Abstract
Regulated gene expression and progeny production are essential for persistent and chronic infection by human pathogens, such as hepatitis B virus (HBV), which affects >400 million people worldwide and is a major cause of liver disease. In this study, we provide the first direct evidence that a liver-specific microRNA, miR-122, binds to a highly conserved HBV pregenomic RNA sequence via base-pairing interactions and inhibits HBV gene expression and replication. The miR-122 target sequence is located at the coding region of the mRNA for the viral polymerase and the 3′ untranslated region of the mRNA for the core protein. In cultured cells, HBV gene expression and replication reduces with increased expression of miR-122, and the expression of miR-122 decreases in the presence of HBV infection and replication. Furthermore, analyses of clinical samples demonstrated an inverse linear correlation in vivo between the miR-122 level and the viral loads in the peripheral blood mononuclear cells of HBV-positive patients. Our results suggest that miR-122 may down-regulate HBV replication by binding to the viral target sequence, contributing to the persistent/chronic infection of HBV, and that HBV-induced modulation of miR-122 expression may represent a mechanism to facilitate viral pathogenesis.—Chen, Y., Shen, A., Rider, P. J., Yu, Y., Wu, K., Mu, Y., Hao, Q, Liu, Y., Gong, H., Zhu, Y., Liu, F., Wu, J. A liver-specific microRNA binds to a highly conserved RNA sequence of hepatitis B virus and negatively regulates viral gene expression and replication.
Keywords: chronic infection, liver disease, hepatocellular carcinoma, viral infection, miR-122, therapeutic agent
Hepatitis b virus (HBV) is a major human pathogen chronically infecting >400 million individuals worldwide (1–3). The hallmark of HBV pathogenesis is persistent and chronic lifelong infection in the liver, which represents a significant risk factor for cirrhosis, liver failure, and hepatocellular carcinoma (HCC; ref. 4). Currently, the molecular mechanism of how HBV establishes persistent infection in human cells is not well understood, although it has been speculated that interactions between specific host factors and HBV proteins are required for the regulation and control of viral infection to achieve a persistent and chronic state. Equally elusive is the nature of the host and viral factors participating in this process. While a preventive vaccine is available, the therapeutic options for chronic HBV infection are limited (2). Understanding the mechanism of how HBV persistent infection is regulated in the liver and developing new compounds and novel strategies against HBV chronic infections are central for treating and preventing HBV-associated diseases.
MicroRNAs (miRNAs; refs. 5–7) are a class of noncoding RNAs of 21 to 22 nt that can be found in animals, plants, and viruses (8–13). MiRNAs primarily function to regulate the expression of specific genes by hybridizing to target mRNAs to modulate their translation and/or stability. There are >1000 miRNA-encoding genes in humans, which have the potential to regulate thousands of protein-encoding genes (11–14). Growing evidence has demonstrated that miRNAs regulate a wide range of biological processes, including development, differentiation, cell proliferation, apoptosis, and immune responses (11–13, 15). Some miRNAs are expressed ubiquitously, whereas others are expressed in a tissue-specific manner (16, 17). For example, miRNA 122 (miR-122) is specifically expressed at a high level in healthy livers, accounting for 70% of the total miRNA population (16, 18), but it is down-regulated in livers with HCC (19). MiR-122 has been shown to play an important role in normal liver functions, such as hepatocyte growth and lipid metabolism, and its natural targets include genes related to the adult liver phenotype and involved in cholesterol biosynthesis (11, 12, 20). Dysregulation of miR-122 expression affects normal liver functions and has been associated with the onset of pathological changes, such as diminished liver metabolism and tumorigenesis (20–22). Recent studies have also shown that miR-122 is essential for hepatitis C virus (HCV) replication by base-pairing with a 5′ untranslated region (UTR) of the viral RNA (23) and that reducing miR-122 expression in chimpanzees led to therapeutic inhibition of HCV infection in vivo (24). However, the roles of miR-122 in HBV infection in the liver have not been extensively studied.
In this study, we provide the first direct evidence that miR-122 directly binds to a region of the HBV pregenomic RNA (pg RNA) and negatively regulates HBV gene expression and replication. The target HBV RNA sequence for miR-122 is highly conserved among all HBV clinical subtypes and is located at the coding region of the mRNA for the viral polymerase and the 3′ UTR of the mRNA coding for the core protein. We show that miR-122 specifically binds to the target HBV sequence via base-pairing interactions and down-regulates HBV polymerase expression in reporter system assays. In cultured cells, HBV replication was inhibited when the level of miR-122 increased, and the level of miR-122 reduced in the presence of HBV replication. Furthermore, there was an inverse linear correlation in vivo between miR-122 levels and viral loads in the peripheral blood mononuclear cells (PBMCs) of patients with HBV infection vs. healthy HBV-negative individuals. Our findings suggest that down-regulation of HBV replication mediated by miR-122 may contribute to HBV persistent/chronic infection, and that the suppression of miR-122 expression by HBV may facilitate viral pathogenesis in the liver. Furthermore, our study provides insight into the development of novel anti-HBV therapeutics by generating miR-122 and its variants for inhibition of HBV infection and replication.
MATERIALS AND METHODS
Ethics statement
All research involving human participants was approved by the Institutional Review Board of the College of Life Sciences, Wuhan University (Wuhan, China), in accordance with the guidelines for the protection of human subjects. Written informed consent was obtained from each participant. No research involving human participants was carried out at the University of California (Berkeley, CA, USA).
Cells, synthetic oligonucleotides, and antibodies
HepG2 and HepG2.2.15 cells (American Type Culture Collection, Manassas, VA, USA) were maintained in DMEM supplemented with 10% heat-inactivated FCS, as described previously (25). The anti-FLAG and anti-β-actin antibodies were purchased from Sigma (St. Louis, MO, USA) and Santa Cruz Biotechnology (Santa Cruz, CA, USA), respectively. Synthetic oligonucleotides miR-122 mimics, miR-122 inhibitor, M1-miR-122 mimics, M2-miR-122 mimics, M3-miR-122 mimics, Ctrl mimics, and Ctrl inhibitor were purchased from RiBo Biotech (Guangzhou, China). The sequences for these oligonucleotides, except miR-122 inhibitor, Ctrl mimics, and Ctrl inhibitor, are listed in Table 1.
Table 1.
