Abstract
Spinal cord injury (SCI) results in permanent loss of motor functions. A significant aspect of the tissue damage and functional loss may be preventable as it occurs, secondary to the trauma. We show that the phospholipase A2 (PLA2) superfamily plays important roles in SCI. PLA2 enzymes hydrolyze membrane glycerophospholipids to yield a free fatty acid and lysophospholipid. Some free fatty acids (arachidonic acid) give rise to eicosanoids that promote inflammation, while some lysophospholipids (lysophosphatidylcholine) cause demyelination. We show in a mouse model of SCI that two cytosolic forms [calcium-dependent PLA2 group IVA (cPLA2 GIVA) and calcium-independent PLA2 group VIA (iPLA2 GVIA)], and a secreted form [secreted PLA2 group IIA (sPLA2 GIIA)] are up-regulated. Using selective inhibitors and null mice, we show that these PLA2s play differing roles. cPLA2 GIVA mediates protection, whereas sPLA2 GIIA and, to a lesser extent, iPLA2 GVIA are detrimental. Furthermore, completely blocking all three PLA2s worsens outcome, while the most beneficial effects are seen by partial inhibition of all three. The partial inhibitor enhances expression of cPLA2 and mediates its beneficial effects via the prostaglandin EP1 receptor. These findings indicate that drugs that inhibit detrimental forms of PLA2 (sPLA2 and iPLA2) and up-regulate the protective form (cPLA2) may be useful for the treatment of SCI.—López-Vales, R., Ghasemlou, N., Redensek, A., Kerr, B. J., Barbayianni, E., Antonopoulou, G., Baskakis, C., Rathore, K. I., Constantinou-Kokotou, V., Stephens, D., Shimizu, T., Dennis, E. A., Kokotos, G., David, S. Phospholipase A2 superfamily members play divergent roles after spinal cord injury.
Keywords: CNS injury, secondary damage, lipid metabolism, prostaglandin receptors
Spinal cord injury (SCI) causes permanent functional deficits due to disruption of spinal pathways and death of neurons and glial cells. The trauma itself causes initial damage to neural tissue, including glia and neurons at the site of injury. This primary damage, however, spreads to regions rostral and caudal to the injury epicenter during the days and weeks after injury. The inflammatory response that occurs after SCI strongly contributes to secondary damage. The cytotoxic effects of immune cells are likely to be mediated via the production of cytokines, chemokines, eicosanoids, proteases, and free radicals, among other factors (1, 2). Reducing inflammation after SCI can therefore be expected to reduce secondary tissue damage and limit functional deficits.
Phospholipase A2 (PLA2) enzymes catalyze the hydrolysis of fatty acids at the sn-2 position in phospholipids and thus give rise to the release of fatty acids, such as arachidonic acid, and the production of lysophospholipids, such as lysophosphatidylcholine (LPC) (3). Several types of PLA2s include both secreted (sPLA2) and intracellular forms, which includes calcium-dependent (cPLA2) and calcium-independent (iPLA2) enzymes (4). Phospholipase A2s are important enzymes involved in membrane turnover. Recent studies, however, have revealed an important multifaceted role for these enzymes in various aspects of inflammation, including in the nervous system, such as in experimental autoimmune encephalomyelitis (EAE; refs. 5–7), brain ischemia (8, 9) and Wallerian degeneration after sciatic nerve injury (10, 11). One way PLA2 can play a role in inflammation is through the arachidonic acid pathway, which is the precursor of proinflammatory eicosanoids, such as prostaglandins, thromboxanes, and leukotrienes. Another way PLA2 can stimulate immune responses is through LPC, which is chemoattractant for monocytes and T cells, activates macrophages, and induces the expression of proinflammatory chemokines and cytokines, and cell adhesion molecules (12–15). Blocking PLA2 might, therefore, be a good therapeutic target to reduce inflammation and prevent tissue loss and demyelination after SCI.
Little is known about the role of PLA2 superfamily members in SCI. Recent studies have reported that cPLA2 GIVA and sPLA2 GIIA are up-regulated after SCI in rats (16, 17). Thus far, the role of sPLA2 was assessed indirectly by intraspinal injection of sPLA2 GIII (from bee venom), into the uninjured, normal spinal cord (16), and in a study that assessed the effects of a nonselective PLA2 inhibitor in SCI over a period of 7 days postinjury (dpi) (18), which blocked both cPLA2 and iPLA2 (19). It is, therefore, not known whether both intracellular forms of PLA2 (cPLA2 and iPLA2) are involved in contributing to SCI pathology and to what extent. In addition, the role of sPLA2 in the injured spinal cord has not been directly examined.
We now provide direct evidence that of the large number of PLA2s comprising the PLA2 superfamily found in mice, the expression of only cPLA2 GIVA, iPLA2 GVIA, and sPLA2 GIIA are increased after spinal cord contusion injury. We also dissected out the contribution of these PLA2 forms in SCI using selective inhibitors against the three different forms of PLA2, as well as two pan-PLA2 inhibitors and the cPLA2-null mouse. We show that cPLA2 GIVA mediates tissue protection after SCI, while sPLA2 GIIA, and to a lesser extent iPLA2 GVIA, contribute to secondary damage and functional loss. These data provide the first clear evidence that different members of the PLA2 superfamily play divergent roles in SCI. We also show that completely blocking all three PLA2s is detrimental to recovery after SCI, while an inhibitor with partial blocking activity is most beneficial.
MATERIALS AND METHODS
Spinal cord contusion and drug administration
Adult (8–10 wk old) female BALB/c (Charles River, Saint-Constant, QC, Canada), cPLA2 GIVA−/− mice, and wild-type littermates were anesthetized with ketamine:xylazine:acepromazine (50:5:1 mg/kg). After performing a laminectomy at the 11th thoracic vertebrae, the exposed spinal cord was contused using the Infinite Horizons Impactor device (Precision Scientific Instrumentation, Lexington, KY, USA). Moderate injuries were made using a force of 50 kdyn, and only animals that had tissue displacements ranging between 400–600 μm were used (20). All surgical procedures were approved by the McGill University Animal Care Committee and followed the guidelines of the Canadian Council on Animal Care.
PLA2 inhibitors
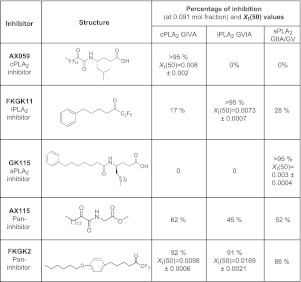
Three types of PLA2 inhibitors were used: 2-oxoamides (AX059 and AX115), fluoroketones (FKGK11 and FKGK2), and an amide (GK115). The 2-oxoamide inhibitors have been extensively characterized (21–23). AX059 is a selective and potent inhibitor of cPLA2 GIVA (Fig. 1) and has been used effectively in vivo (5, 21–25). AX059 exhibits >95% inhibition of cPLA2 at 0.091 mol fraction, while showing 0% inhibition of iPLA2 and sPLA2 (Fig. 1). Its XI(50) value, which is the mole fraction of the inhibitor in the total substrate interface required to inhibit the enzyme by 50%, is 0.008 ± 0.002, indicating high potency. FKGK11 is highly selective for iPLA2 GVIA, showing >95% inhibition of iPLA2 at 0.091 mol fraction, as compared to inhibiting only 17% of cPLA2 and 29% of sPLA2. At the high concentration of substrate used for these assays, values ≤25% are not considered significant. Its XI(50) value (0.0073±0.0007) also indicates that it is a potent inhibitor of iPLA2. FKGK11 has been used effectively in vivo (5, 11). The synthesis and details of the inhibition by this and other fluoroketones used in this work have been published previously (26). The novel amide GK115, which was recently characterized (27), is a highly selective inhibitor of sPLA2 (>95% inhibition at 0.091 mol fraction), while showing no inhibition of cPLA2 and iPLA2 (ref. 27 and Fig. 1). It also has a XI(50) value of 0.003 ± 0.0004, showing high potency (27). In addition, two pan-PLA2 inhibitors were used: one strongly inhibits all three PLA2s (the fluoroketone, FKGK2) while the other partially inhibits all three PLA2s (the 2-oxoamide, AX115). Both these inhibitors (FKGK2 and AX115) have been used previously in vivo experiments (5).
