Abstract
Identification of factors that improve muscle function in boys with Duchenne muscular dystrophy (DMD) could lead to an improved quality of life. To establish a functional in vitro assay for muscle strength, mdx murine myoblasts, the genetic homologue of DMD, were tissue engineered in 96-microwell plates into 3-dimensional muscle constructs with parallel arrays of striated muscle fibers. When electrically stimulated, they generated tetanic forces measured with an automated motion tracking system. Thirty-one compounds of interest as potential treatments for patients with DMD were tested at 3 to 6 concentrations. Eleven of the compounds (insulin-like growth factor-1, creatine, β-hydroxy-β-methylbutyrate, trichostatin A, lisinopril, and 6 from the glucocorticoid family) significantly increased tetanic force relative to placebo-treated controls. The glucocorticoids methylprednisolone, deflazacort, and prednisone increased tetanic forces at low doses (EC50 of 6, 19, and 56 nM, respectively), indicating a direct muscle mechanism by which they may be benefitting DMD patients. The tetanic force assay also identified beneficial compound interactions (arginine plus deflazacort and prednisone plus creatine) as well as deleterious interactions (prednisone plus creatine inhibited by pentoxifylline) of combinatorial therapies taken by some DMD patients. Since mdx muscle in vivo and DMD patients respond in a similar manner to many of these compounds, the in vitro assay will be a useful tool for the rapid identification of new potential treatments for muscle weakness in DMD and other muscle disorders.—Vandenburgh, H., Shansky, J., Benesch-Lee, F., Skelly, K., Spinazzola, J.M., Saponjian, Y., Tseng, B.S. Automated drug screening with contractile muscle tissue engineered from dystrophic myoblasts.
Keywords: tetanic force, glucocorticoids, mdx mouse, dietary supplements, cardiovascular drugs
Tissue engineering of functional organs as a unique tool for in vitro drug screening applications has been envisioned since the field’s early inception (1,2,3). While more complicated than traditional high throughput drug screening (HTS) technologies that use biochemical, gene expression, or single cell assays, the ability to analyze tissue function ex vivo has several advantages. In targeted HTS approaches, it is difficult to predict the ultimate physiological activity of a drug, since all compounds have effects on multiple intracellular second messenger pathways (4). High-content drug screening (HCS) with engineered tissues based on tissue function is the culmination of all the complex interactions of compounds on these intracellular pathways and therefore should be a better predictor of their ultimate in vivo effect at the tissue level. In addition, the engineering of tissues from diseased animal and human cells allows the screening of new potential drug therapies directly against a specific disease’s phenotype. Although HCS with engineered tissues will not replace in vivo drug screening, it should serve as a useful secondary follow-on screen to HTS analyses of large compound banks, reducing the time, cost, and number of animals necessary for in vivo studies.
Physiologically based HCS technologies assaying skeletal muscle function are under development using nematode worms (5, 6), zebrafish (7), and tissue-engineered muscle “organs” (8). They provide a “holistic” analysis of a compound’s effect on the multiple pathways regulating important physiological parameters of muscle such as strength, fatigability, and contraction-induced injury. To be useful as a primary or secondary drug screen for the analysis of hundreds of compounds, the HCS technologies will require adaptation to the computerized robotic liquid-handling hardware and imaging software developed over the past decade for HTS. This automation has revolutionized HTS, allowing the rapid and reproducible screening of large compound banks. HCS automation will be particularly important in the search for new treatments for diseases such as Duchenne muscular dystrophy (DMD), a progressive, lethal, muscle-wasting disease resulting from a defect in the dystrophin gene (9). Many DMD patients take “cocktails” of dozens of compounds daily, the complex interactions of which are unknown. While a cure for DMD will ultimately require correction of the defective gene through either gene- or cell-based therapies, improved therapeutics to attenuate muscle weakness and loss will lead to an enhanced quality and length of life for these patients.
Pharmacological strategies for targeting factors for treating DMD are currently an active area of research. Many compounds tested in the mdx murine model of DMD and/or clinically in patients have either minimal long-term benefits or adverse side effects (reviewed in ref. 10). The most widely accepted medical treatment in DMD is corticosteroids, which have multiple mechanisms of action on skeletal muscle in addition to their primary anti-inflammatory role (5, 11). Combinatorial screening by HCS of multiple drugs that have slight beneficial effects when administered separately in DMD may lead to significant progress in the development of new drug protocols or drug cocktails to help patients with this fatal disease (10, 11). In addition, screening chemical banks of FDA-approved compounds could lead to the rapid identification of new therapeutic targets for DMD.
This study describes the semiautomation of HCS with skeletal muscle tissue engineered from mdx murine myoblasts in standard 96-microwell plate format. Standard HTS liquid-handling hardware and software were adapted to automatically tissue engineer miniature bioartificial muscles (mdx mBAMs), and customized automated HCS hardware and imaging software were designed to measure muscle strength, i.e., tetanic force, generated by the diseased tissues. This is the first report that we are aware of that describes the engineering of tissues with commercially available HTS robotic technology. Compounds of interest as potential strengthening treatments for patients with DMD were tested in a nondestructive manner for their effect on mdx mBAM tetanic force at multiple concentrations over a 3–4 d assay period. A number of the compounds were found to have positive effects in a similar manner to their activity in vivo. The HCS mdx mBAM assay also identified a unique mechanism by which glucocorticoids, a commonly used treatment in DMD patients, might attenuate the muscle weakness that is prevalent in the disease. In addition, combinations of these compounds were found to either enhance or reduce the beneficial effects of other compounds on tetanic force. Tissue engineered contractile tissues may thus be a useful tool for the rapid identification of new compounds for DMD therapy, as well as lead to a better understanding of the complex interactions of the multiple compounds used to improve muscle function, not only in DMD, but potentially in other neuromuscular diseases.
MATERIALS AND METHODS
mdx Myoblast tissue culture
Conditionally immortalized control C57 and mdx myoblast clones were used in these studies and contain a temperature-sensitive immortalizing gene, the tsA58 mutation of SV40 large T antigen, under control of the H-2Kb inducible promoter (12). When induced with interferon-γ (IFN-γ), tsA58 protein is expressed, allowing the cells to proliferate at 33°C. Myoblasts were plated and maintained on gelatin-coated dishes (0.01% gelatin) in H2K proliferation medium [H2KPM: 20% heat-inactivated FBS, 4 mM L-glutamine, 2% v/v chick embryo extract, and pen:strep (100 U/ml:100 μg/ml) in DMEM] at 33°C and 10% CO2 in a humidified incubator. Cultures were fed every 2–3 d with H2KPM, and fresh IFN-γ (20 U/ml) was added to the culture medium at each feeding. Cells were grown to near confluency, harvested with trypsin/EDTA (0.5/0.2 g/L), and centrifuged at 160 g for 5 min. The cell pellet was resuspended in saline for tissue engineering of mBAMs. Under these conditions, the cells doubled every 24–30 h at 33°C and fused with >50% efficiency into multinucleated sarcomeric tropomyosin-positive muscle fibers when switched to 39°C in the absence of IFN-γ. When immunocytochemically stained for dystrophin, the myofibers from the control clones were dystrophin positive, while those from the mdx clone did not express dystrophin, as described previously (12).
