Abstract
We have shown that a single “binge” dose of methamphetamine (Meth) in mice has long-lasting effects on open-field behavior dependent on mouse strain and age. Here we further investigated the impact of genotype and age on tyrosine hydroxylase (TH) loss and dopamine (DA) metabolism due to a high binge dose of Meth (4 × 5 mg/kg × 2 hours × 2 days). Administration of high dose Meth or saline (Sal) to adolescent (PND 40) and adult (PND 80) C57BL/6 (B6), DBA/2 (DBA), and 129S6SvEv/Tac (129) mice was followed by a 1 mg/kg Meth or Sal (control) challenge 40 days later. Striatal and prefrontal cortex tissues were collected one hour following the challenge. Meth-pretreated adolescent B6 and DBA mice exhibited losses in striatal DA concentrations; DBA adolescents also showed losses in striatal 3,4-dihydroxyphenylacetic acid (DOPAC) and increased DA turnover. Pre-exposed B6 and 129 adults demonstrated significant decreases in striatal DA, DOPAC, and increased DA turnover; DBA adults showed significant losses in striatal DA and increased DA turnover. 129 and DBA adults exhibited increases and decreases, respectively, in prefrontal cortex DA. Adult pretreated B6 mice produced significant losses in striatal TH. The results again show age and genotype dependent differences in Meth-induced DA alterations.
Keywords: Methamphetamine, Dopamine, Tyrosine Hydroxylase, Age, Strain Survey
1.1 INTRODUCTION
Methamphetamine (Meth) use has become a large problem in many developed countries. According to the 2005 National Survey on Drug Use and Health, in the United States up to 10.4 million people 12 years and older have reported using the drug at least once in their lives. Chronic Meth users exhibit long term psychological problems and cardiac arrhythmias. Meth psychosis is characterized by hallucinations and delusions, as well as mood and anxiety disorders, and Meth users also exhibit symptoms of social withdrawal, stereotypy and are irrationally hostile (Darke et al., 2008; Harris and Batki, 2000). Abusers of the drug have shown long-term loss to brain regions containing the dopamine transporter (DAT) (Volkow et al., 2001) and the vesicular monoamine transporter-2 (VMAT-2) (Johanson et al., 2006) making the dopaminergic systems a primary target for Meth damage.
As a known neurotoxin to both rodents and humans, Meth has a complex dose-dependent mechanism of action. Neurotoxic doses of Meth can lead to reductions in the number of DAT, increases in DA turnover, long-term loss of tyrosine hydroxylase (TH), the rate limiting step in DA synthesis (Cadet and Brannock, 1998; Davidson et al., 2001; Deng et al., 2001; Fumagalli et al., 1998; Imam et al., 2001a, 2001b; Jayanthi, et al., 2004; Sulzer et al., 2005; Wagner et al., 1980) and loss of VMAT-2, which is responsible for sequestering intracellular DA (Truong et al., 2005). The breakdown of the cascade responsible for DA release and reuptake leads to oxidation of extracellular DA to dopa-quinones, the formation of free radicals, and ultimately neurotoxicity within DA pathways (Acikgoz et al., 2000; Deng et al., 2002, Pubill et al., 2005; Yamamoto et al., 1998).
The age of early Meth use appears to be important in the formation of long-term neuronal damage. Under certain dosing conditions adolescent C57BL/6 mice (B6) are less susceptible to long-term damage caused by Meth in dopaminergic neurons than adult mice (Miller et al., 2000). Neuro-protective mechanisms meant to guard against toxic insults produced by Meth exposure have been shown to decrease throughout a lifetime, a product of the ageing process and oxidative stress-related damage (Cadet and Brannock, 1998; Yamamoto et al., 1998). A study of B6 mice ranging in age from 1 month to 24 months determined that 72 hours following a 4 × 10 mg/kg Meth dosing schedule, only DA is significantly decreased in 1 month old B6 mice while 12 month old B6 mice displayed significant losses to DA, 3,4-dihydroxyphenylacetic acid (DOPAC) and homovanillic acid (HVA). The 2, 5 and 12 month age groups all demonstrated significant increases in GFAP expression 72 hours post dosing while the 1 month animals did not (Miller et al., 2000).
Genetic background is also known to play an important role in the behavioral and physiological responses to Meth administration. The B6 and DBA/2 (DBA) strains vary significantly in their D1R and D2R dopamine receptor densities at 7 weeks of age (Ng et al., 1994), and genotype-dependent effects on the proportion of D2R long vs. short isoforms has been observed (Colelli et al., 2010). Our previous research has highlighted significant Meth-induced behavioral and metabolic differences between adolescent and adult B6, DBA, and 129 mouse strains (Good and Radcliffe, 2011). High dose (i.p. 4 × 5 mg/kg in 2h intervals for 2 days) or low dose (i.p. 2 × 1 mg/kg in 24h intervals) Meth exposure during adolescence (post natal day (PND) 40) or early adulthood (PND 80) in B6, DBA, and 129 genotypes was followed 40 days later with a Meth (1 mg/kg i.p.) or Sal challenge. Open-field behavioral testing was conducted for 1 hour following challenge administration and resulted in strain, age, and dose dependent differences in total distance traveled and vertical activity. Adolescent B6 mice displayed sensitization for high dose Meth-dependent total distance traveled and B6 adults showed tolerance for the vertical activity measure following low-dose pre-exposure. While there was no significant response to either Meth dose in the adolescent DBA animals, adult DBA mice pretreated with a low dose exhibited sensitization for vertical activity. The 129 mice did not exhibit any behavioral changes due to the high or low dose Meth regimens for either age. The 129 genotype did, however, metabolize the 1 mg/kg Meth challenge significantly faster than the B6 and DBA strains (Good and Radcliffe, 2011).
Here we report on further investigation of these behavioral differences by assessing striatal and prefrontal cortex DA concentrations and TH immunoreactivity under the same high dose treatment conditions used for the behavioral studies. Our results indicate that there were decreases in concentrations of DA, DOPAC, and increased DA turnover in response to Meth pretreatment for adult B6, DBA, and 129 mice. Adolescent B6 and DBA animals exhibited significant changes in some measures of DA metabolism following Meth pretreatment, but there was no effect in the 129 adolescents. Only the B6 adults demonstrated significant losses to TH expression.
1.2 METHODS
1.2.1 Animals
Protocols for animal experiments were approved in accordance with federal guidelines on the care and use of laboratory animals by the University of Colorado Denver IACUC committee. Animal experiments were conducted following these federal guidelines.
Male C57BL/6 and DBA/2 mice were obtained from Jackson Laboratories (Bar Harbor, Maine). Male 129S6SvEv/Tac mice were obtained from Taconic Farms (Cranbury, New Jersey). Animals were acclimated to the animal facility at the University of Colorado Health Sciences Center (Denver, CO) for 10 to 12 days before the dosing regimen was initiated. Animals were maintained in an environment of constant temperature and humidity (22° C, 40% humidity) and on a 12h L: 12h D cycle. Mice were housed five per cage.
