Abstract
Laboratory and field studies have shown that ivermectin, a drug that targets invertebrate ligand-gated ion channels (LGICs), is potently active against Anopheles spp. mosquitoes at concentrations present in human blood after standard drug administrations; thus ivermectin holds promise as a mass human-administered endectocide that could help suppress malaria parasite transmission. We evaluated other systemic LGIC-targeting drugs for their activities against the African malaria vector Anopheles gambiae using in vitro blood feeding assays. Eprinomectin, selamectin, moxidectin, and N-tert-butyl nodulisporamide were evaluated as potentially systemic drugs having similar modes of action to ivermectin; all primarily are agonists of invertebrate glutamate-gated chloride ion channels. Additionally, nitenpyram and spinosad were evaluated as systemic drugs that primarily work as agonists of nicotinic acetylcholine receptor channels. Only eprinomectin killed Anopheles gambiae at concentrations that were comparable to ivermectin. At sub-lethal doses, nitenpyram and moxidectin marginally affected mosquito re-blood feeding ability. The macrocyclic lactones, particularly eprinomectin, caused significantly increased knockdown and significantly inhibited recovery in blood fed females. These data are a first step in evaluating drugs that might be eventually combined with, or substituted for ivermectin for future malaria parasite transmission control.
Keywords: mosquito, Anopheles gambiae, malaria, ivermectin, mass drug administration, endectocide, anthelmintic
1. Introduction
Endectocides (drugs that have activity against endo- and ecto- parasites) are widely used in human and animal health. In humans, ivermectin is used in mass drug administrations (MDA) for control of onchocerciasis and lymphatic filariasis (Cupp et al., 2010; Molyneux et al., 2003), and prescribed to individuals for elimination of Strongyloides stercoralis infections (Suputtamongkol et al., 2011) and sometimes used off-label for scabies and lice infestations (Mounsey et al., 2009; Mumcuoglu et al., 1990). Ivermectin (22,23-dihydro-avermectin) is a semi-synthetic 16-membered macrocyclic lactone derived as a fermentation product of Streptomyces avermitilis. This drug is known as an agonist of the glutamate-gated chloride channels (GluCls) of insects (Kane et al., 2000), which can ultimately lead to their paralysis and death. Many studies have demonstrated that therapeutic blood concentrations of ivermectin or the avermectins can be potently active against adult female Anopheles spp. when these mosquitoes ingest blood meals from treated vertebrates. Despite differences in the routes and concentrations administered to different vertebrates in the following studies: Anopheles quadrimaculatus (Gardner et al., 1993; Jones et al., 1992), An. stephensi (Gardner et al., 1993; Iakubovich et al., 1989; Jones et al., 1992; Pampiglioni et al., 1985), Anopheles sacharovi (Iakubovich et al., 1989), Anopheles farauti (Foley et al., 2000), An. punctulatus (Bockarie et al., 1999), and An. gambiae (Chaccour et al., 2010; Fritz et al., 2009; Sylla et al., 2010) have all been shown to be susceptible. Due to variable drug formulations and pharmacokinetics in different vertebrates, the most efficient way to comparatively measure lethal concentrations (LC50) or sub-lethal drug effects of endectocides against adult mosquito species is to add known drug concentrations to in vitro blood meals (Tesh and Guzman, 1990). With this technique, we that found that Anopheles gambiae s.s. G3 strain (LC50 = 22.4 ng/ml) was 27 times more sensitive to ivermectin in a blood meal than Aedes aegypti RexD strain (LC50 = 601.3 ng/ml), that this LC50 against Anopheles gambiae was half the maximal human plasma concentration expected after standard human MDAs, and that sub-lethal drug effects significantly disrupted An. gambiae physiology (Kobylinski et al., 2010). Fritz et al (Fritz et al., 2009) calculated similar LC50 values against An. gambiae s.s. Kisumu strain and An. arabiensis Dongola strain.
Ivermectin MDAs can disrupt malaria parasite transmission, and as a systemic drug, ivermectin should be effective at targeting both endophagic and exophagic malaria vectors. Such MDAs could be highly effective tools for integrated malaria and helminth control if administered more frequently (Foy et al., 2011; Sylla et al., 2010). This human MDA strategy would likely benefit from alternative drugs that might be added to, or substituted for ivermectin to potentially relieve resistance pressure on both mosquitoes and helminths. Eprinomectin and selamectin are also semi-synthetic derivatives of the avermectins, but they have different chemical structures from ivermectin. Eprinomectin (4″-epi-acetylamino-4″-deoxy-avermectin B1) was developed in light of concerns over the milk residue profile of ivermectin-treated dairy cows (Shoop et al., 1996b). Selamectin is a C13-monosaccharide-C5-oxime (Banks et al., 2000) that was developed following reports of unusual IVM sensitivity of collies and the need for a broader spectrum anti-parasitic drug in companion animals (Bishop et al., 2000). Moxidectin is in the milbemycin drug class, which are also 16-membered macrocyclic lactone endectocides related to the avermectins, but they lack a disaccharide substituent at C-13 position of the macrolide ring and are derived from the fermentation broth of Streptomyces cyanogriseus (Takiguchi et al., 1980). Moxidectin is better known for its anthelmintic properties than its insecticidal properties (Shoop et al., 1995). It is being investigated as an alternative treatment for onchocerciasis (Siva, 2009), and it was determined safe in human volunteers at doses of 3 and 36 mg/kg (Cotreau et al., 2003). Nodulisporic acid A is a metabolite of the endophytic fungus Nodulisporium sp. and is structurally related to indole diterpenes. N-tert-butyl nodulisporamide is derivative of a nodulisporic acid A, and was developed as a long-lasting oral systemic ectoparasitocide for flea and tick control in companion animals (Meinke et al., 2009). All of the aforementioned drugs primarily target insect GluCls, but may also exhibit cross activities against γ-amino butyric acid-gated chloride channels (GABA-Cls).
The insect nicotinic acetylcholine receptor channels (nAChRs) are common insecticide targets for drugs such as the neonicotinoids, and some newer drugs targeting this channel might have the capability to also target mosquito channels as oral systemics for humans or other vertebrates. Among these, the neonicotinoid nitenpyram acts as an agonist of nAChRs and is used as a daily oral systemic flea control drug for companion animals (Rust et al., 2003). Spinosad is similarly used as an oral systemic flea control drug, but it has favorable pharmacokinetics in dogs (administered monthly), and the component spinosyns have unique molecular structures and are derived by fermentation of the soil actinomycete Saccharopolyspora spinosa (Snyder et al., 2009; Snyder et al., 2007). Spinosyns are known as agonists of both nAChRs and GABA-Cls (Sparks et al., 2001).