Tissue engineering of mdx mBAMs
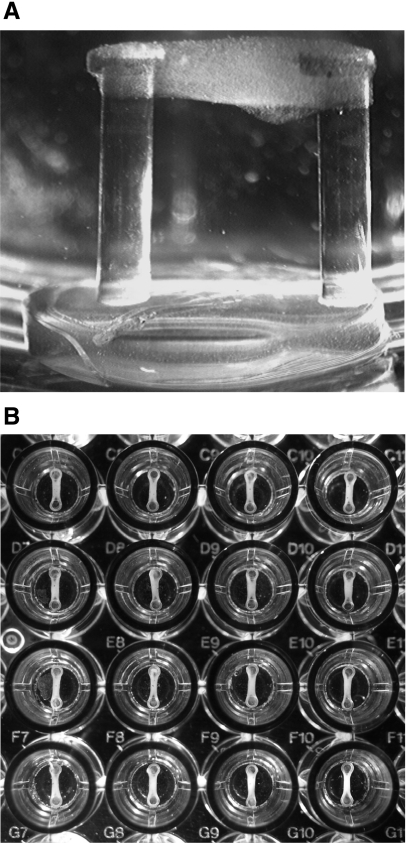
A 96-microwell plate format for tissue engineering mBAMs was developed that enabled the use of a standard robotic liquid-handling system containing 37°C heating stages. Polydimethylsiloxane (PDMS) microwell “plugs” were mold cast using a Sylgard 184 Silicone Elastomer kit (Dow Corning, Midland, MI, USA). Each plug had a 6.0-mm-diameter base and contained two vertical 4.0-mm-tall by 0.8-mm-diameter flexible PDMS microposts spaced 3.5 mm apart (Fig. 1A). Each hydrophobic micropost had a 1.2-mm-diameter hydrophilic cap on top of the hydrophobic microposts to provide a surface for mBAM attachment at the top of the microposts during the tissue-engineering procedure. The plugs were glued sterilely into the bottom of the 7-mm-diameter flat-bottom wells using uncured PDMS.
Figure 1.
mBAMs tissue engineered in a 96-microwell plate. A) Side view of a single mBAM attached to the top of microposts on a plug insert; microposts are 0.8 mm in diameter and 4.0 mm in height. B) Array of mBAMs engineered automatically with a Beckman Biomek 2000 liquid handler. Microwells are 7 mm in diameter.
mBAMs were reproducibly tissue engineered from immortalized control and mdx muscle cells around the caps of the PDMS microposts as described previously for primary murine myoblasts in a prototype manual system (8). The newly designed 96-microwell plates were placed on the deck of a computer-controlled Beckman Coulter Biomek 2000 liquid-handling system (Beckman Coulter, Fullerton CA) in a BioSafety cabinet (Class II Type A/B3; Supplemental Fig. 1). Briefly, immortalized control and mdx cells were suspended in saline and transferred to a 37°C reservoir on the Biomek 2000. A second 37°C reservoir was filled with the appropriate volume of extracellular matrix solution (either 1% collagen Type I or 0.5 mg/ml fibrin) depending on the number of 96-microwell plates to be cast. An 8-tip pipetting tool first dispensed 60 μl of cell suspension (100,000 cells/mBAM) to each well, and then 60 μl of extracellular matrix was added and mixed by aspiration several times. This procedure took ∼7–8 min/plate (Supplemental Video 1).
Immediately after casting, the mBAM plates were transferred to a 37°C, 5% CO2 incubator, and 1 h later (when the cells/matrix had solidified), the Biomek 2000 was used to add 80–100 μl of H2KPM to the top of each well. The cell-matrix mixture gelled rapidly at 37°C and, over 24–48 h, contracted away from the walls of the wells and coalesced around the caps on top of the microposts (Fig. 1B). The mBAMs were maintained for 2 d at 39°C in a humidified 5% CO2 incubator, switched to differentiation medium (H2KDM: 5% HS, 4 mM glutamine, and 100 U/ml penicillin G in DMEM), and maintained in this medium until compound screening was started. The tissue culture media (200 μl/well) were changed daily using the Biomek 2000.
mBAM electrical stimulation and force measurement
At varying times postcasting, the microwell plates were placed onto the 37°C heated stage of a customized myoforce analysis device (MAD) that automatically places electrodes into each well, electrically stimulates the mBAMs, and captures images of the contracting mBAMs with a CMOS camera with a telecentric lens (Supplemental Video 2). MAD is encased in an insulated box so that temperature, CO2 level, and humidity can be maintained in a similar manner to a standard tissue culture incubator. MAD was located in a biosafety cabinet to maintain sterility. Once loaded onto the MAD, the heated stage (connected to an x-y-z translation stage) automatically transported the microwell plate to the stimulating electrodes and electrically stimulated each well for 1–2 s with preselected parameters for tetanic force generation, and 40–60 images of the micropost deflections were captured. mBAMs were electrically stimulated to achieve maximum isotonic tetanic force with electrical stimulus parameters (voltage, frequency, duration, and pulse width) optimized in preliminary experiments (13 V, 40 Hz, 2 s, and 4 ms, respectively). Each well required ∼10 s of test time, and a 96-well plate thus required just under 20 min to assay. A software algorithm written in MatLab automatically determined the maximal micropost deflections in the images and converted micrometers of movement to micronewtons of force. Forces could be calculated with an accuracy of 5–6 μN and are based on the known elastic modulus of PDMS, the micropost height, and the micropost diameter, as described previously (8). After electrical stimulation, the plates were transferred to the Biomek 2000, the tissue culture medium was changed, and the plates were returned to the humidified 5% CO2 incubator for 24 h before retesting in MAD.
Compound testing was initiated on d 9–10 postcasting, when the mBAMs and striated myofibers were well formed and tetanic forces had reached a stable plateau (see Results). The variability of the tetanic force assay in MAD was high (15–20%) when compared with traditional HTS assays, but not unexpected considering the complexity of the tissue-engineering procedure. Each mdx mBAM served as its own control, with changes in tetanic force after exposure to a compound compared with the initial tetanic force generated right before addition of the test compound, i.e., test day 0. Controls (no compound addition) were run in each experiment, and any drift from their test day 0 plateau value over the 3–4 d of the assay was used to correct the response of the test groups by adding or subtracting this amount, which averaged 10–15% in some experiments. With 4 to 6 samples/group, increases in tetanic force ≥30% consistently reached statistical significance (see Results). The majority of negative and positive hits obtained reached >50–100% difference from untreated controls by 3 d of incubation with the test compound and were thus easily identified. The sensitivity of the assay could be improved if necessary by increasing the number of samples per group.
