Abstract
The endothelin (ET) system comprises a family of three isopeptides (ET-1, ET-2 and ET-3) involved in diverse physiological and pathophysiological events. ET-1 is the major renal peptide that exerts its biological activity by binding to ETA and ETB receptors. Both ETA and ETB receptors are expressed by renal microvascular smooth muscle cells where activation causes vasoconstriction. ETB receptors are also expressed by microvascular endothelial cells where leading to vasodilator responses. ET-1 influences preglomerular and postglomerular microvascular tone and thus can significantly influence renal hemodynamics. Alteration of renal ET-1 synthesis and receptor expression has been reported in cardiovascular diseases, and could contribute to renal injury by altering renal microvascular reactivity. In this brief review, we will try to summarize what is known about ET control of renal microvascular function.
Introduction
Since ET was discovered in 1988 [1], three distinct ET isopeptides have been classified as ET-1, ET-2 and ET-3 [2]. Initially ET was considered to be of endothelial origin. It is now clear that ET is synthesized and released from many vascular and non-vascular cell types including renal epithelial cells [3–5]. ET-1 is the predominant isoform produced by the vasculature and is constitutively secreted from the endothelium, where it acts in a paracrine/autocrine manner on adjacent endothelial or vascular smooth muscle cells (VSMC). ET-1 is produced from big ET-1 catalyzed by ET converting enzymes present in endothelial cells and is reportedly the most potent and long-lasting vasoconstrictor [1]. Approximately 80% of secreted ET-1 is released into the basolateral compartment, whereas only a limited amount of ET-1 enters the circulation [6]. Generally, plasma ET-1 concentrations range between 0.5–5 pM in healthy human subjects [4, 7]. Interestingly, levels of immunoreactive ET are higher in the kidney inner medulla than any other organ or tissue [8], implying an important physiological role for ET-1 in regulating renal function.
ET influences diverse physiological and pathophysiological mechanisms by activation of distinct ETA and ETB receptor subtypes [3–5]. In the vasculature, ETA and ETB receptors are expressed by VSMC while endothelial cells mainly express ETB receptors. Activation of ETA and ETB receptors on VSMC increases intracellular Ca2+ concentration ([Ca2+]i), leading to increased vascular tone [4, 5]. Activation of endothelial ETB receptors stimulates release of nitric oxide (NO) and prostacyclin leading to vasodilatation [9]. Additionally, ETB receptors also serve as “clearance receptors” to sequester ET-1 from the plasma [10, 11]. Accordingly, blockade or deletion of ETB receptors tends to elevate ET-1 levels. Competition-binding studies reveal that ETA and ETB receptors have different binding affinities for the three ET isoforms. ETA receptors have a higher affinity for ET-1 and ET-2 and a weaker affinity for ET-3. ETB receptors, on the other hand, display similar affinities for all three ET isopeptides [3]. These receptor binding characteristics have been widely used to identify tissue distribution of receptors.
In the kidney, evidence indicates that ET-1 is produced locally and regulates renal hemodynamics by acting on preglomerular and postglomerular microvascular reactivity. ET-1 is also involved in sodium and water transport, and cell proliferation [4, 5]. Alteration of renal ET-1 levels and receptor expression are implicated in cardiovascular disease development. This review will focus on the role of ET peptides in the renal microcirculation.
ET Receptor Expression in Kidney
Early studies using receptor binding, immunocytochemical and pharmacological tools revealed that almost every renal vascular and tubular cell type expresses ETA and/or ETB receptors but the expression levels vary greatly between different regions of the kidney and across species [4, 5]. In the canine kidney, ETA and ETB receptor ratios average 22:78, 40:60 and 50:50 in cortical, medullary and papillary membranes, respectively [12], while in rat kidneys, the regional ETA/ETB receptor ratios were 50:50, 30:70 and 90:10, respectively [13]. ET receptors are expressed in high levels in the renal medulla particularly in the inner medulla [14, 15]. Positron emission tomography makes it possible to quantify receptor concentration by measuring receptor-bound radioligands in vivo. This approach revealed that medullary ETB receptor expression is double that of the cortex in rabbit kidney [15]. Similarly, ETB receptor expression is nearly four times greater in the human renal medulla than in the cortex (33:9) [15] but the ETA/ETB receptor distribution is similar between the cortex and medulla (30:70) [16].
Little is known regarding how ETA and ETB receptors differentially influence renal vascular reactivity to ET-1. Most studies have employed pharmacological tools to determine the contribution of specific ET receptors in modulating renal microvascular function. Several studies conducted to localize ET receptor expression in renal vessels indicate that both ETA and ETB receptors are present in preglomerular and postglomerular microvessels [17–21]. ETA receptors are present in VSMC while ETB receptors are mainly expressed in vascular and glomerular endothelial cells and are barely detectable in VSMC [17]. Radioligand binding studies show that the proportion of ETA and ETB receptors in membranes of preglomerular vessels is 40:60 in rabbits [18] and 50:50 in rats [19]. In human kidneys, ETA receptors are present in the vasculature throughout the cortex and medulla with low binding densities in vasa recta and glomeruli [20]. Renal and arcuate arteries express a high density of ETA receptors at 90–95% of total ET receptors [21]. Additionally, both ETA and ETB receptors are detected in rat vasa recta but they are located in pericytes and endothelial cells, respectively [17], implying that they might play different roles in regulating medullary perfusion.
