Abstract
Spermatogenesis is sensitive to the chemotherapeutic drug cyclophosphamide, which decreases the patients’ sperm count. Since the recovery of fertility is dependent on regeneration from stem cells, in the present study we evaluated the ability of cyclophosphamide-exposed stem spermatogonia from mice to regenerate spermatogenesis in situ and after transplantation. When seven doses of cyclophosphamide were given at 4-day intervals, the differentiating germ cells were largely eliminated but ~50% of the undifferentiated type A spermatogonia remained. We monitored the recovery and found that sperm production recovered to 64% of control within the time expected. When the cyclophosphamide-surviving spermatogonia were transplanted into recipient mice, recovery of spermatogenesis from the cyclophosphamide-exposed donor cells was observed, but was reduced when compared to cells from cryptorchid donors. Thus, multidose regimens of cyclophosphamide did not eliminate the stem spermatogonia, but resulted in cell loss and residual damage.
Keywords: cyclophosphamide, mice, spermatogonial stem cells, testis
1. Introduction
The seminiferous epithelium is very susceptible to damage caused by toxicants, particularly chemotherapy and radiotherapy used in the treatment of cancer. As a result, young patients cured of cancer often suffer prolonged and sometimes permanent reductions in sperm counts or azoospermia and are infertile.
The most sensitive testicular cells to chemotherapeutic drugs and radiation are the ones that are undergoing constant mitotic activity, which are the differentiating spermatogonia [1–3]. The death of these cells results in maturation depletion of later stages of germ cells and reductions in sperm counts.
The eventual recovery of sperm production depends on the survival of the spermatogonial stem cells and their ability to differentiate to spermatozoa. In rodents, the spermatogonial stem cells have been identified morphologically as individual isolated cells called A single spermatogonia (As) [4]. When these cells divide without complete separation of their cytoplasm, they form A paired spermatogonia (Apr), which undergo further mitotic divisions, forming a chain of cells known as A aligned spermatogonia (Aal). Finally, the Aal spermatogonia can form A1 differentiating spermatogonia, which begin the precisely timed sequence of mitotic divisions and the differentiation process towards the formation of the spermatozoa.
If stem cells survive and their microenvironment is not affected, recovery of spermatogenesis will occur rapidly [2]. However, if some spermatogonial stem cells are killed, as occurs with radiation and some chemotherapeutic agents, recovery is more gradual [5,6]. In mice, it was shown that the interval needed for the recovery of fertility is directly proportional to the level of killing of the spermatogonial stem cells. There is also evidence that these agents can produce damage to the somatic tissue, and in rat testes, even though numerous stem spermatogonia survived the cytotoxic treatment, there was no recovery of the seminiferous epithelium [7,8].
The first methods developed to assess functional damage to the stem cells in the testis relied on quantifying their ability to regenerate spermatogenesis after the toxic insult. These included counts of regenerating tubules or sperm production at the time when the sperm would have been derived from surviving stem spermatogonia [2,9,10]. Although efficient for evaluating damage caused by a cytotoxic agent on the differentiation of stem cells, measurement of these endpoints did not account for possible damage to the somatic microenvironment of the seminiferous epithelium which could reduce the level of germ cell differentiation [8]. More recently, it has been demonstrated that spermatogonial transplantation [11] can be used to assess stem cell numbers and function after cytotoxic treatment, eliminating the possible effects of damage to the endogenous somatic cells. This method was used to determine the decrease in the stem cell number after a single injection of the chemotherapeutic drug busulfan, and the subsequent recovery of stem cell numbers in these mice. Spermatogonial transplantation has also been combined with assessment of undifferentiated A spermatogonia to demonstrate stem cell ablation by busulfan in rhesus monkeys [12].
One chemotherapeutic drug that is widely used for cancer treatments and immunosuppression for nephrotic disorders is cyclophosphamide (CY). This drug causes reduction in patients’ sperm counts during treatment. Recovery from moderate doses of CY is possible, sometimes after a prolonged period of azoospermia; however high doses of CY cause permanent azoospermia in humans [13,14]. To better elucidate the action of CY on spermatogonial stem cell survival and the subsequent recovery of spermatogenesis, studies using experimental animal models systems are needed.
In mice, Lu and Meistrich [2] have studied the comparative effects of single injections of several different chemotherapeutic drugs, given as single doses, on spermatogenesis. One 200-mg/Kg dose of CY totally eliminated differentiating spermatogonia (A2 stage and later) and early preleptotene spermatocytes and this resulted in a decline in testicular sperm count 29 days after treatment. In contrast the undifferentiated A spermatogonia, which include the spermatogonial stem cells, did not appear to be affected by this treatment and testicular sperm count was completely recovered 56 days after treatment.
Similar results were observed in rats. A single injection of CY induced a peak of apoptosis among basally located germ cells within 1 day, primarily in stages where A4 to intermediate spermatogonia were present [15]. However even high doses CY resulted in very little spermatogonial stem cell killing as evidenced by recovery of sperm counts 9 weeks after injection [16].
Based on the above observations of the high sensitivity of rodent differentiating spermatogonia to CY but the relative resistance of the stem cells, we treated mice with multiple doses of CY to eliminate most of the later germ cells and produce an enriched population of stem spermatogonia. We analyzed the damage to the spermatogenic function of the stem spermatogonia in situ, by evaluating endogenous recovery of spermatogenesis in the treated host. We also used germ cell transplantation assay to assess the functional ability of treated stem cells, and determine whether the recovery in the recipient mice is comparable with the results in situ.
2. Materials and Methods
2.1. Animals and treatments
Male mice of the C57BL/6Law strain were used unless otherwise noted. Animals were treated at approximately 8 weeks of age. All procedures were approved by the M. D. Anderson Cancer Center Institutional Animal Care and Use Committee.
Animals received injections of CY (Cytoxan, Bristol-Myers Squibb Co, Princeton, NJ) through oral (p.o., 22G gavage needle) or intraperitoneal (i.p) administration. Control animals received saline injections
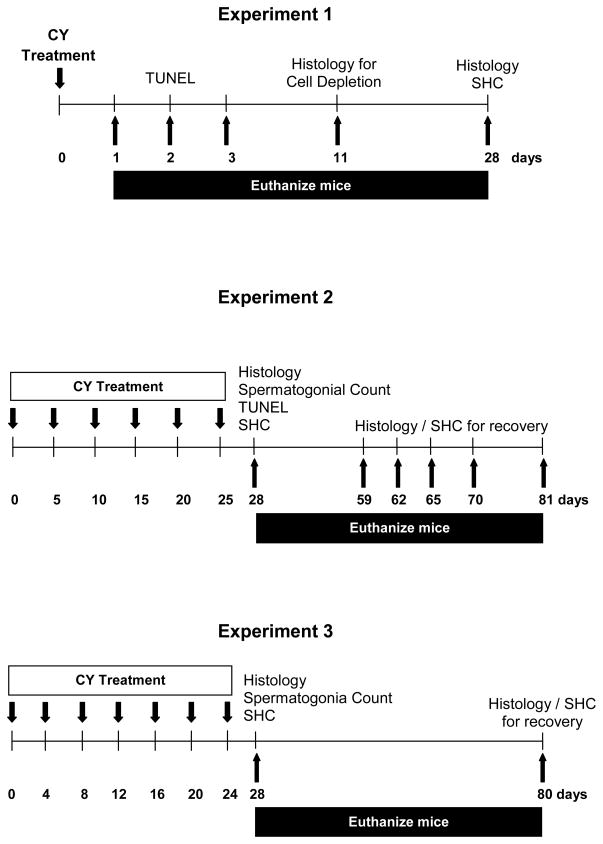
To determine the effect of different doses and routes of injection, in Experiment 1, mice were treated with a single injection of CY (Fig. 1). Animals received single doses of CY at 150 mg/Kg or 200 mg/Kg, either p.o. or i.p. Doses were chosen based on previous results on spermatogonia and animal survival obtained with single i.v. or i.p. injections of CY in C3H mice [2]. Animals were euthanized at 1, 2, 3, 11 and 28 days after administration of the drug, and different parameters were evaluated for each endpoint.
Figure 1.
Schematic time lines of treatments used in three experiments, with end points indicated. Days of administration of cyclophosphamide (CY) are indicated by downward arrows. Days at which subgroups of mice were euthanized are indicated by upward arrows and the endpoints assessed after tissue harvest are indicated above the line. Legend: SHC, sperm head count.
To evaluate the effects of multidose treatments, in Experiment 2, mice were treated with six doses of CY, at a dose of 150 mg/Kg each, based on the results in Expt. 1. The drug was given at 5-day intervals (Fig. 1), through both the p.o. and i.p. routes, over a period of 25 days. The interval between injections was based on the duration of the differentiating spermatogonial stages killed by CY in C3H mice [2]. Animals were euthanized on days 28, 59, 62, 65, 70 and 81 (3, 34, 37, 40, 45, and 56 days after the last injection). The specific time points were chosen based on the time intervals for the different cell types to produce testicular sperm [2,4] (Table 1).
