Abstract
Growing evidence suggests that transcription factor signal transducer and activator of transcription (Stat) 3 may play an important regulatory role during inflammation. However, the function of Stat3 in acute lung injury (ALI) is largely unknown. In the current study, by using an adenoviral vector expressing a dominant-negative Stat3 isoform (Ad-Stat3-EVA), we determined the role of Stat3 in IgG immune complex (IC)-induced inflammatory responses and injury in the lung from C57BL/6J mice. We show that IgG IC-induced DNA binding activity of Stat3 in the lung was significantly inhibited by Stat3-EVA. We demonstrate that both lung vascular permeability (albumin leak) and lung myeloperoxidase accumulation in the Ad-Stat-EVA treated mice were substantially reduced when compared with values in mice receiving control virus (Ad-GFP) during the injury. Furthermore, intratracheal administration of Ad-Stat3-EVA caused significant decreases in the contents of neutrophils, inflammatory cytokines (TNF-α and IL-6), chemokines [keratinocyte cell-derived chemokine, macrophage inflammatory protein (MIP)-1α, and MIP-1β], and complement component C5a in bronchoalveolar lavage fluids. Using Stat3-specific small interfering RNA, we show that knocking down Stat3 expression in alveolar macrophages (MH-S cells) significantly reduced the production of proinflammatory mediators on IgG IC stimulation. These data suggest that Stat3 plays an essential role in the pathogenesis of IgG IC-induced ALI by mediating the acute inflammatory responses in the lung and alveolar macrophages.—Tang, H., Yan, C., Cao, J., Sarma, J. V., Haura, E. B., Wu, M., Gao, H. An essential role for Stat3 in regulating IgG immune complex-induced pulmonary inflammation.
Keywords: signal transducer and activator of transcription, ALI
Signal transducer and activator of transcription (Stat) 3 belongs to a family of 7 different transcription factors that play crucial roles in cytokine signaling. The function of Stat3 has been extensively studied in cell culture systems as well as in vivo. While genetic deletion of Stat3 causes early embryonic lethality, studies from tissue or cell-specific deletion of the Stat3 gene have suggested that Stat3 may play an important role during inflammation (1). Stat3 is expressed in the lung. Its function in lung during inflammation has recently attracted attention. For example, employing both knockout and transgenic approaches, expression of Stat3 in respiratory epithelial cells has been indicated as a key regulator in maintenance of surfactant homeostasis and lung function during hyperoxia-induced acute lung injury (ALI; refs. 2–4). Furthermore, Stat3 has been shown to play a crucial cytoprotective role in epithelial cell survival and maintenance of alveolar structures during the early phases of pulmonary adenoviral infection (5). Recently, Stat3 was reported to play a critical role in regulating surfactant lipid synthesis in both normal and LPS-injured lungs (6). Moreover, in Escherichia coli pneumonia, Stat3 has a beneficial effect by limiting infection and lung injury (7). Stat3 activation has also been reported in other ALI models, such as hemorrhagic shock, gastric acid aspiration, and acute pancreatitis (8, 9). Furthermore, a recent study using small molecule inhibitors linked Stat3 inhibition to the attenuated lung injury induced by LPS (10). These observations suggest that Stat3 may be a common pathway for mediating ALI. Thus, the role of Stat3 during lung inflammation is likely to be complex and begs further investigation.
The IgG immune complex (IC) model in the rodent lung has been extensively employed to investigate the molecular mechanisms of acute lung inflammatory injury (11). In this model, the intra-alveolar deposition of IgG IC activates alveolar macrophages, which results in robust formation of the early response cytokines including TNF-α. The proinflammatory cytokines induce expression of chemokines and adhesion molecules on leukocytes and endothelial/epithelial cells, all of which together induce a strong proinflammatory cascade that causes an acute damage in the lung. Growing evidence indicates that regulation of inflammatory-associated gene expression in the lung are mediated by a highly intricate network of transcription factors, especially NF-κB (11, 12). Interestingly, our recent studies in the IgG IC-induced lung injury model have shown that Stat3 is activated in both alveolar macrophages and whole lung extracts following IgG IC deposition (13). However, the biological relevance for these inductions is not known. In the current study, we determine the functional role of Stat3 in IgG IC-induced ALI. Using an adenoviral vector expressing a dominant-negative Stat3 isoform (Ad-Stat3-EVA) in vivo and a specific siRNA for Stat3 in vitro, we show that Stat3 plays an essential role in the pathogenesis of IgG IC-induced ALI by mediating acute inflammatory responses in the lung and alveolar macrophages.
MATERIALS AND METHODS
Animals and cell culture
In all experiments, specific pathogen-free male C57BL/6 mice obtained from the Jackson Laboratory (Bar Harbor, ME, USA) were used at the age of 8–12 wk (weighing 20–30 g). FcRγ-deficient mice were obtained from Taconic (Germantown, NY, USA). All procedures involving mice were approved by the Animal Care and Use Committee of Harvard Medical School. MH-S cells were obtained from American Type Culture Collection (Manassas, VA, USA). Cells were cultured in RPMI 1640 medium supplemented with 10 mM HEPES, 2 mM l-glutamine, 100 U/ml streptomycin,100 U/ml penicillin, and 10% (v/v) FBS.
Intratracheal administration of adenovirus
The construction of adenoviral vector for Stat3-EVA expression was described previously (14). Recombinant adenoviruses were purified by BD Adeno-X virus purification kit (BD Clontech, Palo Alto, CA, USA) and stored in aliquots at −80°C. The viral stocks were titered using Adeno-X rapid titer kit (BD Clontech). Unless otherwise indicated, 30 μl of adenovirus (5×108 PFU of Ad-Stat3-EVA or Ad-GFP control adenovirus) was administered to mice via an intratracheal injection, using a Hamilton syringe with a sterile 30-gauge needle. After 72 h, lungs were harvested for the analysis of Stat3 DNA binding activity, or mice were used for IgG IC-induced lung injury.
