Abstract
Menthol, the cooling agent in peppermint, is added to almost all commercially available cigarettes. Menthol stimulates olfactory sensations, and interacts with transient receptor potential melastatin 8 (TRPM8) ion channels in cold-sensitive sensory neurons, and transient receptor potential ankyrin 1 (TRPA1), an irritant-sensing channel. It is highly controversial whether menthol in cigarette smoke exerts pharmacological actions affecting smoking behavior. Using plethysmography, we investigated the effects of menthol on the respiratory sensory irritation response in mice elicited by smoke irritants (acrolein, acetic acid, and cyclohexanone). Menthol, at a concentration (16 ppm) lower than in smoke of mentholated cigarettes, immediately abolished the irritation response to acrolein, an agonist of TRPA1, as did eucalyptol (460 ppm), another TRPM8 agonist. Menthol's effects were reversed by a TRPM8 antagonist, AMTB. Menthol's effects were not specific to acrolein, as menthol also attenuated irritation responses to acetic acid, and cyclohexanone, an agonist of the capsaicin receptor, TRPV1. Menthol was efficiently absorbed in the respiratory tract, reaching local concentrations sufficient for activation of sensory TRP channels. These experiments demonstrate that menthol and eucalyptol, through activation of TRPM8, act as potent counterirritants against a broad spectrum of smoke constituents. Through suppression of respiratory irritation, menthol may facilitate smoke inhalation and promote nicotine addiction and smoking-related morbidities.— Willis, D. N., Liu, B., Ha, M. A., Jordt, S.-E., Morris, J. B. Menthol attenuates respiratory irritation responses to multiple cigarette smoke irritants.
Keywords: chemosensation, sensory nerves, TRP channels
Menthol, the cooling agent in peppermint, is present in 90% of commercial cigarettes sold in the United States, including brands that are not marketed as being mentholated (1). Its use as a cigarette smoke additive has been the subject of heated debate, especially following the exemption of menthol from the ban on tobacco flavor additives authorized by the Family Smoking Prevention and Tobacco Control Act (2). The current debate focuses on whether menthol, either through its action as a flavorant or through specific pharmacological effects, affects smoking behavior and aggravates the adverse health effects of smoking (3, 4).
Epidemiological studies indicate that beginning young smokers disproportionally use menthol cigarettes, suggesting a preference for menthol during smoking initiation (5). Menthol smokers show lower smoking cessation success rates (6). While some studies measured higher blood levels of the nicotine metabolite cotinine in menthol smokers, other studies found no differences in this parameter (3, 7, 8). The long-term health effects of menthol smoking are controversial, with some reports detecting increases in esophageal or lung cancer rates, but others failing to establish links to increased morbidity, or even reporting beneficial effects (3, 9, 10).
Inhaled menthol exerts complex olfactory and sensory effects by interacting with olfactory and somatosensory neurons and respiratory tissues (11, 12). Cigarette smoke contains numerous irritants that stimulate chemosensory nerves. Stimulation of these nerves initiates important defense mechanisms, leading to unpleasant burning, tingling, and itching sensations and reflex responses, such as coughing or sneezing, and avoidance behavior (13). Were menthol to inhibit these responses, it would act to facilitate smoke inhalation. Although menthol is widely used in many therapeutic preparations for the treatment of cough, very few controlled studies exist documenting its antitussive activity (14, 15). These studies used a single chemical tussive stimulus (citric acid), but did not examine the effects of menthol on responses to cigarette smoke irritants (14, 15). The mechanisms of menthol's antitussive action are not known and may include effects on respiratory tract secretions, edemagenesis, relief of bronchoconstriction, and/or inhibition of cough-initiating sensory neuronal pathways (15).
The cooling sensation elicited by inhaled menthol is likely mediated via neuronal transient receptor potential melatonin 8 (TRPM8) ion channels expressed in somatosensory neurons innervating the airways (16–19). Menthol is not selective toward TRPM8 and was shown to interact with other sensory targets. In vitro studies indicate that menthol can also act as an agonist or antagonist at the sensory irritant receptor, transient receptor potential ankyrin 1 (TRPA1; refs. 20–22). TRPA1 mediates sensory irritation responses to unsaturated aldehydes in cigarette smoke, terpenes, and many other electrophilic and oxidizing respiratory irritants (23–26, 27, 28, 29). Menthol also activates transient receptor potential vanilloid 3 (TRPV3), another chemosensory TRP ion channel (30).
Currently, little rigorous evidence exists on whether inhaled menthol, at the levels present in cigarette smoke, acts directly on sensory nerves as a counterirritant or irritant and, if so, whether its effects are selective toward specific groups of chemical irritants or irritant receptors. In the present study, we hypothesize that menthol vapor acts as a broad-spectrum counterirritant, diminishing the chemosensory responses to inhaled irritants. To test this hypothesis, we used a mouse model to examine the effects of menthol on neuronally mediated responses to 3 irritants that activate sensory neurons through differing receptor pathways: acrolein, acetic acid, and cyclohexanone. These irritants are present in cigarette smoke (31–33) and cover a diversity of structures, including reactive aldehydes (acrolein), acids (acetic acid), and volatile organic hydrocarbons (cyclohexanone) (33). Acrolein activates chemosensory nerves via the TRPA1 irritant receptor (23); acetic acid and cyclohexanone likely act through acid-sensing ion channels (ASICs), TRPV1 receptors, and other sensory receptor classes (34, 35). Results indicate that menthol acts as a potent broad-based counterirritant in the mouse model, providing objective evidence that the pharmacological actions of inhaled menthol vapor likely act to reduce cigarette smoke-induced irritation and, thereby, may facilitate smoking behaviors.
MATERIALS AND METHODS
Animals
Mice were housed in American Association for Accreditation of Laboratory Animal Care-accredited facilities in standard environmental conditions (12-h light-dark cycle and ∼23°C). All animal procedures were approved by the University of Connecticut and Yale University Institutional Animal Care and Use Committees. Mice were housed over hardwood shavings (Sani-Chip Dry, P. J. Murphy Forest Products, Montville, NJ, USA). Food (Lab Diet; PMI Nutrition International, Brentwood, MO, USA) and tap water were provided ad libitum. Animals were acclimated for at least 2 wk prior to use and were used within 14 wk of arrival. All mice were obtained from Jackson Laboratories (Woods Hole, MA, USA). Respiratory exposure studies were performed in female C57BL/6J mice (8–16 wk), with the exception of female Trpa1−/− mice (B6:129P-Trpa1,tm1Kykw>/J), for which age-matched controls were obtained from the supplier (Jackson Laboratories) Backcrossed Trpv1−/− mice (B6.129×1-Trpv1tm1Jul/J) were used to obtain primary sensory neurons for cell culture.
Respiratory exposures
To assess irritant responsiveness, spontaneously breathing mice were challenged with irritants, and respiratory parameters were monitored in a Buxco (Sharon, CT, USA) double plethysmograph using the Buxco NAM software (36). Exposures were performed by drawing clean- or irritant-laden air into the head space of the double plethysmograph at a flow rate of 0.6 L/min (19). Animals were placed in the plethysmograph for a 10-min acclimatization, 10-min baseline, and then a 15-min exposure period. Trigeminal sensory nerve activation causes the sensory irritation response, characterized by braking at the onset of each expiration (13, 37). The average duration of braking (DB) was measured as 1-min running averages. DB values during exposure for each animal were corrected for that animal's individual baseline value (baseline values were <20 ms). When administered, metyrapone was injected at a dose of 75 mg/kg, s.c. (12.5 mg/ml in distilled water). This dose has been shown to abolish the sensory irritation response to naphthalene and styrene (36). N-(3-aminopropyl)-2-[(3-methylphenyl)methoxy]-N-(2-thienylmethyl)benzamide hydrochloride (AMTB) was injected at a dose of 3 mg/kg, s.c. (0.6 mg/ml in saline), 20 min prior to exposure. This dose has been shown to attenuate hyperactive bladder activity (48).