Figure 1.
Structure, selectivity and potency of the PLA, inhibitors used.
PLA2 inhibitor treatment
Mice were given daily intraperitoneal injections of 2-oxoamide (AX059 and AX115), fluoroketone (FKGK11), or amide (GK115), at a dose of 2 mM in 200 μl, starting 1 h after contusion and for 14 d. Control mice that also had SCI were treated daily with vehicle. These doses have been shown to be effective in our previous studies on peripheral nerve injury (11) and experimental autoimmune encephalomyelitis (5).
Western blotting
Protein was extracted from a 5-mm length of spinal cord tissue containing the lesion site harvested at the same time points that were used for the mRNA work. Protein samples (20 μg) were separated on a 4–12% Bis-Tris gel (Invitrogen, Carlsbad, CA, USA) and transferred onto PVDF membranes (Millipore, Bedford, MA, USA). The membranes were incubated with antibodies against cPLA2 GIVA (Santa Cruz Biotechnology, Santa Cruz, CA, USA), iPLA2 VIA (Cayman Chemical, Ann Arbor, MI, USA), sPLA2 GIIA (Cayman Chemical), COX-2 (BD Transduction, San Jose, CA, USA), 5-LOX, HPGDS (Cayman Chemical), mPGEs-1 (Cayman Chemical), EP1 (Cayman Chemical), EP2 (Cayman Chemical), and EP4 (Cayman Chemical). Bands were detected using chemiluminescence (Western Lightning Chemiluminescence Reagent Plus; PerkinElmer, Wellesley, MA, USA). β-Actin (Sigma-Aldrich, St. Louis, MO, USA) was used to ensure equal loading of samples. Three samples were used for each time point.
Locomotor assessment
Locomotor recovery was evaluated in an open-field test using the Basso mouse scale (BMS; ref. 28), which was developed specifically for locomotor testing after contusion injuries in mice. The BMS analysis of hind-limb movements and coordination was carried out by 2 independent assessors who were masked to the experimental and control groups, and their consensus score was taken. These individuals were trained in Michele Basso's laboratory (Ohio State University, Columbus, OH, USA). The final scores are presented as means ± se. The BMS is a compressed scale with a maximum score of 9 as compared to the 21-point BBB scale for rats. Therefore, small differences in the BMS can account for larger functional differences.
Histology
Mice were perfused with 4% paraformaldehyde in 0.1 M phosphate buffer (PB) at 1, 3, 7, 14, and 28 dpi. A 5-mm length of the spinal cord containing the lesion site was removed, cryoprotected with 30% sucrose in 0.1 M PB, and cut in serial sections (16 μm thick). For double immunofluorescence, sections were incubated with antibodies against cPLA2 GIVA (Santa Cruz Biotechnology), iPLA2 GVIA (Cayman Chemical), or sPLA2 GIIA (Cayman Chemical) and combined with antibodies against Mac-1 (for macrophages/microglia; Serotec, Kidlington, UK), GFAP (for astrocytes; Zymed Labs, Burlingame, CA, USA), CC1 (for oligodendrocytes: Calbiochem, San Diego, CA, USA) and NeuN (for neuron; Chemicon, Temecula, CA, USA). Immunofluorescence labeling for serotonin (5-HT; Sigma-Aldrich) was also performed to assess innervation of serotonergic axons caudal to the lesion. In addition, one series of serial sections of the spinal cord was stained with Luxol fast blue (LFB) histochemistry, which stains myelin, and another series was stained with cresyl violet histochemistry to quantify neuronal loss.
Quantification of histological results
Histological quantification was performed from spinal cord sections harvested at 28 dpi. Tissue sections were viewed with an Axioskop 2 Plus microscope (Zeiss, Oberkochen, Germany), images were captured using a QImaging Retiga 1300 camera (QImaging, Surrey, BC, Canada), and quantification was done using BioQuant Nova Prime image analysis system (BioQuant Image Analysis Corp., Nashville, TN, USA). Tissue sparing was calculated by delineating the GFAP-stained sections. Assessment of myelin sparing was performed by calculating the area occupied by myelin in the lateral white matter. Neuronal survival was assessed by counting the neuron profiles in the ventral horn below the level of the central canal of the spinal cord in tissue sections stained with cresyl violet. Assessment of serotonergic innervation was performed by calculating the area occupied by serotonergic axons in the lateral funiculi and ventral horns of spinal cord sections taken at a distance of 1000 μm caudal to the lesion site.
Statistical analyses
Data are shown as means ± se. Western blot analyses were done using 1-way ANOVA with post hoc Dunnett's test. Statistical analyses of the functional and histological assessments were performed by using 2-way repeated measures ANOVA with post hoc Tukey's test for multiple comparisons. Differences were considered significant at values of P < 0.05.
RESULTS
Screening of the mammalian PLA2s in SCI
Although a total of 27 mammalian PLA2 have been identified in humans, mice, rats, and bovines, we focused our work on the 14 PLA2s that are found in mice. We first assessed the mRNA expression of the 4 intracellular mammalian PLA2s (cPLA2 GIVA, cPLA2 GIVB, iPLA2 GVIA, iPLA2 GVIB) and 10 secreted PLA2s (sPLA2 GIIA, GIIC, IID, GIIE, GIIF, GV, GVII, GX, GXIIA, GIIB) in the uninjured spinal cord, and at 1, 3, 7, 14, 21 and 28 d after contusion injury. Of these 14 mammalian PLA2s examined, the expressions of cPLA2 GIVB, iPLA2 GVIB, sPLA2 GIIC, IID, GIIE, GIIF, GX, and GXIIA were unchanged, while GV, GVII, and GXIIB were undetectable after SCI (data not shown). The mRNA expressions of cPLA2 GIVA, iPLA2 GVIA, and sPLA2 GIIA, which increased after SCI, were further characterized by Western blotting and immunofluorescence, and their functional roles were assessed.
Expression and role of cPLA2 GIVA in SCI
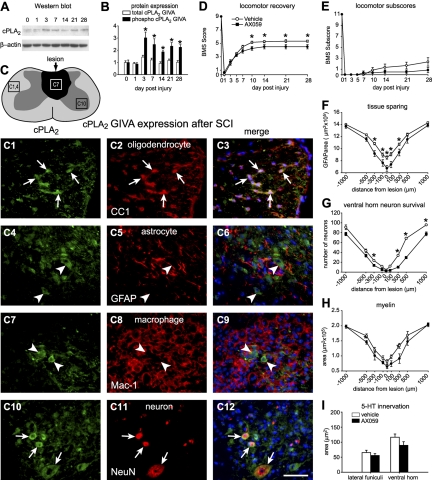
The activation of cPLA2 protein is correlated with phosphorylation of Ser-505 by MAPKs (4). The higher-molecular-mass band on Western blots that corresponds to phospho-cPLA2 is increased from 3 to 28 dpi (Fig. 2A, B). At 3 dpi, cPLA2 is expressed in neurons, particularly the large neurons in the ventral horn, and in some oligodendrocytes located in areas adjacent to the lesion epicenter (Fig. 2C) but not in astrocytes or microglia/macrophages.
Figure 2.
