Abstract
Purpose
American Joint Committee on Cancer (AJCC) staging is used to determine breast cancer prognosis, yet patient survival within each stage shows wide variation. We hypothesized that differences in biology influence this variation and that addition of biologic markers to AJCC staging improves determination of prognosis.
Patients and Methods
We identified a cohort of 3,728 patients who underwent surgery as the first intervention between 1997 and 2006. A Cox proportional hazards model, with backward stepwise exclusion of factors and stratification on pathologic stage (PS), was used to test the significance of adding grade (G), lymphovascular invasion (L), estrogen receptor (ER) status (E), progesterone receptor (PR) status, combined ER and PR status (EP), or combined ER, PR, and human epidermal growth factor receptor 2 status (M). We assigned values of 0 to 2 to these disease-specific survival (DSS) –associated factors and assessed six different staging systems: PS, PS + G, PS + G L, PS + G E, PS + G EP, and PS + G M. We compared 5-year DSS rates, Akaike's information criterion (AIC), and Harrell's concordance index (C-index) between systems. Surveillance, Epidemiology, and End Results data were used as the external validation cohort (n = 26,711).
Results
Median follow-up was 6.5 years, and 5-year DSS rate was 97.4%. The PS + G E status staging system was most precise, with a low AIC (1,931.9) and the highest C-index (0.80). PS + G E status was confirmed to stratify outcomes in internal bootstrapping samples and the external validation cohort.
Conclusion
Our results validate an improved breast cancer staging system that incorporates grade and ER status. We recommend that biologic markers be incorporated into revised versions of the AJCC staging system.
INTRODUCTION
Cancer staging systems are intended to provide information on prognosis and to guide clinicians in treatment planning. Traditionally, breast cancer has been staged using the American Joint Committee on Cancer (AJCC) TNM system. Developed in 1959, this system has been periodically updated to reflect new knowledge regarding the relationship between disease extent and prognosis, ensuring that the system maintains clinical relevance.1 The various possible combinations of tumor, node, and metastasis status are divided into stage groupings for which survival outcomes have been estimated using large cohorts. TNM status is determined for each patient and corresponds to a specific disease stage, used to determine the treatment plan according to guidelines such as those of the National Comprehensive Cancer Network.1 Breast cancer is assigned a clinical stage at initial diagnosis, before surgical intervention, on the basis of physical examination, radiologic studies, and biopsy. Definitive stage is determined after surgery by pathologic evaluation of the primary tumor and regional lymph nodes.2 Although the AJCC staging system is the most widely used classification system for determining breast cancer prognosis, patient survival within each stage shows wide variation.
Recent work investigating the impact of primary tumor histologic grade and biologic tumor markers has indicated the potential for refinement of the AJCC system by inclusion of these factors. Within the last decade, tumor grade has become widely accepted as a powerful indicator of prognosis in breast cancer.3 Most tumor grading systems currently employed are modifications of Black's nuclear grading system.4 Biologic markers routinely assessed in breast cancer specimens include estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2/neu (HER2). Published literature on these markers consistently indicates that ER, PR, and HER2 receptors carry both predictive and prognostic value in patients with breast cancer.5–8 We previously described a novel staging system that takes into account clinical stage, pathologic stage (PS), ER status, and tumor grade to determine a score that correlates with outcome after treatment with neoadjuvant chemotherapy.9 This staging system proved superior to both AJCC clinical staging before treatment and AJCC pathologic staging after chemotherapy in terms of stratifying patients into subgroups with different outcomes. Our novel staging system was validated in both internal and external patient cohorts.10 In the current study, we tested the hypothesis that incorporating biologic tumor markers into the AJCC pathologic staging system would result in more precise determination of prognosis for patients who undergo surgery as the first intervention in their breast cancer treatment.
PATIENTS AND METHODS
We identified patients with invasive breast cancer treated at The University of Texas MD Anderson Cancer Center (Houston, TX) from January 1997 to December 2006. Patients were excluded if they had received neoadjuvant chemotherapy; had stage IV disease; had unknown PS, grade, ER status, or PR status; or had been lost to follow-up within 2 years after surgery. This study was approved by the institutional review board.
Model Building
The clinical end point was disease-specific survival (DSS) calculated from the time of diagnosis to death resulting from breast cancer. Patients not experiencing this end point were censored at last follow-up.
