Abstract
Purpose
Given that smoking affects body mass index (BMI) and survival, stratification by smoking status may be required to determine the true prognostic impact of BMI. Although obesity increases risk for developing esophageal adenocarcinoma (EAC), the prognostic influence of obesity and its potential modification by smoking status is unknown in this disease.
Patients and Methods
All patients (N = 778) underwent potentially curative esophagectomy. BMI was calculated using measured height and weight at surgery and categorized as obese (≥ 30 kg/m2), overweight (25 to 29.9 kg/m2), or normal (18.5 to 24.9 kg/m2). Cigarette smoking was categorized as never or ever. The association of BMI with disease-specific survival (DSS), disease-free survival (DFS), and overall survival (OS) was determined by Cox regression.
Results
Excess BMI was significantly associated with DSS in a manner that differed substantially by smoking status (P for interaction = .023). Among never smokers, obesity was significantly associated with adverse DSS (hazard ratio [HR] = 2.11; 95% CI, 1.31 to 3.43; P = .002), DFS (HR = 2.03; 95% CI, 1.30 to 3.18; P = .002), and OS (HR = 1.97; 95% CI, 1.24 to 3.14; P = .004), as compared with normal weight, after adjusting for covariates. By contrast, among ever smokers, obesity was not prognostic, and overweight status was significantly associated with favorable survival in univariate, but not multivariate, analysis.
Conclusion
Obesity among never smokers was independently associated with two-fold worsening of DSS, DFS, and OS after surgery for EAC, after adjusting for known prognostic factors. These data, in one of the largest reported resected EAC cohorts, are the first to show an adverse prognostic impact of obesity in EAC.
INTRODUCTION
The incidence of esophageal adenocarcinoma, including tumors of the gastroesophageal junction (GEJ) and gastric cardia (collectively referred to as esophageal adenocarcinoma [EAC]1–3), is one of the fastest rising in the United States, paralleling the increase in obesity.4 Despite the use of aggressive therapy consisting primarily of surgical resection, EAC remains highly fatal.5 Among US males, esophageal cancer had the highest death rate among malignancies with increasing mortality trends from 1990 to 2006 and ranks as the fifth leading cause of cancer death in ages 40 to 79 years.6 Although a higher body mass index (BMI) increases the risk of developing EAC, it is unknown whether obesity affects survival in patients with EAC.7–10 Such information could inform patient prognosis and facilitate the development of novel therapeutic strategies. Under the hypotheses that hyperinsulinemia and other metabolic derangements linked to adiposity may promote tumor growth and progression, obesity has been associated with adverse outcome in other cancers, including the pancreas and colon.11–16 Yet, studies in patients with EAC have reported that excess BMI has a null, or even protective, effect on prognosis.
Examination of BMI and survival has increasingly drawn attention to the importance of accounting for cigarette smoking status. Smoking is known to affect BMI and all-cause mortality and has also been shown to attenuate the increase in relative mortality due to excess BMI, with multiple population-based cohort studies showing stronger associations between obesity and mortality in never, as compared with ever, smokers.17–20 Accordingly, the true prognostic impact of BMI may be understood only after stratifying by smoking status or excluding smokers.17–20 The effect of smoking in modulating the association between BMI and mortality may be more pronounced in smoking-related cancers such as EAC, because smoking has been associated with higher risk of developing EAC21 and is common in patients with EAC.22–24
The study of BMI and outcome in EAC has been impeded by several factors. EAC has been conflated with subcardial gastric cancers or esophageal squamous cell carcinomas, both of which are epidemiologically and clinically distinct from EAC.1,2,25 Among patients from the same study, body weight has been ascertained at variable times relative to diagnosis or therapy, or has been estimated, rather than measured. Studies have generally been small in size and number and have infrequently accounted for smoking status.20,23,24,26,27 In addition, EAC study populations have received a diverse set of therapies, commonly including preoperative chemotherapy and/or radiation, which can profoundly affect nutritional intake and BMI.28
To address these questions, we analyzed BMI while stratifying by smoking status in a large cohort of patients with EAC that was homogeneous by histology and limited to the esophagus, GEJ, or gastric cardia. Height and weight were measured at uniform time points relative to diagnosis and surgery. In addition, we limited the cohort to patients who underwent surgical resection before 1998, before perioperative chemotherapy and/or radiotherapy were routinely used for resectable disease at our institution and other US medical centers.29
PATIENTS AND METHODS
Study Cohort
Our study cohort originates from the Mayo Esophageal Cancer Outcomes Database, which has been previously described.30 Briefly, this cohort consists of all adult patients with newly diagnosed, pathologically confirmed adenocarcinoma of the esophagus, GEJ, or gastric cardia who underwent surgical resection with cancer-free margins at Mayo Clinic in Rochester, MN (January 1, 1980, to December 31, 1997). Subcardial gastric cancers and tumors with only nonadenocarcinoma histology were excluded. The database was developed using clinical information systematically extracted from medical records by trained physicians using a standardized abstraction form. Independent data review was performed for quality assurance, and all data entry was checked for accuracy by administrative research staff. A total of 796 patients met all inclusion criteria and comprised the parent study cohort.
