Abstract
Purpose
The type 1 insulin-like growth factor 1 receptor (IGF-1R) has been implicated in the pathogenesis of the Ewing sarcoma family of tumors (ESFT). We conducted a multicenter phase II study of the fully human IGF-1R monoclonal antibody R1507 in patients with recurrent or refractory ESFT.
Patients and Methods
Patients ≥ 2 years of age with refractory or recurrent ESFT received R1507 at doses of 9 mg/kg intravenously one a week or 27 mg/kg intravenously every three weeks. Response was measured by using WHO criteria. Tumor imaging was performed every 6 weeks for 24 weeks and then every 12 weeks.
Results
From December 2007 through April 2010, 115 eligible patients from 31 different institutions were enrolled. The median age was 25 years (range, 8 to 78 years). The location of the primary tumor was bone in 57% of patients and extraskeletal in 43% of patients. A total of 109 patients were treated with R1507 9 mg/kg/wk, and six patients were treated with 27 mg/kg/3 wk. The overall complete response/partial response rate was 10% (95% CI, 4.9% to 16.5%). The median duration of response was 29 weeks (range, 12 to 94 weeks), and the median overall survival was 7.6 months (95% CI, 6 to 9.7 months). Ten of 11 responses were observed in patients who presented with primary bone tumors (P = .016). The most common adverse events of grades 3 to 4 were pain (15%), anemia (8%), thrombocytopenia (7%), and asthenia (5%).
Conclusion
R1507 was a well-tolerated agent that had meaningful and durable benefit in a subgroup of patients with ESFT. The identification of markers that are predictive of a benefit is necessary to fully capitalize on this approach.
INTRODUCTION
The Ewing sarcoma family of tumors (ESFT) comprise a group of malignancies that can arise in bone or soft tissue and are characterized molecularly by rearrangements of the EWS gene.1 Nearly 70% of patients with localized ESFT are cured of the disease when treated with combined-modality therapy that includes multiagent chemotherapy and local control measures such as radiotherapy, surgery, or both.2,3 However, approximately 20% of patients with ESFT present with metastatic disease, and less than 30% of patients are expected to be long-term survivors.1 Importantly, one half of long-term survivors experience long-term treatment related disabilities, which make less toxic effective therapies desirable.4
The type I insulin-like growth factor 1 receptor (IGF-1R) is a tyrosine kinase receptor that activates both mitogenic and antiapoptotic pathways after binding with one of its ligands, insulin-like growth factor 1 (IGF-1) or IGF-2.5 Most Ewing tumor cells have been noted to express increased levels of the IGF-1R or IGF-2 ligand.6 Although no mutations in either IGF ligands or the IGF-1R have been identified in ESFT, epigenetic alterations have been reported, and the loss of imprinting of IGF-2 has been commonly found.7 A specific blockade of the IGF-1R has been shown to inhibit ESFT xenograft tumor growth, including complete and persistent tumor regression in some xenografts, and enhance the activity of chemotherapy in other models.8,9 Thus, the involvement of the IGF pathway in the biology of ESFT makes it an attractive target. R1507 (F. Hoffman–La Roche, Basel, Switzerland), is a fully human IgG1 type monoclonal antibody that is directed against the human IGF-1R.
Responses to IGF-1R antibody were seen on phase I studies of AMG 479 (Amgen, Thousand Oaks, CA),10 figitumumab (Pfizer, New London, CT),11 and R1507.10,12 The initial clinical benefit coupled with the preclinical promise of this signaling pathway led to the joint collaborative program between the Sarcoma Alliance for Research through Collaboration (SARC) and Roche (Nutley, NJ) for the development of R1507 in patients with recurrent sarcoma including ESFT.13–15 This article describes the outcomes of patients with ESFT treated with R1507 in this study.
PATIENTS AND METHODS
Eligibility criteria included a centrally reviewed pathology, age ≥ 2 years, life expectancy of ≥ 6 weeks, Karnofsky/Lansky performance status ≥ 70%, bidimensionally measurable disease by computed tomography or magnetic resonance imaging, signed informed consent, adequate bone marrow, liver, and renal function. A molecular confirmation of ESFT diagnosis was not required for eligibility.
Patients with CNS disease must have been off glucocorticoids for ≥ 4 weeks without neurologic deficit. Patients must have completed previous surgery and systemic or radiation therapy ≥ 3 weeks before enrollment. Patients treated with brain irradiation must have must have completed therapy ≥ 6 weeks before enrollment. Contraception was required in appropriate patients. Exclusion criteria were as follows: significant unrelated systemic illness, poorly controlled diabetes, known hypersensitivity to R1507 or its components, treatment within 2 weeks of study entry with pharmacologic doses of corticosteroids or other immunosuppressive agents, previous therapy with IGF-1R inhibitors, history of solid organ transplantation or other malignancy within 5 years, and patients who were pregnant or breastfeeding.
Patients with ESFT were eligible for participation in one of two cohorts on the basis of the number of previous therapies. Patients in cohort 1 received two or more previous chemotherapy regimens and had ESFT that relapsed less than 24 months from diagnosis. Patients in cohort 2 only received one previous chemotherapy regimen or relapsed greater than 24 months from diagnosis.