1.2.2 Methamphetamine Administration
Methamphetamine hydrochloride (Meth) was obtained from Sigma Aldrich (St. Louis, MO). Meth was dissolved in a 0.9% saline (Sal) solution at concentrations of 0.5 and 0.1 mg/ml and administered at a 0.01 ml/g volume. Pretreatment was administered on post-natal days 40–41 or PND 80–81. The pretreatment consisted of 2 days of 4 × 5 mg/kg Meth or Sal administered in 2 h intervals. Animals were dosed between 0800 and 1730 h. The challenge dose, given 40 days following initial dosing, was a single injection of either 1.0 mg/kg Meth administered at a 0.01 mg/g volume or Sal. Animals were sacrificed via cervical dislocation 60 minutes following administration of the challenge. Treatment groups are defined in Table 1. As our objective was to further investigate our observed genotype and age-dependent behavioral differences, we emulated our previous dosing protocol used for behavioral studies and did not record body temperatures (Good and Radcliffe, 2011). The addition of temperature measurements could have added an additional stress response in the animals and possibly confounded any significant findings.
1.2.3 Tyrosine Hydroxylase Western Blot
Both striatal hemispheres were dissected, frozen in liquid nitrogen and pooled together. Tissues were then homogenized with a lysis buffer (1% SDS, 10 mM Tris-Base, 10 mM EDTA Halt Protease Inhibitor cocktail (Pierce, Rockford, IL), Halt Phosphatase Inhibitor cocktail (Pierce, Rockford, IL)) heated to 90° C. Homogenates were then centrifuged at 13,000 × g for 10 minutes at 25° C. Supernatants were centrifuged again under the same conditions and the resulting supernatant was used for western blot analysis. After protein concentrations were determined by BCA assay (Pierce, Rockford, IL) 10 mg of protein of each sample was combined with Nupage LDS sample buffer (Invitrogen, Carlsbad, CA), and Nupage Reducing agent (Invitrogen, Carlsbad, CA). Denatured samples were loaded onto 1.0 mm Nupage Novex 10% Bis-Tris gels (Invitrogen, Carlsbad, CA). Proteins were then transferred onto PVDF membranes. Membranes were blocked with 5% nonfat dry milk and probed with rabbit anti-TH (1:2,200, Millipore, Temecula, CA) for 1 hour at room temperature with gentle rocking. Membranes were washed with TBS and incubated with HRP-conjugated donkey anti-rabbit (1:70,000, Millipore, Temecula, CA). After washing with TBS, proteins were detected with the use of Western Lighting – ECL (PerkinElmer, Waltham, MA) and exposed on Hyperfilm ECL (GE Healthcare, Piscataway, NJ). Membranes were washed for 1 hour in TBS following protein detection and then probed with rabbit anti-β-actin (1:750, Sigma, St. Louis, MO) as an internal standard for 1 hour at room temperature with gentle rocking. Membranes were washed with TBS and incubated with HRP-conjugated donkey anti-rabbit (1:70,000, Millipore, Temecula, CA). Proteins were again detected using Western Lighting – ECL (PerkinElmer, Waltham, MA) and exposed on Hyperfilm ECL (GE Healthcare, Piscataway, NJ). Densitometry was performed with Image J analysis system, and the relative density of each band was normalized against that of β-actin. All genotype and treatment conditions were represented within each gel.
1.2.4 Measurement of Dopamine and DOPAC
Striatum and prefrontal cortex were dissected 1 hour following challenge administration (1 mg/kg Meth or Sal); both hemispheres were pooled together and immediately frozen in liquid nitrogen. Tissues were then sonicated in ice-cold 0.2 M perchloric acid (1:20 w/v) and centrifuged at 13,000 × g for 15 minutes at 4 °C. The supernatant was analyzed via HPLC equipped with an electrochemical detector (CoulArray system ESA Model 5600), a pump (ESA Model 580) set at 0.6 ml/min, an automatic injector (ESA Model 540) and a catecholamine column (3 μm, 100 × 4.6 mm, Waters). The mobile phase consisted of 100 mM NaH2PO4, 5% methanol, 1mM EDTA and 20 mg/L sodium octyl sulfate (adjusted pH to 3.0 with H3PO4). Electrochemical detector potentials were set at 0/50/150/300 mV (vs. Pd) (Liang and Patel, 2004).
1.2.5 Statistical Analysis
Analysis of DA, DOPAC and DA turnover for the striatum and prefrontal cortex were conducted initially with a 3-way ANOVA. Significant effects of treatment, strain and age were observed for both the striatum and prefrontal cortex. Further analysis was conducted by one-way ANOVA of treatment within each genotype and age group; post-hoc analyses were performed using Tukey’s. Significant treatment group comparisons demonstrated p<0.05. Between-subject effects of strain were examined by one-way ANOVA within each treatment group and age for DA, DOPAC and DA turnover in the striatum and prefrontal cortex. Tukey’s post hoc analyses were performed where relevant; all significant strain comparisons exhibited p<0.05.
Results of the TH investigation were initially analyzed by a two-way ANOVA (treatment group × strain) within each age. This was followed by one-way ANOVA assessment of individual effects of treatment groups within a strain and age; post hoc comparisons were performed with Tukey’s and significant comparisons demonstrated p<0.05. TH immunoreactivity results were analyzed as a percentage of individual within-gel Sal TH expression levels. Each genotype and treatment group condition within an age group was represented in each gel; adolescent and adult samples were considered separate experiments and loaded as separate gels.
1.3 RESULTS
1.3.1 Dopamine and DOPAC Concentrations
These studies were prompted by our previously observed strain and age-dependent behavioral responses to high dose Meth (Good and Radcliffe, 2011). We also determined that the 129 genotype metabolized Meth significantly faster than the B6 or DBA genotypes. As Meth acts on behavior and reward pathways, we began our investigation into possible differences explaining the behaviors by assessing DA and DOPAC concentrations within the mesocorticolimbic pathway. Previous reports assessing age, but not genotype, have determined that adult mice older than PND 90 exhibit long-term deficits to DA and increased DA turnover in the striatum, while adolescent animals do not (Miller et al., 2000). We expected to observe a similar scenario across the genotypes in the striatum and prefrontal cortex, with the exception of the 129 adults as they never showed any Meth pretreatment-induced behavioral changes (Good and Radcliffe, 2011).