Primers and oligonucleotides used in the study
| Name | Sequence |
|---|---|
| HBp-sense | 5′-GCCTGCAGATGCCCCTATCCTATCAACACTTCC-3′ |
| HBp-antisense | 5′-GCAAGCTTTCACGGTGGTCTCCATGCGACGTGC-3′ |
| HBp(adw)-sense | 5′-GCCACCAAATGCCCCTATCTTATCAACACTTCC-3′ |
| HBp(adw)-antisense | 5′-GCAAGCGTTCACGGTGGTCTCCATGCGACGTGC-3′ |
| Mutagenesis primer m1 | 5′-ACACTATTTACACACTGTATGGAAGGCGGGTAT-3′ |
| Mutagenesis primer m2 | 5′-ACACTATTTACACACAGTATGGAAGGCGGGTAT-3′ |
| Mutagenesis primer m3 | 5′-ACACTATTTACACAGAGAATGGAAGGCGGGTAT-3′ |
| HBV qPCR 5′ primer P1 | 5′-AGAAACAACACATAGCGCCTCAT-3′ |
| HBV qPCR 3′ primer P2 | 5′-TGCCCCATGCTGTAGATCTTG-3′ |
| HBV qPCR probe P3 | 5′-TGTGGGTCACCATATTCTTGGG-3′ |
| miR-122 mimics | 5′-UGGAGUGUGACAAUGGUGUUUGU-3′ |
| M1-miR-122 mimics | 5′-UGCAGUGUGACAAUGGUGUUUGU-3′ |
| M2-miR-122 mimics | 5′-UGCUGUGUGACAAUGGUGUUUGU-3′ |
| M3-miR-122 mimics | 5′-UUCUCUGUGACAAUGGUGUUUGU-3′ |
| T | 5′-TTAATCATTACTTCCAAACTAGACACTATTTACACACTCTATGGAAGGCGGGTATATTAT-3′ |
| T(adw) | 5′-TTAATCATTACTTCCAGACGAGACATTATTTACATACTCTTTGGAAGGCGGGTATCTTAT-3′ |
Plasmid constructs
To generate pHBV1.3, the DNA fragment carrying an HBV genome (ayw subtype) was amplified from HepG2.2.15 cells and inserted into pBluescript II (Invitrogen, San Diego, CA, USA). Construct pCMV-luc contained the DNA fragment of the CMV promoter (obtained from pCMV-tag-2B) inserted between the KpnI and XhoI sites of pGL3-basic (Invitrogen). The HBV subtype adw (pHBV1.3 adw) was kindly provided by Dr. Dongping Xu (Beijing 302 Hospital, Beijing, China). To generate construct pCMV-T-luc, the DNA fragment containing the HBV DNA sequence (nt 2738–2760; targeted sequence, T) was obtained from pHBV1.3 and inserted between the XhoI and HindIII sites of pCMV-luc. To generate pCMV-HBP, the coding sequence of the HBV polymerase was amplified using pHBV1.3 as the template with 5′ primer HBp-sense and 3′ primer HBp-antisense (Table 1), respectively, and inserted into pCMV-tag-2B (Invitrogen). The pCMV-HBp(adw) and pCMV-T(adw)-luc were constructed following the same procedures, using pHBV1.3 adw as the template with specific primers (Table 1).
Mutations in the target sequence were generated by site-directed mutagenesis using specific primers listed in Table 1. In these experiments, 2 oligonucleotides that contained the desired mutations were used to prime the full-length DNA of pHBV1.3 in a PCR reaction. The resultant PCR products were treated with restriction enzyme DpnI to remove the original methylated DNA and transformed into Escherichia coli, in which the DNA molecules containing the desired mutations were then recovered. All of the generated constructs were sequenced to confirm the mutations.
Infection and transfection of cells
To carry out transfection, HepG2 or HepG2.2.15 cells were plated in 6-well plates in DMEM and grown to 80% confluence. Cells were transfected with plasmid DNAs or synthetic oligonucleotides, using Lipofectamine 2000 (Invitrogen), according to the manufacturer's instructions. Cells were harvested at 48 h post-transfection, and RNA and protein samples were isolated. Luciferase activities were measured with the dual-luciferase reporter assay system (Promega, Madison, WI, USA). Proteins in the culture supernatants were determined by ELISA using the S- or E-antigen diagnostic kits (Shanghai KeHua Biotech Co., Shanghai, China). Transfection with synthetic oligonucleotides did not exhibit significant cytotoxicity, as revealed by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assays, which showed no significant differences in terms of growth and viability of the cells that were not treated with any oligonucleotides and those that were treated with 10–100 nM miR-122 mimics, miR-122 inhibitor, or the control molecules.
Total RNAs were isolated from cells using TRIzol (Invitrogen) and treated with DNase I to remove the genomic DNA. To isolate capsid-associated viral DNA, cells were homogenized in the lysis buffer (50 mM Tris, pH 7.5; 0.5% Nonidet P-40; 1 mM EDTA; and 100 mM NaCl) and incubated with DNase I at 37°C for 2 h. Viral cores were then precipitated by adding 35 μl of 0.5 M EDTA and 225 μl of 35% polyethylene glycol, followed by centrifugation. The pellet was resuspended in buffer A (10 mM Tris, 100 mM NaCl, 1 mM EDTA, 1% SDS, and 2.5 mg/ml proteinase K) and incubated for 16 h. Viral DNAs released from the lysed cores were extracted with phenol and chloroform, and precipitated with isopropanol.
Quantitative PCR (qPCR) analysis
To determine the levels of capsid-associated DNA, qPCR was performed in a Light Cycler 480 real-time PCR instrument (Roche Diagnostics Ltd. Rotkreuz, Switzerland), using a set of TaqMan real-time PCR primers that contained 5′ primer P1, 3′ primer P2, and probe P3 (listed in Table 1). The PCR reaction was performed as follows: 1 cycle at 50°C for 2 min, 1 cycle at 95°C for 10 min, and 40 cycles at 95°C for 15 s and 60°C for 60 s. Plasmid pHBV1.3 was diluted over a range of 107 to 100 and used as a standard, and all samples were analyzed in triplicate. Real-time PCR-based quantification of miRNAs was performed using the miRNA analysis kits (Applied Biosystems, Foster City, CA, USA), according to the manufacturer's instructions. The levels of miRNAs were normalized to those of the internal control U6 snRNA, using the method as described previously (26). All analyses were repeated 3 times, and the results are the average of triplicate experiments.
Northern and Western blot analyses
The RNA samples were separated in 1% agarose gels that contained 6.5% formaldehyde, transferred to nitrocellulose membranes, hybridized with the [32P]-radiolabeled DNA probes that contained the HBV or human β-actin DNA sequences, and analyzed with autoradiography. The DNA probes were prepared from pHBV1.3 and pβ-actin, respectively.
To study in vitro binding of HBV sequence with miR-122 molecules, in vitro transcribed RNAs containing the mutated HBV polymerase-coding sequences covering the miR-122 target site were synthesized from the HBV constructs generated in this study, separated on 4% polyacrylamide gels that contained 8 M urea, and transferred to membranes. The membranes were hybridized with radiolabeled synthetic wild-type and mutant miR-122 oligonucleotides and DNA probes from pCMV-HBp, and analyzed with autoradiography.
For Western blot analyses, the polypeptides from cell lysates were separated on SDS/12% polyacrylamide gels cross-linked with N,N″-methylenebisacylamide, and transferred electrically to nitrocellulose membranes. We stained the membranes using anti-FLAG and anti-actin antibodies in the presence of a chemiluminescent substrate (SuperSignal chemiluminescent kit; Pierce, Rockford, IL, USA), and analyzed the stained membranes with a LAS-4000 image document instrument (FujiFilm, Tokyo, Japan). Quantitation was performed in the linear range of RNA and protein detection.
Clinical samples
A group of 80 HBV-positive patients (52 males and 28 females, mean age 36.2±13.8 yr), who were admitted to RenMin Hospital of Wuhan University, was recruited for this study. All patients who were confirmed as HBV positive (but negative for HCV, HDV, and HIV) had no concurrent illness and no serological markers suggestive of autoimmune disease. A control group of 80 healthy HBV-negative individuals, who had no history of liver disease and were matched for sex and age (44 males and 36 females, mean age 38.5±12.5 yr) with the patients in the HBV-positive group, was randomly selected from blood donation centers in Wuhan, China.
Statistical analysis
All experiments were reproducible and were carried out in triplicate. Each set of experiments was repeated 3 times and a representative one is shown. Results are presented as means ± sd. Student's t test for paired samples was used to determine statistical significance. Differences were considered statistically significant at a value of P ≤ 0.05.