On the basis of new pharmacokinetic data, with institutional review board/ethics approval, a third group was added to evaluate the efficacy and safety of a different dosing schedule. These patients could have had any number of previous therapies or recurred at any time point, but the age must have been ≥ 2 years and less than 21 years to enrich the evaluation in the pediatric population.
Drug administration.
R1507 was administered intravenously at a dose of 9 mg/kg in 100 mL of normal saline once a week. The third group received a dosing of R1507 27 mg/kg intravenously every 3 weeks. The initial treatment was administered over 90 minutes. In the absence of adverse reactions, subsequent doses of R1507 were administered over 60 minutes.
Laboratory and imaging studies included a baseline physical exam, CBC, chemistries, pregnancy test, ECG, antihuman antibodies, and tumor imaging. Total IGF-1 serum levels were measured by using an enzyme-linked immunosorbent assay at baseline and week 6. PET scans were performed at baseline and were repeated at day 9 and week 18 for responding patients. All imaging was centrally reviewed. Positron emission tomography scan data and extensive pharmacokinetic/pharmacodynamic studies will be reported in subsequent publications. CBC and chemistries were performed once a week for 6 weeks and then every 3 weeks. Tumor imaging was performed every 6 weeks for 24 weeks and then every 12 weeks.
Response to therapy was evaluated by using the WHO criteria. Patients with unconfirmed responses (observed at a single time point without subsequent confirmatory imaging) were not included in the objective response rate.
Off-study criteria included progressive disease, illness that prevented further therapy, unacceptable adverse events, patient withdrawal from the study, greater than 2 weeks that elapsed since the administration of R1507, or missing two or more consecutive doses, death, lost to follow-up, or investigator judgment.
Statistical methods.
ESFT patients were assigned to one of two treatment cohorts. A two-stage design was used in each cohort on the basis of the approach of Green and Dahlberg.16 For cohort 1 the primary end point was progression-free survival (PFS) at 18 weeks from the start of treatment. The planned sample size was 65 patients to allow for 92% power to detect the difference in PFS at 18 weeks between a null hypothesis of 10% versus an alternative of 25% by using a one-sided 2.6%-level test. An interim analysis after 20 patients was planned, with early stopping for negative results if none of the first 20 patients were progression free at 18 weeks. For cohort 2, the primary end point was the response rate by using WHO criteria. A maximum of 35 patients was planned, with early stopping for negative results if there were no responders in the first 20 patients. With this design, the power to detect the difference between a null hypothesis of a 10% response rate and an alternative of 30% was 87% with a one-sided 2%-level test.
Additional pharmacokinetic data were made available after the initiation of the study, and the 27-mg/kg dose of R1507 was considered potentially more favorable. Thus, the protocol was amended to enroll 30 patients age less than 21 years at the every 3-week schedule with a planned safety assessment after the enrollment of six patients. This exploratory cohort was felt to be sufficient to gain adequate toxicity data to allow for the use of this dose and schedule for future planned phase II and III trials at the time.
Overall survival (OS) was measured from the time of study registration to the date of death or was censored at the date of last contact. PFS was measured from the time of study registration to disease progression or death or was censored at the date of last contact. OS and PFS curves were estimated by the method of Kaplan and Meier.17 Demographic and pharmacodynamic variables were related in multivariate regression analyses to response via logistic regression and to OS by using Cox regression.18 All data were analyzed by using the intent-to-treat principle.
RESULTS
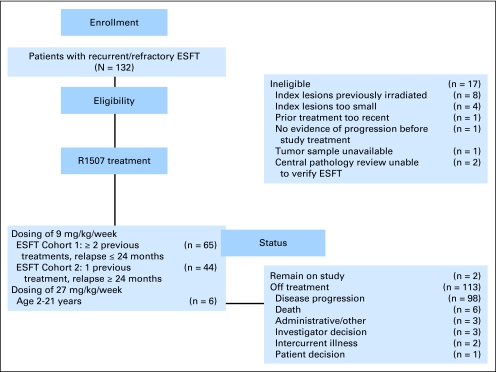
From December 18, 2007 through April 7, 2010, 132 patients were enrolled from 31 different institutions in North America, Europe, and Australia. Seventeen patients (13%) were not eligible as described in Figure 1. The median age at the time of enrollment was 25 years (range, 8 to 78 years). Twenty-three patients (20%) were less than 18 years of age, 54 patients (47%) were between 18 and 30 years of age, and 38 patients (33%) were ≥ 31 years of age. The primary tumor at the time of initial diagnosis in 57% of patients was skeletal, whereas 43% of patients had soft tissue/extraskeletal primary. The median number of previous systemic therapies in patients in cohort 1 was two regimens. The characteristics of patients are depicted in Table 1.
Fig 1.
CONSORT diagram of patients registered in the study. ESFT, Ewing sarcoma family of tumors.
Table 1.
