Abstract
Purpose
NCCTG (North Central Cancer Treatment Group) N9831 is the only randomized phase III trial evaluating trastuzumab added sequentially or used concurrently with chemotherapy in resected stages I to III invasive human epidermal growth factor receptor 2–positive breast cancer.
Patients and Methods
Patients received doxorubicin and cyclophosphamide every 3 weeks for four cycles, followed by paclitaxel weekly for 12 weeks (arm A), paclitaxel plus sequential trastuzumab weekly for 52 weeks (arm B), or paclitaxel plus concurrent trastuzumab for 12 weeks followed by trastuzumab for 40 weeks (arm C). The primary end point was disease-free survival (DFS).
Results
Comparison of arm A (n = 1,087) and arm B (n = 1,097), with 6-year median follow-up and 390 events, revealed 5-year DFS rates of 71.8% and 80.1%, respectively. DFS was significantly increased with trastuzumab added sequentially to paclitaxel (log-rank P < .001; arm B/arm A hazard ratio [HR], 0.69; 95% CI, 0.57 to 0.85). Comparison of arm B (n = 954) and arm C (n = 949), with 6-year median follow-up and 313 events, revealed 5-year DFS rates of 80.1% and 84.4%, respectively. There was an increase in DFS with concurrent trastuzumab and paclitaxel relative to sequential administration (arm C/arm B HR, 0.77; 99.9% CI, 0.53 to 1.11), but the P value (.02) did not cross the prespecified O'Brien-Fleming boundary (.00116) for the interim analysis.
Conclusion
DFS was significantly improved with 52 weeks of trastuzumab added to adjuvant chemotherapy. On the basis of a positive risk-benefit ratio, we recommend that trastuzumab be incorporated into a concurrent regimen with taxane chemotherapy as an important standard-of-care treatment alternative to a sequential regimen.
INTRODUCTION
The human epidermal growth factor receptor-2 (HER2) protein and/or gene are overexpressed or amplified in 19% to 23% of patients with invasive breast cancer.1,2 HER2 positivity is associated with significantly decreased recurrence-free survival and overall survival (OS).3–5 Trastuzumab, a monoclonal antibody targeting HER2, is approved by regulatory agencies as part of treatment in adjuvant or metastatic HER2-positive invasive breast cancer.6–8 In the adjuvant setting, the optimal means of incorporating trastuzumab with chemotherapy, concurrently or sequentially, is debated. Approval by the US Food and Drug Administration (FDA) allows for either approach, whereas in other countries, approval is only for the sequential use of trastuzumab with chemotherapy.8,9
Several adjuvant phase III clinical trials have assessed either sequential or concurrent incorporation of trastuzumab with chemotherapy (Data Supplement), but the NCCTG (North Central Cancer Treatment Group) N9831 trial is the only trial, to the best of our knowledge, prospectively comparing the two different approaches. Specifically, it compares the efficacy and safety of chemotherapy alone (arm A), chemotherapy followed by sequential trastuzumab (arm B), and chemotherapy with concurrent trastuzumab followed by trastuzumab monotherapy (arm C). Results from this pivotal trial, as approved by the study's independent data monitoring committee, are reported herein.
PATIENTS AND METHODS
Eligibility and Enrollment
Eligibility requirements for NCCTG N9831 included primary, operable, and histologically confirmed node-positive or high-risk node-negative invasive stages I to III breast cancer with no evidence of metastases. All tumors must have been removed within 84 days of study registration and found to be HER2 positive by local laboratory testing. Patients undergoing breast-conserving surgery or mastectomy with at least four positive nodes were required to receive radiotherapy after completion of paclitaxel. An additional requirement for enrollment included having left ventricular ejection fraction (LVEF) at or above the lower limit of normal, as defined by the institution.
Eligibility criteria for HER2 positivity was changed in January 2002 because of poor agreement between local laboratory and central study laboratory findings.10,11 During doxorubicin and cyclophosphamide treatment, tumor specimens underwent testing by the central study laboratory and, if necessary, a reference laboratory. Those found to be HER2 positive (immunohistochemistry score of 3+, > 10% circumferential membrane staining, or gene amplified by fluorescent in situ hybridization ratio ≥ 2.0) were eligible to continue on. Otherwise, patients went off study, and future treatment was at the discretion of their physician.
Patients were ineligible if they had locally advanced carcinoma, bilateral invasive carcinoma, previous or current cardiovascular disease, prior anthracycline or taxane therapy, or moderate (grade ≥ 2; National Cancer Institute Common Toxicity Criteria [NCI-CTC] version 2.0) sensory neuropathy.
Participating institutions obtained study approval from their institutional review board and filed assurances with the Department of Health and Human Services. Written informed consent was required for enrollment.
Treatment Regimens
Treatment began with AC (doxorubicin 60 mg/m2 and cyclophosphamide 600 mg/m2) on day 1 every 3 weeks for 12 weeks, followed by either paclitaxel 80 mg/m2 weekly for 12 weeks (arm A); or paclitaxel 80 mg/m2 weekly for 12 weeks and then trastuzumab weekly for 52 weeks, with initial loading dose of 4 mg/kg and subsequent doses of 2 mg/kg/wk (arm B); or paclitaxel 80 mg/m2 plus trastuzumab for 12 weeks with a loading dose of 4 mg/kg with first paclitaxel dose and then 2 mg/kg/wk followed by trastuzumab 2 mg/kg/wk for 40 weeks (arm C). If appropriate, radiotherapy and/or tamoxifen were initiated following completion of paclitaxel. Following the results of the ATAC (Arimidex, Tamoxifen, Alone or in Combination) trial,12 postmenopausal women with estrogen receptor–positive or progesterone receptor–positive disease were permitted to receive an aromatase inhibitor.