RESULTS
HBV-mediated down-regulation of miR-122 expression in human cells
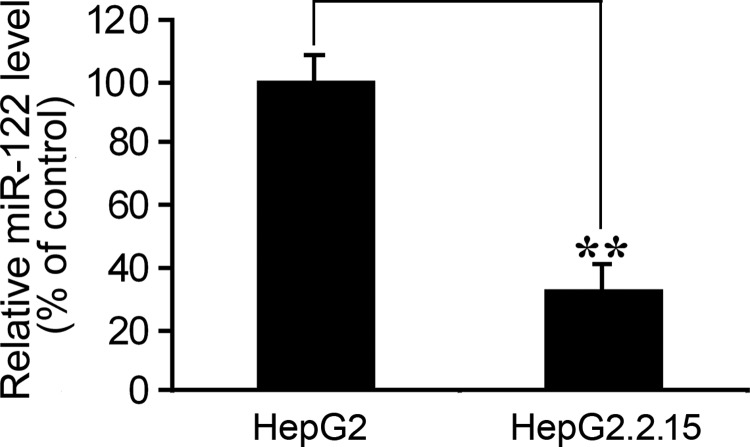
To study the effect of HBV replication on human miR-122 RNA expression, we carried out qRT-PCR analyses and compared the expression of miR-122 in human hepatoma cell lines HepG2 and HepG2.2.15. HepG2 cells expressed a low level of miR-122, and HepG2.2.15 cells were derived from HepG2 and stably contained a transfected full-length genome of HBV (ayw subtype). We used HepG2.2.15 cells as the model system for HBV replication, as these cells constitutively express hepatitis B surface (HBsAg) and E (HBeAg) antigens, and also support full HBV replication (27). The level of miR-122 in HepG2.2.15 cells was found to be lower than that in HepG2 cells by >60% (Fig. 1). These results suggest that the presence of HBV replication and infection may lead to a reduction of miR-122 expression.
Figure 1.
Expression of miR-122 in human HepG2.2.15 and HepG2 cells that stably contained a HBV genome or not, respectively. Levels of miR-122 were quantified by qRT-PCR, using the level of the U6 snRNA as the internal control. Values are means from triplicate experiments and represent the relative levels of miR-122 in HepG2.2.15 cells as a percentage of those in HepG2 cells. Error bars = sd. **P < 0.01.
Inhibition of HBV gene expression and replication by miR-122
We also performed 3 sets of experiments to determine whether modulation of miR-122 expression affects HBV gene expression and replication. To overexpress miR-122, cells were transfected with chemically synthesized miR-122 oligonucleotides, miR-112 mimics. To inactivate miR-122, cells were transfected with miR-122 inhibitor, a synthetic oligonucleotide with exact sequence complementarity to miR-122. Such inhibitor oligonucleotides have been shown to sequester intracellular miRNAs and to inhibit their activity in the RNA-interfering pathway (20, 23). Randomized RNA oligonucleotides, Ctrl mimics and Ctrl inhibitor, were chemically synthesized in parallel with miR-122 mimics and miR-122 inhibitor, respectively, and used as the negative controls. Our MTT assays revealed no significant differences in terms of growth and viability of the cells that were not treated with any oligonucleotides and those cells that were treated with miR-122 mimics, miR-122 inhibitor, and the control molecules, suggesting that the treatment of the synthetic oligonucleotides did not exhibit significant cytotoxicity. Furthermore, the levels of miR-122 in cells transfected with miR-122 mimics and miR-122 inhibitor were found to be increased and decreased, respectively, at 48 h post-transfection, as revealed by qRT-PCR assays (Supplemental Fig. S1). In contrast, transfection of Ctrl mimics and Ctrl inhibitor had no effect on the levels of miR-122 in the transfected cells.
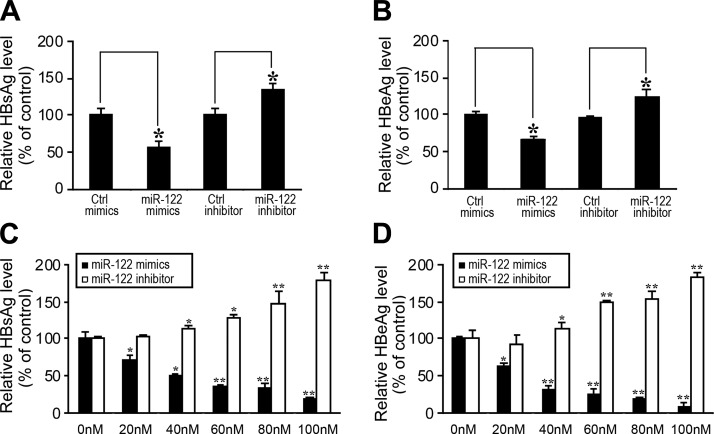
The first set of experiments was carried out to determine whether modulation of miR-122 expression would affect HBV gene expression and production in cells that stably contained a replication-competent HBV DNA. HepG2.2.15 cells were transfected with synthetic miR-122 molecules, miR-122 mimics and the miR-122 inhibitor, and the negative controls, Ctrl mimics and the Ctrl inhibitor. The levels of HBV production were determined by assaying the expression of HBV antigens HBsAg and HBeAg in the supernatants of the cell cultures with ELISA (2). The levels of HBsAg and HBeAg were decreased in cells treated with miR-122 mimics and increased in cells treated with miR-122 inhibitor, while randomized RNA oligonucleotides Ctrl mimics and Ctrl inhibitor had no effect on the expression of HBsAg (Fig. 2A) and HBeAg (Fig. 2B). These results suggest that miR-122 expression may modulate HBV gene expression and production.
Figure 2.
Inhibition of the expression of HBV proteins by miR-122. A, B) HepG2.2.15 cells were transfected with synthetic miR-122 molecules (miR-122 mimics) or the control randomized molecules (Ctrl mimics), and miR-122 inhibitor (miR-122 inhibitor), or the control randomized molecules (Ctrl inhibitor) at a final concentration of 50 nM, respectively. C, D) HepG2 cells were cotransfected with pHBV1.3 and synthetic miR-122 (miR-122 mimics) or miR-122 inhibitor (miR-122 inhibitor) at different concentrations. At 48 h post-transfection, culture supernatants were collected, and the levels of HBsAg (A, C) and HBeAg (B, D) were determined by ELISA. Values are means from triplicate experiments and represent the relative levels of HBsAg and HBeAg in cells treated with different oligonucleotides, as a percentage of those in cells treated with Ctrl mimics (A, B) or in cells not treated with miR-122 mimics (0 nM; (C, D). Error bars = sd. *P < 0.05; **P < 0.01.
The second set of experiments was performed to determine whether modulation of miR-122 expression would similarly affect HBV accumulation in cells newly transfected with replication-competent HBV DNA. We generated construct pHBV1.3, which contained a full-length genomic sequence of HBV (ayw subtype) that is present in HepG2.2.15 cells. HepG2 cells were transfected with pHBV1.3 in the presence or absence of synthetic miR-122 mimics or the miR-122 inhibitor. Transfection of pHBV1.3 in HepG2 cells yielded successful productive replication of HBV, as evidenced by the expression of viral proteins (Fig. 2C, D). The levels of HBsAg (Fig. 2C) and HBeAg (Fig. 2D) were reduced in cells treated with synthetic miR-122 mimics and increased in cells treated with miR-122 inhibitor, respectively, and the response was dose dependent.
