Abstract
Purpose
Patients with Ewing sarcoma (ES) with metastases and those who relapse fare poorly and receive therapies that carry significant toxicity. This phase 1/2 study was conducted to evaluate the efficacy of figitumumab in advanced ES.
Patients and Methods
Patients with sarcoma 10 to 18 years old were enrolled in two dose escalation cohorts (20 and 30 mg/Kg intravenously every 4 weeks) in the phase 1 portion of the study. Patients with ES 10 years old or older were enrolled in the phase 2 portion of the study. The primary phase 2 objective was objective response rate (ORR).
Results
Thirty-one patients with ES (n = 16), osteosarcoma (n = 11), or other sarcomas (n = 4) were enrolled in the phase 1 portion of the study. Dose escalation proceeded to 30 mg/kg every 4 weeks with no dose-limiting toxicity identified. In the phase 2 portion of the study, 107 patients with ES received figitumumab at 30 mg/kg every 4 weeks for a median of 2 cycles (range, 1 to 16). Sixty three percent of phase 2 patients had received at least three prior treatment regimens. Of 106 evaluable patients, 15 had a partial response (ORR, 14.2%) and 25 had stable disease. Median overall survival was 8.9 months. Importantly, patients with a pretreatment circulating free insulin-like growth factor (IGF) -1 lower than 0.65 ng/mL (n = 14) had a median OS of 3.6 months, whereas those with a baseline free IGF-1 ≥ 0.65 ng/mL (n = 84) had a median OS of 10.4 months (P < .001).
Conclusion
Figitumumab had modest activity as single agent in advanced ES. A strong association between pretreatment serum IGF-1 and survival benefit was identified.
INTRODUCTION
Recent efforts have identified sarcoma types driven by specific molecular events.1 Such is the case of the Ewing sarcoma (ES), characterized in 85% of patients by the translocation ES gene/friend leukemia virus integration 1 (EWS/FLI-1).2 EWS/FLI-1 reduces tumor insulin-like growth factor (IGF) binding protein 3 expression, facilitating the bioactivity of IGFs at tumor sites.3 Also, the IGF type 1 receptor (IGF-1R) is required for EWS/FLI-1-mediated transformation,4 and targeting this receptor has been shown to decrease tumor growth, metastasis, vasculogenicity, and angiogenesis of ES.5
Figitumumab (CP-751,871) is a highly specific, fully human, immunoglobulin 2 monoclonal antibody against the IGF-1R.6 In a previous phase 1 study, figitumumab was well tolerated in ES (n = 16) and two of these patients experienced objective responses.7 This study was conducted to investigate the safety and efficacy of figitumumab in ES.
PATIENTS AND METHODS
Patients
Study A4021020 was a multicenter, open label, phase 1/phase 2 clinical trial. The trial population included patients with a histologically confirmed diagnosis of osseous or extraosseous ES including variants with neural differentiation (eg, peripheral neuroectodermal tumors [PNET]). In addition, the phase 1 portion of the study enrolled also patients with relapsed/refractory osteosarcoma, rhabdomyosarcoma, desmoplastic small round cell tumors, and other undifferentiated sarcomas and it was limited to 10- to 18-year-old patients. Other key inclusion/exclusion criteria were: disease state for which there is no known curative therapy or therapy proven to prolong survival with an acceptable quality of life; Eastern Cooperative Oncology Group performance status of 0 to 2 or a Lansky play scale ≥ 50; absolute neutrophil count ≥ 1,000/mL without growth factor support; platelets ≥ 75,000/mL (previous transfusion was allowed); hemoglobin ≥ 8 g/dL (previous transfusion allowed); total bilirubin ≤ 1.5 times the upper limit of normal (ULN) for age (except for Gilbert's syndrome patients); serum ALT ≤ 2.5 × ULN; AST ≤ 2.5 × ULN; serum creatinine ≤ 1.5 × ULN for age; cardiac ejection fraction ≥ 50% or shortening fraction ≥ 28%; at least 1 week from prior radiotherapy provided that it was not at the only site of measurable disease; fully recovered (< grade 1 as defined by the National Cancer Institute Common Toxicity Criteria for Adverse Events, version 3.0 or deemed irreversible) from the acute effects of prior systemic therapy, including 2 weeks since previous chemotherapy (8 weeks for mitomycin or nitrosoureas) and 4 weeks from prior biologic therapy; written voluntary informed consent (adults), parent or legal guardian consent or patient assent/parent permission for minors; no prior anti-IGF-1R therapy; no symptomatic brain metastases, significant active cardiac disease, infection, insulin-dependent diabetes, or concurrent use of high-dose steroids.