Clinical Characteristics of 115 Eligible Patients With Recurrent ESFT
| Characteristic | Overall (N = 115) |
|
|---|---|---|
| No. of Patients | % | |
| Age at study entry, years | ||
| Median | 25 | |
| Minimum-maximum | 8-78 | |
| Age at diagnosis, years | ||
| Median | 21.9 | |
| Minimum-maximum | 1-77 | |
| Age > 18 years | 86 | 75 |
| Sex | ||
| M | 75 | 65 |
| F | 40 | 35 |
| Race | ||
| White | 86 | 75 |
| Asian | 7 | 6 |
| Other | 4 | 3 |
| Not provided* | 18 | 16 |
| Karnofsky/Lansky PS† | ||
| 70 | 25 | 22 |
| 80 | 31 | 27 |
| 90 | 40 | 35 |
| 100 | 18 | 16 |
| Primary tumor location | ||
| Bone | 65 | 57 |
| Soft tissue/extraskeletal | 50 | 43 |
| Time from initial diagnosis to first recurrence, months | ||
| Median | 16.7 | |
| Minimum-maximum | 1.2-175.6 | |
| Time from diagnosis to protocol enrollment, months | ||
| Median | 26.5 | |
| Minimum-maximum | 4.9-176.4 | |
| Sites of metastatic disease at time of enrollment | ||
| Lung | 40 | 35 |
| Bone | 16 | 14 |
| Visceral, other | 3 | 3 |
| Soft tissue | 20 | 17 |
| Multisystem | 36 | 31 |
Abbreviations: ESFT, Ewing sarcoma family of tumors; PS, performance score.
Race was not provided at all sites.
Not reported for one patient.
For cohort 1 (patients with ESFT with ≥ two previous treatments) and cohort 2 (patients with ESFT with one previous treatment regimen), the PFS at 18 weeks was 11% and 10%, and the overall response rate was 9% (no complete response [CR]; six partial response [PRs]) and 9% (one CR; three PRs), respectively (Table 2). Both cohorts were expanded to the second stage on the basis of the interim analysis. For the third group, the overall response rate was 17% (no CR; one PR). After trial inception, it was recognized that there was no distinction for these cohorts in relation to outcome for this study, and thus, additional outcome data in this article was combined for cohorts 1 and 2 as well as for patients treated with the amended dose of 27 mg/kg/3 wk.
Table 2.
Outcomes of Patients by Treatment Dose and Cohort
| Treatment Group | Response(WHO criteria) |
PFS Rate |
PFS (months) |
OS (months) |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PR |
CR |
Overall CR + PR |
18 Weeks |
6 Months |
1 Year |
|||||||||||
| No. | % | No. | % | No. | % | % | 95% CI | % | 95% CI | % | 95% CI | Median | 95% CI | Median | 95% CI | |
| Patients treated with R1507 9 mg/kg/wk | 6 | 9 | 0 | 6 | 9 | 11 | 3.7 to 17.8 | 9 | 2.8 to 15.7 | 2 | 0 to 4.5 | 1.3 | 1.2 to 1.4 | 6.8 | 4.7 to 8.6 | |
| ESFT cohort 1, n = 65 | ||||||||||||||||
| Patients treated with R1507 9 mg/kg/wk | 3 | 7 | 1 | 2.3 | 4 | 9 | 10 | 1.7 to 17.3 | 7 | 0.7 to 13.6 | 5 | 0.2 to 9.4 | 1.4 | 1.2 to 1.7 | 9.7 | 6.3 to 12.5 |
| ESFT cohort 2, n = 44 | ||||||||||||||||
| Patients treated with R1507 27 mg/kg/3 wk, n = 6 | 1 | 17 | 0 | 1 | 17 | 17 | 0 to 46.5 | 17 | 0 to 46.5 | 17 | 0 to 46.5 | 1.9 | 1.3 to 1.9 | Not reached | ||
| All patients | 10 | 8.7 | 1 | 1 | 11 | 10 | 11 | 5.2 to 16.1 | 9 | 3.9 to 13.8 | 3 | 0.4 to 9 | 1.3 | 1.2 to 1.4 | 7.6 | 6 to 9.7 |
Abbreviations: CR, complete response; ESFT, Ewing sarcoma family of tumors; OS, overall survival; PFS, progression-free survival; PR, partial response.
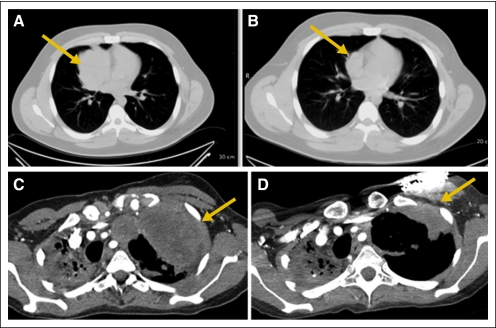
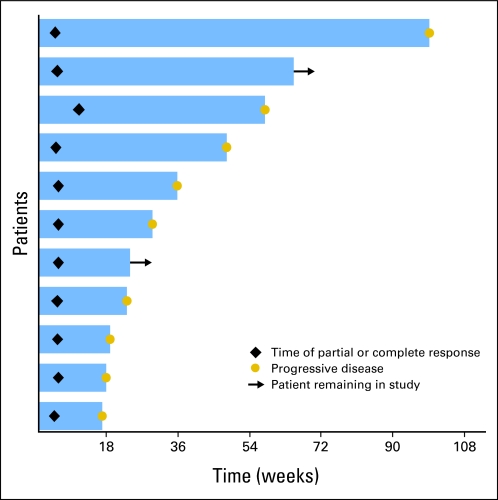
For all patients, the overall response rate was 10% (95% CI, 4.9% to 16.5%; one CR, 10 PRs; Table 2). The median duration of response was 29 weeks (range, 12 to 94 weeks; Appendix Fig A1, online only). In addition, eight patients had an unconfirmed PR that was characterized by a transient decrease in tumor size of ≥ 50% that was not confirmed by repeat imaging. Eighteen patients had stable disease that lasted from 5.6 to 30.1 weeks (median, 11.4 weeks). Several responses were quite dramatic as shown in Figure 4.