ER and PR status were determined with immunohistochemical (IHC) staining and were considered positive if there was staining in more than 10% of cells. Tumors were considered HER2 positive if they were 3+ on IHC staining or 2+ on IHC staining and HER2 amplified (ratio > 2.0) on fluorescence in situ hybridization.11,12 ER, PR, and HER2 were used as surrogate markers to approximate breast cancer subtype: ER and/or PR positive and HER2 negative was considered hormone receptor positive; ER and/or PR positive and HER2 positive was considered hormone receptor and HER2 positive; ER and PR negative and HER2 positive was considered HER2 positive; and ER, PR, and HER2 negative was considered triple negative.13
Because PS is considered the definitive stage, it was used to derive a prognostic model for DSS after surgery. The univariate association of each potential prognostic factor with DSS rate was calculated. We used a Cox proportional hazards model, with backward stepwise exclusion of factors and stratification on PS, to test the significance of adding candidate prognostic factors: modified Black's nuclear grade; presence of lymphovascular invasion (LVI); ER status; PR status; combination of ER and PR; or combination of ER, PR, and HER2. The first multivariate model included grade, LVI, ER, and PR; the second included grade, LVI, and the combination of ER, PR, and HER2; and the third included grade, LVI, and the combination of ER and PR. ER status; combination of ER and PR status; and combination of ER, PR, and HER2 status could not be included in the same model, because they are highly correlated. A prognostic score of 0 to 2 was then assigned to each factor by considering the magnitude of the hazard ratio (HR) and then defining cutoffs. Only independent predictors of DSS (P < .05) were assigned a score. For binary variables, the comparison group with significant impact on DSS was assigned one point. For ordinal variables, the comparison groups determined to have a significant impact on DSS with an HR between 1.1 and 3 were assigned one point, and those variables determined to have an HR between 3.1 and 6 were assigned two points. An overall staging score was calculated by summing scores for the individual independent predictors of DSS. Finally, the overall staging score was used to stratify patients.
Six different staging systems were assessed: first, PS; second, PS and grade; third, PS, grade, and LVI; fourth, PS, grade, and ER status; fifth, PS, grade, and the combination of ER and PR status; and sixth, PS, grade, and the combination of ER, PR, and HER2 status. Model performance was quantified using Harrell's concordance index (C-index).14 The discriminative ability of the model was assessed using the C-index for comparative purposes with the literature as well as with the concordance probability estimate because of the high degree of censoring in the data.15 The concordance probability estimate can range from perfect concordance (1.0) to perfect discordance (0.0). In addition, Akaike's information criterion (AIC) was calculated.16 The AIC takes into account how well the model fits the data and the complexity of the model, thereby reducing the risk of overfitting. After comparisons, the most precise prognostic staging system (ie, one with lowest AIC value and highest C-index) was included the final predictive model. All statistical analyses were performed using R version 2.10.1 (http://www.r-project.org/).
Model Validation
The performance of the final model was internally validated using a bootstrapping technique: 200 resamples were examined, and the ability of the model to discriminate between patients with varying disease stages was calculated. The National Cancer Institute Surveillance, Epidemiology, and End Results (SEER) database was used to externally validate the staging system. Data were obtained from all US cancer registries participating in the SEER program using SEER*Stat version 6.5.2 (http://seer.cancer.gov/seerstat). The geographic scope of the current SEER database has been reported previously.17–19 Patients in the SEER database with invasive breast cancer diagnosed before 2007 were identified. Patients with the following International Classification of Diseases for Oncology (third edition) codes were included: 8521/3 (infiltrating ductal carcinoma), 8522/3 (infiltrating ductal and lobular carcinoma), 8523/3 (infiltrating ductal mixed with other types of carcinoma), and 8524/3 (infiltrating lobular mixed with other types of carcinoma). Patients with stage I to IIIA breast cancer were included. Stage in the SEER database is derived from a combination of clinical and pathologic information. For patients diagnosed before 2004, stage reflects the AJCC third edition, and for those diagnosed in 2004 or later, stage reflects the AJCC sixth edition. Patients with unknown stage, grade, ER status, or PR status and those lost to follow-up within 2 years were excluded. We could not determine whether surgery was the first intervention, because there is no information about neoadjuvant chemotherapy in the SEER database.
RESULTS
Clinicopathologic Characteristics of Initial and External Validation Cohorts
Clinicopathologic characteristics of the initial and external validation cohorts are listed in Table 1. There were differences between the cohorts with respect to all factors compared, including PS, grade, ER status, and PR status.
Table 1.
Clinicopathologic Characteristics of Initial and External Validation Cohorts
| Characteristic | Initial Cohort (n = 3,728) |
External Cohort (n = 26,711) |
P | ||
|---|---|---|---|---|---|
| No. | % | No. | % | ||
| Age, years | < .001 | ||||
| Median | 57 | 60 | |||
| Mean | 57.3 | 60.8 | |||
| Range | 22-99 | 24-99 | |||
| Pathologic stage | < .001 | ||||
| I | 2,309 | 61.9 | 12,930 | 48.4 | |
| IIA | 944 | 25.3 | 7,826 | 29.3 | |
| IIB | 321 | 8.6 | 4,326 | 16.2 | |
| IIIA | 154 | 4.1 | 1,629 | 6.1 | |
| ER status | < .001 | ||||
| Positive | 2,988 | 80.2 | 24,632 | 92.2 | |
| Negative | 740 | 19.8 | 2,079 | 7.8 | |
| PR status | < .001 | ||||
| Positive | 2,444 | 65.6 | 20,273 | 75.9 | |
| Negative | 1,284 | 34.4 | 6,438 | 24.1 | |
| HER2 status* | |||||
| Positive | 508 | 13.6 | |||
| Negative | 2,837 | 76.1 | |||
| Unknown | 383 | 10.3 | |||
| Nuclear grade | < .001 | ||||
| I | 521 | 14.0 | 5,810 | 21.8 | |
| II | 2,006 | 53.8 | 14,512 | 54.3 | |
| III | 1,201 | 32.2 | 6,389 | 23.9 | |
| Adjuvant chemotherapy* | |||||
| Yes | 1,683 | 45.1 | |||
| No | 2,045 | 54.9 | |||
| Adjuvant radiation therapy | .01 | ||||
| Yes | 2,251 | 60.4 | 12,648 | 47.3 | |
| No | 1,477 | 39.6 | 14,063 | 52.7 | |
| Adjuvant hormonal therapy* | |||||
| Yes | 2,565 | 68.8 | |||
| No | 1,163 | 31.2 | |||
| Follow-up time, years | < .001 | ||||
| Median | 6.3 | 5.3 | |||
| Mean | 6.6 | 5.9 | |||
| Range | 0.1-14 | 0.1-17.9 | |||
Abbreviations: ER, estrogen receptor; HER2, human epidermal growth factor receptor 2; PR, progesterone receptor.