Experimental model of IgG IC-induced lung injury
Mice were anesthetized with intraperitoneal ketamine (100 mg/kg body weight; Fort Dodge Animal Health, Fort Dodge, IA, USA) and xylazine (12.5 mg/kg body weight; Ben Venue Laboratories, Benford, OH, USA) for sedation. The trachea was surgically exposed by a midline incision, and 120 μg of rabbit anti-BSA IgG (MP Biomedicals, Solon, OH, USA) in 40 μl of PBS was administered by tracheal puncture with a 30-gauge needle. The incision was closed by surgical clips, and 2 mg of bovine serum albumin (BSA; Sigma-Aldrich, St. Louis, MO, USA) in a volume of 200 μl was injected intravenously immediately thereafter. Control mice received anti-BSA or PBS intratracheally in the absence of an intravenous infusion of BSA.
Assessment of Stat3 activation by EMSA
Nuclear extracts of whole lung tissues were prepared, as described previously (15). Briefly, frozen lungs were homogenized in solution A containing 0.6% (v/v) Nonidet P-40, 150 mM NaCl, 10 mM HEPES (pH 7.9), 1 mM EDTA, 0.5 mM PMSF, and protease inhibitor cocktail (complete protease inhibitors; Roche, Indianapolis, IN, USA). Proteins were extracted from nuclei by incubation at 4°C with vigorous vortexing in solution B containing 420 mM NaCl, 20 mM HEPES (pH 7.9), 1.2 mM MgCl2, 0.2 mM EDTA, 25% (v/v) glycerol, 0.5 mM DTT, 0.5 mM PMSF, and protease inhibitor cocktail (Roche). Protein concentrations were determined by Bio-Rad protein assay (Bio-Rad, Hercules, CA, USA) using BSA as a reference standard. The EMSA probes were double-stranded oligonucleotides containing the Stat3 consensus sequence (5′-GATCCTTCTGGGAATTCCTAGATC-3′; Santa Cruz Biotechnology, Santa Cruz, CA, USA). The probe was end labeled with [γ-32P]ATP (3000 Ci/mmol at 10 mCi/ml; Amersham Biosciences, part of GE Healthcare Biosciences, Piscataway, NJ, USA). DNA-binding reactions were performed at room temperature as described previously (15). Samples were electrophoresed through 5.5% polyacrylamide gels in 1× TBE at 160 V for 2 h, dried under vacuum, and exposed to X-ray film.
Bronchoalveolar lavage (BAL) fluid analysis
BAL fluids were harvested at different time periods after IgG IC deposition for total leukocyte count, differential cell counts, and quantification of chemokine/cytokine production by ELISAs. For differential cell counts, the slides were quantified for neutrophils, macrophages, and lymphocytes by counting a total of 200 cells/slide in randomly selected high-powered fields (×400). BAL levels of TNF-α, IL-6, keratinocyte cell-derived chemokine (KC), macrophage inflammatory protein; (MIP)-1α, MIP-1β, and C5a were determined using ELISA kits (R&D Systems, Minneapolis, MN, USA) according to the instructions of the manufacturer.
Myeloperoxidase (MPO) assay
Lungs were perfused via the right ventricle with 10 ml of sterile PBS, snap-frozen, and stored at −80°C. To measure MPO activity, whole lungs were homogenized and sonicated in 50 mM potassium phosphate buffer containing 0.5% hexadecyltrimethylammonium bromide and 5 mM EDTA. After centrifugation at 12,000 g for 10 min at 4°C, 10 μl of supernatant fluids containing MPO was incubated with 250 μl of 100 mM potassium phosphate buffer containing 5 μg/ml hydrogen peroxide and 167 μg/ml o-dianisidine dihydrochloride (Sigma-Aldrich). The enzymatic activity was determined spectrophotometrically by measuring the kinetics in absorbance at 450 nm over 3 min using a 96-well plate reader (DTX880 Multimode Detector; Beckman, Brea, CA, USA; ref. 16).
Determination of albumin content in BAL fluids
Mouse albumin levels in BAL fluid were measured using a mouse albumin ELISA kit purchased from Bethyl Laboratories (Montgomery, TX, USA). The detection limit for this ELISA was 7 ng/ml.
Morphological assessment of lung injury
At 4 h after IgG IC deposition, lungs were fixed by intratracheal instillation of 0.8 ml of buffered formalin (10%; Fisher Scientific, Fairlawn, NJ, USA) and then further fixed in 10% buffered formalin solution for histological examination to evaluate the lung injury by tissue sectioning and staining with hematoxylin and eosin (H&E).
Stat3-siRNA transfection
MH-S cells were transfected with 600 nM control siRNA or Stat3 siRNA (Santa Cruz Biotechnology) by using Amaxa Nucleofector II device (Lonza Group, Basel, Switzerland), according to the manufacturer's instructions. At 24 h after transfection, the cells were incubated with 100 μg/ml IgG IC for different time periods. Total RNA was then isolated with the Trizol reagent (Invitrogen, Carlsbad, CA, USA) for RT-PCR analysis of Stat3 m RNA expression. Supernatants were collected for ELISA assays.
Detection of Stat3 expression by RT-PCR
RT-PCR was performed with 2 μg of total RNA using the 2-step RT-PCR system (Fisher Scientific, Pittsburgh, PA, USA). The following primers were used for PCR: Stat3, forward primer, 5′-CCCCCGGGCACCTTCCTACT-3′, and reverse primer, 5′-GGGCTCAGCACCTTCACCGTTATT-3′; and GAPDH, forward primer, 5′-GCCTCGTCTCATAGACAAGATG-3′, and reverse primer, 5′-CAGTAGACTCCACGACATAC-3′.
