Abstract
Although the p53 tumor suppressor is relatively well characterized, much less is known about the functions of other members of the p53 family, p73 and p63. Here, we present evidence that in specific pathological conditions caused by exposure of normal cells to bile acids in acidic conditions, p73 protein plays the predominant role in the DNA damage response. These pathological conditions frequently occur during gastric reflux in the human esophagus and are associated with progression to esophageal adenocarcinoma. We found that despite strong DNA damage induced by bile acid exposure, only p73 (but not p53 and p63) is selectively activated in a c-Abl kinase-dependent manner. The activated p73 protein induces DNA damage repair. Using a human DNA repair PCR array, we identified multiple DNA repair genes affected by p73. Two glycosylases involved in base excision repair, SMUG1 and MUTYH, were characterized and found to be transcriptionally regulated by p73 in DNA damage conditions. Using a surgical procedure in mice, which recapitulates bile acid exposure, we found that p73 deficiency is associated with increased DNA damage. These findings were further investigated with organotypic and traditional cell cultures. Collectively our studies demonstrate that p73 plays an important role in the regulation of DNA damage repair.—Zaika, E., Wei, J., Yin, D., Andl, C., Moll, U., El-Rifai, W., Zaika, A. I. p73 protein regulates DNA damage repair.
Keywords: p53, p63, bile acids
Although the overall incidence and mortality rates of many tumors have declined in recent years, the incidence of esophageal adenocarcinoma (EA) is the fastest rising of any tumors in the United States, accounting for a 6-fold increase in the past 3 decades (1). The etiology of EA is strongly linked to gastroesophageal reflux disease (GERD), a chronic pathological condition in which gastric juice, frequently mixed with duodenal bile acids (BAs), enters the esophagus (2). The exposure to gastric acid and BAs causes significant tissue damage and inflammation. If these conditions persist, they may lead to hyperplasia and Barrett's esophagus, the condition in which the normal squamous epithelial cell lining is replaced by metaplastic intestinal type of epithelium. In some patients, these precancerous lesions can further progress to esophageal dysplasia and adenocarcinoma. This sequence of events can be recapitulated in animal models. Mice and rats with surgically induced esophageal reflux develop esophagitis, Barrett's-like metaplasia, and EA (3). The role of BAs in esophageal tumorigenesis deserves special attention, because these natural body metabolites affect esophageal cells in abnormal conditions: low pH and high BA concentration. A peak concentration of total BA can reach 2 mM in the esophagus during a patient's reflux (4). Several studies found that bile and acid reflux induces considerable genotoxic stress. Increased ROS and RNS production, significant oxidative DNA damage, and DNA strand breaks have been shown to follow the exposure of esophageal epithelial cells to bile acids and acid (BA/A) in vitro and in vivo (5–8). Direct measurement of the mutational rate using Big Blue LacI transgenic rats found increased mutational levels (primarily C to T and G to A transitions) in animals with surgically induced BA reflux into the esophagus (9). Based on these studies, the response of esophageal cells to DNA damage induced by GERD has been proposed to play a critical role in EA tumorigenesis (10, 11).
One of the key molecules involved in DNA damage response is the p53 tumor suppressor (12). Following DNA damage, p53 protein is accumulated in the nucleus and activates transcription of multiple p53 target genes, which regulate critical biological processes including apoptosis, cell cycle, and DNA damage repair (13). Recent studies show that other members of the p53 family, p73 and p63, are also involved in response to genotoxic stress (14, 15). These proteins share significant structural and functional similarities with p53. The highest similarity is in the DNA binding domain, where all family members share ∼60% amino acid identity (16). This translates into a significant overlap of their transcription profiles (17, 18). p73 and p63 can bind and activate transcription of p53 target genes, leading to induction of cell cycle arrest or apoptosis (for a review, see ref. 19). However, despite these similarities to p53, many questions remain about biological roles of p73 and p63. In this study we investigated the regulation of the p53 family in conditions of DNA damage associated with BA/A reflux.
MATERIALS AND METHODS
Cells cultures, transfections, retroviral infections, and BA treatment
Primary fetal esophageal fibroblasts, human nontumorous esophageal epithelial cell line HET-1A (20), and p53-null esophageal adenocarcinoma cell line SKGT-4 (21) were cultured in DMEM (Invitrogen, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (Atlanta Biologicals, Lawrenceville, GA, USA). hTERT-immortalized esophageal epithelial EPC2 cells, isolated from normal human esophagus (22), were grown in keratinocyte-SFM (KSFM) medium supplemented with 40 μg/ml bovine pituitary extract and 1.0 ng/ml epidermal growth factor (Invitrogen). Fetal esophageal fibroblasts and EPC2 cells were kindly provided by one of the authors (C.A.). All media were supplemented with 100 μg/ml penicillin and 100 μg/ml streptomycin.
Growth conditions for esophageal epithelial organotypic culture were described previously (22). In brief, 3 × 105 human fetal esophageal fibroblasts were embedded into a layer of Collagen I (Organogenesis, Canton, MA, USA) and Matrigel (BD Biosciences, Franklin Lakes, NJ, USA), mixed at a 3:1 ratio. On day seven, 5 × 105 human EPC2 cells were seeded on top of the contracted matrix. On d 11, cultures were raised to an air-liquid interface to induce differentiation of the epithelium. On d 17, an organotypic culture was submerged into DMEM medium containing 100 μM BA cocktail, pH 4.0, for 3 min. Cells were then recovered in complete DMEM medium with 10% FBS for 1 h, fixed in 10% formalin (VWR, Radnor, PA, USA), and paraffin embedded.
For the generation of cell lines stably expressing human ΔNp73α protein, cells were transfected with vector ΔNp73α-pcDNA3 and selected with G418 (Mediatech, Manassas, VA, USA; ref. 23). For inhibition of p73, either lentiviral transduction with shRNA or transfection with siRNA was used (24). Both approaches targeted the same p73 sequence (5′-GCAATAATCTCTCGCAGTA-3′) found in all p73 isoforms. Cells were transfected with Lipofectamine 2000 (Invitrogen) or PolyJet (SignaGen Laboratories, Rockville, MD, USA) following the manufacturers' protocols.