Fig 4.
Partial responses in patients with Ewing sarcoma family of tumors (ESFT). (A-B) A 17-year-old man with multiple recurrent EFST at (A) baseline and (B) after 6 weeks of treatment with R1507. (C-D) A 29-year-old woman with metastatic ESFT at (C) baseline and (D) after 12 weeks of treatment with R1507. Arrows indicate tumor.
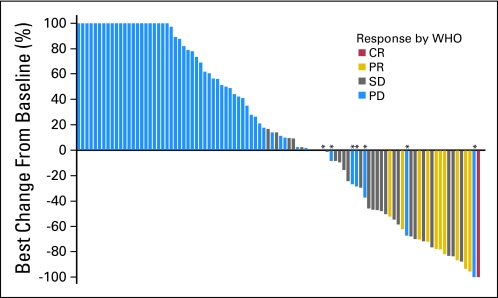
A waterfall plot of the best responses as the percentage decrease in tumor size by using WHO criteria of target lesions is depicted in Figure 2. The median duration of treatment was 6 weeks (range, 1 to 65 weeks).
Fig 2.
Waterfall plot of best change in target-lesion size from baseline. The best change in total lesion size of seven patients from baseline showed an overall decrease in total lesion size but were identified as partial disease (PD) for the following reasons: three patients had new lesions present at week 6, three patients had a target-lesion growth ≥ 25% at week 6, and one patient, despite showing an overall increase in total lesion size at weeks 6 to 18, continued on treatment and resolved to have no lesions present by week 36. CR, complete response; PR, partial response; SD, stable disease.
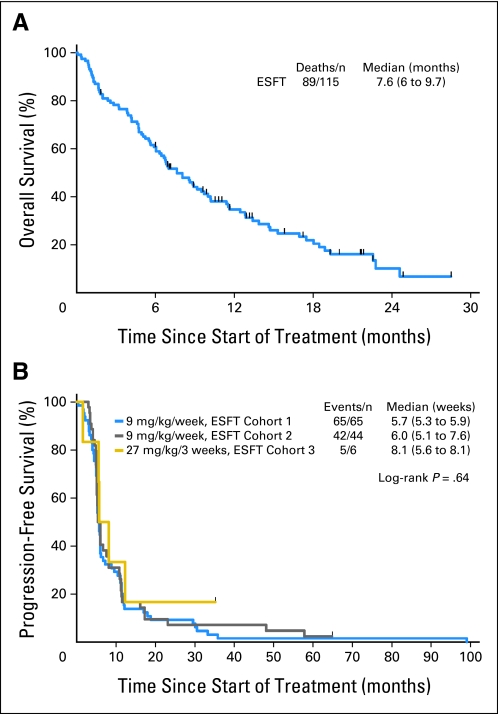
The median OS was 7.6 months (95% CI, 6 to 9.7 months; Fig 3). An analysis of factors predictive of response to R1507 identified that patients who initially presented with bone primaries were more likely to respond to antibody therapy compared with patients who had extraskeletal primary sites (10 of 11 responders had primary in bone; Fisher's exact test P = .016). Site of the tumor (axial v appendicular skeleton) and age did not predict for response.
Fig 3.
Kaplan-Meier estimation of (A) overall survival of all patients treated and (B) progression-free survival by cohort. ESFT, Ewing sarcoma family of tumors.
Adverse events that occurred at a frequency greater than 5% are depicted in Table 3. Grades 3 and 4 toxicities included pain (15%), anemia (8%), thrombocytopenia (7%), and fatigue (5%). Cardiac toxicities were not observed clinically, although specific cardiac imaging was only performed in patients who received the dosing schedule of 27 mg/kg/3 wk . Three patients had grade 3 or 4 hyperglycemia. Of the patients with grade 4 hyperglycemia (glucose > 500 mg/dL), one patient had type 1 diabetes mellitus and hyperglycemia, which was attributed to treatment, and the hyperglycemia of the other patient was attributed to the use of corticosteroids and not to the study treatment.
Table 3.
