Abstract
The nervous system is classically organized into sympathetic and parasympathetic systems acting in opposition to maintain physiological homeostasis. Here, we report that both systems converge in the activation of β2-adrenoceptors of splenic regulatory lymphocytes to control systemic inflammation. Vagus nerve stimulation fails to control serum TNF levels in either β2-knockout or lymphocyte-deficient nude mice. Unlike typical suppressor CD25+ cells, the transfer of CD4+CD25− regulatory lymphocytes reestablishes the anti-inflammatory potential of the vagus nerve and β2-agonists to control inflammation in both β2-knockout and nude mice. β2-Agonists inhibit cytokine production in splenocytes (IC50∼1 μM) and prevent systemic inflammation in wild-type but not in β2-knockout mice. β2-Agonists rescue wild-type mice from established polymicrobial peritonitis in a clinically relevant time frame. Regulatory lymphocytes reestablish the anti-inflammatory potential of β2-agonists to control systemic inflammation, organ damage, and lethal endotoxic shock in β2-knockout mice. These results indicate that β2-adrenoceptors in regulatory lymphocytes are critical for the anti-inflammatory potential of the parasympathetic vagus nerve, and they represent a potential pharmacological target for sepsis. Vida, G., Peña, G., Kanashiro, A., del Rocio Thompson-Bonilla, M., Palange, D., Deitch, E. A., Ulloa, L. β2-Adrenoreceptors of regulatory lymphocytes are essential for vagal neuromodulation of the innate immune system.
Keywords: sepsis, septic shock, parasympathetic nervous system, vagus nerve, inflammation, cytokine
Inflammation is one of the main causes of human morbidity and mortality (1–3). However, immune homeostasis does not arise passively from an absence of inflammatory stimuli, but rather from the mechanisms modulating the immune system, according to the host's physiological needs. Indeed, the regulation of the immune system appears proportional to its complexity, and it can be considered in terms of checkpoints. These regulatory mechanisms are critical for survival, and they represent potential targets for the treatment of inflammatory and infectious diseases. A typical example of the need to control inflammation is sepsis, the third leading cause of death in developed countries (4). Sepsis remains a major clinical challenge because—despite the major advances in critical care—the mortality rate of sepsis is significantly high, ranging from 30 to 70% depending on the organs affected (5). Although new generations of antibiotics are more effective at controlling infections (6), sepsis remains the most common cause of death in hospitalized patients, killing over 250,000 patients and accounting for 9.3% of the overall deaths in the United States annually (4, 6). In addition to infection, sepsis is also characterized by detrimental immune responses that can be even more dangerous than the original infection. Unregulated cytokine production is a life-threatening condition, causing tissue injury, organ damage, and lethal multiple organ failure (1, 3, 7–9). The inhibition of inflammatory cytokines, such as a tumor necrosis factor (TNF), migration inhibitory factor (MIF), or high mobility group box (HMGB)-1, has provided promising results in experimental sepsis (1, 3, 9). However, these cytokines are not specific for sepsis, and the specific inhibition of single cytokines has not been successful in clinical trials for sepsis (10). A plausible explanation is that sepsis is not produced by a single cytokine, and a successful treatment may require a comprehensive strategy to inhibit several, rather than a single cytokine. Thus, recent efforts have focused on understanding the physiological anti-inflammatory mechanisms regulating cytokine production and studying their potential translation for the treatment of sepsis.
Physiological anti-inflammatory mechanisms represent efficient systems selected by evolution to control inflammation (11, 12). Among them, the nervous system plays a critical role orchestrating the immune system, according to changing physiological needs. Although the sympathetic system has been studied for years, only recently has the anti-inflammatory potential of the parasympathetic system been demonstrated (13, 14). The vagus nerve is the principle parasympathetic nerve connecting the central nervous system with peripheral organs (11, 12). Stimulation of the vagus nerve can prevent systemic inflammation, organ damage, and mortality in different experimental settings of critical care, from hemorrhage to sepsis (15). Despite its recent identification, different investigators have already reported that electrical or pharmacological stimulation of the vagus nerve can restrain systemic inflammation in experimental ischemia and reperfusion (16–18), hemorrhage and resuscitation (18), pancreatitis (19), colitis (20), endotoxemia (15, 21), and sepsis (22, 23). In addition to its physiological implications, the vagus nerve mediates the anti-inflammatory potential of multiple clinical treatments, including some nonsteroidal anti-inflammatory drugs, such as semapimod (CNI-1493; currently in clinical trials for Crohn's disease), as well as melanocortin peptides, CCK, and leptin (2, 24–26). Classically, the nervous system has been classified into sympathetic and parasympathetic systems acting in opposition to maintain physiological homeostasis. However, our previous studies suggested that both systems can work together to restrain systemic inflammation in life-threatening conditions, such as sepsis. Our results suggest that acetylcholine released by the vagus nerve in the celiac mesenteric ganglia activates the splenic nerve via postsynaptic α7 nicotinic acetylcholine receptors (27). Despite their clinical and physiological implications, the cellular and molecular mechanisms mediating the anti-inflammatory potential of the vagus and the splenic nerve remain unknown. Here, we report that the parasympathetic vagus nerve controls systemic inflammation through a splenic subpopulation of lymphocytes expressing β2-adrenoceptors. Furthermore, we document that specific β2-agonists can control systemic inflammation and might provide pharmacological advantages against sepsis and septic shock through a mechanism mediated by specific CD4+CD25− regulatory lymphocytes. Thus, our results also reveal a new set of regulatory lymphocytes that is critical for neuromodulation.
MATERIALS AND METHODS
Chemicals and reagents
LPS was purchased from Sigma-Aldrich (Saint Louis, MO, USA) and freshly prepared in PBS (Gibco, Invitrogen, Carlsbad, CA, USA) for every experiment; LPS was dissolved in PBS to get a stock solution of 5 mg/ml. Epinephrine or norepinephrine was given to splenocytes at 30 min before LPS (100 ng/ml). The indicated adrenergic blockers were administered 30 min prior to norepinephrine. Reserpine (5 mg/kg i.p.) was administered at 24 h before the experiment. Fusaric acid (40 mg/kg i.p.) was administered twice at 8 and 4 h before the experiment. The compounds were purchased from Sigma.
Animal experiments
All animal procedures were approved by the Institutional Animal Care and Use Committee of the New Jersey Medical School. Adult male nude mice, B6.Cg-Foxn1<nu>/J, 8–12 wk old, were purchased from the Jackson Laboratory (Bar Harbor, ME, USA); β2AdrR-knockout mice and wild-type littermates (C57BL/6, CD-1), 8–12 wk old, were obtained from Brian Kobilka (Stanford University, Stanford, CA, USA). Sprague-Dawley rats, 8–12 wk old, were purchased from the Jackson Laboratory. Animals were randomly grouped, and investigators were blinded to the experimental treatment. Animals were genotyped by PCR assay using genomic DNA from mouse tails and the Extract-N-Amp Tissue PCR kit (Sigma-Aldrich), as described previously (28). Animals were maintained on a 12-h light-dark cycle, with free access to food and water. Endotoxemia and cecal ligation and puncture (CLP) were performed as previously described in Wang et al. (22).