A) Western blot showing cPLA2 GIVA protein expression after SCI. Samples from uninjured and injured mice show 2 bands corresponding to the phosphorylated (top) and nonphosphorylated (bottom) cPLA2 GIVA forms. B) Quantification of protein levels of cPLA2 GIVA from 1 to 28 d after SCI detected by Western blotting (n=3/time point). Activation of cPLA2 protein is regulated by phosphorylation. Quantification of the 2 protein bands revealed that the activated form of cPLA2 GIVA is significantly increased from 3 to 28 after SCI. C) Line drawing of the contused spinal cord shows the lesion in dark shade. The 3 areas outlined by the rectangles indicate regions from which the micrographs in panels C1–C12 were obtained. Panels show double immunofluorescence images of cPLA2 GIVA (green; C1, C4, C7, C10) colabeled with anti-CC-1 (oligodendrocytes; C2, C3), anti-GFAP (astrocytes; C5, C6), anti-Mac-1 (microglia/macrophages; C8, C9), and NeuN antibody (neurons; C11, C12). Note that cPLA2 GIVA is expressed in oligodendrocytes and neurons (arrows), but not in astrocytes or microglia/macrophages (arrowheads). D, E) Time course of locomotor recovery evaluated using the BMS (D) and locomotor BMS subscores (E). Note that animals treated with AX059 (n=7) show significantly worse motor skills from 10 to 28 d after SCI in the BMS but not in the subscores compared to vehicle-treated mice (n=7). F) Quantification of tissue sparing assessed by staining for GFAP at 28 d after SCI. AX059-treated mice display significant loss of tissue compared with wild-type littermates at the epicenter site and in adjacent areas. G, H) Neuron survival in the ventral horns assessed from tissue sections stained with cresyl violet (G) and myelin sparing in the lateral funiculi assessed from sections stained with Luxol fast blue (H) at 28 d after SCI. Note that animals treated with AX059 display significantly greater loss of neurons and myelin. I) Quantification of serotonergic fibers at 1000 μm caudal to the epicenter 28 d after SCI. Mice treated with AX059 show no effect in sparing of serotonergic fibers. *P < 0.05.
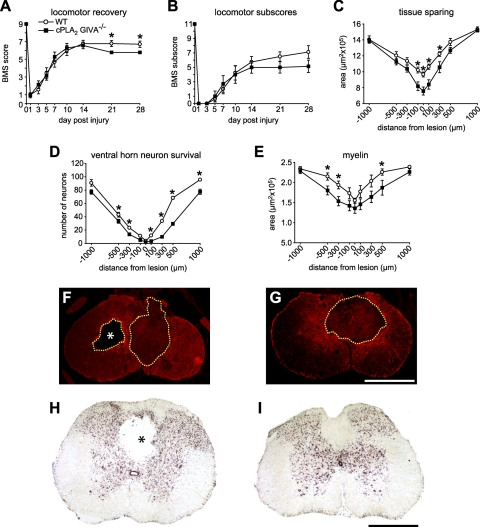
The functional role of cPLA2 in SCI was assessed using AX059, a 2-oxoamide compound, which is a highly potent and selective inhibitor for cPLA2 (Fig. 1 and refs. 5, 21–25). Locomotor recovery was assessed using the 9-point BMS, in which a score of 0 indicates complete paralysis, and a score of 9 represents normal locomotion. To our surprise, locomotor recovery was worse in mice treated with AX059 than in vehicle-treated controls (Fig. 2D, E). Histological analyses revealed that AX059 treatment significantly worsened tissue sparing, neuronal survival, and myelin sparing in areas adjacent to the lesion epicenter (Fig. 2F–H). Similar results were also obtained after inducing SCI in cPLA2 GIVA−/− mice (Fig. 3). Thus, contrary to other models of CNS inflammation, such as brain ischemia or EAE, in which cPLA2 has been shown to cause tissue damage (5, 6, 8, 9, 29), our present data indicate a protective role for cPLA2 after SCI.
Figure 3.
A, B) Time course of locomotor recovery, evaluated using the BMS (A) and locomotor BMS subscores (B) in cPLA2-null mice. Note that animals lacking cPLA2 GIVA (n=7) show significantly worse motor skills at 21 and 28 d after SCI in the BMS but not in the subscores as compared to wild-type littermates (n=7). C) Quantification of tissue sparing assessed by staining for GFAP at 28 d after SCI. cPLA2 GIVA−/− mice display significant loss of tissue compared with wild-type littermates at the epicenter site and in adjacent areas. D, E) Neuron survival in the ventral horns assessed from tissue sections stained with cresyl violet (D) and myelin sparing in the lateral funiculi assessed from sections stained with Luxol fast blue (E) at 28 d after SCI. Note that animals lacking cPLA2 GIVA display significantly greater loss of neurons (D) and myelin (E) in areas adjacent to the epicenter of the lesion. F, G) Representative micrographs showing GFAP staining at the epicenter of the injury in cPLA2 GIVA−/− (F) and wild-type littermates (G) at 28 d after contusion. Note that the loss of tissue (outlined by dashed border) is greater in the cPLA2 GIVA−/−. Furthermore, there is greater evidence of cavitation (asterisk) in the cPLA2-null mice. H, I) Micrographs of cresyl violet-stained spinal cord sections at 1000 μm caudal to the lesion epicenter from cPLA2 GIVA−/− (H) and wild-type littermates (I). Note the presence of a cavitation (asterisk) in cPLA2 GIVA−/− mouse but not the wild-type mouse at this distance from the lesion. Scale bars = 500 μm. *P < 0.05.
Expression and role of iPLA2 GVIA in SCI
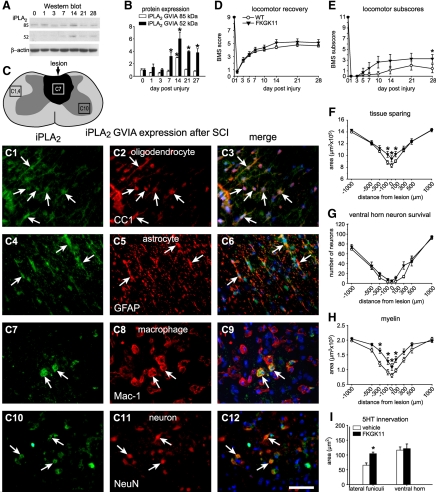
Quantification of iPLA2 GVIA protein expression by Western blotting showed ∼3-fold increase at 14 dpi. iPLA2 has ankyrin-like repeats that negatively control the activity of the enzyme. Removal of these ankyrin-like repeats from the full-length 85-kDa protein results in a smaller 52-kDa form that has enhanced activity (30). We detected the 52-kDa form of iPLA2 after SCI, which increased significantly from 7 to 28 dpi with a peak at 14 dpi (Fig. 4A, B). At 14 d, when its expression peaked after SCI, iPLA2 was expressed mainly in oligodendrocytes but also in some astrocytes, microglia/macrophages, and neurons in the injured cord (Fig. 4C).
Figure 4.
A) Western blot showing iPLA2 GVIA protein expression after SCI. The activated form (52 kDa) is detected from 7 to 28 dpi. B) Quantification of iPLA2 GVIA protein levels from 1 to 28 d after SCI by Western blotting (n=3/time point). The 84-kDa form is significantly increased at 14 dpi. The cleaved form of iPLA2 (∼50 kDa) is significantly increased from 7 to 28 d after SCI, peaking at 14 dpi. C) Line drawing of the contused spinal cord shows the lesion in dark shade. The 3 areas outlined by the rectangles indicate regions from which the micrographs in panels C1–C12 were obtained. Panels illustrate double immunofluorescence images showing colabeling (arrows) of iPLA2 GVIA (green; C1, C4, C7, C10) with anti-CC-1 (oligodendrocytes; C2, C3), anti-GFAP (astrocytes; C5, C6), anti-Mac-1 (microglia/macrophages; C8, C9), and anti-NeuN (neurons; C11, C12). Note that iPLA2 GVIA is mainly expressed in oligodendrocytes but also in some of the other cell types (arrows). D, E) Time course of locomotor recovery evaluated using the BMS (D) and locomotor BMS subscores (E). Treatment with FKGK11 (n=9) results only in significantly better BMS subscores at 28 d after SCI as compared to vehicle-treated mice (n=9; E). These subscores evaluate finer aspects of locomotor control. No differences were seen in the main BMS scores (D). F) Treatment with FKGK11 shows greater tissue sparing at the epicenter and in adjacent areas at 28 d after SCI. G) No differences were seen in the survival of neurons in the ventral horn in mice treated with FKGK11. H) Treatment with FKGK11 results in greater sparing of myelin, assessed by Luxol fast blue staining, in areas adjacent to the lesion epicenter at 28 d after SCI. I) Quantification of serotonergic fibers at 1000 μm caudal to the epicenter at 28 d after SCI. Mice treated with FKGK11 show significantly greater sparing of serotonergic fibers in the lateral funiculi. Scale bar = 50 μm. *P < 0.05.