Adverse Events of Any Attribution That Occurred in > 5% of Patients
| Adverse Event | Toxicity Degree |
Total |
||||
|---|---|---|---|---|---|---|
| 1-2 |
3-4 |
|||||
| No. | % | No. | % | No. | % | |
| Hematologic | ||||||
| Anemia | 9 | 8 | 9 | 8 | 18 | 16 |
| Neutropenia | 7 | 6 | 0 | 7 | 6 | |
| Thrombocytopenia | 4 | 3 | 8 | 7 | 12 | 10 |
| Laboratory abnormalities | ||||||
| Albumin decreased | 7 | 6 | 0 | 7 | 6 | |
| Alkaline phosphatase elevation | 18 | 16 | 2 | 2 | 11 | 10 |
| Bilirubin elevation | 4 | 3 | 2 | 2 | 6 | 5 |
| Blood glucose elevation | 19 | 17 | 3 | 3 | 22 | 19 |
| Creatinine elevation | 7 | 6 | 0 | 7 | 6 | |
| Potassium decreased | 3 | 3 | 4 | 3 | 7 | 6 |
| Phosphate decreased | 7 | 6 | 1 | 1 | 8 | 7 |
| Transaminase elevation | 11 | 10 | 3 | 3 | 14 | 12 |
| Nonhematologic | ||||||
| Arthralgia | 10 | 9 | 1 | 1 | 11 | 10 |
| Appetite decreased | 23 | 20 | 1 | 1 | 24 | 21 |
| Constipation | 17 | 15 | 4 | 3 | 21 | 18 |
| Cough | 13 | 11 | 2 | 2 | 15 | 13 |
| Depression | 6 | 5 | 0 | 6 | 5 | |
| Diarrhea | 22 | 19 | 2 | 2 | 24 | 21 |
| Dyspnea | 16 | 14 | 2 | 2 | 18 | 16 |
| Edema | 5 | 4 | 1 | 1 | 6 | 5 |
| Fever | 20 | 17 | 1 | 1 | 21 | 18 |
| Fatigue | 37 | 32 | 6 | 5 | 43 | 37 |
| Headache | 26 | 23 | 0 | 26 | 23 | |
| Infusion reaction | 7 | 6 | 0 | 7 | 6 | |
| Insomnia | 6 | 5 | 0 | 6 | 5 | |
| Muscle aches | 18 | 16 | 0 | 18 | 16 | |
| Nausea/vomiting | 35 | 30 | 1 | 1 | 36 | 31 |
| Pain | 39 | 34 | 17 | 15 | 56 | 49 |
| Rash | 8 | 7 | 0 | 8 | 7 | |
| Somnolence | 7 | 6 | 1 | 1 | 8 | 7 |
| Weakness | 5 | 4 | 1 | 1 | 6 | 5 |
| Weight decreased | 12 | 10 | 1 | 1 | 13 | 11 |
| Urinary tract Infection | 6 | 5 | 1 | 1 | 7 | 6 |
Multivariate Cox regression analyses of the relationship of demographic and pharmacodynamic variables to OS revealed four factors that were predictive of survival (Appendix Table A1, online only). The evaluation of factors included only patients who contributed serum samples for IGF-1 measurement and, thus, was limited to 80 patients. The primary site in bone (v extraskeletal), Karnofsky/Lansky performance status ≥ 90%, and total IGF-1 ≥ 110 ng/mL were all associated with better survival. In addition, among patients with IGF-1 samples available at week 6 of the study, total IGF-1 ≥ 110 ng/mL at week 6 and a higher percentage increase in total IGF-1 from baseline to week 6 both predicted for survival. Other variables that were considered but not retained in multivariate analyses included age, sex, time from diagnosis to first recurrence, and presence of metastatic disease at study entry. None of these variables were significantly correlated with response as assessed via logistic regression.
DISCUSSION
In this study, we demonstrated that R1507 has modest but meaningful activity and was well tolerated in patients with recurrent or refractory ESFT. In our series, 10% of patients with recurrent or refractory ESFT had clinical responses that lasted for a median duration of greater than 6 months. Our results expanded on results of previous phase I studies of R1507, figitumumab, and AMG 479 and suggested that approximately 10% of patients with refractory ESFT benefited from this therapy.10,12
Although IGF-1R seems to be widely expressed in various sarcomas including ESFT,5,14 only a minority of our patients experienced clinically significant responses to R1507. One report suggested that, the level of expression of IGF-1R varied broadly in rhabdomyosarcoma cell lines, and cells with low levels of IGF-1R expression were unlikely to respond to an IGF-1R blockade.19 Intuitively, if a cancer cell does not have a receptor, it is unlikely to be affected by any interference with that receptor. Furthermore, in a report of breast cancer cell lines, IGF-1R expression and the number of IGF-1 receptors per cell predicted responses in vitro.20 Other factors implicated in resistance to anti–IGF-1R therapy include the overexpression of IGF binding proteins 5 and 6 in breast cancer, IGF binding proteins 3 and 6 in sarcomas, and insulin receptor signaling.21 These observations emphasized the need to develop more reliable biomarkers of response for appropriate patient selection. We are currently completing RNA-expression profiling in a subset of responding and nonresponding patients and will use these samples to perform array comparative genomic hybridization to correlate any expression changes with potential copy-number alterations at the DNA level. Global methylation patterns will also be compared.
Our study also demonstrated that patients who had tumor shrinkage could be categorized into two groups as follows: patients who had lasting objective responses (10% of patients; median, 29 weeks) and patients who had tumor reduction that was short-lived (7% of patients). Reasons for these differences are not well understood. Patients who had a long-lasting response and later developed disease progression likely developed drug resistance, perhaps as a result of a variety of factors including the upregulation of alternative signaling pathways and the reactivation of AKT.19,22 Conceivably, patients who had short-lived responses may not have received a sufficient dose and/or developed drug resistance more rapidly.