Following the release of data from the first joint analysis of NSABP (National Surgical Adjuvant Breast and Bowel Project) B-31 and NCCTG N9831 trials in April 2005, which showed significant clinical benefit with the addition of concurrent trastuzumab to chemotherapy, patients who had been randomly assigned to arm A were allowed to receive trastuzumab if they had an acceptable LVEF level compared with the value at registration and ≤ 6 months had passed since completion of chemotherapy. Patients randomly assigned to arm B were allowed to begin with or switch to trastuzumab concurrently with paclitaxel (Addendum 16 [A16]) and are referred to as post-A16 patients.
Cardiac Considerations
LVEF was assessed by multiple-gated acquisition scanning or echocardiography at registration, completion of AC (3 months), completion of paclitaxel (6 months), 9 months postregistration, and 18 months (arm A and arm C) or 21 months (arm B) postregistration. Detailed guidelines for management according to LVEF measurements and results of the cardiac safety analysis have been published elsewhere.13
Role of Sponsor
The study was conducted under a research and development agreement between Genentech and the NCI. Genentech provided trastuzumab and partial funding support but did not participate in the design, conduct, or analysis of the study; this was carried out by NCCTG. The lead authors wrote the manuscript, which was reviewed by all authors. As supporters of the trial, both Genentech and the NCI were provided with a draft of the manuscript. The authors vouch for the completeness and accuracy of the data.
Statistical Design and Analysis
Patients were randomly assigned to treatment by using a dynamic allocation procedure that balanced the marginal distributions of nodal status and hormone receptor status between treatment arms.14 The primary end point was disease-free survival (DFS), which was defined as the time from random assignment to documentation of the first of any of these events: local, regional, or distant recurrence of breast cancer; contralateral breast cancer; second primary disease; or death resulting from any cause. OS was defined as the time from random assignment to death resulting from any cause.
The trial was designed to allow pairwise comparisons of the treatment strategies with three efficacy interim analyses of each comparison planned when 50%, 67%, and 75% of the expected number of events for that comparison occurred.
The original statistical plan was modified with approval of the NCI and the FDA to take into account the temporary closure of arm C in 2002 because of cardiac safety concerns as well as the release of the joint analysis results (and simultaneous closing of accrual) in April 2005. For the comparison of arm A and arm B, patients randomly assigned to either arm within the year before the release of the joint analysis results contributed data from registration to the date of last follow-up or April 30, 2005, whichever came first. For comparison of arm B and arm C, patients randomly assigned to arm B during the 8.5 months that arm C was closed to accrual were excluded from this comparison; patients randomly assigned to either arm within the 3 months before the release of the joint analysis results contributed data from registration to the date of last follow-up or April 30, 2005, whichever came first. Because of the truncation of follow-up for those patients on arm A who could start trastuzumab and for those patients on arm B who could receive trastuzumab concurrent with paclitaxel, the follow-up period was extended for all other patients to achieve the original goals of the trial. Thus, given this enrollment history, a follow-up period of 4 years, and the original assumptions that the median DFS would be 6.3 years in the poorer of arm A and arm B and 7.3 years in the poorer of arm B and arm C, respectively, an alpha = .01 log-rank test would have an 86% chance of detecting a 25% decrease in the hazard rate with arm C relative to arm A (expected number of events = 647), and an alpha = .03 log-rank test would have an 84% chance of detecting a 22% decrease in the hazard rate with arm B relative to arm C (expected number of events = 647).
The overall distributions of DFS and OS were estimated by using the Kaplan-Meier method. Stratified proportional hazards modeling was used to assess whether DFS or OS differed with respect to treatment. Univariate analyses were conducted on the following strata for their impact on DFS and OS for the comparison of arm A and arm B: age at registration, extent of surgery, hormone receptor status, tumor size, and number of positive nodes.
In October 2009, the NCCTG Independent Data Monitoring Board (IDMC) recommended the release of all NCCTG N9831 study data from the preplanned second interim analysis of the comparison of arm A and arm B, which indicated that the prespecified O'Brien-Fleming boundary had been crossed. Although the statistical boundary had not been reached before the first interim analysis of arm B and arm C, it was also recommended that data from this comparison be released because of a DFS event rate that was lower than anticipated (647 events were expected in 4 years of follow-up; 334 events occurred in 4.5 years of follow-up). Extrapolation based on the rate of events in the trastuzumab arms led to the conclusion that it would have taken at least 10 to 15 additional years of follow-up to reach the number of events originally predicted when the study was written, lending support to the release of data in the context of their relevance to global patient care.
RESULTS
Comparison of Arm A and Arm B: Impact of Adding Sequential Trastuzumab to AC and Paclitaxel
Of 2,448 women randomly assigned to either arm A or arm B of this trial between May 19, 2000, and April 30, 2005, 264 were ineligible (Fig 1A). Patient and tumor characteristics of the remaining 2,184 evaluable patients (1,530 pre-A16 patients; 654 post-A16 patients) are presented in Table 1.
Fig 1.
CONSORT diagram. (A) Second interim analysis of the comparison of arm A and arm B. (B) First interim analysis of the comparison of arm B and arm C. HER2, human epidermal growth factor receptor 2; LLN, lower limit of normal; LVEF, left ventricular ejection fraction; q3wk, once every 3 weeks.