We next assessed the role of iPLA2 after SCI using FKGK11, a fluoroketone compound, which is a highly selective and potent iPLA2 inhibitor (Fig. 1 and refs. 5, 11, 26). Although no differences were seen in the BMS scores after FKGK11 administration (Fig. 4D), the BMS subscores, which rate finer aspects of locomotor control, revealed a significant improvement at d 28 after SCI (Fig. 4E). Inhibiting iPLA2 also resulted in significantly greater tissue and myelin sparing and ∼40% increase in serotonergic (5-HT) fibers in the lateral white matter (Fig. 4F–I). FKGK11, however, had no effect on neuronal survival (Fig. 4G). These data suggest that iPLA2 is likely to have only a minimal detrimental effect after SCI.
Expression and role of sPLA2 GIIA in SCI
sPLA2 GIIA protein was detected at very low levels in the uninjured cord and increased rapidly from 1 to 28 dpi after SCI. Protein levels for sPLA2 were up-regulated from d 1 to 7, and peaked at d 3 (Fig. 5A, B). At 3 and 7 dpi, sPLA2 is seen mainly in astrocytes and oligodendrocytes but also in some neurons and microglia/macrophages (Fig. 5C).
Figure 5.
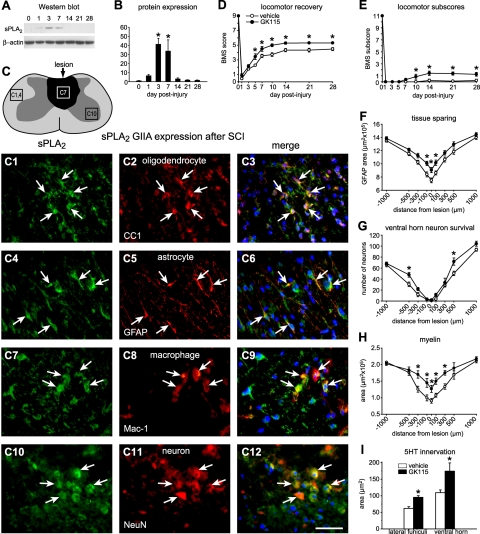
A) Western blot showing sPLA2 GIIA protein expression after SCI. B) Quantification of sPLA2 group GIIA protein levels from 1 to 28 d after SCI, assessed by Western blotting (n=3/time point) shows that expression is significantly increased at 3 and 7 d after SCI. C) Line drawing of the contused spinal cord shows the lesion in dark shade. The 3 areas outlined by the rectangles indicate regions from which the micrographs shown in panels C1–C12 were obtained. Panels illustrate double immunofluorescence images of sPLA2 GIIA (green; C1, C4, C7, C10) colabeled with anti-CC-1 (oligodendrocytes; C2, C3), astrocytes; C5, C6), anti-Mac-1 (microglia/macrophages; C8, C9), and anti-NeuN (neurons; C11, C12). sPLA2 GIIA is mainly expressed in astrocytes and oligodendrocytes, although some microglia/macrophages and neurons also express it (arrows). D) Time course of locomotor recovery evaluated by the BMS scores. Treatment with GK115 (n=8) shows significant improvement in the BMS scores starting from 5 d after SCI as compared to vehicle-treated mice (n=8). E) GK115-treated mice also display improvement in the finer aspects of locomotor control. F) GK115-treated animals show significantly greater tissue sparing at the lesion epicenter and adjacent regions. G) Mice treated with GK115 also have significantly greater neuron survival in the ventral horn in regions located at 500 μm rostral and caudal to the lesion epicenter. H) Mice treated with GK115 show a marked reduction of myelin loss in the epicenter and 300 μm rostral and caudal to the lesion site. I) Serotonergic innervation 1000 μm caudal to the lesion epicenter was also significantly greater in mice treated with GK115. Scale bar = 50 μm. *P < 0.05.
We next assessed the role of sPLA2 after SCI using the novel amide inhibitor GK115, which is highly selective for sPLA2 (Fig. 1 and ref. 27). Daily administration of GK115, starting 1 h after injury, significantly improved locomotor function as evaluated by the BMS score, beginning at 5 dpi until d 28, the longest time period examined (Fig. 5D). In addition, BMS subscores also improved significantly with the GK115 treatment (Fig. 5E). In contrast to these experiments done on BALB/c mice, the sPLA2 inhibitor GK115 did not have any effect on locomotor recovery after SCI in C57BL/6 mice (Supplemental Fig. S1), which have a naturally occurring null mutation of sPLA2GIIA (31). The latter evidence provides additional support that sPLA2 GIIA has detrimental effects after SCI. Histologically, treatment with GK115 resulted in significant tissue protection (Fig. 5F) and myelin sparing (Fig. 5H) at the lesion epicenter and in adjacent areas. GK115 treatment also led to significantly greater neuronal survival in the ventral horn 500 μm rostral and caudal to the lesion epicenter (Fig. 5G), and a 50% increase in serotonergic fibers in the lateral white matter and ventral horns (Fig. 5I).
We also tested the effects of a 2-oxoamide compound, AX115 (Fig. 1), which is a partial pan-inhibitor of cPLA2, iPLA2, and sPLA2 (5, 27). We found that treatment with AX115 after SCI markedly enhanced locomotor recovery and the various histopathological outcome measures examined, including tissue, myelin, and neuronal sparing, and greater serotonergic innervation (Fig. 6). The effects of AX115 are greater than those seen with GK115 (sPLA2 inhibitor) or FKGK11 (iPLA2 inhibitor). This finding suggests that AX115 may not be mediating its effects entirely by partially blocking sPLA2 or iPLA2 but also via some other effects (see below). Interestingly, treatment of SCI with FKGK2, a potent fluoroketone pan-PLA2 inhibitor that almost completely blocks all three PLA2s (Fig. 1 and refs. 5, 26), had detrimental effects in SCI, causing worsening of locomotor recovery, increased tissue damage, and worsening of other histological parameters (Fig. 7).
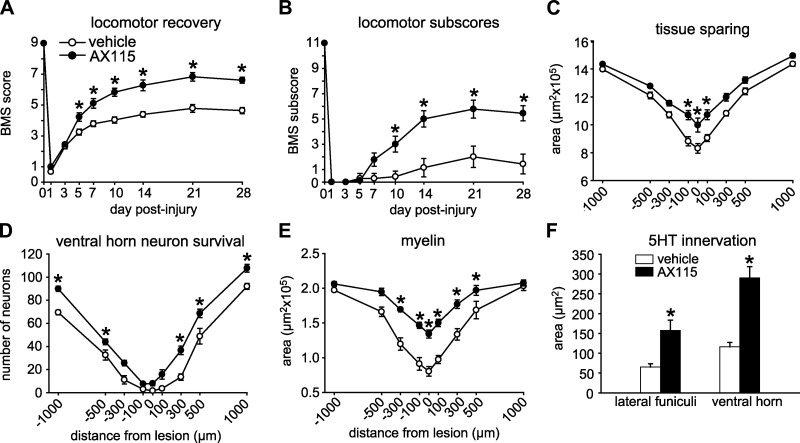
Figure 6.
A) Treatment with AX115 (n=9) shows marked improvement in the BMS scores starting from 5 d after SCI as compared to vehicle-treated mice (n=9). B) AX115-treated mice also display a marked improvement in the finer aspects of locomotor control, showing an increase of 4 points in the BMS subscores. C) AX115-treated animals show a significant amount of tissue sparing at the lesion epicenter and adjacent regions. D) Mice treated with AX115 have significantly greater neuron survival in the ventral horn in regions ranging from 300 to 1000 μm rostral and caudal to the lesion epicenter. E) AX115-treated mice also show a marked reduction of myelin loss in the epicenter and 300 μm rostral and 500 μm caudal to the lesion site. F) Serotonergic innervation 1000 μm caudal to the lesion epicenter is also greater in AX115-treated mice. *P < 0.05.