Statistical analysis
All values are expressed as means ± se. Values of P < 0.05 were considered significant. Data sets were analyzed using Student's t test or 1-way ANOVA, with individual group means being compared with the Student-Newman-Keuls multiple comparison test.
RESULTS
Ad-Stat3-EVA inhibited DNA binding activity of Stat3 in IgG IC- injured lungs
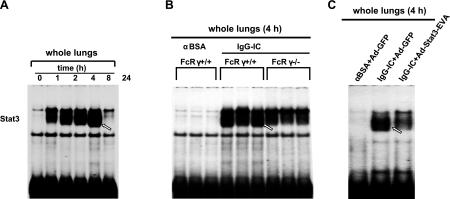
Our previous work in rats had shown that Stat3 was activated in the whole lung during IgG IC-induced lung injury (13). We evaluated the time course of Stat3 activation during IgG IC-induced lung injury in mice by EMSA. As shown in Fig. 1A, at time 0, low levels of constitutive Stat3 in lung nuclear extracts were observed. Stat3 activation was evident by 1 h and became strongest at 4 and 8 h. Subsequently, Stat3 binding activity dramatically decreased at 24 h after IC deposition. Three classes of activating FcγRs have been reported (FcγRI, FcγRIII, and FcγRIV), which share a common FcRγ-chain that is critical for receptor activation (17). Using FcRγ-chain-knockout mice, we then determined that activating FcγRs are involved in Stat3 activation in the inflamed lungs (Fig. 1B). To elucidate the significance of Stat3 activation in IgG IC-injured lungs, we employed an adenoviral vector expressing a dominant-negative Stat3 isoform (Ad-Stat3-EVA) for our study. Stat3-EVA has 2 mutations in the DNA binding site of Stat3, which prevents its binding to DNA but has no effect on tyrosine phosphorylation or dimerization (14). Thus, it can form dimers with endogenous Stat3; however, when expressed in cells, Stat3-EVA attenuates the ability of Stat3 to bind to its binding sites. To determine whether Ad-Stat3-EVA can inhibit Stat3 DNA binding activity in the lungs, we used a dose of 5.0 × 108 PFU for intratracheal virus administration. This dose has been shown to trigger no intrapulmonary inflammatory response (18). As shown in Fig. 1C, no Stat3 activation was detected in control lungs (intratracheally injected with control virus, Ad-GFP, in the absence of IgG IC deposition). A substantially increased DNA binding activity for Stat3 was observed in the IgG IC-injured lungs. However, IgG IC-induced DNA binding activity for Stat3 in the lung was inhibited by Stat3-EVA overexpression. Using different in vitro and in vivo approaches, we have recently excluded the possibility that any of the effects following IgG IC stimulation of cells or deposition in the lungs are based on LPS contamination of the reagents (19). Thus, the activation of Stat3 in the IgG IC-injured lungs was mediated by a genuine agonist effect.
Figure 1.
Effects of Stat3-EVA overexpression on FcγR-dependent Stat3 activation in IgG IC-injured lungs. A) Nuclear extracts from whole lungs harvested at different time points after IgG IC deposition were subjected to EMSA assay for Stat3 DNA binding activity. B) Nuclear extracts from wild-type and FcR γ−/− mouse lungs 4 h after IgG IC deposition were subjected to EMSA assay for Stat3 DNA binding activity. C) Mice received Ad-GFP or Ad-Stat3-EVA 72 h before the lung injury. Nuclear extracts from whole lungs harvested 4 h after IgG IC deposition were subjected to EMSA assay for Stat3 DNA binding activity.
Effects of Ad-Stat3-EVA-mediated Stat3 inhibition on IgG IC-induced lung injury and MPO activity in lung homogenate
After demonstrating the ability of Ad-Stat3-EVA to inhibit the DNA binding activity of Stat3 in the lung, we determined whether Ad-Stat3-EVA can affect IgG IC-induced lung injury. At 72 h after intratracheal administration of Ad-GFP or Ad-Stat3-EVA, IgG IC-mediated lung injury was induced. We examined whether Stat3-EVA overexpression changes the pulmonary vascular permeability after IgG IC deposition by measuring albumin level in the BAL fluids. As shown in Fig. 2A, IgG IC deposition (4 h) caused a significant increase in albumin level in BAL fluids from the mice receiving Ad-GFP. Notably, albumin content in the BAL fluids from the IgG IC-injured mice receiving Ad-Stat3-EVA was significantly reduced (P<0.01).
Figure 2.
Effects of Stat3-EVA overexpression on IgG IC-induced lung injury. Lung vascular permeability and neutrophil accumulation in mice receiving Ad-Stat3-EVA were compared with mice receiving Ad-GFP in the presence (IgG-IC) and absence (Ctrl) of IgG IC deposition. A) Mouse albumin levels in whole lungs were measured as an index for vascular leakage. B) MPO activity in whole lungs was used as an index of neutrophil accumulation in the lungs. Data are represented as means ± se; n = 4–6. *P < 0.05.
We further examined MPO activity, which is a marker for the lung neutrophil burden. As shown in Fig. 2B, MPO activity in lung homogenate significantly increased in injured mice compared with noninjured mice (P<0.01). As with albumin leakage, lung MPO content in mice receiving Ad-Stat3-EVA was significantly lower when compared with values in mice receiving Ad-GFP during the injury (P<0.05).