Compound screening
Stock solutions of test compounds were prepared at a 20× concentration in either DMEM (poloxamer 407, Protandim, and Solu-Medrol); sterile H2O (alanine, arginine, creatine, creatinine, glutamine, glycine, IGF-I, lisinopril, miglustat, pentoxifylline, Haelan 951, AmSport, and Juven); ethanol (prednisone, prednisolone, and trichostatin A); or DMSO (cholesterol, coenzyme Q10, cortisol, deflazacort, flurbiprofen, losartan, resveratrol, sildenafil, ursodeoxycholate, and Vital Detox). Poloxamer 407, AmSport, Vital Detox, deflazacort, losartan, sildenafil, Juven, and Haelan 951 were kind gifts of B.S.T. Haelan 951 was processed by centrifugation at 4°C for 30 min at 20,000 rpm. The supernatant was filtered through a 0.22-μm tube-top filter unit and used as 20× stock. Sources of other compounds were as follows: Protandim and coenzyme Q10 were from General Nutrition Corporation (Pittsburgh, PA, USA), resveratrol was from Biotivia (New York, NY, USA), Solu-Medrol was from Pfizer Inc. (New York, NY, USA), and trichostatin A and miglustat were from Calbiochem (La Jolla, CA, USA). All other compounds were purchased from Sigma-Aldrich (St. Louis, MO, USA). Initial concentrations selected for testing were based on the recommended clinical dose for each compound and then 10-fold greater and 10-fold lesser concentrations were assayed. If any trends were evident in the initial screen, 3 to 4 additional concentrations were tested.
All compound additions were performed using the Biomek 2000. Compounds were diluted into H2KDM, and mBAMs were incubated with the test medium containing the specified concentration of each compound or combination of compounds. Compound additions were repeated daily with medium changes for 3–4 d.
mBAM histological analysis
mBAMs were fixed with 2% formaldehyde in PBS (v/v) for 20 min at room temperature. Tissues were permeabilized with −20°C methanol for 10 min; rinsed with PBS; blocked with 1% BSA and 0.2% Triton in PBS (blocking buffer) for 1 h at room temperature; and then incubated for 1 h with an antibody to sarcomeric tropomyosin (Sigma-Aldrich) diluted 1:100 in blocking buffer. AlexaFluor 488 goat anti-murine secondary antibody (Invitrogen, Eugene OR, USA) was used to detect sarcomeric tropomyosin-positive fibers. Confocal images of BAMs were captured with a Leica TCS SP2 AOBS spectral confocal microscope (Leica Microsystems, Wetzlar, Germany) set to detect GFP fluorescence.
Statistical analysis
The mean ± se of 4 to 8 samples/group was calculated, and t test statistical analyses were performed using SigmaStat software (Systat Software, Inc., Chicago, IL, USA), with a value of P < 0.05 considered statistically significant. Power analyses were performed using GraphPad StatMate 2.00, and EC50 values were determined with GraphPad Prism (GraphPad Software, Inc., San Diego, CA, USA).
RESULTS
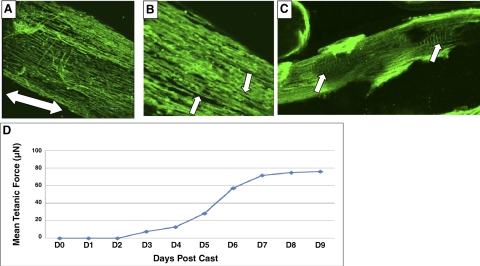
Passive forces within the coalesced extracellular matrix gel aligned the conditionally immortalized myoblasts parallel to each other in the long axis of the mBAM within the first 24–48 h of casting, as previously found for primary murine myoblasts (8). When the mBAMs were then switched to differentiation medium on d 2 postcasting, the myoblasts fused over the next 6–7 d to form aligned and striated muscle fibers (Fig. 2A–C). The tetanic forces that the conditionally immortalized control and mdx mBAMs developed were similar in time required to reach a plateau (7–9 d postcasting) and force generated (40–80 μN) to mBAM tissue engineered from primary murine myoblasts (8; Fig. 2D).
Figure 2.
Differentiation of contractile mdx mBAMs. A) Low-power confocal image showing well-aligned myofibers stained with an antibody to sarcomeric tropomyosin in a d 12 postcasting mdx mBAM. Double-headed arrow indicates long axis of the mdx mBAM. B, C) At higher magnifications, striations are evident (arrows). D) Development of tetanic force with time postcasting. Scale bars = 300 μm (A); 50 μm (C).
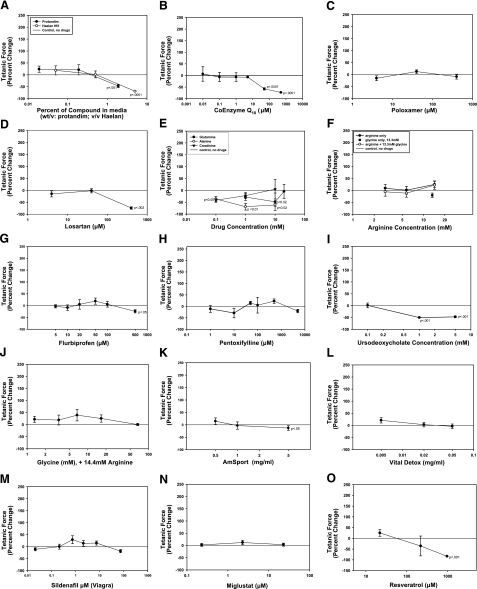
Thirty-one compounds were selected that were either effective in the in vivo mdx murine model for increased muscle strength (13,14,15,16,17) or currently taken by DMD patients for various reasons (personal communications from parents of DMD patients and ref. 18). Each compound was initially tested at 3 doses, and if a trend was evident, 3 to 4 additional doses were tested. Twenty of the compounds tested either were ineffective or significantly decreased tetanic mdx mBAM force at higher concentrations after 2–4 d of incubation (Fig. 3). The compounds ranged from nutraceuticals (Protandim, Haelan 951, AmSport, Vital Detox, and resveratrol; Fig. 3A, K, L, O) to antioxidant coenzyme Q10 (Fig. 3B); the membrane stabilizer poloxamer 407 (Fig. 3C); the antihypertensive drug losartan (Fig. 3D); the creatine metabolite creatinine (Fig. 3E); the amino acids arginine, glycine, alanine, and glutamine (Fig. 3E, F, J); the nonsteriodal anti-inflammatory drug flurbiprofen (Fig. 3G); the glycosphingolipid biosynthesis inhibitor miglustat (Fig. 3N; ref. 19); the phosphodiesterase inhibitor sildenafil (Fig. 3M); and the antifibrotic drugs pentoxifylline and ursodeoxycholic acid (Fig. 3H, I). At high concentrations, 6 of the compounds (Protandim, Haelan 951, coenzyme Q10, alanine, losartan, and ursodeoxycholate) significantly inhibited mdx mBAM tetanic force.