Cells were treated with BA cocktail consisting of a 20 μM equimolar mixture of glycocholic acid, taurocholic acid, glycodeoxycholic acid, glycochenodeoxycholic acid, and dioxycholic acid sodium salts (all reagents were from Sigma-Aldrich, St. Louis, MO, USA); total BA concentration was 100 μM. For cell treatment, BA cocktail was diluted in DMEM, pH 4.0 (BA/A); pH was adjusted with HCl.
Antibodies, immunoprecipitation, and immunofluorescence
Antibodies for the following proteins were used: p73 from Bethyl Laboratories (Montgomery, TX, USA); p63 (4A4) and c-Abl (K-12) from Santa Cruz Biotechnology (Santa Cruz, CA, USA); phospho-c-Abl (Tyr412) from Sigma-Aldrich; phospho-p53 (Ser15), β-actin (13E5), and phospho-histone H2A.X (Ser139) from Cell Signaling (Danvers, MA, USA); phospho-tyrosine (clone 4G10) and 8-oxoguanine (8-OxoG; clone 483.15) from Millipore (Billerica, MA, USA); p53 (Ab-6) and p21 (Ab-1) from Calbiochem (EMD Chemicals, Gibbstown, NJ, USA); PUMA (ab9643) and SMUG1 (ab15716) from Abcam (Cambridge, MA, USA); MUTYH from Novus Biologicals (Littleton, CO, USA); and nonspecific rabbit IgG from Jackson ImmunoResearch (West Grove, PA, USA).
Immunoprecipitation was performed with anti-p73 antibody (Bethyl Laboratories) as described previously (23). Tyrosine phosphorylation of p73 was analyzed by Western blotting.
For immunofluorescence, cells were grown to 50% confluency on chamber slides. For phospho-histone p-H2A.X (Ser139) staining cells were fixed in methanol:aceton mixed at a 1:1 ratio (v/v). For 8-oxoG staining, cells were fixed with 4% paraformaldehyde and treated at room temperature with 10 μg/ml proteinase K for 10 min, 2N HCl for 5 min, and 1 M Tris-base for 10 min to denature DNA. All following steps were the same for 8-oxoG and phospho-histone stainings. After blocking with 10% goat serum (Invitrogen), cells were incubated with the primary antibody for 16–18 h at 4°C, then with the secondary antibody conjugated with AlexaFluor 568 (Invitrogen) for 45 min at room temperature. The intensity of fluorescent signal was measured using the ImageJ software (U.S. National Institutes of Health, Bethesda, MD, USA) in ≥500 cells.
Real-time PCR and chromatin immunoprecipitation (ChIP)
RNA was isolated using the RNeasy kit (Qiagen, Valencia, CA, USA). Total RNA (1 μg) was reverse transcribed with High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Carlsbad, CA, USA). Quantitative real-time PCR was performed using Bio-Rad iCycler thermal cycler and iQ SYBR Green Supermix (Bio-Rad, Hercules, CA, USA). Data are presented as average ± sd.
ChIP was performed with p73 antibody (Bethyl Laboratories) and nonspecific rabbit IgG as negative control in HET-1A cells using the ChIP assay kit (Upstate Biotechnology, Charlottesville, VA, USA), as described previously (25). p73 binding sites were analyzed using the Genomatix MatInspector software (Genomatix Software GmbH, Munich, Germany).
Sequences of primers used for the assessment of p73, MUTYH, and SMUG1 mRNA levels and ChIP analyses are presented in Table 1.
Table 1.
Primers for quantitative real-time PCR and ChIP analyses
| Protein | Primers for real-time PCR |
|---|---|
| TAp73 | 5′-CACGTTTGAGCACCTCTGGA, 5′-GAACTGGGCCATGACAGATG |
| MUTYH | 5′-AGCCTTCTGCCTGTGATGCCTG, 5′-TGGCAACCTGGGTCTGCTGC |
| SMUG1 | 5′-ACCTTTTGGCATGGCCCAGACTG, 5′-GGAGTAAGGTTGCGCCCGCT |
| HPRT1 | 5′-TTGGAAAGGGTGTTTATTCCTCA, 5′-TCCAGCAGGTCAGCAAAGAA |
| Binding site | Primers for ChIP |
|---|---|
| MUTYH (−33) | 5′-GGCAGTGCCCCACACCAGAG, 5′-GGCTCCGGCTGCAAAAGCCT |
| MUTYH (+4254) | 5′-ACGGGGTCTTGCTATGTTGCAAGC, 5′-CTGGACATGCTGGCTCAGGCC |
| SMUG1 (+52) | 5′-CGACTCCCACTGTCCTTAGCGCC, 5′-TCTCGGCTCAGCCCCGACGA |
| SMUG1 (+3941) | 5′-CGGGGTTTCGCCATGTTGGC, 5′-GGCCAGGCATGGTGGCTCAT |
PCR array, comet, and abasic site quantification assays
Comet assay was performed following the protocol described by Speit et al. (26) with small modifications. Briefly, cells were mixed with 0.5% low-melting temperature agarose and applied to a slide precoated with 1% agarose. After cell lysis in the lysis buffer (2.5 M NaCl, 100 mM EDTA, 10 mM Tris, 0.2 M NaOH; 1% Triton and 10% DMSO, pH 10.0), electrophoresis was performed in alkaline conditions (300 mM NaOH and 1 mM EDTA, pH 13). Slides were stained with SYBR Green (Invitrogen) and analyzed using the CometScore software (TriTek, Sumerduck, VA, USA). Typically, tail length was measured in 50–100 randomly selected cells per slide. Experiments were repeated 4 times.
mRNA expression profiles were compared in p73-deficient and control HET-1A cells using the Human DNA Repair RT2 Profiler PCR Array System (SABiosciences, Frederick, MD, USA). Eighty-four key DNA repair genes were assessed.
Abasic sites were quantitated using the OxiSelect Oxidative DNA Damage Quantification Kit (Cell Biolabs, San Diego, CA, USA). DNA for this analysis was purified with DNAzol reagent (Invitrogen) following the manufacturer's protocol.