1.3.2 Striatal DA and DOPAC in Adolescents
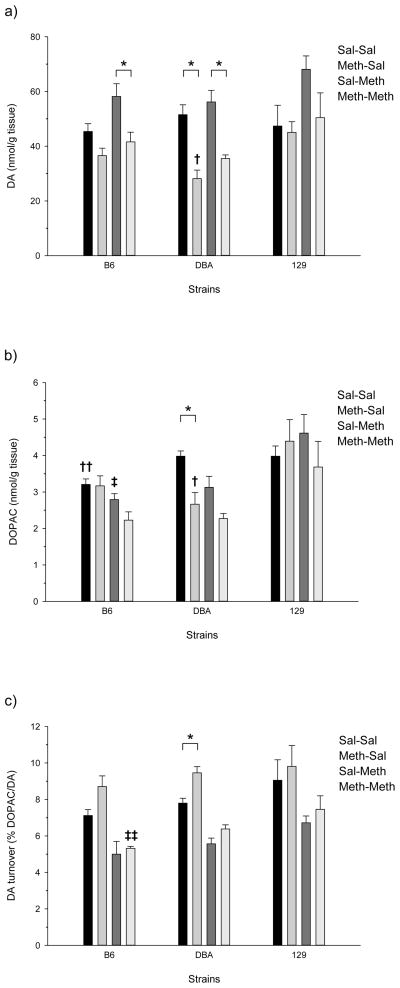
The adolescent responses to high-dose Meth and Meth challenge in the striatum are shown in figure 1. One-way ANOVA of DA, DOPAC and DA turnover for the B6 adolescents showed significant treatment effects for DA (F(3,16) = 6.876, p<0.005), DOPAC (F(3,16) = 4.711, p<0.05), and DA turnover (F(3,16) = 12.615, p<0.001). Post-hoc analysis indicated the B6 adolescents had significant reductions in striatal DA levels for the Meth-Sal/Sal-Meth and Sal-Meth/Meth-Meth treatment group comparisons. There were also significant differences in DOPAC for the Sal-Sal/Meth-Meth and Meth-Sal/Meth-Meth treatment group comparisons, and significantly altered DA turnover for the Sal-Sal/Meth-Sal, Meth-Sal/Sal-Meth, and Meth-Sal/Meth-Meth comparisons.
Figure 1.
Effects of Meth treatment in the striatum of adolescent mice: a) DA concentration; b) DOPAC concentration; and c) DA turnover. Significant differences between treatment groups (within genotype) in DA, DOPAC, or DA turnover are denoted with a bar and an asterisk (p<0.05;Tukey’s); all other significant comparisons between treatment groups are discussed in the results. Significant differences between genotypes (within treatment group) are denoted by crosses (p<0.05; Tukey’s): † (DBA<129, DBA=B6, B6=129); †† (B6<DBA=129); ‡ (B6=DBA<129); ‡‡ (B6<129, B6=DBA, DBA=129).
The Meth pretreatment-induced profile for DBA adolescents was similar to the B6 with a few differences in statistical significance. Adolescent DBA mice showed significant pretreatment-dependent losses to striatal DA and DOPAC which led to increased DA turnover. The effects of the high-dose Meth treatment groups in the DBA adolescent mice were analyzed by one-way ANOVA and produced significant results for DA (F(3,16) = 16.492, p<0.001), DOPAC (F(3,16) = 9.292, p<0.005), and DA turnover (F(3,16) = 33.991, p<0.001) (figure 1). The following adolescent treatment group comparisons for DA levels were found to be significant by post-hoc analysis: Sal-Sal/Meth-Sal, Sal-Sal/Meth-Meth, Meth-Sal/Sal-Meth, and Sal-Meth/Meth-Meth. Post-hoc analysis yielded significant Sal-Sal/Meth-Sal and Sal-Sal/Meth-Meth comparisons for DOPAC levels, and significant DA turnover treatment group comparisons for all but the Sal-Meth/Meth-Meth comparison.
There were no significant treatment effects for 129 adolescents (figure 3). This may have been at least partly due to the apparent greater variation in DA, DOPAC, and DA turnover seen in this strain compared to the others.
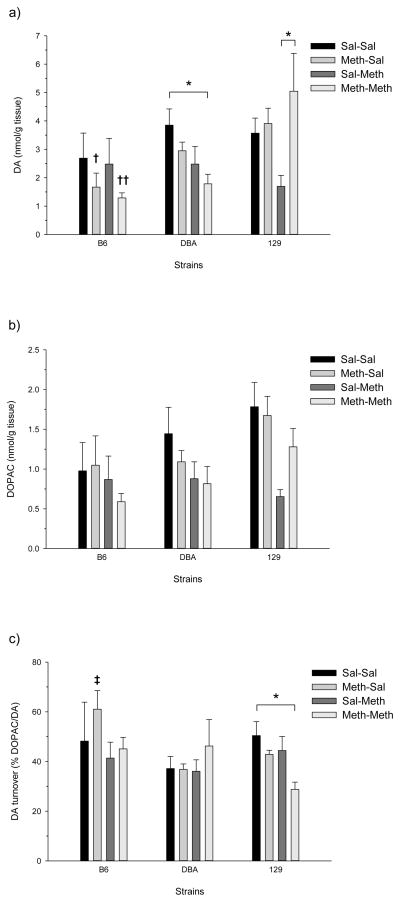
Figure 3.
Effects of Meth treatment in the prefrontal cortex of adolescent mice: a) DA concentration; b) DOPAC concentration; and c) DA turnover. There were no significant differences between treatment groups or between genotypes.
1.3.3 Striatal DA and DOPAC in Adults
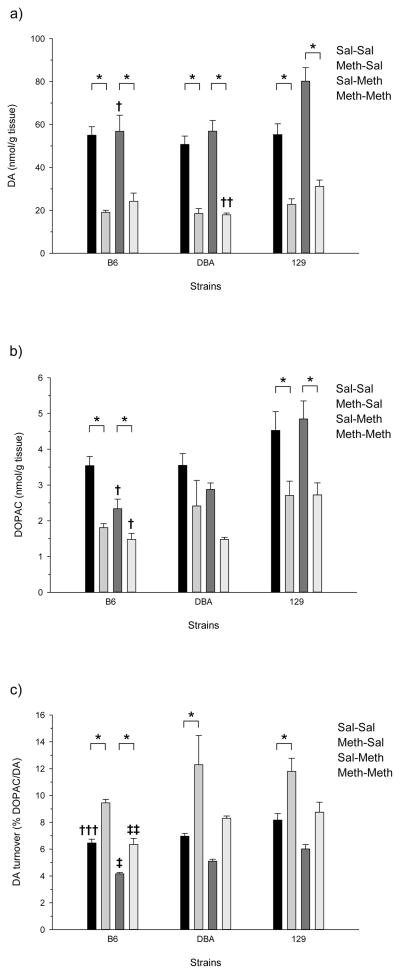
Figure 2 shows adult responses to high-dose Meth and Meth challenge in the striatum. One-way ANOVA of Meth pretreated B6 adult mice demonstrated significant treatment effects for DA (F(3,16) = 18.144, p<0.001), DOPAC (F(3,16) = 18.219, p<0.001), and DA turnover (F(3,16) = 57.731, p<0.001). Post-hoc assessment of the adult B6 results showed significant differences in DA for the Sal-Sal/Meth-Sal, Sal-Sal/Meth-Meth, Meth-Sal/Sal-Meth and Sal-Meth/Meth-Meth treatment comparisons. Tukey’s post-hoc analysis of the adult B6 results also determined that all treatment groups were significantly different from the Sal-Sal treatment group for the DOPAC treatment group comparisons, and that all DA turnover treatment group comparisons, except the Sal-Sal/Meth-Meth comparison, were significant.