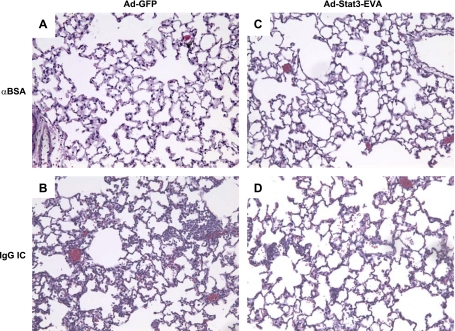
To further determine whether overexpression of Stat3-EVA reduces IgG IC-induced lung injury, histological analyses were performed. As shown in Fig. 3A, C, mice receiving Ad-GFP or Ad-stat3-EVA alone exhibited normal lung architecture with no evidence of inflammation. In the presence of IgG IC, mice receiving Ad-GFP exhibited lung hemorrhage, edema, fibrin deposition, and accumulation of neutrophils (Fig. 3B). In contrast, all of these features were mitigated 4 h after IgG IC deposition in mice receiving Ad-Stat3-EVA (Fig. 3D). These data together indicate that inhibiting Stat3 DNA binding activity provides protection against the development of pulmonary capillary permeability, increased neutrophil recruitment, and lung injury after IgG IC deposition in the lung.
Figure 3.
Effects of Stat3-EVA overexpression on lung histology during IgG IC-induced lung injury. Sections from lungs harvested 72 h after virus administration with or without IgG IC deposition (4 h) were stained with H&E. Shown are representative sections for each condition. Original view: ×200. A) Lung section from mice receiving Ad-GFP absent any other stimulus. B) Lung section from mice receiving Ad-GFP together with IgG IC deposition. C) Lung section from mice receiving Ad-Stat3-EVA absent any other stimulus. D) Lung section from mice receiving Ad-Stat3-EVA together with IgG IC deposition.
Effects of Ad-Stat3-EVA-mediated Stat3 inhibition on leukocyte counts in BAL fluid from IgG IC-injured lungs
BAL fluids were harvested from IgG IC-injured or noninjured lungs at different time periods. As shown in Fig. 4A, the total number of white blood cells in the BAL fluids significantly increased at all time periods (4, 8, and 24 h) examined in mice with injury when compared with control animals. The major cells in BAL fluids from noninjured lungs were mononuclear macrophages and lymphocytes (Fig. 4). In injured lungs, the majority of cells were neutrophils (Fig. 4). Notably, there was a decrease level of neutrophil number in IgG IC-injured mice receiving Ad-Stat3-EVA when compared with the mice receiving Ad-GFP (Fig. 4B). Moreover, there was not a significant change (increase) in the number of macrophages and lymphocytes in BAL fluids from the IgG IC-injured mice receiving Ad-Stat3-EVA as compared with those receiving Ad-GFP (Fig. 4C).
Figure 4.
Effects of Stat3-EVA overexpression on leukocyte counts in BAL fluid from IgG IC-injured lungs. Leukocytes were quantitated in BAL fluids 4, 8, and 24 h after onset of IgG IC-induced lung injury in mice treated with either Ad-GFP or Ad-Stat3-EVA. Leukocytes were also quantitated in BAL fluids from control mice that received αBSA intratracheally together with either Ad-STAT3-EVA or Ad-GFP, but in the absence of intravenously administered BSA. Total numbers of leukocytes (A), neutrophils (B), and macrophages/lymphocytes (C) are shown. Results are expressed as means ± se; n = 3 for control and n = 5 for other time points. *P < 0.05, **P < 0.01.
Effects of Ad-Stat3-EVA-mediated Stat3 inhibition on cytokine/chemokine levels in BAL fluid from IgG IC-injured lungs
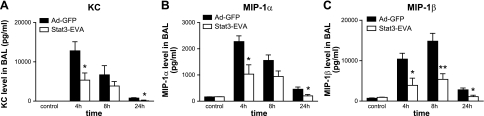
We evaluated the effects of overexpression of Stat3-EVA on levels of proinflammatory cytokines/chemokines in BAL fluids at different time periods after IgG IC-deposition. As shown in Figs. 5 and 6, virus infection alone, in the absence of IC deposition, did not induce an increase in the levels of proinflammatory mediators. In IgG IC-injured lungs infected with Ad-GFP control virus, there were progressive increases in BAL proinflammatory cytokines/chemokines, peaking at 4–8 h, followed by a decline at 24 h. Moreover, Ad-Stat3-EVA administration led to decreased levels of TNF-α, IL-6, KC, MIP-1α, and MIP-1β in IgG IC-injured lungs at all time points examined when compared with mice that were administrated Ad-GFP virus (Figs. 5 and 6), suggesting that Stat3 may play a critical roles for cytokine and chemokine production in IgG IC-injured lungs.
Figure 5.
Effects of Stat3-EVA overexpression on BAL contents of TNF-α (A) and IL-6 (B). Mice received Ad-STAT3-EVA or Ad-GFP 72 h before lung injury. BAL fluids were obtained 4, 8, and 24 h after onset of IgG IC-induced lung injury, and analyzed by ELISAs. Controls received αBSA intratracheally together with either Ad-STAT3-EVA or Ad-GFP but in the absence of intravenously administered BSA. Data are represented as means ± se; n = 3 for control and n = 5 for other time points. *P < 0.05; ***P < 0.001.
Figure 6.
Effects of Stat3-EVA overexpression on BAL contents of KC (A), MIP-1α (B), and MIP-1β (C). Mice received Ad-STAT3-EVA or Ad-GFP 72 h before lung injury. BAL fluids were obtained 4, 8, and 24 h after onset of IgG IC-induced lung injury and analyzed by ELISAs. Controls received αBSA intratracheally together with either Ad-STAT3-EVA or Ad-GFP, but in the absence of intravenously administered BSA. Data are represented as mean ± se; n = 3 for control and n = 5 for other time points. *P < 0.05; **P < 0.01.