Table 1.
Assessment intervals for sperm counts chosen based on predicted recovery of sperm production from different cell types potentially affected by cyclophosphamide.
| Days after last injection | Latest stage of cell capable of producing testicular spermatozoa at indicated timea |
|---|---|
| 34 | A2 spermatogonia, stage IX |
| 37 | Aaligned spermatogonia, stage VI |
| 40 | Apaired spermatogonia, stage I |
| 45 | Asingle spermatogonia, stage VI |
| 56 | Asingle spermatogonia, earlier cycle |
Based on different stage of the seminiferous epithelial cycle [4].
Experiment 3 was an evaluation of multidose regimen with a shorter window between injections, to more effectively eliminate differentiating cells. Mice were treated with seven doses of CY, given p.o. at 150 mg/Kg each. The drug was given with 4-day intervals between doses (Fig. 1), over a period of 24 days. Animals were euthanized on days 28 and 80 (4 and 56 days after last injection, respectively).
For the transplantation assay, male C57BL/6-Tg (CAG-EGFP) mice expressing green fluorescent protein (GFP; Jackson Laboratory, Bar Harbor, ME) were used as donors. Four groups of donors were used: 1) young, 14- to 16-day old mice; 2) untreated adult 8-week old mice; c) surgically cryptorchid adult mice, and 4) adults treated with CY.
The adult cryptorchid mice were 8 weeks old at the time of the surgery, and had their testicles raised by suturing the epididymal fat pad to the upper portion of the lateral abdominal wall [17] for 8 weeks prior being used as donors for the transplantation experiment. Only testicles that weighed less than 30 mg at the time of harvest and appeared healthy (no sign of necrosis) were used for the cell suspension.
Adult CY-treated mice were 8 weeks old at the beginning of the treatment. Mice received CY at a dose of 150 mg/Kg p.o., administered every 4 days over a 24-day period (as in Experiment 3). These animals were used as donors for transplantation 4 days after the last injection of CY. Recipient animals were male C57BL/6Law mice, at approximately 8 weeks of age, treated with 2 doses (1.5 +12 Gy) of ionizing radiation separated by 24 hours [21]. They were used for transplantations 3 to 4 weeks after irradiation.
2.2. Histology and morphological analysis
Testes were weighed, fixed with Bouin’s solution and embedded in paraffin or glycol methacrylate resin. Blocks were sectioned at 5 μm thickness, and sections were stained with periodic acid-Schiff’s (PAS) and hematoxylin.
For animals evaluated 11 days after injection, tubular cross-sections were scored to determine the stage of the seminiferous epithelial cycle, and whether the layers of cell types (A4 spermatogonia to zygotene, pachytene to meiosis, and round to step 12 spermatids) were present in normal amounts (>20 in the case of spermatocytes or spermatids), appreciably reduced (6 to 20), nearly completely absent (1 to 5) or absent (0). After scoring, we were able to calculate the cell types affected by the drug, at the moment of injection 11 days before the analysis, by back-calculation based on the kinetics of germ cell development [2].
In order to distinguish the spermatogonial types in the testes, samples were prepared for high resolution light microscopy [18,19]. Thus, animals were perfusion-fixed with 5% glutaraldehyde in 0.05 M cacodylate buffer (pH 7.4). Testes were removed, weighed and sliced in small slabs. Fragments were post-fixed in osmium tetroxide/potassium ferrocyanide mixture and embedded in Araldite 502 (EMS, Hatfield, PA). One-μm thick sections were obtained from the resin blocks and were stained with toluidine blue-borate for light microscopy studies.
Morphometric studies were performed to obtain the numbers of each different spermatogonial type (Aund, A1, A2, A3, A4, In, B), preleptotene spermatocytes, meiotic spermatocytes, and spermatids per 100 Sertoli cells present in seminiferous tubule cross sections; the Sertoli cells were only counted when the nucleolus was observed. Aund are undifferentiated type A spermatogonia and include the single, paired, and aligned cells. Approximately 30 tubular cross sections were counted per animal, using multiple fragments from both testes. The numbers of each germ cell type, and Sertoli cells was corrected using the Abercrombie formula [20], using the average nuclear diameter of each cell type, calculated after measuring 10 cells, and the average Sertoli cell nucleoli diameter, also calculated after measuring 10 nucleoli.
2.3. TUNEL staining for apoptosis
The testes were fixed with Bouin’s solution and embedded in paraffin. Five-μm sections were obtained and stained with the TUNEL reaction (DeadEnd™ Colorimetric TUNEL system, Promega, Madison, WI) to identify apoptotic cells, followed by PAS/hematoxylin staining.
For those animals analyzed 1 to 3 days after one dose of CY (Expt. 1), the stage of the cycle of the seminiferous epithelium was determined. Only the TUNEL-positive cells found adjacent to the basal membrane were counted, and these cells were consistent with the morphology of spermatogonia or preleptotene spermatocytes. Approximately 10 tubular cross-sections were counted per stage, and the percentage of seminiferous tubules with TUNEL-positive cells was calculated for each stage.
For animals treated with 6 doses of CY (Expt. 2, Fig. 1), staging of the seminiferous epithelium was not possible, instead a spermatogonial apoptotic index was calculated independent of stage. TUNEL-positive cells were counted 3 days after receiving the last dose. The percentage of spermatogonia undergoing apoptosis was calculated by dividing the number of TUNEL-positive spermatogonia by the total number of spermatogonia counted. Approximately 30 tubular cross-sections were counted per animal.
2.4. Sperm head count
In order to calculate the total number of sperm heads per testis [10], the right testis was weighed with the tunica albuginea intact, and placed in 1 ml distilled water. The contents were homogenized, for 30 seconds with a Polytron homogenizer (Brinkmann Instruments, Westbury, NY), keeping the samples on ice. After homogenization, the samples were sonicated for 4 minutes, in a cold water bath using a Branson Sonicator (Heat Systems, Ultrasonic, Plainview, NY). Sperm heads were counted in a hemacytometer with a 40x objective and phase contrast optics.
2.5. Transplantation
To obtain the cell suspensions from the testis, the animals were anesthetized and the testicles were collected and weighed. Donor cell suspensions were obtained by successive enzymatic digestions of GFP mice testes in a 35°C water bath [21], and filtration of the solution obtained through a 40 μm nylon filter. The final germ cell suspensions were adjusted to concentrations of between 2 and 6 × 107 per ml prior to injection into the recipient testes. Approximately 10 μL of the donor cell solution was injected through the efferent ducts.
The recipient animals were euthanized 8 weeks after transplantation and their testes were fixed with 4% paraformaldehyde, and embedded in paraffin. Serial sections 5 μm thick, were obtained and immunohistochemistry for germ cell nuclei antigen 1 (GCNA1, rat monoclonal IgM anti-mouse GCNA1, a gift from Dr George Enders) and green fluorescent protein (GFP, rabbit polyclonal anti-GFP; Cat #2956; Cell Signaling, Danvers, MA) was performed using the ABC Elite kit, appropriate secondary antibodies, and diaminobenzidine (Vector Laboratories, Burlingame, CA) according to the manufacturer’s protocol. Sections were counterstained with hematoxylin. The percentages of tubules with colonies derived from endogenous stem cells (GFP-negative) and from donor stem cells (GFP-positive) were counted in about 30 tubular cross-sections per testis. For comparison between different donor types, the percentages of tubule cross-sections with donor-derived colonies found in each testis were normalized to the average numbers of cells injected.
2.6. Statistical analysis
Data are expressed as mean ± standard error. Groups were compared using ANOVA, followed by Fisher’s LSD or Turkey’s tests when appropriate. Differences were considered to be significant when p<0.05. All analyses were performed with SPSS 15.0 software (SPSS Inc. Chicago, IL).
3. Results
3.1. Effects of a single dose of cyclophosphamide
Initial experiments were performed to characterize the effects of singe injections of cyclophosphamide (Expt. 1, Fig. 1). With both gavage and i.p administration, of either 150 or 200 mg/Kg, there was a transient loss of body weights 3 days after CY treatment, but body weights returned to pre-treatment levels by day 7, and were similar to controls thereafter (data not shown).