Collection of BMI and Smoking Data
Height and weight was measured at Mayo on all patients at the time of esophagectomy. Data on cigarette smoking and weight loss in the year before surgery were prospectively collected by clinical staff on patients undergoing esophagectomy at Mayo. Within 2 weeks before their surgery, patients completed a written institutional questionnaire asking specifically about current31 and past cigarette use and changes in weight. Each patient's responses were verified on the date of questionnaire completion during a visit with their Mayo primary care physician.
Of 796 patients in the parent cohort, nine patients received neoadjuvant chemo- and/or radiotherapy and were excluded. Of remaining patients, nine were underweight (BMI < 18.5 kg/m2) and were excluded. Therefore, 778 patients comprise the current study population.
Clinical End Points and Statistical Analysis
Our primary clinical outcome variable was disease-specific survival (DSS), defined as the time from surgery to death related to EAC, censored at the date of death resulting from postoperative complications or other nonmalignant causes. Secondary end points included disease-free survival (DFS) and overall survival (OS). OS was defined as the time from surgery to death resulting from any cause and DFS as the time from surgery to the first recurrence of index cancer or to all-cause death. Death beyond 5 years was censored.
World Health Organization cut points were used to categorize patients by BMI as very obese (≥ 35 kg/m2), obese (≥ 30.0 to 34.9 kg/m2), overweight (≥ 25.0 to 29.9 kg/m2), and normal weight (≥ 18.5 to 24.9 kg/m2).32 Given the small number of very obese patients, very obese and obese groups were analyzed in combination. Kaplan-Meier and Cox proportional hazards models were used to assess the association between predictor variables and outcomes. Wilcoxon rank sum and χ2 tests were used to compare continuous and categorical variables between groups, respectively. To assess whether the effect of BMI on DSS was modified by smoking status, an interaction term (ie, the product of categorical BMI and smoking status [ever v never]) was included in the Cox model in addition to BMI and smoking status. The primary multivariate Cox model included tumor stage and grade (American Joint Committee on Cancer criteria, 7th edition, 2009). Age, sex, and presurgery weight loss (≥ 10%) were added in subsequent models33–35; given that results were highly stable, multivariate models containing all covariates are reported. For ever smokers, number of pack-years was also included as indicated. Hazard ratios (HRs) with 95% CIs and two-sided P values were reported. P ≤ .05 was considered statistically significant. Analyses were conducted in SAS version 9.1 (SAS Institute, Cary, NC). Study data were collected using REDCap electronic data capture tools hosted at Mayo.36 The study was approved by the Mayo institutional review board.