Table 1.
Patient and Tumor Characteristics
| Characteristic | Cohort for Arm A v Arm B Comparison |
Cohort for Arm B v Arm C Comparison |
||||||
|---|---|---|---|---|---|---|---|---|
| Arm A (n = 1,087) |
Arm B (n = 1,097) |
Arm B (n = 954) |
Arm C (n = 949) |
|||||
| No. | % | No. | % | No. | % | No. | % | |
| Age at random assignment, years | ||||||||
| 18-39 | 191 | 17.6 | 205 | 18.7 | 181 | 19.0 | 148 | 15.6 |
| 40-49 | 362 | 33.3 | 351 | 32.0 | 304 | 31.9 | 324 | 34.1 |
| 50-59 | 365 | 33.6 | 355 | 32.4 | 308 | 32.3 | 300 | 31.6 |
| ≥ 60 | 169 | 15.5 | 186 | 17.0 | 161 | 16.9 | 177 | 18.7 |
| Extent of surgery | ||||||||
| Mastectomy | 660 | 60.7 | 676 | 61.6 | 581 | 60.9 | 586 | 61.7 |
| Breast sparing | 427 | 39.3 | 421 | 38.4 | 373 | 39.1 | 363 | 38.3 |
| Extent of nodal examination | ||||||||
| Sentinel biopsy | 110 | 10.1 | 109 | 9.9 | 106 | 11.1 | 91 | 9.6 |
| Axillary nodal dissection | 977 | 89.9 | 988 | 90.1 | 848 | 88.9 | 858 | 90.4 |
| Tumor size, cm | ||||||||
| ≤ 2.0 | 422 | 38.8 | 444 | 40.5 | 384 | 40.3 | 364 | 38.4 |
| 2.1-4.9 | 561 | 51.6 | 548 | 50.0 | 482 | 50.5 | 507 | 53.4 |
| ≥ 5.0 | 84 | 7.7 | 105 | 9.6 | 88 | 9.2 | 78 | 8.2 |
| Histologically positive nodes | ||||||||
| 0 | 142 | 13.1 | 142 | 12.9 | 142 | 14.9 | 132 | 13.9 |
| 1-3 | 514 | 47.3 | 507 | 46.2 | 435 | 45.6 | 464 | 48.9 |
| 4-9 | 280 | 25.8 | 303 | 27.6 | 253 | 26.5 | 235 | 24.8 |
| ≥ 10 | 151 | 13.9 | 145 | 13.2 | 124 | 13.0 | 118 | 12.4 |
| Tumor grade | ||||||||
| 1 | 13 | 1.2 | 5 | 0.5 | 4 | 0.4 | 13 | 1.4 |
| 2 | 144 | 13.2 | 145 | 13.2 | 134 | 14.0 | 121 | 12.8 |
| 3 | 409 | 37.6 | 451 | 41.1 | 393 | 41.2 | 387 | 40.8 |
| Unknown | 521 | 47.9 | 496 | 45.2 | 423 | 44.3 | 428 | 45.1 |
| Estrogen receptor status | ||||||||
| Positive | 554 | 51.0 | 568 | 51.8 | 495 | 51.9 | 474 | 49.9 |
| Negative | 533 | 49.0 | 529 | 48.2 | 459 | 48.1 | 475 | 50.1 |
Comparison of Treatment Efficacy
Per protocol, post-A16 patients contributed, at most, 1 year of follow-up data to the efficacy evaluation. Median length of follow-up of 1,328 pre-A16 patients who were still alive at analysis was 6 years (range, 1 day to 6 years). Reasons for treatment discontinuation are provided in Table 2.
Table 2.
Reasons for Treatment Discontinuation
| Reason | Arm A v Arm B |
Arm B v Arm C |
||||||
|---|---|---|---|---|---|---|---|---|
| Arm A(n = 1,087) |
Arm B(n = 1,097) |
Arm B(n = 954) |
Arm C(n = 949) |
|||||
| No. | % | No. | % | No. | % | No. | % | |
| Post–Addendum 16 patients who were still receiving treatment when their follow-up was truncated* | 225 | 20.7 | 301 | 27.4 | 83 | 8.7 | 88 | 9.3 |
| Completed planned treatment course | 699 | 64.3 | 528 | 48.1 | 605 | 63.4 | 572 | 60.3 |
| Refusal | 74 | 6.8 | 73 | 6.7 | 69 | 7.2 | 85 | 9.0 |
| Intolerable toxicity | 62 | 5.7 | 43 | 3.9 | 49 | 5.1 | 54 | 5.7 |
| Prohibited from initiating trastuzumab | — | 34 | 3.1 | 28 | 2.9 | 27 | 2.8 | |
| Asymptomatic drop in left ventricular ejection fraction | 0 | 40 | 3.6 | 43 | 4.5 | 66 | 7.0 | |
| Congestive heart failure | 0 | 17 | 1.5 | 16 | 1.7 | 21 | 2.2 | |
| Disease progression | 7 | 0.6 | 31 | 2.8 | 30 | 3.1 | 11 | 1.2 |
| Second primary disease | 0 | 1 | 0.09 | 1 | 0.1 | 1 | 0.1 | |
| Death as a result of: | ||||||||
| Febrile neutropenia/sepsis | 1 | 0.09 | 0 | 0 | 0 | |||
| Pneumonia | 0 | 2 | 0.2 | 2 | 0.2 | 0 | ||
| Pulmonary embolism | 0 | 2 | 0.2 | 1 | 0.1 | 1 | 0.1 | |
| Respiratory distress syndrome | 0 | 1 | 0.09 | 1 | 0.1 | 0 | ||
| Respiratory failure | 0 | 0 | 0 | 1 | 0.1 | |||
| Cardiac arrest | 0 | 1 | 0.09 | 1 | 0.1 | 1 | 0.1 | |
| Indwelling catheter placed in pericardial cavity causing cardiac death | 0 | 1 | 0.09 | 1 | 0.1 | 0 | ||
| Injuries from automobile accident | 0 | 1 | 0.09 | 1 | 0.1 | 0 | ||
| Desire for alternative treatment | 6 | 0.6 | 3 | 0.3 | 3 | 0.3 | 0 | |
| Concurrent disease | 6 | 0.6 | 5 | 0.5 | 5 | 0.5 | 3 | 0.3 |
| Other reasons | 7 | 0.6 | 13 | 1.2 | 15 | 1.6 | 18 | 1.9 |
Follow-up for post–Addendum 16 patients included in these comparisons was truncated at last follow-up or April 30, 2005, whichever came first, per study design.