Not available for external validation cohort.
There were 3,728 patients in the initial cohort and 26,711 in the external validation cohort. For the initial cohort, the 5-year DSS was 97.4% (95% CI, 96.8% to 97.8%). For the external validation cohort, the 5-year DSS was 93.2% (95% CI, 92.9% to 93.5%).
Pathologic and Biologic Marker Staging System
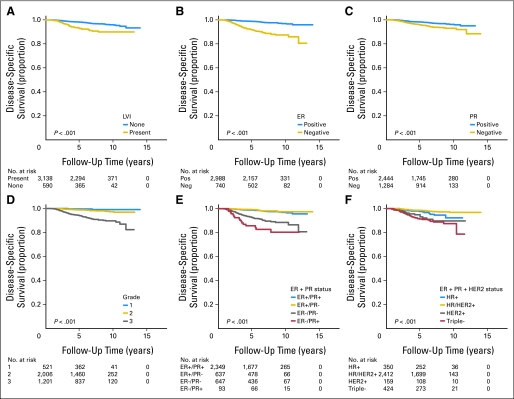
The univariate association of each potential prognostic factor with DSS is shown in Figure 1. The results of univariate and multivariate analyses for clinicopathologic factors associated with DSS in the initial cohort are shown in Table 2. ER status, combination of ER and PR status, and combination of ER, PR, and HER2 status could not be included in the same model, because they are known to be highly correlated. The first multivariate analysis indicated that grade 3 tumor, presence of LVI, and ER- and PR-negative disease were additional independent risk factors (Table 2); the second indicated that grade 3 tumor, presence of LVI, and triple-negative subtype were additional independent risk factors; and the third indicated that grade 3 tumor, negative ER and PR status, and negative ER and positive PR status were additional independent risk factors. Patients with negative ER and positive PR status (2.5%) had worse DSS compared with those with other ER/PR subtypes. In addition, within each PS, patients with grade 3 tumors fared worse than those with grade 1 and 2 tumors.
Fig 1.
Kaplan-Meier survival plots with risk tables demonstrating association between predictor variables and disease-specific survival in patients with invasive breast cancer treated with surgery as first intervention. (A) Lymphovascular invasion (LVI); (B) estrogen receptor (ER) status; (C) progesterone receptor (PR) status; (D) modified Black's nuclear grade; (E) ER and PR status; (F) ER, PR, and human epidermal growth factor receptor 2 (HER2) status. Log-rank test is provided for each comparison. HR, hormone receptor.
Table 2.