The effects of menthol and eucalyptol were examined on respiratory responses to 3 irritant vapors: acrolein, acetic acid, and cyclohexanone. Racemic menthol was used for initial studies. The final experiment used l-menthol. Acrolein, acetic acid, cyclohexanone, and eucalyptol atmospheres were generated by flash evaporation (36). Menthol atmospheres were generated by passing air over two flasks in a series, each containing crystalline menthol. Airborne concentrations of acrolein, cyclohexanone, menthol, or eucalyptol were measured by gas chromatography using a Varian 3800 gas chromatograph (Varian, Sugarland, TX, USA), 15 m DB-WAX column (Agilent Technologies, Santa Clara, CA, USA), and flame ionization detection. Samples were injected every 3 min to provide continuous discrete sampling (36). Standard curves were generated by evaporation of known amounts of compound in a glass container, allowing >1 h for evaporation, and sampling of the air with the sample train used for plethysmograph sampling. Acetic acid concentrations were measured by drawing air samples through 2 midget impingers running in series containing 10 ml of distilled water each and analysis for acetate content by HPLC (Waters 600; Waters. Milford, MA, USA) with ultraviolet detection (210 nm; Shimadzu SPD-20A; Shimadzu, Columbia, MD, USA) using a mobile phase of 95:5 0.1% phosphoric acid:acetonitrile (38). Standard curves were prepared by analysis of solutions containing known amounts of acetic acid. Within an exposure group, the sd for exposure concentrations among the individual animals was ≤10% of the mean.
Dosimetry
Upper respiratory tract (URT; defined as all regions of the respiratory tract anterior to the larynx) uptake efficiency was measured, as described previously (39). Briefly, following the onset of urethane-induced anesthesia (1.3 g/kg, i.p.), a tracheostomy was performed, and an endotracheal tube was inserted anteriorly until its tip was forward to the larynx and was then tied in place. The surgically prepared anesthetized animal was placed in a nose-only inhalation exposure chamber in a supine position, and the endotracheal tube was connected to a stainless-steel air sampling line. Chamber laden air was drawn through the surgically isolated URT at a constant flow rate of 25 ml/min (within the physiological range of the mouse) for a period of 60 min. Vapor concentration was measured by gas chromatography in chamber (inspired) air and air exiting the URT, with uptake efficiency being calculated from the difference between these concentrations (39) Dosimetry studies were performed at two target concentrations: 16 ppm, to approximate the concentration used in the irritant studies, and 1 ppm, the lowest concentration that was practicable.
Airway uptake of inspired vapors is strongly dependent on the blood:air partition coefficient (40, 41). The mouse blood:air partition coefficient for menthol was measured by standard vial equilibration techniques (42) using pooled blood samples, each obtained from 2–4 mice, were used. Blood was used on the day of collection and was stored on ice prior to use.
Cell culture and Ca2+ imaging
Dorsal root ganglia from adult wild-type or TRPV1-knockout mice were dissected and dissociated by 1-h incubation in 0.28 WU/ml Liberase Blendzyme 1 (Roche, Mannheim, Germany), followed by washes with Hank's buffered saline, trituration, and straining (70 μm; Falcon, Billerica, MA, USA). Neurons were cultured in Neurobasal-A medium (Invitrogen, Carlsbad, CA, USA) with B-27 supplement, 0.5 mM glutamine and 50 ng/ml nerve growth factor (Calbiochem; Merck, Darmstadt, Germany) on 8-well chambered coverglass or 35-mm dishes (Nunc, Roskilde, Denmark) coated with polylysine (Sigma, St. Louis, MO, USA) and laminin (Invitrogen). Human embryonic kidney (HEK)-293t cells for Ca2+ imaging were cultured and transfected with mouse TRPV1, TRPM8, or TRPA1 cDNAs, or empty vector controls (pcDNA3.1; Invitrogen). Ca2+-imaging experiments were performed in modified standard Ringer's bath solution: 140 mM NaCl, 5 mM KCl, 2 mM CaCl2, 2 mM MgCl2, 10 mM HEPES-NaOH, and 8 mM glucose (pH 7.4), 315–320 mosmol. Cultured neurons and HEK293t cells were loaded in modified Ringer's with 10 μM Fura-2-AM (Calbiochem) and 0.02% Pluronic F127 (BASF, Mount Olive, NJ, USA) for 45 min, and subsequently washed and imaged in glucose-free modified Ringer's solution. Fura-2 emission ratios were obtained with alternating 0.100-ms exposures at 340 and 380 nm. For population analysis, responsiveness of neurons was defined by a 20% increase in Fura-2 ratio following agonist application.
Chemicals
Racemic menthol (99% purity), l-menthol (99% purity,) acrolein (90% purity), eucalyptol (99% purity), cyclohexanone, and 2-methyl-1,2-di-3-pyridyl-1-propanone (metyrapone; 98% purity) were obtained from Sigma-Aldrich (St. Louis, MO, USA). AMTB (99% purity) was obtained from Tocris Bioscience (Ellisville, MO, USA). Glacial acetic acid was obtained from Fisher Scientific (Springfield, NJ, USA).
Statistics
Data were analyzed by ANOVA or repeated-measures ANOVA (time course studies) using Statistica (Statsoft, Tulsa, OK, USA) or Origin (OriginLab, Northampton, MA, USA) software. When appropriate, individual groups were compared by Newman-Keuls test. A value of P < 0.05 was required for significance. Data are presented as means ± se.
RESULTS
Menthol is efficiently absorbed and metabolized in the upper airways of mice
The Tobacco Product Scientific Advisory Committee report estimates the menthol concentration in mentholated cigarette smoke is 8 μM, equivalent to ∼200 ppm (3). Since some menthol may be absorbed on smoke particulates, and the smoke is diluted by air during smoking, the free menthol vapor concentration in smoke of menthol cigarettes is likely lower. On the basis of pilot studies, the current experiments used concentrations of ≤20 ppm. Highly soluble vapors are absorbed efficiently in the upper airways. However, it is unclear whether menthol is absorbed in quantities sufficient to elicit pharmacological effects on sensory nerve endings in airway tissue (41).
Vapor solubility, as indicated by the blood air partition coefficient, was measured using 6 pooled mouse blood samples and averaged 1074 ± 38. Uptake of menthol in the URT and the potential influence of local metabolism were measured next. Nasal tissues in mice and humans express cytochrome P450 monooxygenase (CYP450) and are metabolically active (43, 44). Our previous work has shown that in situ URT metabolism of chemical vapors enhances local uptake and modifies irritant activities (41, 45, 46). Therefore, URT uptake was measured in control mice and mice pretreated with the CYP450 inhibitor metyrapone (47). Two menthol concentrations (1 and 18 ppm) were used because in situ metabolism has been shown to exert greater effects on URT absorption at low compared to high concentrations (45, 46). Menthol was absorbed with high efficiency, with uptake averaging 65% or greater depending on the experimental group (Table 1). Menthol was absorbed with greater efficiency at the low- compared to high-exposure concentration. In mice treated with metyrapone, uptake efficiency was significantly lower than in control mice at the low- but not the high-exposure concentration (Table 1).
Table 1.
URT menthol uptake efficiency
| Inspired concentration (ppm) | Control (%) | Metyrapone (%) |
|---|---|---|
| 1.3 | 90.3 ± 1.1b | 83.3 ± 1.8c |
| 18.3 | 66.3 ± 2.8a | 70.2 ± 2.2a |
Data are presented as means ± se; n = 4/group. Uptake efficiency (expressed as a percentage) was measured in the surgically isolated URT of urethane-anesthetized mice at a flow rate of 25 ml/min. Data were analyzed by ANOVA followed by Newman-Keuls test, groups with differing letter superscripts differ at the P < 0.05 level.