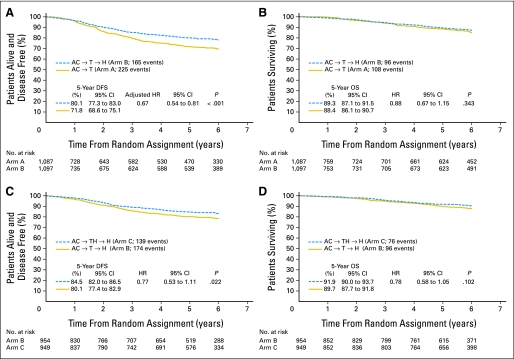
Clinical outcomes, including number and sites of first disease events, are listed in Table 3. Five-year DFS rates were estimated at 71.8% (95% CI, 68.6% to 75.1%) on arm A and 80.1% (95% CI, 77.3% to 83.0%) on arm B. The risk of disease progression was significantly decreased with the addition of trastuzumab following AC and then paclitaxel (log-rank P < .001; arm B/arm A HR, 0.69; 95% CI, 0.57 to 0.85). Univariate analysis showed that decreased DFS was associated with age younger than 40 years or older than 59 years (P = .006), estrogen receptor–negative disease (P < .001), tumor size (P < .001), and a greater number of positive lymph nodes (P < .001). After adjusting for these four factors, risk of disease progression was still found to be significantly decreased with the addition of trastuzumab following AC and then paclitaxel (P < .001; adjusted arm B/arm A HR, 0.67; 95% CI, 0.54 to 0.81; Fig 2A).
Table 3.
Clinical Outcomes and Sites of First Disease-Free Survival Event
| Outcome | Arm A v Arm B |
Arm B v Arm C |
||||||
|---|---|---|---|---|---|---|---|---|
| Arm A(n = 1,087) |
Arm B (n = 1,097) |
Arm B(n = 954) |
Arm C(n = 949) |
|||||
| No. | % | No. | % | No. | % | No. | % | |
| Alive without a disease event | 862 | 79.3 | 932 | 85.0 | 780 | 81.8 | 810 | 85.4 |
| Any event | 225 | 20.7 | 165 | 15.0 | 174 | 18.2 | 139 | 14.6 |
| Locoregional recurrence | 35 | 3.2 | 23 | 2.1 | 27 | 2.8 | 24 | 2.5 |
| Distant recurrence | 148 | 13.6 | 94 | 8.6 | 99 | 10.4 | 70 | 7.4 |
| Locoregional/distant recurrence | 10 | 0.9 | 13 | 1.2 | 11 | 1.2 | 8 | 0.8 |
| Second primary | 25 | 2.3 | 22 | 2.0 | 24 | 2.5 | 27 | 2.8 |
| Locoregional or distant recurrence with a second primary | 2 | 0.2 | 1 | 0.09 | 1 | 0.1 | 0 | 0.0 |
| Death | 5 | 0.5 | 12 | 1.1 | 12 | 1.3 | 10 | 1.1 |
Fig 2.
Kaplan-Meier curves showing (A) disease-free survival (DFS) and (B) overall survival (OS) for the comparison of arm A and arm B and (C) DFS and (D) OS for the comparison of arm B and arm C. Hazard ratios (HRs; with 95% CIs and P values) for pairwise comparisons were obtained by using stratified proportional hazards modeling. AC, doxorubicin and cyclophosphamide; H, trastuzumab; T, paclitaxel.
With 108 deaths on arm A and 96 deaths on arm B, the estimated 5-year OS rate for arm A was 88.4% (95% CI, 86.1% to 90.7%; Fig 2B), and the rate for arm B was 89.3% (95% CI, 87.1% to 91.5%), with an arm B/arm A HR of 0.88 (95% CI, 0.67 to 1.15; log-rank P = .343).
Adverse Events
The most common adverse events (NCI-CTC version 2.0) among the 2,064 trial patients during AC were grade ≥ 4 neutropenia (26.9%), grade ≥ 4 leukopenia (7.6%), and grade ≥ 3 nausea (5.1%); among the 1,443 pre-A16 patients during paclitaxel alone, they were grade ≥ 2 neurosensory difficulties (19.0%), grade ≥ 2 myalgia (9.3%), grade ≥ 2 arthralgia (8.5%), and grade ≥ 2 nail changes (5.9%); and among the 677 pre-A16 patients on arm B during trastuzumab alone, they were grade ≥ 2 LVEF changes (9.7%), grade ≥ 2 neurosensory difficulties (9.0%), grade ≥ 2 arthralgia (6.4%), and grade ≥ 2 myalgia (5.0%). The most common adverse events reported by these patients are presented in Appendix Table A1 (online only).