Univariate and Multivariate Analyses for Clinicopathologic Factors Associated With DSS in Initial Cohort
| Factor | No. of Events | 5-Year DSS (%) | Univariate Analysis |
Multivariate Analysis |
Assigned Points | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 |
2 |
3 |
|||||||||
| HR | P | HR | P | HR | P | HR | P | ||||
| Pathologic stage | |||||||||||
| I | 43 | 98.8 | Referent | Referent | Referent | Referent | 0 | ||||
| IIA | 49 | 96.3 | 2.9 | < .001 | 2.3 | < .001 | 2.42 | < .001 | 2.26 | < .001 | 1 |
| IIB | 22 | 94.5 | 3.9 | < .001 | 2.6 | < .001 | 2.65 | .001 | 2.58 | < .001 | 1 |
| IIIA | 19 | 88.6 | 7.7 | < .001 | 5.1 | < .001 | 5.92 | < .001 | 5.02 | < .001 | 2 |
| Nuclear grade | |||||||||||
| I | 3 | 99.6 | Referent | Referent | Referent | Referent | 0 | ||||
| II | 34 | 98.8 | 2.8 | .09 | 2.1 | .2 | 2.22 | .2 | 2.09 | .2 | 0 |
| III | 96 | 94.0 | 13.8 | < .001 | 5.1 | .003 | 5.97 | .003 | 5.29 | .006 | 1 |
| ER status | |||||||||||
| Positive | 57 | 98.8 | Referent | Referent | 0 | ||||||
| Negative | 76 | 91.6 | 5.6 | < .001 | 3.7 | < .001 | 1 | ||||
| PR status | |||||||||||
| Positive | 63 | 98.2 | Referent | Referent | |||||||
| Negative | 70 | 95.8 | 2.0 | < .001 | 0.50 | .006 | |||||
| HER2 status | |||||||||||
| Positive | 29 | 96.2 | Referent | ||||||||
| Negative | 92 | 97.4 | 0.6 | .02 | NS | ||||||
| ER and PR status | |||||||||||
| ER and PR positive | 47 | 98.7 | Referent | Referent | 0 | ||||||
| ER positive, PR negative | 10 | 99.2 | 0.8 | .5 | 0.76 | .4 | 0 | ||||
| ER and PR negative | 60 | 92.5 | 4.8 | < .001 | 2.64 | < .001 | 1 | ||||
| ER negative, PR positive | 16 | 85.8 | 8.7 | < .001 | 5.11 | < .001 | 2 | ||||
| ER, PR, and HER2 status | |||||||||||
| Hormone receptor positive | 16 | 97.6 | Referent | Referent | 0 | ||||||
| Hormone receptor and HER2 positive | 50 | 98.5 | 0.5 | .01 | 0.79 | .4 | 0 | ||||
| HER2 positive | 13 | 93.1 | 1.9 | .09 | 1.27 | .5 | 0 | ||||
| Triple negative | 42 | 91.3 | 2.4 | .003 | 2.20 | .009 | 1 | ||||
| Presence of LVI | |||||||||||
| No | 87 | 98.2 | Referent | Referent | Referent | 0 | |||||
| Yes | 46 | 92.9 | 3.2 | < .001 | 1.8 | .004 | 1.72 | .009 | NS | 1 | |
Abbreviations: DSS, disease-specific survival; ER, estrogen receptor; HER2, human epidermal growth factor receptor 2; HR, hazard ratio; LVI, lymphovascular invasion; NS, not significant; PR, progesterone receptor.
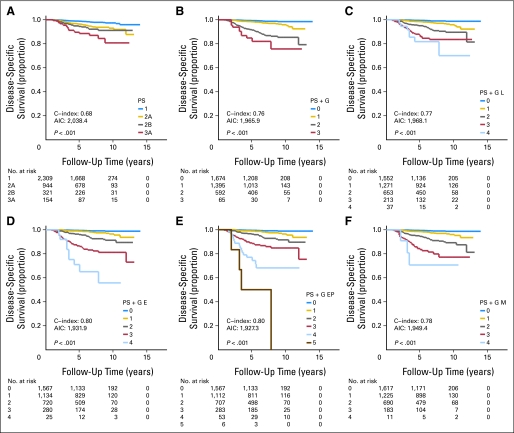
Points assigned for the various predictors of DSS by using the methods described (except grade) to create the overall staging score are listed in Table 2. For the category of tumor grade, grade 3 was assigned one point, with an associated HR greater than 5. The reason for this designation was that lower grades (eg, grade 2) were noted to be insignificant, with HRs greater than 2, and it was reasoned that overall, this variable is of less significance despite the magnitude of the HR for a single category. Five-year DSS and the C-index for each proposed staging system are shown in Figure 2. The staging system that included PS, grade, and ER status (PS + G E staging system) had the lowest AIC and highest C-index (0.80), and the staging system that included PS, grade, and combined ER and PR status (PS + G EP staging system) and allowed for expansion of the staging system into more distinct subgroups (Figs 2D, 2E). These two staging systems showed good validation on bootstrapping (C-index, 0.80; bootstrap validated, 0.79; concordance probability estimate, 0.71; bootstrap validated, 0.69).
Fig 2.
Kaplan-Meier survival plots with risk tables demonstrating association between different staging systems and disease-specific survival in patients with invasive breast cancer treated with surgery as first intervention. (A) Pathologic stage (PS); (B) PS plus nuclear grade (PS + G); (C) PS plus nuclear grade plus lymphovascular invasion status (PS + G L); (D) PS plus grade plus estrogen receptor (ER) status (PS + G E); (E) PS plus grade plus the combination of ER and progesterone receptor (PR) status (PS + G EP); (F) PS plus grade plus combination of ER, PR, and human epidermal growth factor receptor 2 status (PS + G M). Log-rank test is provided for each comparison. AIC, Akaike's information criterion; C-index, Harrell's concordance index.
Table 3 summarizes 5-year DSS for the initial and external validation cohorts stratified according to PS + G E and PS + G EP score. Survival differences were noted between the initial and external cohorts for patients with PS + G E and PS + G EP scores of 2 or 3. In the external validation cohort, PS + G E and PS + G EP staging facilitated categorization of patients into more refined subgroups than did pathologic staging, and the patterns of prediction of DSS were similar to those demonstrated in the initial cohort (Fig 3).
Table 3.