Menthol inhibits the respiratory irritation response to acrolein, a major tobacco smoke irritant
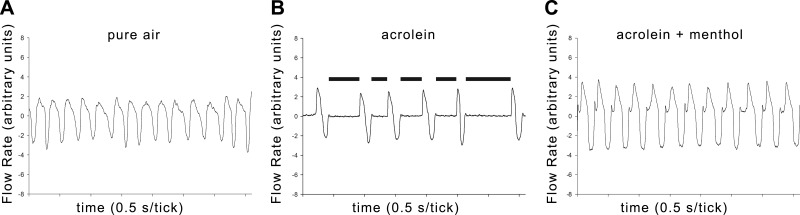
Chemosensory responses to vaporized irritants were examined in mice by measuring the magnitude of the respiratory sensory irritation response. This response is mediated directly by trigeminal nerve stimulation and is characterized by a prolonged pause (termed braking) due to glottal closing at the onset of each expiration (13, 37). Acrolein was used as the primary model irritant in this study. Acrolein is a potent irritant in cigarette smoke that induces sensory irritation in the mouse and cough in humans (38, 48). Acrolein (2 ppm) induced a marked sensory irritation response, as indicated by a large increase in the DB, readily apparent in the tracings of breathing patterns (Figs. 1A, B and 2). The DB was significantly elevated during min 1–15 (P<0.05 Newman-Keuls test), and a response of >200 ms was sustained throughout min 5–14 of exposure.
Figure 1.
Breathing patterns of mice at baseline, during acrolein exposure, or during coexposure with acrolein and menthol. Representative recording of digitized respiratory flow signals obtained from mice during exposure to clean air (A), acrolein (B) or menthol-acrolein (C) after metyrapone pretreatment (see text). The 0 point on the flow axis separates inspiration (downward) from expiration (upward). Each tick mark represents 0.5 s. Breathing frequency was ∼240, 100, and 200 breaths/min in clean air, acrolein, and acrolein-menthol, respectively. The prolonged DB, indicated by black bars, at the onset of expiration during acrolein exposure is readily apparent.
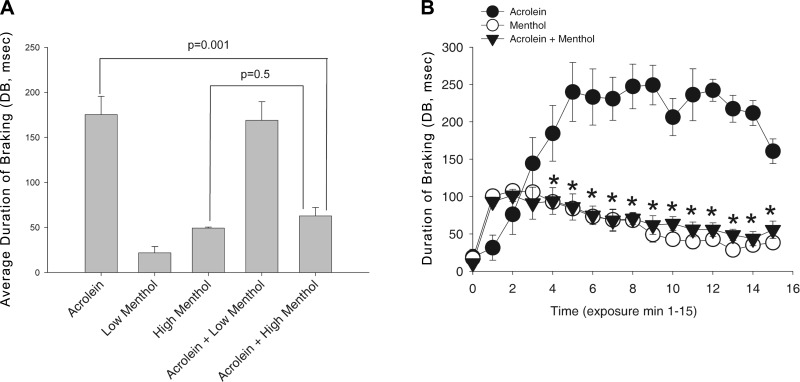
Figure 2.
Effect of menthol vapor on the respiratory irritation response to acrolein. A) Average DB response during 15-min exposure to acrolein (1.9 ppm), menthol (4.1 or 16 ppm), or the combination. Response in the acrolein and acrolein-menthol groups did not differ (P=0.07) at the low menthol concentration; at the high menthol concentration, the response in the acrolein-menthol group was significantly attenuated and not different from the response to menthol alone. P values are indicated. B) Time course of the DB response in the high concentration menthol study. In all groups, DB was increased over baseline during exposure. As indicated by the asterisks, from min 3 to 15 of exposure the response in the combined group was less than in the acrolein group (P<0.05). Data are shown as means ± se; 4–6 mice/group.
Next, we investigated the effects of menthol (4 and 16 ppm) on acrolein (2 ppm)-induced sensory irritation. At 4 ppm, menthol did not attenuate the irritant response to acrolein (Fig. 2A). At 16 ppm, the response in the combined group was virtually identical to the response in the menthol-alone group, indicating that menthol essentially blocked the irritant response to acrolein (Figs. 1B, C and 2B). Examination of the time course revealed that the counterirritation effect was not transient. Except for the start of exposure, the irritation response throughout the entire exposure in the combined group was identical to that in the control group of mice exposed to menthol alone (16 ppm; Fig. 2B). Thus, counterirritant effects of menthol in this mouse model are apparent at concentrations below or equal to those present in mentholated cigarette smoke.
Menthol exposure causes mild respiratory irritation, depending on CYP450 metabolism and TRPA1
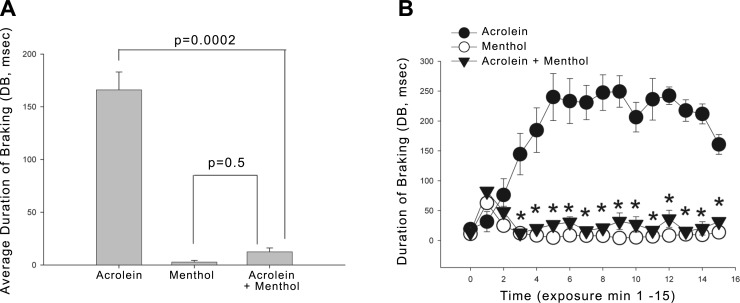
While the animals' responses to acrolein were much greater, we consistently observed that menthol vapor by itself (16 ppm) caused a mild irritation response, leading to a small but significant elevation of DB over the preexposure value throughout the entire exposure (Fig. 2). We wished to determine whether menthol irritation was due to menthol parent compound, or to CYP450 metabolites of menthol. This is particularly relevant as in situ nasal metabolism of menthol occurs, as evidenced by the decreased absorption efficiency in metyrapone-pretreated mice. We observed that menthol irritation was almost completely absent in mice pretreated with metyrapone (Table 2 and Fig. 3). Metyrapone-injected mice showed only a transient increase in DB, visible in min 1 and 2 of exposure (Fig. 3B). Metyrapone by itself was without effect (P>0.05) on the preexposure breathing frequency, minute ventilation, or DB. Metyrapone also did not affect the magnitude and kinetics of the irritation response to acrolein (Fig. 3).
Table 2.
Sensory irritation responses to 16 ppm menthol vapor
| Parameter | TRPA1 |
CYP450 inhibition |
||
|---|---|---|---|---|
| Wild-type +/+ | Knockout −/− | Control | Metyrapone | |
| DB (ms) | 37 ± 6 | 7 ± 3* | 43 ± 7 | 6 ± 3* |
Data are presented as average DB during the 15-min exposure and are shown as means ± se. Each group contained 4–6 mice. The measured menthol vapor concentration averaged 16 ± 2 ppm.
P < 0.05 vs. respective control.
Figure 3.
Effect of menthol vapor on respiratory irritation by acrolein in metyrapone-treated mice. A) Average DB during exposure to 2.1 ppm acrolein, 16 ppm menthol, and the combination. All mice were pretreated with metyrapone to inhibit nasal CYP450 metabolism. Coexposure with menthol significantly inhibited irritation to levels observed with menthol alone. P values are indicated. B) Time course of DB. DB was increased over baseline throughout acrolein exposure, but only during min 1–2 of exposure in the other groups. As indicated by the asterisks, from min 2 to 15 of exposure, response in the combined group was smaller than in the acrolein group (P<0.05). Data are shown as means ± se; 4–6 mice/group.
In a previous study, we found that the irritant effects of some CYP450-metabolized organic vapors (styrene and naphthalene) were mediated by the neuronal irritant receptor TRPA1, which is likely targeted by oxidized metabolites of these chemicals(36). Since menthol's mild irritant effects depended on CYP450 metabolism, we tested whether these effects are also mediated by TRPA1. We exposed Trpa1−/− mice to menthol and compared their responses to those in wild-type animals. Menthol-induced irritation was essentially absent in Trpa1−/− mice (Table 2). Control Ca2+ imaging experiments in HEK293 cells showed that metyrapone (20 μM) neither inhibited nor activated TRPA1 channels (not shown). These data provide evidence that menthol-induced sensory irritation is due to activation of trigeminal TRPA1 channels by a CYP450-generated metabolite.