Endotoxemia
Briefly, endotoxin (Escherichia coli LPS 0111:B4; Sigma-Aldrich) was dissolved in sterile, pyrogen-free PBS, and sonicated for 30 min immediately before use. Animals received an LD50 dose of LPS (6 mg/kg) and splenocytes were given LPS in a concentration of 100 ng/ml. Both are standard procedures extensively published in the literature.
CLP
Animals were anesthetized with ketamine (75 mg/kg i.m.; Fort Dodge, Fort Dodge, IA, USA) and xylazine (20 mg/kg; i.m.; Boehringer Ingelheim, St. Joseph, MO, USA) and subjected to CLP with an average 50% natural mortality, as we have previously described (22). Briefly, animals were subjected to abdominal incision and ligation of the cecum at 5.0 mm from the cecal tip away from the ileocecal valve. The ligated cecal stump was punctured once with a 22-gauge needle, and stool was extruded (∼1 mm) to ascertain patency of puncture. Abdominal wound was closed in two layers, peritoneum and fascia separately, to prevent leakage of fluid. All animals received antibiotic (0.9 mg/kg enrofloxacine, s.c.) dissolved in 0.9% normal saline (20 ml/kg, s.c.) immediately after surgery and every 12 h for 3 d.
Vagus nerve stimulation (VNS)
Cervical VNS was performed as we previously described (15). Briefly, a small incision was made to explore and identify the right cervical vagus nerve to perform surgical vagotomy, 48 h prior to the experimental procedure. Platinum electrodes were placed across the vagal trunk for electrical stimulation. The platinum electrode was connected to the stimulation device (STM 150; Biopac Systems, Goleta, CA, USA) controlled by the AcqKnowledge software (Biopac Systems). Vagus stimulation was applied for 10 min at 5 V. Control animals underwent sham surgery.
Splenocytes
Spleens were mechanically disrupted with a 100-μm pore cell strainer (BD Falcon; Becton Dickinson, Bedford, MA, USA), erythrocytes were lysed in a lyse solution (Gentra Systems, Minneapolis, MN, USA) for 10 min, and intact cells were washed twice in PBS. Splenocytes were incubated with different dosage of epinephrine or norepinephrine from Sigma for 30 min before giving LPS (100 ng/ml, E. coli 0111:B4; Sigma). Cytokines were measured in the conditioned supernatant by ELISA.
Isolation of lymphocyte populations
Suppressor lymphocytes were isolated following the manufacturer's guidelines. Pan T cells and CD4+CD25+ suppressor T-cell isolation kits were obtained from Miltenyi Biotec (Auburn, CA, USA).
Lymphocyte transfer
CD3+CD4+CD25+ or CD3+CD4+CD25− lymphocytes were resuspended in 0.9% sodium chloride solution, and 1.5 × 106 cells were injected intraperitoneally at 24 h before the experimental challenge.
Cytokine analyses
Blood was collected at the indicated time points, allowed to clot for 2 h at room temperature, and centrifuged for 15 min at 2000 g. TNF concentration was analyzed by TNF ELISA kit (eBioscience, San Diego, CA, USA) following the manufacturer's instructions. TNF was analyzed in the serum at 90 min and at 3 h in the conditioned medium after LPS challenge. HMGB1 was analyzed as described previously in Wang et al. (22). HMGB1 assay was performed by Western blot using rabbit anti-mouse HMGB1 polyclonal antibody (0.4 μg/ml; Ab 18256; Abcam, Cambridge, MA, USA). Blood catecholamines were determined by ELISA (Rocky Mountain Diagnostics, Colorado Springs, CO, USA), as described previously (27).
Statistical analyses
All data in the figures and text are expressed as means ± sd. Statistical analyses were performed using 1-way ANOVA with multiple pairwise comparisons with the Bonferroni's adjustment for multiple hypothesis testing. Normality and homogeneity of variance were confirmed. ANOVA was used to compare all treatments and specific pair-wise comparisons as stated in the experiments. The Mann-Whitney U test (a subclass of Student's t test) was used to compare mean values between two experimental groups. Statistical analyses of survival were determined using the log-rank test. Values of P < 0.05 were considered statistically significant.
RESULTS
Norepinephrine inhibits cytokine production in splenocytes via the β2-adrenoceptors
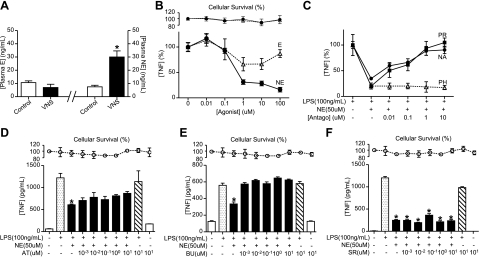
Our previous studies indicated that the vagus nerve controls systemic inflammation in sepsis through a mechanism mediated by the splenic nerve (27). First, we analyzed the induction of catecholamines by the vagus nerve. VNS increases the levels of norepinephrine but not of epinephrine (Fig. 1A). The anti-inflammatory potential of both catecholamines was analyzed in primary cultures of splenocytes (Fig. 1B). Norepinephrine is significantly more efficient than epinephrine at inhibiting LPS-induced TNF production in primary culture of splenocytes. Norepinephrine inhibits LPS-induced TNF production with an EC50 ∼ 0.7 μM. Trypan blue exclusion studies and MTT assays indicate that the inhibition of TNF production is not due to a cellular cytotoxicity. The receptors mediating the anti-inflammatory potential of norepinephrine were analyzed by using the classic antagonists (Fig. 1C). The anti-inflammatory potential of norepinephrine is prevented by β-blockers (propranolol and nadolol) but not by the α-antagonist phentolamine. Specific β-receptors were analyzed by using selective antagonists for β1 (atenolol, betaxolol)-, β2 (butoxamine, ICI 118,551)-, or β3 (SR59230A)-adrenoceptors (29–33). Neither β1- nor β3-blockers prevented the anti-inflammatory potential of norepinephrine in splenocytes (Fig. 1D, F). However, β2-blockers efficiently inhibited the anti-inflammatory potential of norepinephrine in primary culture of splenocytes (Fig. 1E). Together, these results suggest that the anti-inflammatory potential of norepinephrine in splenocytes is mediated by β2-adrenoceptors, and thus this receptor may contribute to the anti-inflammatory potential of the vagus nerve in sepsis.
Figure 1.