Comparison of Arm B and Arm C: Timing of Trastuzumab (concurrent with or after paclitaxel)
Of 2,111 women randomly assigned to either arm B or arm C between May 19, 2000, and January 23, 2002, and between September 1, 2002, and April 30, 2005 (when enrollment was opened to both treatment arms), 208 patients were ineligible (Fig 1B). Patient and tumor characteristics of the remaining 1,903 evaluable patients (1,731 pre-A16 patients; 172 post-A16 patients) are presented in Table 1.
Clinical Outcomes
Per protocol, post-A16 patients completed, at most, 4 months of follow-up prior to the arm B versus arm C comparison. The median length of follow-up of the 1,559 pre-A16 patients who are still alive at analysis is 6 years (range, 1 day to 6 years). Reasons for treatment discontinuation are provided in Table 2.
Clinical outcomes, including number of and sites of first disease events, are presented in Table 3. The 5-year DFS rate for arm B was estimated at 80.1% (95% CI, 77.4% to 82.9%) and for arm C, it was estimated at 84.4% (95% CI, 82.0% to 86.5%; Fig 2C). When testing whether DFS differed with respect to the timing of trastuzumab addition to AC then paclitaxel, the log-rank P value was .0216, which did not cross the prespecified O'Brien-Fleming boundary of significance (.00116) at this first interim analysis. However, this P value indicates a trend toward an increase in DFS with the addition of trastuzumab to paclitaxel relative to its addition following paclitaxel (arm C/arm B HR, 0.77; 99.9% CI, 0.53 to 1.11; P = .0216).
With 96 deaths on arm B and 76 deaths on arm C, there was an insufficient number of events to address whether survival differs among these treatments (arm C/arm B HR, 0.78; 95% CI, 0.58 to 1.05; log-rank P = .102). The 5-year OS rate for arm B was estimated at 89.7% (95% CI, 87.7% to 91.8%) and for arm C, it was estimated at 91.9% (95% CI, 90.0% to 93.7%; Fig 2D).
Adverse Events
The most common adverse events among the 1,924 patients during AC were grade ≥ 4 neutropenia (29.1%), grade ≥ 4 leukopenia (7.5%), and grade ≥ 3 febrile neutropenia (5.5%). The most common events during paclitaxel alone for the 799 pre-A16 patients on arm B and during paclitaxel + trastuzumab for the 739 pre-A16 patients on arm C were grade ≥ 2 neurosensory difficulties (arm B, 18.9%; arm C, 12.7%), grade ≥ 2 neuromotor difficulties (arm B, 5.1%; arm C, 2.3%), grade ≥ 2 arthralgia (arm B, 8.0%; arm C, 6.8%), grade ≥ 2 myalgia (arm B, 8.5%; arm C, 7.6%), and grade ≥ 2 nail changes (arm B, 6.9%; arm C, 6.1%). The most common events among 1,496 pre-A16 patients (arm B, n = 771; arm C, n = 725) during trastuzumab alone were grade ≥ 2 LVEF changes (arm B, 10.0%; arm C, 11.4%), grade ≥ 2 neurosensory difficulties (arm B, 9.6%; arm C, 8.8%), and grade ≥ 2 arthralgia (arm B, 6.0%; arm C, 7.3%). The most common adverse events reported by these patients are presented in Appendix Table A1 (online only).
DISCUSSION
These results from the second planned interim analysis of arm A versus arm B in the NCCTG N9831 trial with a median follow-up of 6 years provide further evidence that the addition of 1 year of adjuvant trastuzumab significantly improves DFS compared with chemotherapy alone in patients with resected HER2-positive breast cancer. This DFS advantage was maintained after accounting for patient and tumor characteristics. Comparison of arm B and arm C with a median follow-up of 6 years indicates that although trastuzumab added sequentially to chemotherapy improves DFS, there is a strong trend toward a better outcome with concurrent trastuzumab relative to sequential administration with chemotherapy.
The National Surgical Adjuvant Breast and Bowel Project B-31 (NSABP B-31)15,16 and the Breast Cancer International Research Group 006 (BCIRG-006)17 trials evaluated concurrent trastuzumab relative to chemotherapy alone, whereas the Breast International Group 01-01 (HERA; BIG 01-01)18,19 and the Federation Nationale des Centres de Lutte Contre le Cancer (FNCLCC-PACS-04)20 trials evaluated sequential trastuzumab relative to chemotherapy alone. The specific regimens used in these trials are presented in Appendix Table A2 (online only). Similarities in trial design and conduct enabled the NSABP B-31 and NCCTG N9831 study teams to gain approval from the FDA and NCI Cancer Therapy Evaluation Program (CTEP) for a joint analysis plan to assess the impact of the addition of concurrent trastuzumab to chemotherapy. The first preplanned interim analysis after the 395th event was reported (55% of the total number events needed for final analysis) found a statistically significant improvement in DFS (52% reduction in risk of a disease event; P < .001), with a trend toward improved OS (33% reduction in the risk of death; P = .015).15 These analyses were repeated after the 779th event occurred with similar results.16
BCIRG-006 further supported the use of trastuzumab administered concurrently with chemotherapy in the adjuvant setting.17 With median follow-up of 5.4 years, both the AC-TH arm (trastuzumab [H] administered concurrently with docetaxel [T] after doxorubicin [A] and cyclophosphamide [C]) and the TCpH arm (H administered concurrently with T and carboplatin [Cp]) were found to improve DFS (AC-TH: HR, 0.64; 95% CI, 0.53 to 0.78; P < .001; TCpH: HR, 0.75; 95% CI, 0.63 to 0.90; P = .040) and OS (AC-TH: HR, 0.63; 95% CI, 0.48 to 0.81; P < .001; TCpH: HR, 0.77; 95% CI, 0.60 to 0.99; P = .038) relative to AC-T.