This study was designed to comparatively evaluate the systemic drugs ivermectin, eprinomectin, selamectin, moxidectin, N-tert-butyl nodulisporamide, nitenpyram and spinosad for activity against the African malaria vector Anopheles gambiae. The LC50 of each drug was calculated from serially diluted drug concentrations fed to mosquitoes through in vitro blood meals. Previous reports observed lethargy and poor coordination of mosquitoes ingesting sub-lethal concentrations of ivermectin. This study also attempted to quantify these sub-lethal drug effects by testing mosquitoes that ingested the LC25 and LC5 of each drug.
2. Materials and methods
2.1 Mosquitoes
Anopheles gambiae G3 strain (origin, The Gambia) were raised at 28–31°C, 80% relative humidity, and a 14:10 light dark cycle. Larvae were raised on a diet of ground fish food and adults were allowed access to water and 10% sucrose ad libitum.
2.2. Drugs
Nodulisporic acid A and N-tert-butyl-nodulisporamide technical grade powders were both generously provided by Merck & Co., Inc. (Rahway, NJ, USA), but N-tert-butyl nodulisporamide was used in all assays reported, because the latter drug was found to be significantly more lethal and caused more significant sub-lethal effects. Spinosad technical grade powder was generously provided by Elanco Animal Health (Greenfield, IN). Selamectin technical grade powder was generously provided by Pfizer, Inc. (Groton, CT). Ivermectin, nitenpyram and eprinomectin technical grade powders were purchased from Sigma-Aldrich (St. Louis, MO). Drug powders were dissolved in dimethylsulfoxide (DMSO) to 10 mg/ml, and aliquots of this stock were frozen at −20°C. We could not obtain moxidectin as a technical grade powder, therefore we purchased the commercially available Cydectin® (Fort Dodge Animal Health, Fort Dodge, IA), which was a 10 mg/ml solution in an unreported vehicle.
2.3. In vitro blood feed and LC50 determinations
Blood feeds and LC50 assays were performed and analyzed as described previously (Kobylinski et al., 2010). Briefly, serial dilutions of drugs were added to blood in membrane feeders; moxidectin solution and the other drug aliquots dissolved in DMSO were diluted in phosphate buffered saline (PBS) to five different concentrations per drug, at which point 10 μl was mixed with 990 μl human blood. These mixtures were then blood fed to mosquitoes between 2 and 8 days post emergence. Fully engorged mosquitoes were immediately sorted on chilled glass plates after the blood feed, then returned to clean 4 liter cages with access to water and 10% sucrose, and monitored for survival daily over 5 days. At least three replicates containing at least 50 mosquitoes each were performed with each drug. A non-linear mixed model with probit analysis was used to calculate the LC50 as described previously (Kobylinski et al., 2010).
2.4. Mosquito re-blood feeding assays
Re-blood feeding assays were performed as previously described (Kobylinski et al., 2010). Briefly, age-matched adult cohort females were given a blood meal containing the LC25 and LC5 of each drug; 990 μl of human blood with 10 μl of drug mixture. Vehicle-only controls were fed blood containing 10 μl of a PBS-DMSO mixture equivalent to the volumes found in each matched drug concentration. Moxidectin controls were fed blood mixed with 10 μl PBS alone. Mosquitoes were blood fed at 2 days post emergence, and 10 fully engorged mosquitoes per replicate were placed into separate organdy-covered 50 ml tubes with access to water. Every 24 hours, the mosquitoes were offered a human arm on which to blood feed for 5 min. Each replicate proceeded until all mosquitoes either re-blood fed on the human arm or died. Three replicates per drug concentration were analyzed. Re-blood feeding curves of the cumulative percent re-blood feeding over time were constructed. Mosquitoes that died instead of re-blood feeding were treated as censored data and curves were analyzed by LogRank analysis (Mantel-Haenszel method; proportional hazards model) and the hazard ratio with 95% confidence intervals using Prism (GraphPad Software, Inc.).
2.5. Initial knockdown assays
For each assay, 20 newly-emerged cohort female An. gambiae s.s. G3 strain mosquitoes per replicate were placed in tall 8 L volume cages (one rectangular 4 L cage sealed onto the top of another 4 L cage, scored on the inside) and held with access to water and 10% sucrose until 2 days post emergence. At this age, they were offered 990 μl freshly-drawn human blood spiked with 10 μl of drug corresponding to the LC25 and LC5, or 10 μl of vehicle-only control solution. Immediately prior to the blood feed, sticky fly paper (Olson Products, Inc, Medina, OH, USA) was lined on the bottom of the cage. Newly blood fed mosquitoes blood typically rest on the sides of the cages. Those that blood fed and then landed on the bottom of the cage (as opposed to resting on the scored sides) were trapped by the fly paper and defined as ‘knocked-down’. Knockdown rates of blood fed mosquitoes were calculated at 1hr, 3hrs and 24hrs post blood feed. Four replicates were performed for each drug concentration tested, and drug groups were compared to vehicle-matched controls by LogRank analysis (Mantel-Haenszel method; proportional hazards model) and the hazard ratio with 95% confidence intervals using Prism (GraphPad Software, Inc.).
2.6. Recovery assays
For each assay, newly-emerged cohort female An. gambiae s.s. G3 strain mosquitoes were placed in 4 L volume cages and held with access to water and 10% sucrose until 2 days post emergence. Then they were offered 990 μl freshly-drawn human blood spiked with 10 μl of drug corresponding to the LC25 and LC5, or 10 μl of vehicle-only control solution. Immediately following the blood feed, 20 fully-engorged blood fed females were placed in the bottom chamber of a Mosquito Breeder (BioQuip Products, Inc., Rancho Dominguez, USA), which consists of an emergence cone leading into a top chamber. A wet cotton ball and raisins were placed in the sides of the top chamber. Mosquitoes able to recover from the toxic effects of a sub-lethal concentration blood meal were able sense the food and water source and use coordinated flight to pass through the emergence cone and gain access to water and sugar in the top chamber. The proportion ‘recovered’ was defined by the proportion of mosquitoes in the top chamber and calculated at 1hr, 3hrs, 24hrs and 48hrs post-blood feed. Control mosquitoes demonstrated near 100% recovery rates by 24hrs. Three replicates were performed for each drug concentration tested, and drug groups were compared to matched controls by LogRank analysis (Mantel-Haenszel method; proportional hazards model) and the hazard ratio with 95% confidence intervals using Prism (GraphPad Software, Inc.).