Broad-spectrum counterirritant effects of menthol and eucalyptol
The mechanism of the counterirritant effects of menthol may differ from that for its sensory irritation effects. To determine whether the counterirritant effects of menthol were due to the parent compound or a metabolite, mice were pretreated with metyrapone and exposed to 2 ppm acrolein, 16 ppm menthol, or the combination. (Metyrapone was without effect on the sensory irritation response to acrolein, P > 0.1, data not shown). While acrolein produced a marked irritant response, essentially no response was observed in animals exposed to both acrolein and menthol, providing strong evidence that the parent compound, menthol, is the active counterirritant (Fig. 3). The time course of the response (Fig. 3B) indicates that the counterirritant effects of menthol were immediate and lasted the duration of the exposure.
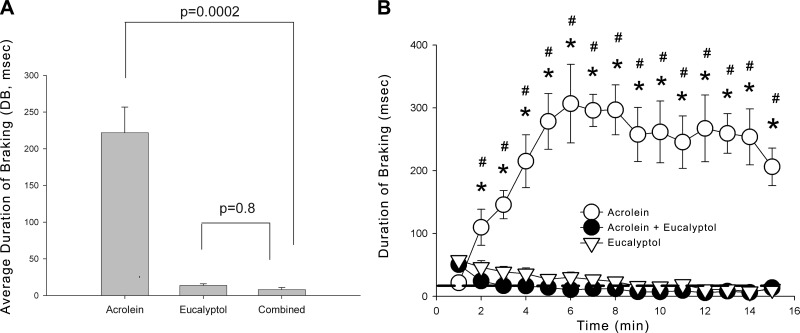
The goals of the next experiments were 2-fold: to determine whether the counterirritation was specific to menthol itself or could be produced by another TRPM8 agonist, eucalyptol (16); and to determine whether the counterirritant effects were specific to irritants, which acted through TRPA1 only or were more generalized in nature. Like menthol, eucalyptol (460 ppm) caused minimal, if any, sensory irritation, but abolished the sensory irritation response to acrolein (Fig. 4). At a concentration of 45 ppm, eucalyptol was without effect on acrolein irritation (data not shown).
Figure 4.
Effect of eucalyptol vapor on respiratory irritation by acrolein. A) Average DB response during exposure to 2.1 ppm acrolein, 464 ppm eucalyptol, or combined vapors. Coexposure with eucalyptol significantly attenuated respiratory irritation to levels observed with eucalyptol alone. P values are indicated; 5 mice/group. B) Time course of DB in the same groups of mice shown in A. DB was increased over baseline throughout acrolein exposure. Coexposure with eucalyptol immediately and completely inhibited the respiratory irritation response. Eucalyptol alone did not cause any increase in DB. *P < 0.05 vs. acrolein + eucalyptol; #P < 0.05 vs. eucalyptol. Data are shown as means ± se.
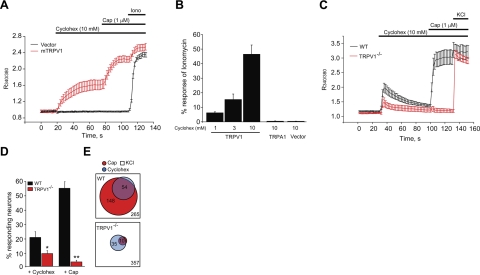
In addition to electrophilic irritants, such as acrolein, cigarette smoke contains acidic and volatile organic irritant constituents targeting diverse sensory receptor pathways. We examined the effects of menthol and eucalyptol on the irritation responses elicited by acetic acid and cyclohexanone, chosen as representatives for these classes of smoke irritants. Acetic acid excites sensory neurons through diverse receptor targets. Cyclohexanone was shown to activate TRPV1, the capsaicin receptor, in heterologous cells (35). Because the compound was tested neither on other TRP channels nor in primary neurons, we examined its specificity using fluorescent Ca2+-imaging in heterologously expressing HEK293t cells and cultured sensory neurons. Recordings from HEK293t cells confirmed that cyclohexanone is specific for TRPV1, and did not activate TRPA1, even at a very high concentration of 10 mM (Fig. 5A, B). Compared to capsaicin (1 μM), cyclohexanone (10 mM) was a weak TRPV1 agonist (Fig. 5A). Recordings in cultured sensory neurons of wild-type mice showed that cyclohexanone (10 mM)-activated Ca2+ influx within a population of capsaicin-sensitive neurons and that the majority of this response depended on Trpv1 (Fig. 5C, D). In neurons dissociated from Trpv1−/− mice, the number of cyclohexanone-sensitive cells was diminished by ∼60%. Cyclohexanone activated only very small Ca2+ transients in the remaining cells (Fig. 5C).
Figure 5.
Cyclohexanone, a tobacco smoke irritant, activates TRPV1 channels in sensory neurons. A) Ca2+ imaging experiments showing averaged amplitudes of Fura-2 ratios in HEK 293 cells expressing mouse TRPV1 (mTRPV1, red) or pCDNA3.1 vector control (vector, black) in response to applications of cyclohexanone (cyclohex, 10 mM), capsaicin (cap, 1 μM), and ionomycin (iono, 1.5 μM). B) Steady-state Ca2+ responses in TRPV1-, TRPA1- or vector-transfected HEK 293 cells to cyclohexanone, measured 1 min after application at the indicated concentrations. Responses are indicated as percentage ratio of ionomycin response. C) Ca2+ responses in cyclohexanone-sensitive DRG neurons from wild-type and Trpv1−/− mice superfused with cyclohexanone (10 mM), capsaicin (1 μM), and KCl (40 mM). Averages of 20 to 30 neurons are shown for each group. Trpv1−/− neurons show strongly diminished Ca2+ transients. Only the neurons that showed a minimum 20% increase in Fura-2 ratio above baseline on agonist applications are included. D) Population analysis of cyclohexanone-sensitive cultured primary sensory neurons derived from wild-type and Trpv1−/− mice. Experiments were performed as in C. Bar graph displays percentage of K+-sensitive cells responding to the given stimulus (cyclohexanone, 10 mM; or capsaicin 1 μM). Responsiveness was defined by a 20% increase in Fura-2 ratio above baseline following agonist application. *P < 0.05; **P < 0.01. E) Venn diagrams of cyclohexanone and capsaicin-responsive sensory neurons from wild-type (top panel) or Trpv1−/− mice (bottom panel). A total of 265 neurons from wild-type mice, identified through K+ responsiveness, were analyzed from 8 fields. Of these neurons, 54 were responsive to cyclohexanone (10 mM) and 148 were responsive to capsaicin (1 μM). A large majority of cyclohexanone-sensitive neurons (n=50) were contained within the capsaicin-sensitive population.
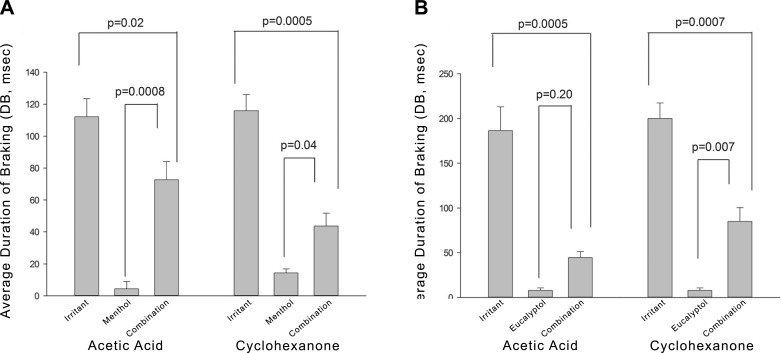
To examine the effects of menthol and eucalyptol on acetic acid or cyclohexanone irritation, mice to be exposed to menthol were pretreated with metyrapone to focus on the effects of parent menthol. Eucalyptol-exposed mice were not pretreated with metyrapone because eucalyptol did not produce sensory irritation. Menthol partially attenuated the irritation response to both 150 ppm acetic acid and 1500 ppm cyclohexanone (Fig. 6A), indicating that its counterirritant effects are likely generalized in nature. Menthol also produced significant attenuation of the irritant responses to acetic acid and cyclcohexanone in animals not pretreated with metyrapone (data not shown). Eucalyptol, at an exposure concentration of 450 ppm, attenuated the irritation responses to both acetic acid and cyclohexanone, and, particularly for acetic acid, more strongly than menthol (Fig. 6B).