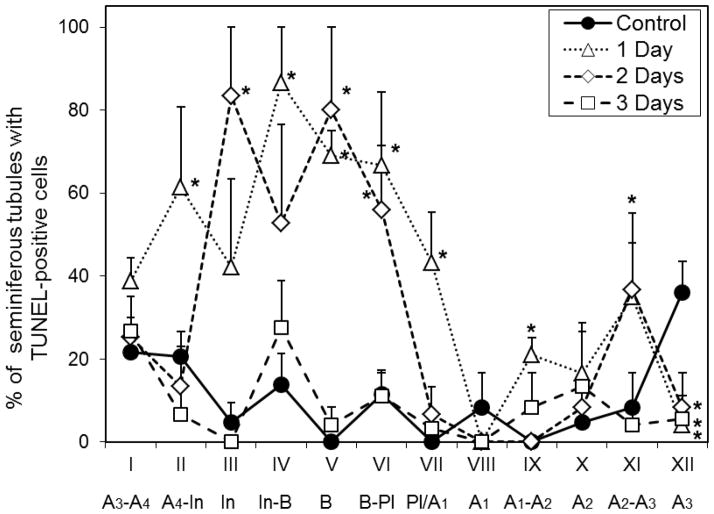
Detailed analysis of cell loss was performed with the 150 mg/Kg dose administered p.o. The TUNEL analysis revealed that CY increased the incidence of apoptotic cells 1 and 2 days after administration (Fig. 2). These increases were observed, especially in tubules in stages II–VII of the cycle of the seminiferous epithelium which contain intermediate or B spermatogonia and preleptotene spermatocytes, which means that the cells that were A4 to B spermatogonia at the time of injection were sensitive to induction of apoptosis. These are the stages during which the largest numbers of mitotically active spermatogonia are present in the testis. The apoptosis returned to the basal level on the third day after injection, indicating that the cells that underwent apoptosis were cleared from the tissue during the first two days after exposure to CY.
Figure 2.
Percentage of tubules with TUNEL-positive cells for each stage of seminiferous epithelium, at 1, 2 and 3 days after injection in mice that received one dose of cyclophosphamide (150 mg/Kg p.o.). Values expressed as Mean ± SEM (N=3 for each endpoint, and control group). Asterisks indicate statistically significant differences from control (p<0.05). The differentiating spermatogonial cell types or preleptotene spermatocytes (Pl) present at each stage are indicated below the stage.
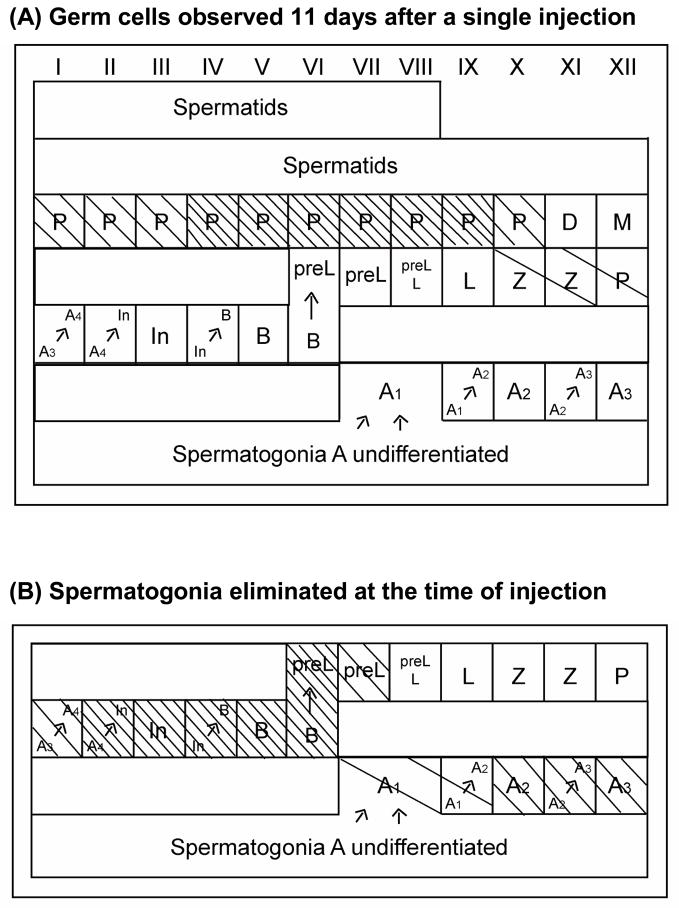
At 11 days after injection, most tubules showed partial or complete absence of spermatocytes in meiotic prophase (Fig. 3B). Pachytenes were completely absent from stages IV to IX, and nearly completely absent in stages I to III and stage X (Fig. 4A). Zygotenes and early pachytenes in stage XII were markedly reduced. Back-calculation based on the kinetics of spermatogenesis (Fig. 4B) revealed that this dose of CY specifically killed the differentiating spermatogonia A1–A4, In and B spermatogonia and the newly formed preleptotene cells, with the A4 to B spermatogonia being the most sensitive. Both earlier (Aund spermatogonia) and later stage cells (spermatocytes in meiotic prophase), did not appear to be appreciably affected by this dose. These results agree with the analysis of apoptotic cells showing that the spermatogonia that are undergoing active proliferation and mitotic divisions are the main target of CY cell killing.
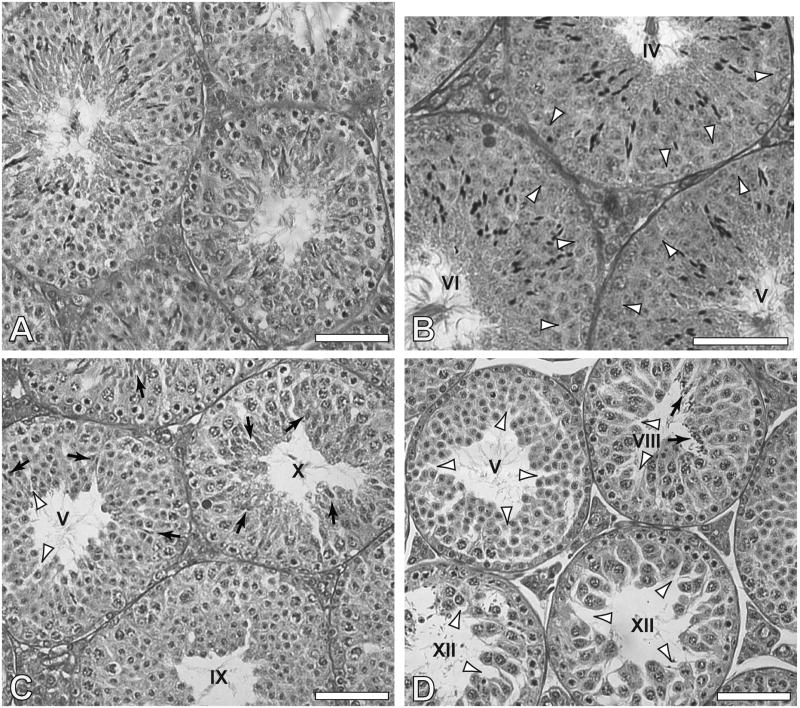
Figure 3.
Seminiferous tubule cross-sections of testes of mice that received saline injection (A), and those that received one injection of cyclophosphamide, p.o. at 150 mg/Kg, 11 days previously (B), or 28 days previously (C, D). Arrowheads: areas where spermatocytes (B) or elongated spermatids (C, D) are missing; Arrows: areas where there are remaining elongating and condensed spermatids; Stages of seminiferous epithelium are indicated in the lumen of the tubules. (A,C,D) paraffin embedded; (B) methacrylate embedded. Bars indicate 70 micrometers.
Figure 4.
Germ cells organized in the twelve stages of seminiferous epithelium cycle. Stages/germ cells that are affected by the cyclophosphamide are shaded.
(A) Cells observed in testes 11 days after p.o. CY injection at 150 mg/Kg. (B) Calculated cells affected at time of injection. Arrows indicate mitotic divisions and spermatogonial differentiation. A1, A2, A3, A4, In and B, differentiating spermatogonia; preL, preleptotene; L, leptotene; Z, zygotene; P, pachytene; D, diplotene; M, meiotic divisions. □ Appreciably reduced; ▧ Nearly completely absent; □ Absent.
In order to evaluate the relative effectiveness of these treatments on depletion of differentiating spermatogonia (types A1-B) mice were treated with single doses of CY, of either 150 mg/Kg or 200 mg/Kg, both given p.o. and i.p. and spermatogenesis was evaluated 28 days later. This is the time point at which late spermatids would be derived from the surviving spermatogonia.
Histological analysis of the seminiferous epithelium (Fig. 3C), 28 days after injection with 150 mg/Kg p.o., showed that the spermatogonia, spermatocytes, and early spermatids regenerate quite well from surviving undifferentiated spermatogonia. Although it was common to see appreciable numbers of elongating and condensing spermatids in tubules at stages IX–XII, most of late elongated spermatids are absent (stages II–VIII). In contrast, with treatments using 200 mg/Kg p.o. and 150 or 200 mg/Kg i.p, most of the tubules at stages IX–XII had reduced numbers of elongating and condensing spermatids (Fig. 3D). These results are consistent with the moderate 30% losses in testis weights observed (Table 2). In addition, the testis weight data showed that intraperitoneal injections of CY seemed to be slightly more effective at reducing the numbers of recovering cells than did gavage administrations.