3. Results
3.1. LC50 determinations
There were significant differences in the concentration of each drug needed to kill 50% of blood feeding mosquitoes (Table 1). Of the drugs tested, only eprinomectin had LC50 values comparable to ivermectin. Nitenpyram exhibited the most potent toxicity of the oral systemic nAChR agonists (LC50 = 111 ng/ml [64.4, 222.1]).
Table 1.
LC50 determination of systemic endectocides blood fed to Anopheles gambiae s.s. G3 strain mosquitoes.
| Drug | Mode of Action | LC50 (ng/ml) [95% fiducial limits] |
|---|---|---|
| Ivermectin1 | GluCl agonist | 22.4 [18.0, 26.9] |
| Eprinomectin | GluCl agonist | 23.6 [19.3, 26.7] |
| Selamectin | GluCl agonist | 277 [214.6, 336.2] |
| Moxidectin | GluCl & GABA-Cl agonist | 2789 [1525, 4430] |
| N-tert-butyl nodulisporamide | GluCl agonist | 760 [645.6, 886.8] |
| Nitenpyram | nAChR agonist | 111 [64.4, 222.1] |
| Spinosad | nAChR & GABA-Cl agonist | 461 [405.9, 510.7] |
Data from Kobylinski et al (Kobylinski et al., 2010) and presented here for comparative purposes.
GluCl – glutamate-gated chloride channel; GABA-Cl – γ-amino butyric acid-gated chloride channel; nAChR – nicotinic acetylcholine receptor channel.
3.2. Re-blood feeding assays
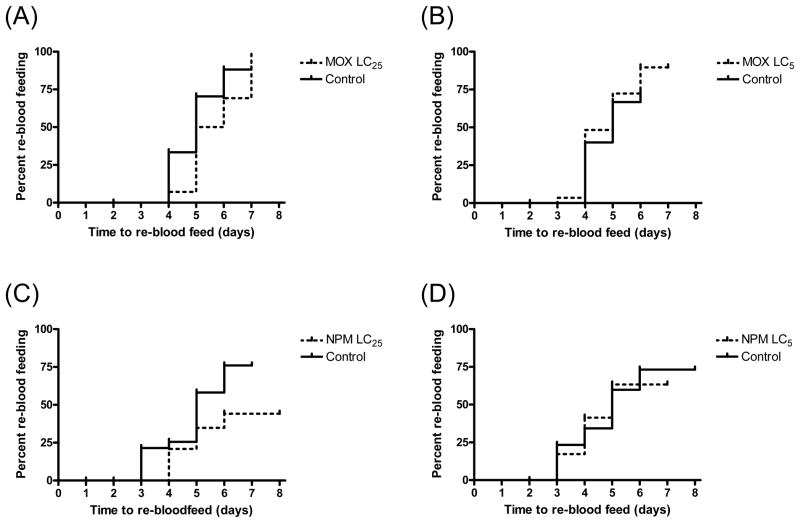
When administered at sub-lethal concentrations, nitenpyram and moxidectin were the only drugs to marginally affect re-blood feeding rates, but only at the LC25 concentrations (Fig 1). LC25 nitenpyram (78.65 ng/ml) compared to vehicle-only control; P = 0.058, Hazard ratio = 0.5032 [0.185, 1.029]. LC25 moxidectin (1001 ng/ml) compared to vehicle-only control; P = 0.0562, Hazard ratio = 0.6564 [0.2125, 1.02]. All other drugs at LC25 and LC5 concentrations failed to affect mosquito re-blood feeding (data not shown).
Figure 1.
Re-blood feeding frequency of An. gambiae s.s. G3 strain after a primary blood meal that contained moxidectin (MOX, A & B) or nitenpyram (NPM, C & D). Re-blood feeding frequency was assessed for 2 days post-emergence. Mosquitoes ingested blood containing either the LC25 (panels A & C) or the LC5 (panels B & D) of each drug and were compared to mosquitoes ingested vehicle-only control blood meals. Censored data are marked by upticks on the graph curves.
3.3. Knockdown assays
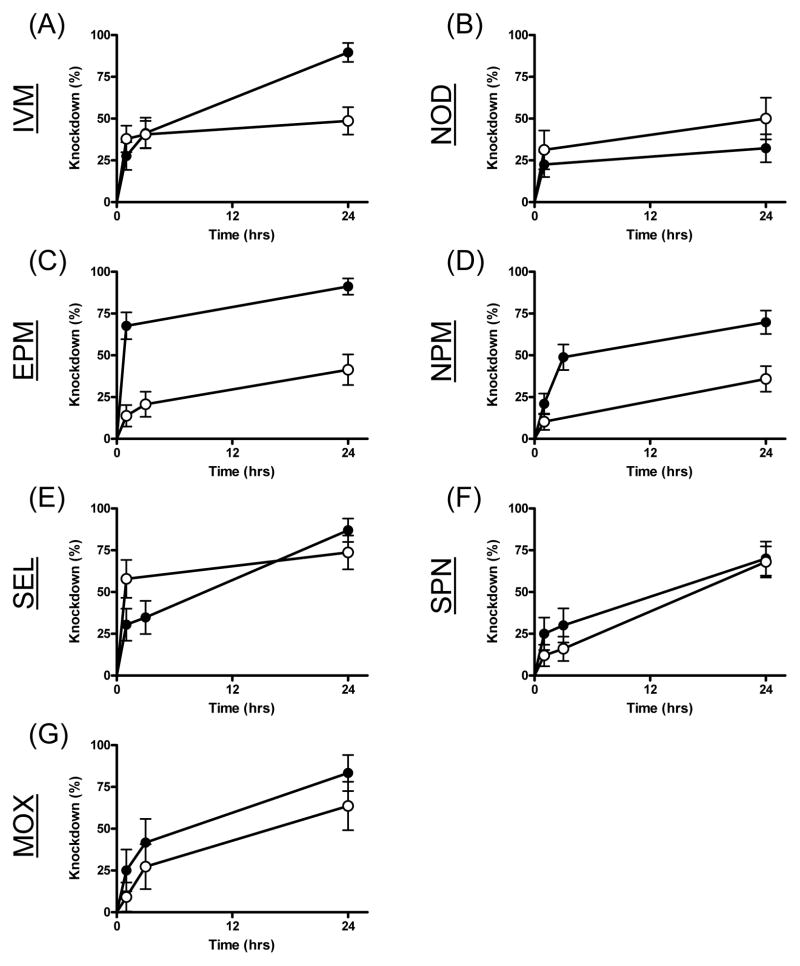
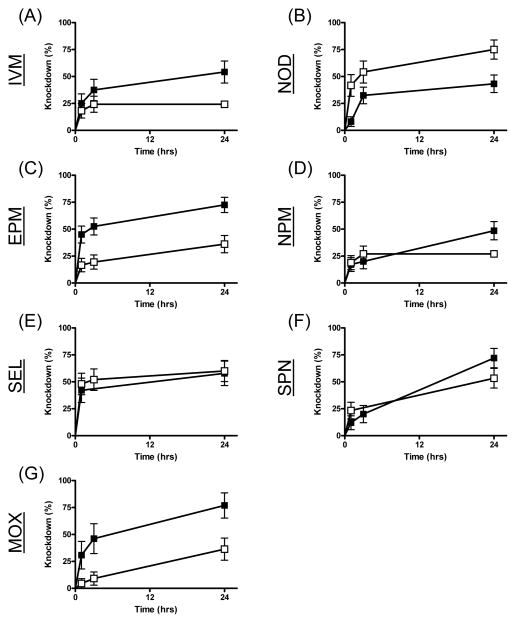
Sub-lethal concentrations of some drugs caused a noticeable knockdown effect; mosquitoes were often observed to be either lethargic or twitching, exhibiting uncoordinated flight, and resting on the bottom cages post-blood feeding. We quantified this effect in two phases: a) initial knockdown and b) recovery from toxicity. Of the drugs tested, nitenpyram, ivermectin, eprinomectin and moxidectin exhibited significant knockdown differences relative to controls (Table 2, Figs 2 & 3). Both sub-lethal concentrations of ivermectin and eprinomectin caused significant knockdown, however, the knockdown effect of eprinomectin was much more rapid, occurring in the first hour after the blood meal was ingested compared to ivermectin, whereas the knockdown effect was not apparent until 24hrs after the blood meal. The LC25 of nitenpyram and the LC5 of moxidectin caused knockdown effects, but neither did at the other sub-lethal drug concentration. The significant P value from the LC5 N-tert-butyl nodulisporamide concentrations is not relevant because it is an inverse result (Hazard ratio <1) due to an unusual number of control mosquitoes that stuck to the fly paper.