Figure 3.
Compounds that did not increase mdx mBAM tetanic force included Protandim and Haelan 951 (A); coenzyme Q10 (B); poloxamer (C); losartan (D); alanine, glutamine, and creatinine (E); arginine, glycine, and arginine plus glycine (F, J); flurbiprofen (G); pentoxifylline (H); ursodeoxycholate (I); AmSport (K); Vital Detox (L); sildenafil (M); miglustat (N); and resveratrol (O). mdx mBAMs were cast on d 0, maintained in growth medium for 1–2 d, and switched to differentiation medium for 8–9 d when mBAM tetanic force had plateaued. mBAMs were then incubated for 3–4 d with the various compounds, and tetanic force was measured in MAD. Each point represents the mean ± se of 4–6 samples/group. Results are expressed as percentage change in tetanic force from initial tetanic force for each mBAM. Untreated control mdx mBAMs were run in each experiment, and any drift from their test day 0 plateau value was used to correct the response of the test groups as described in Materials and Methods.
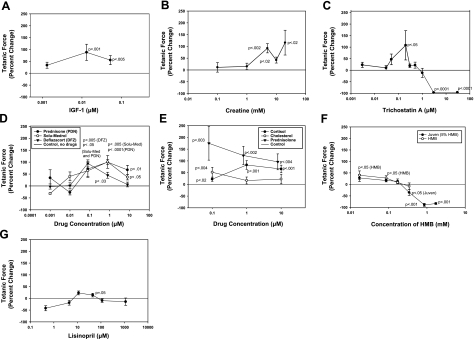
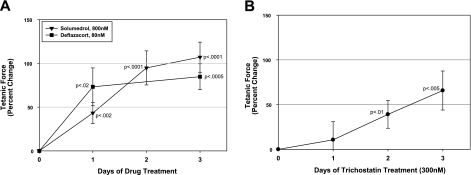
Eleven compounds directly increased mdx mBAM tetanic force, including the growth factor IGF-I (Fig. 4A), the energy source creatine (Fig. 4B), the histone deacetylase inhibitor trichostatin A (Fig. 4C), and the anti-inflammatory glucocorticoids prednisone, Solu-Medrol (methylprednisolone), deflazacort, prednisolone, cortisol, and their parent compound, cholesterol (Fig. 4D, E). Trichostatin A at 0.2 μM resulted in a large increase in mdx mBAM tetanic force (108%) but had a very narrow window of activity and was highly inhibitory at higher concentrations (>3 μM; Fig. 4C). All of the glucocorticoids significantly increased mdx mBAM tetanic force at 0.1 μM and were active through 10 μM, with the exception of deflazacort, which showed a steeper decline in activity at high doses (Fig. 4D, E). Half-maximal effective concentration (EC50) values for methylprednisolone, deflazacort, and prednisone for tetanic force increases were 6, 19, and 56 nM, respectively. The nutraceutical Juven increased mdx mBAM tetanic force, and this appeared to be due to one of its ingredients, β-hydroxy-β-methylbutyrate, which showed a similar dose-response curve (Fig. 4F). The angiotensin-converting enzyme inhibitor lisinopril also significantly increased tetanic force (Fig. 4G). The glucocorticoids methylprednisolone (Solu-Medrol) and deflazacort at 0.80 and 0.080 μM, respectively, rapidly increased mdx mBAM tetanic force within 24 h of addition (Fig. 5A), while trichostatin A showed a slower rate of activity, requiring several days to reach statistical significance (Fig. 5B). Four of the positive compounds (IGF-I, methylprednisolone, deflazacort, and prednisone) were tested with nondystrophic control mBAMs, and they showed a similar positive effect at similar doses (data not shown).
Figure 4.
Compounds that increased mdx mBAM tetanic force included IGF-I (A); creatine (B); trichostatin A (C); prednisone, Solu-Medrol, and deflazacort (D); prednisolone, cortisol, and cholesterol (E); Juven and β-hydroxy-β-methylbutyrate (HDM) (F); and lisinopril (G). mdx mBAMs were cast on d 0, maintained in growth medium for 2 d, and switched to differentiation medium for 8–9 d when mBAM tetanic force had plateaued. mBAMs were then incubated for 3–4 d with the various compounds, and tetanic force was measured in MAD. Each point represents the mean ± se of 4–6 samples/group. Results are expressed as percentage change in tetanic force from initial tetanic force for each mBAM. Untreated control mdx BAMs were run in each experiment, and any drift from their test day 0 plateau value was used to correct the response of the test groups as described in Materials and Methods.
Figure 5.
Time course of effect of glucocorticoids (A) and trichostatin A (B) on mdx mBAM tetanic force. Experiments were performed as outlined in Fig. 4. Each point represents the mean ± se of 4–6 samples/group. Results are expressed as percentage change in tetanic force from initial tetanic force for each mBAM.
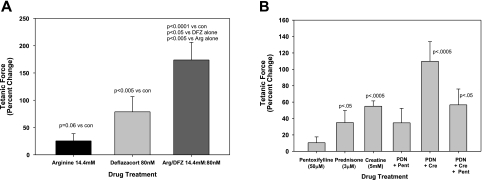
Over 50 compounds have been tested individually that improve skeletal muscle function in the mdx murine model, but none (with the exception of the glucocorticoids) have been found to be effective enough to move into common clinical practice for DMD. Combination of two or more of these factors has been proposed to be an attractive alternative approach to treating DMD (10, 11), and several drug combinations have been shown to be more effective than their individual components in the in vivo mdx murine model (13, 20). To investigate whether similar combinatorial effects could be seen in vitro, mdx mBAM tetanic forces were measured when incubated with arginine, a creatine precursor, or arginine plus deflazacort, a combination shown to have an additive effect in vivo in mdx mice (20). Arginine at the highest concentration tested (14.4 mM) had a marginal effect on mdx mBAM tetanic force (Fig. 3F), but nearly doubled the increase in tetanic force seen with the optimal concentration of deflazacort (0.08 μM; Fig. 6A). Similar synergistic interactions occurred with another glucocorticoid (prednisone), when combined with creatine as an energy source (Fig. 6B). Conversely, a recent clinical trial showed no improvement in muscle strength in prednisone-treated DMD patients who were also treated for 12 mo with the antifibrotic drug pentoxifylline (21); likewise, in mdx mBAMs, there was no improvement seen in prednisone-stimulated tetanic force with pentoxifylline (Fig. 6B). Interestingly, pentoxifylline completely inhibited the synergistic effect seen with prednisone and creatine (Fig. 6B). Similarly, another antifibrotic drug, ursodeoxycholic acid (Fig. 3I) at 100 μM, completely blocked the synergistic effect of prednisolone (0.8 μM) and creatine (5 mM) on mdx mBAM tetanic force (data not shown).
Figure 6.