Table 2.
Testis weight and sperm head count per testis 28 days the start of treatment. Treatment was given as a single dose (Expt. 1) or with 6 doses given at 5-day intervals (Expt. 2) or 7 doses at 4-day intervals (Expt. 3) (N=3).
| Testis Weight | Sperm Head Count | |
|---|---|---|
| Control | 83.8 ± 2.6 a | 16.7 ± 1.9 ×106 a |
| Expt. 1 | ||
| 150 p.o. | 67.8 ± 3.3 b | 1.9 ± 0.2 ×106 b,c |
| 150 i.p. | 59.9 ± 3.6 c | 1.2 ± 0.05 ×106 c |
| 200 p.o. | 63.0 ± 0.8 b,c | 1.0 ± 0.02 ×106 c |
| 200 i.p. | 57.8 ± 0.5 c | 1.1 ± 0.2 ×106 c |
| Expt. 2 | ||
| 150 p.o./5 days | 31.3 ± 2.8 d | 2.2 ± 0.3 ×106 b |
| 150 i.p./5 days | 30.3 ± 3.3 d | 2.9 ± 0.1 ×106 b |
| Expt. 3 | ||
| 150 p.o./4 days | 30.5 ± 1.1 d | 2.9 ± 0.3 ×106 b |
Different letters indicate statistically significant differences among all groups (p<0.05).
The testicular sperm head counts (Table 2) revealed a large decrease in the numbers of sperm heads present in treated animals. Treatment with 150 mg/Kg p.o. produced a 10-fold decline in sperm counts. Treatments with 200 mg/Kg by both routes and 150 mg/Kg i.p produced about 15-fold declines.
3.1.2 Acute effects of multiple injections of cyclophosphamide
To evaluate the effects of multiple doses of CY, which should eliminate most differentiating germ cells, animals received 6 doses of 150 mg/Kg with a 5-day interval between injections over a span of 25 days, through either the p.o. or i.p. routes (Expt. 2, Fig. 1), or with 7 doses p.o. with a 4-day interval (Expt. 3). There were minor toxic effects of the p.o. administration as the mice lost 0.7 ± 0.2 g body weight with the 5-day intervals and 0.9 ± 0.4 g with the 4-day interval, compared to a 1.9 ± 0.4 g weight gain in sham-treated controls. However there was more severe toxicity in those treated i.p. as there was a 2.3 ± 0.2 g loss of body weight at the end of the treatment
All of the multiple injection regimens caused more severe depletion in the seminiferous epithelium than did the single dose treatments. The testis weights in animals treated with multiple doses, 28 days after the first injection, were about 30 mg compared to 60–68 mg with single doses (Table 2). Although mice treated with multiple doses had markedly reduced sperm head counts compared to controls, they were higher than in animals treated with a single dose of CY.
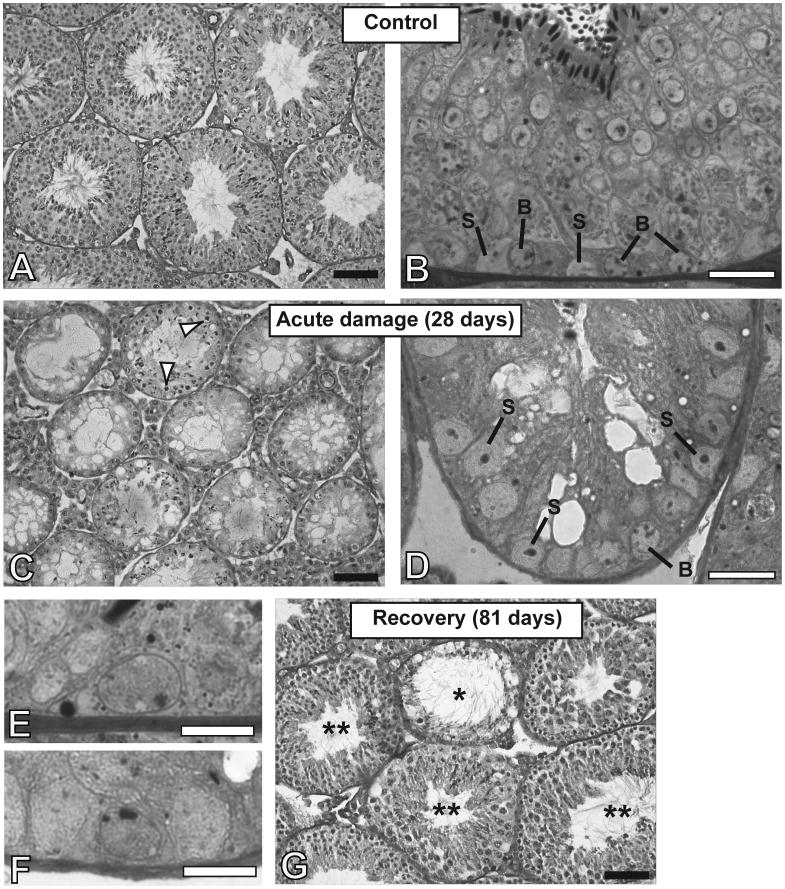
Histological analyses showed major depletion of the seminiferous epithelium 28 days after first injection (Fig. 5C). At this time point there was severe seminiferous tubular atrophy with all three treatment regimens. There was a very dramatic reduction of most spermatocytes and spermatids, although a few could still be observed. The presence of elongating/mature spermatids (Fig. 5C) could be a result of delayed release in the damaged seminiferous epithelium and might explain the greater number of sperm heads observed in testicular homogenates of animals receiving multidose treatments. In addition, there were some differentiated spermatogonia (Fig. 5D) that survived or recovered from earlier stage spermatogonia in the 3 days from the last injection, as well as undifferentiated spermatogonia (Fig. 5F) that had the same morphology as those from control animals (Fig. 5E). Nearly all of the spermatogonia appeared normal and viable, for all groups. The apoptotic index calculated for animals treated with 6 injections showed that only 2.4±1.4% of the spermatogonia were undergoing apoptosis, which was similar to the percentage in control animals (data not shown). Thus any apoptosis that was induced by the last CY injection 3 days previously had been completed, consistent with what was observed after a single injection of CY.
Figure 5.
Seminiferous tubule cross-sections of mice that received saline injections (A,B) showing Sertoli cells (S) and type B differentiating spermatogonia. (C,D), Seminiferous tubules of mice that received multiples doses of cyclophosphamide (Expt. 2 -150 mg/Kg p.o., 6 doses, at 5-day intervals), 28 days after first injection showing severe damage to the germinal epithelium at the end of the treatment. The tubules contain predominantly Sertoli cells (S). Some remaining spermatocytes (arrowheads) and spermatids are observed as well as some type B (“B”) and type A spermatogonia. (E,F) Undifferentiated type A spermatogonia in the control and the treated animals, respectively. (G) Seminiferous tubules of the treated animals, 81 days after first injection, showing recovery in most of the tubules (**), but some tubules are still damaged (*). Tissues in A, C, and G were Bouin’s fixed, paraffin embedded and stained with PAS-hematoxylin. Those in B, D, E, and F were prepared for HRLM by glutaraldehyde fixation, araldite embedding, and toluidine blue staining. Black bars represent 70 micrometers, and white bars represent 20 micrometers (B,D) or 10 micrometers (E,F).
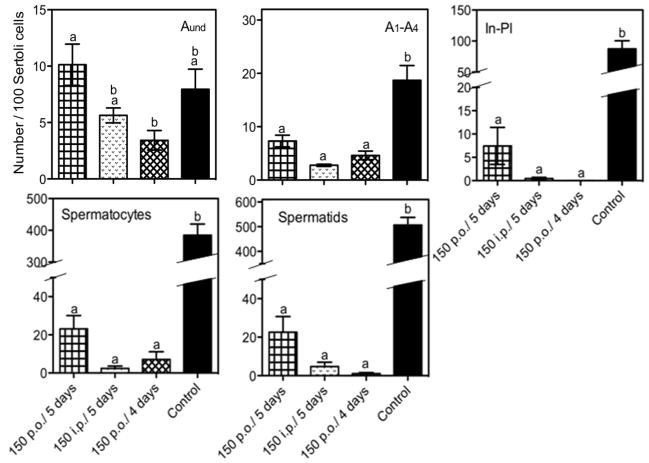
Quantitative counts of cells from the histological material showed that, at 28 days, all multidose regimens produced large decreases in the numbers of differentiating spermatogonia, spermatocytes and spermatids (Fig. 6). Analysis of the numbers of differentiating cells following the 6-dose/5-day interval regimens showed that, when given p.o., there were still some intermediate spermatogonia to preleptotene spermatocytes (7% of control), meiotic spermatocytes (5% of control), and spermatids (4% of control) present. However, numbers of these cells were effectively reduced to about 1% of control by giving the same dose i.p., or giving p.o. doses with a shorter interval.