Effects of ET on Renal Hemodynamics
The renal microcirculatory system is a unique portal circulation characterized by two independently regulated resistance vessels (afferent and efferent arterioles) in series straddling the glomerular capillaries. The endothelial cells release a variety of vasodilators and vasoconstrictors in response to physical and chemical stimuli, shear stress or stretch. These vasoactive substances modulate the glomerular microcirculation through autocrine and/or paracrine mechanisms to influence afferent and efferent arteriolar resistance and hence renal hemodynamics. Thus, the balance of vascular resistance conferred by afferent and efferent arterioles is a crucial factor in determining overall glomerular hemodynamics.
A role for ET in regulating the renal microcirculation has been substantiated by numerous in vivo and in vitro studies in different animal models. The kidney is highly sensitive to exogenous ET-1 compared to other organs. Almost all in vivo studies show that infusion of exogenous ET-1 produces a marked and prolonged renal vasoconstriction marked by profound reductions in renal blood flow (RBF) and glomerular filtration rate (GFR) reflecting increased renal vascular resistance [12, 13, 22–29]. However, the relative contribution of ET receptors to the ET-1-mediated vasoconstriction is variable among species and results are controversial. In canine kidneys, ET-1-induced reduction of RBF and GFR was abolished by ETA receptor blockade [12], implicating ETA receptors in regulating renal vascular reactivity. Furthermore, infusion of a highly selective ETB receptor agonist, sarafotoxin 6c (S6c), markedly increased urine flow and sodium excretion, but had little effect on RBF and GFR [12], indicating that ETB receptors primarily regulate tubular transports compared to vascular function in dog kidneys. ET-1 is also powerful vasoconstrictor in rabbit kidneys [24, 25]. Interestingly however, ET-1-mediated decreases in rabbit RBF reflected cortical vasoconstriction because medullary perfusion increased during ET-1 infusion [25]. The ET-1-induced reduction of cortical perfusion was reversed by ETA receptor blockade but enhanced by ETB receptor blockade. These studies indicate that ET-1-induced renal vasoconstriction in dogs and rabbits is primarily mediated by ETA receptors.
In contrast to dogs and rabbits, many in vivo studies in rats indicate that both ETA and ETB receptors mediate renal vasoconstriction induced by ET-1, but ETB receptors may play a more prominent role [13, 26–29]. Intravenous infusion of ET-1 in anesthetized rats usually decreases RBF and GFR. This is accompanied by a transient reduction in blood pressure followed by a sustained pressor response. ETA receptor antagonists abrogated the pressor effects of ET-1 but had little effect on the renal hemodynamic response [26, 29]. Others showed that ET-1 and the ETB receptor ligand or agonist (ET-3 or S6c) elicited a similar reduction in RBF [27–29]. The ET-1-induced reduction of RBF was partially blocked by ETA receptor antagonists and completely abolished by a combined ETA/B receptor blockade [28, 29]. Collectively, these findings indicate that both ETA and ETB receptors are involved in renal microvascular response to ET-1, but ETB receptors play a predominant role in rats. Moreover, the ability of ET-1 to decrease RBF was exaggerated by selective ETB receptor blockade with BQ-788 [28], suggesting that ET-1 elicits both vasoconstrictor and vasodilator effects. The vasodilator component could reflect ETB receptor-dependent release of vasodilators or reduced ET-1 clearance due to ETB receptor blockade.
Effects of ET on Renal Microvascular Reactivity in vitro
While the whole kidney studies provide in vivo evidence supporting ET-1 as a regulatory of renal vascular reactivity, the data are often confounded by concomitant changes in mean arterial pressure and released circulating factors. Furthermore, the distribution of ETA and ETB receptors in different microvascular segments compromises interpretation of which ET receptors are responsible for ET-1’s overall effect and to what degree they contribute to ET-1-induced alteration of renal hemodynamics. Accordingly, many in vitro models have been used to provide site-specific information. Studies have frequently utilized partially resected kidney models, with or without hydronephrosis, or isolated renal microvessels to directly visualize ET’s effects on afferent and efferent arteriolar diameters.