Combinatorial effects of glucocorticoids and other compounds. mdx mBAMs were cast and maintained as described in Figs. 3 and 4. A) On d 8–9 after casting, the mBAMs were incubated for 3–4 d with either arginine (arg), deflazacort (DFZ), or a combination of both compounds, and tetanic force was measured. B) In a separate experiment, prednisone (PDN) and creatine (Cre) increased tetanic force in an additive manner, but the effect was inhibited by pentoxifylline (Pent). Each point represents the mean ± se of 4–6 samples/group. Results are expressed as percentage change in tetanic force from initial tetanic force for each mBAM.
DISCUSSION
DMD is a progressive, lethal, muscle-wasting disease resulting from a defect in the dystrophin gene (9). It affects 1 in 3500 boys worldwide who are wheelchair bound by their early teens and normally die in their late teens or early 20s. Dystrophin is a large cytoskeletal protein that provides structural support to the cell membrane of the muscle fibers, and exercise-induced stress to dystrophin-defective fibers damages their integrity and ability to generate force (22,23,24,25,26). More recently, dystrophin has been shown to also be capable of signal transduction via second messenger pathways relevant to muscle growth in both dystrophic muscle (27) and other muscle-wasting disorders (28).
Potential compounds to attenuate loss of muscle strength in DMD include those that stimulate muscle protein synthesis (anabolic steroids and recombinant growth factors), inhibit muscle protein degradation (protease inhibitors), reduce inflammation (glucocorticoids), modulate cytokine levels (antitumor necrosis factor-α and nitric oxide stimulators), or stimulate muscle stem-cell proliferation/fusion into muscle fibers (antimyostatins). The compounds that increased muscle strength in vivo also increased tetanic force in the in vitro mdx mBAM assay: IGF-I (14), creatine (29), trichostatin A (15), and glucocorticoids (30). Of the nutraceuticals tested, only the two that have been clinically proven to increase muscle strength (creatine and β-hyroxy-β-methylbutyrate) (31) tested positive in the mdx mBAM assay. Interestingly, angiotensin-converting enzyme inhibitors such as lisinopril, which is taken by older DMD patients to treat heart conditions (32) and was positive in the in vitro mdx mBAM assay, have been proposed as a treatment for muscle weakness in frail elderly patients (33).
To date, only glucocorticoids (e.g., prednisone and deflazacort) are in regular use for DMD patients, since they have been clinically proven to increase muscle strength in long-term clinical trials (34). These results are somewhat surprising, since it is well known that high doses of the glucocorticoid dexamethasone can lead to skeletal muscle atrophy by increasing proteolysis of myosin heavy chain in nondiseased muscle cells (35, 36). The mechanisms for the clinical benefit of glucocorticoids to the DMD patient are therefore uncertain, and there is evidence that glucocorticoids may have direct beneficial effects on skeletal muscle cells outside of their well-known anti-inflammatory action (5, 11). Although their primary target in skeletal muscle disease is thought to be via this anti-inflammatory activity, our results suggest that the glucocorticoids might directly increase muscle strength, therefore acting outside of the inflammatory mechanism. This is consistent with the recent in vivo findings of Golumbek et al.(37) where prednisolone was effective in increasing muscle strength in immunodeficient mdx RAG2−/− mice that do not produce T cells, B cells, or immunoglobulins. Glucocorticoids have been shown to affect numerous pathways in skeletal muscle, including accelerated myoblast fusion, altered calcium transport, prevention of oxidative damage, membrane stabilization (reviewed in ref. 38), and utrophin up-regulation (39). Since the effect of the glucocorticoids on increasing mdx mBAM strength is evident within 24 h of addition, it is unlikely that their mode of action is through myoblast fusion. In contrast, the increase in mdx mBAM tetanic force induced by trichostatin A required several days of drug exposure and therefore might involve the fusion of myoblasts to new or existing myofibers (15). Since the antioxidative compounds Protandim, coenzyme Q10, and Vital Detox, as well as the membrane stabilizer poloxamer, were ineffective in increasing mdx mBAM tetanic force, it is unlikely that the glucocorticoids are acting through either of these pathways. The pathways by which glucocorticoids directly increase tetanic force generation may involve calcium flux alterations (40), since the EC50 for tetanic force increases with glucocorticoids found in the mdx mBAMs are similar to the EC50 for glucocorticoid-induced calcium flux alterations in skeletal muscle fibers (41). However, the effects of glucocorticoids on dystrophin-negative muscle fibers are likely to be quite complex, since they have been shown to induce unique changes in the expression of >140 genes in dystrophic muscle (42). This shows the difficulty in identifying which pathways might be relevant for the beneficial effects of glucocorticoids in DMD and an important advantage of compound screening based on muscle physiology rather than targeting individual genes or second messenger pathways. Identification of the physiologically important mechanisms for glucocorticoid action on muscle force generation may lead to new targeted therapeutic approaches for DMD that improve muscle strength.
Many DMD patients take dozens of compounds per day in an attempt to attenuate muscle weakness, and the complex interactions of these are poorly understood. The amino acids that have been shown to increase muscle strength in the mdx murine model [alanine, glutamine (14), arginine (20), and glycine (43)] were ineffective in improving mdx mBAM tetanic forces. It was therefore surprising that there was a strong synergism in mdx mBAM tetanic force with arginine plus deflazacort, as previously found in vivo(20). The current model for this synergism is that the functional muscle gain in mdx mice occurs by a reduction in exercise-induced muscle damage in dystrophin-negative muscle fibers through cellular pathways such as increased levels of utrophin, calcineurin/nuclear factor of activated T cells, nitric oxide, and/or satellite cell activation (20). The results of the current study suggest an additional potential pathway for this synergism with arginine or creatine, an increased energy supply (44). The ability of pentoxifylline or ursodeoxycholic acids to inhibit this synergism, while having no effect on glucocorticoid-induced force increase, may be related to their general phosphodiesterase inhibition activities and regulation of cytoskeleton-linked calcium channels (40) and/or energy metabolism (45).