Study Design and Procedures
The phase 1 portion of the study enrolled two dosing cohorts with at least three patients/cohort testing the safety and tolerability of figitumumab at doses of 20 and 30 mg/kg every 4 weeks. An additional dose, identical to the one given on cycle 1 day 1, was given on cycle 1 day 2. Figitumumab at 30 mg/kg every 4 weeks was the phase 2 regimen. Patients continued figitumumab treatment until progression, unacceptable toxicity, or withdrawal of consent. Based on experimental data supporting the combination of figitumumab with rapamycin in ES,8 patients who would be otherwise discontinued from the study due to disease progression, or who experienced less than a partial response to figitumumab at cycle 4 and beyond, were eligible to receive salvage therapy with oral rapamycin (2 to 4 mg/d) in combination with figitumumab. No objective responses were seen in these patients and this portion of the study will be reported elsewhere. Use of concomitant supportive therapies was allowed. Patients requiring dose reduction of figitumumab received the reduced dose for the remainder of the study. A maximum of two dose reductions were allowed. Cycle delays of up to 8 weeks from the last dose were permitted for recovery from adverse events.
Toxicities were assessed using National Cancer Institute Common Toxicity Criteria for Adverse Events, version 3.0. Dose-limiting toxicities (DLTs) included the following figitumumab-related cycle 1 adverse events (AEs). Any grade ≥ 3 AEs despite optimal supportive care except grade 3 isolated gamma glutamyl transferase (GGT) elevation and grade 3 asthenia, grade ≥ 3 GGT elevation with ALT and AST higher than 2.5 × ULN, grade 4 GGT elevation, grade 4 asthenia, and grade ≥ 3 hyperglycemia not manageable with antidiabetic medication. Before enrollment, at each treatment visit, and at treatment discontinuation, patients underwent safety laboratory testing (ie, blood chemistry, hematology) and were queried for AEs and concomitant medication use.
During the phase 1 portion of the study, blood samples for pharmacokinetic (PK) analysis of figitumumab were collected at preinfusion (within 2 hours before day 1 figitumumab dosing) in cycles 1, 2, 4, 5, and 6; at 1-hour post infusion on cycle 1 day 2 and cycle 5 day 1; on day 8 of cycles 1 and 5; and, whenever possible, at 28 and 150 days after the last figitumumab dose. In patients enrolled into the phase 2 study portion, blood samples for figitumumab PK were collected at preinfusion (within 2 hours before day 1 dosing) in cycles 1, 4, 5, and 6; at 1 hour postinfusion on cycle 5 day 1; and at 28 and 150 days after the last figitumumab dose. Figitumumab PK was analyzed as described previously.9
Pharmacodynamic serum markers investigated included growth hormone, IGF-1 (total and free), glucose, and insulin. Samples, obtained after fasting, were taken at each treatment cycle starting at cycle 1 day 1. Provision of tissue for the analysis of IGF-1R and related molecules as well as EWS translocations was voluntary.
Tumor assessments were conducted at baseline and repeated every 8 weeks until disease progression. Disease status was assessed by investigator according to Response Evaluation Criteria in Solid Tumors (RECIST).
The study protocol was approved by institutional review boards/ethics committees and it was conducted in accordance with local regulations.
Statistical Analysis
The primary end point of the phase 2 portion of the study was objective response according to RECIST. The null hypothesis was 10% ORR. The planned sample size was 100 evaluable patients. This design had a significance level of 0.04% and 87% power to detect a true ORR of 20%. The Kaplan-Meier method was used to estimate medians for progression-free survival (PFS) and overall survival (OS). Cox proportional hazards regression analysis was used to test biomarker associations with efficacy end points. Statistical analysis was conducted using R 2.11.1 Software (R Foundation for Statistical Computing, Vienna, Austria; http://www.r-project.org/).
RESULTS
Study Population
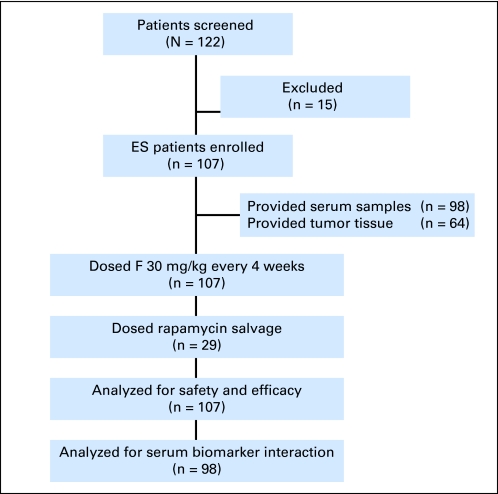
The safety and tolerability of figitumumab in adult patients have been well established.10 Since the peak incidence of ES occurs in adolescents,2 the phase 1 portion of the study focused on those patients and enrolled only 10- to 18-year-old patients (n = 31). In the phase 2 portion of the study, 107 patients ≥ 10 years of age with a histologically confirmed diagnosis of ES were enrolled between July 2008 and October 2009 (Fig 1). Demographic characteristics of the study patients are summarized in Table 1.
Fig 1.
Phase 2 schema. ES, Ewing sarcoma; F, figitumumab.
Table 1.