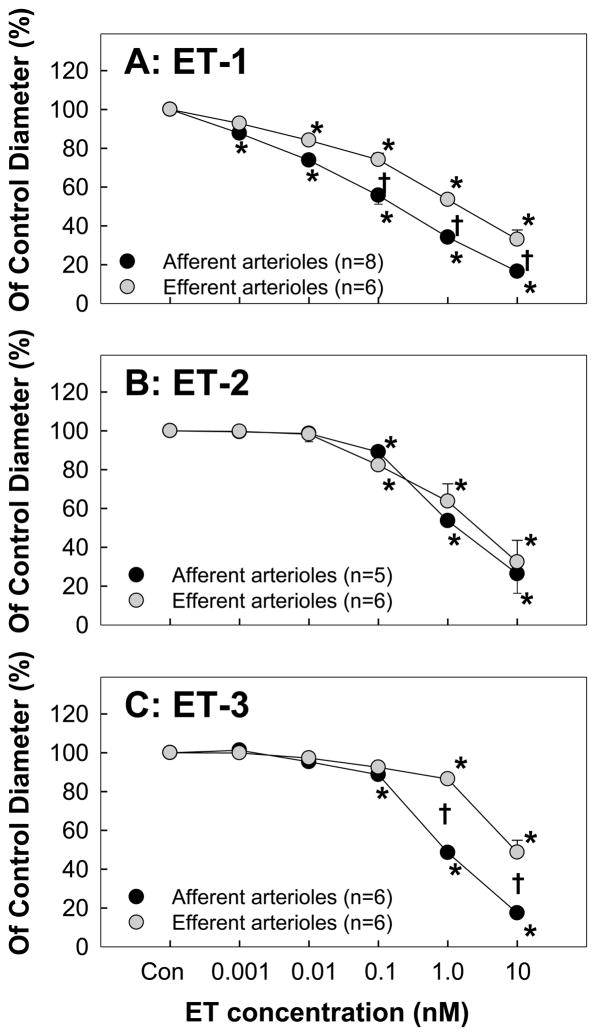
The in vitro blood-perfused juxtamedullary nephron preparation is frequently used for direct assessment of renal microvascular reactivity while maintaining an intact association between the vascular and tubular elements [30]. ET-1, ET-2 and ET-3 all evoke concentration-dependent vasoconstriction of afferent and efferent arterioles [31–34] (Figure 1). ET-1 vasoconstricts afferent and efferent arterioles at concentrations of 1 and 10 pM, respectively [32]. ET-1 vasoconstricted afferent arterioles more effectively than efferent arterioles such that afferent diameter declined by 83% of control compared to 67% for efferent arterioles at a 10 nM concentration. Similar to ET-1, ET-3 also yielded a greater vasoconstrictor response in afferent than efferent arterioles. ET-2 however, elicited similar degrees of vasoconstriction for afferent and efferent arterioles. The ET-1 concentration curve in afferent arterioles was significantly shifted to the right by pre-treatment with ETA or ETB receptor blockers and completely abolished by combined ETA/B blockers, indicating the vasoconstrictor property of both ET receptors at preglomerular microvessels. In contrast, ETA receptor blockade in efferent arterioles converted ET-1-induced vasoconstriction to vasodilation at low concentrations (0.01–0.1 nM) while vasoconstriction was retained at higher concentrations (1–10 nM), suggesting that ETB receptors can elicit vasodilation and vasoconstriction of efferent arterioles. The dual effect of ETB receptors in efferent arterioles was confirmed using S6c, which yielded efferent arteriolar vasodilation under control conditions but vasoconstriction during an ETB receptor blockade with A-192621 [32]. These observations suggest that during ETA receptor blockade, low ET-1 concentrations mainly stimulate endothelial ETB receptors, probably by releasing NO or prostacyclin, while higher concentrations of ET-1 shift to activating ETB receptors on VSMC in efferent arterioles.
Figure 1. Renal microvascular responses to endothelin (ET) peptides.
Kidneys were superfused with increasing concentrations of ET-1 (A), ET-2 (B) or ET-3 (C) from 1.0 pM to 10 nM (5 minutes for each concentration) while perfusion pressure was maintained at 100 mmHg. Afferent arteriolar diameters (black symbols) and efferent arteriolar diameters (gray symbols) were measured at 12 s intervals and calculated from the average of all measurements obtained during the final 2 minutes of each 5-minute treatment period. Data are expressed as percent of the control diameter. Values are mean ± SEM. *P<0.05 vs. control diameter in same group; †P<0.05 vs. control rats at same perfusion pressure. Figure is adapted from [32].
Preferential ET-1-mediated vasoconstriction of rat afferent arterioles is demonstrated using different experimental approaches [23, 29, 35–37]. For example, micropuncture studies in Munich-wistar rats showed that ET-1 reduced single nephron GFR with significant increases in afferent and efferent arteriolar resistance, but particularly in afferent arterioles [35]. This is consistent with studies in hydronephrotic rat kidneys showing that ET-1 caused substantial afferent arteriolar vasoconstriction but only modest efferent arteriolar vasoconstriction [23, 36]. Alternatively, other studies indicated that efferent arterioles are more sensitive to ET-1 than afferent arterioles [22, 38]. Glomerular capillary pressure, as assessed by measuring stop-flow pressure, was significantly increased in response to ET, which was associated with a greater increase in efferent arteriolar resistance than in afferent arteriolar resistance [22]. Studies in isolated-perfused microvessels showed that the EC50 of ET-1 was lower in efferent arterioles (5.7 pM) compared to afferent arterioles (52 pM) [38], suggesting that ET-1 is a more potent vasoconstrictor of efferent arterioles. The explanation for the discrepancies among these studies is unclear but may reflect differences between experimental conditions.