DSS Outcomes by Stage According to PS + G E and PS + G EP Staging Systems
| Score | Initial Cohort (n = 3,728) |
External Cohort (n = 26,711) |
P | ||||||
|---|---|---|---|---|---|---|---|---|---|
| No. of Patients | No. of Events | 5-Year DSS (%) | 95% CI | No. of Patients | No. of Events | 5-Year DSS (%) | 95% CI | ||
| PS + G E | 3,728 | 133 | 26,711 | 2,131 | |||||
| 0 | 1,567 | 12 | 99.5 | 98.9 to 99.7 | 10,237 | 234 | 98.5 | 98.2 to 98.7 | NS |
| 1 | 1,134 | 24 | 98.9 | 98.0 to 99.4 | 10,567 | 739 | 95.2 | 94.7 to 95.6 | NS |
| 2 | 720 | 43 | 96.1 | 94.3 to 97.3 | 4,538 | 734 | 86.3 | 85.2 to 87.4 | < .001 |
| 3 | 280 | 45 | 86.2 | 81.4 to 89.8 | 1,424 | 364 | 72.2 | 69.3 to 74.8 | < .001 |
| 4 | 25 | 9 | 65.2 | 41.7 to 81.1 | 127 | 60 | 54.2 | 44.3 to 63.1 | NS |
| C-index | 0.80 | ||||||||
| CPE | 0.71 | ||||||||
| PS + G EP | |||||||||
| 0 | 1,567 | 12 | 99.4 | 98.9 to 99.7 | 10,237 | 234 | 98.5 | 98.2 to 98.7 | NS |
| 1 | 1,112 | 24 | 98.9 | 98.0 to 99.4 | 10,443 | 733 | 95.2 | 94.7 to 95.6 | NS |
| 2 | 707 | 41 | 96.3 | 94.5 to 97.5 | 4,526 | 722 | 86.3 | 85.2 to 87.4 | < .001 |
| 3 | 283 | 37 | 88.4 | 83.9 to 91.7 | 1,294 | 362 | 73.9 | 71.2 to 76.4 | < .001 |
| 4 | 53 | 15 | 74.3 | 59.7 to 84.3 | 197 | 72 | 68.2 | 60.8 to 77.5 | NS |
| 5 | 6 | 4 | 50.0 | 11.1 to 80.3 | 14 | 8 | 53.6 | 23.8 to 76.2 | NS |
| C-index | 0.80 | ||||||||
| CPE | 0.71 | ||||||||
Abbreviations: C-index, Harrell's concordance index; CPE, concordance probability estimate; DSS, disease-specific survival; E, estrogen receptor status; EP, estrogen and progesterone status; G, grade; NS, not significant; PS, pathologic stage.
Fig 3.
Kaplan-Meier survival plots with risk tables of disease-specific survival for (A) subgroups of external validation cohort defined using American Joint Committee on Cancer staging; (B) scores based on pathologic stage plus grade plus estrogen receptor status (PS + G E); (C) scores based on pathologic stage plus grade plus combination of estrogen and progesterone receptor status (PS + G EP).
DISCUSSION
A major challenge to the development of cancer staging systems is the rapid evolution of cancer biology and identification of additional biologic factors that predict outcome and response to treatment with more accuracy than tumor size and nodal status.20,21 The development of superior staging systems for patients with invasive breast cancer has been the focus of several studies.3,22–25 In the current analysis, when patients were restaged with grade and ER status along with PS, we observed improved discrimination between stages with respect to DSS. Both PS + G E and PS + G EP staging systems refine assessment of prognosis of patients with breast cancer using variables routinely assessed at standard pathologic examination; therefore, these novel staging systems can be easily implemented in clinical practice.
In agreement with other published studies, we found that higher PS, grade 3 tumor, negative ER, and presence of LVI were adverse prognostic factors.4,24–26 Lundin et al27 suggested that omission of histologic grade from clinical decision making may result in overuse of adjuvant therapies. Rakha et al25 suggested that grade was a strong predictor of outcome in patients and should be incorporated into a breast cancer staging system.3 Tumor grade is already part of the staging systems for prostate cancer, soft tissue sarcomas, and certain bone tumors, and Wasif et al28 recently suggested that grade be incorporated into the AJCC staging system for pancreatic cancer. The AJCC has not included tumor grade in the breast cancer staging system for a number of reasons. First, some have questioned whether grade adds value in patients with small tumors.1 We found that grade maintained its utility even in patients with small tumors, consistent with the results of other investigations.25,26 Second, the applicability of tumor grade to nonductal histologic subtypes has been questioned. Recent evidence suggests that grade is also prognostic in invasive lobular cancers.29
The value of LVI status in determining prognosis has been debated. Although some have argued that LVI adds little to predicting outcome in patients with lymph node–positive disease, others have found that LVI is associated with worse outcome in node-negative patients independent of tumor size and grade.30 In our study, survival differences between patients in different stages decreased and AIC increased when we incorporated LVI into the PS + G staging system, and LVI was a significant predictor of worse DSS in univariate and multivariate analyses.