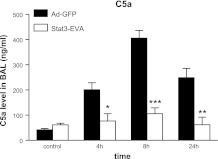
Effects of Ad-Stat3-EVA-mediated Stat3 inhibition on C5a level in BAL fluid from IgG IC-injured lungs
Previous studies have demonstrated that C5a plays an essential role for the full production of TNF-α, lung injury, and neutrophil accumulation in IgG IC induced lung injury (20, 21). We thus investigated whether overexpression of Stat3-EVA regulates the IgG IC-induced C5a production in the BAL fluids. As shown in Fig. 7, Ad-Stat3-EVA alone, in the absence of IC deposition, did not induce an increase in the C5a level. In IgG IC-injured lung with Ad-GFP present, C5a level in BAL fluids was significantly elevated (Fig. 7). Notably, Ad-Stat3-EVA administration led to a significantly decreased level of C5a in BAL fluids at all time points examined, when compared with mice that received Ad-GFP.
Figure 7.
Effects of Stat3-EVA overexpression on C5a content in BAL fluid. Mice received Ad-STAT3-EVA or Ad-GFP 72 h before lung injury. BAL fluids were obtained 4, 8, and 24 h after onset of IgG IC-induced lung injury, and analyzed by ELISA for C5a content. Controls received αBSA intratracheally together with either Ad-STAT3-EVA or Ad-GFP but in the absence of intravenously administered BSA. Data are represented as means ± se; n = 3 for control and n = 5 for other time points. *P < 0.05; **P < 0.01; ***P < 0.001.
Stat3-siRNA inhibited cytokine/chemokine secretion from macrophages when incubated with IgG IC
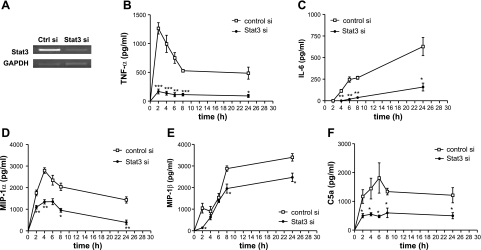
Our previous study suggested that Stat3 activation in the IgG IC-injured lung was macrophage dependent (13). Therefore, we sought to determine the effects of Stat3 deficiency on the secretion of proinflammatory mediators from IgG-IC-stimulated alveolar macrophages. We first showed that Stat3 expression was efficiently down-regulated by siRNA specific for Stat3, in MH-S, an alveolar macrophage cell line, by RT-PCR (Fig. 8A). We next showed that secretions of TNF-α, IL-6, MIP-1α, and MIP-1β were all significantly induced from IgG IC-stimulated MH-S cells. Moreover, on IgG IC treatment, silencing Stat3 led to a significant decrease in IgG IC-induced secretion of TNF-α, IL-6, MIP-1α, and MIP-1β from MH-S cells at all time points when compared with control siRNA-treated cells (Fig. 8B–E). C5a can be generated from IC-stimulated macrophages (22). We thus examined the effect of Stat3 deficiency on the C5a secretion from IgG-IC-stimulated alveolar macrophages. As shown in Fig. 8F, IgG IC-stimulated C5a production from MH-S cells was significantly reduced when Stat3 expression was down-regulated by siRNA.
Figure 8.
Effects of Stat3 silencing on the production of inflammatory mediators in IgG IC-stimulated MH-S cells. A) MH-S cells were transiently transfected with control siRNA or Stat3-specific siRNA. At 24 h after transfection, the cells were harvested for RNA isolation, and RT-PCR was performed by using primers for Stat3 and GAPDH, respectively. B–F) MH-S cells were transiently transfected with control siRNA or Stat3 siRNA. 24 h later, the cells were incubated with 100 μg /ml IgG IC for the indicated time periods. Levels of TNF-α (B), IL-6 (C), MIP-1α (D), MIP-1β (E), and C5a (F) in the supernatants were assayed by ELISA. Data are represented as means ± se; n = 4. *P < 0.05; **P < 0.01; ***P < 0.001.
DISCUSSION
The pathogenesis of lung injury in response to IgG IC deposition is initiated by the production of early response cytokines such as TNF-α and the complement activation product C5a, resulting in induction of proinflammatory mediators including CXC and CC chemokines, and up-regulation of vascular adhesion molecules, followed by neutrophil migration into the interstitial and alveolar compartments. All these events constitute a strong proinflammatory cascade. Several transcription factors, such as NF-κB, AP-1, and CCAAT/enhancer-binding proteins, are activated following IgG IC stimulation and have been suggested to be involved in IC-associated inflammatory responses (13, 23–25). However, these studies are either indirect or correlative. Our previous studies in the IgG IC-induced lung injury model have shown that Stat3 is activated in both alveolar macrophages and whole lung extracts following IgG IC deposition (13). In the current study, we show that pretreatment with Ad-Stat3-EVA, an adenoviral vector expressing a dominant-negative Stat3 isoform, significantly inhibits IgG IC-induced lung injury as defined by reduced albumin leakage into lung, MPO content, and histology change in the lung, suggesting that Stat3 is a key regulator of IgG IC-induced inflammatory responses in the lung.