The pathophysiology of DMD is extremely complicated, involving a multitude of organs and tissues in the body. The loss of the dystrophin protein can affect not only the muscle fibers directly but also the supporting connective tissue, its vasculature, and innervation (46). Compound screening with tissue-engineered muscle constructs as performed in the present study will therefore have significant limitations as to its overall relevance in predicting a drug’s effect in vivo. In mBAMs the muscle fibers are noninnervated and not fully differentiated, expressing a mixture of embryonic, neonatal, and adult myosin isoforms (8). The striated myofibers in the mBAMs are thus of a more neonatal than adult fiber type and may prove to be a better predictor of compounds for use in younger DMD patients, who may have different therapeutic requirements than older patients (47). The surrounding extracellular environment in mBAMs is also quite different from in vivo muscle, with a lack of blood vessels and complex extracellular matrix components. Beneficial compounds acting as antifibrotic agents (e.g., pentoxifylline or ursodeoxycholic acid; refs. 21, 48), through the vasculature (e.g., losartan and sildenafil; refs. 17, 49), or on the inflammatory response (e.g., flurbiprofen; ref. 50) will thus be missed in the in vitro mBAM assay. Amino acid supplements, including glutamine, alanine, arginine, glycine, and AmSport (an enzymatic protein hydrolysate of amino acids), may be effective in vivo but ineffective in vitro, since the tissue culture medium has been optimized previously for cell growth. They may also be deleterious in the in vivo environment in the presence of an immune system (51). Other compounds, such as those aimed at minimizing muscle contraction-induced membrane damage (e.g., poloxamer; ref. 52) will also be missed with the tetanic force assay. The mBAM screening technology is currently being expanded to measure other physiological parameters, such as muscle contraction rate, relaxation rate, muscle fatigue/damage, and recovery from fatigue/damage. Finally, the mdx murine myofiber tissue may not be the ideal representative model for DMD, since its disease phenotype is quite different than in humans, and expansion of the mBAM screening technology to tissues engineered from immortalized human myoblasts (53) may be a better predictor of the clinical outcome of a compound.
In summary, the in vitro mdx mBAM assay has been validated as a reasonable semiautomated physiological screen for compounds that may improve muscle strength in DMD patients. Defining unfavorable compounds also has great value for the DMD community. The tissue-engineered mdx mBAMs do not exactly replicate an adult muscle or the in vivo environment; but they can serve as an important rapid and cost-effective bridge between HTS biochemical or cell-based screening methods as performed by others (39, 54, 55) and in vivo animal testing. Hopefully, this will lead to new treatments to improve the quality of life of the DMD patient.
Supplementary Material
Acknowledgments
We thank Dr. Terry Partridge (Children’s National Medical Center, Washington, DC, USA) for providing the conditionally immortalized control and mdx skeletal muscle cells. This work was supported by the Slater Technology Fund; the National Institutes of Health (AG-029705, NS-059098, and AR-052308); the National Science Foundation (award 0724445); the Hood, Sharp, and Jett Foundations; and Charley’s Fund. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the authors and not necessarily those of the granting agencies. H.V. and B.T. designed the experiments and wrote the paper; J.S., K.S., and J.M.S. performed the tissue engineering, compound screening, and force measurements; F.B.-L. developed the casting molds for the micropost plugs, wrote the LabView and MatLab software, and built the MAD device; and Y.S. performed histological analyses.
References
- Vandenburgh H. H., Swasdison S., Karlisch P. Computer aided mechanogenesis of skeletal muscle organs from single cells in vitro. FASEB J. 1991;5:2860–2867. doi: 10.1096/fasebj.5.13.1916108. [DOI] [PubMed] [Google Scholar]
- Langer R., Vacanti J. P. Tissue engineering. Science. 1993;260:920–926. doi: 10.1126/science.8493529. [DOI] [PubMed] [Google Scholar]
- Khademhosseini A., Langer R., Borenstein J., Vacanti J. P. Microscale technologies for tissue engineering and biology. Proc Natl Acad Sci U S A. 2006;103:2480–2487. doi: 10.1073/pnas.0507681102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terstappen G. C., Schlupen C., Raggiaschi R., Gaviraghi G. Target deconvolution strategies in drug discovery. Nat Rev Drug Discov. 2007;6:891–903. doi: 10.1038/nrd2410. [DOI] [PubMed] [Google Scholar]
- Gaud A., Simon J. M., Witzel T., Carre-Pierrat M., Wermuth C. G., Segalat L. Prednisone reduces muscle degeneration in dystrophin-deficient Caenorhabditis elegans. Neuromuscul Disord. 2004;14:365–370. doi: 10.1016/j.nmd.2004.02.011. [DOI] [PubMed] [Google Scholar]
- Catoire H., Pasco M. Y., Abu-Baker A., Holbert S., Tourette C., Brais B., Rouleau G. A., Parker J. A., Neri C. Sirtuin inhibition protects from the polyalanine muscular dystrophy protein PABPN1. Human Mol Genetics. 2008;17:2108–2117. doi: 10.1093/hmg/ddn109. [DOI] [PubMed] [Google Scholar]
- Steffen L. S., Guyon J. R., Vogel E. D., Beltre R., Pusack T. J., Zhou Y., Zon L. I., Kunkel L. M. Zebrafish orthologs of human muscular dystrophy genes. BMC Genomics. 2007;8:79. doi: 10.1186/1471-2164-8-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenburgh H., Shansky J., Benesch-Lee F., Barbata V., Reid J., Thorrez L., Valentini R., Crawford G. A drug screening platform based on the contractility of tissue engineered muscle. Muscle Nerve. 2008;37:438–447. doi: 10.1002/mus.20931. [DOI] [PubMed] [Google Scholar]
- Matsumura K., Campbell K. P. Dystrophin-glycoprotein complex: its role in the molecular pathogenesis of muscular dystrophies. Muscle Nerve. 1994;17:2–15. doi: 10.1002/mus.880170103. [DOI] [PubMed] [Google Scholar]
- Khurana T. S., Davies K. E. Pharmacological strategies for muscular dystrophy. Nat Rev Drug Discov. 2003;2:379–390. doi: 10.1038/nrd1085. [DOI] [PubMed] [Google Scholar]
- Tidball J. G., Wehling-Henricks M. Evolving therapeutic strategies for Duchenne muscular dystrophy: targeting downstream events. Pediatr Res. 2004;56:831–841. doi: 10.1203/01.PDR.0000145578.01985.D0. [DOI] [PubMed] [Google Scholar]