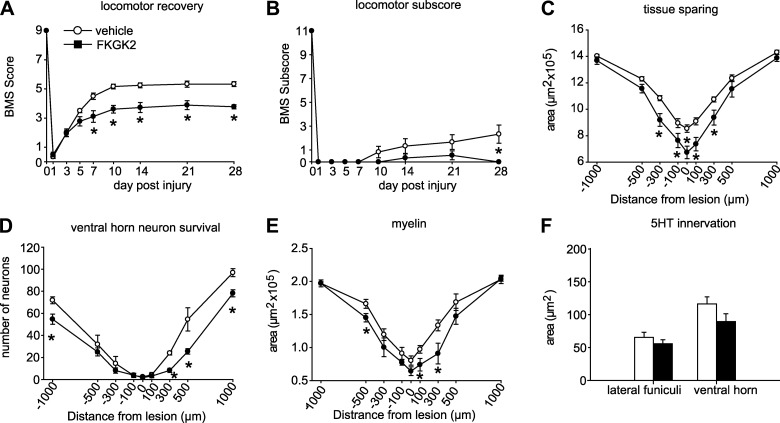
Figure 7.
Effects of treatment with the strong pan-PLA2 inhibitor (FKGK2) on locomotor and histological changes after SCI. A) Locomotor recovery evaluated using the BMS analysis shows that FKGK2-treated mice have markedly poor locomotor recovery starting as early as 7 d after SCI. B) The finer aspects of locomotor control assessed with the BMS subscore analysis also showed worse outcome in mice treated with FKGK2 at 28 d after SCI. C–F) Mice treated with FKGK2 also showed significantly less tissue sparing (C), less neuronal survival (D), less spared myelin at and adjacent to the epicenter (E), and no difference in serotonergic innervation (F) at 1000 μm caudal to the lesion. n = 9/group. *P < 0.05.
Potential crosstalk between PLA2s mediates protection after SCI via the prostaglandin EP1 receptor
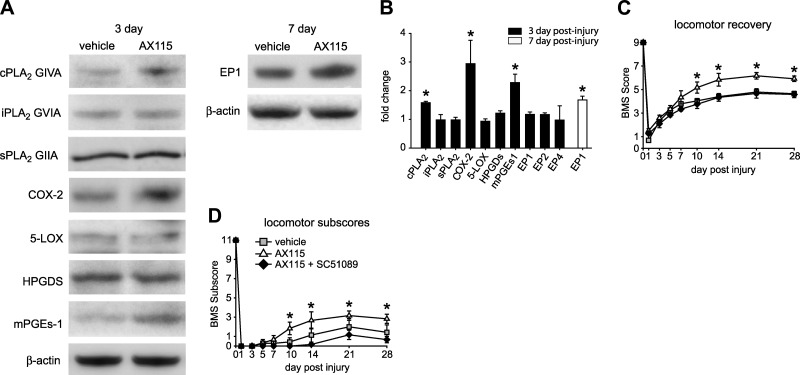
As AX115 was most effective in promoting locomotor recovery and neuroprotection, we sought to assess potential mechanisms by which it could mediate these effects. Because of evidence of crosstalk between different forms of PLA2 (4, 32), we first assessed whether AX115 modulates the protein levels of cPLA2, iPLA2, and sPLA2. After contusion injury, mice were treated with AX115, and the spinal cords were taken at 3 and 7 dpi for Western blot analysis for PLA2s and their downstream enzymes. Interestingly, the protein level of cPLA2 but not iPLA2 or sPLA2 is increased in the injured spinal cord of mice treated with AX115 at 3 dpi (Fig. 8A, B). We next examined the expression of two enzymes downstream of cPLA2, namely cyclooxygenase-2 (COX-2) and 5-lipoxygenase (5-LOX). The expression of COX-2, but not 5-LOX, was increased in AX115-treated mice at 3 dpi (Fig. 8A, B), suggesting higher prostaglandin (PG) production in the spinal cord of mice treated with AX115. We therefore assessed the expression of the synthases that regulate prostaglandin E2 and D2 expression, namely microsomal-PGE2 (mPGEs-1) and hematopeitic-PGD2 synthase (HPGDS). We found that mPGEs-1, but not HPGDS, is increased in the lesioned spinal cord of AX115-treated mice at 3 dpi (Fig. 8A, B), which suggests higher PGE2 in the spinal cord of mice treated with AX115. As PGE2 acts on 4 G-protein receptors (EP1–4), we assessed whether any changes occurred in the expression at the protein level of the three EP receptors, which showed increases in mRNA after SCI (EP1, EP2, and EP4; data not shown). The injured spinal cord showed very high levels of expression of EP1 but very low levels for EP2 and EP4. Furthermore, treatment with AX115 resulted in increased protein levels for EP1 at 7dpi (Fig. 8A, B), while levels for EP2 and EP4 did not change (data not shown). These data suggest that the protective effects of AX115 in SCI may be mediated, at least in part, via PGE2 and EP1. To confirm these observations, we next assessed the effects of AX115 in combination with an EP1 antagonist (SC51089) on locomotor recovery after SCI. The beneficial effect of AX115 on locomotor control was blocked when EP1 was inhibited (Fig. 8C, D). EP1 antagonist treatment alone did not cause any change in locomotor recovery (data not shown). As the free acid metabolite of AX115 inhibits sPLA2, these results suggest that sPLA2 may negatively regulate cPLA2 expression and modulate further downstream pathway (COX-2, mPGEs-1 and EP1) that mediate protection after SCI.
Figure 8.
A, B) Effect of AX115 on the pathways downstream of PLA2 after SCI. A) Western blots of spinal cord tissue taken 3 and 7 d after SCI. B) Western blot analysis of spinal cord tissue taken 3 d after SCI (n=3/time point) shows that in mice treated with AX115, cPLA2 GIVA is increased ∼60% as compared to vehicle-treated mice, while iPLA2 GVIA and sPLA2 GIIA showed no change. At the same time point, COX-2 and mPGEs-1 were increased >2-fold in mice treated with AX115. Similar results were also observed at 7 dpi (data not shown). No changes were found in the expression of 5-LOX, and HPGDS. No changes were found in iPLA2, sPLA2, 5-LOX, or HPGDS at 7 dpi either (data not shown). These results suggest that AX115 appears to have an effect on the cPLA2, COX-2, prostaglandin E pathway. EP1 receptor expression showed a delayed increase of ∼50% at 7 dpi in mice treated with AX115, but no differences were detected at 3 dpi. No changes in expression were seen in EP2 and EP4. C, D) Treatment with SC51089, an EP1 antagonist, significantly reduced the beneficial effects of AX115 on locomotor recovery starting from d 7 onwards, as seen with the BMS analysis (C), and from d 10 onwards in the BMS subscores (D) (vehicle n=9; AX115 n=7; AX115 + SC51089 n=7). *P < 0.05.
DISCUSSION
In this study, we have characterized the expression and role of different members of the PLA2 superfamily in SCI. We show that of the 14 PLA2 forms expressed in mice, only cPLA2 GIVA, iPLA2 GVIA, and sPLA2 GIIA are up-regulated after SCI. We also provide evidence that these three PLA2 forms have differing roles in SCI.
Phospholipase A2s play important roles in several inflammatory conditions in the central and peripheral nervous system (5, 6, 8, 10, 11). One way this enzyme can play a role in inflammation is through the release of fatty acids from phospholipids, such as the release of arachidonic acid, a precursor of proinflammatory eicosanoids. The principal pathways of arachidonic acid metabolism are the lipoxygenase pathway, which produces a collection of leukotrienes, and the cyclooxygenase pathway that produces prostaglandins and thromboxanes (33). Several lines of evidence suggest an involvement of arachidonic acid and its metabolites in the pathophysiology of SCI. Intravenous administration of arachidonic acid increased functional deficits after SCI (34). In addition, lipoxygenase gene deletion or administration of selective COX-2 inhibitors resulted in enhanced locomotor recovery and tissue sparing after SCI (35–38). However, these interventions block only some parts of the pathway downstream of arachidonic acid. In addition, one of the metabolites of PLA2 action is the production of lysophophatidylcholine a potent demyelinating agent and one capable of inducing proinflammatory chemokine and cytokine expression (12, 13). Therefore, blocking the appropriate PLA2s rather than one of the downstream enzymes or metabolites may be a more effective therapeutic target for SCI.