Neutrophil transmigration into the alveolar compartment and lung interstitium plays a key role in the development of ALI. There is evidence from this study that Stat3 appears to be an important regulator in neutrophil recruitment into the lung since inhibition of Stat3 DNA binding activity by Ad-Stat3-EVA results in a significant decrease of neutrophils in BAL fluids. However, the molecular mechanisms by which Stat3 regulates neutrophil migrating into IgG IC-injured lungs are not clear. One possible mechanism is that of the modulation of inflammatory cytokines. Both TNF-α and IL-6 are inflammatory cytokines that play an important role in the development of ALI by inducing the expression of adhesive interactions between endothelial cells and leukocytes (21). However, when we compared the expression of intercellular adhesion molecule (ICAM)-1 and vascular cell adhesion molecule (VCAM)-1 in Ad-GFP-treated and Ad-Stat3-EVA-treated lungs in the presence of IgG IC deposition, we found that Ad-Stat3-EVA treatment has no significant effect on the ICAM-1 and VCAM-1 expression as compared with Ad-GFP treatment (data not shown). These results suggest that Ad-Stat3-EVA might affect neutrophil adhesion to the lung vascular endothelium and/or their subsequent transmigration by modulating alternative adhesion pathways independent of ICAM-1 and VCAM-1. Supporting this observation, Crockett et al. (26) reported that in a cecal ligation-puncture-induced sepsis model, gene deletion of P-selectin and ICAM-1 does not inhibit neutrophil infiltration into peritoneal cavity.
Another possible mechanism of Stat3 involvement in neutrophil infiltration is its regulation on chemokine production. Both CXC and CC chemokines are involved in IgG IC-induced lung injury (11). CXC chemokines (MIP-2 and KC) play a pivotal role by directly chemoattracting neutrophils along a chemotactic gradient into the alveolar compartment and lung interstitium (27). CC chemokines (MIP-1α and MIP-1β) seem to function as autocrine stimulators of alveolar macrophages, priming them to respond to inflammatory stimuli with enhanced production of proinflammatory cytokines such as TNF-α (28, 29). Our current finding that intratracheal administration of Ad-Stat3-EVA caused significant decreases in the contents of KC, MIP-1α, and MIP-1β supports the above hypothesis. Interestingly, using a murine model with conditional Stat3 deletion in bone marrow, Nguyen-Jackson et al. (30) recently demonstrated that Stat3 controls the neutrophil migratory response to CXCR2 ligands (MIP-2 and KC) by inducing CXCR2 expression and via modulation of CXCR2 signal transduction. Taken together, these data suggest that Stat3 mediates neutrophil infiltration into the lungs by regulating chemokine production and chemokine signaling. Interestingly, although we found that Ad-Stat3-EVA could also lead to a decrease of BAL MIP-2 production, the reduction is not significant (data not shown). Furthermore, Chouchakova et al. (31) showed that FcγRIII-mediated production of TNF-α induces IC alveolitis independently of MIP-2 and KC generation. Therefore, it would be interesting to investigate how and the exact molecular mechanisms whereby MIP-2 and KC regulate IgG IC-induced lung inflammatory responses.
Probably, the most interesting finding in this study is that with inhibition of Stat3 DNA binding activity, there was a reduction in the level of the neutrophil chemotactic peptide C5a. Compelling evidence showed that C5a is required for the IgG IC-induced inflammatory responses in the lung (11, 32). Both C5-deficient mice and in vivo blockade of C5a showed a significant decrease in vascular permeability and sharply diminished neutrophil accumulation (21, 33). The protective effects of anti-C5a were related to its ability to suppress lung macrophage production of TNF-α (21, 33). Furthermore, C5a plays a critical role in regulating the expression of the inhibitory/activating FcγR II/III receptor pair (20, 34, 35). The molecular mechanism whereby Stat3 regulates C5a production remains to be investigated. Interestingly, it has been demonstrated that Stat3 is involved in the IL-6-induced up-regulation of CCAAT/enhancer-binding protein δ and β gene promoters (32). Furthermore, in the CCAAT/enhancer-binding protein β-deficient mice, induction of complement 3 (C3) was totally impaired (36). Thus, it is tempting to speculate that Stat3 may induce C3/C5 production through CCAAT/enhancer-binding protein-mediated pathways.
Alveolar macrophages play a major role in IgG IC-induced lung injury (11, 29). Our previous studies (13) have shown that Stat3 activation in alveolar macrophages in vivo occurs before Stat3 activation in whole lung tissues following IgG IC deposition. Furthermore, IgG IC-induced Stat3 activation is significantly reduced by depletion of alveolar macrophages in the lung (13). It has also been demonstrated that C5a can be generated by IC-stimulated macrophages (22). In our current study, we show that knocking down Stat3 expression in alveolar macrophage cells significantly reduced the levels of TNF-α, IL-6, MIP-1α, and MIP-1β as well as C5a on IgG IC stimulation. These data suggest that Stat3 is involved in the production of proinflammatory mediators from alveolar macrophages, which initiate the early responses in lung following IgG IC deposition.
In summary, our studies provide evidence that Stat3 plays a significant role in the pathogenesis of IgG IC-induced ALI. Furthermore, our data suggest that Stat3 is an important regulator of C5a production during IC responses. Although Stat3 activation has been reported in several acute lung diseases, little is known about the functional role of Stat3 in the lung and the molecular mechanisms exerted by Stat3 to regulate acute lung inflammatory responses. Furthermore, there is no clear understanding of exactly how Stat3 activation is regulated in the lung. The net effect of Stat3 activation in various acute lung diseases may be dependent on under what disease condition and when and in what cell type Stat3 has been induced. Thus, identification of Stat3-regulated downstream mediators (including cytokines/chemokines and C5a shown in the current study) will lead to a better understanding how Stat3 regulates acute lung inflammatory responses. This knowledge may help identify new targets for therapeutic intervention in lung diseases.
Acknowledgments
The authors thank Dr. Peter A. Ward (University of Michigan, Ann Arbor, MI, USA) and Dr. Richard C. Schwartz (Michigan State University, Lansing, MI, USA) for constant encouragement and advice. The authors also thank Dr. Wei Zhang for preparing the adenovirus.
