Abstract
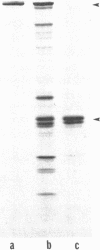
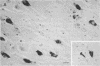
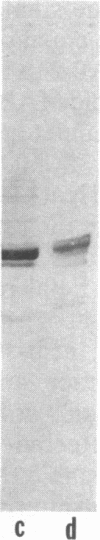
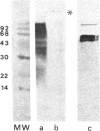
The detailed protein composition of the paired helical filaments (PHF) that accumulate in human neurons in aging and Alzheimer disease is unknown. However, the identity of certain components has been surmised by using immunocytochemical techniques. Whereas PHF share epitopes with neurofilament proteins and microtubule-associated protein (MAP) 2, we report evidence that the MAP tau (tau) appears to be their major antigenic component. Immunization of rabbits with NaDodSO4-extracted, partially purified PHF (free of normal cytoskeletal elements, including tau) consistently produces antibodies to tau but not, for example, to neurofilaments. Such PHF antibodies label all of the heterogeneous fetal and mature forms of tau from rat and human brain. Absorption of PHF antisera with heat-stable MAPs (rich in tau) results in almost complete loss of staining of neurofibrillary tangles (NFT) in human brain sections. An affinity-purified antibody to tau specifically labels NFT and the neurites of senile plaques in human brain sections as well as NaDodSO4-extracted NFT. tau-Immunoreactive NFT frequently extend into the apical dendrites of pyramidal neurons, suggesting an aberrant intracellular locus for this axonal protein. tau and PHF antibodies label tau proteins identically on electrophoretic transfer blots and stain the gel-excluded protein representing NaDodSO4-insoluble PHF in homogenates of human brain. The progressive accumulation of altered tau protein in neurons in Alzheimer disease may result in instability of microtubules, consequent loss of effective transport of molecules and organelles, and, ultimately, neuronal death.
Full text
PDF

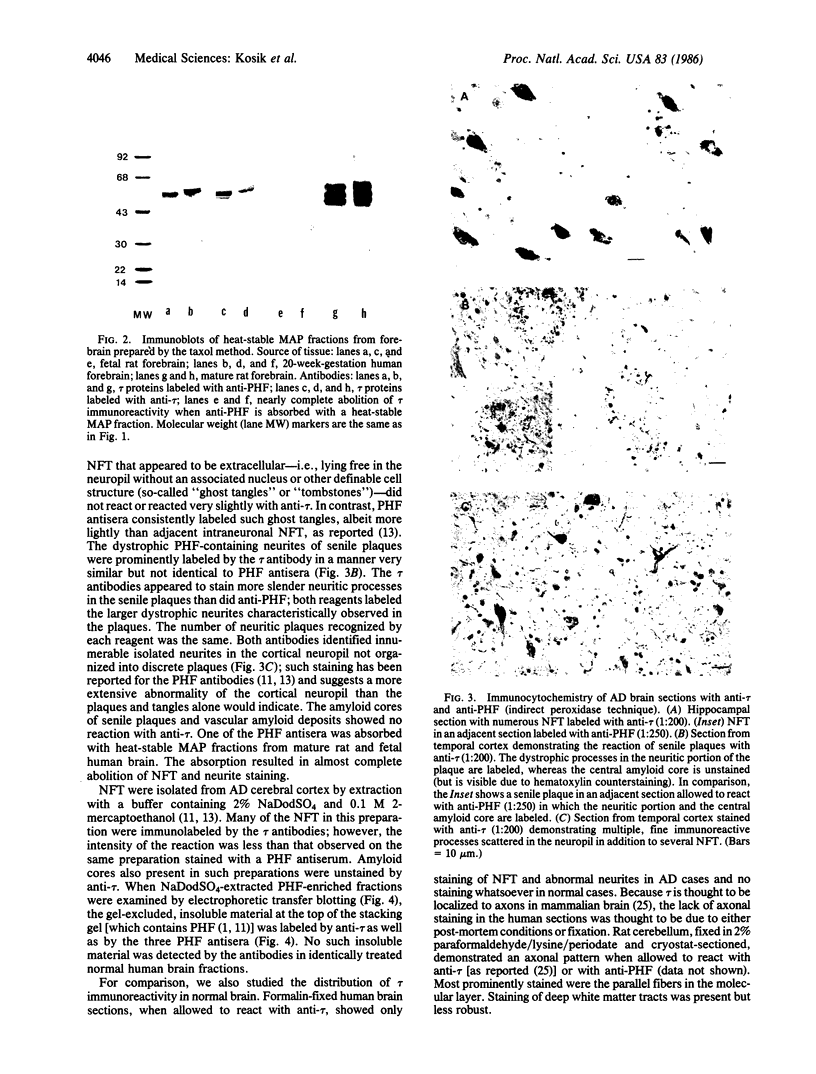


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderton B. H., Breinburg D., Downes M. J., Green P. J., Tomlinson B. E., Ulrich J., Wood J. N., Kahn J. Monoclonal antibodies show that neurofibrillary tangles and neurofilaments share antigenic determinants. Nature. 1982 Jul 1;298(5869):84–86. doi: 10.1038/298084a0. [DOI] [PubMed] [Google Scholar]
- Binder L. I., Frankfurter A., Kim H., Caceres A., Payne M. R., Rebhun L. I. Heterogeneity of microtubule-associated protein 2 during rat brain development. Proc Natl Acad Sci U S A. 1984 Sep;81(17):5613–5617. doi: 10.1073/pnas.81.17.5613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder L. I., Frankfurter A., Rebhun L. I. The distribution of tau in the mammalian central nervous system. J Cell Biol. 1985 Oct;101(4):1371–1378. doi: 10.1083/jcb.101.4.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brion J. P., Couck A. M., Passareiro E., Flament-Durand J. Neurofibrillary tangles of Alzheimer's disease: an immunohistochemical study. J Submicrosc Cytol. 1985 Jan;17(1):89–96. [PubMed] [Google Scholar]