Two groups have attempted previously to assess the role of PLA2 in SCI. One reported that when a nonmammalian form of PLA2 found in bee venom (sPLA2 GIII) was injected directly into the normal uninjured rat spinal cord, it caused demyelination, tissue damage, and functional impairment (16, 39). The researchers also showed similar effects with intraspinal injection of melittin into the normal uninjured spinal cord. Melittin is the principal active component of bee venom and is a stimulator of secreted phospholipase A2. In other work, they also showed in cell culture that recombinant human sPLA2 GIIA induced a dose-dependent cytotoxicity of oligodendrocyte precursors (17). Another group assessed the effects of arachidonyl trifluoromethyl ketone (AACOCF3), a nonselective PLA2 inhibitor, for 1 wk after SCI in rats and reported a modest effect on locomotor recovery and reduction of neuronal and oligodendrocyte loss (18). As mentioned previously, AACOCF3 is a nonselective inhibitor that blocks both cPLA2 and iPLA2 (19). Our work now shows that cPLA2 and iPLA2 play divergent roles in SCI.
Among the PLA2 superfamily members, cPLA2 shows the strongest preference for arachidonic acid at the sn-2 position in phospholipids. cPLA2-null mice fail to generate arachidonic acid metabolites after brain injury (8, 9). We also showed previously that the cPLA2 inhibitor, AX059, blocked the hydrolysis of arachidonic acid from phospholipids in EAE (5). Several studies have reported a deleterious role for cPLA2 in CNS ischemia (8, 9), EAE (5–7), and Alzheimer disease (40). In contrast to the detrimental effect of cPLA2 in these neuroinflammatory conditions, in the present study we show the opposite in SCI, namely, that cPLA2 plays a beneficial role. Our data revealed that mice treated with AX059, a selective and potent inhibitor for cPLA2, as well as cPLA2-null mice, showed greater locomotor deficits and tissue loss after SCI. The cPLA2 inhibitor and cPLA2-null mice line used here were also used in the earlier studies on EAE (5) and brain ischemia (9), respectively, pointing to the striking difference in the role of cPLA2 in cerebral ischemia, EAE and SCI. We found that cPLA2 was expressed in neurons and oligodendrocytes in the spinal cord after injury, which is in agreement with an earlier report showing cPLA2 expression in neurons and oligodendrocytes in rats after SCI (16). We found that cPLA2-null mice and wild-type mice treated with AX059 displayed greater neuronal and myelin loss after injury, suggesting that inhibition or deletion of cPLA2 make these cells more vulnerable. Thus, contrary to other models of CNS disorders in which cPLA2 contributes to tissue damage (6, 8, 9, 29), our present data indicate a protective role for cPLA2 after SCI. Interestingly, cPLA2 has previously been shown to be required for the survival of cortical and hippocampal neurons in vitro (41) and to exert a protective role in autoimmune diabetes in mice (42) and in human embryonic kidney cells after calcium overexposure (43).
Another novel finding of this study is that iPLA2 GVIA is up-regulated after SCI. We recently reported that iPLA2 is involved in the onset and progression of EAE and that treatment with FKGK11, a potent and highly selective iPLA2 inhibitor, ameliorated demyelination and progression of the disease (5). We show here using the same iPLA2 inhibitor that iPLA2 appears to play a minor detrimental role in SCI. Although mice treated with FKGK11 showed only an effect in the locomotor BMS subscore, the histological outcomes showed clear improvements as compared to controls. Our data also revealed that iPLA2 is expressed mainly in oligodendrocytes after lesion and that treatment with FKGK11 reduced myelin loss after SCI. The molecular mechanisms involved in iPLA2 cytotoxicity are not known at present. However, Lauber et al. (30) showed that LPC generated by the actions of the lower-molecular-mass forms of iPLA2 (52 kDa), but not via cPLA2, acts as an “eat me” signal for macrophages. In the present study, we observed the expression of the 52-kDa form of iPLA2 starting at 7 dpi, which may suggest that LPC might also be generated by iPLA2 after SCI. Evidence indicates that LPC, which acts as a potent demyelinating agent (12, 13, 44), also induces the expression of chemokines, cytokines, and cell adhesion molecules that are important for the recruitment and activation of immune cells into the CNS (12–15). Our recent study on the role of intracellular PLA2s in myelin breakdown and Wallerian degeneration after sciatic nerve injury revealed that blocking iPLA2 with the FKGK11 inhibitor reduces macrophage recruitment and myelin clearance, and lowers the expression of proinflammatory cytokines (11). In addition, LPC has high affinity to C-reactive protein and IgM antibodies leading to the activation of the complement pathway (10), which is involved in secondary damage after SCI (45). Although further studies are needed to fully elucidate the detrimental effects of iPLA2, these data suggest that LPC generated by the lower-molecular-mass forms of iPLA2 might be responsible for demyelination after SCI. Therefore, iPLA2, in contrast to cPLA2, is likely to contribute to some of the secondary damage in SCI. This finding may also explain the modest effect reported previously after treatment with AACOCF3 in SCI in rats (18). AACOCF3 is a nonselective inhibitor that blocks both cPLA2 and iPLA2 (19). Our results suggest that AACOCF3 would block not only the detrimental effects of iPLA2 but also the beneficial effects of cPLA2.
We found that the expression of sPLA2 GIIA is increased at the protein level by Western blot analysis between d 1 and 7 after lesion. Our data now provide the first direct evidence that sPLA2 GIIA that is up-regulated in the injured mouse spinal cord contributes to secondary damage and functional loss after SCI. This was shown with the treatments with the sPLA2 selective inhibitor GK115 on BALB/c mice. In addition, this inhibitor did not have any effect on locomotor recovery after SCI in the C57BL/6 mouse strain, which has a naturally occurring null mutation of sPLA2 GIIA (31).
An important finding is that the inhibitor that partially blocks all three PLA2s at about the 50% level (AX115) yielded the most beneficial effects in enhancing tissue protection and neuronal, myelin, and axonal sparing, as well as locomotor recovery after SCI. Interestingly, although AX115 partially blocks sPLA2 and iPLA2 activity, it also appeared to enhance the expression of cPLA2, which we have shown has protective or beneficial effects in SCI. However, complete blocking of all three PLA2s by FKGK2 was detrimental. Our studies revealed that AX115 led to an up-regulation of cPLA2, which is beneficial in SCI. Previous studies in rat ovalbumin-induced bronchoconstriction have shown that sPLA2 can negatively regulate the expression of cPLA2 (32). Our results with AX115 may, therefore, be due at least in part to its ability to block sPLA2, which negatively regulates cPLA2 expression. We also found the cPLA2 downstream pathway involved in AX115-mediated protection was dependent on COX-2, mPGES-1, and EP1 receptor signaling, since the beneficial effects of AX115 were blocked with an EP1 antagonist. In agreement with these results, a previous work also showed that the protective effects of cPLA2 observed in autoimmune diabetes were also dependent on PGE2 (42). Moreover, the protective effects of cPLA2 in human embryonic kidney cells to calcium overexposure were also dependent on prostaglandins (43). In contrast, in a model of cerebral ischemia, cPLA2 and PGE2 via EP1 receptor signaling exacerbates tissue damage (46, 47), pointing to the diversity of biological effects of the cPLA2-PGE2 pathway in different CNS injuries.