Figure 2.
Effects of Meth treatment in the striatum of adult mice: a) DA concentration; b) DOPAC concentration; and c) DA turnover. Significant differences between treatment groups (within genotype) in DA, DOPAC, or DA turnover are denoted with a bar and an asterisk (p<0.05; Tukey’s); all other significant comparisons between treatment groups are discussed in the results. Significant differences between genotypes (within treatment group) are denoted by crosses (p<0.05; Tukey’s): † (B6=DBA<129); †† (DBA<129, DBA=B6, B6=129); ‡‡‡ (B6<129, B6=DBA, DBA=129); ‡ (B6<DBA<129); ‡‡ (B6<DBA=129).
Adult DBA mice showed significant losses to striatal DA and increased DA turnover, but, unlike the adolescents, not in DOPAC (figure 2). One-way ANOVA of the DBA adults demonstrated significant alterations in striatal DA levels (F(3,16) = 37.120, p<0.001), DOPAC (F(3,16) = 4.579, p<0.05), and DA turnover (F(3,16) = 7.695, p<0.005). Tukey’s post hoc analysis of DBA adult striatal DA showed significant differences between the Sal-Sal/Meth-Sal, Sal-Sal/Meth-Meth, Meth-Sal/Sal-Meth, and Sal-Meth/Meth-Meth treatment groups. Post hoc analysis also showed that only the Sal-Sal/Meth-Meth comparison exhibited significantly different DOPAC levels, and significant differences in DA turnover were observed between the Sal-Sal/Meth-Sal and Meth-Sal/Sal-Meth treatment groups.
Unlike the adolescents, 129 adults exhibited significant losses to DA, DOPAC and increased DA turnover. One-way ANOVA of the 129 genotype produced significant findings for the adult striatal levels of DA (F(3,16) = 33.003, p<0.001), DOPAC (F(3,16) = 6.494, p<0.005), and DA turnover (F(3,16) = 12.354, p<0.001) (figure 2). Post-hoc analysis of the 129 adult results showed the Sal-Sal/Meth-Sal, Sal-Sal/Sal-Meth, Sal-Sal/Meth-Meth, Meth-Sal/Sal-Meth, and Sal-Meth/Meth-Meth treatment group comparisons were significant for levels of DA. Significant differences in striatal DOPAC were observed for the Sal-Sal/Meth-Sal, Sal-Sal/Meth-Meth, Meth-Sal/Sal-Meth, and Sal-Meth/Meth-Meth treatment groups. DA turnover was significantly different between the Sal-Sal/Meth-Sal, Meth-Sal/Sal-Meth, and Meth-Sal/Meth-Meth treatment groups as determined by Tukey’s; the Sal-Meth/Meth-Meth comparison was almost significant (p=0.051).
1.3.4 Prefrontal Cortex DA and DOPAC in Adolescents
Figure 3 shows DA, DOPAC, and DA turnover from the prefrontal cortex of the three strains following high-dose Meth pretreatment and/or a Meth challenge. There were no significant differences observed for any of the measure in any of the strains.
1.3.5 Prefrontal Cortex DA and DOPAC in Adults
Figure 4 shows DA, DOPAC, and DA turnover from the prefrontal cortex of adult mice. None of the DA measures were found to be significantly different as a result of Meth treatment in the B6 and only DA levels were significantly affected in the DBA (one-way ANOVA; F(3,16) = 3.948, p<0.05). Post-hoc assessment of DA levels in the DBA showed a single significant difference: less DA in the Meth-Meth treatment group compared to the Sal-Sal group (figure 4c). The 129 adults showed significant effects for DA (F(3,20) = 3.098, p=0.05), DOPAC (F(3,20) = 4.900, p<0.05), and DA turnover (F(3,20) = 4.460, p<0.05) (figure 4). Post-hoc evaluation of DA levels showed a significant difference for the Sal-Meth/Meth-Meth treatment group comparison, as well as significant differences for the DOPAC Sal-Sal/Sal-Meth and Meth-Sal/Sal-Meth treatment group comparisons. DA turnover in the 129 was significantly higher in the Sal-Sal group compared to the Meth-Meth group.
Figure 4.
Effects of Meth treatment in the prefrontal cortex of adult mice: a) DA concentration; b) DOPAC concentration; and c) DA turnover. Significant differences between treatment groups (within genotype) in DA, DOPAC, or DA turnover are denoted with a bar and an asterisk (p<0.05;Tukey’s); all other significant comparisons between treatment groups are discussed in the results. Significant differences between genotypes (within treatment group) are denoted by crosses (p<0.05; Tukey’s): † (B6<129, B6=DBA, DBA=129); †† (B6=DBA<129); ‡ (B6>DBA=129).
1.3.6 Genotype Effects
It is clear that the three strains responded differently to high-dose Meth in several instances. For example, Meth caused a significant reduction in adolescent striatal DOPAC concentrations in the DBA but not in the B6 (Sal-Sal vs. Meth-Sal; figure 1b). Another striking example was the effect on adult DA levels in the prefrontal cortex (figure 4a). The B6 and DBA showed non-significant reductions in DA in the Sal-Meth compared to the Meth-Meth group. In contrast, a significant increase in DA was observed in the 129 strain.
A more formal analysis of genotype effects was conducted within each treatment and age group for between-strain effects on DA, DOPAC, and DA turnover in the striatum and prefrontal cortex (one-way ANOVA followed by Tukey’s post hoc). Significant results are indicated by crosses in figures 1 through 4 and described in the following.
Among both adolescents and adults, there were 12 instances for which there was a significant strain effect in the striatum (figures 1 and 2). In all but two of these cases, the 129 showed higher DA, DOPAC, and DA turnover than either the B6 or DBA strains, sometimes both. One-way ANOVA indicated significant genotype effects in adolescent striatum for DA (Meth-Sal: F(2,14) = 6.619, p<0.05), DOPAC (Sal-Sal: F(2,14) = 4.961, p<0.05; Meth-Sal: F(2,14) = 4.605, p<0.05; Sal-Meth: F(2,14) = 7.489, p<0.01), and DA turnover (Meth-Meth: F(2,14) = 5.585, p<0.05). There was a significant effect of genotype the adult striatum for DA (Sal-Meth: F(2,14) = 4.508, p<0.05; Meth-Meth: F(2,14) = 5.507, p<0.05), DOPAC (Sal-Meth: F(2,14) = 14.469, p<0.01; Meth-Meth: F(2,14) = 10.576, p<0.005), and DA turnover (Sal-Sal: F(2,14) = 6.549, p<0.05; Sal-Meth: F(2,14) = 18.507, p<0.001; Meth-Meth: F(2,14) = 6.238, p<0.05). The results of the post hoc analyses are presented in the figure legends.