Figure 6.
Effects of menthol or eucalyptol vapor on respiratory irritation responses elicited by acetic acid or cyclohexanone vapors. A) Average DB response during exposure to 149 ppm acetic acid or 1483 ppm cyclohexanone with and without 17 ppm menthol vapor. Menthol significantly attenuated responses to both exposures. All mice were pretreated with metyrapone to focus on the effect of parent menthol. B) Average DB response during exposure to 110 ppm acetic acid or 1472 ppm cyclohexanone with or without coexposure to 464 ppm eucalyptol. Eucalyptol significantly attenuated responses to both irritants. Mice were not pretreated with metyrapone. P values are indicated.
Diminished menthol counterirritant action in mice treated with a TRPM8 antagonist
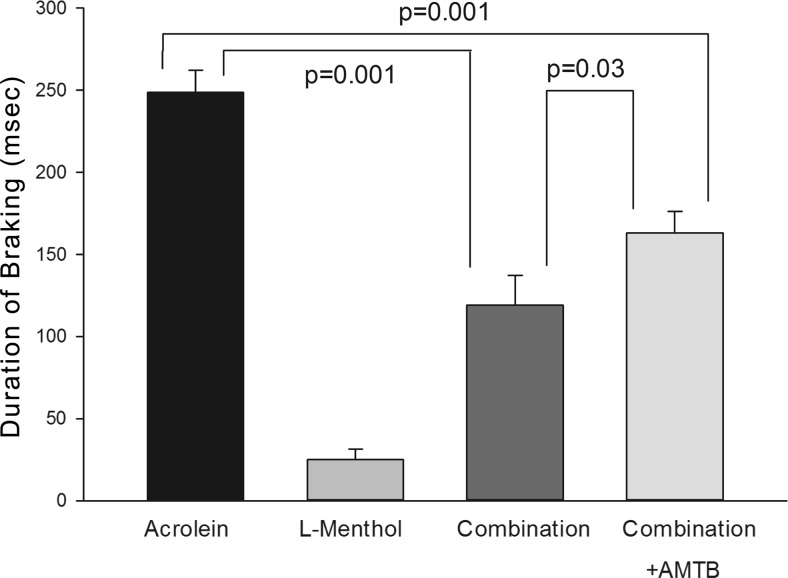
The final experiments were designed to investigate the role of TRPM8 in menthol-induced counterirritation. Since l-menthol is the most active enantiomer at the TRPM8 receptor, these studies used l-menthol rather than racemic menthol. In addition, the effects of the TRPM8 antagonist AMTB (49) on the response to acrolein alone, menthol alone, and the combination were examined. These studies used an l-menthol target concentration of 6 ppm. The concentration was significantly less than the 16 ppm used for racemic menthol and was selected, on the basis of pilot experiments, to produce significant attenuation of the acrolein response. AMTB, administered at a dose shown to inhibit TRPM8 in rats in vivo (3 mg/kg, s.c.; ref. 49), was without effect on the response to acrolein vapor alone or menthol vapor alone (P>0.1 in both cases). Similar to the effects of racemic menthol, l-menthol strongly attenuated the sensory irritation response to acrolein (Fig. 7). This effect was significantly diminished in animals treated with AMTB, the TRPM8 antagonist (3 mg/kg, s.c.). Control experiments in HEK293t cells showed that AMTB inhibited menthol-activated murine TRPM8 channels, but not menthol- or acrolein-activated mTRPA1 channels, confirming specificity of this compound for TRPM8 receptors.
Figure 7.
Effects of l-menthol and the TRPM8 antagonist, AMTB, on respiratory irritation responses elicited by acrolein. Average DB response during exposure to 2.2 ppm acrolein with and without 7 ppm l-menthol. l-Menthol concentration averaged 6.4, 7.6, and 6.8 ppm in the l-menthol, combination and combination+AMTB groups, respectively (P>0.1). AMTB (3 mg/kg, s.c.) did not affect the response in the acrolein or l-menthol groups; therefore, data from nontreated and treated-animals were pooled for these groups. l-Menthol significantly attenuated the response to acrolein, while AMTB significantly diminished the menthol-induced attenuation of the irritant response to acrolein.
DISCUSSION
In our present study, we demonstrate that menthol has broad-spectrum counterirritant activity in mice, inhibiting respiratory irritation responses to tobacco smoke irritants activating diverse sensory neuronal receptors. Inspired menthol vapor was absorbed with high efficiency in the URT, indicating it was effectively delivered to that site during inhalation exposure. The partition coefficient of menthol is high, commensurate with effective absorption in the URT (41). Precise estimation of the menthol concentration in respiratory tract tissues during inhalation exposure will require dosimetry modeling (41, 42). An initial estimate can be made, however, from the dosimetry data obtained in this study. The minute ventilation observed in the plethysmograph in this study was ∼50 ml/min. At a menthol concentration of 16 ppm (0.65 μM), the inspired burden was 0.03 nmol/min. Assuming 70% uptake (Table 1) and a nasal blood flow of 1% of the cardiac output of the mouse (50, 51), the average nasal tissue concentration would be 150 μM if all of the deposited menthol remained within body tissues. This is likely an overestimation, as some vapor is lost to the airstream during exhalation by desorption (52, 53), but the estimate does suggest that tissue concentrations are sufficiently high to stimulate the mouse TRPM8 receptor, and not inhibit mouse TRPA1 (16, 21, 22, 54). That menthol absorption was diminished by pretreatment with the CYP450 inhibitor metyrapone provides strong evidence that it was metabolized within that site (41). The URT dosimetry patterns that were observed for menthol were typical of those for soluble metabolized vapors. Such vapors (e.g., styrene and naphthalene; refs. 45, 46) demonstrate decreased absorption efficiencies in animals pretreated with metyrapone with the effect of inhibitors being significantly more pronounced at low compared to high exposure concentrations. This is attributed to saturation of in situ metabolism at high exposure concentrations. That menthol is metabolized within nasal tissues raises the possibility that its effects may be due to parent menthol itself and/or a metabolite.
The inhibition of menthol sensory irritation by metyrapone suggests that a CYP450 metabolite of menthol is a mild sensory irritant. The absence of sensory irritation in Trpa1−/− mice suggests that the metabolite likely acts through TRPA1. In this regard, menthol appears to act identically to naphthalene and styrene, which are thought to induce sensory irritation via interaction of CYP450-generated electrophilic metabolites with TRPA1 (36). The structure of the menthol metabolite is not known; however, since TRPA1 is sensitive to electrophiles, it is likely that the metabolite is electrophilic and/or easily converted to an electrophilic moiety. The involvement of CYP450 in the metabolism of menthol has not been reported before and may be restricted to specific tissues, such as respiratory epithelia. Previous studies have shown that menthol is eliminated by glucuronidation (55). While glucuronidation products may be pharmacologically active, a glucuronide metabolite is not a likely agonist for the TRPA1 receptor (20, 23, 25), and the effects of metyrapone are thought to be specific for CYP450 (56). Notably, in contrast to in vitro results (21, 22), the current results provide no evidence that parent menthol interacts with TRPA1 in vivo. In metyrapone-pretreated animals, menthol only induces a small transient irritant response at the onset of exposure. This response was observed in Trpa1−/− mice, indicating that it was not TRPA1 mediated. This response likely represents a nonspecific response to a change in atmospheres, as it is observed when one clean air line is swapped with another and also observed at the end of exposure when irritant-laden air is swapped with clean air (57).