Norepinephrine inhibits cytokine production in splenocytes via the β2-adrenoceptors. A) Adult male mice underwent sham surgery (control) or VNS. Epinephrine (E) and norepinephrine (NE) plasma levels were analyzed by ELISA at 15 min after stimulation. *P < 0.01 vs. control; Mann-Whitney U test (n=4/group). B) Primary cultures of splenocytes were treated with LPS (100 ng/ml) and E or NE. C) Primary cultures of splenocytes were treated with β-blockers, propranolol (PR) and nadolol (NA), or α-blocker, phentolamine (PH), 30 min prior to LPS and NE. D–F) Primary cultures of splenocytes were treated with β1-blocker, atenolol (AT; D), β2-blocker, butoxamine (BU; E), or β3-blocker, SR59230A (SR; F). In all the experiments, blockers were administered 30 min prior to LPS and NE, and TNF concentrations were analyzed in the conditioned medium at 3 h after treatment. Top panels represent cell survival as determined by MTT assay. *P < 0.01; 1-way ANOVA with Bonferroni's corrections (n=4).
Vagus nerve inhibits serum TNF levels in endotoxemia via the β2-adrenoceptors
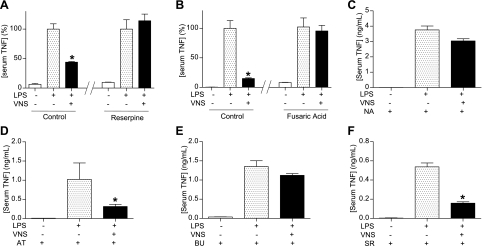
The contribution of norepinephrine to the anti-inflammatory potential of the vagus nerve was analyzed by using specific inhibitors. Reserpine is a classical inhibitor of the vesicular monoamine transporter that inhibits the release of norepinephrine from peripheral sympathetic nerve endings. Fusaric acid inhibits the production of norepinephrine by targeting the dopamine β-hydroxylase. Both reserpine and fusaric acid prevented the anti-inflammatory potential of the vagus nerve to inhibit serum TNF induced in endotoxemia (Fig. 2A, B). The contribution of specific adrenergic receptors was analyzed in vivo by using the characteristic adrenergic blockers. Similar to that described in primary culture of splenocytes, the anti-inflammatory potential of the vagus nerve was prevented by the β-blocker nadolol (Fig. 2C). Specific β-receptors were analyzed by using the selective β-blockers. Neither the β1-blocker atenolol nor the β3-blocker SR59230A prevented the anti-inflammatory potential of the vagus nerve (Fig. 2D, F). However, the β2-blocker butoxamine efficiently inhibited the anti-inflammatory potential of the vagus nerve (Fig. 2E). Together, these results suggest that the anti-inflammatory potential of the vagus nerve is mediated by β2-adrenoceptors.
Figure 2.
The vagus nerve inhibits systemic inflammation via β2-adrenoceptors. Adult male mice were treated with reserpine (5 mg/kg i.p.; A); fusaric acid (40 mg/kg i.p.; B); β-blocker, nadolol (NA; 15 mg/kg i.p.; C); β1-blocker, atenolol (AT; 15 mg/kg i.p.; D); β2-blocker, butoxamine (BU; 15 mg/kg i.p.; E); or β3-blocker, SR59230A (SR; 15 mg/kg i.p.; F), 30 min prior to VNS or sham surgery. Sham surgery or VNS was performed during 10 min before the LPS challenge. *P < 0.01 vs. LPS; 1-way ANOVA with Bonferroni's corrections (n=4).
The anti-inflammatory potential of the vagus nerve is mediated by the β2-adrenoceptors of CD3+CD4+CD25− lymphocytes
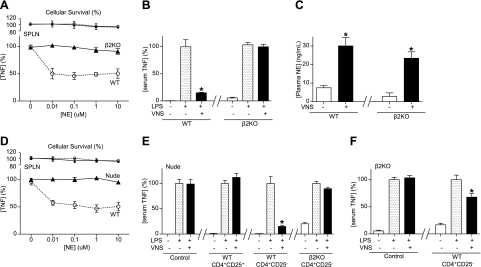
The contribution of the β2-adrenoceptors to the anti-inflammatory potential of norepinephrine was also confirmed in primary culture of splenocytes from β2-knockout mice. Norepinephrine inhibited LPS-induced TNF production in the wild-type mice but not in the splenocytes from the β2-knockout mice (Fig. 3A). Trypan blue exclusion studies and MTT assays indicated that the inhibition of TNF production was not secondary to the cellular cytotoxicity. In vivo, VNS significantly attenuates LPS-induced serum TNF levels in the control wild-type but not in the β2-knockout mice (Fig. 3B). This effect is not due to a defect of the vagus nerve to activate norepinephrine release from the splenic nerve. VNS induces statistically similar levels of norepinephrine in both control wild-type and β2-knockout mice (Fig. 3C). Because our previous studies suggested that nude mice may be more susceptible to sepsis, we analyzed whether norepinephrine inhibits TNF production in primary culture of splenocytes from lymphocyte-deficient nude mice. Norepinephrine inhibited TNF production in primary culture of splenocytes from wild-type mice but not in those from nude mice (Fig. 3D). This cellular mechanism was also confirmed in vivo by performing VNS in nude mice. VNS inhibited serum TNF levels in wild-type but not in nude mice (Fig. 3E). The contribution of suppressor lymphocytes was analyzed by transferring typical suppressor (CD3+CD4+CD25+) lymphocytes and the respective counterpart control CD3+CD4+CD25− lymphocytes into nude mice. The transfer of typical suppressor CD3+CD4+CD25+ lymphocytes into nude mice failed to reestablish the anti-inflammatory potential of the vagus nerve (Fig. 3E). However, the transfer of wild-type CD3+CD4+CD25− lymphocytes into nude mice reestablished the anti-inflammatory potential of the vagus nerve (Fig. 3E). FACS studies confirmed that our CD3+CD4+CD25− lymphocytes were neither CD25+ nor Foxp3+ with >98% purity. These effects were specifically mediated by the β2-adrenoceptors, because the transfer of CD3+CD4+CD25− lymphocytes from β2-knockout mice failed to reestablish the anti-inflammatory potential of the vagus nerve. Conversely, the implications of these cells were also confirmed in the β2-knockout mice. The transfer of wild-type CD3+CD4+CD25− lymphocytes reestablished the anti-inflammatory potential of the vagus nerve in β2-knockout mice (Fig. 3F). Together, these results suggest that the anti-inflammatory potential of the vagus nerve is mediated by the β2-adrenoceptors of CD3+CD4+CD25− lymphocytes. These results also suggest that selective β2-agonists may mimic the anti-inflammatory potential of the vagus nerve and provide a pharmacologic advantage against sepsis.
Figure 3.
Wild-type CD3+CD4+CD25− lymphocytes reestablish neuromodulation in β2-knockout mice. A) Primary cultures of splenocytes (SPLN) from wild-type (WT) or β2-knockout (β2KO) mice were treated with norepinephrine (NE), 30 min before LPS challenge (100 ng/ml). Top panels represent cell survival as determined by MTT assay. B) WT or β2KO mice underwent sham surgery or VNS during 10 min before endotoxemia (LPS; 6 mg/kg i.p). Serum TNF levels were analyzed by ELISA at 90 min after LPS. C) WT or β2KO mice underwent sham surgery or VNS, and plasma levels of NE were analyzed at 15 min after stimulation. *P < 0.01 vs. control; Mann-Whitney U test (n=4/group). D) Primary SPLN cultures from WT or lymphocyte-deficient nude (Nude) mice were treated with NE, 30 min before LPS challenge (100 ng/ml). TNF concentrations were analyzed in the conditioned medium at 3 h after the LPS stimulation. Top panels represent cell survival as determined by MTT assay. E, F) Lymphocyte-deficient nude mice (E) or β2KO mice (F) received vehicle (control), classical suppressor CD3+CD4+CD25+, or CD3+CD4+CD25− splenocytes from WT or β2KO mice. Animals underwent sham surgery or VNS during 10 min before endotoxemia. Serum TNF levels were analyzed by ELISA at 90 min post-LPS. *P < 0.01 vs. LPS; 1-way ANOVA with Bonferroni's corrections (n=4).