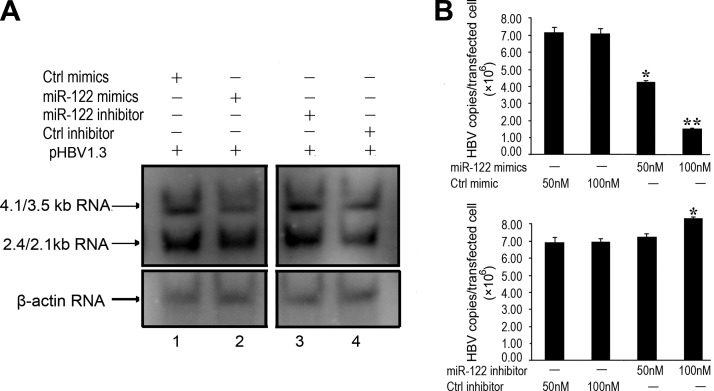
To further confirm the inhibitory effects of miR-122 on HBV gene expression and production, the third set of experiments was carried out to examine the levels of HBV RNA transcripts and genomic DNA in HepG2 cells that were cotransfected with pHBV1.3 and miR-122 mimics or miR-122 inhibitor. Specific HBV transcripts of 4.1/3.5 and 2.4/2.1 kb, which represent the pre-C mRNA/pregenomic RNA and pre-S/L mRNA, respectively (2), were predominantly detected in cells transfected with pHBV1.3 by Northern blot analysis. Levels of these transcripts were found to be lower in cells treated with synthetic miR-122 mimics and higher in cells with miR-122 inhibitor (Fig. 3A). Similarly, the levels of intracellular capsid-associated viral DNA in these cells, as determined by qPCR, were lower in cells treated with miR-122 mimics and higher in cells treated with miR-122 inhibitor, in a dose-dependent fashion (Fig. 3B). The control randomized RNA oligonucleotides, Ctrl mimics and Ctrl inhibitor, had no effect on HBV gene expression and production (Figs. 2 and 3). These results suggest that up- and down-regulation of the miR-122 level may lead to a decrease and increase of HBV gene expression and production, respectively.
Figure 3.
Inhibition of HBV RNA expression and DNA replication by miR-122. A) Northern blot analysis of RNAs isolated from HepG2 cells transfected with pHBV1.3 in the presence of synthetic miR-122 (miR-122 mimics; lane 2) or its randomized control (Ctrl mimics; lane 1), and miR-122 inhibitor (miR-122 inhibitor; lane 3) or its inhibitor control (Ctrl inhibitor; lane 4). At 48 h post-transfection, RNA samples were isolated from cells, separated on denaturing gels, transferred to membranes, hybridized with a 32P-labeled HBV DNA probe, and analyzed with autoradiography, using human β-actin mRNA as the loading control. B) Levels of capsid-associated viral DNA in cells determined by qPCR. At 48 h post-transfection, HBV capsid-associated DNAs were isolated from HepG2 cells that were transfected with pHBV1.3 DNA and different concentrations of synthetic oligonucleotides. Values are means from triplicate experiments. Error bars = sd. *P < 0.05; **P < 0.01.
Targeting the mRNA coding for HBV polymerase by miR-122
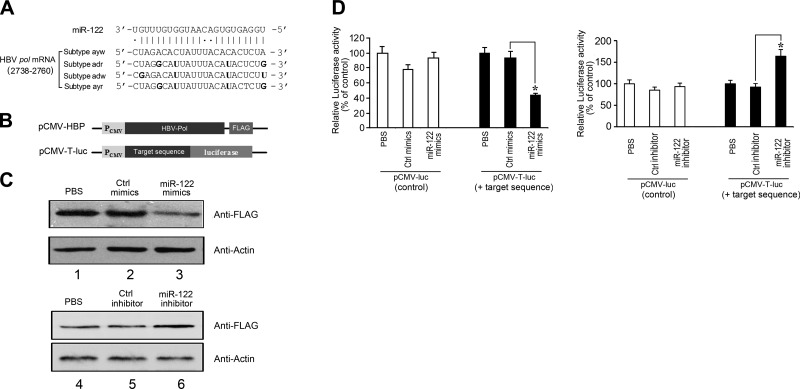
MiRNAs generally assert their functions by hybridizing to target mRNAs and modulating their expression (11–13, 15). It is conceivable that miR-122 inhibits HBV gene expression and production by directly base pairing with a HBV mRNA or pregenomic RNA sequence. Several targets for miR-122, including specific human genes and an HCV genomic RNA region, have been identified (20, 23). However, whether miR-122 targets an HBV RNA transcript has not been reported. We searched the HBV genomic sequence for potential RNA duplex formation with miR-122 using the RNA22, miRanda, and TargetScan algorithms (28, 29). These analyses predicted stable pairing of miR-122 with an HBV sequence (nt 2738 to 2760; Fig. 4A). This sequence is within the overlapping region of HBV pre-C mRNA and pregenomic RNA (pg RNA), which functions as the template for HBV genomic DNA synthesis and as a bicistronic mRNA encoding both the core protein and polymerase (pol) (2). Indeed, this sequence is located within the coding region of the mRNA for the polymerase and the 3′ UTR of the mRNA for the core protein.
Figure 4.
Targeting of a highly conserved HBV RNA sequence within the coding region of the viral polymerase mRNA by miR-122. A) Schematic representation of the proposed interactions of miR-122 to its target sequence, which is highly conserved among 4 HBV subtypes and is within the coding region of the viral polymerase mRNA. Numbers indicate relative position to the 5′ terminus of HBV genome. Positions that are not completely conserved are in bold. B) Schematic representation of constructs pCMV-HBP and pCMV-T-luc. C) Inhibition of HBV polymerase expression by miR-122, as detected by Western blot analysis. HepG2 cells were transfected with pCMV-HBP in the absence of synthetic oligonucleotides (PBS, lanes 1 and 4), and in the presence of miR-122 (miR-122 mimics, lane 3), miR-122 inhibitor (miR-122 inhibitor, lane 6), or the randomized control (Ctrl mimics, lane 2; Ctrl inhibitor, lane 5). Proteins were analyzed with an anti-FLAG antibody using the level of β-actin as the internal control. D) Expression of firefly luciferase in cells transfected with plasmids pCMV-T-luc and pCMV-luc, which contained the target HBV sequence of miR-122 or not, respectively. Cells were transfected in the absence of any synthetic oligonucleotides or in the presence of miR-122 mimics, miR-122 inhibitor, Ctrl mimics, and Ctrl inhibitor. Values are means from triplicate experiments and represent relative levels of luciferase activity in cells transfected with pCMV-luc and pCMV-T-luc in the presence of different oligonucleotides, as a percentage of those in cells transfected with pCMV-luc and pCMV-T-luc in the absence of synthetic oligonucleotides, respectively. Firefly luciferase activity was normalized on Renilla luciferase activity of the cotransfected pRL vector. Error bars = sd. *P < 0.05.
Extensive base-pairing interactions were predicted between miR-122 and the target HBV (subtype ayw) sequence. Sixteen of the 23 positions in this HBV sequence potentially form canonical Watson-Crick base pairing with miR-122, and 4 of the remaining 7 positions may interact with miR-122 via Wobble (G:U) base-pairing interactions (Fig. 4A). Furthermore, this viral sequence is highly conserved among all HBV clinical subtypes whose sequences are currently available (2). Indeed, 18 of the 23 positions are completely conserved, and 3 of the remaining 5 positions that are not completely conserved are found to change to nucleotides that form Wobble interactions (e.g., G-U) with miR-122, suggesting strong preservation of base-pairing interactions (Fig. 4A). The miR-122 sequence is completely conserved in humans, and no mutations in miR-122 have been reported (30, 31). The remarkable level of sequence conservation and complementarity suggests that direct base-pairing interactions between miR-122 and the target HBV sequence are important for the function of miR-122 and HBV infection.