At concentrations lower than that in mentholated cigarette smoke, menthol vapor abolished the sensory irritation response to acrolein, a potent cigarette smoke irritant that acts through the TRPA1 receptor (23). Menthol also significantly attenuated the irritation responses to acetic acid and cyclohexanone, two other cigarette smoke irritants representative for acidic or organic hydrocarbon smoke vapor constituents. Acetic acid activates sensory neurons through diverse receptor targets, including ASICs, 2-pore potassium channels, proton-sensitive G-protein-coupled receptors, adenosine receptors, or P2X receptors (34, 58, 59). In vivo studies have shown that respiratory irritation responses to acetic acid are independent from TRPV1 and TRPA1, since Trpv1−/− and Trpa1−/− mice showed normal respiratory irritation responses to this irritant in our previous studies (27, 60). Our studies confirm that cyclohexanone is a TRPV1 agonist and also indicate that it is not an agonist at the TRPA1 receptor. In vitro studies have shown that, at high concentrations, menthol is an antagonist of murine TRPA1 channels (21, 22). However, this effect is not sufficient to explain our experimental results. TRPA1 antagonism may contribute to the counterirritant effects of menthol against acrolein but cannot explain the counterirritant effects against cyclohexanone and acetic acid. The broad-based counterirritancy effects of menthol are likely reflective of effects exerted through other receptor pathways. The response we measured, sensory irritation, is a direct neuronally mediated response (13, 37), and, unlike cough, is not influenced by airway secretions, edemagenesis, and/or bronchoconstriction (15). Therefore, the current studies provide strong evidence that counterirritation is most likely reflective of direct neuronal effects of menthol (and eucalyptol). To our knowledge, a direct effect of menthol vapor on respiratory tract chemosensory responsiveness to inhaled irritants in vivo has not been demonstrated previously.
The counterirritant properties of menthol appear to be due to parent menthol itself rather than a CYP450 metabolite, as counterirritation was fully apparent in metyrapone-pretreated mice. Thus, the direct stimulation of sensory nerves (e.g., the sensory irritant response) and the counterirritation are likely due to differing molecules (e.g., metabolite vs. parent menthol). Both menthol and eucalyptol are TRPM8 agonists, and TRPM8 stimulation is thought to exert analgesic and/or local anesthetic effects (61), suggesting that this pathway may be involved in the counterirritancy. Moreover, both menthol and eucalyptol exerted the same pattern of counterirritant effectiveness, with maximal effectiveness for acrolein and lesser effects for acetic acid and cyclohexanone. Eucalyptol appears less potent than menthol, as 45 ppm eucalyptol was without effect, whereas 16 ppm menthol abolished the response to acrolein. This is consistent with the Kd values of menthol and eucalyptol with the TRPM8 receptor (16, 54). Moreover, l-menthol, the active enantiomer at TRPM8, exerts more potent counterirritant effects than racemic menthol (Figs. 2 vs. 7). Notably, the TRPM8 antagonist AMTB was without effect on the response to acrolein alone or l-menthol alone but significantly attenuated the counterirritant effects of l-menthol on acrolein irritation. In total, these data strongly implicate a role for TRPM8 pathways in the counterirritant effects of menthol.
The mechanism through which TRPM8 activation in trigeminal nerve endings leads to inhibition of respiratory irritation responses is unknown. It is possible that this mechanism is related to mechanisms mediating the analgesic effects of menthol in models of pain. Studies in a neuropathic pain model suggested that topical menthol application leads to central inhibition of nociceptor input from DRG in the spinal cord (61). Other studies suggest that menthol causes presynaptic inhibition of sensory fibers coexpressing TRPM8 with proalgesic receptors (62). On the basis of these findings, both peripheral and central mechanisms of menthol inhibition of the respiratory irritation response need to be explored. Differences in neuronal distributions of irritant receptors, and in the contributions of peripheral and central inhibitory mechanisms, may explain the differences in efficacies of menthol and eucalyptol inhibition of sensory irritation by the 3 irritant vapors observed in the present study.
Menthol, at a level lower than that present in mentholated cigarette smoke, attenuated the irritant response to multiple cigarette smoke irritants with widely varying chemical structures and biological properties. This is a novel finding and suggests that assertions of the tobacco industry that chemosensory (as opposed to flavoring) effects of menthol on smoking behavior are nonexistent may be premature; to quote: “experience with cigarette product development indicates no special role of menthol … particularly with respect to the chemosensory properties (primarily irritation)” (4). Our current study relied on a widely used, long-standing mouse model of airway sensory neuronal responsiveness, sensory irritation (13, 63). Sensory irritant sensitivity in the mouse bioassay correlates well with that observed in the human (37, 63). Effects observed in this model, particularly for acrolein, are predictive of the sensory neuronal effects observed in humans, including cough (48). Direct comparisons of the current mouse data to the smoking human may be difficult because of differences in exposure protocols (intermittent exposure during smoking, continuous for the current mouse study) and the use of exposures to single irritants. While our study covered the majority of chemical classes of smoke irritants, menthol's effects on respiratory irritation by smoke, a complex mixture of irritants and particulates, may be different and needs to be further examined. Nevertheless, the current results provide objective evidence that menthol is present in pharmacologically effective counterirritant concentrations in mentholated cigarette smoke. Intriguingly, two recent studies identified TRPA1 as a target for sensory neuronal activation by particulates (64, 65). In our hands, menthol had the most potent effects on TRPA1-induced respiratory irritation (by acrolein), and may thus also diminish sensory irritation by smoke particulates targeting this receptor. By decreasing the unpleasant chemosensory responses to the irritants present in cigarette smoke through direct interaction with chemosensory nerves, menthol may facilitate smoke inhalation, thereby promoting the development of nicotine addiction and smoking-related morbidities. These findings support the conclusion of the Tobacco Products Scientific Advisory Committee of the Food and Drug Administration (3), stating that menthol may act to increase the numbers of smokers.
Supplementary Material
Acknowledgments
This work was supported in part by U.S. National Institutes of Health grants R01HL105365 (to J.B.M. and S.E.J.) and R01ES015056 (to S.E.J.), and by the American Asthma Foundation (07-0212 to S.E.J.).