Table 2.
Knockdown and recovery assay results from sub-lethal drug concentrations blood fed to An. gambiae s.s. G3 strain mosquitoes.
| Drug | Knockdown | Recovery | ||||||
|---|---|---|---|---|---|---|---|---|
| LC25 | LC5 | LC25 | LC5 | |||||
| P | Hazard [95% CI] | P | Hazard [95% CI] | P | Hazard [95% CI] | P | Hazard [95% CI] | |
| Ivermectin | 0.015 | 1.821 [1.192, 5.129] | 0.0321 | 2.365 [1.092, 7.221] | < 0.0001 | 0.411 [0.377, 0.158] | < 0.0001 | 0.573 [0.06, 0.272] |
| Eprinomectin | < 0.0001 | 3.052 [2.834, 13.75] | 0.008 | 2.486 [1.654, 6.93] | < 0.0001 | 0.057 [0.037, 0.142] | < 0.0001 | 0.099 [0.045, 0.174] |
| Selamectin | 0.8793 | 0.963 [0.351, 2.45] | 0.8082 | 0.927 [0.333, 2.358] | < 0.0001 | 0.133 [0.049, 0.173] | < 0.0001 | 0.333 [0.109, 0.363] |
| Moxidectin | 0.2643 | 1.54 [0.596, 6.611] | 0.0061 | 2.956 [1.588. 16.05] | < 0.0001 | 0.057 [0.037, 0.142] | < 0.0001 | 0.099 [0.045, 0.174] |
| N-tert-butyl nodulisporamide | 0.2531 | 0.621 [0.174, 1.584] | 0.0061 | 0.446 [0.143, 0.724] | 0.4135 | 0.911 [0.368, 1.508] | 0.231 | 0.894 [0.27, 1.371] |
| Nitenpyram | 0.0006 | 2.557 [1.671, 6.499] | 0.1301 | 1.731 [0.83, 4.269] | < 0.0001 | 0.12 [0.044, 0.146] | 0.007 | 0.573 [0.267, 0.812] |
| Spinosad | 0.5474 | 1.176 [0.516, 3.487] | 0.3292 | 1.303 [0.652, 3.583] | 0.2479 | 1.128 [0.76, 2.897] | 0.1987 | 0.883 [0.315, 1.272] |
P is the result of the LogRank chi square test of the survival curves (drug compared matched vehicle-only control), and significant tests (P <0.05) are designated by bold font. Hazard = Hazard ratio; CI = confidence interval
Figure 2.
Knockdown measurements of An. gambiae s.s. G3 strain over 24 hours following ingestion of the LC25 of seven different drugs. Mosquitoes were blood fed ivermectin (IVM, A), N-tert-butyl nodulisporamide (NOD, B), eprinomectin (EPM, C), nitenpyram (NPM, D), selamectin (SEL, E), spinosad (SPN, F) and moxidectin (MOX, G). Curves representing mosquitoes that ingested drug in their blood meals are marked with solid circles (●), while curves representing mosquitoes that ingested vehicle-only control blood meals are marked with open circles (○).
Figure 3.
Knockdown measurements of An. gambiae s.s. G3 strain over 24 hours following ingestion of the LC5 of seven different drugs. Mosquitoes were blood fed ivermectin (IVM, A), N-tert-butyl nodulisporamide (NOD, B), eprinomectin (EPM, C), nitenpyram (NPM, D), selamectin (SEL, E), spinosad (SPN, F) and moxidectin (MOX, G). Curves representing mosquitoes that ingested drug in their blood meals are marked with solid squares (■), while curves representing mosquitoes that ingested vehicle-only control blood meals are marked with open circles (□).