In the HERA BIG 01-01 trial,18,19 patients received neoadjuvant or adjuvant chemotherapy and radiotherapy before enrollment. With a median follow-up of 4 years, that trial (n = 1,566) showed a significant reduction in the risk of disease progression with 1 year of trastuzumab relative to observation (HR, 0.76; 95% CI, 0.66 to 0.87; P < .001).19 In the FNCLCC-PACS-0420 trial, patients with HER2-positive disease (n = 528 of 3,010 enrolled) were randomly assigned to trastuzumab or observation after anthracycline-based chemotherapy with or without docetaxel. With a median follow-up of 3 years, there was a nonsignificant reduction in the risk of relapse with 1 year of sequential trastuzumab relative to observation (HR, 0.86; 95% CI, 0.61 to 1.22; P = .41).
Although the findings of HERA BIG 01-01and FNCLCC-PACS-04 regarding the sequential addition of trastuzumab to chemotherapy were contradictory, our NCCTG N9831 study supports the DFS benefit of sequential treatment as seen in HERA BIG 01-01 but suggests that better outcomes may be provided by a concurrent approach. Paclitaxel with trastuzumab after AC did not appear to have a significantly worse safety profile compared with sequential administration, including cardiac safety, further supporting the use of the concurrent regimen.
The data for this analysis were released (sanctioned by the IDMC, the NCI, and the study team) following a much lower than anticipated DFS event rate after a long period of follow-up. It was determined that the early data release was the correct approach for patient care because it was estimated that it would have taken more than 10 years to reach the number of originally prespecified events. The comparison of sequential and concurrent trastuzumab regimens was based on a first planned interim analysis of DFS. Future interim analyses for this trial should help to clarify any OS advantage of trastuzumab over chemotherapy alone (including concurrent v sequential therapy) in the adjuvant setting. On the basis of the positive risk-benefit ratio shown by the current analysis of NCCTG N9831 for the primary end point of DFS, we recommend that trastuzumab be incorporated into a concurrent regimen (instead of sequential) with taxane chemotherapy.
Supplementary Material
Appendix
Table A1.
Common Adverse Events Reported*
| Adverse Event | Arm A v Arm B Comparison (%)† |
Arm B v Arm C Comparison (%)† |
||
|---|---|---|---|---|
| Arm A (n = 1,087) | Arm B (n = 1,097) | Arm B (n = 954) | Arm C (n = 949) | |
| Grade ≥ 4 hematologic toxicities | ||||
| Leukopenia | 8.6 | 7.2 | 7.2 | 8.2 |
| Neutropenia | 26.5 | 27.5 | 26.7 | 30.6 |
| Prespecified grade ≥ 2 nonhematologic toxicities | ||||
| Arthralgia | 7.7 | 10.0 | 11.6 | 8.5 |
| Left ventricular ejection fraction | 2.1 | 7.9 | 9.7 | 10.7 |
| Myalgia | 7.1 | 10.7 | 11.7 | 5.4 |
| Nail changes | 4.0 | 5.4 | 7.7 | 4.7 |
| Neuromotor difficulties | 3.0 | 5.1 | 6.1 | 2.3 |
| Neurosensory difficulties | 13.4 | 14.67 | 19.7 | 8.7 |
| Other grade ≥ 3 nonhematologic toxicities | ||||
| Nausea | 5.4 | 4.9 | 4.7 | 5.5 |
| Febrile neutropenia | 4.1 | 4.3 | 3.7 | 6.6 |
Reporting requirements included grade ≥ 4 hematologic toxicities, grade ≥ 2 neuropathies, nail changes, myalgias, arthralgias, and other grade ≥ 3 nonhematologic toxicities.
Adverse events were recorded at the end of each phase of treatment (doxorubicin and cyclophosphamide, paclitaxel ± trastuzumab, trastuzumab alone). Thus, for 656 patients in the arm A varm B comparison who were enrolled after April, 25, 2004, only adverse events reported at completion of doxorubicin and cyclophosphamide phase are reported; for patients in the arm B v arm C comparison who were enrolled after January 25, 2005, only adverse events reported at completion of doxorubicin and cyclophosphamide phase are reported.
Table A2.