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
REFERENCES
- 1. Klausner K. (2010) Menthol cigarettes and the initiation of smoking: a white paper. http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/TobaccoProductsScientificAdvisoryCommittee/UCM228401.pdf
- 2. U.S. Congress House Committee on Energy and Commerce Subcommittee on Health (2008) The Family Smoking Prevention and Tobacco Control Act: hearing before the Subcommittee on Health of the Committee on Energy and Commerce, House of Representatives, One-Hundred Tenth Congress, first session, on H.R. 1108, October 3, 2007, U.S. Government Printing Office, Washington, DC [Google Scholar]
- 3. Tobacco Products Scientific Advisory Committee (2011) Menthol cigarettes and public health: review of the scientific evidence and recommendations. http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/TobaccoProductsScientificAdvisoryCommittee/UCM247689.pdf
- 4. Tobacco Industry Stakeholders (2011) Menthol cigarettes: no disproportionate impact on public health. http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/TobaccoProductsScientificAdvisoryCommittee/UCM249320.pdf
- 5. Rising J., Wasson-Blader K. (2011) Menthol and initiation of cigarette smoking. Tob. Induc. Dis. 9(Suppl. 1), S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Levy D., Blackman K., Tauras J., Chaloupka F., Villanti A., Niaura R., Vallone D., Abrams D. (2011) Quit attempts and quit rates among menthol and nonmenthol smokers in the United States. Am J. Public Health 101, 1241–1247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Williams J. M., Gandhi K. K., Steinberg M. L., Foulds J., Ziedonis D. M., Benowitz N. L. (2007) Higher nicotine and carbon monoxide levels in menthol cigarette smokers with and without schizophrenia. Nicotine Tob. Res. 9, 873–881 [DOI] [PubMed] [Google Scholar]
- 8. Muscat J. E., Chen G., Knipe A., Stellman S. D., Lazarus P., Richie J. P., Jr. (2009) Effects of menthol on tobacco smoke exposure, nicotine dependence, and NNAL glucuronidation. Cancer Epidemiol. Biomarkers Prev. 18, 35–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mendiondo M. S., Alexander L. A., Crawford T. (2010) Health profile differences for menthol and non-menthol smokers: findings from the National Health Interview Survey. Addiction 105(Suppl. 1), 124–140 [DOI] [PubMed] [Google Scholar]
- 10. Blot W. J., Cohen S. S., Aldrich M., McLaughlin J. K., Hargreaves M. K., Signorello L. B. (2011) Lung cancer risk among smokers of menthol cigarettes. J. Natl. Cancer Inst. 103, 810–816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Eccles R., Jawad M. S., Morris S. (1989) Olfactory and trigeminal thresholds and nasal resistance to airflow. Acta Otolaryngol. 108, 268–273 [DOI] [PubMed] [Google Scholar]
- 12. Lawrence D., Cadman B., Hoffman A. C. (2011) Sensory properties of menthol and smoking topography. Tob. Induc. Dis. 9(Suppl. 1), S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Alarie Y. (1973) Sensory irritation by airborne chemicals. CRC Crit. Rev. Toxicol. 2, 299–363 [DOI] [PubMed] [Google Scholar]
- 14. Morice A. H., Marshall A. E., Higgins K. S., Grattan T. J. (1994) Effect of inhaled menthol on citric acid induced cough in normal subjects. Thorax 49, 1024–1026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Laude E. A., Morice A. H., Grattan T. J. (1994) The antitussive effects of menthol, camphor and cineole in conscious guinea-pigs. Pulm. Pharmacol. 7, 179–184 [DOI] [PubMed] [Google Scholar]
- 16. McKemy D. D., Neuhausser W. M., Julius D. (2002) Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature 416, 52–58 [DOI] [PubMed] [Google Scholar]
- 17. Peier A. M., Moqrich A., Hergarden A. C., Reeve A. J., Andersson D. A., Story G. M., Earley T. J., Dragoni I., McIntyre P., Bevan S., Patapoutian A. (2002) A TRP channel that senses cold stimuli and menthol. Cell 108, 705–715 [DOI] [PubMed] [Google Scholar]
- 18. Bautista D. M., Siemens J., Glazer J. M., Tsuruda P. R., Basbaum A. I., Stucky C. L., Jordt S. E., Julius D. (2007) The menthol receptor TRPM8 is the principal detector of environmental cold. Nature 448, 204–208 [DOI] [PubMed] [Google Scholar]
- 19. McCoy D. D., Knowlton W. M., McKemy D. D. (2011) Scraping through the ice: Uncovering the role of TRPM8 in cold transduction. Am. J. Physiol. Regul. Integr. Comp. Physiol. 300, R1278–R1287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jordt S. E., Bautista D. M., Chuang H. H., McKemy D. D., Zygmunt P. M., Hogestatt E. D., Meng I. D., Julius D. (2004) Mustard oils and cannabinoids excite sensory nerve fibres through the TRP channel ANKTM1. Nature 427, 260–265 [DOI] [PubMed] [Google Scholar]
- 21. Macpherson L. J., Hwang S. W., Miyamoto T., Dubin A. E., Patapoutian A., Story G. M. (2006) More than cool: promiscuous relationships of menthol and other sensory compounds. Mol. Cell. Neurosci. 32, 335–343 [DOI] [PubMed] [Google Scholar]
- 22. Karashima Y., Damann N., Prenen J., Talavera K., Segal A., Voets T., Nilius B. (2007) Bimodal action of menthol on the transient receptor potential channel TRPA1. J. Neurosci. 27, 9874–9884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bautista D. M., Jordt S. E., Nikai T., Tsuruda P. R., Read A. J., Poblete J., Yamoah E. N., Basbaum A. I., Julius D. (2006) TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell 124, 1269–1282 [DOI] [PubMed] [Google Scholar]
- 24. Andre E., Campi B., Materazzi S., Trevisani M., Amadesi S., Massi D., Creminon C., Vaksman N., Nassini R., Civelli M., Baraldi P. G., Poole D. P., Bunnett N. W., Geppetti P., Patacchini R. (2008) Cigarette smoke-induced neurogenic inflammation is mediated by alpha, beta-unsaturated aldehydes and the TRPA1 receptor in rodents. J. Clin. Invest. 118, 2574–2582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bessac B. F., Sivula M., von Hehn C. A., Caceres A. I., Escalera J., Jordt S. E. (2009) Transient receptor potential ankyrin 1 antagonists block the noxious effects of toxic industrial isocyanates and tear gases. FASEB J. 23, 1102–1114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Escalera J., von Hehn C. A., Bessac B. F., Sivula M., Jordt S. E. (2008) TRPA1 mediates the noxious effects of natural sesquiterpene deterrents. J. Biol. Chem. 283, 24136–24144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bessac B. F., Sivula M., von Hehn C. A., Escalera J., Cohn L., Jordt S. E. (2008) TRPA1 is a major oxidant sensor in murine airway sensory neurons. J. Clin. Invest. 118, 1899–1910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bessac B. F., Jordt S. E. (2008) Breathtaking TRP channels: TRPA1 and TRPV1 in airway chemosensation and reflex control. Physiology (Bethesda) 23, 360–370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lin Y. S., Hsu C. C., Bien M. Y., Hsu H. C., Weng H. T., Kou Y. R. (2010) Activations of TRPA1 and P2X receptors are important in ROS-mediated stimulation of capsaicin-sensitive lung vagal afferents by cigarette smoke in rats. J. Appl. Physiol. 108, 1293–1303 [DOI] [PubMed] [Google Scholar]
- 30. Vogt-Eisele A. K., Weber K., Sherkheli M. A., Vielhaber G., Panten J., Gisselmann G., Hatt H. (2007) Monoterpenoid agonists of TRPV3. Br. J. Pharmacol. 151, 530–540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bi X., Sheng G., Feng Y., Fu J., Xie J. (2005) Gas- and particulate-phase specific tracer and toxic organic compounds in environmental tobacco smoke. Chemosphere 61, 1512–1522 [DOI] [PubMed] [Google Scholar]
- 32. Perfetti R. (2009) Aldehydes and ketones. In The Chemical Components of Tobacco and Tobacco Smoke (Rodgman A, Perfetti T. A. eds) pp. 215–315, Taylor & Francis, Oxford, UK [Google Scholar]