3.4. Recovery assays
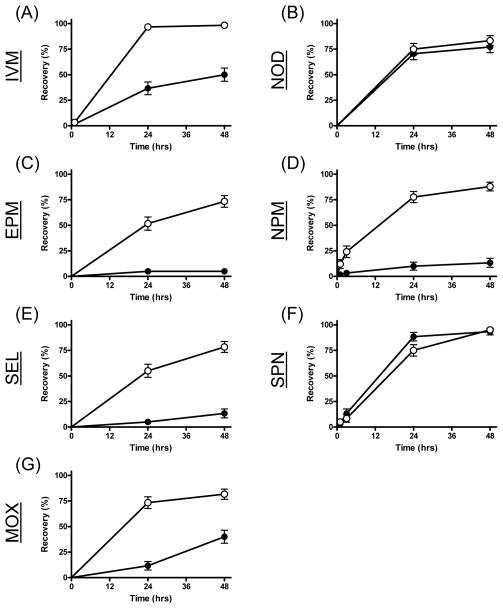
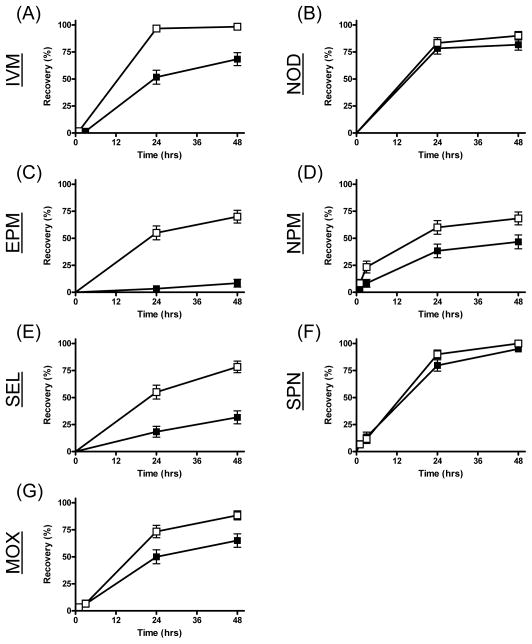
Significant inhibited recovery was observed from mosquitoes that ingested both concentrations of each avermectin derivative (Table 2, Figs 4 & 5). The strongest effect was observed for eprinomectin, where 93% of mosquitoes ingesting the lowest concentration (LC5) failed to recover after 48 hrs (Fig 5). Both concentrations of nitenpyram also significantly inhibited mosquito recovery, but the effect was stronger with the LC25. Neither spinosad nor N-tert-butyl nodulisporamide affected recovery of An. gambiae at either sub-lethal drug concentration.
Figure 4.
Recovery measurements of An. gambiae s.s. G3 strain over 48 hours following ingestion of the LC25 of seven different drugs. Mosquitoes were blood fed ivermectin (IVM, A), N-tert-butyl nodulisporamide (NOD, B), eprinomectin (EPM, C), nitenpyram (NPM, D), selamectin (SEL, E), spinosad (SPN, F) and moxidectin (MOX, G). Curves representing mosquitoes that ingested drug in their blood meals are marked with solid circles (●), while curves representing mosquitoes that ingested vehicle-only control blood meals are marked with open circles (○).
Figure 5.
Recovery measurements of An. gambiae s.s. G3 strain over 48 hours following ingestion of the LC5 of seven different drugs. Mosquitoes were blood fed ivermectin (IVM, A), N-tert-butyl nodulisporamide (NOD, B), eprinomectin (EPM, C), nitenpyram (NPM, D), selamectin (SEL, E), spinosad (SPN, F) and moxidectin (MOX, G). Curves representing mosquitoes that ingested drug in their blood meals are marked with solid squares (■), while curves representing mosquitoes that ingested vehicle-only control blood meals are marked with open circles (□).
4. Discussion
These experiments provide a comparative evaluation of systemic endectocides for their effectiveness against the African malaria vector Anopheles gambiae s.s. Derivatives of macrocyclic lactones are highly variable in their affects against this mosquito. Of the four tested, only eprinomectin compared favorably with ivermectin for mortality and sub-lethal affects on mosquito physiology at similar concentrations. The systemic neonicotinoid nitenpyram can kill Anopheles gambiae and sub-lethal concentrations can cause knockdown and inhibit their recovery, but only at ~5 times the concentration of ivermectin and eprinomectin. The newer and unique drugs spinosad and N-tert-butyl nodulisporamide were largely ineffective against Anopheles gambiae. In general, the drug efficacies did not correspond to differences in the drugs’ mode of actions. These data should be useful for future drug discovery efforts and malaria control programs seeking to expand the tools for malaria parasite transmission control.
Of the drugs tested, only ivermectin is approved for human use in MDA campaigns, although moxidectin has recently been tested as a replacement for ivermectin in onchocerciasis control (Siva, 2009). In general, the discovery of novel systemic endectocides and ectoparasiticides is driven by the veterinary pharmaceutical industry seeking new compounds for flea, tick and endoparasite control. Anopheles gambiae is highly anthropophilic (Sylla et al., 2010), therefore any drug with favorable activity against this vector would need to be evaluated for efficacy and safety in humans. To enhance its utility in humans, any efficacious new drug for malaria parasite transmission control would best be simultaneously evaluated for activity against human helminths, particularly the soil transmitted helminths (Foy et al., 2011). Alternatively, most of these drugs have been approved for veterinary use, and some have shown similar activity against the cow-biting species An. arabiensis (Fritz et al., In press). Applying these drugs to cattle or other livestock in areas dominated by zoophagic vectors may simultaneously control endo- and ectoparasites of the livestock and malaria parasite transmission to nearby humans.
The in vitro blood feeding assays we employed are useful for direct comparative evaluations of these drugs against colonized mosquitoes without the added variable of pharmacokinetic differences in vertebrates. All of these drugs, and not their metabolites, have direct activity against their molecular targets. With the exception of ivermectin and moxidectin, the pharmacokinetics of the other drugs have not been reported in humans, and so we compared the sub-lethal concentrations (LC25 and LC5) of each drug in our experiments. Pharmacokinetic differences between these drugs in a human would likely exacerbate the differences we observed in drug activity against Anopheles gambiae. Indeed, eprinomectin was designed as an avermectin derivative with reduced excretion into the milk of dairy cows (Shoop et al., 1996b). Likewise, nodulisporic acid was developed as single dose, longer lasting systemic ectoparasiticide for flea and tick control (Meinke et al., 2002).