Significant between-subject effects of genotype were also observed in the prefrontal cortex, although only in adult animals and not to the extent that was seen in the striatum. Adults demonstrated significant effects of genotype for DA (Meth-Sa: F(2,15) = 5.744, p<0.05; Meth-Meth: F(2,16) = 5.865, p<0.05) and DA turnover (Meth-Sal: F(2,15) = 8.026, p<0.01). The results of the post hoc analyses are presented in the figure legends.
1.3.5 Striatal Tyrosine Hydroxylase Expression
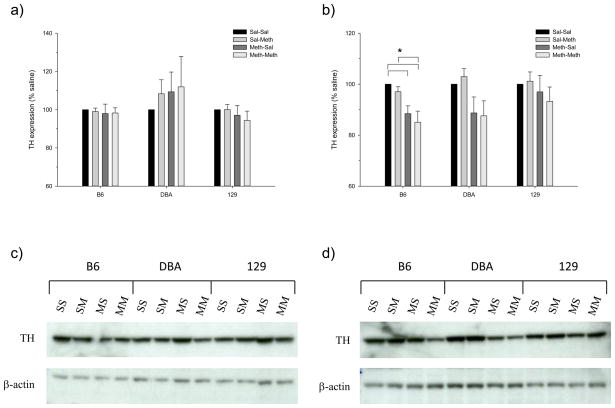
The significant losses to striatal DA, DOPAC and increased DA turnover led to the question of whether decreases in striatal TH expression were driving these deficits. We hypothesized that our dosing regimen would produce long-term losses to TH expression in the striatum for all of the adult genotypes, but less so for the adolescents, especially the adolescent 129. We thus examined TH expression by western blotting in the striatum of an independent group of mice that had been treated identically as in the DA studies. As shown in figure 5, the adolescents of all strains, as well as the DBA and 129 adults, did not show significant long-term losses to TH expression. Assessment of the between-subject effects of Meth treatment group on TH expression within age and strain found a significant difference for the B6 only (F(3,28) = 6.167, p<0.005) (figure 5b). Post-hoc analysis of B6 pretreated adults showed significant losses of TH for the Meth-Sal and Meth-Meth treatment groups when compared to Sal-Sal; the Meth-Meth treatment group also expressed significantly less TH than the Sal-Meth group. Although not quite significant (p=0.052), the adult DBA showed a similar pattern of TH expression as the adult B6; i.e., the Meth-Sal and Meth-Meth groups were both lower than the Sal-Sal and Sal-Meth groups. Representative western blots are shown in figures 5c and 5d. Note in particular the difference in intensity between the Sal-Meth and Meth-Meth groups in the adult B6 and DBA (figure 5d).
Figure 5.
Effects of Meth treatment on TH expression in the striatum of (a) adolescent and (b) adult mice. Significant differences between treatment groups (within genotype) are indicated with a bar and an asterisk (p<0.05; Tukey’s). Representative blots of TH immuneoreactivity are shown for (c) adolescents and (d) adults (SS=Sal-Sal; SM=Sal-Meth; MS=Meth-Sal; MM=Meth-Meth).
1.4 DISCUSSION
As with our behavioral study (Good and Radcliffe, 2011), high dose Meth-induced pretreatment effects on DA and TH were strain- and age-dependent. The DA studies also demonstrated brain region-specific effects. Under our treatment conditions the following significant Meth pretreatment-induced DA and DOPAC findings were observed: 1) adult 129 mice displayed DA and DOPAC deficits in the striatum; 2) adult 129 mice exhibited a significant increase in prefrontal cortex DA; 3) adolescent B6 mice exhibited decreases in striatal DA concentrations; 4) B6 adults demonstrated significant reductions in DA and DOPAC concentrations within the striatum; 5) adolescent DBA mice showed losses to striatal DA and DOPAC; and 6) DBA adults demonstrated significant decreases in striatal DA concentrations. With these results in mind, one would hypothesize that each group displaying significant changes to DA concentrations would also exhibit losses to TH. However, only the B6 adults demonstrated a significant loss of TH. The DBA and 129 adult DA deficits, in spite of no significant reductions to TH expression, indicate that we have yet to explore all of the variables affecting DA within these genotypes. Possible explanations may include Meth-induced changes to signaling cascades regulating DA, and enzymes responsible for DA metabolism, such as monoamine oxidase (MAO) or c-O-methyl transferase (COMT).
Age-dependent deficits to the DA system have been seen in rodent models with adolescent animals consistently displaying fewer to no neurotoxic effects of Meth. (Miller et al., 2000; Truong et al., 2005). Rats ages PND 40 and 90 were administered a dosing regimen of 4 × 10 mg/kg s.c. in 2-hour intervals. DA, DAT and TH activity were all significantly reduced 7 days following dosing in the PND 90 rats but not the PND 40 group (Kokoshka et al., 2000). In addition, DAT activity was assessed in both age groups 1 hour following either a single 15 mg/kg Meth administration or 4 × 10 mg/kg s.c. in 2-hour intervals; the PND 90 animals consistently showed significant reductions in DAT activity. A dose dependent decrease in catecholamine concentrations was also shown across a wider range of ages (1, 6 and 12 months) 4 days following Meth administration of 5, 10, 20 or 40 mg/kg in rats (Imam and Ali, 2001a). In this study, the 1 month old rats were slightly less sensitive to the loss in DA. These results are generally consistent with our own results in that there was less striatal DA depletion in adults than adolescents in the B6 and 129. The DBA showed a robust depletion at both ages, although it appeared to be slightly less in the adolescents.
Under our experimental conditions, TH expression followed a different pattern than the Meth-induced changes to catecholamine concentrations. Adult B6 and DBA animals produced significant and almost significant losses (respectively) of TH due to the Meth pretreatment, while adult 129 mice and adolescents of all strains displayed no changes to TH expression levels. Our observation that there were no changes to TH but significant decreases in DA for the adolescents has been previously reported in the rat caudate-putamen (Pereira et al., 2006). Using both single (20 mg/kg) injection and multiple injection (4 × 5 mg/kg × 2 hours) dosing schedules it was observed that at either 1 or 24 hours following dosing there was a significant increase in DA turnover, but no loss of TH. While the time course utilized was significantly shorter than our 40 day study, the doses were comparable to one day of our pretreatment, giving us an idea of what may be occurring during the early stages after dosing. However, in CD-1 mice it was determined that TH loss did not begin to appear until 16 hours after a bolus 30 mg/kg injection, and the decrease in TH did not stabilize until 48 hours (Zhu et al., 2005). The loss of TH at 16 hours was only significant in the dorsal striatum; loss of TH in the ventral striatum was not shown to be significant until 48 hours post dose. TH deficits remained at the 48 hour levels through the 72 hour time point (Zhu et al., 2005). The long-term effects of a single day of i.p. 4 × 7.5 mg/kg Meth, given every 2 hours, were assessed in male BALB/C mice (age 12–14 weeks) at 10 days, 3 months and 5 months post Meth administration. The mice demonstrated apparent decreases in DA after 10 days and increased tunel staining 3 days post Meth exposure. However, DA levels recovered to control levels by the 3 and 5 month time points (Krasnova et al., 2009). It is unknown whether a recovery in DA levels would occur in the B6, DBA or 129 strains as our final time point was only 40 days after initial dosing. It seems that a higher dose of Meth is needed to produce the early loss of TH, and that DA levels may recover after several months. We can conclude that in adult mice the two day regimen is sufficient at producing the TH loss in the B6 genotype.