This research was supported by U.S. National Institutes of Health grants 5R01-HL-092905-04 and 3R01-HL-092905-02S1 (to H.G.) and the U.S. Department of Agriculture, Agricultural Research Service (ARS) program, CRIS 5450-51000-046-00D (to J.C.). The authors declare no competing financial interests.
REFERENCES
- 1. Fu X. Y. (2006) STAT3 in immune responses and inflammatory bowel diseases. Cell. Res. 16, 214–219 [DOI] [PubMed] [Google Scholar]
- 2. Hokuto I., Ikegami M., Yoshida M., Takeda K., Akira S., Perl A. K., Hull W. M., Wert S. E., Whitsett J. A. (2004) Stat-3 is required for pulmonary homeostasis during hyperoxia. J. Clin. Invest. 113, 28–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lian X., Qin Y., Hossain S. A., Yang L., White A., Xu H., Shipley J. M., Li T., Senior R. M., Du H., Yan C. (2005) Overexpression of Stat3C in pulmonary epithelium protects against hyperoxic lung injury. J. Immunol. 174, 7250–7256 [DOI] [PubMed] [Google Scholar]
- 4. Zhang X., Shan P., Jiang G., Zhang S. S., Otterbein L. E., Fu X. Y., Lee P. J. (2006) Endothelial STAT3 is essential for the protective effects of HO-1 in oxidant-induced lung injury. FASEB J. 20, 2156–2158 [DOI] [PubMed] [Google Scholar]
- 5. Matsuzaki Y., Xu Y., Ikegami M., Besnard V., Park K. S., Hull W. M., Wert S. E., Whitsett J. A. (2006) Stat3 is required for cytoprotection of the respiratory epithelium during adenoviral infection. J. Immunol. 177, 527–537 [DOI] [PubMed] [Google Scholar]
- 6. Ikegami M., Falcone A., Whitsett J. A. (2008) STAT-3 regulates surfactant phospholipid homeostasis in normal lung and during endotoxin-mediated lung injury. J. Appl. Physiol. 104, 1753–1760 [DOI] [PubMed] [Google Scholar]
- 7. Quinton L. J., Jones M. R., Robson B. E., Simms B. T., Whitsett J. A., Mizgerd J. P. (2008) Alveolar epithelial STAT3, IL-6 family cytokines, and host defense during Escherichia coli pneumonia. Am. J. Respir. Cell Mol. Biol. 38, 699–706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Meng Z. H., Dyer K., Billiar T. R., Tweardy D. J. (2001) Essential role for IL-6 in postresuscitation inflammation in hemorrhagic shock. Am. J. Physiol. Cell. Physiol. 280, C343–C351 [DOI] [PubMed] [Google Scholar]
- 9. Severgnini M., Takahashi S., Rozo L. M., Homer R. J., Kuhn C., Jhung J. W., Perides G., Steer M., Hassoun P. M., Fanburg B. L., Cochran B. H., Simon A. R. (2004) Activation of the STAT pathway in acute lung injury. Am. J. Physiol. Lung Cell. Mol. Physiol. 286, L1282–L1292 [DOI] [PubMed] [Google Scholar]
- 10. Severgnini M., Takahashi S., Tu P., Perides G., Homer R. J., Jhung J. W., Bhavsar D., Cochran B. H., Simon A. R. (2005) Inhibition of the Src and Jak kinases protects against lipopolysaccharide-induced acute lung injury. Am. J. Respir. Crit. Care Med. 171, 858–867 [DOI] [PubMed] [Google Scholar]
- 11. Gao H., Neff T., Ward P. A. (2006) Regulation of lung inflammation in the model of IgG immune-complex injury. Annu. Rev. Pathol. 1, 215–242 [DOI] [PubMed] [Google Scholar]
- 12. Fan J., Ye R. D., Malik A. B. (2001) Transcriptional mechanisms of acute lung injury. Am. J. Physiol. Lung Cell. Mol. Physiol. 281, L1037–L1050 [DOI] [PubMed] [Google Scholar]
- 13. Gao H., Guo R. F., Speyer C. L., Reuben J., Neff T. A., Hoesel L. M., Riedemann N. C., McClintock S. D., Sarma J. V., Van Rooijen N., Zetoune F. S., Ward P. A. (2004) Stat3 activation in acute lung injury. J. Immunol. 172, 7703–7712 [DOI] [PubMed] [Google Scholar]
- 14. Song L., Turkson J., Karras J. G., Jove R., Haura E. B. (2003) Activation of Stat3 by receptor tyrosine kinases and cytokines regulates survival in human non-small cell carcinoma cells. Oncogene 22, 4150–4165 [DOI] [PubMed] [Google Scholar]
- 15. Gao H., Hoesel L. M., Guo R. F., Rancilio N. J., Sarma J. V., Ward P. A. (2006) Adenoviral-mediated overexpression of SOCS3 enhances IgG immune complex-induced acute lung injury. J. Immunol. 177, 612–620 [DOI] [PubMed] [Google Scholar]
- 16. Bradley P. P., Priebat D. A., Christensen R. D., Rothstein G. (1982) Measurement of cutaneous inflammation: estimation of neutrophil content with an enzyme marker. J. Invest. Dermatol. 78, 206–209 [DOI] [PubMed] [Google Scholar]
- 17. Nimmerjahn F., Ravetch J. V. (2008) Fcgamma receptors as regulators of immune responses. Nat. Rev. Immunol. 8, 34–47 [DOI] [PubMed] [Google Scholar]