β2-Adrenoceptors in regulatory lymphocytes control systemic inflammation and hemodynamics, and “rescue” mice from established polymicrobial sepsis
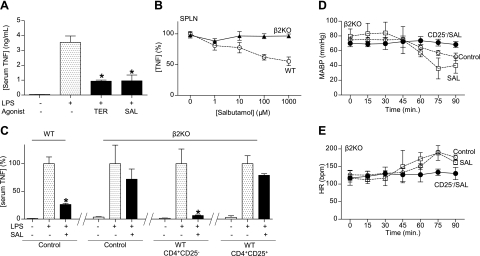
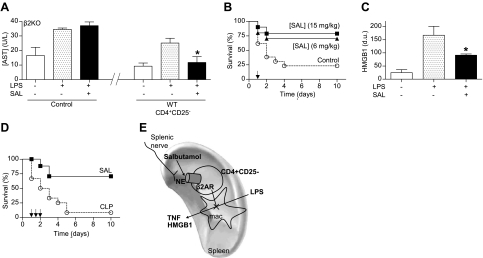
First, we analyzed the anti-inflammatory potential of the most characteristic β2-agonists, including salbutamol, terbutaline, and formoterol, in splenocytes. All agonists inhibited LPS-induced TNF production in a concentration-dependent manner (data not shown). Formoterol was associated with a significant cytotoxicity at high concentrations. The anti-inflammatory potential of both β2-agonists was also confirmed in vivo during experimental endotoxemia. Again, both salbutamol and terbutaline had a similar efficiency at inhibiting serum TNF levels by over 75% in endotoxemia (Fig. 4A). Salbutamol and terbutaline have statistically similar effects, but salbutamol has been widely described in the literature. The specificity of salbutamol was confirmed in primary culture of splenocytes from wild-type and β2-knockout mice (Fig. 4B). The specificity of salbutamol was also confirmed in vivo. Salbutamol inhibited serum TNF levels in wild-type but not in the β2-knockout mice. Furthermore, the transfer of CD3+CD4+CD25− lymphocytes from wild-type mice into β2-knockout mice reestablished the anti-inflammatory potential of salbutamol in β2-knockout mice (Fig. 4C). Similar to that described for the vagus nerve, typical suppressor CD3+CD4+CD25+ lymphocytes failed to reestablish the anti-inflammatory potential of salbutamol in β2-knockout mice (Fig. 4C). Since catecholamines are used to treat the hemodynamic consequences of septic shock (31), we wonder whether those effects are specifically mediated by the β2-adrenoceptors of the CD3+CD4+CD25− lymphocytes or whether salbutamol requires other β2-adrenoceptors in the cardiovascular system. β2-Knockout mice treated with either vehicle or salbutamol manifested the typical hypotensive and tachycardic hemodynamic responses to endotoxemia. Significantly, the transfer of CD3+CD4+ CD25− lymphocytes allowed the β2AR-agonist to prevent these hemodynamic responses and to preserve normal physiological hemodynamics (Fig. 4D, E). In addition, in this endotoxemia model, the β2-adrenoceptors in CD3+CD4+CD25− lymphocytes also prevented organ injury, as determined by aspartate transaminase (Fig. 5A). However, while salbutamol failed to prevent serum increases of aspartate transaminase in β2-knockout mice, the transfer of CD3+ CD4+CD25− lymphocytes restored the ability of the β2AR-agonist to prevent the increase of aspartate transaminase (Fig. 5A). In addition to organ damage, treatment with β2AR-agonist protected the animals from lethal endotoxemia and improved survival by >65%, even when treatment was started at 24 h after the endotoxin challenge (Fig. 5B). Survival was analyzed for 2 wk, and no late deaths were observed, suggesting that β2-agonist induces a lasting protection and does not merely delay the onset of death. In addition to TNF, we also analyzed “late” inflammatory mediators, because their inhibition can provide a wider therapeutic time window for the treatment of sepsis. Since bacterial endotoxin activates macrophages to secrete HMGB1 into the extracellular milieu at ∼20 h after stimulation (34–36), we analyzed whether salbutamol inhibits HMGB1 secretion. β2AR-agonist inhibited serum HMGB1 levels, even when the treatment was started at 24 h after the endotoxin challenge (Fig. 5C). In these conditions, treatment was started long after the TNF response; thus, the inhibition of serum HMGB1 levels was not secondary to an early inhibition of TNF production. The effects of salbutamol were also analyzed in polymicrobial sepsis induced by CLP, a more clinically relevant experimental model of sepsis. In this model, polymicrobial peritonitis is induced by the cecal puncture, and necrotic tissue is induced by the cecal ligation. Treatment with salbutamol started at 24 h after the septic challenge rescued the animals from CLP and improved survival by >50% (Fig. 5D). Around 10–15% of the animals started to die at this time; thus, this was the latest time point possible to analyze serum cytokines, while all of the animals remained alive. β2AR-agonist also attenuated the clinical manifestations of sepsis, including lethargy, diarrhea, piloerection, and huddling. Animals were monitored for 2 wk, and no late deaths were observed, indicating that salbutamol conferred a lasting protection, reversing the pathogenesis of sepsis in a clinically realistic time frame. Together, these results indicated that norepinephrine, released by the splenic nerve, activated the β2-adrenoceptors of the CD3+CD4+CD25− lymphocytes (Fig. 5E). Of note, our results mimic the histological organization of the spleen (37); noradrenergic nerve fibers are mainly located in the periarterial lymphoid sheath (PALS), where they make synaptic-like connections with T lymphocytes located in the vicinity of B cells and macrophages (38–40). From a pharmacological perspective, the activation of the β2-adrenoceptors of the CD3+CD4+CD25− lymphocytes can prevent systemic inflammation, organ injury, and mortality in experimental sepsis.
Figure 4.