To test this hypothesis and determine whether miR-122 targets the HBV polymerase, we generated an expression construct, pCMV-HBP, in which the HBV polymerase open-reading frame (ORF) was tagged with a FLAG epitope sequence at its 3′ terminus and under the control of the CMV promoter (Fig. 4B). HepG2 cells were transfected with pCMV-HBP and then treated with synthetic miR-122 molecules, miR-122 mimics and the miR-122 inhibitor, or control randomized oligonucleotides, Ctrl mimics and the Ctrl inhibitor. As shown in Western blot analysis (Fig. 4C), the levels of the FLAG-tagged polymerase decreased and increased on treatment of miR-122 mimics and miR-122 inhibitor, respectively, whereas the control Ctrl mimics and Ctrl inhibitor had no effect, suggesting that miR-122 inhibits HBV polymerase expression.
To further determine whether the sequence at nt 2738–2760 of the HBV genome was responsible for miR-122-mediated inhibition of HBV polymerase expression, we inserted the target sequence upstream from the firefly luciferase reporter gene of plasmid pCMV-luc to generate construct pCMV-T-luc (Fig. 4B). The construct was introduced into HepG2 cells together with a control Renilla luciferase-expressing plasmid (pRL) as a loading control. Luciferase activity was measured in the presence of cotransfected synthetic miR-122 mimics and miR-122 inhibitor, or negative control Ctrl mimics and Ctrl inhibitor. Reporter gene expression, measured as luciferase activity, was similar in cells treated with the reporter construct pCMV-T- luc alone or with the negative control Ctrl mimics and Ctrl inhibitor, but it was reduced and increased in cells transfected with pCMV-T-luc and treated with miR-122 mimics and miR-122 inhibitor, respectively (Fig. 4D, right panel). In contrast, treatment with miR-122 mimics and miR-122 inhibitor had no effect on the level of luciferase activity in cells transfected with control empty vector pCMV-luc, which did not contain the target HBV sequence of miR-122 (Fig. 4D, left panel). These results suggest that miR-122 may down-regulate HBV polymerase expression by interacting with the pol mRNA sequence coding for nt 2738–2760 of HBV genome and that this regulation does not require other HBV sequences or factors.
Every other HBV subtype isolate (e.g., subtype adw) shown in Fig. 4A, besides ayw subtype, has a uridine at position 2745 within the miR-122 binding region. To determine whether the miR-122-mediated effect on HBV (ayw subtype) infection is also conserved in these subtypes, experiments similar to those described in Figs. 2–4 were carried out to study the gene expression and replication of HBV adw subtype in HepG2 cells in the presence and absence of miR-122 mimics, miR-122 inhibitors, Ctrl mimics, and Ctrl inhibitor. In cells transfected with construct pHBV1.3 adw that contained a full-length genomic sequence of HBV adw subtype, the levels of HBsAg and HBeAg were reduced in cells treated with miR-122 mimics and increased in cells treated with miR-122 inhibitor, respectively, and the response was dose dependent (Supplemental Fig. S2A). Furthermore, levels of the HBV 4.1/3.5 and 2.4/2.1 kb transcripts (Supplemental Fig. S2B) and intracellular capsid-associated viral DNA (Supplemental Fig. S2C) were found to be lower in cells treated with miR-122 mimics and higher in cells with miR-122 inhibitor. In cells transfected with pCMV-HBP(adw) containing the FLAG-tagged pol ORF of HBV adw subtype (Supplemental Fig. S3A), levels of the FLAG-tagged polymerase decreased and increased on treatment with miR-122 mimics and miR-122 inhibitor, respectively (Supplemental Fig. S3B).
To further determine whether the sequence at nt 2738–2760 of the HBV (adw subtype) genome was responsible for miR-122-mediated inhibition of the polymerase expression, we inserted this sequence upstream from the firefly luciferase reporter gene of plasmid pCMV-luc to generate construct pCMV-T-luc(adw) (Supplemental Fig. S3A). Reporter gene expression, measured as luciferase activity, was reduced and increased in cells transfected with pCMV-T-luc(adw) and treated with miR-122 mimics and miR-122 inhibitor, respectively (Supplemental Fig. S3C). These results suggest that miR-122 may interact with the pol mRNA sequence coding for nt 2738–2760 of the genomes of HBV ayw and adw subtype isolates and negatively regulate the gene expression and replication of both subtypes.
Binding the target HBV RNA sequence via base-pairing interactions to regulate HBV infection by miR-122
To investigate the potential interactions between miR-122 and its target HBV pol mRNA sequence and study the role of such interactions in viral infection, we introduced mutations in the “seed region” of miR-122 (nt 2–8; refs. 28, 29) and its corresponding positions at the target sequence of HBV, and tested the effect of mutated miR-122 molecules on HBV gene expression and production. Point mutations were introduced to miR-122 (G3A4→C3U4) and pol (U2757C2758→A2757G2758) to generate mutant M1-miR-122 and m1-HBV, respectively (Fig. 5A). HepG2 cells were cotransfected with the wild-type (wt-HBV) or mutated pHBV1.3 (m1-HBV), and synthetic wild-type (miR-122 mimics) or mutated miR-122 molecules (M1-miR-122 mimics), and the effects of miR-122 on HBV gene expression and production were studied by assaying HBsAg/HBeAg expression with ELISA. Similar levels of HBV genomic DNA (Fig. 5B), as well as HBsAg and HBeAg (Fig. 5C, D), were found in cells transfected with wt-HBV and m1-HBV in the absence of any oligonucleotides (PBS). These observations suggest that the mutation (U2757C2758→A2757G2758) may have no significant effect on the polymerase activity or HBV gene expression/production.
Figure 5.
Interactions of miR-122 with its HBV target sequence. A) Schematic representation of the proposed interactions of miR-122 to the target HBV RNA sequence and of compensatory base pair changes. “Seed match” region of miR-122 is underscored; mutations are in bold and boxed. B) Levels of capsid-associated viral DNA in cells, determined by qPCR. At 48 h post-transfection, HBV capsid-associated DNAs were isolated from HepG2 cells that were transfected with the wild-type (wt-HBV) or mutated pHBV1.3 DNA (m1-HBV). Values are means from triplicate experiments. Error bars = sd. C, D) Levels of HBsAg (C) and HBeAg (D), as determined by ELISA, in the supernatants of cultures of HepG2 cells transfected with wt-HBV or m1-HBV in the absence of any oligonucleotides (PBS) or in the presence of control randomized oligonucleotides (Ctrl mimics), and wild-type (miR-122 mimics) and mutant miR-122 (M1-miR-122 mimics). Values represent relative levels of HBsAg (C) and HBeAg (D) in cells transfected with wt- or m1-HBV and treated with oligonucleotides, as a percentage of those in control cells (PBS). Error bars = sd. *P < 0.05; ▴P > 0.05. E) Binding of miR-122 to its HBV target sequence in vitro. In vitro transcribed RNAs that contained wt-HBV and m1-HBV were separated on denaturing gels, transferred to membranes, and hybridized with radiolabeled synthetic miR-122 mimics (lanes 1 and 2) and M1-miR-122 mimics (lanes 3 and 4) and a HBV DNA probe (antisense-HBV; lanes 5 and 6), and analyzed with autoradiography.
Consistent with the results described in Fig. 2C, D, synthetic wild-type miR-122 mimics inhibited the HBsAg/HBeAg expression from wt-HBV, and control randomized oligonucleotides had no effect on HBV gene expression (Fig. 5C, D). In contrast, the HBsAg/HBeAg expression of wt-HBV was not repressed by synthetic mutant M1-miR-122 mimics, while wild-type miR-122 mimics did not significantly affect the protein expression of the mutated m1-HBV. Repression of HBV HBsAg/HBeAg expression by miR-122 was restored using m1-HBV and M1-miR-122 mimics, which contained the compensatory mutations (Fig. 5C, D). To further validate the interactions between miR-122 and its target HBV sequence, two additional pairs of compensatory mutations were introduced to miR-122 (G2G3A4G5→U2C3U4C5 and G3→C3) and pol (C2756U2757C2758U2759→G2756A2757G2758A2759 and C2758→ G2758) to generate mutants M2- and M3-miR-122 and m2- and m3-HBV, respectively (Fig. 5A). Repression of the HBsAg/HBeAg expression of m2- and m3-HBV was restored only by using synthetic mutated M2- and M3-miR-122 mimics, respectively, which contained compensatory mutations (Supplemental Fig. S4). These results suggest that miR-122 inhibits HBV gene expression by interacting with the target sequence coding for nt 2738–2760 of the HBV genome via base-pairing interactions.