The LC50 and re-blood feeding assays are helpful in predicting endectocide affects against the two most important components of vectorial capacity the daily probability of mosquito survival (p) and the daily probability of a mosquito feeding on a human (a) (Black and Moore, 2005). However, field data on the affects of ivermectin human MDA on wild An. gambiae survival demonstrated at least 3 fold greater efficacy over laboratory predictions using these assays, despite incomplete MDA coverage (Sylla et al., 2010). Factors likely contributing to this enhanced field effect are knockdown and inhibited recovery of mosquitoes, neither of which has been previously quantified for endectocides.
Knockdown in the field after ingesting a sub-lethal drug concentration would contribute to mortality by exposing freshly blood fed mosquitoes to desiccation and to ants, spiders, or other predators common in African houses. Inhibited recovery from a blood meal containing a sub-lethal drug concentration would contribute to mortality by preventing mosquitoes from escaping the dwelling through door gaps and open eaves in order to seek water and plant nectar to prevent desiccation and acquire nutrients. We felt the need to design our own knockdown assay instead of using the standard WHO knockdown bioassay (World Health Organization, 2006), because the latter is a test designed for evaluating the affects of volatile pesticides impregnated on filter papers against unfed mosquitoes. Our knockdown assay, while effective, was less robust than the recovery assay. We employed two-times taller than normal, rectangle-shaped cages (32 cm height × 16 cm width × 16 cm length) with scored sides for the former assay to limit the number of control mosquitoes that naturally landed on the fly paper after imbibing their blood meal. Regardless of this precaution, random flying behavior would sometimes cause non-intoxicated mosquitoes to touch the bottom and get stuck to the fly paper. In contrast, nearly all control mosquitoes from the recovery assays reached the top chamber through the emergence cone and accessed the sugar and water, while many drug-fed mosquitoes failed this task. Despite the insectary being held at 80% relative humidity during the recovery experiments, drug-affected mosquitoes that failed to acquire direct access to water and sugar following the blood meal began to die on the bottom of the chamber between 24–48 hrs, probably from a combination of drug toxicity, desiccation and depleted energy reserves. From these results, it is reasonable to postulate that wild intoxicated mosquitoes would have even more difficulty escaping eaves or gaps in dwellings and subsequently difficulty foraging in the harsher natural environment to access needed water and possibly sugar sources. The recovery assay we developed should be a simple quantifiable test for mosquito intoxication in the field that would complement the direct mortality assays we have previously employed (Sylla et al., 2010).
The highly variable efficacy of each drug against An. gambiae, despite structural or target site commonalities of some drugs, is currently not understood. The crystal structure of ivermectin complexed to the glutamate-gated chloride channel of Caenorhabditis elegans was recently elucidated (Hibbs and Gouaux, 2011). Lipophilic ivermectin integrates into the plasma membrane and buries itself between the transmembrane domains of each channel’s subunit proteins, stabilizing the open conformation of the channel. Differential side chains of the avermectin derivatives could alter this molecular interaction specifically with the An. gambiae GluCl. Alternatively, differential drug efficacies could depend on the specificities of An. gambiae detoxification enzymes, such as various cytochrome P450s (Stevenson et al., 2011), or xenobiotic transporters such as P-glycoprotein (Buss and Callaghan, 2008), for each drug. Regardless of the reasons for variable efficacy, it may be unlikely that any drug with a LC50 significantly greater than ivermectin could be expected to act as ivermectin’s supplement or substitute for malaria parasite transmission control unless the drug was exceptionally safe at the higher required concentrations. An offsetting factor would be if the substitute drug had superior bioavailabilty in human sera following oral or alternative administration.
None of the alternative drugs we tested, other than moxidectin, have been examined for safety or bioavailability in humans. In the absence of such data, we can only directly compare LC50s and sub-lethal effects, and these comparisons suggest eprinomectin, and possibly nitenpyram, might be considered alternatives to ivermectin for malaria parasite transmission control if they were proven safe for humans. Nitenypyram has the advantage of being a different compound (a neonicotinoid) and targeting a different channel (nAChRs) than ivermectin, and its acute oral toxicity for vertebrates (rat oral LC50 = 1680 mg/kg) (Yu, 2008) is high enough for the drug to be marketed as an oral flea control product for dogs and cats. To its detriment, approximately a 5-fold higher concentration relative to ivermectin would be needed in human blood to elicit a comparable mortality effect against An. gambiae, it has a very short half-life in dogs and cats, and nAChR vertebrate orthologues can be agonized by the drug (Tomizawa and Casida, 2003). Also, neonicotinoids are not known as anthelmintics. Eprinomectin should be considered as an alternative to ivermectin for malaria parasite transmission control. The drug has almost identical mortality effects against An. gambiae, but causes faster mosquito intoxication than ivermectin. Eprinomectin’s pharmacokinetics are also favorable because there is less excretion in dairy cattle milk (Shoop et al., 1996a), which might carry over to human pharmacokinetics and ultimately allow its distribution to newly lactating mothers during MDAs. Lastly eprinomectin has excellent broad spectrum activity against veterinary helminths and ectoparasites (Hoste et al., 2004; Shoop et al., 1996b), and it is reasonable to speculate that this activity would carry over to human helminths. One detriment might be that cross-resistance to ivermectin would limit eprinomectin’s efficacy, although this remains speculative until tested through bioassays. Ultimately, eprinomectin should be considered for evaluation in human clinical trials for dual activity against human helminths and malaria-transmitting mosquitoes.