Demographic Characteristics
| Characteristic | Patients by Phase |
|||
|---|---|---|---|---|
| 1 (n = 31) |
2 (n = 107) |
|||
| No. | % | No. | % | |
| Age, years | ||||
| Median | 16 | 18 | ||
| Range | 11-18 | 10-62 | ||
| Sex | ||||
| Male | 20 | 64.5 | 67 | 62.6 |
| Female | 11 | 35.5 | 40 | 37.4 |
| ECOG performance status | ||||
| 0 | 16 | 51.6 | 53 | 49.5 |
| 1 | 12 | 38.7 | 44 | 41.1 |
| 2 | 3 | 9.7 | 10 | 9.3 |
| Clinical stage at study entry | ||||
| Local recurrence | — | — | 3 | 2.8 |
| Metastatic | 31 | 100 | 104 | 97.2 |
| Tumor type | ||||
| ES | 16 | 51.6 | 107 | 100 |
| Osteosarcoma | 11 | 35.5 | — | — |
| Other sarcoma | 4 | 12.9 | — | — |
| Prior chemotherapy regimens | ||||
| 1 | 4 | 12.9 | 13 | 12.1 |
| 2 | 7 | 22.6 | 27 | 25.2 |
| 3 | 4 | 12.9 | 23 | 21.5 |
| ≥ 4 | 16 | 51.6 | 44 | 41.1 |
| Prior radiotherapy | 22 | 71.0 | 94 | 87.9 |
| Prior surgery | 31 | 100 | 101 | 94.4 |
Abbreviations: ECOG, Eastern Cooperative Oncology Group; ES, Ewing sarcoma.
Safety and Tolerability
In the phase 1 portion of the study, patients received figitumumab intravenously every 4 weeks in 2 dose escalation cohorts at 20 (n = 4) and 30 mg/kg (n = 8). The 30 mg/kg cohort was expanded with an additional 19 patients. Median numbers of treatment cycles were 2.5 (range, 1 to 12) and 2 (range, 1 to 13) for 20 and 30 mg/kg, respectively. No cycle 1 DLTs were identified. Grade ≥ 3 figitumumab-related AEs observed at cycle 2 and beyond included: grade 4 tumor pain (n = 1) and grade 3 lymphopenia, thrombocytopenia, fatigue, GGT elevation, headache, dizziness, and dyspnea (n = 1, each).
In the phase 2 portion of the study, patients received a median of 2 cycles (range, 1 to 16) of figitumumab at 30 mg/kg every 4 weeks. There were three AE-related dose reductions: one instance of grade 2 headache, one instance of grade 2 weight loss, and one instance of grade 3 thrombocytopenia. There was one dose omission due to grade 2 hyperglycemia that resolved with antidiabetes management. There were no grade 5 treatment related AEs reported. Table 2 displays all treatment emergent AEs that occurred with a frequency higher than 10% in phase 2 patients, irrespectively of causality. In addition, there were two grade 4 AEs attributed to figitumumab—decrease appetite and leukemia. The case of leukemia, M2 with t (8;21) (q22;q22), presented after one cycle of figitumumab therapy in a patient treated before study entry with two multiagent chemotherapy regimens that included doxorubicin and etoposide. Two additional cases of leukemia (grade 4) were reported on study. One event that was attributed to rapamycin in combination with figitumumab, appeared in a patient that received one cycle of figitumumab followed by two cycles of figitumumab and rapamycin. The second case was attributed to prior etoposide treatment in a patient that received seven cycles of figitumumab. Three cases of grade 3 hyperglycemia, three cases of grade 3 liver enzymes increase, one case of grade 3 mucositis, and two cases of grade 3 neutropenia were also attributed to figitumumab treatment by investigator.
Table 2.