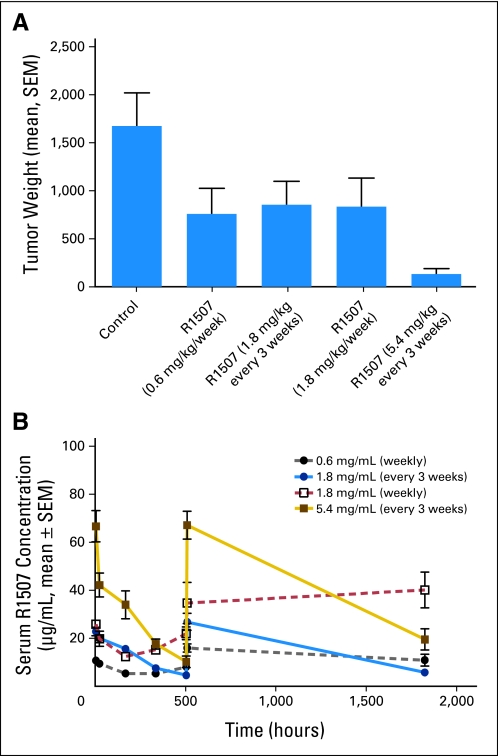
The drug dosing in this study deserves mention. The majority of patients on this study received R1507 9 mg/kg/wk. This was based on phase I studies that demonstrated that, in lung cancer, doses of R1507 3 or 9 mg/kg/wk could achieve a median half-life of 8 to 190 days and trough concentrations that exceeded 0.02 mg/mL, which was expected to saturate greater than 90% of receptors. The study moved forward on the basis of this initial data as well as responses seen in the phase I study. Ongoing pharmacokinetic studies performed by Roche during trial enrollment by using Ewing xenograft tumors indicated that higher serum peak levels were more important than the area under the curve when antibody doses were suboptimal. With insufficient dosing, the increase of IGF serum concentration on treatment as well as the concentration of autocrine IGF in the tumor may also determine the time point at which the antibody dose is too low to completely block binding of the ligand to the receptor. Preclinical ESFT xenograft models that used different treatment schedules demonstrated that a dosage of R1507 every third week was more efficacious than the same dose divided once a week (see Appendix Fig A2A, online only). The analysis of drug serum levels revealed that the goal trough level of 0.02 mg/mL was insufficient for maximum tumor response and that only higher peak levels in the three weekly dosing schedules could compensate for this (Appendix Fig A2B, online only).
Given these finding, if we had been able to complete accrual to the dosing schedule of 27 mg/kg/3 wk, perhaps we may have been able to observe improved patient outcomes at this dose. For business reasons, Roche decided to halt the development of R1507 and consequently terminated accrual to the ongoing study, which prevented additional exploration of this dosing schedule.
The design of this study was a collaborative effort between SARC and Roche, and ESFT patients were divided into two distinct cohorts on the basis of the number of previous therapies and time of relapse. Initially, it was believed that the natural history and response to treatment may have been unique to each cohort. Although the statistical plan was based on these distinctions, on final analysis, it was discovered that there was no significant difference in outcomes of either of these groups of patients. Thus, the majority of results were presented in this study as a combined aggregate of these patients. We now recognize that the number of previous therapies did not predict a response or lack of response to the targeted drug therapy in this population.
There were characteristics of the population treated in this study that deserve mention. For example, the median age at time of diagnosis was 21 years with only 20% of patients younger than 18 years of age at study entry and 43% of patients with soft tissue/extraskeletal primaries at diagnosis. In contrast, in studies of newly diagnosed patients with ESFT performed by the Children's Oncology Group, approximately 20% of patients had soft tissue primaries, and the median age at diagnosis was 12 years. This study reported an older population than usually reported on by pediatric groups. The presence of a soft-tissue primary tumor was associated with increased age at diagnosis. On multivariate analysis, the location of the primary tumor within the bone was predictive of both response and OS. Traditionally, skeletal ESFT and extraskeletal ESFT have been grouped together because of histology and fusion gene similarities, and no therapy has been shown to work in one subset versus another. Now, we recognize that there may be a therapy that indeed is preferential to ESFT of bony origin. Of note, although younger patients are more likely to have a primary tumor site that originates in bone, on multivariate analysis, age did not predict for response or survival.
In summary, R1507 had modest clinical activity in unselected patients with recurrent ESFT. Although the response rates observed in our study with single agent R1507 were similar to those reported with other single-agent monoclonal antibodies, such as the initial phase II report of trastuzumab for breast cancer, we have yet to identify the population who could potentially benefit from this class of drugs.23 If a predictive marker can be identified for this pathway, there can be a significant positive clinical impact on a subset of patients with ESFT treated with IGF-1R–targeted therapy. If we can increase this subset of patients by using optimal drug dosing and understanding, and perhaps even preventing, resistance mechanisms, the impact could be even greater.
Although the development of R1507 was halted, additional testing of anti–IGF-1R treatment in ESFT is warranted. SARC investigators are exploring avenues to secure other agents or partner to purchase one of these compounds.