Figure 6.
Relative numbers of different types of germ cells per 100 Sertoli cells in tubules of animals treated with 150 mg/Kg dose of cyclophosphamide p.o. and i.p., every 5 days, and 150 mg/Kg p.o., every 4 days determined by HRLM. Legend: In-Pl, differentiating In and type B spermatogonia plus preleptotene spermatocytes. Aund, undifferentiated spermatogonia type A; A1–A4, differentiating type A spermatogonia. Values expressed as Mean ± SEM (N=3). Different letters indicate statistically significant differences among all groups (p<0.05).
In contrast to the large decreases of the numbers of differentiating germ cells, there was at most a small effect of multiple doses of CY on the numbers of undifferentiated type A spermatogonia (Fig. 5F and Fig. 6). There was no effect of giving 6 doses of CY p.o. at 150 mg/Kg with a 5-day interval on these cells. There were trends towards reductions in undifferentiated type A spermatogonial numbers with i.p. injection or the 7-dose/4-day interval p.o. treatment, and they were at most about 50%. Although the treatments with 150 mg/Kg i.p. (5-day intervals) and with 150 mg/Kg p.o. (4-day intervals) produced very similar germ cell counts, the i.p. regimen produced a greater weight loss during treatment, suggesting that this treatment may cause additional systemic damage.
3.3. Endogenous recovery of spermatogenesis from surviving stem cells
Since there were remaining stem cells at the end of the treatment, despite the nearly complete tubular atrophy, it gave us an opportunity to assess the time course of the recovery from this depleted state. The recovery of the seminiferous epithelium after the end of treatment was evaluated at different times after the multidose treatment regimens.
Qualitative histological analysis performed 56 days after the last injection (80/81 days after the first injection), when the surviving stem spermatogonia should be producing spermatozoa, showed recovery of most of the seminiferous epithelium (Fig. 5G), although there were still some damaged seminiferous tubules, deficient in germ cells, for all treatment regimens.
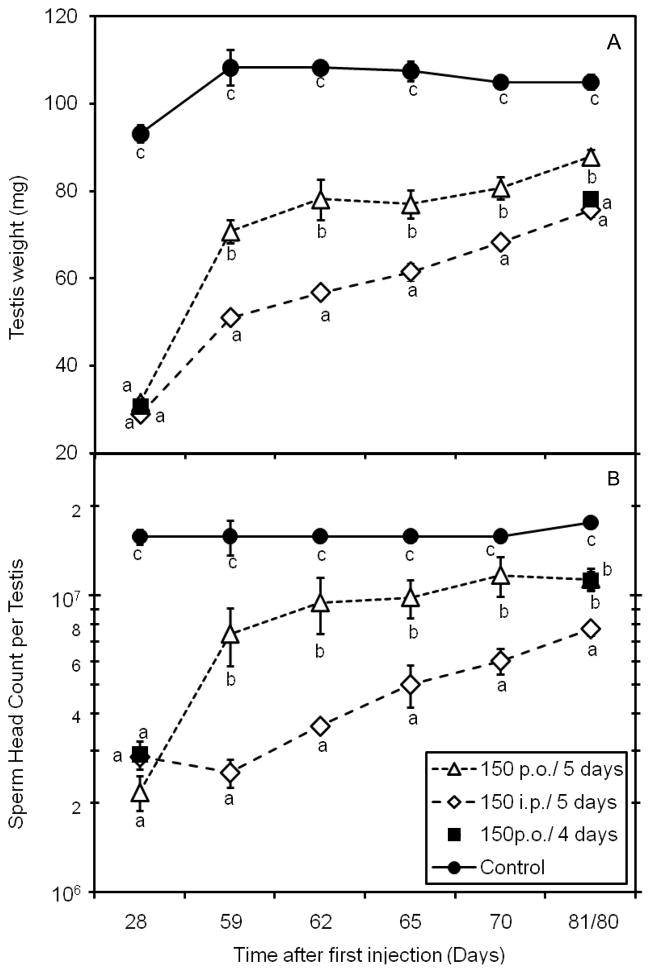
The recovery of spermatogenesis was quantified by measurements of testis weight and sperm head count (Fig. 7). Both parameters steadily increased after the end of the treatment. At 56 days after the last injection among the animals receiving 6 treatments at 5-day intervals, those receiving i.p. injections only reached testis weights and sperm head counts that were 70% and 40% of control, respectively, whereas those receiving p.o. administration had significantly greater recoveries reaching 80% and 64% of control, respectively. It was noted that none of the treated animals had reached the values measured for control animals. Thus we conclude that recovery of spermatogenesis following p.o. treatment, although incomplete, is better than after i.p. treatment. Comparing the p.o. treatments, 7 administrations given 4 days apart (Fig. 1, Expt 3) showed recovery of testis weight to 74% of control, observed 56 days after the last injection (80 days after first injection), lower than those receiving 6 treatments at 5-day intervals. However, the numbers of sperm produced, which should be more directly a measure of recovery of spermatogenesis from the surviving stem cells, at this time point were approximately the same (64% of control) (Fig. 7B); therefore the surviving stem cells seem to be effectively differentiating even after the more intense treatment regimen.
Figure 7.
A) Testis weight and B) sperm head count per testis for mice treated with multiple doses of cyclophosphamide (150 mg/Kg p.o. or i.p.), starting 28 days after initiation of treatment, showing the recovery of spermatogenesis. Note only 28-day and 80-day points were obtained for Expt. 3 (p.o doses at 4 day intervals) A sham-treated control group is shown for comparison. Times are given on the X-axis from the time of the first injection. Values expressed as Mean ± SEM (N=3). Different letters indicate statistically significant differences among all groups (p<0.05).
3.4. Functional evaluation of cyclophosphamide-treated stem cells
Next we tested whether the stem cells that survived CY treatment, and were able to produce recovery of spermatogenesis in situ in the host testis, were fully functional after germ cell transplantation. For this purpose, the animals were treated with 7 doses of 150 mg/Kg p.o. with 4-day intervals between injections, which we found to be an efficient treatment for eliminating differentiating germ cells, with only limited toxicity, and a seemingly modest effect on the numbers of undifferentiated type A spermatogonia. We compared the efficiency of transplantation with known donor models of spermatogonial transplantation, including prepubertal mice and cryptorchid mice, which have a high reported concentration of stem cells, and untreated adult mice, which have a low reported concentration of stem cells.
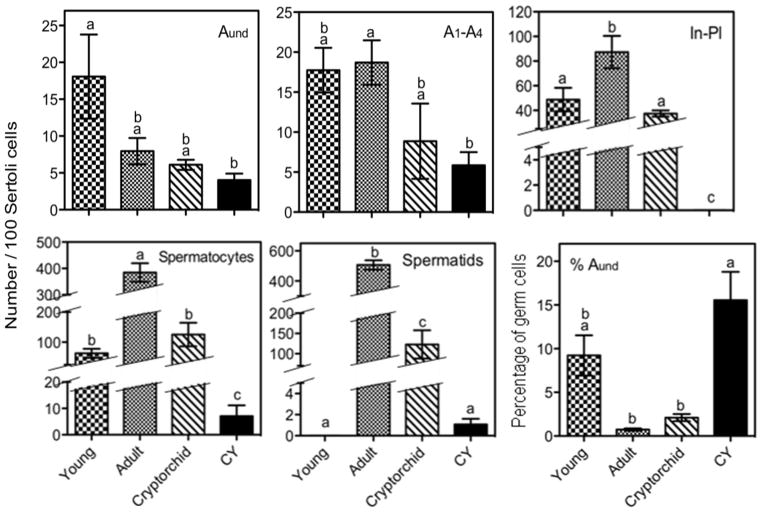
To obtain a prediction of the relative numbers of stem cells in the cell suspension from donor animals, we counted the numbers of different types of germ cells in the prepubertal, adult, cryptorchid, and CY-treated testes fixed in glutaraldehyde for HRLM (Fig. 8). The total numbers of type A undifferentiated spermatogonia per Sertoli cell were not significantly different among all the adult groups, but were lower for adult mice treated with CY than for young animals The adult mice had the highest numbers of later spermatogonia (In and B) and preleptotene spermatocytes and very high numbers of spermatocytes and spermatids. The cryptorchid B6 mice showed significantly reduced numbers of In spermatogonia to preleptotene spermatocytes and 3- to 4-fold reductions in the numbers of later germ cells, such as spermatocytes and spermatids. However, we have rarely been able to achieve the complete loss of these differentiated cells as reported by Nishimune and Aizawa [17]. Immature mice had nearly as many spermatogonia per Sertoli cell as the adult but lower numbers of spermatocytes and no spermatids. The CY-treated mice had the lowest number of differentiating germ cells at all stages and very markedly reduced numbers of cells from the intermediate spermatogonia through spermatids. These results taken together demonstrate that the percentage of germ cells that were of type Aund spermatogonia was highest for CY animals (Fig. 8), because these animals had fewer differentiated germ cells than all the other three groups. Even if we include the number of Sertoli cells, which could also be in the cell suspension, in the counts, the percentage of tubule cells that are Aund, although highest for the young animals, would still be next highest for the CY-treated ones but lower in cryptorchid mice. However, the yield of Sertoli cells in cell suspensions may vary with age of animals.