Randomized, Open-Label, Phase III Trials Investigating Adjuvant Trastuzumab Therapy in Patients With HER2-Positive Early Breast Cancer
| Study | Key Inclusion Criteria | Treatment Regimen |
|---|---|---|
| Concurrent studies | ||
| BCIRG-006 | Node-positive or high-risk node-negative, chemotherapy-naive | Doxorubicin 60 mg/m2 and cyclophosphamide 600 mg/m2 every 3 weeks × four cycles → docetaxel 100 mg/m2 every 3 weeks × four cycles + concurrent trastuzumab 6 mg/kg loading dose, then 2 mg/kg/wk × 11 cycles → trastuzumab 6 mg/kg every 3 weeks (total trastuzumab treatment of 1 year) |
| Docetaxel-carboplatin 75 mg/m2/AUC 6 every 3 weeks × six cycles + concurrent trastuzumab 4 mg/kg loading dose, then 2 mg/kg/wk × 18 cycles → trastuzumab 6 mg/kg every 3 weeks × 13 cycles (total trastuzumab treatment of 1 year) | ||
| Doxorubicin 60 mg/m2 and cyclophosphamide 600 mg/m2 every 3 weeks × four cycles → docetaxel 100 mg/m2 every 3 weeks × four cycles | ||
| NSABP B-31 | Node-positive, chemotherapy-naive | Doxorubicin 60 mg/m2 and cyclophosphamide 600 mg/m2 every 3 weeks × four cycles → paclitaxel 175 mg/m2 every 3 weeks × four cycles (or 80 mg/m2 once per week × 12 cycles) + concurrent trastuzumab 4 mg/kg loading dose with cycle 1 of paclitaxel 2 mg/kg once per week × 51 cycles (total trastuzumab treatment of 1 year) |
| Doxorubicin 60 mg/m2 and cyclophosphamide 600 mg/m2 every 3 weeks × four cycles → PAC 175 mg/m2 every 3 weeks × four cycles (80 mg/m2 once per week × 12 cycles) | ||
| Sequential studies | ||
| HERA (BIG 01-01) | Node-positive or high-risk node-negative, adjuvant and/or neoadjuvant chemotherapy with or without radiotherapy | Trastuzumab 8 mg/kg loading dose week 1 → trastuzumab 6 mg/kg every 3 weeks × 2 years |
| Trastuzumab 8 mg/kg loading dose week 1 → trastuzumab 6 mg/kg every 3 weeks × 1 year | ||
| Observation alone | ||
| Fluorouracil 500 mg/m2, epirubicin 100 mg/m2, and cyclophosphamide 500 mg/m2 every 3 weeks × six cycles → radiotherapy → trastuzumab 6 mg/kg every 3 weeks × 1 year (8 mg/kg loading dose) | ||
| FNCLCC-PACS-04* | Node-positive, chemotherapy-naive | Epirubicin 75 mg/m2 and docetaxel 75 mg/m2 every 3 weeks × six cycles → radiotherapy → trastuzumab 6 mg/kg every 3 weeks × 1 year (8 mg/kg loading dose) |
| Fluorouracil 500 mg/m2, epirubicin 100 mg/m2, and cyclophosphamide 500 mg/m2 every 3 weeks × six cycles → radiotherapy → observation | ||
| Epirubicin 75 mg/m2 and docetaxel 75 mg/m2 every 3 weeks × six cycles → radiotherapy → observation | ||
| Sequential v concurrent study | ||
| NCCTG N9831 | Node-positive or high-risk node-negative, chemotherapy-naive | Doxorubicin 60 mg/m2 and cyclophosphamide 600 mg/m2 every 3 weeks × four cycles → paclitaxel 80 mg/m2 once per week × 12 cycles |
| Doxorubicin 60 mg/m2 and cyclophosphamide 600 mg/m2 every 3 weeks × four cycles → paclitaxel 80 mg/m2 once per week × 12 cycles + concurrent trastuzumab 2 mg/kg once per week × 52 weeks (4 mg/kg loading dose) | ||
| Doxorubicin 60 mg/m2 and cyclophosphamide 600 mg/m2 every 3 weeks × four cycles → paclitaxel 80 mg/m2 once per week × 12 weeks → trastuzumab 2 mg/kg once per week × 52 weeks (4 mg/kg loading dose) |
Abbreviations: AUC, area under curve; BCIRG-006, Breast Cancer International Research Group 006 (trial); FNCLCC-PACS-04, Federation Nationale des Centres de Lutte Contre le Cancer PACS-04 (trial); HER2, human epidermal growth factor receptor 2; HERA (BIG 01-01), Breast International Group 01-01 (trial); NCCTG N9831, North Central Cancer Treatment Group N9831 (trial); NSABP B-31, National Surgical Adjuvant Breast and Bowel Project B-31 (trial); PAC, paclitaxel.
Treatment arms shown are for the HER2-positive subgroup.