ER, PR, and HER2 status have long been known to have both predictive and prognostic value in breast cancer, and combinations of these receptors have been correlated with distinct genomic signatures.8,13 As use of targeted therapy increases, it becomes important to classify patients according to biomarker profiles for which specific treatment protocols are available.31–33 In our study, on univariate analysis, ER-negative status, PR-negative status, and HER2-positive status were significant adverse factors. However, when both ER and PR were incorporated into a multivariate model, PR-negative disease was associated with better DSS. We studied the combination of ER and PR status in a multivariate model and found that patients with negative ER and positive PR status had worse DSS than patients with positive ER and positive PR status or negative ER and negative PR status, consistent with a report by Rakha et al.34 The PS + G E and PS + G EP staging systems shared the highest C-index (0.80), and there was no statistical difference in AIC, even though the PS + G EP staging system had the lowest AIC value (1,927.3 v 1,931.9). However, because of the small number of patients with ER-negative and PR-positive status (2.5%), the group with a PS + G EP score of five had a small sample size (n = 6 for initial cohort; n = 14 for external cohort). As a result, it was not possible to identify significant differences between the values. A larger cohort with negative ER and positive PR status is required to validate the survival impact for this subset. On the basis of the results from our study, we recommend use of the PS + G E instead of the PS + G EP staging system. Breast cancers with different IHC receptor profiles (hormone receptor positive, hormone receptor and HER2 positive, HER2 positive, and triple negative) have been associated with significantly different prognoses in patients treated both with and without adjuvant endocrine therapy.13 However, in our study, we found that survival differences decreased and AIC increased when we incorporated combined ER, PR, and HER2 status into the PS + G staging system. One caveat is that the dates of inclusion in this study largely predate the routine use of trastuzumab for patients with HER2-overexpressing disease. Our model, therefore, was not able to capture the benefit of trastuzumab therapy. Thus, these staging systems are not applicable to such patients, and future work will need to be performed to develop similar staging systems appropriate for this population.
The PS + G E staging system was developed with an initial cohort and validated using an external cohort (SEER). Internal validation confirmed the robustness of the model (C-index dropped slightly from 0.80 to 0.79, and concordance probability estimate from 0.71 to 0.69 after bootstrapping), and the C-index of 0.8 represents acceptable concordance. For comparison, a majority of C-indices for other cancer prediction models (eg, pancreatic, colorectal, gastric, and prostate cancers) are from 0.61 to 0.80.35–39 Despite differences in the distribution of pathologic factors in the initial and external cohorts, the PS + G E staging system stratified patients in the external cohort into more refined prognostic subgroups than the current AJCC staging system. Together, these findings suggest that the PS + G E staging system has excellent discrimination and broad applicability and can be generalized to other institutions with patient populations and/or practice patterns not identical to those at MD Anderson.
This study has several limitations. First, it was performed using retrospectively collected data, and treatment was not assigned in a randomized fashion. Second, we used population-based data as the external cohort. SEER data are checked regularly for discrepancies and reportedly have 95% accuracy; however, the possibility of coding errors remains. Furthermore, we cannot account for variability among SEER regions in pathology protocols used to assess grade and ER status or for interobserver variability among pathologists. Finally, SEER lacks information on chemotherapy, so we were unable to exclude patients treated with neoadjuvant chemotherapy, which may explain the discrepancy between groups when PS + G E score was two or three. However, we demonstrated that the addition of grade and ER status makes sense given their significant and independent association with survival, and they can simply be added to the existing pathologic staging system. In other words, the novel PS + G E staging system does not create a more complex staging system but rather builds on the existing AJCC staging system.
In conclusion, our results show that tumor grade and ER status are significant, independent predictors of DSS after primary surgery. We also confirmed that the PS + G E staging system improves discrimination among patient subgroups with respect to DSS. These findings may have implications for decision making regarding adjuvant therapy and risk stratification of patients entering clinical trials. Importantly, the PS + G E score can readily be determined using data available in clinical and pathologic records. For grade and ER to be integrated into the AJCC staging system, a national effort is needed to standardize their assessment so that both are reproducible, and intra- and interpathologist variability is minimized.
Acknowledgment
We thank Stephanie Deming, BA, Department of Scientific Publications, The University of Texas MD Anderson Cancer Center, for editorial assistance.