RESULTS
Overall Study Population
The study population (N = 778) is from the Mayo Esophageal Cancer Outcomes Database as described.30 Baseline clinicopathologic characteristics are shown in Table 1. In brief, all patients had adenocarcinomas and underwent surgery at Mayo with cancer-free margins. Most patients were male (89%) and had locally advanced tumor stage (80% were IIB to IIIC). By anatomic site, 279 tumors (36%) were esophageal, 458 (59%) were GEJ, and 40 (5%) were limited to the gastric cardia. The nine patients who were underweight were excluded. No patient received neoadjuvant therapy, and 625 patients (80%) did not receive postoperative adjuvant therapy. Postoperative chemotherapy alone, radiotherapy alone, and concurrent chemoradiotherapy was delivered in 27 (3.5%), 23 (3.0%), and 53 (6.8%) patients, respectively; adjuvant therapy status was unknown in 50 patients (6.3%). Chemotherapy agents delivered in various combinations included fluorouracil (n = 67), doxorubicin (n = 27), cisplatin (n = 7), mitomycin (n = 5), carboplatin (n = 1), etoposide (n = 1), and not recorded or other (n = 7). Ivor-Lewis, transhiatal, and other surgeries were performed in 546 (70%), 112 (14%), and 120 (15%) patients, respectively. Median follow-up for assessment of vital status and disease recurrence was 12.9 and 5.0 years, respectively.
Table 1.
Baseline Characteristics at Surgery by Smoking Status in Patients With Esophageal Adenocarcinoma
| Characteristic | Overall (N = 778) |
Never Smokers(n = 236) |
Ever Smokers(n = 542) |
P | |||
|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | ||
| Sex | |||||||
| Male | 692 | 89 | 192 | 81 | 500 | 92 | < .001 |
| Female | 86 | 11 | 44 | 19 | 42 | 8 | |
| Age, years | < .001 | ||||||
| Median | 65 | 68 | 64 | ||||
| Range | 22–89 | 22–89 | 30–88 | ||||
| Stage | |||||||
| IIIC | 127 | 17 | 38 | 16 | 89 | 17 | .138 |
| IIIB | 173 | 23 | 56 | 24 | 117 | 22 | |
| IIIA | 160 | 21 | 40 | 17 | 120 | 23 | |
| IIB | 144 | 19 | 55 | 24 | 89 | 17 | |
| IA to IIA | 156 | 20 | 44 | 19 | 112 | 22 | |
| Grade | |||||||
| 4 | 300 | 39 | 84 | 36 | 216 | 41 | .225 |
| 1 to 3 | 467 | 61 | 150 | 64 | 317 | 59 | |
| Weight loss in the year preceding surgery | |||||||
| ≥ 10% | 174 | 22 | 60 | 25 | 114 | 21 | .177 |
| < 10% | 604 | 78 | 176 | 75 | 428 | 79 | |
| Body mass index | |||||||
| Obese | 171 | 22 | 46 | 19 | 125 | 23 | .192 |
| Overweight | 348 | 45 | 117 | 50 | 231 | 43 | |
| Normal | 259 | 33 | 73 | 31 | 186 | 34 | |
Smoking status, and measured height and weight at surgery, was available for all patients. Among never smokers (n = 236), 46 (19%) were obese, 117 (50%) were overweight, and 73 (31%) had normal weight. Among ever smokers (n = 542; median 37 pack-years [range, 1 to 280]), 125 (23%) were obese, 231 (43%) were overweight, and 186 (34%) had normal weight. Never (v ever) smoking status was associated with older age and female sex, consistent with prior literature37,38 (Table 1).