Figure 8.
Relative numbers of different types of germ cells per 100 Sertoli cells, and percentage of germ cells that are A undifferentiated spermatogonia in seminiferous tubules of young mice, and adults with no treatment, cryptorchidization, or treatment with cyclophosphamide (CY), determined by HRLM. Samples were prepared from mice that were of similar ages and received the same treatment as donor animals. Legend: In-Pl, differentiating In and type B spermatogonia plus preleptotene spermatocytes. Aund, undifferentiated type A spermatogonia; A1–A4, differentiating type A spermatogonia. Values expressed as Mean ± SEM (N=4). Different letters indicate statistically significant differences among all groups (p<0.05).
The number of cells obtained per testis in the cell suspension preparation from mice treated with CY, was similar to what was observed for cryptorchid animals, but was lower than the numbers observed for young animals (Table 3). The greater yield of cells from the immature than from the CY-treated or cryptorchid mice, may be a result of more Sertoli cells in the suspension, because they have not yet formed tight junctions, whereas the Sertoli cells from adult mice (including the cryptorchid and CY-treated), have tight junctions and many are damaged and lost during the cell suspension preparation.
Table 3.
Testis weight of donor animals, and number of cells obtained in tubule cell suspensions per testis. (N=5 for young, adult and CY; N=10 for cryptorchid, after exclusion of testes >30 mg).
| Donor | Testis Weight (mg) | Cells/Testis (×106) |
|---|---|---|
| Young | 11.5 | 3.4 |
| Adult | 104.0 | 21.2 |
| Adult/Cryptorchid | 27.1 | 1.2 |
| Adult/Cyclophosphamide | 29.9 | 1.3 |
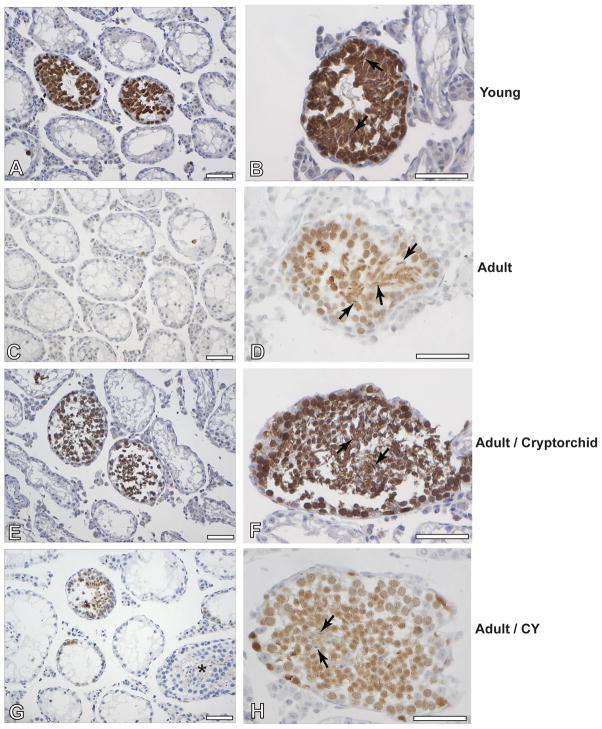
We injected similar numbers of cells from the cell suspensions (Table 4), obtained from animals of each of the four groups, through the efferent ducts, in order to produce colonization of recipient testis after transplantation. Histological analysis of the testes of recipient animals (Fig. 9) harvested 8 weeks after transplantation were performed by immunostaining for GCNA-1, in order to detect differentiation of the germ cells, and for GFP, to distinguish endogenous and donor colonies, among the GCNA-1-positive tubular cross-sections. There was recovery of spermatogenesis from endogenous stem cells in about 3 to 4% of the tubules of recipient animals with no significant differences between animals transplanted with cells from the different donor groups. Donor cells from all sources used were capable of colonizing the recipient testis and to differentiate, forming elongated spermatids (Fig. 9B, D, F and H); there were no apparent differences in the stages of differentiation or the quality of colonies produced between the different donor animals. The percentages of tubules that contained GFP positive colonies (donor derived) were assessed and normalized based on the numbers of cells injected in each group during the transplantation (Table 4). The results showed that the prepubertal mice and adult cryptorchid mice had the highest concentrations of functional stem cells in their testes. Despite the higher concentration of Aund spermatogonia and presumably stem cells present in their cell suspensions (Fig. 8), cells from CY-treated donors resulted in significantly lower levels of colonization than did those from cryptorchid mice. These results suggest that the loss of functional stem cells might have been greater than what the in situ recovery indicated. Nevertheless, the cells from CY-treated mice still appeared to induce proportionally more colonization than did those from untreated adult mice.
Table 4.
Numbers of cells injected and recovery of spermatogenesis in the recipient testes. The percentages of tubules are given for recovery of endogenous spermatogenesis (GFP-negative), donor colonies (GFP+), and donor colonies normalized to the average number of cells injected are shown. Values expressed as Mean ± SEM (N=10 testes for young, CY and cryptorchid; N=7 testes for adult).
| Source of donor cells | Numbers of viable cells injected | Tubules showing endogenous recovery (%) | Tubules showing donor (GFP+) recovery (%) | Donor recovery normalized to average number of cells injected |
|---|---|---|---|---|
| Young | 0.19 ± 0.01 ×106 | 3.4 ± 0.8 | 13.8 ± 3.5 a | 15.0 ± 3.6 a |
| Adult | 0.16 ± 0.02 ×106 | 4.6 ± 0.8 | 0.5 ± 0.2 b | 0.5 ± 0.3 b |
| Cryptorchid | 0.15 ± 0.01 ×106 | 2.9 ± 0.6 | 6.7 ± 1.8 a | 7.5 ± 2.0 a |
| Cyclophosphamide | 0.19 ± 0.01 ×106 | 4.7 ± 1.1 | 2.4 ± 0.8 b | 2.4 ± 0.8 b |
Different letters indicate statistically significant differences among all groups (p<0.05).
Figure 9.
Histological and immunohistochemical analysis of seminiferous tubules of the recipient mice transplanted with cells from prepubertal donors (A,B), adult donors (C,D), adult cryptorchid donors (E,F) and adults treated with cyclophosphamide (CY) (G,H), at 8 weeks after transplantation. Slides were immunostained with antibodies to GFP and visualized with the diaminobenzidine chromophore (brown). An endogenous colony is indicated by “*” and donor colonies are stained brown. At higher magnification (B,D,F,H) the production of elongated spermatids (arrows) at this time point is observed in donor colonies. White bars indicate 70 micrometers.
NOTE: This figure is intended for color reproduction on the web and in print.
4. Discussion
In the present study we observed that multidose regimens of treatment with CY are capable of depleting the differentiated cells in the testis, while maintaining the population of undifferentiated spermatogonia, which includes the stem cell population. Even with an aggressive regimen, such as 7 doses of 150 mg/Kg p.o., given at 4-day intervals, the Aund spermatogonia were present at ~50% of the number in the control group. These results are consistent with and extend other studies in the literature on rodents showing the sensitivity of differentiating spermatogonia but relative resistance of stem cells to a wide variety of CY regimens [2,15,22–24]. This is in contrast to some other chemotherapeutic drugs, such as busulfan, doxorubicin, thiotepa, and procarbazine that effectively kill the stem spermatogonia as well as differentiating spermatogonia [2,25–27]. The enrichment of undifferentiated spermatogonia by CY-treatment could potentially be used as an initial step in the purification of these cells for further molecular characterization of the effects of CY.
The presence of healthy looking Aund spermatogonia at the end of the treatment regimen is the source of the subsequent recovery of spermatogenesis in the treated testes. Already at 45 days after last injection, before the surviving As had time to go through further self–renewal divisions and still produce late spermatids, the sperm produced from these stem cells were at approximately 63% of the control levels. Although we did not analyze sperm function or DNA damage, in rats, treatment with CY induces double-strand DNA breaks, and affects the quality of the spermatozoa, resulting in post implantation fetal loss [28,29]. Hence, it is probable that the sperm produced by the CY-treated mice from surviving stem cells, display some level of functional and DNA damage.