Footnotes
Supported in part by the National Cancer Institute through Cancer Center Support Grant No. CA016672 to the MD Anderson Cancer Center.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Min Yi, Elizabeth A. Mittendorf, Janice N. Cormier, Thomas A. Buchholz, Karl Bilimoria, Aysegul A. Sahin, Gabriel N. Hortobagyi, Henry M. Kuerer, Kelly K. Hunt
Financial support: Kelly K. Hunt
Administrative support: Gabriel N. Hortobagyi, Kelly K. Hunt
Provision of study materials or patients: Gabriel N. Hortobagyi, Kelly K. Hunt
Collection and assembly of data: Min Yi, Jaime R. Crow, Kelly K. Hunt
Data analysis and interpretation: Min Yi, Janice N. Cormier, Karl Bilimoria, Gabriel N. Hortobagyi, Ana Maria Gonzalez-Angulo, Sheng Luo, Aman U. Buzdar, Kelly K. Hunt
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Edge SB, Byrd DR, Compton CC. AJCC Cancer Staging Manual. ed 7. Chicago, IL: Springer; 2009. [Google Scholar]
- 2.Jeruss JS, Mittendorf EA, Tucker SL, et al. Staging of breast cancer in the neoadjuvant setting. Cancer Res. 2008;68:6477–6481. doi: 10.1158/0008-5472.CAN-07-6520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rakha EA, Reis-Filho JS, Baehner F, et al. Breast cancer prognostic classification in the molecular era: The role of histological grade. Breast Cancer Res. 2010;12:207. doi: 10.1186/bcr2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Elston CW, Ellis IO. Pathological prognostic factors in breast cancer: I. The value of histological grade in breast cancer—Experience from a large study with long-term follow-up. Histopathology. 1991;19:403–410. doi: 10.1111/j.1365-2559.1991.tb00229.x. [DOI] [PubMed] [Google Scholar]
- 5.Calza S, Hall P, Auer G, et al. Intrinsic molecular signature of breast cancer in a population-based cohort of 412 patients. Breast Cancer Res. 2006;8:R34. doi: 10.1186/bcr1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harigopal M, Barlow WE, Tedeschi G, et al. Multiplexed assessment of the Southwest Oncology Group-directed Intergroup Breast Cancer Trial S9313 by AQUA shows that both high and low levels of HER2 are associated with poor outcome. Am J Pathol. 2010;176:1639–1647. doi: 10.2353/ajpath.2010.090711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ross JS. Multigene classifiers, prognostic factors, and predictors of breast cancer clinical outcome. Adv Anat Pathol. 2009;16:204–215. doi: 10.1097/PAP.0b013e3181a9d4bf. [DOI] [PubMed] [Google Scholar]
- 8.Wang Y, Klijn JG, Zhang Y, et al. Gene-expression profiles to predict distant metastasis of lymph-node-negative primary breast cancer. Lancet. 2005;365:671–679. doi: 10.1016/S0140-6736(05)17947-1. [DOI] [PubMed] [Google Scholar]
- 9.Jeruss JS, Mittendorf EA, Tucker SL, et al. Combined use of clinical and pathologic staging variables to define outcomes for breast cancer patients treated with neoadjuvant therapy. J Clin Oncol. 2008;26:246–252. doi: 10.1200/JCO.2007.11.5352. [DOI] [PubMed] [Google Scholar]
- 10.Mittendorf EA, Jeruss JS, Tucker SL, et al. Validation of a novel staging system for disease-specific survival in patients with breast cancer treated with neoadjuvant chemotherapy. J Clin Oncol. 2011;29:1956–1962. doi: 10.1200/JCO.2010.31.8469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Piccart-Gebhart MJ, Procter M, Leyland-Jones B, et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005;353:1659–1672. doi: 10.1056/NEJMoa052306. [DOI] [PubMed] [Google Scholar]
- 12.Romond EH, Perez EA, Bryant J, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353:1673–1684. doi: 10.1056/NEJMoa052122. [DOI] [PubMed] [Google Scholar]
- 13.Nguyen PL, Taghian AG, Katz MS, et al. Breast cancer subtype approximated by estrogen receptor, progesterone receptor, and HER-2 is associated with local and distant recurrence after breast-conserving therapy. J Clin Oncol. 2008;26:2373–2378. doi: 10.1200/JCO.2007.14.4287. [DOI] [PubMed] [Google Scholar]
- 14.Harrell FE, Jr, Califf RM, Pryor DB, et al. Evaluating the yield of medical tests. JAMA. 1982;247:2543–2546. [PubMed] [Google Scholar]
- 15.Gonen M, Heller G. Concordance probability and discriminatory power in proportional hazards regression. Biometrika. 2005;92:965–970. [Google Scholar]
- 16.Akaike H. New look at statistical-model identification. IEEE Trans Automat Contr. 1974;19:716–723. [Google Scholar]
- 17.Leggett MD, Chen SL, Schneider PD, et al. Prognostic value of lymph node yield and metastatic lymph node ratio in medullary thyroid carcinoma. Ann Surg Oncol. 2008;15:2493–2499. doi: 10.1245/s10434-008-0022-z. [DOI] [PubMed] [Google Scholar]
- 18.Martinez SR, Robbins AS, Meyers FJ, et al. Racial and ethnic differences in treatment and survival among adults with primary extremity soft-tissue sarcoma. Cancer. 2008;112:1162–1168. doi: 10.1002/cncr.23261. [DOI] [PubMed] [Google Scholar]