- Morgan J. E., Beauchamp J. R., Pagel C. N., Peckham M., Ataliotis P., Jat P. S., Noble M. D., Farmer K., Partridge T. A. Myogenic cell lines derived from transgenic mice carrying a thermolabile T antigen: a model system for the derivation of tissue-specific and mutation-specific cell lines. Dev Biol. 1994;162:486–498. doi: 10.1006/dbio.1994.1103. [DOI] [PubMed] [Google Scholar]
- Payne E. T., Yasuda N., Bourgeois J. M., Devries M. C., Rodriguez M. C., Yousuf J., Tarnopolsky M. A. Nutritional therapy improves function and complements corticosteroid intervention in mdx mice. Muscle Nerve. 2006;33:66–77. doi: 10.1002/mus.20436. [DOI] [PubMed] [Google Scholar]
- Granchelli J. A., Pollina C., Hudecki M. S. Pre-clinical screening of drugs using the mdx mouse. Neuromusc Disord. 2000;10:235–239. doi: 10.1016/s0960-8966(99)00126-1. [DOI] [PubMed] [Google Scholar]
- Minetti G. C., Colussi C., Adami R., Serra C., Mozzetta C., Parente V., Fortuni S., Straino S., Sampaolesi M., Di Padova M., Illi B., Gallinari P., Steinkuhler C., Capogrossi M. C., Sartorelli V., Bottinelli R., Gaetano C., Puri P. L. Functional and morphological recovery of dystrophic muscles in mice treated with deacetylase inhibitors. Nat Med. 2006;12:1147–1150. doi: 10.1038/nm1479. [DOI] [PubMed] [Google Scholar]
- Schertzer J. D., Ryall J. G., Lynch G. S. Systemic administration of IGF-I enhances oxidative status and reduces contraction-induced injury in skeletal muscles of mdx dystrophic mice. Am J Physiol Endocrinol Metab. 2006;291:E499–E505. doi: 10.1152/ajpendo.00101.2006. [DOI] [PubMed] [Google Scholar]
- Cohn R. D., van Erp C., Habashi J. P., Soleimani A. A., Klein E. C., Lisi M. T., Gamradt M., Ap Rhys C. M., Holm T. M., Loeys B. L., Ramirez F., Judge D. P., Ward C. W., Dietz H. C. Angiotensin II type 1 receptor blockade attenuates TGF-beta-induced failure of muscle regeneration in multiple myopathic states. Nat Med. 2007;13:204–210. doi: 10.1038/nm1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciafaloni E., Moxley R. T. Treatment options for Duchenne muscular dystrophy. Curr Treat Options Neurol. 2008;10:86–93. doi: 10.1007/s11940-008-0010-4. [DOI] [PubMed] [Google Scholar]
- Steen M. S., Adams M. E., Tesch Y., Froehner S. C. Amelioration of muscular dystrophy by transgenic expression of Niemann-Pick C1. Mol Biol Cell. 2009;20:146–152. doi: 10.1091/mbc.E08-08-0811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archer J. D., Vargas C. C., Anderson J. E. Persistent and improved functional gain in mdx dystrophic mice after treatment with L-arginine and deflazacort. FASEB J. 2006;20:738–740. doi: 10.1096/fj.05-4821fje. [DOI] [PubMed] [Google Scholar]
- Escolar D. M., Gorni K., Tesi-Rocha A. C., Mah J., Nevo Y., Korengberg A., Kolski H., Bertorini T., Connolly A., Kuntz N., Zimmerman A., Morgenroth L., Arrieta A., Mayhew J., Florence J., Nei L., Hu F., Henricson E., Leshner R., Dubrovsky A. Pentoxifylline treatment fails to rescue muscle strength and function deterioration in prednisone-treated Duchenne muscular dystrophy (DMD) Neuromuscul Disord. 2008;18:824. [Google Scholar]
- Yeung E. W., Head S. I., Allen D. G. Gadolinium reduces short-term stretch-induced muscle damage in isolated mdx mouse muscle fibres. J Physiol. 2003;552:449–458. doi: 10.1113/jphysiol.2003.047373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco-Obregon A., Lansman J. B. Changes in mechanosensitive channel gating following mechanical stimulation in skeletal muscle myotubes from the mdx mouse. J Physiol. 2002;539:391–407. doi: 10.1113/jphysiol.2001.013043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moens P., Baatsen P. H., Marechal G. Increased susceptibility of EDL muscles from mdx mice to damage induced by contractions with stretch. J Muscle Res Cell Motil. 1993;14:446–451. doi: 10.1007/BF00121296. [DOI] [PubMed] [Google Scholar]
- Sampaolesi M., Yoshida T., Iwata Y., Hanada H., Shigekawa M. Stretch-induced cell damage in sarcoglycan-deficient myotubes. Pflügers Arch. 2001;442:161–170. doi: 10.1007/s004240100516. [DOI] [PubMed] [Google Scholar]
- Petrof B. J., Shrager J. B., Stedman H. H., Kelly A. M., Sweeney H. L. Dystrophin protects the sarcolemma from stresses developed during muscle contraction. Proc Natl Acad Sci U S A. 1993;90:3710–3714. doi: 10.1073/pnas.90.8.3710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A., Khandelwal N., Malya R., Reid M. B., Boriek A. M. Loss of dystrophin causes aberrant mechanotransduction in skeletal muscle fibers. FASEB J. 2004;18:102–113. doi: 10.1096/fj.03-0453com. [DOI] [PubMed] [Google Scholar]
- Acharyya S., Butchbach M. E., Sahenk Z., Wang H., Saji M., Carathers M., Ringel M. D., Skipworth R. J., Fearon K. C., Hollingsworth M. A., Muscarella P., Burghes A. H., Rafael-Fortney J. A., Guttridge D. C. Dystrophin glycoprotein complex dysfunction: a regulatory link between muscular dystrophy and cancer cachexia. Cancer Cell. 2005;8:421–432. doi: 10.1016/j.ccr.2005.10.004. [DOI] [PubMed] [Google Scholar]
- Tarnopolsky M. A., Mahoney D. J., Vajsar J., Rodriguez C., Doherty T. J., Roy B. D., Biggar D. Creatine monohydrate enhances strength and body composition in Duchenne muscular dystrophy. Neurology. 2004;62:1771–1777. doi: 10.1212/01.wnl.0000125178.18862.9d. [DOI] [PubMed] [Google Scholar]
- Hudecki M. S., Pollina C. M., Granchelli J. A., Daly M. K., Byrnes T., Wang J. C., Hsiao J. C. Strength and endurance in the therapeutic evaluation of prednisolone-treated MDX mice. Res Commun Chem Pathol Pharmacol. 1993;79:45–60. [PubMed] [Google Scholar]
- Nissen S. L., Sharp R. L. Effect of dietary supplements on lean mass and strength gains with resistance exercise: a meta-analysis. J Appl Physiol. 2003;94:651–659. doi: 10.1152/japplphysiol.00755.2002. [DOI] [PubMed] [Google Scholar]
- McNally E. M. Duchenne muscular dystrophy: how bad is the heart? Heart. 2008;94:976–977. doi: 10.1136/hrt.2007.138461. [DOI] [PubMed] [Google Scholar]
- Sumukadas D., Struthers A. D., McMurdo M. E. Sarcopenia–a potential target for angiotensin-converting enzyme inhibition? Gerontology. 2006;52:237–242. doi: 10.1159/000093656. [DOI] [PubMed] [Google Scholar]