Surgical procedures and immunohistochemistry
Mouse strains deficient for p73 were described previously (27). Esophagojejunostomy was performed on 5 wild-type and 5 p73 heterozygous mice. The proximal jejunum was anastomosed to the lower esophagus in 8-wk-old mice, according to the protocol approved by the Vanderbilt University Animal Care and Use Committee. Mice were euthanized 15 wk after surgery, and the lower esophagus was analyzed by immunohistochemistry as described previously (28).
Statistical analysis
Statistical analysis was performed using the Student's t test or Wilcoxon rank sum test. Results are expressed as averages ± se, if not specifically indicated. Results were considered significant at values of P < 0.05.
RESULTS
p73 is up-regulated by BA/A treatment
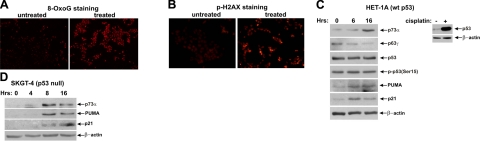
As a prelude to defining the role of the p53 family, we first sought to characterize DNA damage induced by BA/A in our model system of interest. Nontumorous esophageal HET-1A cells were treated with a single dose of 100 μM BA cocktail, pH 4.0, for 30 min, and DNA damage was analyzed by immunofluorescence using 8-OxoG and phospho-H2A.X(S139) antibodies. The concentration and composition of the BA cocktail were selected based on previously published measurements and mimic a typical, physiological episode of gastroesophageal reflux (6). This short exposure to BA/A led to significant oxidative DNA damage and DNA strand breaks in esophageal cells, as detected with antibodies against 8-oxoguanine and phosphorylated histone H2A.X(S139) (Fig. 1A, B). Increased DNA damage was also found in another nontumorous esophageal cell line, EPC2 (data not shown). Since all members of the p53 family (p53, p73, and p63) are induced and activated by DNA damage, we decided to analyze these proteins after BA/A treatment. Surprisingly, we did not find accumulation of the p53 protein by DNA damage induced by BA/A. An increased phosphorylation of p53 at serine 15, which is considered to be a central event during the induction of p53 by DNA damage, was also undetected (Fig. 1C). As an additional control, HET-1A cells were treated with a commonly used DNA-damaging drug, cisplatin, which induces p53. We found strong up-regulation of p53 following the cisplatin treatment, showing that the p53 pathway remains intact in these cells (Fig. 1C, right panel). Interestingly, p63 levels were decreased by BA/A. At 16 h after treatment with BA/A, the p63 protein was barely detectable by Western blotting (Fig. 1C). In contrast, TAp73 was strongly induced by BA/A in HET-1A and EPC2 cells in a time-dependent manner (Fig. 1C and Supplemental Fig. S1A). Since p53 could affect p73 regulation, we next repeated this experiment in SKGT-4 esophageal cells, which lack p53 expression. Similar to EPC2 and HET-1A cells, p73 was significantly up-regulated by the BA/A treatment in SKGT-4 cells, showing that p73 induction is independent of p53 (Fig. 1D). Moreover, the p73 protein was not only up-regulated but also activated by the BA/A treatment. Our analysis found a strong induction of known p73/p53 targets, p21/WAF1 and PUMA, after BA/A treatment in esophageal cells (Fig. 1C, D). Taken together, our data show that p73, but not p53 or p63, is induced by BA/A treatment.
Figure 1.
Exposure to BAs at low pH leads to extensive DNA damage and up-regulation of p73. A) Representative immunofluorescent staining for 8-oxoG in HET-1A cells before and after treatment with 100 μM BA/A cocktail, pH 4.0, for 30 min. B) Representative immunofluorescent staining for phospho-histone p-H2A.X in HET-1A cells treated with 100 μM BA cocktail, pH 4.0, for 30 min, and then recovered in complete medium without BAs for 1 h. C, D) Western blot analyses of total cell extracts from HET-1A (C) and SKGT-4 (D) cells collected at the indicated time after treatment with 100 μM BA cocktail, pH 4.0, for 30 min or after 16 h incubation with 5 μM cisplatin.
Regulation of p73 by BA/A treatment
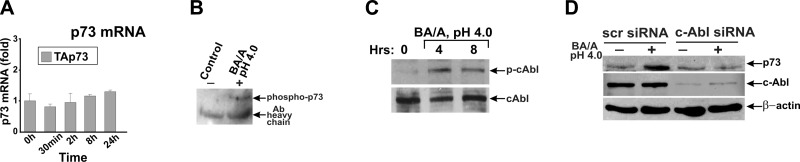
To investigate how the p73 protein is regulated, we asked whether BA/A treatment alters the p73 gene transcription. As shown in Fig. 2A, the p73 mRNA levels were not elevated in esophageal cells, indicating that the p73 protein is up-regulated by post-translational mechanisms. Previously, it has been reported that the p73 protein can be regulated by tyrosine phosphorylation induced by c-Abl protein kinase (29–31). To explore whether c-Abl is activated by BA/A treatment, we analyzed the phosphorylation of c-Abl at tyrosine 412. The phosphorylation at this residue stimulates kinase activity of c-Abl. Our analyses show that BA/A treatment activates c-Abl tyrosine kinase and induces tyrosine phosphorylation of p73 (Fig. 2B, C). To confirm the role of c-Abl kinase in the regulation of p73, we down-regulated c-Abl with specific siRNA and treated these cells with a BA/A cocktail. We found that cells deficient in c-Abl expression completely lost the ability to induce p73, showing that the p73 protein is up-regulated in a c-Abl-dependent manner (Fig. 2D).
Figure 2.
c-Abl tyrosine kinase regulates p73 after BA/A treatment. A) Real-time PCR analysis of p73 mRNA collected from HET-1A cells after 30 min treatment with 100 μM BA cocktail, pH 4.0. Cells were collected at the indicated time. B) Tyrosine phosphorylation of p73 is increased after BA/A treatment in HET-1A cells. p73 was immunoprecipitated with p73-specific antibody and analyzed by Western blotting with p-Tyr antibody. C) Western blot analysis of c-Abl kinase phosphorylation. Phosphorylation of c-Abl at Tyr 412 is increased after BA/A treatment in HET-1A cells. D) Induction of p73 is inhibited by siRNA against c-Abl in HET-1A cells.