- 19.Chen SL, Martinez SR. The survival impact of the choice of surgical procedure after ipsilateral breast cancer recurrence. Am J Surg. 2008;196:495–499. doi: 10.1016/j.amjsurg.2008.06.018. [DOI] [PubMed] [Google Scholar]
- 20.Park YH, Lee SJ, Cho EY, et al. Clinical relevance of TNM staging system according to breast cancer subtypes. Ann Oncol. 2011;22:1554–1560. doi: 10.1093/annonc/mdq617. [DOI] [PubMed] [Google Scholar]
- 21.Veronesi U, Zurrida S, Viale G, et al. Rethinking TNM: A breast cancer classification to guide treatment and facilitate research. Breast J. 2009;15:291–295. doi: 10.1111/j.1524-4741.2009.00719.x. [DOI] [PubMed] [Google Scholar]
- 22.Baak JP, Van Dop H, Kurver PH, et al. The value of morphometry to classic prognosticators in breast cancer. Cancer. 1985;56:374–382. doi: 10.1002/1097-0142(19850715)56:2<374::aid-cncr2820560229>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 23.Chevallier B, Mosseri V, Dauce JP, et al. A prognostic score in histological node negative breast cancer. Br J Cancer. 1990;61:436–440. doi: 10.1038/bjc.1990.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.D'Eredita G, Giardina C, Martellotta M, et al. Prognostic factors in breast cancer: The predictive value of the Nottingham Prognostic Index in patients with a long-term follow-up that were treated in a single institution. Eur J Cancer. 2001;37:591–596. doi: 10.1016/s0959-8049(00)00435-4. [DOI] [PubMed] [Google Scholar]
- 25.Rakha EA, El-Sayed ME, Lee AH, et al. Prognostic significance of Nottingham histologic grade in invasive breast carcinoma. J Clin Oncol. 2008;26:3153–3158. doi: 10.1200/JCO.2007.15.5986. [DOI] [PubMed] [Google Scholar]
- 26.Frkovic-Grazio S, Bracko M. Long term prognostic value of Nottingham histological grade and its components in early (pT1N0M0) breast carcinoma. J Clin Pathol. 2002;55:88–92. doi: 10.1136/jcp.55.2.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lundin J, Lundin M, Holli K, et al. Omission of histologic grading from clinical decision making may result in overuse of adjuvant therapies in breast cancer: Results from a nationwide study. J Clin Oncol. 2001;19:28–36. doi: 10.1200/JCO.2001.19.1.28. [DOI] [PubMed] [Google Scholar]
- 28.Wasif N, Ko CY, Farrell J, et al. Impact of tumor grade on prognosis in pancreatic cancer: Should we include grade in AJCC staging? Ann Surg Oncol. 2010;17:2312–2320. doi: 10.1245/s10434-010-1071-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rakha EA, El-Sayed ME, Menon S, et al. Histologic grading is an independent prognostic factor in invasive lobular carcinoma of the breast. Breast Cancer Res Treat. 2008;111:121–127. doi: 10.1007/s10549-007-9768-4. [DOI] [PubMed] [Google Scholar]
- 30.Mohammed RA, Martin SG, Mahmmod AM, et al. Objective assessment of lymphatic and blood vascular invasion in lymph node-negative breast carcinoma: Findings from a large case series with long-term follow-up. J Pathol. 2011;223:358–365. doi: 10.1002/path.2810. [DOI] [PubMed] [Google Scholar]
- 31.Brenton JD, Carey LA, Ahmed AA, et al. Molecular classification and molecular forecasting of breast cancer: Ready for clinical application? J Clin Oncol. 2005;23:7350–7360. doi: 10.1200/JCO.2005.03.3845. [DOI] [PubMed] [Google Scholar]
- 32.Perou CM, Sørlie T, Eisen MB, et al. Molecular portraits of human breast tumours. Nature. 2000;406:747–752. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 33.Sørlie T, Perou CM, Tibshirani R, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci U S A. 2001;98:10869–10874. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rakha EA, El-Sayed ME, Green AR, et al. Biologic and clinical characteristics of breast cancer with single hormone receptor positive phenotype. J Clin Oncol. 2007;25:4772–4778. doi: 10.1200/JCO.2007.12.2747. [DOI] [PubMed] [Google Scholar]
- 35.Brennan MF, Kattan MW, Klimstra D, et al. Prognostic nomogram for patients undergoing resection for adenocarcinoma of the pancreas. Ann Surg. 2004;240:293–298. doi: 10.1097/01.sla.0000133125.85489.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kattan MW, Karpeh MS, Mazumdar M, et al. Postoperative nomogram for disease-specific survival after an R0 resection for gastric carcinoma. J Clin Oncol. 2003;21:3647–3650. doi: 10.1200/JCO.2003.01.240. [DOI] [PubMed] [Google Scholar]
- 37.Kattan MW, Wheeler TM, Scardino PT. Postoperative nomogram for disease recurrence after radical prostatectomy for prostate cancer. J Clin Oncol. 1999;17:1499–1507. doi: 10.1200/JCO.1999.17.5.1499. [DOI] [PubMed] [Google Scholar]
- 38.Stephenson AJ, Scardino PT, Eastham JA, et al. Postoperative nomogram predicting the 10-year probability of prostate cancer recurrence after radical prostatectomy. J Clin Oncol. 2005;23:7005–7012. doi: 10.1200/JCO.2005.01.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weiser MR, Landmann RG, Kattan MW, et al. Individualized prediction of colon cancer recurrence using a nomogram. J Clin Oncol. 2008;26:380–385. doi: 10.1200/JCO.2007.14.1291. [DOI] [PubMed] [Google Scholar]