The aforementioned studies of ET-1 on renal microvascular reactivity mostly employed pharmacological tools to establish the contribution of ET receptors. Genetic approaches provide useful strategies to determine specific gene or protein functions. Although several ET-1 or ETB receptor knockout mouse models have been developed, only one study was conducted to assess the distribution of ET receptors in ET-1-induced renal microvascular responsiveness in vascular ETB receptor deficient mice and its wild-type control mice [39]. The results as performed in isolated-perfused renal microvessels indicate that ET-1 more potently vasoconstricts afferent arterioles compared to efferent arterioles, and that ET-1-induced vasoconstriction involves activation of ETA receptors in afferent arterioles but both ETA and ETB receptors in efferent arterioles [39]. The relative contribution of ET receptors in ET-1-induced responses in mouse renal microvessels is different from rats. Interestingly, endothelium specific ET-1 knockout mice exhibit 10–12 mmHg lower mean arterial pressure [40] , suggesting a pivotal role of endogenous endothelium-derived ET receptor system in control of blood pressure and maintenance of vascular tone under physiological conditions. How these changes affect renal hemodynamics remains to be determined.
ET in Medullary Microcirculation
Medullary perfusion is supplied by descending vasa recta (DVR) arising from juxtamedullary efferent arterioles [41]. Given the potent vasoconstrictor effect of ET-1 in juxtamedullary nephron microvasculature and expression of ETA and ETB receptors in vasa recta [17], it is not surprising that ET is also involved in regulating medullary microcirculation. Elegant studies using isolated-perfused DVR establish ET as a potent vasoconstrictor of DVR [41]. ET-1, ET-2 and ET-3 elicit potent vasoconstriction with a rank order of potency of ET-1>ET-2>ET-3. DVR diameter started to decrease with 0.01 pM ET-1 and almost completely collapsed at 100 pM. ET-1-induced vasoconstriction was attenuated by an ETA antagonist or a mixed ETA/B receptor antagonist whereas ET-3-induced vasoconstriction was only blocked by an ETB receptor antagonist, suggesting the dual involvement of ETA and ETB receptors. These observations however, seem to contradict rabbit studies where infusion of ET-1 increases medullary blood flow with concurrent reduction in total RBF and cortical blood flow [25]. Conflicting outcomes probably reflect species variation (rats versus rabbits) and different experimental conditions (in vitro versus in vivo). The intense vasoconstriction of DVR by ET-1 suggests a potentially important role of ET-1 for the development of renal injury in cases where the renal ET system is upregulated [41].
Endogenous ET-1 in Regulating Renal Microvascular Tone
While both in vivo and in vitro studies provide compelling evidence that ET-1 regulates renal hemodynamics by influencing preglomerular and postglomerular microvascular tone, there is conflicting evidence establishing that ET-1 regulates renal vascular tone under basal conditions. Infusion of ETA receptor antagonists did not change RBF or GFR in conscious rats [9, 26, 42], implying that ETA receptors may not regulate renal hemodynamics under basal conditions. In contrast, combined ETA/B receptor blockade with bosentan markedly reduced glomerular capillary pressure with a significant increase in preglomerular resistance while postglomerular resistance remained unchanged [42]. ETB receptor blockade with RES-701–1 or BQ-788 significantly decreased RBF without affecting arterial blood pressure [9, 13]. Reduction of RBF was prevented by pre-treatment with L-NAME (NOS inhibitor) and ibuprofen (cyclo-oxygenase inhibitor). These findings suggest that endogenous ET-1 contributes to basal renal vascular resistance by ETB receptor-dependent release of NO and/or prostacyclin. Overall, these studies imply that ETB receptor-mediated vasodilation may influence renal microcirculatory function under physiological conditions. For example, exogenous ET-1 or S6c normally vasoconstricts normal rat afferent arterioles [32], however, when rats are fed a high salt diet, ET-1 and S6c-mediated vasoconstriction is attenuated and even converted to vasodilation at low ET-1 concentrations. ETB receptor expression in preglomerular microvessels is increased during salt loading [34]. Accordingly, salt-induced upregulation of ETB receptor expression may counteract ETA and ETB receptor activation in VSMC and facilitate sodium excretion by increasing RBF. The physiological mechanisms behind salt-induced increases in ETB receptor expression need further investigation.
ET-induced Intracellular Signaling in Renal Microvessels
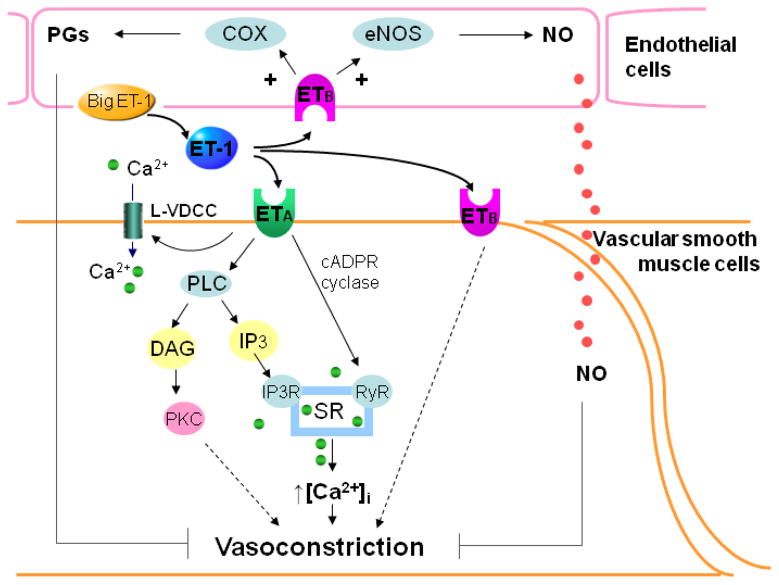
Both ETA and ETB receptors belong to the G-protein coupled receptor family. ET receptor activation is coupled to many second messenger pathways including phospholipase-C, phospholipase-D, protein kinase-C, mitogen activated protein kinases, cytosolic Ca2+ and tyrosine kinase [4, 5]. Application of ET-1 to VSMC increases [Ca2+]i via influx of extracellular Ca2+ and mobilization of Ca2+ from intracellular stores [43]. ETA receptors are coupled with activation of phospholipase-C to generate diacylglycerol and inositol triphosphate, which in turn stimulates Ca2+ release from intracellular stores, ultimately causing vasoconstriction (Figure 2). In contrast, activation of ETB receptors in endothelial cells stimulates release of NO and/or prostacyclin, which increase cGMP and cAMP formation leading to vasodilation. Downstream signaling of ETB receptor-activation in VSMC still remains uncertain.
