISP-3 in human seminal plasma. Biochem Biophys Res Commun. 2005;333:555–561. doi: 10.1016/j.bbrc.2005.05.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turk V., Bode W. The cystatins: protein inhibitors of cysteine proteinases. FEBS Lett. 1991;285:213–219. doi: 10.1016/0014-5793(91)80804-c. [DOI] [PubMed] [Google Scholar]
- Vray B., Hartmann S., Hoebeke J. Immunomodulatory properties of cystatins. Cell Mol Life Sci. 2002;59:1503–1512. doi: 10.1007/s00018-002-8525-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorck L. Proteinase inhibition, immunoglobulin-binding proteins and a novel antimicrobial principle. Mol Microbiol. 1990;4:1439–1442. doi: 10.1111/j.1365-2958.1990.tb02054.x. [DOI] [PubMed] [Google Scholar]
- Christopher-Hennings J., Nelson E. A., Althouse G. C., Lunney J. Comparative antiviral and proviral factors in semen and vaccines for preventing viral dissemination from the male reproductive tract and semen. Anim Health Res Rev. 2008:1–11. doi: 10.1017/S1466252307001387. [DOI] [PubMed] [Google Scholar]
- Wawer M. J., Gray R. H., Sewankambo N. K., Serwadda D., Li X., Laeyendecker O., Kiwanuka N., Kigozi G., Kiddugavu M., Lutalo T., Nalugoda F., Wabwire-Mangen F., Meehan M. P., Quinn T. C. Rates of HIV-1 transmission per coital act, by stage of HIV-1 infection, in Rakai, Uganda. J Infect Dis. 2005;191:1403–1409. doi: 10.1086/429411. [DOI] [PubMed] [Google Scholar]