In summary, our results show that 3 members of the PLA2 superfamily (cPLA2 GIVA, iPLA2 GVIA, and sPLA2 GIIA) play divergent roles in SCI. Our data reveal that the different PLA2s have distinct and even opposite effects in SCI. Our work also suggests that drugs such as AX115 that inhibit detrimental forms of PLA2 (sPLA2 and iPLA2) and up-regulate protective ones (cPLA2) may be useful candidates for the treatment of acute SCI.
Supplementary Material
Acknowledgments
The authors thank Hiba Kazak and Eva Santos-Nogueira for technical assistance and Margaret Attiwell for help with preparing the illustrations.
This work was supported by grants from the Canadian Institutes of Health Research (CIHR) and the Wings for Life (Austria) to S.D.; from the European Social Fund and National Resources (EPEAEK program) to G.K.; and from the U.S. National Institutes of Health (RO1 GM20508 and R01 GM64611) to E.A.D. R.L.V. was the recipient of a CIHR postdoctoral fellowship.
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
REFERENCES
- 1. Popovich P. G., Longbrake E. E. (2008) Can the immune system be harnessed to repair the CNS? Nat. Rev. 9, 481–493 [DOI] [PubMed] [Google Scholar]
- 2. Kwon B. K., Fisher C. G., Dvorak M. F., Tetzlaff W. (2005) Strategies to promote neural repair and regeneration after spinal cord injury. Spine 30, S3–S13 [DOI] [PubMed] [Google Scholar]
- 3. Murakami M., Nakatani Y., Atsumi G., Inoue K., Kudo I. (1997) Regulatory functions of phospholipase A2. Crit. Rev. Immunol. 17, 225–283 [DOI] [PubMed] [Google Scholar]
- 4. Murakami M., Kudo I. (2001) Diversity and regulatory functions of mammalian secretory phospholipase A2s. Adv. Immunol. 77, 163–194 [DOI] [PubMed] [Google Scholar]
- 5. Kalyvas A., Baskakis C., Magrioti V., Constantinou-Kokotou V., Stephens D., Lopez-Vales R., Lu J. Q., Yong V. W., Dennis E. A., Kokotos G., David S. (2009) Differing roles for members of the phospholipase A2 superfamily in experimental autoimmune encephalomyelitis. Brain 132(Pt. 5), 1221–1235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kalyvas A., David S. (2004) Cytosolic phospholipase A2 plays a key role in the pathogenesis of multiple sclerosis-like disease. Neuron 41, 323–335 [DOI] [PubMed] [Google Scholar]
- 7. Marusic S., Leach M. W., Pelker J. W., Azoitei M. L., Uozumi N., Cui J., Shen M. W., DeClercq C. M., Miyashiro J. S., Carito B. A., Thakker P., Simmons D. L., Leonard J. P., Shimizu T., Clark J. D. (2005) Cytosolic phospholipase A2 alpha-deficient mice are resistant to experimental autoimmune encephalomyelitis. J. Exp. Med. 202, 841–851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bonventre J. V., Huang Z., Taheri M. R., O'Leary E., Li E., Moskowitz M. A., Sapirstein A. (1997) Reduced fertility and postischaemic brain injury in mice deficient in cytosolic phospholipase A2. Nature 390, 622–625 [DOI] [PubMed] [Google Scholar]
- 9. Tabuchi S., Uozumi N., Ishii S., Shimizu Y., Watanabe T., Shimizu T. (2003) Mice deficient in cytosolic phospholipase A2 are less susceptible to cerebral ischemia/reperfusion injury. Acta Neurochir. 86, 169–172 [DOI] [PubMed] [Google Scholar]
- 10. De S., Trigueros M. A., Kalyvas A., David S. (2003) Phospholipase A2 plays an important role in myelin breakdown and phagocytosis during wallerian degeneration. Mol. Cell. Neurosci. 24, 753–765 [DOI] [PubMed] [Google Scholar]
- 11. Lopez-Vales R., Navarro X., Shimizu T., Baskakis C., Kokotos G., Constantinou-Kokotou V., Stephens D., Dennis E. A., David S. (2008) Intracellular phospholipase A(2) group IVA and group VIA play important roles in wallerian degeneration and axon regeneration after peripheral nerve injury. Brain 131, 2620–2631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ousman S. S., David S. (2000) Lysophosphatidylcholine induces rapid recruitment and activation of macrophages in the adult mouse spinal cord. Glia 30, 92–104 [PubMed] [Google Scholar]
- 13. Ousman S. S., David S. (2001) MIP-1alpha, MCP-1, GM-CSF, and TNF-alpha control the immune cell response that mediates rapid phagocytosis of myelin from the adult mouse spinal cord. J. Neurosci. 21, 4649–4656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ryborg A. K., Deleuran B., Sogaard H., Kragballe K. (2000) Intracutaneous injection of lysophosphatidylcholine induces skin inflammation and accumulation of leukocytes. Acta Derm. Venereol. 80, 242–246 [DOI] [PubMed] [Google Scholar]
- 15. Ryborg A. K., Deleuran B., Thestrup-Pedersen K., Kragballe K. (1994) Lysophosphatidylcholine: a chemoattractant to human T lymphocytes. Arch. Dermatol. Res. 286, 462–465 [DOI] [PubMed] [Google Scholar]
- 16. Liu N. K., Zhang Y. P., Titsworth W. L., Jiang X., Han S., Lu P. H., Shields C. B., Xu X. M. (2006) A novel role of phospholipase A2 in mediating spinal cord secondary injury. Ann. Neurol. 59, 606–619 [DOI] [PubMed] [Google Scholar]
- 17. Titsworth W. L., Cheng X., Ke Y., Deng L., Burckardt K. A., Pendleton C., Liu N. K., Shao H., Cao Q. L., Xu X. M. (2009) Differential expression of sPLA2 following spinal cord injury and a functional role for sPLA2-IIA in mediating oligodendrocyte death. Glia 57, 1521–1537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Huang W., Bhavsar A., Ward R. E., Hall J. C., Priestley J. V., Michael-Titus A. T. (2009) Arachidonyl trifluoromethyl ketone is neuroprotective after spinal cord injury. J. Neurotrauma 26, 1429–1434 [DOI] [PubMed] [Google Scholar]
- 19. Ghomashchi F., Loo R. W., Balsinde J., Bartoli F., Apitz-Castro R., Clark J. D., Dennis E. A., Gelb M. H. (1999) Trifluoromethyl ketones and fluoromethyl phosphonates as selective inhibitors of group IV and VI phospholipases A2: structure-function studies with vesicle, micelle, and membrane assays. Biochim. Biophys. Acta 1420, 45–56 [DOI] [PubMed] [Google Scholar]
- 20. Ghasemlou N., Kerr B. J., David S. (2005) Tissue displacement and impact force are important contributors to outcome after spinal cord contusion injury. Exp. Neurol. 196, 9–17 [DOI] [PubMed] [Google Scholar]
- 21. Kokotos G., Six D. A., Loukas V., Smith T., Constantinou-Kokotou V., Hadjipavlou-Litina D., Kotsovolou S., Chiou A., Beltzner C. C., Dennis E. A. (2004) Inhibition of group IVA cytosolic phospholipase A2 by novel 2-oxoamides in vitro, in cells, and in vivo. J. Med. Chem. 47, 3615–3628 [DOI] [PubMed] [Google Scholar]
- 22. Stephens D., Barbayianni E., Constantinou-Kokotou V., Peristeraki A., Six D. A., Cooper J., Harkewicz R., Deems R. A., Dennis E. A., Kokotos G. (2006) Differential inhibition of group IVA and group VIA phospholipases A2 by 2-oxoamides. J. Med. Chem. 49, 2821–2828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Six D. A., Barbayianni E., Loukas V., Constantinou-Kokotou V., Hadjipavlou-Litina D., Stephens D., Wong A. C., Magrioti V., Moutevelis-Minakakis P., Baker S. F., Dennis E. A., Kokotos G. (2007) Structure-activity relationship of 2-oxoamide inhibition of group IVA cytosolic phospholipase A2 and group V secreted phospholipase A2. J. Med. Chem. 50, 4222–4235 [DOI] [PubMed] [Google Scholar]