Figure 2. Intracellular ET pathway in renal preglomerular microvessels.
ET-1 is produced from big ET-1 catalyzed by the ET converting enzyme from endothelial cells. Activation of ETA receptors in vascular smooth muscle cells increases intracellular calcium concentration ([Ca2+]i) via influx of extracellular Ca2+ through activating L-type voltage-dependent Ca2+ channels (L-VDCC) and mobilization of Ca2+ from sarcoplasmic reticulum (SR) through activating inositol triphosphate (IP3) pathway and cyclic adenine diphosphate ribose (cADPR) cyclase/ryanodine receptor (RyR) pathways. Activation of endothelial ETB receptors releases nitric oxide (NO) and prostaglandins (PGs) causing vasodilation. Activation of ETB receptors in vascular smooth muscle cells mediates vasoconstriction via as yet undefined intracellular mechanisms (Dashed line)
In the kidney, L-type voltage-dependent Ca2+ channels (L-VDCC) are important for regulating basal vascular tone, and preglomerular microvascular responses to many vasoconstrictors [44]. However, there are conflicting results whether ET-1-induced vasoconstriction involves L-VDCC signaling. Several in vivo and in vitro studies indicate that L-VDCC blockade had little effect on ET-1 or ETB receptor-mediated renal vasoconstriction [33, 43, 45], while others found that ET-1 increased [Ca2+]i via L-VDCC-dependent influx of extracellular Ca2+ in afferent arterioles [23, 46], but not in efferent arterioles [46]. Studies in freshly isolated preglomerular microvascular VSMC found that ET-1 evoked a biphasic increase in [Ca2+]i, with a rapid initial increase followed by a sustained plateau [43]. The sustained plateau was eliminated by superfusion with Ca2+-free medium while the initial peak was unaltered, indicating that ET-1 increases [Ca2+]i in preglomerular microvascular VSMC by stimulating Ca2+ release from intracellular stores and Ca2+ influx from extracellular fluid. In contrast, ET-3 or S6c only evoked small and monophasic increases in [Ca2+]i [33, 43]. Subsequent studies revealed that ET-1-induced increases in [Ca2+]i were unchanged by blockade of L-VDCC with diltilazem or Ni2+. This was confirmed with both in vivo and in vitro studies. Nifedipine only slightly attenuated the reduction of RBF when ET-1 or S6c were applied at very high doses while ET-1-induced juxtamedullary afferent arteriolar vasoconstriction was slightly inhibited by diltilazem only at very low ET-1 concentrations (1 and 10 pM) [33]. Although L-VDCC inhibition led to slightly different observations in these two experimental settings, the results suggest that L-VDCC do not play a critical role in ET-1 and ETB receptor-mediated afferent arteriolar vasoconstriction in rats. Alternatively, studies in hydronephrotic rat kidney showed that nifedipine completely eliminated ET-1-induced afferent arteriolar vasoconstriction, indicating a prominent role for L-VDCC in ET-1-mediated afferent arteriolar responses [23].
Recent studies indicate that cyclic adenine diphosphate ribose (cADPR) cyclase/ryanodine receptor-mediated Ca2+ signaling pathways contribute to ET-1-mediated vasoconstriction in afferent arterioles [47]. Blockade of ribosyl cyclase with nicotinamide reduced ET-1-mediated [Ca2+]i signaling by 60% in preglomerular microvascular VSMC. This effect is largely due to stimulation of NADPH oxidase by ET-1 via ETA receptor activation because application of tempol, apocynin, or a specific cADPR cyclase inhibitor attenuated [Ca2+]i signaling induced by ET-1 but not by S6c. These studies suggest that the superoxide-NADPH oxidase pathway contributes to the preglomerular microvascular response to ET-1 through cADPR.
Collectively, ET-1-mediated vasoconstriction of afferent arterioles is coupled with activation of ETA and ETB receptors with a predominant role of ETA receptors in dogs, rabbits and mice, and a predominant role of ETB receptors in rats. ET-1-mediated vasoresponsiveness in efferent arterioles seems to be exclusively coupled to ETB receptors except in mice. Low ET-1 concentrations activate endothelial ETB receptors leading to vasodilation while high ET-1 concentrations activate ETB receptors expressed in VSMC evoking vasoconstriction. Additionally, endogenous ET-1 may contribute to control of basal renal vascular resistance via a vasodilator influence of ETB receptors in rats. The magnitude of ET-1-mediated vasoresponsiveness depends upon the segmental distribution of ET receptor expression as well as species variation. Different responses of afferent and efferent arterioles to ET-1 may certainly have a distinct impact on glomerular capillary pressure and hence alter glomerular hemodynamics. Any changes in ET receptor expression or renal ET-1 levels may play a crucial role in renal injury under pathological conditions by impairing renal hemodynamics.