- Burgoyne R. D., Cumming R. Ontogeny of microtubule-associated protein 2 in rat cerebellum: differential expression of the doublet polypeptides. Neuroscience. 1984 Jan;11(1):156–167. doi: 10.1016/0306-4522(84)90220-3. [DOI] [PubMed] [Google Scholar]
- Cork L. C., Sternberger N. H., Sternberger L. A., Casanova M. F., Struble R. G., Price D. L. Phosphorylated neurofilament antigens in neurofibrillary tangles in Alzheimer's disease. J Neuropathol Exp Neurol. 1986 Jan;45(1):56–64. doi: 10.1097/00005072-198601000-00005. [DOI] [PubMed] [Google Scholar]
- Drubin D. G., Caput D., Kirschner M. W. Studies on the expression of the microtubule-associated protein, tau, during mouse brain development, with newly isolated complementary DNA probes. J Cell Biol. 1984 Mar;98(3):1090–1097. doi: 10.1083/jcb.98.3.1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francon J., Lennon A. M., Fellous A., Mareck A., Pierre M., Nunez J. Heterogeneity of microtubule-associated proteins and brain development. Eur J Biochem. 1982 Dec 15;129(2):465–471. doi: 10.1111/j.1432-1033.1982.tb07072.x. [DOI] [PubMed] [Google Scholar]
- Garruto R. M., Fukatsu R., Yanagihara R., Gajdusek D. C., Hook G., Fiori C. E. Imaging of calcium and aluminum in neurofibrillary tangle-bearing neurons in parkinsonism-dementia of Guam. Proc Natl Acad Sci U S A. 1984 Mar;81(6):1875–1879. doi: 10.1073/pnas.81.6.1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geddes J. W., Monaghan D. T., Cotman C. W., Lott I. T., Kim R. C., Chui H. C. Plasticity of hippocampal circuitry in Alzheimer's disease. Science. 1985 Dec 6;230(4730):1179–1181. doi: 10.1126/science.4071042. [DOI] [PubMed] [Google Scholar]
- Gonatas N. K., Anderson W., Evangelista I. The contribution of altered synapses in the senile plaque: an electron microscopic study in Alzheimer's dementia. J Neuropathol Exp Neurol. 1967 Jan;26(1):25–39. doi: 10.1097/00005072-196701000-00003. [DOI] [PubMed] [Google Scholar]
- Herzog W., Weber K. Fractionation of brain microtubule-associated proteins. Isolation of two different proteins which stimulate tubulin polymerization in vitro. Eur J Biochem. 1978 Dec 1;92(1):1–8. doi: 10.1111/j.1432-1033.1978.tb12716.x. [DOI] [PubMed] [Google Scholar]
- Ihara Y., Abraham C., Selkoe D. J. Antibodies to paired helical filaments in Alzheimer's disease do not recognize normal brain proteins. Nature. 1983 Aug 25;304(5928):727–730. doi: 10.1038/304727a0. [DOI] [PubMed] [Google Scholar]