Treatment Emergent Adverse Events of All Causality With a Frequency > 10% in Phase 2 Patients
| Adverse Event (n = 107) | Grade |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 |
2 |
3 |
4 |
All |
||||||
| No. | % | No. | % | No. | % | No. | % | No. | % | |
| Fatigue | 13 | 12.1 | 12 | 11.2 | 2 | 1.9 | 0 | 27 | 25.2 | |
| Decreased appetite | 13 | 12.1 | 8 | 7.5 | 1 | 0.9 | 1 | 0.9 | 23 | 21.5 |
| Headache | 11 | 10.3 | 7 | 6.5 | 2 | 1.9 | 0 | 20 | 18.7 | |
| Muscle spasm | 13 | 12.1 | 5 | 4.7 | 2 | 1.9 | 0 | 20 | 18.7 | |
| Back pain | 3 | 2.8 | 10 | 9.3 | 5 | 4.7 | 1 | 0.9 | 19 | 17.8 |
| Constipation | 10 | 9.3 | 8 | 7.5 | 1 | 0.9 | 0 | 19 | 17.8 | |
| Dyspnea | 7 | 6.5 | 7 | 6.5 | 3 | 2.8 | 1 | 0.9 | 19* | 17.8 |
| Vomiting | 9 | 8.4 | 7 | 6.5 | 3 | 2.8 | 0 | 19 | 17.8 | |
| Cough | 13 | 12.1 | 4 | 3.7 | 0 | 0 | 17 | 15.9 | ||
| Nausea | 9 | 8.4 | 6 | 5.6 | 1 | 0.9 | 0 | 16 | 15 | |
| Pyrexia | 14 | 13.1 | 2 | 1.9 | 0 | 0 | 16 | 15 | ||
| Chest pain | 3 | 2.8 | 8 | 7.5 | 3 | 2.8 | 1 | 0.9 | 15 | 14 |
| Diarrhea | 14 | 13.1 | 1 | 0.9 | 0 | 0 | 15 | 14 | ||
| Anemia | 1 | 0.9 | 8 | 7.5 | 3 | 2.8 | 2 | 1.9 | 14 | 13.1 |
| Ocular hyperemia | 12 | 11.2 | 2 | 1.9 | 0 | 0 | 14 | 13.1 | ||
| Asthenia | 8 | 7.5 | 4 | 3.7 | 1 | 0.9 | 0 | 13 | 12.1 | |
| Hyperglycemia | 7 | 6.5 | 3 | 2.8 | 3 | 2.8 | 0 | 13 | 12.1 | |
| Weight decrease | 7 | 6.5 | 6 | 5.6 | 0 | 0 | 13 | 12.1 | ||
| Thrombocytopenia | 6 | 5.6 | 1 | 0.9 | 2 | 1.9 | 3 | 2.8 | 12 | 11.2 |
| Arthralgia | 5 | 4.7 | 3 | 2.8 | 3 | 2.8 | 0 | 11 | 10.3 | |
| Musculoskeletal pain | 4 | 3.7 | 7 | 6.5 | 1 | 0.9 | 0 | 11 | 10.3 | |
One grade 5 event of dyspnea attributed to disease progression was reported.
Efficacy End Points
One partial response (RECIST) was observed in the phase 1 portion of the study in a patient with ES. In the phase 2 portion, of the 106 patients evaluable for objective response, 15 had partial responses (ORR, 14.2%; 95% CI, 8.1% to 22.3%) and 25 stable disease. Twelve of 15 objective responses were seen at cycle 2. Median duration of response was 4.7 (95% CI, 3.7 to 6.1) months. There was no apparent difference in response rate by age group (10 to 18 v > 18 years) or clinical stage (local v metastatic recurrence). Median number of prior systemic therapies in responders was 2 (range of 1 to 5).
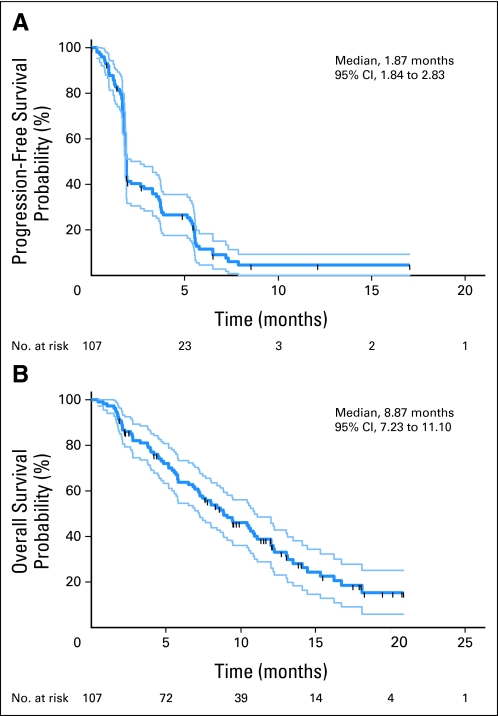
PFS and OS were investigated as secondary end points. Figure 2 shows the Kaplan-Meier plots for PFS and OS for patients enrolled in the phase 2 portion of the study. Median PFS and OS were respectively 1.9 (95% CI, 1.8 to 2.8) and 8.9 (95% CI, 7.2 to 11.1) months (n = 107). Exploratory analyses were conducted by age group, clinical stage, presence of lung, bone metastases or both, or number of prior systemic therapy regimens. No significant differences were observed.
Fig 2.
Kaplan-Meier curves for (A) progression-free survival and (B) overall survival. 95% CI boundaries are for all phase 2 study patients.
PK Findings
At the time of the preparation of this article, PK data were available from 51 patients: four patients who received figitumumab at 20 mg/kg and 47 at 30 mg/kg. PK parameters are summarized in Table 3. Of note, the maximum figitumumab concentration (Cmax) and the area under the plasma concentration-time curve within the 4-week dose interval (AUC0-d28) in cycle 1 post loading dose were similar to those at steady-state (cycle 5). Although the sample size was small, it is noteworthy to indicate also that PK parameters were similar between the 10 to 18 years and the older than 18 years age group. At 30 mg/kg, the cycle 5 Cmax was 944 ± 353 mg/L (± standard deviation) in patients 10 to 18 years old (n = 24) whereas a Cmax of 843 ± 353 mg/L was observed in those older than 18 years (n = 8).