- 33. Stedman R. L. (1968) The chemical composition of tobacco and tobacco smoke. Chem. Rev. 68, 153–207 [DOI] [PubMed] [Google Scholar]
- 34. Deval E., Gasull X., Noel J., Salinas M., Baron A., Diochot S., Lingueglia E. (2010) Acid-sensing ion channels (ASICs): pharmacology and implication in pain. Pharmacol. Ther. 128, 549–558 [DOI] [PubMed] [Google Scholar]
- 35. Silver W. L., Clapp T. R., Stone L. M., Kinnamon S. C. (2006) TRPV1 receptors and nasal trigeminal chemesthesis. Chem. Senses 31, 807–812 [DOI] [PubMed] [Google Scholar]
- 36. Lanosa M. J., Willis D. N., Jordt S., Morris J. B. (2010) Role of metabolic activation and the TRPA1 receptor in the sensory irritation response to styrene and naphthalene. Toxicol. Sci. 115, 589–595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Vijayaraghavan R., Schapper M., Thompson R., Stock M. F., Alarie Y. (1993) Characteristic modification of the breathing pattern of mice to evaluate the effects of airborne chemicals on the respiratory tract. Arch. Toxicol. 67, 478–490 [DOI] [PubMed] [Google Scholar]
- 38. Morris J. B., Symanowicz P. T., Olsen J. E., Thrall R. S., Cloutier M. M., Hubbard A. K. (2003) Immediate sensory nerve-mediated respiratory responses to irritants in healthy and allergic airway-diseased mice. J. Appl. Physiol. 94, 1563–1571 [DOI] [PubMed] [Google Scholar]
- 39. Morris J. B. (1999) A method for measuring upper respiratory tract vapor uptake and its applicability to quantitative inhalation risk assessment. Inhal. Toxicol. 11, 943–965 [DOI] [PubMed] [Google Scholar]
- 40. Medinsky M. A., Kimbell J. S., Morris J. B., Gerde P., Overton J. H. (1993) Advances in biologically based models for respiratory tract uptake of inhaled volatiles. Fundam. Appl. Toxicol. 20, 265–272 [DOI] [PubMed] [Google Scholar]
- 41. Morris J. B., Kimbell J. S., Asgharian B. (2010) Upper airway dosimetry of gases, vapors and particulate matter in rodents. In Toxicology of the Nose and Upper Airway (Morris J. B., Shusterman D. J. eds), pp. 99–115, Informa Healthcare, New York [Google Scholar]
- 42. Morris J. B., Hubbs A. F. (2009) Inhalation dosimetry of diacetyl and butyric acid, two components of butter flavoring vapors. Toxicol. Sci. 108, 173–183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Thornton-Manning J. R., Dahl A. R. (1997) Metabolic capacity of nasal tissue interspecies comparisons of xenobiotic-metabolizing enzymes. Mutat. Res. 380, 43–59 [DOI] [PubMed] [Google Scholar]
- 44. Su T., Bao Z., Zhang Q. Y., Smith T. J., Hong J. Y., Ding X. (2000) Human cytochrome P450 CYP2A13: predominant expression in the respiratory tract and its high efficiency metabolic activation of a tobacco-specific carcinogen, 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone. Cancer Res. 60, 5074–5079 [PubMed] [Google Scholar]
- 45. Morris J. B., Buckpitt A. R. (2009) Upper respiratory tract uptake of naphthalene. Toxicol. Sci. 111, 383–391 [DOI] [PubMed] [Google Scholar]
- 46. Morris J. B. (2000) Uptake of styrene in the upper respiratory tract of the CD mouse and Sprague-Dawley rat. Toxicol. Sci. 54, 222–228 [DOI] [PubMed] [Google Scholar]
- 47. Rossi M. (1983) Structural studies of metyrapone: a potent inhibitor of cytochrome P-450. J. Med. Chem. 26, 1246–1252 [DOI] [PubMed] [Google Scholar]
- 48. Agency for Toxic Substances and Disease Registry (2007) Toxicological Profile for Acrolein, U.S. Department of Health and Human Services, Washington, DC [Google Scholar]
- 49. Lashinger E. S., Steiginga M. S., Hieble J. P., Leon L. A., Gardner S. D., Nagilla R., Davenport E. A., Hoffman B. E., Laping N. J., Su X. (2008) AMTB, a TRPM8 channel blocker: evidence in rats for activity in overactive bladder and painful bladder syndrome. Am. J. Physiol. Renal Physiol. 295, F803–F810 [DOI] [PubMed] [Google Scholar]
- 50. Stott W. T., Dryzga M. D., Ramsey J. C. (1983) Blood-flow distribution in the mouse. J. Appl. Toxicol. 3, 310–312 [DOI] [PubMed] [Google Scholar]
- 51. Brown R. P., Delp M. D., Lindstedt S. L., Rhomberg L. R., Beliles R. P. (1997) Physiological parameter values for physiologically based pharmacokinetic models. Toxicol. Ind. Health 13, 407–484 [DOI] [PubMed] [Google Scholar]
- 52. Gerde P., Dahl A. R. (1991) A model for the uptake of inhaled vapors in the nose of the dog during cyclic breathing. Toxicol. Appl. Pharmacol. 109, 276–288 [DOI] [PubMed] [Google Scholar]
- 53. Johanson G. (1991) Modelling of respiratory exchange of polar solvents. Ann. Occup. Hyg. 35, 323–339 [DOI] [PubMed] [Google Scholar]
- 54. Behrendt H. J., Germann T., Gillen C., Hatt H., Jostock R. (2004) Characterization of the mouse cold-menthol receptor TRPM8 and vanilloid receptor type-1 VR1 using a fluorometric imaging plate reader (FLIPR) assay. Br. J. Pharmacol. 141, 737–745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Yamaguchi T., Caldwell J., Farmer P. B. (1994) Metabolic fate of [3H]-l-menthol in the rat. Drug Metab. Dispos. 22, 616–624 [PubMed] [Google Scholar]
- 56. Igaz P., Tombol Z., Szabo P. M., Liko I., Racz K. (2008) Steroid biosynthesis inhibitors in the therapy of hypercortisolism: theory and practice. Curr. Med. Chem. 15, 2734–2747 [DOI] [PubMed] [Google Scholar]
- 57. Desesa C. R., Vaughan R. P., Lanosa M. J., Fontaine K. G., Morris J. B. (2008) Sulfur-containing malodorant vapors enhance responsiveness to the sensory irritant capsaicin. Toxicol. Sci. 104, 198–209 [DOI] [PubMed] [Google Scholar]
- 58. Honore P., Mikusa J., Bianchi B., McDonald H., Cartmell J., Faltynek C., Jarvis M. F. (2002) TNP-ATP, a potent P2X3 receptor antagonist, blocks acetic acid-induced abdominal constriction in mice: comparison with reference analgesics. Pain 96, 99–105 [DOI] [PubMed] [Google Scholar]
- 59. Vaughan R. P., Szewczyk M. T., Jr., Lanosa M. J., Desesa C. R., Gianutsos G., Morris J. B. (2006) Adenosine sensory transduction pathways contribute to activation of the sensory irritation response to inspired irritant vapors. Toxicol. Sci. 93, 411–421 [DOI] [PubMed] [Google Scholar]
- 60. Symanowicz P. T., Gianutsos G., Morris J. B. (2004) Lack of role for the vanilloid receptor in response to several inspired irritant air pollutants in the C57Bl/6J mouse. Neurosci. Lett. 362, 150–153 [DOI] [PubMed] [Google Scholar]
- 61. Proudfoot C. J., Garry E. M., Cottrell D. F., Rosie R., Anderson H., Robertson D. C., Fleetwood-Walker S. M., Mitchell R. (2006) Analgesia mediated by the TRPM8 cold receptor in chronic neuropathic pain. Curr. Biol. 16, 1591–1605 [DOI] [PubMed] [Google Scholar]
- 62. Harrington A. M., Hughes P. A., Martin C. M., Yang J., Castro J., Isaacs N. J., Ashley Blackshaw L., Brierley S. M. (2011) A novel role for TRPM8 in visceral afferent function. Pain 152, 1459–1468 [DOI] [PubMed] [Google Scholar]
- 63. Nielsen G. D., Wolkoff P., Alarie Y. (2007) Sensory irritation: risk assessment approaches. Regul. Toxicol. Pharmacol. 48, 6–18 [DOI] [PubMed] [Google Scholar]
- 64. Deering-Rice C. E., Romero E. G., Shapiro D., Hughen R. W., Light A. R., Yost G. S., Veranth J. M., Reilly C. A. (2011) Electrophilic components of diesel exhaust particles (DEP) activate transient receptor potential ankyrin-1 (TRPA1): a probable mechanism of acute pulmonary toxicity for DEP. Chem. Res. Toxicol. 24, 950–959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Hazari M. S., Haykal-Coates N., Winsett D. W., Krantz Q. T., King C., Costa D. L., Farraj A. K. (2011) TRPA1 and sympathetic activation contribute to increased risk of triggered cardiac arrhythmias in hypertensive rats exposed to diesel exhaust. Environ. Health Perspect. 119, 951–957 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.