Acknowledgments
This works was supported by NIH grant R21 AI079528, Grand Challenges Explorations grant 51995 from the Bill and Melinda Gates Foundation, and CRC grant 1686174 from Colorado State University.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Banks BJ, Bishop BF, Evans NA, Gibson SP, Goudie AC, Gration KA, Pacey MS, Perry DA, Witty MJ. Avermectins and flea control: structure-activity relationships and the selection of selamectin for development as an endectocide for companion animals. Bioorg Med Chem. 2000;8:2017–2025. doi: 10.1016/s0968-0896(00)00120-6. [DOI] [PubMed] [Google Scholar]
- Bishop BF, Bruce CI, Evans NA, Goudie AC, Gration KA, Gibson SP, Pacey MS, Perry DA, Walshe ND, Witty MJ. Selamectin: a novel broad-spectrum endectocide for dogs and cats. Vet Parasitol. 2000;91:163–176. doi: 10.1016/s0304-4017(00)00289-2. [DOI] [PubMed] [Google Scholar]
- Black WCI, Moore CG. Population Biology as a Tool to Study Vector-Borne Diseases. In: Marquardt WC, editor. Biology of Disease Vectors. 2. Elsevier Academic Press; San Diego, CA: 2005. pp. 187–206. [Google Scholar]
- Bockarie MJ, Hii JL, Alexander ND, Bockarie F, Dagoro H, Kazura JW, Alpers MP. Mass treatment with ivermectin for filariasis control in Papua New Guinea: impact on mosquito survival. Med Vet Entomol. 1999;13:120–123. doi: 10.1046/j.1365-2915.1999.00159.x. [DOI] [PubMed] [Google Scholar]
- Buss DS, Callaghan A. Interaction of pesticides with p-glycoprotein and other ABC proteins:A survey of the possible importance to insecticide, herbicide and fungicide resistance. Pesticide Biochemistry and Physiology. 2008;90:141–153. [Google Scholar]
- Chaccour C, Lines J, Whitty CJ. Effect of ivermectin on Anopheles gambiae mosquitoes fed on humans: the potential of oral insecticides in malaria control. J Infect Dis. 2010;202:113–116. doi: 10.1086/653208. [DOI] [PubMed] [Google Scholar]
- Cotreau MM, Warren S, Ryan JL, Fleckenstein L, Vanapalli SR, Brown KR, Rock D, Chen CY, Schwertschlag US. The antiparasitic moxidectin: safety, tolerability, and pharmacokinetics in humans. J Clin Pharmacol. 2003;43:1108–1115. doi: 10.1177/0091270003257456. [DOI] [PubMed] [Google Scholar]
- Cupp EW, Sauerbrey M, Richards F. Elimination of human onchocerciasis: History of progress and current feasibility using ivermectin (Mectizan((R))) monotherapy. Acta Trop. 2010 doi: 10.1016/j.actatropica.2010.08.009. [DOI] [PubMed] [Google Scholar]
- Foley DH, Bryan JH, Lawrence GW. The potential of ivermectin to control the malaria vector Anopheles farauti. Trans R Soc Trop Med Hyg. 2000;94:625–628. doi: 10.1016/s0035-9203(00)90211-6. [DOI] [PubMed] [Google Scholar]
- Foy BD, Kobylinski KC, Silva IM, Rasgon JL, Sylla M. Endectocides for malaria control. Trends Parasitol. 2011;27:423–428. doi: 10.1016/j.pt.2011.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritz ML, Siegert PY, Walker ED, Bayoh MN, Vulule JR, Miller JR. Toxicity of bloodmeals from ivermectin-treated cattle to Anopheles gambiae s.l. Ann Trop Med Parasitol. 2009;103:539–547. doi: 10.1179/000349809X12459740922138. [DOI] [PubMed] [Google Scholar]
- Fritz ML, Walker ED, Miller JR. Lethal and sublethal effects of avermectin/milbemycin parasiticides on the African malaria vector, Anopheles arabiensis. J Med Entomol. doi: 10.1603/me11098. In press. [DOI] [PubMed] [Google Scholar]
- Gardner K, Meisch MV, Meek CL, Biven WS. Effects of ivermectin in canine blood on Anopheles quadrimaculatus, Aedes albopictus and Culex salinarius. J Am Mosq Control Assoc. 1993;9:400–402. [PubMed] [Google Scholar]
- Hibbs RE, Gouaux E. Principles of activation and permeation in an anion selective Cys-loop receptor. Nature. 2011 doi: 10.1038/nature10139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoste H, Lespine A, Lemercier P, Alvinerie M, Jacquiet P, Dorchies P. Efficacy of eprinomectin pour-on against gastrointestinal nematodes and the nasal bot fly (Oestrus ovis) in sheep. Vet Rec. 2004;154:782–785. doi: 10.1136/vr.154.25.782. [DOI] [PubMed] [Google Scholar]
- Iakubovich VI, Zakharova NF, Alekseev AN, Alekseev EA. Evaluation of ivermectin on blood-sucking mosquitoes. Medicinskaya Parazitologia Moskva. 1989;3:60–64. [PubMed] [Google Scholar]
- Jones JW, Meisch MV, Meek CL, Bivin WS. Lethal effects of ivermectin on Anopheles quadrimaculatus. J Am Mosq Control Assoc. 1992;8:278–280. [PubMed] [Google Scholar]
- Kane NS, Hirschberg B, Qian S, Hunt D, Thomas B, Brochu R, Ludmerer SW, Zheng Y, Smith M, Arena JP, Cohen CJ, Schmatz D, Warmke J, Cully DF. Drug-resistant Drosophila indicate glutamate-gated chloride channels are targets for the antiparasitics nodulisporic acid and ivermectin. Proc Natl Acad Sci U S A. 2000;97:13949–13954. doi: 10.1073/pnas.240464697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobylinski KC, Deus KM, Butters MP, Hongyu T, Gray M, da Silva IM, Sylla M, Foy BD. The effect of oral anthelmintics on the survivorship and re-feeding frequency of anthropophilic mosquito disease vectors. Acta Trop. 2010;116:119–126. doi: 10.1016/j.actatropica.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinke PT, Colletti SL, Fisher MH, Wyvratt MJ, Shih TL, Ayer MB, Li C, Lim J, Ok D, Salva S, Warmke LM, Zakson M, Michael BF, Demontigny P, Ostlind DA, Fink D, Drag M, Schmatz DM, Shoop WL. Discovery of the development candidate N-tert-butyl nodulisporamide: a safe and efficacious once monthly oral agent for the control of fleas and ticks on companion animals. J Med Chem. 2009;52:3505–3515. doi: 10.1021/jm801334v. [DOI] [PubMed] [Google Scholar]