BMI, Smoking, and Survival
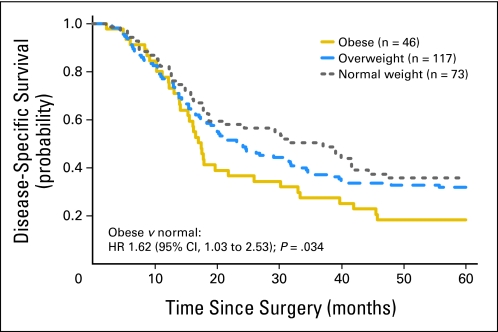
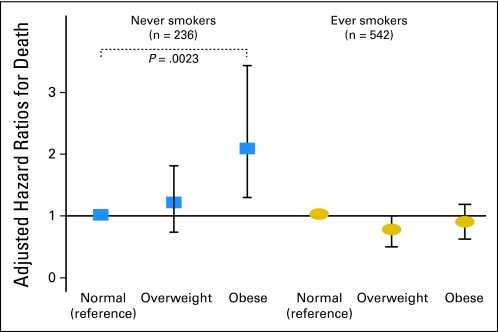
For DSS, the BMI × smoking interaction term was significant (P = .023), indicating that the prognostic impact of excess BMI differed significantly on the basis of smoking status. Among never smokers (n = 236), univariate analysis revealed that obese patients had significantly shorter DSS as compared with normal-weight patients (HR = 1.62; 95% CI, 1.03 to 2.53; P = .034; Fig 1). Overweight patients did not have significantly different DSS as compared with normal-weight patients. In this regard, among never smokers, only 18% of obese patients had not died as a result of EAC 5 years after surgery, as compared with 32% of overweight and 36% of normal weight patients (Tables 2 and 3). Other covariates were not associated with DSS (Table 2). For DFS, among never smokers, obesity was associated with worse DFS (HR = 1.48; 95% CI, 0.97 to 2.25), as compared with normal weight, though this association did not reach statistical significance (P = .066). Overweight status did not confer worse DFS as compared with normal weight. Univariate results were similar for OS (data not shown). In multivariable analysis among never smokers, obesity was significantly associated with adverse DSS (HR = 2.11; 95% CI, 1.31 to 3.43; P = .002), DFS (HR = 2.03; 95% CI, 1.30 to 3.18; P = .002), and OS (HR = 1.97; 95% CI, 1.24 to 3.14; P = .004), as compared with normal weight, after adjusting for tumor stage, grade, age, presurgical weight loss, and sex (Table 3, Fig 2). By contrast, overweight status was not significantly associated with adverse DSS, DFS, or OS, as compared with normal weight among never smokers (Table 3, Fig 2). Results were similar after adjustment for the presence of Barrett's esophagus and surgery type (data not shown).
Fig 1.
Disease-specific survival in never smokers after curative resection for esophageal adenocarcinoma according to body mass index at the time of surgery (n = 236). HR, hazard ratio.
Table 2.
Disease-Specific Survival According to Clinicopathologic Variables in Univariate and Multivariate Analysis Among Never Smokers (n = 236)
| Clinicopathologic Variable | Univariate |
Multivariate* |
||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |
| Sex | ||||||
| Male | 1.57 | 1.00 to 2.47 | .049 | 1.25 | 0.76 to 2.05 | .370 |
| Female | Ref | Ref | ||||
| Age, per year increase | 1.00 | 0.99 to 1.02 | .841 | 1.01 | 0.99 to 1.02 | .378 |
| Stage | ||||||
| IIIC | 14.16 | 6.73 to 29.81 | < .0001 | 11.98 | 5.60 to 25.63 | < .0001 |
| IIIB | 10.55 | 5.11 to 21.79 | < .0001 | 11.19 | 5.32 to 23.54 | < .0001 |
| IIIA | 7.96 | 3.81 to 16.64 | < .0001 | 7.57 | 3.59 to 15.98 | < .0001 |
| IIB | 3.96 | 1.80 to 8.30 | .0003 | 4.12 | 1.96 to 8.66 | .0002 |
| IA to IIA | Ref | Ref | ||||
| Grade | ||||||
| 4 | 1.61 | 1.17 to 2.22 | .0038 | 1.17 | 0.83 to 1.65 | .374 |
| 1 to 3 | Ref | Ref | ||||
| Weight loss in the year preceding surgery | ||||||
| ≥ 10% | 1.68 | 1.19 to 2.38 | .0035 | 1.38 | 0.96 to 1.99 | .081 |
| < 10% | Ref | Ref | ||||
| Body mass index | ||||||
| Obese | 1.62 | 1.03 to 2.53 | .034 | 2.11 | 1.31 to 3.43 | .0023 |
| Overweight | 1.17 | 0.81 to 1.71 | .403 | 1.27 | 0.86 to 1.88 | .231 |
| Normal | Ref | Ref | ||||
Abbreviations: HR, hazard ratio; Ref, reference (HR = 1.0).
Adjusted for all variables shown in table.
Table 3.