Wild-type CD3+CD4+CD25− lymphocytes reestablish the hemodynamics and anti-inflammatory potential of β2-agonist in β2-knockout mice. A) Adult male wild-type mice were treated with specific β2-agonists terbutaline (TER) or salbutamol (SAL), 30 min before the LPS challenge (LPS; 6 mg/kg i.p.). B) Primary culture of splenocytes from wild-type (WT) or β2-knockout (β2KO) mice were treated with SAL, 30 min before LPS challenge (100 ng/ml). TNF concentration was analyzed by ELISA in the conditioned medium at 3 h poststimulation. C) Adult male WT or β2KO mice were treated with vehicle (control), CD3+CD4+CD25− or CD3+CD4+CD25+ lymphocytes from WT mice. Mice were treated with β2-agonist SAL (15 mg/kg i.p.), 30 min prior the endotoxemia. Serum TNF levels were analyzed by ELISA at 90 min after LPS. *P < 0.01 vs. LPS; 1-way ANOVA with Bonferroni's corrections (n=4). D, E) Adult β2KO mice received vehicle (control) or CD3+CD4+CD25− (CD25−) lymphocytes from WT mice, and then treated with β2-agonist SAL (15 mg/kg i.p.). Mean arterial blood pressure (MABP; D) and heart rate (HR; E) were recorded.
Figure 5.
β2-Agonist prevents organ damage and rescues mice from established polymicrobial peritonitis. A) Adult β2-knockout (β2KO) mice were given vehicle (control), or CD3+CD4+CD25− lymphocytes from wild-type (WT) mice with vehicle or β2-agonist salbutamol (SAL; 15 mg/kg i.p.). Serum aspartate transaminase (AST) levels were analyzed at 90 min after LPS. *P < 0.01 vs. LPS; 1-way ANOVA with Bonferroni's corrections (n=4). B) Adult male mice were treated with β2-agonist SAL. Survival was represented in a Kaplan-Meier analysis (n=20/group; P<0.01, survival log-rank test vs. control). Arrow represents the single dose of vehicle (control) or SAL (6 or 15 mg/kg i.p.) given 24 h after LPS challenge. C) Serum HMGB1 levels were analyzed by Western blot at 44 h after LPS challenge. Graph represents densitometric units (d.u.) from scanning of the film. *P < 0.01 vs. LPS; 1-way ANOVA with Bonferroni's corrections (n=5/group). D) Arrows represent 3 doses of vehicle (control) or SAL (15 mg/kg i.p.) started 24 h after CLP, and given every 12 h. Survival was analyzed and represented in a Kaplan-Meier analysis (n=15/group; P<0.01, survival log-rank test vs. control). E) VNS leads to the activation of the splenic nerve and subsequent release of norepinephrine (NE) in the spleen. Both NE (splenic nerve) and β2AR-agonists (salbutamol) inhibit cytokine production in the spleen and systemic inflammation in experimental sepsis through a mechanism specifically mediated by the β2-adrenoceptors of CD3+CD4+CD25− lymphocytes. This cellular mechanism resembles the innervation of the periarterial lymphoid sheath (PALS), where the splenic nerve makes synaptic-like connections with T lymphocytes located in the vicinity of macrophages (37).
DISCUSSION
Physiological anti-inflammatory mechanisms represent efficient systems selected by evolution to preserve immune homeostasis. Although the sympathetic system has been studied for years, only recently has the anti-inflammatory potential of the parasympathetic system been demonstrated (13, 14). Our studies indicated that the parasympathetic vagus nerve controls systemic inflammation in sepsis by inhibiting cytokine production in the spleen (15). This is important because overzealous cytokine production in the spleen can be more dangerous than the original insult, leading to deleterious inflammatory responses and lethal organ failure both in sepsis and after trauma (15). This potentially dangerous splenic cytokine response is consistent with a recent clinical study showing that patients with severe trauma who underwent splenectomy had significantly shorter hospital length of stay and better secondary outcomes than patients who were managed nonoperatively or with splenorrhaphy (41). Despite its clinical implications, the multidisciplinary physiological and cellular mechanisms modulating innate immune responses to infection and trauma remain uncertain. Our study indicates that the vagus nerve inhibits systemic inflammation in sepsis through a mechanism specifically mediated by the β2-adrenoceptors in CD3+CD4+CD25− splenic lymphocytes. These results concur with previous studies suggesting that the anti-inflammatory potential of the vagus nerve can be mediated by the splenic nerve (27, 42). The present study is now the molecular evidence of a physiological connection between the parasympathetic and sympathetic systems that function together to control innate immune responses.
Either pharmacological or genetic inhibition of the β2-adrenoceptors abrogates the anti-inflammatory potential of the vagus nerve to control systemic inflammation in sepsis. It is significant that one specific member of the adrenoceptors, which are classically recognized as typical “sympathetic” receptors, is an essential component for the anti-inflammatory potential of the parasympathetic vagus nerve. These results represent a conceptual advantage, because the nervous system is classically described as being composed of sympathetic and parasympathetic systems that act in opposition to each other to maintain physiological homeostasis. However, these current results suggest that both systems work together to restrain systemic inflammation in life-threatening conditions such as sepsis, with both systems converging on the activation of the β2-adrenoceptors. Consequently, this is the first known adrenergic receptor documented to be involved in the anti-inflammatory potential of the parasympathetic system. The specificity of this mechanism is also quite significant, taking into consideration the diversity of the family of adrenoceptors. Adrenergic receptors are members of the 7-membrane-spanning family of G-protein-coupled receptors, comprise 9 subtypes, including 6 α (α1A, α1B, α1D, α2A, α2B, α2C) and 3 β (β1-AR, β2-AR, β3-AR) adrenoceptors (43, 44). This variety of receptors appears to account for the complex biological activity of catecholamines in different cell lines (45–47). We also confirmed the role of the β2-adrenoceptors in the anti-inflammatory potential of the vagus nerve by using the β2-knockout mice. These mice lack some typical responses to β-agonists, such as isoproterenol, but they have a normal phenotype and physiology, including normal blood pressure, respiration, and heart rate (28). These results are similar to those reported for the α7 nicotinic acetylcholine (ACh) receptors. α7nACh receptors also contribute to the anti-inflammatory potential of the vagus nerve, and the α7nAChR-knockout mice also have an apparent normal phenotype and physiology (27, 48). Our previous studies suggested that ACh released by the vagus nerve in the celiac mesenteric ganglia activates postsynaptic α7nAChR of the splenic nerve leading to the release of norepinephrine in the spleen (27). Although previous studies have analyzed the potential of catecholamines in specific cell lines, our results in vivo indicate that both the anti-inflammatory potential of the vagus nerve and norepinephrine involve two specific cellular processes: TNF-producing cells representing splenic macrophages (42); and CD3+CD4+CD25− splenic lymphocytes, which reestablish the anti-inflammatory potential of the vagus nerve in nude and β2-knockout mice. It is noteworthy that these results appear to follow the innervations and histological organization of the spleen. Noradrenergic nerves distribute with the vascular system and innervate the periarteriolar lymphatic sheath, the marginal sinus, and the parafollicular zone (37). These innervations are particularly numerous in the periarteriolar lymphoid sheath, a predominant T-lymphocyte region, where nerves make synaptic-like connections with T lymphocytes located in the vicinity of the marginal zone and metallophilic macrophages (38). In contrast, these innervations do not appear to establish a direct synaptic connection with splenic macrophages. In this sense, our results appear to suggest a new set of regulatory lymphocytes. Previous studies described the classic T-suppressor lymphocytes as CD3+CD4+ lymphocytes characterized by the expression of IL-2Rα (CD25+) and the transcription factor Foxp3. These suppressor lymphocytes do not require any activation, and they constitutively inhibit cytokine production from macrophages (49). Yet, we found that classic T-suppressor lymphocytes failed to reestablish the anti-inflammatory potential of the vagus nerve in nude mice. Unlike these classic T-suppressor lymphocytes (49, 50), our results indicate that neuroimmunomodulation (both the parasympathetic vagus nerve and the sympathetic splenic nerve) requires a different set of regulatory lymphocytes, which do not express IL-2Rα (CD25) or Foxp3, and they are not constitutive suppressor cells. In normal conditions, these CD25− regulatory lymphocytes do not affect cytokine production in splenocytes by themselves. However, they reestablished the anti-inflammatory potential of the vagus nerve in nude mice, suggesting that norepinephrine activates these regulatory lymphocytes to inhibit the splenocytes from the nude mice. This mechanism is specific because the typical suppressor CD3+CD4+CD25+ lymphocytes fail to reestablish the anti-inflammatory potential of the vagus nerve. Indeed, the transfer of CD3+CD4+CD25− lymphocytes is sufficient to reestablish anti-inflammatory neuromodulation in nude mice, even in the absence of the classic CD3+CD4+CD25+ suppressor T cells.