Despite the survival of the stem cells and their active function for recovery of spermatogenesis in situ in the testis, these cells seemed to have some loss of function when assayed by transplantation. When comparing the groups of donors used in the transplant experiment, notwithstanding the fact that the CY-treated testis had the highest concentration of Aund cells as a percentage of germ cells, the ability of the stem cells to produce colonies upon transplantation of a given number of cells was only 16% of that of the suspensions from immature testes and 32% of that from cryptorchid testes. Concerning the results observed for young mice as donors, it may be difficult to quantitatively compare with the CY-treated as the yield of cells was much higher per mg-testis for the young animals, a likely result of greater recovery of these Sertoli cells in suspension. Inclusion of immature Sertoli cells in the suspension that was transplanted could possibly provide additional niche function for development of spermatogenesis [30,31], although we were not able to detect GFP-stained Sertoli cells in the recipient mice.
However comparisons can be made between the cryptorchid and CY-treated animals as they appear to be very similar in many respects. The testis weights and yields of cells per testis were the same for both groups and the numbers of Aund per Sertoli cell were similar. Since both models were derived from treating already adult mice, the somatic cell types and structure should be largely the same. Yet although the percentage of germ cells that were Aund spermatogonia was ~7 times higher in the CY-treated animals than in the cryptorchid ones, the number of tubules with colonies generated by CY treated donors was only 32% of the colonization produced by cryptorchid cells. Although the initial collagenase digestion resulted in the removal of most of the interstitial cells, and loss of many Sertoli cells during the digestion of tubules [32], some of these cells might be present in the suspension. Even if all of the Sertoli cells were recovered in the suspension, the concentration of Aund spermatogonia, calculated from the data in Fig. 8, would still be 2.2-fold higher in the suspensions from the CY-treated mice than from the cryptorchid ones, and we expected at least 2.2 times the number of stem-cell derived colonies upon transplantation. Thus the yield of colonies from the CY-treated mice is at most 14% (32% ÷ 2.2) of what would be expected based on data from cryptorchid donors, further supporting the conclusion that the stem cells from the former were functionally impaired.
There is general agreement that the stem cells are contained within the population of Aund spermatogonia. There is one report in the literature that c-kit-positive spermatogonia are capable of colonizing recipient testes [33], implying that these must be differentiated spermatogonia. If this were true, then the reduced number of differentiated spermatogonia in the CY-treated mice could possibly account for the lower transplantation efficiency. However, c-kit expression begins in late stage Aal [34] so the colonization ability of the c-kit positive cells could be due to Aal, which are undifferentiated spermatogonia, or possibly early stage differentiated type A cells. However the numbers of A1 spermatogonia are not reduced by the CY-treatment (data not shown), so this cannot explain the lower colonizing ability of the cells from CY-treated mice.
The histology method we used, high-resolution light microscopy of tissue sections, permits identification of the Aund spermatogonia but does not show their chain sizes or provide other markers. The stem cell pool that contributes to steady state spermatogenesis is largely contained within a subpopulation of As spermatogonia, however the Apr and Aal can occasionally contribute to the stem cell pool by fragmentation [34]. These chains are considered to be potential stem cells and contribute more markedly to the self-renewing stem cells during recovery from cell depletion, as occurs after treatment with a chemotherapy agent such as busulfan or after transplantation [35]. Although we have not determined the distribution of As, Apr, and Aal within these Aund spermatogonia, we can conclude that the percentage of these that are stem cells should be at least as high as in the untreated testis. Since the treatment involves 7 CY doses, the presence of appreciable numbers of Apr and Aal cells, which constitute the bulk of the Aund population, at 4 days after the last treatment indicates that their progenitors, the stem cells, must have survived the first 6 injections and hence should have survived the seventh injection. The lower colonizing efficiency of cells from the CY-treated mice than from the cryptorchid mice may reflect differences within the Aund compartment. Further studies characterizing the clone size and stem and differentiation markers in the surviving Aund population from cryptorchid and CY-treated mice might further elucidate reasons for the differences in stem cell functional abilities.
It is also possible that each CY-treatment is killing a fraction of the stem cells, but the loss is compensated by increased clone fragmentation and increased self-renewal that occurs after cell loss [36]. Sperm production could be maintained after recovery from CY-treatment, despite killing of stem cells, by increased division of the Aal and reduction of differentiated type A spermatogonial apoptosis [37]. It is also possible that an increased recovery of sperm production from stem cells in situ in CY-treated mice could be due to loss of checkpoint control due to damage to the Sertoli cells by CY treatment, resulting in enhanced progression of spermatogenesis in situ, but production of damaged germ cells [28]. Thus the transplantation assay may be more directly measuring the loss of surviving functional stem cells, whereas endogenous recovery is not sensitive to small to moderate losses in stem cell numbers. Thus, the less efficient transplantation from the CY-treated mice actually reflects lower numbers of functional stem cells than in the cryptorchid mice.
Another possible reason for the apparent poorer recovery of spermatogenesis from transplanted spermatogonia from CY-treated mice than from spermatogonia from cryptorchid mice, or from the CY-treated spermatogonia left in situ, is that spermatogonia from CY-treated mice have some defect in homing to the niche in the recipient testes. Defects in homing have been observed in stem spermatogonia having a low expression of β1-integrin [38].
In summary, the stem spermatogonia in mice seem to survive multiple doses of CY, and very pronounced in situ recovery is observed. Still there is evidence for some loss of stem cells or residual damage to them when they are isolated from the testis and transplanted, but the specific reason why it occurs still remains to be elucidated. The observation of stem cell loss and damage in CY-treated mice may provide methods for further understanding of the apparently greater sensitivity of the human testis to prolonged azoospermia after CY treatment [14,39].
Research Highlights.
Effects of cyclophosphamide in stem spermatogonia.
Recovery of spermatogenesis evaluated in situ and after transplantation.
Stem cells showed good recovery in situ.
Poor recovery after transplantation.
Cyclophosphamide caused stem cell depletion and functional damage.
Acknowledgments
The present study was funded by the NIH, the Florence M. Thomas Professorship in Cancer Research, and by the program CAPES-UT, established between the University of Texas and the Brazilian agency CAPES. We thank Shan Shao, Nalini Patel and Kuriakose Abraham, from the MDACC, and Maria Luiza Silva, from UFMG, for their technical assistance with histological preparations and immunostaining. We thank Dr G. Enders for kindly supplying anti GCNA1 antibody and Dr R. Behringer for opening his laboratory for making glass pipettes.
Abbreviations
- CY
cyclophosphamide
- p.o
per oral
- i.p
intraperitoneal
- GCNA 1
germ cell nuclei antigen 1
- Expt
experiment
- SHC
sperm head count
- HRLM
high resolution light microscopy
- Pl and preL
preleptotene spermatocytes
Footnotes
The authors declare that there are no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Ana Luiza Drumond, Email: abock@bcm.edu.
Connie C. Weng, Email: ccweng@mdanderson.org.
Gensheng Wang, Email: gwang@lrri.org.
Helio Chiarini-Garcia, Email: chiarini@icb.ufmg.br.
Leticia Eras-Garcia, Email: lets_eg@yahoo.com.br.
Marvin L. Meistrich, Email: mesitrich@mdanderson.org.