Acknowledgment
Presented in part at the 45th Annual Meeting of the American Society of Clinical Oncology (ASCO), May 29-June 2, 2009, Orlando, FL, and 46th Annual Meeting of ASCO, June 4-8, 2010, Chicago, IL.
Appendix
Table A1.
Multivariate Cox Regression Analyses of Overall Survival in Patients With Available Serum Samples
| Characteristic | No. | % | HR | 95% CI | P* |
|---|---|---|---|---|---|
| Primary site in bone | 42 of 80 | 53 | 0.48 | 0.27 to 0.85 | .012 |
| KPS ≥ 90% | 41 of 80 | 51 | 0.58 | 0.34 to 0.99 | .047 |
| Total IGF-1 ≥ 110 ng/mL (baseline) | 68 of 80 | 85 | 0.27 | 0.13 to 0.55 | < .001 |
| Total IGF-1 ≥ 110 ng/mL at 6 weeks | 42 of 46 | 91 | 0.03 | 0.01 to 0.13 | < .001 |
| Percentage change in total IGF-1 from baseline to 6 weeks | 46 | 0.96 | 0.93 to 0.98 | .001 |
Abbreviations: HR, hazard ratio; IGF, insulin-like growth factor; KPS, Karnofsky performance status.
Wald χ2 test in Cox regression.
Fig A1.
Response duration among 11 patients who achieved a partial response or a complete response.
Fig A2.
Preclinical modeling of dose response in RD-ES xenograft tumors. (A) Xenograft tumors were exposed to R1507 at different concentrations and schedules. Decreased tumor weight was noted with treatment with R1507 at the dosing of 5.4 mg/kg/3 wk compared with the dosing of 1.8 mg/kg/wk, although the cumulative dose of drug administered was the same. (B) R1507 serum levels with different treatment schedules are shown. Higher peak levels were seen with the dosing of 5.4 mg/kg/3 wk.
Footnotes
Supported by F. Hoffman–La Roche, Basel, Switzerland.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information can be found for the following: NCT00642941.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: Denise K. Reinke, Sarcoma Alliance for Research through Collaboration Study (SARC; C); Klaus-Peter Kuenkele, Roche Diagnostics (C) Consultant or Advisory Role: Alberto S. Pappo, Ziopharm (C); Robert S. Benjamin, Eli Lilly (C), Amgen (C), Merck (C); Laurence H. Baker, Merck (U), The Hope Foundation (U) Stock Ownership: Robert S. Benjamin, Merck, Pfizer, Johnson and Johnson Honoraria: Robert S. Benjamin, Novartis, Jansen Research Funding: Shreyaskumar R. Patel, SARC; Sant P. Chawla, SARC; Robert G. Maki, SARC; Rashmi Chugh, SARC; Scott M. Schuetze, SARC; Robert S. Benjamin, SARC, Genentech, Amgen Expert Testimony: None Other Remuneration: Robert S. Benjamin, Jansen, Novartis, SARC
AUTHOR CONTRIBUTIONS
Conception and design: Alberto S. Pappo, Shreyaskumar R. Patel, John Crowley, Denise K. Reinke, Robert G. Maki, Paul A. Meyers, Robert S. Benjamin, Lee J. Helman, Laurence H. Baker
Administrative support: Denise K. Reinke, Rashmi Chugh
Provision of study materials or patients: Denise K. Reinke, Guy C. Toner, Robert G. Maki, Paul A. Meyers, Scott M. Schuetze, Heribert Juergens, Birgit Geoerger, Lee J. Helman
Collection and assembly of data: Alberto S. Pappo, Shreyaskumar R. Patel, John Crowley, Denise K. Reinke, Robert G. Maki, Rashmi Chugh, Kristen N. Ganjoo, Scott M. Schuetze, Michael G. Leahy, Birgit Geoerger, Lee J. Helman
Data analysis and interpretation: Alberto S. Pappo, Shreyaskumar R. Patel, John Crowley, Denise K. Reinke, Klaus-Peter Kuenkele, Sant P. Chawla, Guy C. Toner, Rashmi Chugh, Kristen N. Ganjoo, Heribert Juergens, Lee J. Helman, Laurence H. Baker
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Balamuth NJ, Womer RB. Ewing's sarcoma. Lancet Oncol. 2010;11:184–192. doi: 10.1016/S1470-2045(09)70286-4. [DOI] [PubMed] [Google Scholar]
- 2.Granowetter L, Womer R, Devidas M, et al. Dose-intensified compared with standard chemotherapy for nonmetastatic Ewing sarcoma family of tumors: A Children's Oncology Group Study. J Clin Oncol. 2009;27:2536–2541. doi: 10.1200/JCO.2008.19.1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grier HE, Krailo MD, Tarbell NJ, et al. Addition of ifosfamide and etoposide to standard chemotherapy for Ewing's sarcoma and primitive neuroectodermal tumor of bone. N Engl J Med. 2003;348:694–701. doi: 10.1056/NEJMoa020890. [DOI] [PubMed] [Google Scholar]