Our results indicate that specific activation of the β2-adrenoceptors is sufficient to inhibit cytokine production in splenocytes, prevent systemic inflammation, and improve survival in lethal endotoxemia. Previous studies indicate that β-agonists can induce proinflammatory or anti-inflammatory effects depending on the cell line (47, 51–53). Our in vivo results concur with clinical studies, indicating that inhibitory β2-gene polymorphism is associated with increased organ dysfunction and higher mortality in septic shock (54). The AA genotype of ADRB2 rs1042717, identifying homozygotes for the CysGlyGln haplotype, makes the receptor more susceptible to desensitization and, therefore, less responsive to β2-agonists (54). Since recent studies suggest that catecholamines can modulate cardiovascular responses to LPS (31), we also wondered whether the specific β2-adrenoceptors of the splenic regulatory lymphocytes could control hemodynamic responses during septic shock. Our results indicate that the β2-adrenoceptors of the splenic CD3+CD4+CD25− regulatory lymphocytes are necessary and sufficient to control hemodynamics responses. Similar to that described in the patients with the AA genotype, salbutamol fails to inhibit serum TNF levels in the β2-knockout mice, and it thereby fails to prevent septic shock. The transfer of wild-type splenic CD3+CD4+CD25− lymphocytes is sufficient to reestablish the anti-inflammatory potential of salbutamol and to prevent septic shock in the β2-knockout mice, even in the absence of β2-adrenoceptors in the cardiovascular system or any other organ or system.
Our study suggests that the therapeutic potential of the β2-agonists is due to their potential to inhibit the production of inflammatory and cardiodepressant factors. The use of several alternative experimental models provides additional strong evidence for the modulatory role of the vagus nerve and the implications of the β2-agonists. In addition to septic shock and endotoxemia, our results also indicate that salbutamol can provide a therapeutic potential in polymicrobial peritonitis. In addition to TNF, our results indicate that β2-agonists also inhibit other inflammatory and cardiodepressant factors, such as high mobility group box (HMGB1). HMGB1 is a characteristic inflammatory factor contributing to severe sepsis, epithelial cell permeability, abrupt cardiac standstill, and cardiopulmonary collapse (3, 35, 36, 55). HMGB1 represents a novel family of inflammatory cytokines composed of intracellular proteins that, when present in the extracellular milieu, are recognized by the innate immune system as necrotic markers or damage-associated molecular pattern (DAMP; refs. 35, 55). In this sense, HMGB1 is a characteristic late mediator of severe sepsis that correlates with tissue damage, and its expression mimics the late mortality kinetic (34). A remarkable implication of these results is that β2-agonists not only prevent, but can rescue mice from lethal sepsis, even when the treatment is started long after the production of early inflammatory and cardiodepressant factors, such as TNF. This conclusion is based on the observation that β2-agonist administration improves survival in polymicrobial sepsis, even when the treatment is started 24 h after the challenge. Since ∼15% of the animals normally die at 24–30 h, this is the latest time point possible to start a treatment or to analyze the pathogenesis, while all the animals remain alive. By comparison with other targets, such as the administration of anti-TNF antibodies after cecal perforation increased mortality (56), while antimacrophage migration inhibitory factor antibodies are ineffective if administered at 8 h after the induction of peritonitis (57). The therapeutic window for β2-agonists is also significantly wider than for lysophosphatidylcholine, which is only effective when treatment occurs within 10 h after cecal puncture. These results open the potential of harnessing β2-agonists for the treatment of sepsis, even in a clinically realistic time frame.
Acknowledgments
The authors thank Dr. Brian Kobilka (Stanford University, Stanford, CA, USA) for his collaboration with the β2KO mice.
G.V. was supported by the Hungarian Rosztoczy Foundation, A.K. was supported by the Brazilian Fundação ao Amparo à Pesquisa do Estado de São Paulo (FAPESP), and M.R.T. was supported by the Mexican National Council for Science and Technology (CONACyT). L.U. is supported by the faculty program of the Department of Surgery of the New Jersey Medical School, and the U.S. National Institutes of Health (RO1-GM084125).