It was hypothesized that the B6 adolescents would have a greater change in striatal DA levels than the DBA or 129 genotypes as the B6 showed long-term behavioral sensitization due to high-dose Meth pretreatment (Good and Radcliffe, 2011). There was, in fact, little effect of a high-dose Meth pretreatment in the 129, but the DBA, if anything showed a greater deficit than the B6. The adult behavioral responses to high dose Meth also cannot be explained by the DA results; i.e., all three strains showed a significant and long-lasting change in DA levels, but the only behavioral pretreatment effect observed in our previous study was sensitization for stereotypy counts in the DBA adults (unpublished results). Previous strain surveys have primarily investigated Meth-induced behavioral differences between the B6 and DBA genotypes in addition to the dd, ICR, BALB/c, and C3H/He strains (Kuribara et al., 1989; Grisel et al., 1997; Phillips et al., 1994). While no studies include a direct comparison of the B6, DBA and 129 strains for specific toxicity responses to Meth, a handful of reports do exist for various comparisons of the three genotypes looking at amphetamine (AMPH) and cocaine (COC) induced DA responses. Administration of AMPH in either 2.5 mg/kg or 5 mg/kg doses in B6 and 129S2/DvHsd animals aged 8 to 10 weeks resulted in the B6 showing greater locomotor activity and AMPH-induced striatal DA efflux (Chen et al., 2007). In this same study, it was found that the B6 and 129S2/DvHsd strains did not show any significant differences between the strains for striatal DA content, surface and total DAT expression, and DAT activity (Chen et al., 2007). In another study, a different result was found following administration of acute COC. B6 as well as DBA animals were shown to have significant differences from the 129/Sv-ter strain for basal DA uptake as well as a greater increase in dialysate concentration of DA in response to 5, 10 and 20 mg/kg acute COC (He and Shippenberg, 2000) which is opposite of the AMPH study. The discrepancy could be due to either different mechanisms of action of the two drugs, use of different 129 substrains, or possibly to different ages of the mice, although the He and Shippenberg (2000) study did not list the ages of their subjects. One consistent finding is that the strains all had similar baseline levels of DA which was also the case in our study. In addition, the 129 consistently seems to respond differently than the other strains; i.e., DA changes in adult B6 and DBA mice were similar while the 129 appeared to be more responsive, at least to the Meth challenge.
A recent report indicated that the D2R is necessary for many measures of toxicity that result from high dose Meth. The D2R null mutant mouse showed little effect of a Meth dosing schedule similar to the high dose regimen that we used, including very little effect on striatal DA levels and TH immunoreactivity (Granado et al., 2011). These authors concluded that D2R activation is an important mediator of Meth neurotoxicity. This raises an important question about the extent to which genetic variation in D2R density may have contributed to our observed genotype-dependent responses to high-dose Meth. It has been well established that inbred mouse strains show considerable variation in D2R density (e.g., Jones et al., 1999; Kanes et al., 1993). A comparison of the B6 to the 129 (substrain not listed) found no difference in striatal D2R density (Yoo et al., 2010); however, the B6 consistently has exhibited higher D2R density in the striatum when compared to the DBA at ages ranging from 42 to 63 days (Erwin et al., 1993; Kanes et al., 1993; Cabib et al., 1998; Ng et al., 1994; Boehme et al., 1982). Based on these naturally occurring D2R differences and the findings of Granado et al. (2011), one couls speculate that the DBA would be more protected from Meth toxicity than the B6 or 129; however, this was not the case. As mentioned above, DBA adolescents, if anything, had a greater toxic response than the B6 and 129 as measured by reduction in DA levels; the adult response was similar in the strains, although the 129 appeared to have a greater overall reduction. The picture is complicated by the differential expression of the short and long forms of the D2R (D2L, D2S) which have distinct as well as overlapping functions (Lindgren et al., 2003; Usiello et al., 2000; Centonze et al., 2004) and are differentially expressed in the B6 and DBA strains with the D2L/D2S ratio greater in the B6 (Colelli et al., 2010). Interestingly, D2L mRNA was up-regulated in amphetamine-sensitized B6 mice (Giordano et al., 2006), although the role of the D2R isoforms in amphetamine toxicity – Meth or otherwise – has not been examined.
Further explanation of the observed metabolic and behavioral differences produced by our study variables will require us to probe deeper into the Meth-induced toxic effects on DA metabolism, the effects of DA release due to Meth, and genotype specific Meth-induced alterations to other monoamine systems and brain regions. B6 adolescents exhibited significant decreases in DA, but no changes to DOPAC or DA turnover. B6 adults, however, showed a consistent loss of DA, DOPAC and increased DA turnover and loss of TH. DBA adolescents exhibited a loss of DA, DOPAC and an increased DA turnover for the Sal-Sal/Meth-Sal comparison and only a loss of DA for the Sal-Meth/Meth-Meth comparison. These strain and age differences may result from a decrease in MAO activity, as Meth is a known acute inhibitor of MAO function (Suzuki et al., 1980, Egashira and Yamanaka, 1993). It is apparent that further investigation into the toxic effects of Meth on the DA system, particularly the enzymes responsible for DA metabolism, will be necessary to better understand our behavioral results as well as the current DA metabolism and TH results.
Highlights.
We examined the long-term effects of high dose methamphetamine on DA and TH in several strains of mice at adolescence and adulthood.
Strain- and age-dependent effects were observed for DA levels and turnover, and for TH levels.
These changes may have important implications for methamphetamine addiction.