- 4.Ginsberg JP, Goodman P, Leisenring W, et al. Long-term survivors of childhood Ewing sarcoma: Report from the childhood cancer survivor study. J Natl Cancer Inst. 2010;102:1272–1283. doi: 10.1093/jnci/djq278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim SY, Toretsky JA, Scher D, et al. The role of IGF-1R in pediatric malignancies. Oncologist. 2009;14:83–91. doi: 10.1634/theoncologist.2008-0189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.LeRoith D, Werner H, Beitner-Johnson D, et al. Molecular and cellular aspects of the insulin-like growth factor I receptor. Endocr Rev. 1995;16:143–163. doi: 10.1210/edrv-16-2-143. [DOI] [PubMed] [Google Scholar]
- 7.Zhan S, Shapiro DN, Helman LJ. Loss of imprinting of IGF2 in Ewing's sarcoma. Oncogene. 1995;11:2503–2507. [PubMed] [Google Scholar]
- 8.Scotlandi K, Benini S, Nanni P, et al. Blockage of insulin-like growth factor-I receptor inhibits the growth of Ewing's sarcoma in athymic mice. Cancer Res. 1998;58:4127–4131. [PubMed] [Google Scholar]
- 9.Benini S, Manara MC, Baldini N, et al. Inhibition of insulin-like growth factor I receptor increases the antitumor activity of doxorubicin and vincristine against Ewing's sarcoma cells. Clin Cancer Res. 2001;7:1790–1797. [PubMed] [Google Scholar]
- 10.Tolcher AW, Sarantopoulos J, Patnaik A, et al. Phase I, pharmacokinetic, and pharmacodynamic study of AMG 479, a fully human monoclonal antibody to insulin-like growth factor receptor 1. J Clin Oncol. 2009;27:5800–5807. doi: 10.1200/JCO.2009.23.6745. [DOI] [PubMed] [Google Scholar]
- 11.Olmos D, Postel-Vinay S, Molife LR, et al. Safety, pharmacokinetics, and preliminary activity of the anti-IGF-1R antibody figitumumab (CP-751,871) in patients with sarcoma and Ewing's sarcoma: A phase 1 expansion cohort study. Lancet Oncol. 2010;11:129–135. doi: 10.1016/S1470-2045(09)70354-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kurzrock R, Patnaik A, Aisner J, et al. A phase I study of weekly R1507, a human monoclonal antibody insulin-like growth factor-I receptor antagonist, in patients with advanced solid tumors. Clin Cancer Res. 2010;16:2458–2465. doi: 10.1158/1078-0432.CCR-09-3220. [DOI] [PubMed] [Google Scholar]
- 13.Toretsky JA, Kalebic T, Blakesley V, et al. The insulin-like growth factor-I receptor is required for EWS/FLI-1 transformation of fibroblasts. J Biol Chem. 1997;272:30822–30827. doi: 10.1074/jbc.272.49.30822. [DOI] [PubMed] [Google Scholar]
- 14.Kim SY, Wan X, Helman LJ. Targeting IGF-1R in the treatment of sarcomas: Past, present and future. Bull Cancer. 2009;96:E52–60. doi: 10.1684/bdc.2009.0915. [DOI] [PubMed] [Google Scholar]
- 15.Kurmasheva RT, Houghton PJ. IGF-I mediated survival pathways in normal and malignant cells. Biochim Biophys Acta. 2006;1766:1–22. doi: 10.1016/j.bbcan.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 16.Green SJ, Dahlberg S. Planned versus attained design in phase II clinical trials. Stat Med. 1992;11:853–862. doi: 10.1002/sim.4780110703. [DOI] [PubMed] [Google Scholar]
- 17.Kaplan AL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 18.Cox DR. Regression models and life tables. J Royal Stat Soc Series B. 1972;34:187–202. [Google Scholar]
- 19.Cao L, Yu Y, Darko I, et al. Addiction to elevated insulin-like growth factor I receptor and initial modulation of the AKT pathway define the responsiveness of rhabdomyosarcoma to the targeting antibody. Cancer Res. 2008;68:8039–8048. doi: 10.1158/0008-5472.CAN-08-1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zha J, O'Brien C, Savage H, et al. Molecular predictors of response to a humanized anti-insulin-like growth factor-I receptor monoclonal antibody in breast and colorectal cancer. Mol Cancer Ther. 2009;8:2110–2121. doi: 10.1158/1535-7163.MCT-09-0381. [DOI] [PubMed] [Google Scholar]
- 21.Hendrickson AW, Haluska P. Resistance pathways relevant to insulin-like growth factor-1 receptor-targeted therapy. Curr Opin Investig Drugs. 2009;10:1032–1040. [PubMed] [Google Scholar]
- 22.Huang F, Greer A, Hurlburt W, et al. The mechanisms of differential sensitivity to an insulin-like growth factor-1 receptor inhibitor (BMS-536924) and rationale for combining with EGFR/HER2 inhibitors. Cancer Res. 2009;69:161–170. doi: 10.1158/0008-5472.CAN-08-0835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baselga J, Tripathy D, Mendelsohn J, et al. Phase II study of weekly intravenous trastuzumab (Herceptin) in patients with HER2/neu-overexpressing metastatic breast cancer. Semin Oncol. 1999;26(4 suppl 12):78–83. [PubMed] [Google Scholar]