Inhibition of p73 induces DNA damage
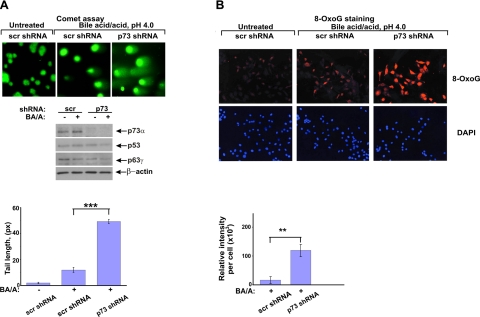
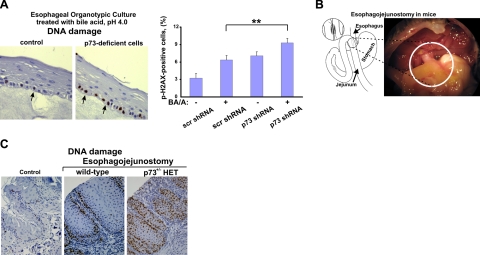
Since DNA damage associated with GERD plays a critical role in promoting tumorigenesis, we investigated whether p73 affects DNA damage response in esophageal cells. Here, p73 was down-regulated with specific shRNA against p73 or by overexpression of the dominant-negative isoform, ΔNp73α, in HET-1A and EPC2 esophageal cells. Preliminary testing found that p73 shRNA efficiently inhibits p73 but did not affect p53 and p63 proteins (Fig. 3A, middle panel). The p73-deficient and control cells, which expressed scrambled shRNA, were then treated with BA/A as described above, and DNA damage was measured by comet assay. As shown in Fig. 3A and Supplemental Fig. S1B, p73-deficient cells are significantly more susceptible to DNA damage induced by BA/A treatment compared with control cells. We also found that p73 inhibition increases oxidative DNA damage induced by BA/A or hydrogen peroxide treatment (Fig. 3B and data not shown). Next, we investigated the role of p73 in the setting of human esophageal organotypic cultures. Using special growth conditions (22) and a feeder layer of primary esophageal fibroblasts isolated from the human esophagus, we induced the differentiation of EPC2 cells into multilayer squamous epithelium that is morphologically similar to the native esophageal tissue. This approach better recapitulates normal esophageal biology than traditional cell cultures and provides a significant experimental flexibility for studies of DNA damage in the esophagus. The established organotypic cultures of control and p73-deficient cells were treated with a single dose of 100 μM BA cocktail dissolved in acidic growth medium (pH 4.0) for 3 min. These conditions mimic a typical physiological episode of gastroesophageal reflux (6). DNA damage was measured by staining for phosphorylated histone p-H2A.X (Ser129) 1 h after treatment. Consistent with our findings, p73-deficient cells were significantly more susceptible to DNA damage than control cells (Fig. 4A). Interestingly, esophageal cells in the basal layer, where p73 is expressed in the normal esophagus, were primarily damaged (32). To explore the role of p73 in vivo, we established a surgical mouse model of esophageal reflux injury. For this purpose, a section of the mouse jejunum was excised 4 cm distal to the ligament of Treitz and then anastomosed to distal esophagus (Fig. 4B). This procedure leads to an increased reflux to the esophagus. Using our colony of p73-deficient mice, esophagojejunostomy was performed on two groups of p73+/− heterozygous mice and p73+/+ control wild-type mice (n=10). Analyzing experimental animals by immunohistochemistry with p-H2A.X (Ser139) antibody 15 wk after surgery, we found that although esophageal reflux caused inflammation and DNA damage in both groups of animals, DNA damage was significantly higher in the esophagus of p73+/− heterozygous than control wild-type mice with surgeries (Fig. 4C).
Figure 3.
p73 down-regulation results in increased DNA damage. A) Analysis of DNA damage by comet assay in p73-deficient and control HET-1A cells. Cells were stably transfected with p73 or scrambled shRNA and treated with 100 μM BA cocktail, pH 4.0. Blots show the effect of p73 shRNA on p73, p63, and p53. Graph shows measurements of comet tail lengths. B) Immunofluorescent staining for 8-oxoG in HET-1A cells treated as above. Specific fluorescence per cell was measured using the ImageJ software. Fluorescence intensity in untreated cells was set at 0. **P < 0.01; ***P < 0.001.
Figure 4.
p73-deficiency leads to increased DNA damage in vivo. A) Analysis of DNA damage in organotypic culture. Organotypic cultures of p73-deficient and control EPC2 cells were treated with 100 μM BA cocktail, pH 4.0, for 3 min, recovered for 1 h, and then analyzed by immunohistochemistry with phospho-histone (p-H2A.X) antibody. DNA damage was significantly higher in p73-deficient cells compared to control cells. Arrows show nuclei positive for phosphorylated histone p-H2A.X. Graph shows percentage of p-H2A.X-positive cells. **P < 0.01. B) Scheme and microphotograph show the surgical procedure (esophagojejunostomy) used to induce reflux in mice. C) Representative immunohistochemical staining for DNA damage (p-H2A.X) in the esophaguses of control wild-type and p73 heterozygous mice sacrificed at 15 wk after surgery. Control panel shows a representative image of the esophagus from the control group of mice without surgery.
Mechanisms of DNA damage regulation
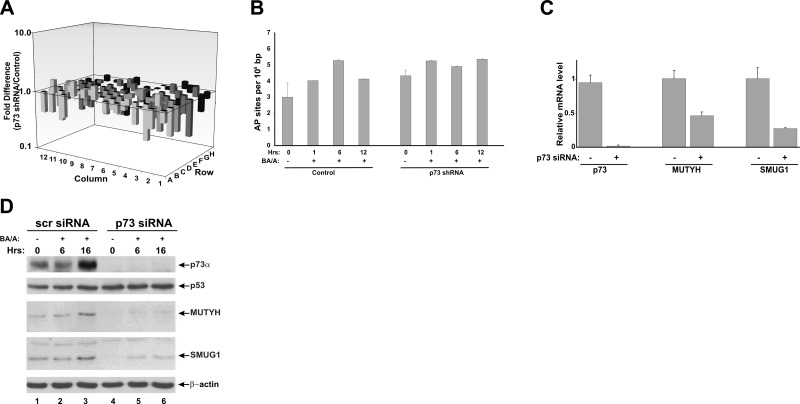
To investigate the molecular mechanisms associated with the p73 function, we carried out analyses of multiple DNA repair genes using the PCR array platform from SABiosciences in HET-1A esophageal cells. We found that down-regulation of p73 with shRNA leads to inhibition of several genes involved in DNA damage repair (Fig. 5A). Genes significantly affected by p73 are shown in Table 2. Since esophageal reflux causes strong oxidative DNA damage, we focused our studies on base excision repair (BER), which primarily corrects this type of DNA damage. To confirm further the effect of p73 on BER, we analyzed the formation of DNA abasic sites in p73-deficient and control cells after BA/A treatment. Control cells showed a complex dynamic change in the generation of abasic sites with a significant increase in 6 h and a decrease in 12 h after treatment with BA/A (Fig. 5B). In contrast, AP sites were not removed efficiently in p73 deficient cells, further supporting our data that p73-deficient cells are characterized by DNA repair defects. A similar dynamic of AP site generation was also seen after treatment of HET-1A cells with hydrogen peroxide (data not shown).