Acknowledgments
The authors would like to acknowledge grant support from National Institutes of Health (PO1 HL095499) for EW Inscho and the American Heart Association (10SDG3770010) for Z Guan.
References
- 1.Yanagisawa M, Kurihara H, Kimura S, Tomobe Y, Kobayashi M, Mitsui Y, Yazaki Y, Goto K, Masaki T. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature. 1988;332:411–415. doi: 10.1038/332411a0. [DOI] [PubMed] [Google Scholar]
- 2.Inoue A, Yanagisawa M, Kimura S, Kasuya Y, Miyauchi T, Goto K, Masaki T. The human endothelin family: three structurally and pharmacologically distinct isopeptides predicted by three separate genes. Proc Natl Acad Sci U S A. 1989;86:2863–2867. doi: 10.1073/pnas.86.8.2863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sakurai T, Yanagisawa M, Takuwa Y, Miyazaki H, Kimura S, Goto K, Masaki T. Cloning of a cDNA encoding a non-isopeptide-selective subtype of the endothelin receptor. Nature. 1990;348:732–735. doi: 10.1038/348732a0. [DOI] [PubMed] [Google Scholar]
- 4.Simonson MS. Endothelins: multifunctional renal peptides. Physiol Rev. 1993;73:375–411. doi: 10.1152/physrev.1993.73.2.375. [DOI] [PubMed] [Google Scholar]
- 5.Kohan DE, Rossi NF, Inscho EW, Pollock DM. Regulation of blood pressure and salt homeostasis by endothelin. Physiol Rev. 2011;91:1–77. doi: 10.1152/physrev.00060.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wagner OF, Christ G, Wojta J, Vierhapper H, Parzer S, Nowotny PJ, Schneider B, Waldhausl W, Binder BR. Polar secretion of endothelin-1 by cultured endothelial cells. J Biol Chem. 1992;267:16066–16068. [PubMed] [Google Scholar]
- 7.Treiber FA, Jackson RW, Davis H, Pollock JS, Kapuku G, Mensah GA, Pollock DM. Racial differences in endothelin-1 at rest and in response to acute stress in adolescent males. Hypertension. 2000;35:722–725. doi: 10.1161/01.hyp.35.3.722. [DOI] [PubMed] [Google Scholar]
- 8.Kitamura K, Tanaka T, Kato J, Ogawa T, Eto T, Tanaka K. Immunoreactive endothelin in rat kidney inner medulla: marked decrease in spontaneously hypertensive rats. Biochem Biophys Res Commun. 1989;162:38–44. doi: 10.1016/0006-291x(89)91958-x. [DOI] [PubMed] [Google Scholar]
- 9.Matsuura T, Miura K, Ebara T, Yukimura T, Yamanaka S, Kim S, Iwao H. Renal vascular effects of the selective endothelin receptor antagonists in anaesthetized rats. Br J Pharmacol. 1997;122:81–86. doi: 10.1038/sj.bjp.0701349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fukuroda T, Fujikawa T, Ozaki S, Ishikawa K, Yano M, Nishikibe M. Clearance of circulating endothelin-1 by ETB receptors in rats. Biochem Biophys Res Commun. 1994;199:1461–1465. doi: 10.1006/bbrc.1994.1395. [DOI] [PubMed] [Google Scholar]
- 11.Bagnall AJ, Kelland NF, Gulliver-Sloan F, Davenport AP, Gray GA, Yanagisawa M, Webb DJ, Kotelevtsev YV. Deletion of endothelial cell endothelin B receptors does not affect blood pressure or sensitivity to salt. Hypertension. 2006;48:286–293. doi: 10.1161/01.HYP.0000229907.58470.4c. [DOI] [PubMed] [Google Scholar]
- 12.Brooks DP, DePalma PD, Pullen M, Nambi P. Characterization of canine renal endothelin receptor subtypes and their function. J Pharmacol Exp Ther. 1994;268:1091–1097. [PubMed] [Google Scholar]
- 13.Gellai M, Fletcher T, Pullen M, Nambi P. Evidence for the existence of endothelin-B receptor subtypes and their physiological roles in the rat. Am J Physiol Regul Integr Comp Physiol. 1996;271:R254–261. doi: 10.1152/ajpregu.1996.271.1.R254. [DOI] [PubMed] [Google Scholar]
- 14.Waeber C, Hoyer D, Palacios JM. Similar distribution of [125I]sarafotoxin-6b and [125I]endothelin-1, -2, -3 binding sites in the human kidney. Eur J Pharmacol. 1990;176:233–236. doi: 10.1016/0014-2999(90)90534-d. [DOI] [PubMed] [Google Scholar]
- 15.Johnstrom P, Rudd JH, Richards HK, Fryer TD, Clark JC, Weissberg PL, Pickard JD, Davenport AP. Imaging endothelin ETB receptors using [18F]-BQ3020: in vitro characterization and positron emission tomography (microPET) Exp Biol Med. 2006;231:736–740. [PubMed] [Google Scholar]