To further determine whether miR-122 directly binds to the target HBV sequence, in vitro transcribed RNAs that coded for the pol ORF sequence and contained mutations at the miR-122 target sequence were separated on denaturing gels, transferred to membranes, and incubated with labeled synthetic miR-122 molecules carrying different mutations. Synthetic wild-type miR-122 mimics appeared to bind to the wt-HBV, as hybridized signals were detected and colocalized with the in vitro transcribed RNAs on the membranes (Fig. 5E, compare lanes 1 and 5). In contrast, no binding was detected between miR-122 mimics and the sequence of m1-HBV, which contained point mutations (U2757C2758→A2757G2758) at the pol mRNA, or between the wt-HBV sequence and synthetic mutated M1-miR-122 mimics, which contained point mutations (G3A4→C3U4; Fig. 5E, lanes 2 and 3). Binding was only restored using M1-miR-122 mimics and m1-HBV, which contained the compensatory mutations (Fig. 5E, lane 4). As negative control, randomized oligonucleotides Ctrl mimics exhibited no binding to the HBV sequence. As positive control, a HBV DNA probe (antisense-HBV) could bind to both the wt-HBV and m1-HBV sequence (Fig. 5E, lanes 5 and 6). Similar results were also observed between mutant M2- and M3-miR-122 mimics, and the sequence of mutant m2- and m3-HBV. These results suggest that miR-122 may directly and specifically bind to the target HBV sequence via base-pairing interactions.
Correlation of miR-122 level with HBV infection in vivo
It is reasonable to suggest that there would be an inverse correlation between the levels of miR-122 and the HBV infection in vivo, based on our results in cultured cells in vitro. To determine whether this is the case, we used qRT-PCR to determine the levels of miR-122 in PBMCs of 80 HBV-positive patients (52 males and 28 females, mean age 36.2±13.8 yr) and 80 healthy HBV-negative individuals (44 males and 36 females, mean age 38.5±12.5 yr) (Table 2). Our results indicated that the levels of miR-122 were significantly lower (P<0.001) in HBV-positive patients (187.6±45.9 copies/ml) in comparison with healthy HBV-negative individuals (308.0±79.9 copies/ml) (Table 2).
Table 2.
Characteristics of HBV-positive patients and healthy HBV-negative individuals enrolled in this study
| Characteristic | HBV-negative | HBV-positive | P |
|---|---|---|---|
| Group size (n) | 80 | 80 | |
| Age (yr) | 38.5 ± 12.7 | 36.2 ± 13.8 | 0.89 |
| Gender (male/female) | 44/36 | 52/28 | 0.95 |
| Race or ethnic group Asian [n (%)] | 80 (100) | 80 (100) | 1.00 |
| HBeAg (+/−) | 0/80 | 32/48 | |
| HBV genotype (b/c) | NA | 26/54 | |
| WBC (cells/μl) | 3415 ± 820 | 7402 ± 7240 | |
| ALT (U/L) | <30 | 156.9 ± 174.9 | |
| HBV DNA (copies/ml) | <500 | 9.9E+05 ± 4.7E+05 | |
| miR122 level (copies/ml) | 308.0 ± 79.9 | 187.6 ± 45.9 | <0.001 |
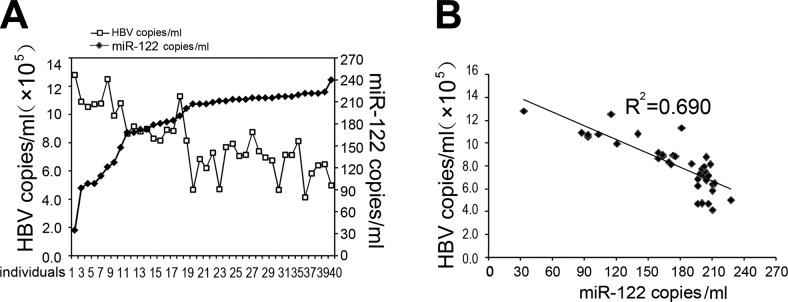
The levels of viral loads, measured as numbers of copies of HBV genomic DNA by qPCR, were also assayed and compared to the levels of miR-122 in 40 HBV-positive patients. These results showed that the levels of miR-122 decreased as viral loads increased (Fig. 6A). When the levels of miR-122 were plotted against viral loads, there was linear inverse correlation between miR-122 expression and HBV replication, with a coefficient of 0.69 (Fig. 6B). These in vivo observations further support our results in vitro in cell culture, which suggested that the presence of HBV replication and infection inhibited miR-122 expression and that miR-122 negatively regulated HBV gene expression and production.
Figure 6.
Correlation of miR-122 expression and HBV infection in clinical samples. A) Levels of miR-122 (♦) and numbers of HBV genome copies (□) in PBMCs isolated from 40 HBV-positive patients. B) Correlation of the levels of miR-122 and numbers of HBV genome copies in PBMCs isolated from the 40 HBV-positive patients. Levels of miR-122 and numbers of HBV genome copies were determined by qRT-PCR and qPCR, respectively. Values for miR-122 represent numbers of miR-122 copies, as compared to a standard synthetic wild-type miR-122 (miR-122 mimic) oligonucleotide measured in vitro. All experiments were repeated 3 times.
DISCUSSION
It is generally believed that HBV, due to its small genome size (∼3 kb) and coding capacity, requires extensive networks of host factors to facilitate successful infection and pathogenesis. In particular, the unique, chronic, and persistent infection of HBV in the liver demands tight regulation of its infection by specific host factors in a tissue-specific manner in the hepatic tissues. Our study here provides the first direct evidence that miR-122, the most abundant miRNA in the liver, negatively regulates HBV gene expression and replication by binding to a highly conserved region of HBV pregenomic RNA, which is also a bicistronic mRNA encoding the viral polymerase and core protein.
One of the most fundamental issues in HBV biology is how HBV maintains persistent and chronic infection in the liver tissues and cells. Several host factors, including immune-associated proteins, have been linked with the susceptibility to and pathogenesis of persistent HBV infection (32, 33). Little is currently known about how HBV replication is controlled or maintained in a delicate balance at a persistent state in a single infected cell. Our finding that miR-122 expression negatively regulates HBV gene expression/replication suggests that miR-122 may participate in regulating and maintaining HBV persistent infection in hepatic cells. MiR-122, a liver-specific miRNA, is expressed at a high level in the hepatic tissues, reaching ∼70% of the total miRNA population (16, 18). MiR-122-mediated inhibition of HBV infection requires specific interactions between miR-122 and the viral polymerase mRNA region covering nt 2738–2760 of the HBV genome. This HBV region is highly conserved among all viral subtypes worldwide whose sequences have been determined, while miR-122 is completely conserved in humans (30, 31). The remarkable level of sequence conservation and complementarity in both the miR-122 and its target HBV sequence highlights the significance of their specific interactions in HBV infection. This is further supported by our mutagenesis results. Mutations at miR-122 or its target HBV sequence, in the case of a double- or single-base substitution (e.g., M1-, and M3-MIR122 and m1-, and m3-HBV), disrupted the binding interaction and significantly diminished miR-122-mediated regulation of HBV gene expression and replication. It is reasonable to suggest that the conservation of this region is required because miR-122-mediated modulation of HBV infection and vice versa is essential for HBV infection, although we can not completely rule out the possibility that these nucleotides are conserved for other unidentified functions that are important for HBV infection in vivo.