Figure 5.
p73 regulates enzymes involved in DNA damage repair. A) Analysis of DNA repair genes by PCR array. Bar graph represents a fold difference between mRNA expression of 84 DNA repair genes in p73-deficient and control HET-1A cells. Genes significantly affected by p73 are shown in Table 2. B) Quantification of abasic sites (AP) in p73-deficient and control HET-1A cells treated with 200 μM BA/A for 1 h. Cells were collected at the indicated time points. C) Real-time PCR analysis of p73, MUTYH, and SMUG1 mRNA in cells transfected with p73 or scrambled siRNA. D) Analysis of MUTYH and SMUG1 proteins by Western blotting in HET-1A cells transfected with p73 or scrambled siRNA for 48 h. Cells were treated with 100 μM BA cocktail, pH 4.0, for 30 min, and then cell lysates were collected at the indicated time points.
Table 2.
PCR array
| Gene | mRNA change (fold) |
|---|---|
| APEX2 | −2.07 |
| DDB1 | −2.28 |
| DDB2 | −2.85 |
| ERCC1 | −3.96 |
| ERCC1 | −2.32 |
| FEN1 | −3.32 |
| LIG1 | −2.58 |
| LIG3 | −2.29 |
| MMS19 | −2.06 |
| MUTYH | −2.77 |
| NEIL1 | −3.21 |
| NEIL2 | −2.20 |
| PARP1 | −2.61 |
| PARP3 | −2.11 |
| PNKP | −4.18 |
| POLL | −2.54 |
| RAD51L3 | −2.60 |
| SMUG1 | −3.67 |
| TOP3A | −2.98 |
| TOP3B | −3.55 |
| TREX1 | −2.00 |
| XAB2 | −2.62 |
| XRCC1 | −2.41 |
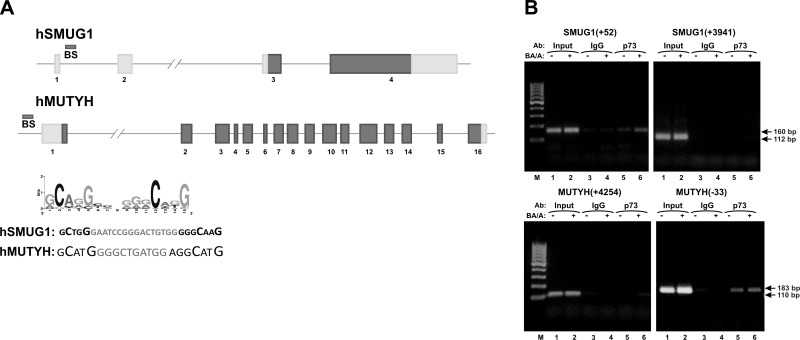
Among identified proteins involved in BER (Table 2), we focused on two DNA glycosylases involved in the repair of oxidized and deaminated nucleotides, MUTYH and SMUG1. These enzymes were not previously associated with the p73 function. Figure 5C shows that down-regulation of p73 leads to a decrease of MUTYH and SMUG1 mRNA levels. p73 inhibition also down-regulates both MUTYH and SMUG1 proteins (Fig. 5D, compare lanes 4–6 with lanes 1–3). Notably, in control cells expressing p73, these proteins are induced by BA/A treatment (Fig. 5D). To determine how p73 regulates these genes, we analyzed them for potential p73 binding sites using the Genomatix MatInspector software. We also employed recently published data on the putative binding motif for p73 shown in Fig. 6A (33). Several potential binding sites for p73 were identified in the promoter and intron regions of the MUTYH and SMUG1 genes. To investigate whether p73 regulates transcription directly, we conducted ChIP analysis. The identified binding sites were tested, and the ChIP analysis demonstrated that p73 directly binds to the promoter region of the MUTYH gene at position −33 and in the first intron of SMUG1 at position +52 (Fig. 6B). Sequences of potential binding sites are shown in Fig. 6A.
Figure 6.
p73 regulates expression of SMUG1 and MUTYH genes. A) Architecture of human SMUG1 and MUTYH genes. BS bars show the positions of p73 binding sites. Consensus binding motif for p73 (33) and sequences of p73 binding sites in hSMUG1 and hMUTYH genes are shown. B) ChIP analysis of p73 binding to SMUG1 and MUTYH genes at 4 h after treatment with 100 μM BA/A for 30 min. Several potential binding sites were tested. p73 directly binds to the promoter region of the MUTYH gene at position −33 and in the first intron of SMUG1 at position +52.