- Kosik K. S., Duffy L. K., Dowling M. M., Abraham C., McCluskey A., Selkoe D. J. Microtubule-associated protein 2: monoclonal antibodies demonstrate the selective incorporation of certain epitopes into Alzheimer neurofibrillary tangles. Proc Natl Acad Sci U S A. 1984 Dec;81(24):7941–7945. doi: 10.1073/pnas.81.24.7941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mareck A., Fellous A., Francon J., Nunez J. Changes in composition and activity of microtubule-associated proteins during brain development. Nature. 1980 Mar 27;284(5754):353–355. doi: 10.1038/284353a0. [DOI] [PubMed] [Google Scholar]
- McLean I. W., Nakane P. K. Periodate-lysine-paraformaldehyde fixative. A new fixation for immunoelectron microscopy. J Histochem Cytochem. 1974 Dec;22(12):1077–1083. doi: 10.1177/22.12.1077. [DOI] [PubMed] [Google Scholar]
- Perl D. P., Brody A. R. Alzheimer's disease: X-ray spectrometric evidence of aluminum accumulation in neurofibrillary tangle-bearing neurons. Science. 1980 Apr 18;208(4441):297–299. doi: 10.1126/science.7367858. [DOI] [PubMed] [Google Scholar]
- Perl D. P., Gajdusek D. C., Garruto R. M., Yanagihara R. T., Gibbs C. J. Intraneuronal aluminum accumulation in amyotrophic lateral sclerosis and Parkinsonism-dementia of Guam. Science. 1982 Sep 10;217(4564):1053–1055. doi: 10.1126/science.7112111. [DOI] [PubMed] [Google Scholar]
- Perry G., Rizzuto N., Autilio-Gambetti L., Gambetti P. Paired helical filaments from Alzheimer disease patients contain cytoskeletal components. Proc Natl Acad Sci U S A. 1985 Jun;82(11):3916–3920. doi: 10.1073/pnas.82.11.3916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry G., Selkoe D. J., Block B. R., Stewart D., Autilio-Gambetti L., Gambetti P. Electron microscopic localization of Alzheimer neurofibrillary tangle components recognized by an antiserum to paired helical filaments. J Neuropathol Exp Neurol. 1986 Mar;45(2):161–168. doi: 10.1097/00005072-198603000-00006. [DOI] [PubMed] [Google Scholar]
- Probst A., Basler V., Bron B., Ulrich J. Neuritic plaques in senile dementia of Alzheimer type: a Golgi analysis in the hippocampal region. Brain Res. 1983 Jun 6;268(2):249–254. doi: 10.1016/0006-8993(83)90490-0. [DOI] [PubMed] [Google Scholar]
- Rasool C. G., Abraham C., Anderton B. H., Haugh M., Kahn J., Selkoe D. J. Alzheimer's disease: immunoreactivity of neurofibrillary tangles with anti-neurofilament and anti-paired helical filament antibodies. Brain Res. 1984 Sep 24;310(2):249–260. doi: 10.1016/0006-8993(84)90148-3. [DOI] [PubMed] [Google Scholar]
- Rasool C. G., Selkoe D. J. Alzheimer's disease: exposure of neurofilament immunoreactivity in SDS-insoluble paired helical filaments. Brain Res. 1984 Nov 19;322(1):194–198. doi: 10.1016/0006-8993(84)91205-8. [DOI] [PubMed] [Google Scholar]
- Selkoe D. J., Abraham C. R., Podlisny M. B., Duffy L. K. Isolation of low-molecular-weight proteins from amyloid plaque fibers in Alzheimer's disease. J Neurochem. 1986 Jun;46(6):1820–1834. doi: 10.1111/j.1471-4159.1986.tb08501.x. [DOI] [PubMed] [Google Scholar]