Acknowledgments
We are grateful for our support from the following: R01 AA016957 (R.A.R.); RO1 NS045748-06 (M.P.); NIDA Training Grant DA017637-05 (R.L.G.)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
1.6 References
- Açikgöz O, Gönenç S, Kayatekin BM, Pekçetin C, Uysal N, Dayi A, Semin I, Güre A. The effects of single dose of methamphetamine on lipid peroxidation levels in the rat striatum and prefrontal cortex. Eur Neuropsychopharmacol. 2000;10:415–8. doi: 10.1016/s0924-977x(00)00103-6. [DOI] [PubMed] [Google Scholar]
- Boehme RE, Ciaranello RD. Genetic control of dopamine and serotonin receptors in brain regions of inbred mice. Brain Res. 1983;266:51–65. doi: 10.1016/0006-8993(83)91308-2. [DOI] [PubMed] [Google Scholar]
- Cabib S, Giardino L, Calza L, Zanni M, Mele A, Puglisi-Allegra S. Stress promotes major changes in dopamine receptor densities within the mesoaccumbens and nigrostriatal systems. Neurosci. 1998;84:193–200. doi: 10.1016/s0306-4522(97)00468-5. [DOI] [PubMed] [Google Scholar]
- Cadet JL, Brannock C. Free radicals and the pathobiology of brain dopamine systems. Neurochem Int. 1998;32:117–31. doi: 10.1016/s0197-0186(97)00031-4. [DOI] [PubMed] [Google Scholar]
- Centonze D, Gubellini P, Usiello A, Rossi S, Tscherter A, Bracci E, Erbs E, Tognazzi N, Bernardi G, Pisani A, Calabresi P, Borrelli E. Differential contribution of dopamine D2S and D2L receptors in the modulation of glutamate and GABA transmission in the striatum. Neurosci. 2004;129:157–66. doi: 10.1016/j.neuroscience.2004.07.043. [DOI] [PubMed] [Google Scholar]
- Chen R, Zhang M, Park S, Gnegy ME. C57BL/6J mice show greater amphetamine-induced locomotor activation and dopamine efflux in the striatum than 129S2/SvHsd mice. Pharmacol Biochem Behav. 2007;87:158–63. doi: 10.1016/j.pbb.2007.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colelli V, Fiorenza MT, Conversi D, Orsini C, Cabib S. Strain-specific proportion of the two isoforms of the dopamine D2 receptor in the mouse striatum: associated neural and behavioral phenotypes. Genes Brain Behav. 2010;9:703–11. doi: 10.1111/j.1601-183X.2010.00604.x. [DOI] [PubMed] [Google Scholar]
- Darke S, Kaye S, McKetin R, Duflou J. Major physical and psychological harms of methamphetamine use. Drug Alcohol Rev. 2008;27:253–62. doi: 10.1080/09595230801923702. [DOI] [PubMed] [Google Scholar]
- Davidson C, Gow AJ, Lee TH, Ellinwood EH. Methamphetamine neurotoxicity: necrotic and apoptotic mechanisms and relevance to human abuse and treatment. Brain Res Brain Res Rev. 2001;36:1–22. doi: 10.1016/s0165-0173(01)00054-6. [DOI] [PubMed] [Google Scholar]
- Deng X, Wang Y, Chou J, Cadet JL. Methamphetamine causes widespread apoptosis in the mouse brain: evidence from using an improved TUNEL histochemical method. Brain Res Mol Brain Res. 2001;93:64–9. doi: 10.1016/s0169-328x(01)00184-x. [DOI] [PubMed] [Google Scholar]
- Deng X, Cai NS, McCoy MT, Chen W, Trush MA, Cadet JL. Methamphetamine induced apoptosis in an immortalized rat striatal cell line by activating the mitochondrial cell death pathway. Neuropharmacology. 2002;42:837–45. doi: 10.1016/s0028-3908(02)00034-5. [DOI] [PubMed] [Google Scholar]
- Egashira T, Yamanaka Y. Changes in monoamine oxidase activity in mouse brain associated with d-methamphetamine dependence and withdrawal. Biochem Pharmacol. 1993;46:609–14. doi: 10.1016/0006-2952(93)90545-8. [DOI] [PubMed] [Google Scholar]
- Erwin VG, Womer DE, Campbell AD, Jones BC. Pharmacogenetics of cocaine: II. Mesocorticolimbic and striatal dopamine and cocaine receptors in C57BL and DBA mice. Pharmacogenetics. 1993;3:189–96. doi: 10.1097/00008571-199308000-00003. [DOI] [PubMed] [Google Scholar]
- Fumagalli F, Gainetdinov RR, Valenzano KJ, Caron MG. Role of dopamine transporter in methamphetamine-induced neurotoxicity: evidence from mice lacking the transporter. J Neurosci. 1998;18:4861–9. doi: 10.1523/JNEUROSCI.18-13-04861.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giordano TP, Satpute SS, Striessnig J, Kosofsky BE, Rajadhyaksha AM. Up-regulation of dopamine D2L mRNA levels in the ventral tegmental area and dorsal striatum of amphetamine-sensitized C57BL/6 mice: role of Cav1. 3 L-type Ca2+ channels. J Neurochem. 2006;99:1197–1206. doi: 10.1111/j.1471-4159.2006.04186.x. [DOI] [PubMed] [Google Scholar]
- Good RL, Radcliffe RA. Methamphetamine-induced locomotor changes are dependent on age, dose and genotype. Pharmacol Biochem Behav. 2011;98:101–11. doi: 10.1016/j.pbb.2010.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granado N, Ares-Santos S, Oliva, O’Shea E, Martin ED, Colado MI, Moratalla R. Dopamine D2-receptor knockout mice are protected against dopaminergic neurotoxicity induced by methamphetamine or MDMA. Neurobiol Disease. 2011;42:391–403. doi: 10.1016/j.nbd.2011.01.033. [DOI] [PubMed] [Google Scholar]
- Grisel JE, Belknap JK, O’Toole LA, Helms ML, Wenger CD, Crabbe JC. Quantitative trait loci affecting methamphetamine responses in BXD recombinant inbred mouse strains. J Neurosci. 1997;17:745–54. doi: 10.1523/JNEUROSCI.17-02-00745.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris D, Batki SL. Stimulant psychosis: symptom profile and acute clinical course. Am J Addict. 2000;9:28–37. doi: 10.1080/10550490050172209. [DOI] [PubMed] [Google Scholar]
- He M, Shippenberg TS. Strain differences in basal and cocaine-evoked dopamine dynamics in mouse striatum. J Pharmacol Exp Ther. 2000;293:121–7. [PubMed] [Google Scholar]
- Imam SZ, Ali SF. Aging increases the susceptibility to methamphetamine-induced dopaminergic neurotoxicity in rats: correlation with peroxynitrite production and hyperthermia. J Neurochem. 2001a;78:952–9. doi: 10.1046/j.1471-4159.2001.00477.x. [DOI] [PubMed] [Google Scholar]
- Imam SZ, el-Yazal J, Newport GD, Itzhak Y, Cadet JL, Slikker W, Jr, Ali SF. Methamphetamine-induced dopaminergic neurotoxicity: role of peroxynitrite and neuroprotective role of antioxidants and peroxynitrite decomposition catalysts. Ann NY Acad Sci. 2001b;939:366–80. doi: 10.1111/j.1749-6632.2001.tb03646.x. [DOI] [PubMed] [Google Scholar]