- 16.Nambi P, Pullen M, Wu HL, Aiyar N, Ohlstein EH, Edwards RM. Identification of endothelin receptor subtypes in human renal cortex and medulla using subtype-selective ligands. Endocrinology. 1992;131:1081–1086. doi: 10.1210/endo.131.3.1324149. [DOI] [PubMed] [Google Scholar]
- 17.Wendel M, Knels L, Kummer W, Koch T. Distribution of endothelin receptor subtypes ETA and ETB in the rat kidney. J Histochem Cytochem. 2006;54:1193–1203. doi: 10.1369/jhc.5A6888.2006. [DOI] [PubMed] [Google Scholar]
- 18.Edwards RM, Trizna W. Characterization of 125I-endothelin-1 binding to rat and rabbit renal microvasculature. J Pharmacol Exp Ther. 1995;274:1084–1089. [PubMed] [Google Scholar]
- 19.De Leon H, Garcia R. Characterization of endothelin receptor subtypes in isolated rat renal preglomerular microvessels. Regul Pept. 1995;60:1–8. doi: 10.1016/0167-0115(95)00112-1. [DOI] [PubMed] [Google Scholar]
- 20.Kuc R, Davenport AP. Comparison of endothelin-A and endothelin-B receptor distribution visualized by radioligand binding versus immunocytochemical localization using subtype selective antisera. J Cardiovasc Pharmacol. 2004;44 (Suppl 1):S224–226. doi: 10.1097/01.fjc.0000166260.35099.d5. [DOI] [PubMed] [Google Scholar]
- 21.Davenport AP, Kuc RE, Maguire JJ, Harland SP. ETA receptors predominate in the human vasculature and mediate constriction. J Cardiovasc Pharmacol. 1995;26 (Suppl 3):S265–267. [PubMed] [Google Scholar]
- 22.King AJ, Brenner BM, Anderson S. Endothelin: a potent renal and systemic vasoconstrictor peptide. Am J Physiol Renal Physiol. 1989;256:F1051–1058. doi: 10.1152/ajprenal.1989.256.6.F1051. [DOI] [PubMed] [Google Scholar]
- 23.Loutzenhiser R, Epstein M, Hayashi K, Horton C. Direct visualization of effects of endothelin on the renal microvasculature. Am J Physiol Renal Physiol. 1990;258:F61–68. doi: 10.1152/ajprenal.1990.258.1.F61. [DOI] [PubMed] [Google Scholar]
- 24.Evans RG, Bergstrom G, Cotterill E, Anderson WP. Renal haemodynamic effects of endothelin-1 and the ETA/ETB antagonist TAK-044 in anaesthetized rabbits. J Hypertens. 1998;16:1897–1905. doi: 10.1097/00004872-199816121-00008. [DOI] [PubMed] [Google Scholar]
- 25.Evans RG, Madden AC, Oliver JJ, Lewis TV. Effects of ETA- and ETB-receptor antagonists on regional kidney blood flow, and responses to intravenous endothelin-1, in anaesthetized rabbits. J Hypertens. 2001;19:1789–1799. doi: 10.1097/00004872-200110000-00013. [DOI] [PubMed] [Google Scholar]
- 26.Pollock DM, Opgenorth TJ. Evidence for endothelin-induced renal vasoconstriction independent of ETA receptor activation. Am J Physiol Regul Integr Comp Physiol. 1993;264:R222–226. doi: 10.1152/ajpregu.1993.264.1.R222. [DOI] [PubMed] [Google Scholar]
- 27.Cristol JP, Warner TD, Thiemermann C, Vane JR. Mediation via different receptors of the vasoconstrictor effects of endothelins and sarafotoxins in the systemic circulation and renal vasculature of the anaesthetized rat. Br J Pharmacol. 1993;108:776–779. doi: 10.1111/j.1476-5381.1993.tb12877.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Just A, Olson AJ, Arendshorst WJ. Dual constrictor and dilator actions of ETB receptors in the rat renal microcirculation: interactions with ETA receptors. Am J Physiol Renal Physiol. 2004;286:F660–668. doi: 10.1152/ajprenal.00368.2003. [DOI] [PubMed] [Google Scholar]
- 29.Wellings RP, Corder R, Warner TD, Cristol JP, Thiemermann C, Vane JR. Evidence from receptor antagonists of an important role for ETB receptor-mediated vasoconstrictor effects of endothelin-1 in the rat kidney. Br J Pharmacol. 1994;111:515–520. doi: 10.1111/j.1476-5381.1994.tb14767.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Casellas D, Navar LG. In vitro perfusion of juxtamedullary nephrons in rats. Am J Physiol Renal Physiol. 1984;246:F349–358. doi: 10.1152/ajprenal.1984.246.3.F349. [DOI] [PubMed] [Google Scholar]
- 31.Imig JD, Pham BT, LeBlanc EA, Reddy KM, Falck JR, Inscho EW. Cytochrome P450 and cyclooxygenase metabolites contribute to the endothelin-1 afferent arteriolar vasoconstrictor and calcium responses. Hypertension. 2000;35:307–312. doi: 10.1161/01.hyp.35.1.307. [DOI] [PubMed] [Google Scholar]