- Meinke PT, Smith MM, Shoop WL. Nodulisporic acid: its chemistry and biology. Curr Top Med Chem. 2002;2:655–674. doi: 10.2174/1568026023393714. [DOI] [PubMed] [Google Scholar]
- Molyneux DH, Bradley M, Hoerauf A, Kyelem D, Taylor MJ. Mass drug treatment for lymphatic filariasis and onchocerciasis. Trends Parasitol. 2003;19:516–522. doi: 10.1016/j.pt.2003.09.004. [DOI] [PubMed] [Google Scholar]
- Mounsey KE, Holt DC, McCarthy JS, Currie BJ, Walton SF. Longitudinal evidence of increasing in vitro tolerance of scabies mites to ivermectin in scabies-endemic communities. Arch Dermatol. 2009;145:840–841. doi: 10.1001/archdermatol.2009.125. [DOI] [PubMed] [Google Scholar]
- Mumcuoglu KY, Miller J, Rosen LJ, Galun R. Systemic activity of ivermectin on the human body louse (Anoplura: Pediculidae) J Med Entomol. 1990;27:72–75. doi: 10.1093/jmedent/27.1.72. [DOI] [PubMed] [Google Scholar]
- World Health Organization. Guidelines for testing mosquito adulticides for indoor residual spraying and treatment of mosquito nets, Control of neglected tropical diseases; WHO Pesticide evaluation scheme. World Health Organization; Geneva: 2006. p. 60. [Google Scholar]
- Pampiglioni S, Majori G, Petrangeli G, Romi R. Avermectins, MK-933 and MK-936, for mosquito control. Trans R Soc Trop Med Hyg. 1985;79:797–799. doi: 10.1016/0035-9203(85)90121-x. [DOI] [PubMed] [Google Scholar]
- Rust MK, Waggoner MM, Hinkle NC, Stansfield D, Barnett S. Efficacy and longevity of nitenpyram against adult cat fleas (Siphonaptera: Pulicidae) J Med Entomol. 2003;40:678–681. doi: 10.1603/0022-2585-40.5.678. [DOI] [PubMed] [Google Scholar]
- Shoop WL, DeMontigny P, Fink DW, Williams JB, Egerton JR, Mrozik H, Fisher MH, Skelly BJ, Turner MJ. Efficacy in sheep and pharmacokinetics in cattle that led to the selection of eprinomectin as a topical endectocide for cattle. Int J Parasitol. 1996a;26:1227–1235. doi: 10.1016/s0020-7519(96)00122-1. [DOI] [PubMed] [Google Scholar]
- Shoop WL, Egerton JR, Eary CH, Haines HW, Michael BF, Mrozik H, Eskola P, Fisher MH, Slayton L, Ostlind DA, Skelly BJ, Fulton RK, Barth D, Costa S, Gregory LM, Campbell WC, Seward RL, Turner MJ. Eprinomectin: a novel avermectin for use as a topical endectocide for cattle. Int J Parasitol. 1996b;26:1237–1242. doi: 10.1016/s0020-7519(96)00123-3. [DOI] [PubMed] [Google Scholar]
- Shoop WL, Mrozik H, Fisher MH. Structure and activity of avermectins and milbemycins in animal health. Vet Parasitol. 1995;59:139–156. doi: 10.1016/0304-4017(94)00743-v. [DOI] [PubMed] [Google Scholar]
- Siva N. WHO researchers start trial on a new drug for river blindness. Bmj. 2009;339:b2755. doi: 10.1136/bmj.b2755. [DOI] [PubMed] [Google Scholar]
- Snyder DE, Cruthers LR, Slone RL. Preliminary study on the acaricidal efficacy of spinosad administered orally to dogs infested with the brown dog tick, Rhipicephalus sanguineus (Latreille, 1806) (Acari: Ixodidae) Vet Parasitol. 2009;166:131–135. doi: 10.1016/j.vetpar.2009.07.046. [DOI] [PubMed] [Google Scholar]
- Snyder DE, Meyer J, Zimmermann AG, Qiao M, Gissendanner SJ, Cruthers LR, Slone RL, Young DR. Preliminary studies on the effectiveness of the novel pulicide, spinosad, for the treatment and control of fleas on dogs. Vet Parasitol. 2007;150:345–351. doi: 10.1016/j.vetpar.2007.09.011. [DOI] [PubMed] [Google Scholar]
- Sparks TC, Crouse GD, Durst G. Natural products as insecticides: the biology, biochemistry and quantitative structure-activity relationships of spinosyns and spinosoids. Pest Manag Sci. 2001;57:896–905. doi: 10.1002/ps.358. [DOI] [PubMed] [Google Scholar]
- Stevenson BJ, Bibby J, Pignatelli P, Muangnoicharoen S, O’Neill PM, Lian LY, Muller P, Nikou D, Steven A, Hemingway J, Sutcliffe MJ, Paine MJ. Cytochrome P450 6M2 from the malaria vector Anopheles gambiae metabolizes pyrethroids: Sequential metabolism of deltamethrin revealed. Insect Biochem Mol Biol. 2011 doi: 10.1016/j.ibmb.2011.02.003. [DOI] [PubMed] [Google Scholar]
- Suputtamongkol Y, Premasathian N, Bhumimuang K, Waywa D, Nilganuwong S, Karuphong E, Anekthananon T, Wanachiwanawin D, Silpasakorn S. Efficacy and Safety of Single and Double Doses of Ivermectin versus 7-Day High Dose Albendazole for Chronic Strongyloidiasis. PLoS Negl Trop Dis. 2011;5:e1044. doi: 10.1371/journal.pntd.0001044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sylla M, Kobylinski KC, Gray M, Chapman PL, Sarr MD, Rasgon JL, Foy BD. Mass drug administration of ivermectin in south-eastern Senegal reduces the survivorship of wild-caught, blood fed malaria vectors. Malar J. 2010;9:365. doi: 10.1186/1475-2875-9-365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takiguchi Y, Mishima H, Okuda M, Terao M, Aoki A, Fukuda R. Milbemycins, a new family of macrolide antibiotics: fermentation, isolation and physico-chemical properties. J Antibiot (Tokyo) 1980;33:1120–1127. doi: 10.7164/antibiotics.33.1120. [DOI] [PubMed] [Google Scholar]
- Tesh RB, Guzman H. Mortality and infertility in adult mosquitoes after the ingestion of blood containing ivermectin. Am J Trop Med Hyg. 1990;43:229–233. doi: 10.4269/ajtmh.1990.43.229. [DOI] [PubMed] [Google Scholar]
- Tomizawa M, Casida JE. Selective toxicity of neonicotinoids attributable to specificity of insect and mammalian nicotinic receptors. Annu Rev Entomol. 2003;48:339–364. doi: 10.1146/annurev.ento.48.091801.112731. [DOI] [PubMed] [Google Scholar]
- Yu SJ. The Toxicology and Biochemistry of Insecticides. CRC Press; Boca Raton, FL: 2008. [Google Scholar]