DISCUSSION
Symptoms of gastroesophageal reflux disease affect 10%–20% of the population in the Western world (34). At the molecular level, these pathological conditions are associated with excessive oxidative DNA damage, increased mutation rate, and genomic instability (5–8). We also observed that even short exposure to BA/A causes strong genotoxic stress. DNA damage caused by BA/A can be easily detected by staining for 8-oxoguanine, phospho-histone H2A.X, or comet assay. Interestingly, DNA damage induced by BA/A treatment affected the p53 family in a unique way. We found that among members of the p53 family, p73 is the only protein directly induced by BA/A. This is a somewhat unexpected result, given that all members of the p53 protein family (p53, p73, and p63) are up-regulated and activated by multiple genotoxic stresses and that BA/A treatment induces strong DNA damage. We found that p73 up-regulation is independent of p53 but dependent on c-Abl tyrosine kinase. Our studies demonstrated that BA/A treatment induces c-Abl kinase, which phosphorylates and activates p73. Down-regulation of c-Abl abrogates the induction of p73 by BA/A. We did not observe any significant changes in activity or protein levels of p53 after BA/A treatment, though other genotoxic agents strongly induce p53 in the same cells. Previous studies found that deoxycholic acid can suppress p53 by inducing its proteasomal degradation in colon cancer cells (35). A lower concentration of BA used in our experiments, which was selected based on physiological levels in the esophagus, may explain the differences in these studies. Interestingly, BA/A treatment significantly down-regulated the p63 protein. A similar observation was recently made by Roman et al. (36), who found a suppressive effect of BA on p63. Taken together, these data emphasize different roles of p53, p73, and p63 in various genotoxic stresses.
Studies conducted by others and us suggest that p73 is inactivated in cancer, although the mechanisms of its inactivation are different from those of p53. In contrast to p53, p73 is rarely mutated in human tumors. Loss of heterozygosity of the p73 gene and hypermethylation of the p73 promoter has been reported in human esophageal and gastric tumors (37–39). An additional critical mechanism of p73 inactivation includes up-regulation of dominant-negative isoforms of p73, which can collectively be referred to as ΔN or ΔTA isoforms (23, 27, 40–42). These isoforms are frequently overexpressed in human tumors, including esophageal and gastric adenocarcinomas, and their up-regulation is associated with poor survival of patients with cancer (41, 43, 44). Interestingly, ΔNp73 is increased during the early stages of esophageal tumorigenesis and can be detected in the Barrett's esophagus. With this in mind, we inhibited p73 with shRNA or by overexpression of ΔNp73 and studied DNA damage using traditional or organotypic esophageal cell cultures. In both cases, p73-deficient cells were significantly more susceptible to DNA damage induced by BA/A or hydrogen peroxide. These findings were further supported by our experiments in vivo. To mimic GERD conditions, esophagojejunostomy was performed on mice. This surgical procedure allows a constant delivery of BAs into the esophagus through the site of esophagojejunal anastomosis. We found that p73-deficient mice with surgery are characterized by increased DNA damage compared with wild-type control.
Our studies show that the p73 deficiency is mechanistically associated with the defects in DNA damage repair. Using human DNA repair PCR array, we identified multiple DNA repair genes affected by p73. Among them are novel targets as well as previously characterized targets for p53, FEN1, and DDB2 (45, 46). Given the important role of oxidative DNA damage played in esophageal tumorigenesis, we focused our studies on BER-related genes and identified two novel transcriptional targets of p73, SMUG1 and MUTYH. Both of these enzymes are glycosylases that repair oxidized and deaminated nucleotides. SMUG1 removes uracil, 5-hydroxyuracil, and several other nucleotide derivatives from DNA. MYTUH excises 2-hydroxyadenine incorporated opposite guanine and adenine incorporated opposite 8-oxoG. If left unrepaired, these DNA lesions will lead to transitions and transversions. Accordingly, SMUG1 and MUTYH deficiencies are mutagenic and cancerogenic. Patients with MUTYH-associated polyposis, which is characterized by germline mutation in the MUTYH gene, are prone to develop colorectal and other extracolonic tumors, including upper gastrointestinal tumors (47). Down-regulation of SMUG1 is a typical feature of sporadic gastric tumors characterized by DNA repair defects (48).
We found that p73 directly binds and regulates SMUG1 and MUTYH transcription. However, we cannot exclude an indirect effect of p73 on other targets identified by our DNA repair PCR array. Additional studies are needed to further characterize these targets. It has been recently reported that p73 transcriptionally regulates Mre11, RAD51, and BRCA2 in mouse embryonic fibroblasts (49). These proteins are involved in the repair of DNA double-strand breaks. These targets were not identified in our PCR array analysis; this suggests that cell type or tissue differences may play a role. Another member of the RAD51 family, RAD51L3, was found to be affected by p73 in esophageal cells.
In summary, we found that the TAp73 protein is induced by BA/A treatment in a c-Abl-dependent manner. The activation of p73, in turn, leads to the up-regulation of p73 targets involved in the repair of DNA damage (Fig. 7). Collectively, our studies support the concept that p73 may play a tumor suppressor role in the esophagus. Inactivation of p73 by ΔNp73 or other mechanisms can potentially increase DNA damage and mutational rate. This is particularly important during the early stages of tumorigenesis when increased levels of mutations can contribute to tumor formation.
Figure 7.
Schematic representation of the p53 family regulation in conditions of gastroesophageal reflux.
Supplementary Material
Acknowledgments
This work was supported by the U.S. National Cancer Institute, grants NIH R01CA138833, NIH R01CA108956, and NIH R01CA106176. The authors thank Dr. P. Williams for valuable discussion of the manuscript.