- 18. Sun L., Guo R. F., Gao H., Sarma J. V., Zetoune F. S., Ward P. A. (2009) Attenuation of IgG immune complex-induced acute lung injury by silencing C5aR in lung epithelial cells. FASEB J. 23, 3808–3818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rittirsch D., Flierl M. A., Day D. E., Nadeau B. A., Zetoune F. S., Sarma J. V., Werner C. M., Wanner G. A., Simmen H. P., Huber-Lang M. S., Ward P. A. (2009) Cross-talk between TLR4 and FcgammaReceptorIII (CD16) pathways. PLoS Pathog. 5, e1000464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shushakova N., Skokowa J., Schulman J., Baumann U., Zwirner J., Schmidt R. E., Gessner J. E. (2002) C5a anaphylatoxin is a major regulator of activating versus inhibitory FcgammaRs in immune complex-induced lung disease. J. Clin. Invest. 110, 1823–1830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mulligan M. S., Vaporciyan A. A., Miyasaka M., Tamatani T., Ward P. A. (1993) Tumor necrosis factor alpha regulates in vivo intrapulmonary expression of ICAM-1. Am. J. Pathol. 142, 1739–1749 [PMC free article] [PubMed] [Google Scholar]
- 22. Kumar V., Ali S. R., Konrad S., Zwirner J., Verbeek J. S., Schmidt R. E., Gessner J. E. (2006) Cell-derived anaphylatoxins as key mediators of antibody-dependent type II autoimmunity in mice. J. Clin. Invest. 116, 512–520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fernandez N., Renedo M., Garcia-Rodriguez C., Sanchez Crespo M. (2002) Activation of monocytic cells through Fc gamma receptors induces the expression of macrophage-inflammatory protein (MIP)-1 alpha, MIP-1 beta, and RANTES. J. Immunol. 169, 3321–3328 [DOI] [PubMed] [Google Scholar]
- 24. Guo R. F., Lentsch A. B., Sarma J. V., Sun L., Riedemann N. C., McClintock S. D., McGuire S. R., Van Rooijen N., Ward P. A. (2002) Activator protein-1 activation in acute lung injury. Am. J. Pathol. 161, 275–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fernandez N., Renedo M., Sanchez Crespo M. (2002) FcgammaR receptors activate MAP kinase and up-regulate the cyclooxygenase pathway without increasing arachidonic acid release in monocytic cells. Eur. J. Immunol. 32, 383–392 [DOI] [PubMed] [Google Scholar]
- 26. Crockett E. T., Remelius C., Hess K., Al-Ghawi H. (2004) Gene deletion of P-Selectin and ICAM-1 does not inhibit neutrophil infiltration into peritoneal cavity following cecal ligation-puncture. BMC Clin. Pathol. 4, 2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Shanley T. P., Schmal H., Warner R. L., Schmid E., Friedl H. P., Ward P. A. (1997) Requirement for C-X-C chemokines (macrophage inflammatory protein-2 and cytokine-induced neutrophil chemoattractant) in IgG immune complex-induced lung injury. J. Immunol. 158, 3439–3448 [PubMed] [Google Scholar]
- 28. Shanley T. P., Schmal H., Friedl H. P., Jones M. L., Ward P. A. (1995) Role of macrophage inflammatory protein-1 alpha (MIP-1 alpha) in acute lung injury in rats. J. Immunol. 154, 4793–4802 [PubMed] [Google Scholar]
- 29. Lentsch A. B., Czermak B. J., Bless N. M., Van Rooijen N., Ward P. A. (1999) Essential role of alveolar macrophages in intrapulmonary activation of NF-kappaB. Am. J. Respir. Cell Mol. Biol. 20, 692–698 [DOI] [PubMed] [Google Scholar]
- 30. Nguyen-Jackson H., Panopoulos A. D., Zhang H., Li H. S., Watowich S. S. (2010) STAT3 controls the neutrophil migratory response to CXCR2 ligands by direct activation of G-CSF-induced CXCR2 expression and via modulation of CXCR2 signal transduction. Blood 115, 3354–3363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chouchakova N., Skokowa J., Baumann U., Tschernig T., Philippens K. M., Nieswandt B., Schmidt R. E., Gessner J. E. (2001) Fc gamma RIII-mediated production of TNF-alpha induces immune complex alveolitis independently of CXC chemokine generation. J. Immunol. 166, 5193–5200 [DOI] [PubMed] [Google Scholar]
- 32. Cantwell C. A., Sterneck E., Johnson P. F. (1998) Interleukin-6-specific activation of the C/EBPdelta gene in hepatocytes is mediated by Stat3 and Sp1. Mol. Cell. Biol. 18, 2108–2117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Larsen G. L., Mitchell B. C., Henson P. M. (1981) The pulmonary response of C5 sufficient and deficient mice to immune complexes. Am. Rev. Respir. Dis. 123, 434–439 [DOI] [PubMed] [Google Scholar]
- 34. Ravetch J. V., Clynes R. A. (1998) Divergent roles for Fc receptors and complement in vivo. Annu. Rev. Immunol. 16, 421–432 [DOI] [PubMed] [Google Scholar]
- 35. Trcka J., Moroi Y., Clynes R. A., Goldberg S. M., Bergtold A., Perales M. A., Ma M., Ferrone C. R., Carroll M. C., Ravetch J. V., Houghton A. N. (2002) Redundant and alternative roles for activating Fc receptors and complement in an antibody-dependent model of autoimmune vitiligo. Immunity 16, 861–868 [DOI] [PubMed] [Google Scholar]
- 36. Cappelletti M., Alonzi T., Fattori E., Libert C., Poli V. (1996) C/EBPbeta is required for the late phases of acute phase genes induction in the liver and for tumour necrosis factor-alpha, but not Interleukin-6, regulation. Cell Death Differ. 3, 29–35 [PubMed] [Google Scholar]