Table 3.
Plasma Pharmacokinetic Parameters
| Cycle by Dose (mg/kg) | Cmax (mg/L) |
AUC(0-d28) (mg × hr/L) |
||||
|---|---|---|---|---|---|---|
| No. of Patients | Mean | SD | No. of Patients | Mean | SD | |
| 20 | ||||||
| 1* | 4 | 545 | 216 | 3 | 206,000 | 74,900 |
| 5 | 2 | 533 | 997† | 1 | 427,000 | |
| 30 | ||||||
| 1* | 25 | 1,040 | 279 | 19 | 364,000 | 76,000 |
| 5 | 32 | 918 | 350 | 7 | 326,000 | 138,000 |
Abbreviations: Cmax, maximum plasma concentration within a cycle; AUC(0-d28), area under the plasma concentration-time curve within the dosing interval of 28 days; SD, standard deviation.
A loading dose consisting of two doses on 2 consecutive days was administered in cycle 1.
Individual values.
Pharmacodynamic Findings
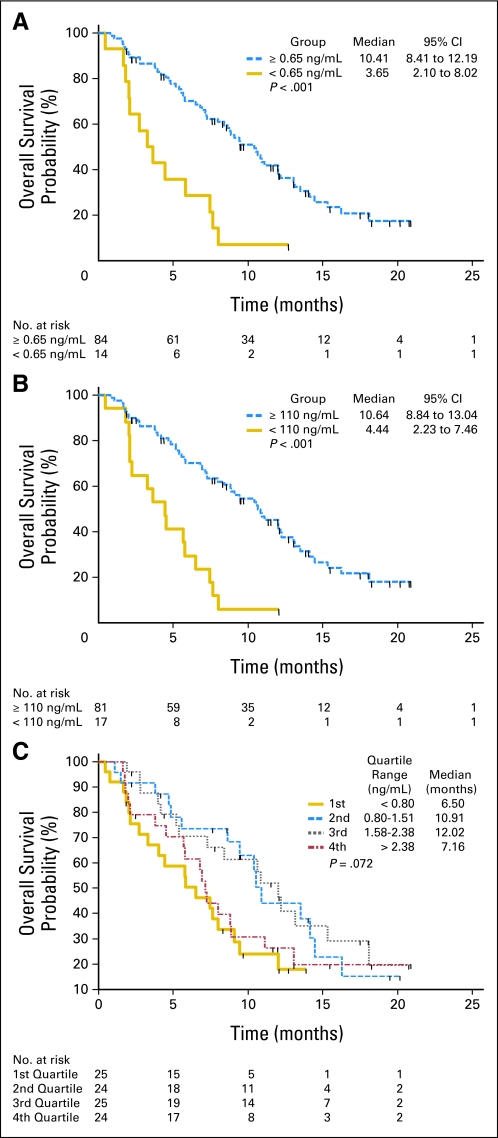
Sixty-five tumor tissue samples and 98 serum samples were provided in the phase 2 portion of the study. The characterization of IGF-1R expression and related proteins in the tumor samples is currently ongoing and will be described elsewhere. No association between translocation type and efficacy end point was identified. Of the 10 patients with objective response for whom translocation information was available, nine had the EWS-FLI (type I or II) translocation and one had EWS-ERG. Of the 37 nonresponders with available karyotype information, 32 had EWS-FLI and five had EWS-ERG. Pretreatment (cycle 1 day 1) serum samples were analyzed for total and free IGF-1 content. Median and 95% reference intervals for serum free and total IGF-1 were 1.55 (95% CI, 0.34 to 6.14) and 188.50 ng/mL (95% CI, 60.36 to 482.0), respectively. The association of these parameters with efficacy end points was investigated. No significant relationship between ORR and serum marker levels was observed. A late improvement of PFS in patients with elevated free IGF-1 was observed (log-rank P < .001) but the effect on median PFS was minor. In contrast, analysis of OS by baseline serum marker criteria revealed a number of key observations. Significant biomarker/treatment outcome associations were seen for free IGF-1 in the 0.4 to 0.85 ng/mL range and for total IGF-1 in the 60 to 130 ng/mL range, with the highest P values in the Cox hazards model observed for 0.65 ng/mL of free IGF-1 and 110 ng/mL of total IGF-1. Free IGF-1 was the most discriminating factor. Patients with a pretreatment circulating free IGF-1 level lower than 0.65 ng/mL (n = 14) had a median OS of 3.65 months (95% CI, 2.1 to 8.0) whereas those with baseline free IGF-1 concentration ≥ 0.65 ng/mL (n = 84) had a median OS of 10.4 (95% CI, 8.4 to 12.2) months (Fig 3A). The estimated OS hazard ratio between these free IGF-1 defined populations was 0.30 (95% CI, 0.16 to 0.56; log-rank P < .001). Similarly, patients with a pretreatment circulating total IGF-1 lower than 110 ng/mL (n = 17) had a median OS of 4.4 months (95% CI, 2.2 to 7.5) whereas those with a baseline total IGF-I ≥ 110 ng/mL (n = 81) had a median OS of 10.6 months (95% CI, 8.8 to 13.0; Fig 3B). The estimated OS hazard ratio between the total IGF-1 defined populations was 0.28 (95% CI, 0.15 to 0.51; log-rank P < .001). The agreement between both classification criteria (free IGF-1 < 0.65 or ≥ 0.65 ng/mL, and total IGF-1 < 110 or ≥ 110 ng/mL) was good (concordance kappa = 0.6) but the use of both criteria did not appear to improve the diagnostic value of using the free IGF-1 test alone (not shown). Also, the association of free IGF-1 to survival benefit did not appear to be the result of differences in baseline characteristics. Patients with baseline free IGF-1 levels lower than or ≥ 0.65 ng/mL had similar median age (19 v 18 years), prior therapy (35.7% v 42.9% received four or more previous regimens). Both subsets had similar rate of metastatic disease (100% v 96.4%) and tumor burden (57.1% v 72.6% had only one or two tumor lesions).