REFERENCES
- 1. Nathan C. (2002) Points of control in inflammation. Nature 420, 846–852 [DOI] [PubMed] [Google Scholar]
- 2. Ulloa L. (2005) The vagus nerve and the nicotinic anti-inflammatory pathway. Nat. Rev. 4, 673–684 [DOI] [PubMed] [Google Scholar]
- 3. Ulloa L., Tracey K. J. (2005) The “cytokine profile”: a code for sepsis. Trends Mol. Med. 11, 56–63 [DOI] [PubMed] [Google Scholar]
- 4. Sands K. E., Bates D. W., Lanken P. N., Graman P. S., Hibberd P. L., Kahn K. L., Parsonnet J., Panzer R., Orav E. J., Snydman D. R. (1997) Epidemiology of sepsis syndrome in 8 academic medical centers. Academic Medical Center Consortium Sepsis Project Working Group. JAMA 278, 234–240 [PubMed] [Google Scholar]
- 5. Angus D. C. (2007) Caring for the critically ill patient: challenges and opportunities. JAMA 298, 456–458 [DOI] [PubMed] [Google Scholar]
- 6. Angus D. C., Linde-Zwirble W. T., Lidicker J., Clermont G., Carcillo J., Pinsky M. R. (2001) Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit. Care Med. 29, 1303–1310 [DOI] [PubMed] [Google Scholar]
- 7. Tracey K. J., Fong Y., Hesse D. G., Manogue K. R., Lee A. T., Kuo G. C., Lowry S. F., Cerami A. (1987) Anti-cachectin/TNF monoclonal antibodies prevent septic shock during lethal bacteraemia. Nature 330, 662–664 [DOI] [PubMed] [Google Scholar]
- 8. Riedemann N. C., Guo R. F., Ward P. A. (2003) Novel strategies for the treatment of sepsis. Nat. Med. 9, 517–524 [DOI] [PubMed] [Google Scholar]
- 9. Ulloa L., Brunner M., Ramos L., Deitch E. A. (2009) Scientific and clinical challenges in sepsis. Curr. Pharm. Des. 15, 1918–1935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Abraham E., Laterre P. F., Garbino J., Pingleton S., Butler T., Dugernier T., Margolis B., Kudsk K., Zimmerli W., Anderson P., Reynaert M., Lew D., Lesslauer W., Passe S., Cooper P., Burdeska A., Modi M., Leighton A., Salgo M., Van der Auwera P. (2001) Lenercept (p55 tumor necrosis factor receptor fusion protein) in severe sepsis and early septic shock: a randomized, double-blind, placebo-controlled, multicenter phase III trial with 1,342 patients. Crit. Care Med. 29, 503–510 [DOI] [PubMed] [Google Scholar]
- 11. Steinman L. (2004) Elaborate interactions between the immune and nervous systems. Nat. Immunol. 5, 575–581 [DOI] [PubMed] [Google Scholar]
- 12. Sternberg E. M. (2006) Neural regulation of innate immunity: a coordinated nonspecific host response to pathogens. Nat. Rev. Immunol. 6, 318–328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tracey K. J. (2009) Reflex control of immunity. Nat. Rev. Immunol. 9, 418–428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tracey K. J. (2010) Understanding immunity requires more than immunology. Nat. Immunol. 11, 561–564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Huston J. M., Ochani M., Rosas-Ballina M., Liao H., Ochani K., Pavlov V. A., Gallowitsch-Puerta M., Ashok M., Czura C. J., Foxwell B., Tracey K. J., Ulloa L. (2006) Splenectomy inactivates the cholinergic antiinflammatory pathway during lethal endotoxemia and polymicrobial sepsis. J. Exp. Med. 203, 1623–1628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bernik T. R., Friedman S. G., Ochani M., DiRaimo R., Susarla S., Czura C. J., Tracey K. J. (2002) Cholinergic antiinflammatory pathway inhibition of tumor necrosis factor during ischemia reperfusion. J. Vasc. Surg. 36, 1231–1236 [DOI] [PubMed] [Google Scholar]
- 17. Altavilla D., Guarini S., Bitto A., Mioni C., Giuliani D., Bigiani A., Squadrito G., Minutoli L., Venuti F. S., Messineo F., De Meo V., Bazzani C., Squadrito F. (2006) Activation of the cholinergic anti-inflammatory pathway reduces NF-kappab activation, blunts TNF-alpha production, and protects againts splanchic artery occlusion shock. Shock 25, 500–506 [DOI] [PubMed] [Google Scholar]
- 18. Cai B., Chen F., Ji Y., Kiss L., DeJonge W., Szabo C., Deitch E., Ulloa L. (2009) Alpha7 cholinergic-agonist prevents systemic inflammation and improves survival during resuscitation. J. Cell. Mol. Med. 13, 3774–3785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Van Westerloo D. J., Giebelen I. A., Florquin S., Bruno M. J., Larosa G. J., Ulloa L., Tracey K. J., van der Poll T. (2006) The vagus nerve and nicotinic receptors modulate experimental pancreatitis severity in mice. Gastroenterology 130, 1822–1830 [DOI] [PubMed] [Google Scholar]
- 20. Pullan R. D., Rhodes J., Ganesh S., Mani V., Morris J. S., Williams G. T., Newcombe R. G., Russell M. A., Feyerabend C., Thomas G. A., Sawe U. (1994) Transdermal nicotine for active ulcerative colitis. N. Engl. J. Med. 330, 811–815 [DOI] [PubMed] [Google Scholar]
- 21. Borovikova L. V., Ivanova S., Zhang M., Yang H., Botchkina G. I., Watkins L. R., Wang H., Abumrad N., Eaton J. W., Tracey K. J. (2000) Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature 405, 458–462 [DOI] [PubMed] [Google Scholar]
- 22. Wang H., Liao H., Ochani M., Justiniani M., Lin X., Yang L., Al-Abed Y., Wang H., Metz C., Miller E. J., Tracey K. J., Ulloa L. (2004) Cholinergic agonists inhibit HMGB1 release and improve survival in experimental sepsis. Nat. Med. 10, 1216–1221 [DOI] [PubMed] [Google Scholar]
- 23. Van Westerloo D. J., Giebelen I. A., Florquin S., Daalhuisen J., Bruno M. J., de Vos A. F., Tracey K. J., van der Poll T. (2005) The cholinergic anti-inflammatory pathway regulates the host response during septic peritonitis. J. Infect. Dis. 191, 2138–2148 [DOI] [PubMed] [Google Scholar]
- 24. De Jonge W. J., Ulloa L. (2007) The alpha7 nicotinic acetylcholine receptor as a pharmacological target for inflammation. Brit. J. Pharmacol. 151, 915–929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bernik T. R., Friedman S. G., Ochani M., DiRaimo R., Ulloa L., Yang H., Sudan S., Czura C. J., Ivanova S. M., Tracey K. J. (2002) Pharmacological stimulation of the cholinergic antiinflammatory pathway. J. Exp. Med. 195, 781–788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Flood J. F., Smith G. E., Morley J. E. (1987) Modulation of memory processing by cholecystokinin: dependence on the vagus nerve. Science 236, 832–834 [DOI] [PubMed] [Google Scholar]
- 27. Vida G., Peña G., Deitch E. A., Ulloa L. (2011) Alpha7-nicotinic receptor mediates vagal induction of splenic norepinephrine. J. Immunol. 186, 4340–4346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chruscinski A. J., Rohrer D. K., Schauble E., Desai K. H., Bernstein D., Kobilka B. K. (1999) Targeted disruption of the beta2 adrenergic receptor gene. J. Biol. Chem. 274, 16694–16700 [DOI] [PubMed] [Google Scholar]