- 24. Kokotos G., Kotsovolou S., Six D. A., Constantinou-Kokotou V., Beltzner C. C., Dennis E. A. (2002) Novel 2-oxoamide inhibitors of human group IVA phospholipase A(2). J. Med. Chem. 45, 2891–2893 [DOI] [PubMed] [Google Scholar]
- 25. Yaksh T. L., Kokotos G., Svensson C. I., Stephens D., Kokotos C. G., Fitzsimmons B., Hadjipavlou-Litina D., Hua X. Y., Dennis E. A. (2006) Systemic and intrathecal effects of a novel series of phospholipase A2 inhibitors on hyperalgesia and spinal prostaglandin E2 release. J. Pharmacol. Exper. Ther. 316, 466–475 [DOI] [PubMed] [Google Scholar]
- 26. Baskakis C., Magrioti V., Cotton N., Stephens D., Constantinou-Kokotou V., Dennis E. A., Kokotos G. (2008) Synthesis of polyfluoro ketones for selective inhibition of human phospholipase A2 enzymes. J. Med. Chem. 51, 8027–8037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Antonopoulou G., Barbayianni E., Magrioti V., Cotton N., Stephens D., Constantinou-Kokotou V., Dennis E. A., Kokotos G. (2008) Structure-activity relationships of natural and non-natural amino acid-based amide and 2-oxoamide inhibitors of human phospholipase A(2) enzymes. Bioorg. Med. Chem. 16, 10257–10269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Basso D. M., Fisher L. C., Anderson A. J., Jakeman L. B., McTigue D. M., Popovich P. G. (2006) Basso mouse scale for locomotion detects differences in recovery after spinal cord injury in five common mouse strains. J. Neurotrauma 23, 635–659 [DOI] [PubMed] [Google Scholar]
- 29. Arai K., Nakatani Y., Kudo I., Nishiyama N., Matsuki N. (2001) Phospholipase A2 mediates ischemic injury in the hippocampus: a regional difference of neuronal vulnerability. Eur. J. Neurosci. 13, 2319–2323 [DOI] [PubMed] [Google Scholar]
- 30. Lauber K., Bohn E., Krober S. M., Xiao Y. J., Blumenthal S. G., Lindemann R. K., Marini P., Wiedig C., Zobywalski A., Baksh S., Xu Y., Autenrieth I. B., Schulze-Osthoff K., Belka C., Stuhler G., Wesselborg S. (2003) Apoptotic cells induce migration of phagocytes via caspase-3-mediated release of a lipid attraction signal. Cell 113, 717–730 [DOI] [PubMed] [Google Scholar]
- 31. Kennedy B. P., Payette P., Mudgett J., Vadas P., Pruzanski W., Kwan M., Tang C., Rancourt D. E., Cromlish W. A. (1995) A natural disruption of the secretory group II phospholipase A2 gene in inbred mouse strains. J. Biol. Chem. 270, 22378–22385 [DOI] [PubMed] [Google Scholar]
- 32. Offer S., Yedgar S., Schwob O., Krimsky M., Bibi H., Eliraz A., Madar Z., Shoseyov D. (2005) Negative feedback between secretory and cytosolic phospholipase A2 and their opposing roles in ovalbumin-induced bronchoconstriction in rats. Am. J. Physiol. 288, L523–L529 [DOI] [PubMed] [Google Scholar]
- 33. Rocha P. N., Plumb T. J., Coffman T. M. (2003) Eicosanoids: lipid mediators of inflammation in transplantation. Springer Semin. Immunopathol. 25, 215–227 [DOI] [PubMed] [Google Scholar]
- 34. Huang W. L., King V. R., Curran O. E., Dyall S. C., Ward R. E., Lal N., Priestley J. V., Michael-Titus A. T. (2007) A combination of intravenous and dietary docosahexaenoic acid significantly improves outcome after spinal cord injury. Brain 130, 3004–3019 [DOI] [PubMed] [Google Scholar]
- 35. Genovese T., Mazzon E., Rossi A., Di Paola R., Cannavo G., Muia C., Crisafulli C., Bramanti P., Sautebin L., Cuzzocrea S. (2005) Involvement of 5-lipoxygenase in spinal cord injury. J. Neuroimmunol. 166, 55–64 [DOI] [PubMed] [Google Scholar]
- 36. Hains B. C., Yucra J. A., Hulsebosch C. E. (2001) Reduction of pathological and behavioral deficits following spinal cord contusion injury with the selective cyclooxygenase-2 inhibitor NS-398. J. Neurotrauma 18, 409–423 [DOI] [PubMed] [Google Scholar]
- 37. Lopez-Vales R., Garcia-Alias G., Guzman-Lenis M. S., Fores J., Casas C., Navarro X., Verdu E. (2006) Effects of COX-2 and iNOS inhibitors alone or in combination with olfactory ensheathing cell grafts after spinal cord injury. Spine 31, 1100–1106 [DOI] [PubMed] [Google Scholar]
- 38. Resnick D. K., Graham S. H., Dixon C. E., Marion D. W. (1998) Role of cyclooxygenase 2 in acute spinal cord injury. J. Neurotrauma 15, 1005–1013 [DOI] [PubMed] [Google Scholar]
- 39. Titsworth W. L., Onifer S. M., Liu N. K., Xu X. M. (2007) Focal phospholipases A2 group III injections induce cervical white matter injury and functional deficits with delayed recovery concomitant with Schwann cell remyelination. Exper. Neurol. 207, 150–162 [DOI] [PubMed] [Google Scholar]
- 40. Sanchez-Mejia R. O., Newman J. W., Toh S., Yu G. Q., Zhou Y., Halabisky B., Cisse M., Scearce-Levie K., Cheng I. H., Gan L., Palop J. J., Bonventre J. V., Mucke L. (2008) Phospholipase A2 reduction ameliorates cognitive deficits in a mouse model of Alzheimer's disease. Nat. Neurosci. 11, 1311–1318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Forlenza O. V., Mendes C. T., Marie S. K., Gattaz W. F. (2007) Inhibition of phospholipase A2 reduces neurite outgrowth and neuronal viability. Prostaglandins Leukot. Essent. Fatty Acids 76, 47–55 [DOI] [PubMed] [Google Scholar]
- 42. Oikawa Y., Yamato E., Tashiro F., Yamamoto M., Uozumi N., Shimada A., Shimizu T., Miyazaki J. (2005) Protective role for cytosolic phospholipase A2alpha in autoimmune diabetes of mice. FEBS Lett. 579, 3975–3978 [DOI] [PubMed] [Google Scholar]
- 43. Casas J., Gijon M. A., Vigo A. G., Crespo M. S., Balsinde J., Balboa M. A. (2006) Overexpression of cytosolic group IVA phospholipase A2 protects cells from Ca2+-dependent death. J. Biol. Chem. 281, 6106–6116 [DOI] [PubMed] [Google Scholar]
- 44. Hall S. M., Gregson N. A. (1971) The in vivo and ultrastructural effects of injection of lysophosphatidyl choline into myelinated peripheral nerve fibres of the adult mouse. J. Cell Sci. 9, 769–789 [DOI] [PubMed] [Google Scholar]
- 45. Qiao F., Atkinson C., Song H., Pannu R., Singh I., Tomlinson S. (2006) Complement plays an important role in spinal cord injury and represents a therapeutic target for improving recovery following trauma. Am. J. Pathol. 169, 1039–1047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kawano T., Anrather J., Zhou P., Park L., Wang G., Frys K. A., Kunz A., Cho S., Orio M., Iadecola C. (2006) Prostaglandin E2 EP1 receptors: downstream effectors of COX-2 neurotoxicity. Nat. Med. 12, 225–229 [DOI] [PubMed] [Google Scholar]
- 47. Ikeda-Matsuo Y., Ota A., Fukada T., Uematsu S., Akira S., Sasaki Y. (2006) Microsomal prostaglandin E synthase-1 is a critical factor of stroke-reperfusion injury. Proc. Natl. Acad. Sci. U. S. A. 103, 11790–11795 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.