Fig 3.
Association of free and total insulin-like growth factor (IGF) with patient survival. (A) Kaplan-Meier plots for overall survival (OS) of patients with a baseline (cycle 1 day predose) free IGF-1 lower than 0.65 ng/mL (n = 14) versus ≥ 0.65 ng/mL (n = 84). (B) Kaplan-Meier plots for OS of patients with a baseline total IGF-1 lower than 110 ng/mL (n = 17) versus ≥ 110 ng/mL (n = 81). (C) Kaplan-Meier plots for OS by quartile of baseline (cycle 1 day predose) free IGF-1 levels.
The OS of patient cohorts defined by free IGF-1 quartiles was also investigated (Fig 3C). As expected, the lowest median OS (6.50 months) was observed in patients with the lowest free IGF-1 levels (first free IGF-1 quartile, < 0.8 ng/mL, 6.5 months). However, of note, patients with very high levels of free IGF-1 (fourth free IGF-1 quartile, > 2.3 ng/mL, 7.16 months) appeared to have a reduced clinical benefit related to those in the third or second free IGF-1 quartile (95% CI, 10.91 to 12.02 months), suggesting that while a threshold level of free IGF-1 may be needed to observe clinical benefit from figitumumab, excess free IGF-1 could be deleterious. Increases in growth hormone, total and free IGF-1, and insulin levels during the course of figitumumab treatment were observed as it has been described previously in other figitumumab studies.9,10 These results will be described elsewhere in detail.
DISCUSSION
Our study shows that figitumumab is well tolerated and has modest activity as a single agent in patients with relapsed and/or refractory ES. The response rate of 14.2% in addition to the considerable number of patients with stable disease (n = 25), and the median survival of 8.9 months, suggest that further evaluation of figitumumab would be of interest, especially if we consider the extremely poor outcome of these patients, the lack of new active agents, and high drug tolerability. While treatment was overall well tolerated, the observation of three events of leukemia in the current study is concerning. Cases of figitumumab-related leukemia have not been described previously in other types of cancer, and the risk for myelodysplasia and hematologic malignancies in chemotherapy-treated patients with ES is well known, suggesting that these events were likely related to prior treatment.11–13
Research to help identify the subsets of patients who could benefit from figitumumab treatment is crucial. An important observation of the study resulted from the analysis of OS with respect to pretreatment levels of total and free IGF-1. Patients with elevated free or total IGF-1 had significantly longer OS than those with low serum biomarker concentrations. Recent meta-analyses have confirmed that IGF-1 is a negative prognostic factor for multiple carcinomas, including those of prostate, breast, and colon.14,15 There is also evidence that high circulating IGF-1 levels increase the risk of cancer-related death.16 Peak ES incidence coincides with the lifetime highest levels of IGF-1 observed during puberty.17 Furthermore, a trend toward increased ES survival has been reported in patients with a high circulating IGF binding protein 3 to IGF-1 ratio, suggesting that high IGF-1 bioactivity may be associated to worse ES prognosis.18 In the absence of a control arm, we could not confirm a predictive value for high IGF-1 levels in figitumumab-treated patients with ES. However, our results are consistent with prior observations in other tumor types. For example, in a phase II clinical trial of paclitaxel and carboplatin (PC) with or without figitumumab (10 to 20 mg/kg every 3 weeks) in advanced non–small-cell lung cancer, patients with pretreatment free IGF-1 higher than 0.7 ng/mL (n = 46) had median PFS times of 2.63 months (PC alone), 3.97 months (PC + figitumumab 10 mg/kg) and 6.53 months (PC + figitumumab 20 mg/kg; P < .001), while no significant difference in PFS among treatment cohorts was observed in patients with baseline free IGF-1 ≤ 0.7 ng/mL (n = 64).19 An additional intriguing finding was the lower than expected OS observed in patients with very high levels of free IGF-1. This observation requires verification in larger series. Of note, figitumumab and IGF-1 have similar affinities for the IGF-1R and excess free IGF-1 has been shown experimentally to reverse the inhibitory effects of an anti-IGF-1R antibody.20,21 Thus, it is plausible that very high levels of free IGF-1 could compete with the ability of figitumumab to inhibit the IGF-1R. This could be of relevance to adolescent patients who could require higher figitumumab doses or the use of growth hormone antagonists to decrease overall IGF-1 levels.22 The latter hypothesis is being tested in a combination study of figitumumab and pegvisomant. Overall, our results support the need of further research of circulating IGF-1 and its bioactivity in figitumumab treated patients, in addition to conventional tumor-based biomarkers.