- 32.Inscho EW, Imig JD, Cook AK, Pollock DM. ETA and ETB receptors differentially modulate afferent and efferent arteriolar responses to endothelin. Br J Pharmacol. 2005;146:1019–1026. doi: 10.1038/sj.bjp.0706412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pollock DM, Jenkins JM, Cook AK, Imig JD, Inscho EW. L-type calcium channels in the renal microcirculatory response to endothelin. Am J Physiol Renal Physiol. 2005;288:F771–777. doi: 10.1152/ajprenal.00315.2004. [DOI] [PubMed] [Google Scholar]
- 34.Schneider MP, Inscho EW, Pollock DM. Attenuated vasoconstrictor responses to endothelin in afferent arterioles during a high-salt diet. Am J Physiol Renal Physiol. 2007;292:F1208–1214. doi: 10.1152/ajprenal.00280.2006. [DOI] [PubMed] [Google Scholar]
- 35.Kon V, Yoshioka T, Fogo A, Ichikawa I. Glomerular actions of endothelin in vivo. J Clin Invest. 1989;83:1762–1767. doi: 10.1172/JCI114079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Takenaka T, Forster H, Epstein M. Protein kinase C and calcium channel activation as determinants of renal vasoconstriction by angiotensin II and endothelin. Circ Res. 1993;73:743–750. doi: 10.1161/01.res.73.4.743. [DOI] [PubMed] [Google Scholar]
- 37.Saito M, Homma S, Yamatsu I, Sato M, Ohshima N. Visualization of renal microcirculation in isolated Munich-Wistar rat kidneys: effects of endothelin-1 on renal hemodynamic activity. Jpn J Pharmacol. 1994;66:221–229. doi: 10.1254/jjp.66.221. [DOI] [PubMed] [Google Scholar]
- 38.Lanese DM, Yuan BH, McMurtry IF, Conger JD. Comparative sensitivities of isolated rat renal arterioles to endothelin. Am J Physiol Renal Physiol. 1992;263:F894–899. doi: 10.1152/ajprenal.1992.263.5.F894. [DOI] [PubMed] [Google Scholar]
- 39.Schildroth J, Rettig-Zimmermann J, Kalk P, Steege A, Fahling M, Sendeski M, Paliege A, Lai EY, Bachmann S, Persson PB, Hocher B, Patzak A. Endothelin type A and B receptors in the control of afferent and efferent arterioles in mice. Nephrol Dial Transplant. 2011;26:779–789. doi: 10.1093/ndt/gfq534. [DOI] [PubMed] [Google Scholar]
- 40.Kisanuki YY, Emoto N, Ohuchi T, Widyantoro B, Yagi K, Nakayama K, Kedzierski RM, Hammer RE, Yanagisawa H, Williams SC, Richardson JA, Suzuki T, Yanagisawa M. Low blood pressure in endothelial cell-specific endothelin 1 knockout mice. Hypertension. 2010;56:121–128. doi: 10.1161/HYPERTENSIONAHA.109.138701. [DOI] [PubMed] [Google Scholar]
- 41.Pallone TL, Zhang Z, Rhinehart K. Physiology of the renal medullary microcirculation. Am J Physiol Renal Physiol. 2003;284:F253–266. doi: 10.1152/ajprenal.00304.2002. [DOI] [PubMed] [Google Scholar]
- 42.Qiu C, Samsell L, Baylis C. Actions of endogenous endothelin on glomerular hemodynamics in the rat. Am J Physiol Regul Integr Comp Physiol. 1995;269:R469–473. doi: 10.1152/ajpregu.1995.269.2.R469. [DOI] [PubMed] [Google Scholar]
- 43.Schroeder AC, Imig JD, LeBlanc EA, Pham BT, Pollock DM, Inscho EW. Endothelin-mediated calcium signaling in preglomerular smooth muscle cells. Hypertension. 2000;35:280–286. doi: 10.1161/01.hyp.35.1.280. [DOI] [PubMed] [Google Scholar]
- 44.Navar LG, Arendshorst WJ, Pallone TL, Inscho EW, Imig JD, Bell PD. Microcirculation. In: Tuma RFD, Wa LK, editors. Handbook of Physiology. Vol. 550. San Diego: Elsevier; 2008. pp. F720–683. [Google Scholar]
- 45.Fretschner M, Endlich K, Gulbins E, Lang RE, Schlottmann K, Steinhausen M. Effects of endothelin on the renal microcirculation of the split hydronephrotic rat kidney. Ren Physiol Biochem. 1991;14:112–127. doi: 10.1159/000173394. [DOI] [PubMed] [Google Scholar]
- 46.Edwards RM, Trizna W, Ohlstein EH. Renal microvascular effects of endothelin. Am J Physiol Renal Physiol. 1990;259:F217–221. doi: 10.1152/ajprenal.1990.259.2.F217. [DOI] [PubMed] [Google Scholar]
- 47.Fellner SK, Arendshorst W. Endothelin-A and -B receptors, superoxide, and Ca2+ signaling in afferent arterioles. Am J Physiol Renal Physiol. 2007;292:F175–184. doi: 10.1152/ajprenal.00050.2006. [DOI] [PubMed] [Google Scholar]