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
REFERENCES
- 1. Jemal A., Siegel R., Xu J., Ward E. (2010) Cancer statistics, 2010. CA Cancer J. Clin. 60, 277–300 [DOI] [PubMed] [Google Scholar]
- 2. Melhado R., Alderson D., Tucker O. (2010) The changing face of esophageal cancer. Cancer 2, 1379–1404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Li Y., Martin R. C., 2nd. (2007) Reflux injury of esophageal mucosa: experimental studies in animal models of esophagitis, Barrett's esophagus and esophageal adenocarcinoma. Dis. Esophagus 20, 372–378 [DOI] [PubMed] [Google Scholar]
- 4. Kauer W. K., Peters J. H., DeMeester T. R., Feussner H., Ireland A. P., Stein H. J., Siewert R. J. (1997) Composition and concentration of bile acid reflux into the esophagus of patients with gastroesophageal reflux disease. Surgery 122, 874–881 [DOI] [PubMed] [Google Scholar]
- 5. Chen X., Ding Y. W., Yang G., Bondoc F., Lee M. J., Yang C. S. (2000) Oxidative damage in an esophageal adenocarcinoma model with rats. Carcinogenesis 21, 257–263 [DOI] [PubMed] [Google Scholar]
- 6. Dvorak K., Payne C. M., Chavarria M., Ramsey L., Dvorakova B., Bernstein H., Holubec H., Sampliner R. E., Guy N., Condon A., Bernstein C., Green S. B., Prasad A., Garewal H. S. (2007) Bile acids in combination with low pH induce oxidative stress and oxidative DNA damage: relevance to the pathogenesis of Barrett's oesophagus. Gut 56, 763–771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhang H. Y., Hormi-Carver K., Zhang X., Spechler S. J., Souza R. F. (2009) In benign Barrett's epithelial cells, acid exposure generates reactive oxygen species that cause DNA double-strand breaks. Cancer Res. 69, 9083–9089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Maley C. C., Reid B. J. (2005) Natural selection in neoplastic progression of Barrett's esophagus. Semin. Cancer Biol. 15, 474–483 [DOI] [PubMed] [Google Scholar]
- 9. Theisen J., Peters J. H., Fein M., Hughes M., Hagen J. A., Demeester S. R., Demeester T. R., Laird P. W. (2005) The mutagenic potential of duodenoesophageal reflux. Ann. Surg. 241, 63–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bernstein H., Bernstein C., Payne C. M., Dvorak K. (2009) Bile acids as endogenous etiologic agents in gastrointestinal cancer. World J. Gastroenterol. 15, 3329–3340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Souza R. F. (2002) Molecular and biologic basis of upper gastrointestinal malignancy-esophageal carcinoma. Surg. Oncol. Clin, N. Am. 11, 257–272, viii [DOI] [PubMed] [Google Scholar]
- 12. Lane D., Levine A. (2010) p53 research: the past thirty years and the next thirty years. Cold Spring Harbor Perspect. Biol. 2, a000893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. El-Deiry W. S. (1998) Regulation of p53 downstream genes. Semin. Cancer Biol. 8, 345–357 [DOI] [PubMed] [Google Scholar]
- 14. Wang W., Kim S. H., El-Deiry W. S. (2006) Small-molecule modulators of p53 family signaling and antitumor effects in p53-deficient human colon tumor xenografts. Proc. Natl. Acad. Sci. U. S. A. 103, 11003–11008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lin K. W., Nam S. Y., Toh W. H., Dulloo I., Sabapathy K. (2004) Multiple stress signals induce p73beta accumulation. Neoplasia 6, 546–557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Belyi V. A., Levine A. J. (2009) One billion years of p53/p63/p73 evolution. Proc. Natl. Acad. Sci. U. S. A. 106, 17609–17610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fontemaggi G., Kela I., Amariglio N., Rechavi G., Krishnamurthy J., Strano S., Sacchi A., Givol D., Blandino G. (2002) Identification of direct p73 target genes combining DNA microarray and chromatin immunoprecipitation analyses. J. Biol. Chem. 277, 43359–43368 [DOI] [PubMed] [Google Scholar]
- 18. Perez C. A., Ott J., Mays D. J., Pietenpol J. A. (2007) p63 consensus DNA-binding site: identification, analysis and application into a p63MH algorithm. Oncogene 26, 7363–7370 [DOI] [PubMed] [Google Scholar]
- 19. Vilgelm A., El-Rifai W., Zaika A. (2008) Therapeutic prospects for p73 and p63: rising from the shadow of p53. Drug Resist. Update 11, 152–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Stoner G. D., Kaighn M. E., Reddel R. R., Resau J. H., Bowman D., Naito Z., Matsukura N., You M., Galati A. J., Harris C. C. (1991) Establishment and characterization of SV40 T-antigen immortalized human esophageal epithelial cells. Cancer Res. 51, 365–371 [PubMed] [Google Scholar]
- 21. Altorki N., Schwartz G. K., Blundell M., Davis B. M., Kelsen D. P., Albino A. P. (1993) Characterization of cell lines established from human gastric-esophageal adenocarcinomas. Biologic phenotype and invasion potential. Cancer 72, 649–657 [DOI] [PubMed] [Google Scholar]
- 22. Andl C. D., Mizushima T., Nakagawa H., Oyama K., Harada H., Chruma K., Herlyn M., Rustgi A. K. (2003) Epidermal growth factor receptor mediates increased cell proliferation, migration, and aggregation in esophageal keratinocytes in vitro and in vivo. J. Biol. Chem. 278, 1824–1830 [DOI] [PubMed] [Google Scholar]
- 23. Zaika A. I., Slade N., Erster S. H., Sansome C., Joseph T. W., Pearl M., Chalas E., Moll U. M. (2002) DeltaNp73, a dominant-negative inhibitor of wild-type p53 and TAp73, is up-regulated in human tumors. J. Exp. Med. 196, 765–780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Vilgelm A. E., Washington M. K., Wei J., Chen H., Prassolov V. S., Zaika A. I. (2009) Interactions of the p53 protein family in cellular stress response in gastrointestinal tumors. Mol. Cancer Ther. 9, 693–705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tomkova K., El-Rifai W., Vilgelm A., Kelly M. C., Wang T. C., Zaika A. I. (2006) The gastrin gene promoter is regulated by p73 isoforms in tumor cells. Oncogene 25, 6032–6036 [DOI] [PubMed] [Google Scholar]
- 26. Speit G., Haupter S., Hartmann A. (1998) Evaluation of the genotoxic properties of paraquat in V79 Chinese hamster cells. Mutat. Res. 412, 187–193 [DOI] [PubMed] [Google Scholar]