- Selkoe D. J., Ihara Y., Salazar F. J. Alzheimer's disease: insolubility of partially purified paired helical filaments in sodium dodecyl sulfate and urea. Science. 1982 Mar 5;215(4537):1243–1245. doi: 10.1126/science.6120571. [DOI] [PubMed] [Google Scholar]
- Sharma R. K., Desai R., Thompson T. R., Wang J. H. Purification of the heat-stable inhibitor protein of the Ca2+-activated cyclic nucleotide phosphodiesterase by affinity chromatography. Can J Biochem. 1978 Jun;56(6):598–604. doi: 10.1139/o78-090. [DOI] [PubMed] [Google Scholar]
- Siegel N., Haug A. Aluminum interaction with calmodulin. Evidence for altered structure and function from optical and enzymatic studies. Biochim Biophys Acta. 1983 Apr 14;744(1):36–45. doi: 10.1016/0167-4838(83)90337-0. [DOI] [PubMed] [Google Scholar]
- Skene J. H., Willard M. Changes in axonally transported proteins during axon regeneration in toad retinal ganglion cells. J Cell Biol. 1981 Apr;89(1):86–95. doi: 10.1083/jcb.89.1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternberger N. H., Sternberger L. A., Ulrich J. Aberrant neurofilament phosphorylation in Alzheimer disease. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4274–4276. doi: 10.1073/pnas.82.12.4274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TERRY R. D., GONATAS N. K., WEISS M. ULTRASTRUCTURAL STUDIES IN ALZHEIMER'S PRESENILE DEMENTIA. Am J Pathol. 1964 Feb;44:269–297. [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallee R. B. A taxol-dependent procedure for the isolation of microtubules and microtubule-associated proteins (MAPs). J Cell Biol. 1982 Feb;92(2):435–442. doi: 10.1083/jcb.92.2.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G. P., Grundke-Iqbal I., Kascsak R. J., Iqbal K., Wisniewski H. M. Alzheimer neurofibrillary tangles: monoclonal antibodies to inherent antigen(s). Acta Neuropathol. 1984;62(4):268–275. doi: 10.1007/BF00687608. [DOI] [PubMed] [Google Scholar]
- Wood J. G., Mirra S. S., Pollock N. J., Binder L. I. Neurofibrillary tangles of Alzheimer disease share antigenic determinants with the axonal microtubule-associated protein tau (tau) Proc Natl Acad Sci U S A. 1986 Jun;83(11):4040–4043. doi: 10.1073/pnas.83.11.4040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen S. H., Crowe A., Dickson D. W. Monoclonal antibodies to Alzheimer neurofibrillary tangles. 1. Identification of polypeptides. Am J Pathol. 1985 Aug;120(2):282–291. [PMC free article] [PubMed] [Google Scholar]
- Yen S. H., Gaskin F., Fu S. M. Neurofibrillary tangles in senile dementia of the Alzheimer type share an antigenic determinant with intermediate filaments of the vimentin class. Am J Pathol. 1983 Dec;113(3):373–381. [PMC free article] [PubMed] [Google Scholar]