References
- 1.Dym M, Clermont Y. Role of spermatogonia in the repair of the seminiferous epithelium following x-irradiation of the rat testis. Am J Anat. 1970;128:265–82. doi: 10.1002/aja.1001280302. [DOI] [PubMed] [Google Scholar]
- 2.Lu CC, Meistrich ML. Cytotoxic effects of chemotherapeutic drugs on mouse testis cell. Cancer Res. 1979;39:3575–82. [PubMed] [Google Scholar]
- 3.Kangasniemi M, Veromaa TI, Kulmala J, Kaipia A, Parvinen M. Toppari 1. DNA-flow cytometry of defined stages of rat seminiferous epithelium: effects of 3 Gv of high-energy X-irradiation. J Androl. 1990;11:312–7. [PubMed] [Google Scholar]
- 4.de Rooij DG. Stem cells in the testis. Int J Exp Pathol. 1998;79:67–80. doi: 10.1046/j.1365-2613.1998.t01-1-00057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meistrich ML, Hunter NR, Suzuki N, Trostle PK, Withers HR. Gradual regeneration of mouse testicular stem cells after exposure to ionizing radiation. Radiat Res. 1978;74:349–62. [PubMed] [Google Scholar]
- 6.Meistrich ML. Quantitative relation between testicular stem cell survival, sperm production, and fertility in the mouse after treatment with different cytotoxic agents. J Androl. 1982;3:58–68. [Google Scholar]
- 7.Kangasniemi M, Huhtaniemi I, Meistrich ML. Failure of spermatogenesis to recover despite the presence of A spermatogonia in the irradiated LBNF1 rat. Biol Reprod. 1996;54:1200–8. doi: 10.1095/biolreprod54.6.1200. [DOI] [PubMed] [Google Scholar]
- 8.Zhang Z, Shao S, Meistrich ML. The radiation-induced block in spermatogonial differentiation is due to damage to the somatic environment, not the germ cells. J Cell Physiol. 2007;211:149–58. doi: 10.1002/jcp.20910. [DOI] [PubMed] [Google Scholar]
- 9.de Ruiter-Bootsma AL, Kramer MF, de Rooij DG. Response of stem cells in the mouse testis to fission neutrons of 1 MeV mean energy and 300 kV x rays. Methodology, doseresponse studies, relative biological effectiveness. Radiat Res. 1976;67:56–68. [PubMed] [Google Scholar]
- 10.Meistrich ML, Van Beek M. Spermatogonial stem cells: Assessing their survival and ability to produce differentiated cells. In: Chapin RE, Heindel JJ, editors. Methods in Toxicology. 3A. San Diego: Academic Press Inc; 1993. pp. 106–23. [Google Scholar]
- 11.Brinster RL, Zimmermann JW. Spermatogenesis following male germ-cell transplantation. Dev Biol. 1994;91:11298–302. doi: 10.1073/pnas.91.24.11298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hermann BP, Sukhwani M, Lin CC, Sheng Y, Tomko J, Rodriguez M, et al. Characterization, cryopreservation, and ablation of spermatogonial stem cells in adult rhesus macaques. Stem Cells. 2007;25:2330–8. doi: 10.1634/stemcells.2007-0143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pryzant RM, Meistrich ML, Wislon G, Brown B, McLaughlin P. Long-term reduction in sperm count after chemotherapy with and without radiation therapy for non-hodgkin’s lymphomas. J Clin Oncol. 1993;11:239–47. doi: 10.1200/JCO.1993.11.2.239. [DOI] [PubMed] [Google Scholar]
- 14.Fukutani K, Ishida H, Shinohara M, Minowada S, Niijima T, Hijikata K, et al. Supression of spermatogenesis in patients with behçet’s disease treated with cyclophosphamide and colchicine. Fertil Steril. 1981;36:76–80. doi: 10.1016/s0015-0282(16)45622-0. [DOI] [PubMed] [Google Scholar]
- 15.Cai L, Hales BF, Robaire B. Induction of apoptosis in the germ cells of adult male rats after exposure to cyclophosphamide. Biol Reprod. 1997;56:1490–7. doi: 10.1095/biolreprod56.6.1490. [DOI] [PubMed] [Google Scholar]
- 16.Meistrich ML, Parchri N, Wilson G, Kurdoglu B, Kangasniemi M. Hormonal protection from cyclophosphamide-induced inactivation of rat stem spermatogonia. J Androl. 1995;16:334–41. [PubMed] [Google Scholar]
- 17.Nishimune Y, Aizawa S. Temperature sensitivity of DNA synthesis in mouse testicular germ cells in vitro. Exp Cell Res. 1978;113:403–8. doi: 10.1016/0014-4827(78)90381-6. [DOI] [PubMed] [Google Scholar]
- 18.Chiarini-Garcia H, Russell LD. High resolution light microscopic characterization of mouse spermatogonia. Biol Reprod. 2001;65:1170–8. doi: 10.1095/biolreprod65.4.1170. [DOI] [PubMed] [Google Scholar]
- 19.Chiarini-Garcia H, Meistrich ML. High resolution light microscopic characterization of spermatogonia. In: Hou SX, Singh SR, editors. Germline Stem Cells. New York: Humana Press Inc; pp. 95–107. [DOI] [PubMed] [Google Scholar]
- 20.Abercrombie M. Estimation of nuclear population from microtome sections. Anat Rec. 1946;94:239–47. doi: 10.1002/ar.1090940210. [DOI] [PubMed] [Google Scholar]
- 21.Zhang Z, Shao S, Meistrich ML. Irradiated mouse testes efficiently support spermatogenesis derived from donor germ cells of mice and rats. J Androl. 2006;27:365–75. doi: 10.2164/jandrol.05179. [DOI] [PubMed] [Google Scholar]
- 22.Karashima T, Zalatnai A, Schally AV. Protective effects of analogs of luteinizing hormone-releasing hormone against chemotherapy-induced testicular damage in rats. Proc Natl Acad Sci USA. 1988;85:2329–33. doi: 10.1073/pnas.85.7.2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Velez de Ia Calle JF, de Queiroz F, Gamier DH, Kercret H, Folliot R, Jegou B. Reproductive effects of the anticancer drug cyclophosphamide in male rats at different ages. Arch Androl. 1989;22:251–63. doi: 10.3109/01485018908986781. [DOI] [PubMed] [Google Scholar]
- 24.Russell LD, Russell JA. Short-term morphological response of the rat testis to administration of five chemotherapeutic agents. Am J Anat. 1991;192:142–68. doi: 10.1002/aja.1001920205. [DOI] [PubMed] [Google Scholar]
- 25.Meistrich ML, Finch M, da Cunha MF, Hacker U, Au WW. Damaging effects of fourteen chemotherapeutic drugs on mouse testis cells. Cancer Res. 1982;42:122–31. [PubMed] [Google Scholar]
- 26.Brinster RL, Avarbock MR. Germline transmission of donor haplotype following spermatogonial transplantation. Proc Natl Acad Sci USA. 1994;91:11303–7. doi: 10.1073/pnas.91.24.11303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bucci LR, Meistrich ML. Effects of busulfan on murine spermatogenesis: cytotoxicity, sterility, sperm abnormalities and dominant lethal mutations. Mutat Res. 1987;176:159–268. doi: 10.1016/0027-5107(87)90057-1. [DOI] [PubMed] [Google Scholar]
- 28.Aguilar-Mahecha A, Hales BF, Robaire B. Effects of acute and chronic cyclophosphamide treatment on meiotic progression and the induction of DNA double-strand breaks in rat spermatocytes. Biol Reprod. 2005;72:1297–304. doi: 10.1095/biolreprod.104.038620. [DOI] [PubMed] [Google Scholar]
- 29.Vaisheva F, Delbes G, Hales BF, Robaire B. Effects of the chemotherapeutic agents for non-Hodgkin lymphoma, cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP), on the male rat reproductive system and progeny outcome. J Androl. 2007;28:578–87. doi: 10.2164/jandrol.106.002428. [DOI] [PubMed] [Google Scholar]
- 30.Kanatsu-Shinohara M, Miki H, Inoue K, Ogonuki N, Toyokuni S, Ogura A, Shinohara T. Germline niche transplantation restores fertility in infertile mice. Hum Reprod. 2005;20:2376–82. doi: 10.1093/humrep/dei096. [DOI] [PubMed] [Google Scholar]
- 31.Zhang Z, Shao S, Shetty G, Meistrich M. Donor Sertoli cells transplanted into irradiated rat testes stimulate partial recovery of endogenous spermatogenesis. Reprod. 2009;137:497–508. doi: 10.1530/REP-08-0120. [DOI] [PubMed] [Google Scholar]
- 32.Meistrich ML, Bruce WR, Clermont Y. Cellular composition of fractions of mouse testis cells following velocity sedimentation separation. Exp Cell Res. 1973;79:213–27. [PubMed] [Google Scholar]
- 33.Barroca V, Lassalle B, Coureuil M, Louis JP, Le Page F, Testart J, et al. Mouse differentiating spermatogonia can generate germinal stem cells in vivo. Nat Cell Biol. 2008;11:190–201. doi: 10.1038/ncb1826. [DOI] [PubMed] [Google Scholar]
- 34.Nakagawa T, Sharma M, Nabeshima Y, Braun RE, Yoshida S. Functional hierarchy and reversibility within the murine spermatogenis stem cell compartment. Science. 2010;328:62–7. doi: 10.1126/science.1182868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nakagawa T, Nabeshima Y, Yoshida S. Functional identification of the actual and potential stem cell compartments in mouse spermatogenesis. Dev Cell. 2007;12:195–206. doi: 10.1016/j.devcel.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 36.de Rooij DG. Regulation of the proliferation of spermatogonial stem cells. J Cell Sci Suppl. 1988;10:181–94. doi: 10.1242/jcs.1988.supplement_10.14. [DOI] [PubMed] [Google Scholar]
- 37.de Rooij DG. Proliferation and differentiation of spermatogonial stem cells. Reproduction. 2001;121:347–54. doi: 10.1530/rep.0.1210347. [DOI] [PubMed] [Google Scholar]
- 38.Kanatsu-Shinohara M, Takehashi M, Takashima S, Lee J, Morimoto H, Chuma S, et al. Homing of mouse spermatogonial stem cells to germline niche depends on β1-integrin. Cell stem cell. 2008;3:533–42. doi: 10.1016/j.stem.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 39.Hsu AC, Folami AO, Bain J, Rance CP. Gonadal function in males treated with cyclophosphamide for nephrotic syndrome. Fertil Steril. 1979;31:173–8. doi: 10.1016/s0015-0282(16)43818-5. [DOI] [PubMed] [Google Scholar]