- 29. Oberbeck R., Kobbe P. (2009) Beta-adrenergic antagonists: indications and potential immunomodulatory side effects in the critically ill. Curr. Med. Chem. 16, 1082–1090 [DOI] [PubMed] [Google Scholar]
- 30. Crassous P. A., Denis C., Paris H., Senard J. M. (2007) Interest of alpha2-adrenergic agonists and antagonists in clinical practice: background, facts and perspectives. Curr. Top. Med. Chem. 7, 187–194 [DOI] [PubMed] [Google Scholar]
- 31. De Montmollin E., Aboab J., Mansart A., Annane D. (2009) Bench-to-bedside review: Beta-adrenergic modulation in sepsis. [Online] Crit. Care 13, 230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Javed U., Deedwania P. C. (2009) Beta-adrenergic blockers for chronic heart failure. Cardiol. Rev. 17, 287–292 [DOI] [PubMed] [Google Scholar]
- 33. Ortega V. E., Peters S. P. (2010) Beta-2 adrenergic agonists: focus on safety and benefits versus risks. Curr. Opin. Pharmacol. 10, 246–253 [DOI] [PubMed] [Google Scholar]
- 34. Wang H., Bloom O., Zhang M., Vishnubhakat J. M., Ombrellino M., Che J., Frazier A., Yang H., Ivanova S., Borovikova L., Manogue K. R., Faist E., Abraham E., Andersson J., Andersson U., Molina P. E., Abumrad N. N., Sama A., Tracey K. J. (1999) HMG-1 as a late mediator of endotoxin lethality in mice. Science 285, 248–251 [DOI] [PubMed] [Google Scholar]
- 35. Lotze M. T., Tracey K. J. (2005) High-mobility group box 1 protein (HMGB1): nuclear weapon in the immune arsenal. Nat. Rev. Immunol. 5, 331–342 [DOI] [PubMed] [Google Scholar]
- 36. Ulloa L., Messmer D. (2006) High-mobility group box 1 (HMGB1) protein: friend and foe. Cytokine Growth Factor Rev. 17, 189–201 [DOI] [PubMed] [Google Scholar]
- 37. Mebius R. E., Kraal G. (2005) Structure and function of the spleen. Nat. Rev. Immunol. 5, 606–616 [DOI] [PubMed] [Google Scholar]
- 38. Felten D. L., Felten S. Y., Bellinger D. L., Carlson S. L., Ackerman K. D., Madden K. S., Olschowki J. A., Livnat S. (1987) Noradrenergic sympathetic neural interactions with the immune system: structure and function. Immunol. Rev. 100, 225–260 [DOI] [PubMed] [Google Scholar]
- 39. Felten S. Y., Madden K. S., Bellinger D. L., Kruszewska B., Moynihan J. A., Felten D. L. (1998) The role of the sympathetic nervous system in the modulation of immune responses. Adv. Pharmacol. 42, 583–587 [DOI] [PubMed] [Google Scholar]
- 40. Straub R. H., Westermann J., Scholmerich J., Falk W. (1998) Dialogue between the CNS and the immune system in lymphoid organs. Immunol. Today 19, 409–413 [DOI] [PubMed] [Google Scholar]
- 41. Crandall M., Shapiro M. B., West M. A. (2009) Does splenectomy protect against immune-mediated complications in blunt trauma patients? Mol. Med. 15, 263–267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Rosas-Ballina M., Ochani M., Parrish W. R., Ochani K., Harris Y. T., Huston J. M., Chavan S., Tracey K. J. (2008) Splenic nerve is required for cholinergic antiinflammatory pathway control of TNF in endotoxemia. Proc. Natl. Acad. Sci. U. S. A. 105, 11008–11013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kobilka B. (1992) Adrenergic receptors as models for G protein-coupled receptors. Annu. Rev. Neurosci. 15, 87–114 [DOI] [PubMed] [Google Scholar]
- 44. Small K. M., McGraw D. W., Liggett S. B. (2003) Pharmacology and physiology of human adrenergic receptor polymorphisms. Annu. Rev. Pharmacol. Toxicol. 43, 381–411 [DOI] [PubMed] [Google Scholar]
- 45. Sanders V. M., Kavelaars A. (2007) Adrenergic regulation of immunity. Phychoneuroimmunology 1, 63–83 [Google Scholar]
- 46. Levine J. D., Coderre T. J., Helms C., Basbaum A. I. (1988) Beta 2-adrenergic mechanisms in experimental arthritis. Proc. Natl. Acad. Sci. U. S. A. 85, 4553–4556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. van der Poll T., Jansen J., Endert E., Sauerwein H. P., van Deventer S. J. (1994) Noradrenaline inhibits lipopolysaccharide-induced tumor necrosis factor and interleukin 6 production in human whole blood. Infect. Immun. 62, 2046–2050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wang H., Yu M., Ochani M., Amella C. A., Tanovic M., Susarla S., Li J. H., Wang H., Yang H., Ulloa L., Al-Abed Y., Czura C. J., Tracey K. J. (2003) Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation. Nature 421, 384–388 [DOI] [PubMed] [Google Scholar]
- 49. Venet F., Chung C. S., Monneret G., Huang X., Horner B., Garber M., Ayala A. (2008) Regulatory T-cell populations in sepsis and trauma. J. Leukocyte Biol. 83, 523–535 [DOI] [PubMed] [Google Scholar]
- 50. O'Mahony C., van der Kleij H., Bienenstock J., Shanahan F., O'Mahony L. (2009) Loss of vagal anti-inflammatory effect: in vivo visualization and adoptive transfer. Am. J. Physiol. Regul. Integr. Comp. Physiol. 297, R1118–R1126 [DOI] [PubMed] [Google Scholar]
- 51. Hasko G., Szabo C., Nemeth Z. H., Salzman A. L., Vizi E. S. (1998) Stimulation of beta-adrenoceptors inhibits endotoxin-induced IL-12 production in normal and IL-10 deficient mice. J. Neuroimmunol. 88, 57–61 [DOI] [PubMed] [Google Scholar]
- 52. Eijkelkamp N., Cobelens P. M., Sanders V. M., Heijnen C. J., Kavelaars A. (2004) Tissue-specific effects of the beta 2-adrenergic agonist salbutamol on LPS-induced IFN-gamma, IL-10 and TGF-beta responses in vivo. J. Neuroimmunol. 150, 3–9 [DOI] [PubMed] [Google Scholar]
- 53. Nakamura A., Imaizumi A., Yanagawa Y., Kohsaka T., Johns E. J. (2004) beta(2)-Adrenoceptor activation attenuates endotoxin-induced acute renal failure. J. Am. Soc. Nephrol. 15, 316–325 [DOI] [PubMed] [Google Scholar]
- 54. Nakada T. A., Russell J. A., Boyd J. H., Aguirre-Hernandez R., Thain K. R., Thair S. A., Nakada E., McConechy M., Walley K. R. (2010) β2-Adrenergic receptor gene polymorphism is associated with mortality in septic shock. Am. J. Respir. Crit. Care. Med. 181, 143–149 [DOI] [PubMed] [Google Scholar]
- 55. Seong S. Y., Matzinger P. (2004) Hydrophobicity: an ancient damage-associated molecular pattern that initiates innate immune responses. Nat. Rev. Immunol. 4, 469–478 [DOI] [PubMed] [Google Scholar]
- 56. Eskandari M. K., Bolgos G., Miller C., Nguyen D. T., DeForge L. E., Remick D. G. (1992) Anti-tumor necrosis factor antibody therapy fails to prevent lethality after cecal ligation and puncture or endotoxemia. J. Immunol. 148, 2724–2730 [PubMed] [Google Scholar]
- 57. Calandra T., Echtenacher B., Roy D. L., Pugin J., Metz C. N., Hultner L., Heumann D., Mannel D., Bucala R., Glauser M. P. (2000) Protection from septic shock by neutralization of macrophage migration inhibitory factor. Nat. Med. 6, 164–170 [DOI] [PubMed] [Google Scholar]