- Bonifati M. D., Ruzza G., Bonometto P., Berardinelli A., Gorni K., Orcesi S., Lanzi G., Angelini C. A multicenter, double-blind, randomized trial of deflazacort versus prednisone in Duchenne muscular dystrophy. Muscle Nerve. 2000;23:1344–1347. doi: 10.1002/1097-4598(200009)23:9<1344::aid-mus4>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Chromiak J. A., Vandenburgh H. H. Glucocorticoid-induced skeletal muscle atrophy in vitro is attenuated by mechanical stimulation. Am J Physiol Cell Physiol. 1992;262:C1471–C1477. doi: 10.1152/ajpcell.1992.262.6.C1471. [DOI] [PubMed] [Google Scholar]
- Clarke B. A., Drujan D., Willis M. S., Murphy L. O., Corpina R. A., Burova E., Rakhilin S. V., Stitt T. N., Patterson C., Latres E., Glass D. J. The E3 Ligase MuRF1 degrades myosin heavy chain protein in dexamethasone-treated skeletal muscle. Cell Metab. 2007;6:376–385. doi: 10.1016/j.cmet.2007.09.009. [DOI] [PubMed] [Google Scholar]
- Golumbek P. T., Keeling R. M., Connolly A. M. Strength and corticosteroid responsiveness of mdx mice is unchanged by RAG2 gene knockout. Neuromusc Disord. 2007;17:376–384. doi: 10.1016/j.nmd.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Leijendekker W. J., Passaquin A.-C., Metzinger L., Ruegg U. T. Regulation of cytosolic calcium in skeletal muscle cells of the mdx mouse under conditions of stress. Br J Pharmacol. 1996;118:611–616. doi: 10.1111/j.1476-5381.1996.tb15445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courdier-Fruh I., Barman L., Briguet A., Meier T. Glucocorticoid-mediated regulation of utrophin levels in human muscle fibers. Neuromuscul Disord. 2002;12(Suppl. 1):S95–S104. doi: 10.1016/s0960-8966(02)00089-5. [DOI] [PubMed] [Google Scholar]
- Johnson B. D., Scheuer T., Catterall W. A. Convergent regulation of skeletal muscle Ca2+ channels by dystrophin, the actin cytoskeleton, and cAMP-dependent protein kinase. Proc Natl Acad Sci U S A. 2005;102:4191–4196. doi: 10.1073/pnas.0409695102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzinger L., Passaquin A. C., Leijendekker W. J., Poindron P., Ruegg U. T. Modulation by prednisolone of calcium handling in skeletal muscle cells. Br J Pharmacol. 1995;116:2811–2816. doi: 10.1111/j.1476-5381.1995.tb15930.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher I., Abraham D., Bouri K., Hoffman E. P., Muntoni F., Morgan J. Prednisolone-induced changes in dystrophic skeletal muscle. FASEB J. 2005;19:834–836. doi: 10.1096/fj.04-2511fje. [DOI] [PubMed] [Google Scholar]
- Passaquin A.-C., Renard M., Kay L., Challet C., Mokhtarian A., Wallimann T., Ruegg U. T. Creatine supplementation reduces skeletal muscle degeneration and enhances mitochondrial function in mdx mice. Neuromusc Disord. 2002;12:174–182. doi: 10.1016/s0960-8966(01)00273-5. [DOI] [PubMed] [Google Scholar]
- McClure W. C., Rabon R. E., Ogawa H., Tseng B. S. Upregulation of the creatine synthetic pathway in skeletal muscles of mature mdx mice. Neuromuscul Disord. 2007;17:639–650. doi: 10.1016/j.nmd.2007.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellinger A. M., Mongillo M., Marks A. R. Stressed out: the skeletal muscle ryanodine receptor as a target of stress. J Clin Invest. 2008;118:445–453. doi: 10.1172/JCI34006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlain J. S., Rando T. A. Informa HealthCare; New York: Duchenne Muscular Dystrophy: Advances in Therapeutics. 2006 [Google Scholar]
- Grounds M. D. Two-tiered hypotheses for Duchenne muscular dystrophy. Cell Mol Life Sci. 2008;65:1621–1625. doi: 10.1007/s00018-008-7574-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel A. L., Bledsoe C., Lavin J., Gatti F., Berge J., Millman G., Turin E., Winders W. T., Rutter J., Palmeiri B., Carlson C. G. Treatment with inhibitors of the NF-kappaB pathway improves whole body tension development in the mdx mouse. Neuromuscul Disord. 2008;19:131–139. doi: 10.1016/j.nmd.2008.10.006. [DOI] [PubMed] [Google Scholar]
- Asai A., Sahani N., Kaneki M., Ouchi Y., Martyn J. A., Yasuhara S. E. Primary role of functional ischemia, quantitative evidence for the two-hit mechanism, and phosphodiesterase-5 inhibitor therapy in mouse muscular dystrophy. PLoS ONE. 2007;2:e806. doi: 10.1371/journal.pone.0000806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunelli S., Sciorati C., D'Antona G., Innocenzi A., Covarello D., Galvez B. G., Perrotta C., Monopoli A., Sanvito F., Bottinelli R., Ongini E., Cossu G., Clementi E. Nitric oxide release combined with nonsteroidal anti-inflammatory activity prevents muscular dystrophy pathology and enhances stem cell therapy. Proc Natl Acad Sci U S A. 2007;104:264–269. doi: 10.1073/pnas.0608277104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villalta S. A., Nguyen H. X., Deng B., Gotoh T., Tidball J. G. Shifts in macrophage phenotypes and macrophage competition for arginine metabolism affect the severity of muscle pathology in muscular dystrophy. Hum Mol Genet. 2009;18:482–496. doi: 10.1093/hmg/ddn376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng R., Metzger J. M., Claflin D. R., Faulkner J. A. Poloxamer 188 reduces the contraction-induced force decline in lumbrical muscles from mdx mice. Am J Physiol Cell Physiol. 2008;295:C146–C150. doi: 10.1152/ajpcell.00017.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu C. H., Mouly V., Cooper R. N., Mamchaoui K., Bigot A., Shay J. W., Di Santo J. P., Butler-Browne G. S., Wright W. E. Cellular senescence in human myoblasts is overcome by human telomerase reverse transcriptase and cyclin-dependent kinase 4: consequences in aging muscle and therapeutic strategies for muscular dystrophies. Aging Cell. 2007;6:515–523. doi: 10.1111/j.1474-9726.2007.00306.x. [DOI] [PubMed] [Google Scholar]
- Tanaka M., Bateman R., Rauh D., Vaisberg E., Ramachandani S., Zhang C., Hansen K. C., Burlingame A. L., Trautman J. K., Shokat K. M., Adams C. L. An unbiased cell morphology based screen for new, biologically active small molecules. PLoS Biol. 2005;3:e128. doi: 10.1371/journal.pbio.0030128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch E. M., Barton E. R., Zhuo J., Tomizawa Y., Friesen W. J., Trifillis P., Paushkin S., Patel M., Trotta C. R., Hwang S., Wilde R. G., Karp G., Takasugi J., Chen G., Jones S., Ren H., Moon Y. C., Corson D., Turpoff A. A., Campbell J. A., Conn M. M., Khan A., Almstead N. G., Hedrick J., Mollin A., Risher N., Weetall M., Yeh S., Branstrom A. A., Colacino J. M., Babiak J., Ju W. D., Hirawat S., Northcutt V. J., Miller L. L., Spatrick P., He F., Kawana M., Feng H., Jacobson A., Peltz S. W., Sweeney H. L. PTC124 targets genetic disorders caused by nonsense mutations. Nature. 2007;447:87–91. doi: 10.1038/nature05756. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.