Our study was primarily carried out using the HepG2/HepG2.2.15 cell culture system. HepG2.2.15, which is derived from HepG2 and stably contains a transfected full-length genome of HBV (ayw subtype), has been extensively used as a cell culture model for HBV-persistent replication and infection (27). The availability of HepG2.2.15 in combination with HepG2 cells provides an excellent cell culture model to study the effect of miR-122 on the replication of HBV genome that is stably and transiently expressed in a human cell. While HepG2 cells express a low level of miR-122, the level of miR-122 that is available to bind to HBV can be easily modulated to be increased and decreased with the transfection of miR-122 mimics and miR-122 inhibitors, respectively (Supplemental Fig. S1). In contrast, the high level of miR-122 in other cell culture systems, such as in Huh7 cells (23), makes it difficult to further increase the level of miR-122 in these cells with the transfection of miR-122 mimics in our experiments. It will be important to investigate the miR-122 effect on HBV gene expression and replication in cells expressing different levels of miR-122 (e.g., Huh7 and PBMCs) and in different contexts of HBV infection. These studies will help elucidate the exact roles of miR-122 in HBV replication and pathogenesis.
Modulation of miR-122 expression in the presence of HBV replication, as revealed in our study both in vitro and in clinical samples, may play an important role in HBV pathogenesis. Several studies have identified the role of miR-122 in diverse aspects of hepatic function (11, 12), including hepatocyte growth and neoplastic transformation, lipid metabolism, and HCV replication (20–22). It is conceivable that the suppression of miR-122 expression induced by HBV may have a significant effect on normal liver functions, leading to pathogenic changes, such as diminished liver metabolism and tumorigenesis. Thus, HBV-mediated modulation of miR-122 expression may represent another mechanism of viral pathogenesis, independent from the roles of individual HBV-encoded proteins.
Our analyses of the clinical samples suggest that the level of miR-122 decreased as the viral load of HBV increased. However, we cannot completely exclude the possibility that the miR-122 levels decreased due to the liver damage/loss of hepatocytes and not necessarily due to HBV replication per se, given the large difference in the ALT levels between healthy individuals and HBV-positive patients (Table 2). Further analyses will be carried out to determine the correlation between miR-122 levels and viral loads in vivo.
The pregenomic RNA (pg RNA), which is the mRNA encoding the viral polymerase, is a bicistronic mRNA that also encodes the core protein (2). Thus, the target sequence of miR-122 is located at the region of the polymerase mRNA coding for the terminal domain, as well as at the 3′ UTR of the core protein mRNA. Our results with the reporter system assays suggested that miR-122 down-regulated the protein expression of the polymerase by specifically interacting with the target sequence. Unlike most of the binding regions for miRNAs, which are either at the 3′ or 5′ UTR of the target mRNAs (11–13), miR-122 binds to the coding sequence of HBV pol mRNA. It is possible that miR-122 may inhibit polymerase expression by modulating its mRNA stability and translation. Meanwhile, it is conceivable that the binding of miR-122 to this region may also affect the core protein mRNA stability and translation, as well as the stability of pg RNA, thereby leading to inhibition of HBV gene expression/production. Our observations that a single- or double-base substitution disrupted the binding between miR-122 and its target HBV sequence and diminished the miR-122-mediated regulation of HBV gene expression and production suggest that unique RNA conformations or structures may be formed to achieve successful binding. Further studies, such as detailed mutagenesis analyses coupled with biochemical characterization, are needed to determine the mechanism of how miR-122 achieves efficient binding and reducing the levels of HBV polymerase and, possibly, other viral components.
The HBV sequence targeted by miR-122 may represent an ideal target for modulating the level of HBV production since it serves as a part of the coding region of the mRNA for the viral polymerase, a part of the 3′ UTR of the mRNA for the core protein, and a part of the pg RNA, all of which are essential for viral replication (2). Targeting this highly conserved region may represent the best strategy for precise control of viral replication, as required for HBV persistent infection and pathogenesis, as it will shut down both the viral essential stoichiometric (e.g., pg RNA and core protein) and enzymatic functions (e.g., polymerase) simultaneously. This is consistent with our observations that miR-122 inhibits HBV protein expression, RNA transcription, and genomic DNA replication (Figs. 2–4). As a consequence of overall inhibition of viral replication, the expression of many viral proteins, such as HBsAg, is reduced, even though their mRNAs may not overlap with the miR-122 binding site and are not directly targeted by miR-122. Furthermore, our results raise the possibility of developing miR-122 or its mimics or variants for blocking HBV infection and treating HBV-associated diseases. While miR-122 plays important roles in several cellular processes, modulation of miR-122 expression may not exhibit significant side effects or toxicity in vivo, as shown in a recent study using miR-122 inhibitors for treatment of HCV infection in chimpanzees (24). Further studies will provide significant insights into the role of miR-122, as well as other human factors, in HBV infection and greatly facilitate the development of new compounds and novel strategies for the treatment and prevention of HBV infection.
Supplementary Material
Acknowledgments
The authors are indebted to Gerry Abenes, Naresh Sunkara, and Ed Yang for technical assistance and valuable suggestions.
This work has been supported by research grants from the National Mega Project on Major Infectious Diseases Prevention and Treatment (2008ZX10002-009), National Basic Research Program of China (973 Program; 2011CB504800 and 2012CB518900), National Mega Project on Major Drug Development (2009ZX09301-012), Major State Basic Research Development Program of China (2005CB522901 and 2007CB512803), National Natural Science Foundation of China (30730001, 81171525, 30570070, 31100128, and 81030031), and Program for Changjiang Scholars and Innovative Research Team in Universities (IRT0745). This research has been supported by grants from the U.S. National Institutes of Health (AI041927, AI050468, and DE014842).