Acknowledgment
We thank A.M. Flanagan, MB, BCH, BAO, FRCPath, PhD, Royal National Orthopaedic Hospital, Stanmore, United Kingdom, for the analysis of Ewing sarcoma gene translocations.
Footnotes
Supported by Pfizer, by Public Health Service Grant No. ES015704, by the Department of Health's National Institute for Health Research Biomedical Research Centres funding scheme, and by the European Consortium for Innovative Therapies for Children with Cancer.
Presented in part at the 35th European Society of Medical Oncology Congress, Milan, Italy, October 8-10, 2010.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information can be found for the following: NCT00560235
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: Donghua Yin, Pfizer (C); Tao Wang, Pfizer (C); Stephanie Green, Pfizer (C); Luisa Paccagnella, Pfizer (C); Antonio Gualberto, Pfizer (C) Consultant or Advisory Role: None Stock Ownership: Donghua Yin, Pfizer; Tao Wang, Pfizer; Stephanie Green, Pfizer; Luisa Paccagnella, Pfizer Honoraria: None Research Funding: Heribert Juergens, Pfizer; Najat C. Daw, Pfizer; Stefano Ferrari, Pfizer; Milena Villarroel, Pfizer; Jeremy Whelan, Pfizer; Uta Dirksen, Pfizer Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Heribert Juergens, Najat C. Daw, Donghua Yin, Stephanie Green, Luisa Paccagnella, Antonio Gualberto
Provision of study materials or patients: Najat C. Daw, Isabelle Aerts, Uta Dirksen
Collection and assembly of data: Heribert Juergens, Najat C. Daw, Birgit Geoerger, Stefano Ferrari, Milena Villarroel, Isabelle Aerts, Jeremy Whelan, Uta Dirksen, Mary L. Hixon, Donghua Yin, Tao Wang, Stephanie Green, Luisa Paccagnella, Antonio Gualberto
Data analysis and interpretation: Heribert Juergens, Uta Dirksen, Mary L. Hixon, Donghua Yin, Tao Wang, Stephanie Green, Luisa Paccagnella, Antonio Gualberto
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Helman LJ, Meltzer P. Mechanisms of sarcoma development. Nat Rev Cancer. 2003;3:685–694. doi: 10.1038/nrc1168. [DOI] [PubMed] [Google Scholar]
- 2.Fletcher CDM, Unni KK, Mertens F. World Health Organisation Classification of Tumours: Pathology and Genetics of Tumours of Soft Tissue and Bone. Lyon, France: IARC Press; 2002. [Google Scholar]
- 3.Prieur A, Tirode F, Cohen P, et al. EWS/FLI-1 silencing and geneprofiling of Ewing cells reveal downstream oncogenic pathways and a crucial role for repression of insulin-like growth factor binding protein 3. Mol Cell Biol. 2004;24:7275–7283. doi: 10.1128/MCB.24.16.7275-7283.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Toretsky JA, Kalebic T, Blakesley V, et al. The insulin-like growth factor-I receptor is required for EWS/FLI-1 transformation of fibroblasts. J Biol Chem. 1997;272:30822–30827. doi: 10.1074/jbc.272.49.30822. [DOI] [PubMed] [Google Scholar]
- 5.Olmos D, Tan DS, Jones RL, et al. Biological rationale and current clinical experience with anti-insulin-like growth factor 1 receptor monoclonal antibodies in treating sarcoma: Twenty years from the bench to the bedside. Cancer J. 2010;16:183–194. doi: 10.1097/PPO.0b013e3181dbebf9. [DOI] [PubMed] [Google Scholar]
- 6.Gualberto A. Figitumumab (CP-751,871) for cancer therapy. Expert Opin Biol Ther. 2010;10:575–585. doi: 10.1517/14712591003689980. [DOI] [PubMed] [Google Scholar]
- 7.Olmos D, Postel-Vinay S, Molife LR, et al. Safety, pharmacokinetics, and preliminary activity of the anti-IGF-1R antibody figitumumab (CP-751,871) in patients with sarcoma and Ewing's sarcoma: A phase 1 expansion cohort study. Lancet Oncol. 2010;11:129–135. doi: 10.1016/S1470-2045(09)70354-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kurmasheva RT, Dudkin L, Billups C, et al. The insulin-like growth factor-1 receptor-targeting antibody, CP-751,871, suppresses tumor-derived VEGF and synergizes with rapamycin in models of childhood sarcoma. Cancer Res. 2009;69:7662–7671. doi: 10.1158/0008-5472.CAN-09-1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lacy MQ, Alsina M, Fonseca R, et al. Phase I, pharmacokinetic and pharmacodynamic study of the anti-insulinlike growth factor type 1 Receptor monoclonal antibody CP-751,871 in patients with multiple myeloma. J Clin Oncol. 2008;26:3196–3203. doi: 10.1200/JCO.2007.15.9319. [DOI] [PubMed] [Google Scholar]