Footnotes
See accompanying editorial on page 4474; listen to the podcast by Dr Pritchard at www.jco.org/podcast
Support by Grant No. CA25224 from National Institutes of Health, by the Breast Cancer Research Foundation, and by Grant No. 35-03 from Genentech, which also provided copyediting services.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information can be found for the following: NCT00005970.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: Peter A. Kaufman, Genentech (C) Stock Ownership: None Honoraria: None Research Funding: Edith A. Perez, Genentech, sanofi-aventis; Vera J. Suman, Genentech, Breast Cancer Research Foundation; Julie R. Gralow, Genentech, Novartis, Amgen, Roche; Peter A. Kaufman, Genentech Expert Testimony: None Other Remuneration: Robert B. Jenkins, Abbott Molecular
AUTHOR CONTRIBUTIONS
Conception and design: All authors
Financial support: Edith A. Perez
Administrative support: Edith A. Perez, Robert B. Jenkins
Provision of study materials or patients: Edith A. Perez, James N. Ingle, Robert B. Jenkins
Collection and assembly of data: Edith A. Perez, Vera J. Suman, Daniel W. Visscher, Beiyun Chen, Robert B. Jenkins
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Sjögren S, Inganäs M, Lindgren A, et al. Prognostic and predictive value of c-erbB-2 overexpression in primary breast cancer, alone and in combination with other prognostic markers. J Clin Oncol. 1998;16:462–469. doi: 10.1200/JCO.1998.16.2.462. [DOI] [PubMed] [Google Scholar]
- 2.Owens MA, Horten BC, Da Silva MM. HER2 amplification ratios by fluorescence in situ hybridization and correlation with immunohistochemistry in a cohort of 6556 breast cancer tissues. Clin Breast Cancer. 2004;5:63–69. doi: 10.3816/cbc.2004.n.011. [DOI] [PubMed] [Google Scholar]
- 3.Paik S, Hazan R, Fisher ER, et al. Pathologic findings from the National Surgical Adjuvant Breast and Bowel Project: Prognostic significance of erbB-2 protein overexpression in primary breast cancer. J Clin Oncol. 1990;8:103–112. doi: 10.1200/JCO.1990.8.1.103. [DOI] [PubMed] [Google Scholar]
- 4.Dawood S, Broglio K, Buzdar AU, et al. Prognosis of women with metastatic breast cancer by HER2 status and trastuzumab treatment: An institutional-based review. J Clin Oncol. 2010;28:92–98. doi: 10.1200/JCO.2008.19.9844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gabos Z, Sinha R, Hanson J, et al. Prognostic significance of human epidermal growth factor receptor positivity for the development of brain metastasis after newly diagnosed breast cancer. J Clin Oncol. 2006;24:5658–5663. doi: 10.1200/JCO.2006.07.0250. [DOI] [PubMed] [Google Scholar]
- 6.Albanell J, Bellmunt J, Molina R, et al. Node-negative breast cancers with p53(-)/HER2-neu(-) status may identify women with very good prognosis. Anticancer Res. 1996;16:1027–1032. [PubMed] [Google Scholar]
- 7.Hudis CA. Trastuzumab: Mechanism of action and use in clinical practice. N Engl J Med. 2007;357:39–51. doi: 10.1056/NEJMra043186. [DOI] [PubMed] [Google Scholar]
- 8.Herceptin European Summary of Product Characteristics. http://www.emea.europa.eu/humandocs/Humans/EPAR/herceptin/herceptin.htm.
- 9.Genentech. Herceptin package insert. South San Francisco, CA: Genentech; 2009. Mar, [Google Scholar]
- 10.Roche PC, Suman VJ, Jenkins RB, et al. Concordance between local and central laboratory HER2 testing in the breast intergroup trial N9831. J Natl Cancer Inst. 2002;94:855–857. doi: 10.1093/jnci/94.11.855. [DOI] [PubMed] [Google Scholar]
- 11.Perez EA, Suman VJ, Davidson NE, et al. HER2 testing by local, central, and reference laboratories in specimens from the North Central Cancer Treatment Group N9831 intergroup adjuvant trial. J Clin Oncol. 2006;24:3032–3038. doi: 10.1200/JCO.2005.03.4744. [DOI] [PubMed] [Google Scholar]
- 12.Howell A, Cuzick J, Baum M, et al. Results of the ATAC (Arimidex, Tamoxifen, Alone or in Combination) trial after completion of 5 years' adjuvant treatment for breast cancer. Lancet. 2005;365:60–62. doi: 10.1016/S0140-6736(04)17666-6. [DOI] [PubMed] [Google Scholar]
- 13.Perez EA, Suman VJ, Davidson NE, et al. Cardiac safety analysis of doxorubicin and cyclophosphamide followed by paclitaxel with or without trastuzumab in the North Central Cancer Treatment Group N9831 adjuvant breast cancer trial. J Clin Oncol. 2008;26:1231–1238. doi: 10.1200/JCO.2007.13.5467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pocock SJ, Simon R. Sequential treatment assignment with balancing for prognostic factors in the controlled clinical trial. Biometrics. 1975;31:103–115. [PubMed] [Google Scholar]
- 15.Romond EH, Perez EA, Bryant J, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353:1673–1684. doi: 10.1056/NEJMoa052122. [DOI] [PubMed] [Google Scholar]
- 16.Perez EA, Romond EH, Suman VJ, et al. Four-year follow-up of trastuzumab plus adjuvant chemotherapy for operable human epidermal growth factor receptor 2–positive breast cancer: Joint analysis of data from NCCTG N9831 and NSABP B-31. J Clin Oncol. 2011;29:3366–3373. doi: 10.1200/JCO.2011.35.0868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Slamon DJ, Eiermann W, Robert N, et al. Phase III randomized trial comparing doxorubicin and cyclophosphamide followed by docetaxel (AC→T) with doxorubicin and cyclophosphamide followed by docetaxel and trastuzumab (AC→TH) with docetaxel, carboplatin and trastuzumab (TCH) in Her2neu positive early breast cancer patients: BCIRG 006 study. Cancer Res. 2009;69:500s. abstr 62. [Google Scholar]
- 18.Smith I, Procter M, Gelber RD, et al. 2-year follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer: A randomised controlled trial. Lancet. 2007;369:29–36. doi: 10.1016/S0140-6736(07)60028-2. [DOI] [PubMed] [Google Scholar]
- 19.Gianni L. Update of the HERA trial at 4 years'median follow-up. Oral presentation at the 11th International St. Gallen Breast Cancer Conference; March 11-14, 2009; St. Gallen, Switzerland. http://www.senology.it/SGallen.pdf. [Google Scholar]
- 20.Spielmann M, Roché H, Delozier T, et al. Trastuzumab for patients with axillary-node-positive breast cancer: Results of the FNCLCC-PACS 04 trial. J Clin Oncol. 2009;27:6129–6134. doi: 10.1200/JCO.2009.23.0946. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.