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
REFERENCES
- 1. Parkin D. M., Pisani P., Ferlay J. (1999) Global cancer statistics. CA Cancer J. Clin. 49, 33–64, 31 [DOI] [PubMed] [Google Scholar]
- 2. Seegar C., Zoulim F., Mason W. S. (2007) Hepadnaviruses. In Fields Virology (Knipe D. M., Howley P. M., Griffin D. E., Martin M. A., Lamb R. A., Roizman B., Straus S. E. eds) pp. 2978–3029, Lippincott-William & Wilkins, Philadelphia [Google Scholar]
- 3. Belongia E. A., Costa J., Gareen I. F., Grem J. L., Inadomi J. M., Kern E. R., McHugh J. A., Petersen G. M., Rein M. F., Sorrell M. F., Strader D. B., Trotter H. T. (2008) NIH consensus development statement on management of hepatitis B. NIH Consens. State Sci. Statements 25, 1–29 [PubMed] [Google Scholar]
- 4. Ganem D., Varmus H. E. (1987) The molecular biology of the hepatitis B viruses. Annu. Rev. Biochem. 56, 651–693 [DOI] [PubMed] [Google Scholar]
- 5. Lagos-Quintana M., Rauhut R., Lendeckel W., Tuschl T. (2001) Identification of novel genes coding for small expressed RNAs. Science 294, 853–858 [DOI] [PubMed] [Google Scholar]
- 6. Lau N. C., Lim L. P., Weinstein E. G., Bartel D. P. (2001) An abundant class of tiny RNAs with probable regulatory roles in Caenorhabditis elegans. Science 294, 858–862 [DOI] [PubMed] [Google Scholar]
- 7. Lee R. C., Ambros V. (2001) An extensive class of small RNAs in Caenorhabditis elegans. Science 294, 862–864 [DOI] [PubMed] [Google Scholar]
- 8. Bennasser Y., Le S. Y., Yeung M. L., Jeang K. T. (2004) HIV-1 encoded candidate micro-RNAs and their cellular targets. Retrovirology 1, 43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pfeffer S., Zavolan M., Grasser F. A., Chien M., Russo J. J., Ju J., John B., Enright A. J., Marks D., Sander C., Tuschl T. (2004) Identification of virus-encoded microRNAs. Science 304, 734–736 [DOI] [PubMed] [Google Scholar]
- 10. Sullivan C. S., Grundhoff A. T., Tevethia S., Pipas J. M., Ganem D. (2005) SV40-encoded microRNAs regulate viral gene expression and reduce susceptibility to cytotoxic T cells. Nature 435, 682–686 [DOI] [PubMed] [Google Scholar]
- 11. Ghildiyal M., Zamore P. D. (2009) Small silencing RNAs: an expanding universe. Nat. Rev. Genet. 10, 94–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Moazed D. (2009) Small RNAs in transcriptional gene silencing and genome defence. Nature 457, 413–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Skalsky R. L., Cullen B. R. (2010) Viruses, microRNAs, and host interactions. Annu. Rev. Microbiol. 64, 123–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jackson R. J., Standart N. (2007) How do microRNAs regulate gene expression? Sci. STKE 2007, re1 [DOI] [PubMed] [Google Scholar]
- 15. Sullivan C. S., Ganem D. (2005) MicroRNAs and viral infection. Mol. Cell 20, 3–7 [DOI] [PubMed] [Google Scholar]
- 16. Lagos-Quintana M., Rauhut R., Yalcin A., Meyer J., Lendeckel W., Tuschl T. (2002) Identification of tissue-specific microRNAs from mouse. Curr. Biol. 12, 735–739 [DOI] [PubMed] [Google Scholar]
- 17. Sempere L. F., Freemantle S., Pitha-Rowe I., Moss E., Dmitrovsky E., Ambros V. (2004) Expression profiling of mammalian microRNAs uncovers a subset of brain-expressed microRNAs with possible roles in murine and human neuronal differentiation. Genome Biol. 5, R13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chang J., Nicolas E., Marks D., Sander C., Lerro A., Buendia M. A., Xu C., Mason W. S., Moloshok T., Bort R., Zaret K. S., Taylor J. M. (2004) miR-122, a mammalian liver-specific microRNA, is processed from hcr mRNA and may downregulate the high affinity cationic amino acid transporter CAT-1. RNA Biol. 1, 106–113 [DOI] [PubMed] [Google Scholar]
- 19. Kutay H., Bai S., Datta J., Motiwala T., Pogribny I., Frankel W., Jacob S. T., Ghoshal K. (2006) Downregulation of miR-122 in the rodent and human hepatocellular carcinomas. J. Cell. Biochem. 99, 671–678 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 20. Krutzfeldt J., Rajewsky N., Braich R., Rajeev K. G., Tuschl T., Manoharan M., Stoffel M. (2005) Silencing of microRNAs in vivo with ‘antagomirs’. Nature 438, 685–689 [DOI] [PubMed] [Google Scholar]
- 21. Bai S., Nasser M. W., Wang B., Hsu S. H., Datta J., Kutay H., Yadav A., Nuovo G., Kumar P., Ghoshal K. (2009) MicroRNA-122 inhibits tumorigenic properties of hepatocellular carcinoma cells and sensitizes these cells to sorafenib. J. Biol. Chem. 284, 32015–32027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tsai W. C., Hsu P. W., Lai T. C., Chau G. Y., Lin C. W., Chen C. M., Lin C. D., Liao Y. L., Wang J. L., Chau Y. P., Hsu M. T., Hsiao M., Huang H. D., Tsou A. P. (2009) MicroRNA-122, a tumor suppressor microRNA that regulates intrahepatic metastasis of hepatocellular carcinoma. Hepatology 49, 1571–1582 [DOI] [PubMed] [Google Scholar]
- 23. Jopling C. L., Yi M., Lancaster A. M., Lemon S. M., Sarnow P. (2005) Modulation of hepatitis C virus RNA abundance by a liver-specific MicroRNA. Science 309, 1577–1581 [DOI] [PubMed] [Google Scholar]
- 24. Lanford R. E., Hildebrandt-Eriksen E. S., Petri A., Persson R., Lindow M., Munk M. E., Kauppinen S., Orum H. (2010) Therapeutic silencing of microRNA-122 in primates with chronic hepatitis C virus infection. Science 327, 198–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wu K. L., Zhang X., Zhang J., Yang Y., Mu Y. X., Liu M., Lu L., Li Y., Zhu Y., Wu J. (2005) Inhibition of hepatitis B virus gene expression by single and dual small interfering RNA treatment. Virus Res. 112, 100–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Livak K. J., Schmittgen T. D. (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 25, 402–408 [DOI] [PubMed] [Google Scholar]
- 27. Sells M. A., Chen M. L., Acs G. (1987) Production of hepatitis B virus particles in Hep G2 cells transfected with cloned hepatitis B virus DNA. Proc. Natl. Acad. Sci. U. S. A. 84, 1005–1009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lewis B. P., Shih I. H., Jones-Rhoades M. W., Bartel D. P., Burge C. B. (2003) Prediction of mammalian microRNA targets. Cell 115, 787–798 [DOI] [PubMed] [Google Scholar]
- 29. Lewis B. P., Burge C. B., Bartel D. P. (2005) Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell 120, 15–20 [DOI] [PubMed] [Google Scholar]
- 30. Fu H., Tie Y., Xu C., Zhang Z., Zhu J., Shi Y., Jiang H., Sun Z., Zheng X. (2005) Identification of human fetal liver miRNAs by a novel method. FEBS Lett. 579, 3849–3854 [DOI] [PubMed] [Google Scholar]
- 31. Landgraf P., Rusu M., Sheridan R., Sewer A., Iovino N., Aravin A., Pfeffer S., Rice A., Kamphorst A. O., Landthaler M., Lin C., Socci N. D., Hermida L., Fulci V., Chiaretti S., Foa R., Schliwka J., Fuchs U., Novosel A., Muller R. U., Schermer B., Bissels U., Inman J., Phan Q., Chien M., Weir D. B., Choksi R., De Vita G., Frezzetti D., Trompeter H. I., Hornung V., Teng G., Hartmann G., Palkovits M., Di Lauro R., Wernet P., Macino G., Rogler C. E., Nagle J. W., Ju J., Papavasiliou F. N., Benzing T., Lichter P., Tam W., Brownstein M. J., Bosio A., Borkhardt A., Russo J. J., Sander C., Zavolan M., Tuschl T. (2007) A mammalian microRNA expression atlas based on small RNA library sequencing. Cell 129, 1401–1414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ramezani A., Hasanjani Roshan M. R., Kalantar E., Eslamifar A., Banifazl M., Taeb J., Aghakhani A., Gachkar L., Velayati A. A. (2008) Association of human leukocyte antigen polymorphism with outcomes of hepatitis B virus infection. J. Gastroenterol. Hepatol. 23, 1716–1721 [DOI] [PubMed] [Google Scholar]
- 33. Tan A. T., Loggi E., Boni C., Chia A., Gehring A. J., Sastry K. S., Goh V., Fisicaro P., Andreone P., Brander C., Lim S. G., Ferrari C., Bihl F., Bertoletti A. (2008) Host ethnicity and virus genotype shape the hepatitis B virus-specific T-cell repertoire. J. Virol. 82, 10986–10997 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.