- 10.Haluska P, Shaw HM, Batzel GN, et al. Phase I dose escalation study of the anti insulin-like growth factor-I receptor monoclonal antibody CP-751,871 in patients with refractory solid tumors. Clin Cancer Res. 2007;13:5834–5840. doi: 10.1158/1078-0432.CCR-07-1118. [DOI] [PubMed] [Google Scholar]
- 11.Bhatia S, Krailo MD, Chen Z, et al. Therapy-related myelodysplasia and acute myeloid leukemia after Ewing sarcoma and primitive neuroectodermal tumor of bone: A report from the Children's Oncology Group. Blood. 2007;109:46–51. doi: 10.1182/blood-2006-01-023101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rihani R, Bazzeh F, Faqih N, et al. Secondary hematopoietic malignancies in survivors of childhood cancer: An analysis of 111 cases from the Surveillance, Epidemiology, and End Result-9 registry. Cancer. 2010;116:4385–4394. doi: 10.1002/cncr.25313. [DOI] [PubMed] [Google Scholar]
- 13.Friedman DL, Whitton J, Leisenring W, et al. Subsequent neoplasms in 5-year survivors of childhood cancer: The Childhood Cancer Survivor Study. J Natl Cancer Inst. 2010;102:1083–1095. doi: 10.1093/jnci/djq238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Endogenous Hormones and Breast Cancer Collaborative Group. Key TJ, Appleby PN, Reeves GK, et al. Insulin-like growth factor 1 (IGF1), IGF binding protein 3 (IGFBP3), and breast cancer risk: Pooled individual data analysis of 17 prospective studies. Lancet Oncol. 2010;11:530–542. doi: 10.1016/S1470-2045(10)70095-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pollak M. Insulin and insulin-like growth factor signaling in neoplasia. Nat Rev Cancer. 2008;8:915–928. doi: 10.1038/nrc2536. [DOI] [PubMed] [Google Scholar]
- 16.Major JM, Laughlin GA, Kritz-Silverstein D, et al. Insulin-like growth factor-I and cancer mortality in older men. J Clin Endocrinol Metab. 2010;95:1054–1059. doi: 10.1210/jc.2009-1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Juul A, Flyvbjerg A, Frystyk J, et al. Serum concentrations of free and total insulin-like growth factor-I, IGF binding proteins -1 and -3 and IGFBP-3 protease activity in boys with normal or precocious puberty. Clin Endocrinol (Oxf) 1996;44:515–523. doi: 10.1046/j.1365-2265.1996.711531.x. [DOI] [PubMed] [Google Scholar]
- 18.Toretsky JA, Steinberg SM, Thakar M, et al. Insulin-like growth factor type 1 (IGF-1) and IGF binding protein-3 in patients with Ewing sarcoma family of tumors. Cancer. 2001;92:2941–2947. doi: 10.1002/1097-0142(20011201)92:11<2941::aid-cncr10072>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 19.Hixon ML, Gualberto A, Demers L, et al. Correlation of plasma levels of free insulin-like growth factor 1 and clinical benefit of the IGF-IR inhibitor figitumumab (CP- 751, 871) J Clin Oncol. 2009;27:155s. abstr 3539. [Google Scholar]
- 20.Cohen BD, Baker DA, Soderstrom C, et al. Combination therapy enhances the inhibition of tumor growth with the fully human anti-type 1 insulin-like growth factor receptor monoclonal antibody CP-751,871. Clin Cancer Res. 2005;11:2063–2073. doi: 10.1158/1078-0432.CCR-04-1070. [DOI] [PubMed] [Google Scholar]
- 21.Arteaga CL, Osborne CK. Growth inhibition of human breast cancer cells in vitro with an antibody against the type I somatomedin receptor. Cancer Res. 1989;49:6237–6241. [PubMed] [Google Scholar]
- 22.Gualberto A, Pollak M. Emerging role of insulin-like growth factor receptor inhibitors in oncology: Early clinical trial results and future directions. Oncogene. 2009;28:3009–3021. doi: 10.1038/onc.2009.172. [DOI] [PubMed] [Google Scholar]