- Jayanthi S, Deng X, Noailles PH, Ladenheim B, Cadet JL. Methamphetamine induces neuronal apoptosis via cross-talks between endoplasmic reticulum and mitochondria-dependent death cascades. FASEB J. 2004;18:238–51. doi: 10.1096/fj.03-0295com. [DOI] [PubMed] [Google Scholar]
- Johnason CE, Frey KA, Lundahl LH, Keenan P, Lockhart N, Roll J, Galloway GP, Koeppe RA, Kilbourn MR, Robbins T, Schuster CR. Cognitive function and nigrostriatal markers in abstinent methamphetamine users. Psychopharmacology (Berl) 2006;185:327–38. doi: 10.1007/s00213-006-0330-6. [DOI] [PubMed] [Google Scholar]
- Jones BC, Tarantino LM, Rodriguez LA, Reed CL, McClearn GE, Plomin R, Erwin VG. Quantitative-trait loci analysis of cocaine-related behaviours and neurochemistry. Pharmacogenetics. 1999;9:607–17. [PubMed] [Google Scholar]
- Kanes SJ, Hitzemann BA, Hitzemann RJ. On the relationship between D2 receptor density and neuroleptic-induced catalepsy among eight inbred strains of mice. J Pharmacol Exp Ther. 1993;267:538–547. [PubMed] [Google Scholar]
- Kokoshka JM, Fleckenstein AE, Wilkins DG, Hanson GR. Age-dependent differential responses of monoaminergic systems to high doses of methamphetamine. J Neurochem. 2000;75:2095–102. doi: 10.1046/j.1471-4159.2000.0752095.x. [DOI] [PubMed] [Google Scholar]
- Krasnova IN, Hodges AB, Ladenheim B, Rhoades R, Phillip CG, Cesena A, Ivanova E, Hohmann CF, Cadet JL. Methamphetamine treatment causes delayed decrease in novelty-induced locomotor activity in mice. Neurosci Res. 2009;65:160–5. doi: 10.1016/j.neures.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuribara H, Tadokoro S. Reverse tolerance to ambulation-increasing effects of methamphetamine and morphine in 6 mouse strains. Jpn J Pharmacol. 1989;49:197–203. doi: 10.1254/jjp.49.197. [DOI] [PubMed] [Google Scholar]
- Liang LP, Patel M. Iron-sulfur enzyme mediated mitochondrial superoxide toxicity in experimental Parkinson’s disease. J Neurochem. 2004;90:1076–84. doi: 10.1111/j.1471-4159.2004.02567.x. [DOI] [PubMed] [Google Scholar]
- Lindgren N, Usiello A, Goiny M, Haycock J, Erbs E, Greengard P, Hökfelt T, Borrelli E, Fisone G. Distinct roles of dopamine D2L and D2S receptor isoforms in the regulation of protein phosphorylation at presynaptic and postsynaptic sites. P Natl Acad Sci USA. 2003;100:4305–4309. doi: 10.1073/pnas.0730708100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller DB, O’Callaghan JP, Ali SF. Age as a susceptibility factor in the striatal dopaminergic neurotoxicity observed in the mouse following substituted amphetamine exposure. Ann NY Acad Sci. 2000;914:194–207. doi: 10.1111/j.1749-6632.2000.tb05196.x. [DOI] [PubMed] [Google Scholar]
- Ng GY, O’Dowd BF, George SR. Genotypic differences in brain dopamine receptor function in the DBA/2J and C57BL/6J inbred mouse strains. Eur J Pharmacol. 1994;269:349–64. doi: 10.1016/0922-4106(94)90043-4. [DOI] [PubMed] [Google Scholar]
- Pereira FC, Lourenco ES, Borges F, Morgadinho T, Ribeiro CF, Macedo TR, Ali SF. Single or multiple injections of methamphetamine increased dopamine turnover but did not decrease tyrosine hydroxylase levels or cleave caspase-3 in caudate-putamen. Synapse. 2006;60:185–93. doi: 10.1002/syn.20285. [DOI] [PubMed] [Google Scholar]
- Phillips TJ, Dickinson S, Burkart-Kasch S. Behavioral sensitization to drug stimulant effects in C57BL/6J and DBA/2J inbred mice. Behav Neurosci. 1994;108:789–803. doi: 10.1037//0735-7044.108.4.789. [DOI] [PubMed] [Google Scholar]
- Pubill D, Chipana C, Camins A, Pallas M, Camarasa J, Escubedo E. Free radical production induced by methamphetamine in rat striatal synaptosomes. Toxicol Appl Pharmacol. 2005;204:57–68. doi: 10.1016/j.taap.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration (SAMHSA) NSDUH Series H-30. DHHS Pub No. SMA 06-4194. Rockville, MD: DHHS; 2006. Results from the 2005 National Survey on Drug Use and Health. [Google Scholar]
- Sulzer D, Sonders MS, Poulsen NW, Gallia A. Mechanisms of neurotransmitter release by amphetamines: a review. Prog Neurobiol. 2005;75:406–33. doi: 10.1016/j.pneurobio.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Suzuki O, Hattori H, Asano M, Oya M, Katsumata Y. Inhibition of monoamine oxidase by d-methamphetamine. Biochem Pharmacol. 1980;29:2071–3. doi: 10.1016/0006-2952(80)90493-1. [DOI] [PubMed] [Google Scholar]
- Truong JG, Wilkins DG, Baudys J, Crouch DJ, Johnson-Davis KL, Gibb JW, Hanson GR, Fleckenstein AE. Age-dependent methamphetamine-induced alterations in vesicular monoamine transporter-2 function: Implications for neurotoxicity. J Pharmacol Exp Ther. 2005;314:1087–92. doi: 10.1124/jpet.105.085951. [DOI] [PubMed] [Google Scholar]
- Usiello A, Baik JH, Rougé-Pont F, Picetti R, Dierich A, LeMeur M, Piazza PV, Borrelli E. Distinct functions of the two isoforms of dopamine D2 receptors. Nature. 2000;408:199–203. doi: 10.1038/35041572. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Chang L, Wang GJ, Fowler JS, Franceschi D, Sedler M, Gatley SJ, Miller E, Hitzemann R, Ding YS, Logan J. Loss of dopamine transporters in methamphetamine abusers recovers with protracted abstinence. J Neurosci. 2001;21:9414–18. doi: 10.1523/JNEUROSCI.21-23-09414.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner GC, Ricaurte GA, Seiden LS, Schuster CR, Miller RJ, Westley J. Long-lasting depletions of striatal dopamine and loss of dopamine uptake sites following repeated administration of methamphetamine. Brain Res. 1980;181:151–60. doi: 10.1016/0006-8993(80)91265-2. [DOI] [PubMed] [Google Scholar]
- Yamamoto BK, Zhu W. The effects of methamphetamine on the production of free radicals and oxidative stress. J Pharmacol Exp Ther. 1998;287:107–14. [PubMed] [Google Scholar]
- Yoo JH, Bailey A, Ansonoff M, Pintar JE, Matifas A, Kieffer BL, Kitchen I. Lack of genotype effect on D1, D2 receptors and dopamine transporter binding in triple MOP-, DOP-, and KOP-opioid receptor knockout mice of three different genetic backgrounds. Synapse. 2010;64:520–527. doi: 10.1002/syn.20757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu JPQ, Xu W, Angulo JA. Disparity in the temporal appearance of methamphetamine induced apoptosis and depletion of dopamine terminal markers in the striatum of mice. Brain Res. 2005;1049:171–181. doi: 10.1016/j.brainres.2005.04.089. [DOI] [PMC free article] [PubMed] [Google Scholar]