- 27. Yang A., Walker N., Bronson R., Kaghad M., Oosterwegel M., Bonnin J., Vagner C., Bonnet H., Dikkes P., Sharpe A., McKeon F., Caput D. (2000) p73-deficient mice have neurological, pheromonal and inflammatory defects but lack spontaneous tumours. Nature (London) 404, 99–103 [DOI] [PubMed] [Google Scholar]
- 28. Wei J., O'Brien D., Vilgelm A., Piazuelo M. B., Correa P., Washington M. K., El-Rifai W., Peek R. M., Zaika A. (2008) Interaction of Helicobacter pylori with gastric epithelial cells is mediated by the p53 protein family. Gastroenterology 134, 1412–1423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Agami R., Blandino G., Oren M., Shaul Y. (1999) Interaction of c-Abl and p73alpha and their collaboration to induce apoptosis. Nature (London) 399, 809–813 [DOI] [PubMed] [Google Scholar]
- 30. Gong J. G., Costanzo A., Yang H. Q., Melino G., Kaelin W. G., Jr., Levrero M., Wang J. Y. (1999) The tyrosine kinase c-Abl regulates p73 in apoptotic response to cisplatin-induced DNA damage. Nature (London) 399, 806–809 [DOI] [PubMed] [Google Scholar]
- 31. Yuan Z. M., Shioya H., Ishiko T., Sun X., Gu J., Huang Y. Y., Lu H., Kharbanda S., Weichselbaum R., Kufe D. (1999) p73 is regulated by tyrosine kinase c-Abl in the apoptotic response to DNA damage. Nature (London) 399, 814–817 [DOI] [PubMed] [Google Scholar]
- 32. Masuda N., Kato H., Nakajima T., Sano T., Kashiwabara K., Oyama T., Kuwano H. (2003) Synergistic decline in expressions of p73 and p21 with invasion in esophageal cancers. Cancer Sci. 94, 612–617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yang A., Zhu Z., Kettenbach A., Kapranov P., McKeon F., Gingeras T. R., Struhl K. (2010) Genome-wide mapping indicates that p73 and p63 co-occupy target sites and have similar DNA-binding profiles in vivo. PloS ONE 5, e11572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Dent J., El-Serag H. B., Wallander M. A., Johansson S. (2005) Epidemiology of gastro-oesophageal reflux disease: a systematic review. Gut 54, 710–717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Qiao D., Gaitonde S. V., Qi W., Martinez J. D. (2001) Deoxycholic acid suppresses p53 by stimulating proteasome-mediated p53 protein degradation. Carcinogenesis 22, 957–964 [DOI] [PubMed] [Google Scholar]
- 36. Roman S., Petre A., Thepot A., Hautefeuille A., Scoazec J. Y., Mion F., Hainaut P. (2007) Downregulation of p63 upon exposure to bile salts and acid in normal and cancer esophageal cells in culture. Am. J. Physiol. Gastrointest. Liver Physiol. 293, G45–G53 [DOI] [PubMed] [Google Scholar]
- 37. Bernal C., Vargas M., Ossandon F., Santibanez E., Urrutia J., Luengo V., Zavala L. F., Backhouse C., Palma M., Argandona J., Aguayo F., Corvalan A. (2008) DNA methylation profile in diffuse type gastric cancer: evidence for hypermethylation of the BRCA1 promoter region in early-onset gastric carcinogenesis. Biol. Res. 41, 303–315 [PubMed] [Google Scholar]
- 38. Cai Y. C., Yang G. Y., Nie Y., Wang L. D., Zhao X., Song Y. L., Seril D. N., Liao J., Xing E. P., Yang C. S. (2000) Molecular alterations of p73 in human esophageal squamous cell carcinomas: loss of heterozygosity occurs frequently; loss of imprinting and elevation of p73 expression may be related to defective p53. Carcinogenesis 21, 683–689 [DOI] [PubMed] [Google Scholar]
- 39. Yokozaki H., Shitara Y., Fujimoto J., Hiyama T., Yasui W., Tahara E. (1999) Alterations of p73 preferentially occur in gastric adenocarcinomas with foveolar epithelial phenotype. Int. J. Cancer 83, 192–196 [DOI] [PubMed] [Google Scholar]
- 40. Vilgelm A., Wei J. X., Piazuelo M. B., Washington M. K., Prassolov V., El-Rifai W., Zaika A. (2008) DeltaNp73alpha regulates MDR1 expression by inhibiting p53 function. Oncogene 27, 2170–2176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Vilgelm A. E., Hong S. M., Washington M. K., Wei J., Chen H., El-Rifai W., Zaika A. (2010) Characterization of DeltaNp73 expression and regulation in gastric and esophageal tumors. Oncogene 29, 5861–5868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Stiewe T., Theseling C. C., Putzer B. M. (2002) Transactivation-deficient Delta TA-p73 inhibits p53 by direct competition for DNA binding: implications for tumorigenesis. J. Biol. Chem. 277, 14177–14185 [DOI] [PubMed] [Google Scholar]
- 43. Casciano I., Mazzocco K., Boni L., Pagnan G., Banelli B., Allemanni G., Ponzoni M., Tonini G. P., Romani M. (2002) Expression of DeltaNp73 is a molecular marker for adverse outcome in neuroblastoma patients. Cell Death Differ. 9, 246–251 [DOI] [PubMed] [Google Scholar]
- 44. Concin N., Hofstetter G., Berger A., Gehmacher A., Reimer D., Watrowski R., Tong D., Schuster E., Hefler L., Heim K., Mueller-Holzner E., Marth C., Moll U. M., Zeimet A. G., Zeillinger R. (2005) Clinical relevance of dominant-negative p73 isoforms for responsiveness to chemotherapy and survival in ovarian cancer: evidence for a crucial p53–p73 cross-talk in vivo. Clin. Cancer Res. 11, 8372–8383 [DOI] [PubMed] [Google Scholar]
- 45. Christmann M., Tomicic M. T., Origer J., Kaina B. (2005) Fen1 is induced p53 dependently and involved in the recovery from UV-light-induced replication inhibition. Oncogene 24, 8304–8313 [DOI] [PubMed] [Google Scholar]
- 46. Tan T., Chu G. (2002) p53 Binds and activates the xeroderma pigmentosum DDB2 gene in humans but not mice. Mol. Cell. Biol. 22, 3247–3254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Poulsen M. L., Bisgaard M. L. (2008) MUTYH associated polyposis (MAP). Curr. Genomics 9, 420–435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. D'Errico M., de Rinaldis E., Blasi M. F., Viti V., Falchetti M., Calcagnile A., Sera F., Saieva C., Ottini L., Palli D., Palombo F., Giuliani A., Dogliotti E. (2009) Genome-wide expression profile of sporadic gastric cancers with microsatellite instability. Eur. J. Cancer 45, 461–469 [DOI] [PubMed] [Google Scholar]
- 49. Lin Y. L., Sengupta S., Gurdziel K., Bell G. W., Jacks T., Flores E. R. (2009) p63 and p73 transcriptionally regulate genes involved in DNA repair. PLoS Genet. 5, e1000680 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.