Survival According to Body Mass Index Stratified by Smoking Status in Multivariable Models Adjusted for Age, Stage, Grade, Weight Loss,and Sex (N = 778)
| Body Mass Index | No. | Disease-Specific Survival |
Disease-Free Survival |
Overall Survival |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Event-Free Rate, % | HR | 95% CI | P | Event-Free Rate, % | HR | 95% CI | P | Event-Free Rate, % | HR | 95% CI | P | ||
| Never smokers (n = 236) | |||||||||||||
| Obese | 46 | 18 | 2.11 | 1.31 to 3.43 | .002 | 15 | 2.03 | 1.30 to 3.18 | .002 | 17 | 1.97 | 1.24 to 3.14 | .004 |
| Overweight | 117 | 32 | 1.27 | 0.86 to 1.88 | NS | 28 | 1.16 | 0.81 to 1.67 | NS | 30 | 1.17 | 0.81 to 1.70 | NS |
| Normal | 73 | 36 | Ref | 28 | Ref | 32 | Ref | ||||||
| Ever smokers (n = 542) | |||||||||||||
| Obese | 125 | 33 | 0.90 | 0.67 to 1.20 | NS | 27 | 1.00 | 0.76 to 1.33 | NS | 30 | 0.90 | 0.68 to 1.20 | NS |
| Overweight | 231 | 39 | 0.78 | 0.61 to 1.02 | NS | 34 | 0.87 | 0.68 to 1.11 | NS | 37 | 0.80 | 0.62 to 1.02 | NS |
| Normal | 186 | 28 | Ref | 23 | Ref | 26 | Ref | ||||||
Abbreviations: HR, hazard ratio; NS, not statistically significant; Ref, reference (HR = 1.0).
Fig 2.
Adjusted hazard ratios (HRs) for disease-specific survival after curative resection in 778 patients with esophageal adenocarcinoma according to body mass index (BMI) at the time of surgery, stratified by smoking status (P value for interaction between BMI and smoking status = .023). HRs are adjusted for tumor stage, grade, age, presurgical weight loss, and sex; 95% CIs and only significant P values (< .05) are shown.
In univariate analysis among ever smokers (n = 542), overweight status was significantly associated with longer DSS (HR = 0.72; 95% CI, 0.57 to 0.92; P = .008), DFS (HR = 0.76; 95% CI, 0.60 to 0.96; P = .020), and OS (HR = 0.74; 95% CI, 0.58 to 0.94; P = .012), as compared with normal weight. Obese patients did not have significantly different DSS, DFS, or OS as compared with normal-weight patients. In this regard, among ever smokers, 39% of overweight patients had not died as a result of EAC 5 years after surgery, as compared with 33% of obese and 28% of normal weight patients (Table 3). However, in multivariable analysis among ever smokers, the association of overweight status with DSS, DFS, and OS showed only marginal statistical significance. Among ever smokers, obesity was not associated with survival in multivariate analysis (Table 3, Fig 2). For all analyses among ever smokers, results were similar after adjustment for pack-years (data not shown).
Sensitivity analysis in the subgroup of patients (n = 625) who did not receive postoperative chemo- or radiotherapy yielded stable results: Among never smokers, obesity was associated with significantly worse DSS, as compared with normal weight (adjusted HR = 1.98; 95% CI, 1.15 to 3.42; P = .014).
DISCUSSION
Cigarette smoking status has been shown to attenuate the effect of BMI on mortality in prospective cohort studies, including death from multiple types of cancer.17–20 However, smoking status has been infrequently accounted for in studies of EAC patients.26,27,39 Therefore, we examined the potential interaction between BMI and smoking in a large cohort of patients with EAC who underwent potentially curative esophagectomy. We found that the prognostic impact of excess BMI significantly differed on the basis of smoking status (P for interaction = .023). This interaction indicates that stratification by or exclusion of smokers is necessary to determine the true prognostic impact of excess BMI and that simply adjusting for smoking as a confounder may fail to reveal this association in EAC. In never smokers, we found that obesity was associated with a two-fold increase in risk of death owing to EAC after adjusting for covariates. This adverse impact was limited to never smokers. By contrast, obesity was not prognostic among ever smokers and, in these patients, overweight status was shown to confer a protective effect of borderline statistical significance. Multiple studies have shown a favorable prognosis in overweight subjects,40,41 particularly in men,20 which may be related to limitations of BMI versus direct measurements of body fat.42 Among never—but not ever—smokers, obesity doubled the risk of EAC recurrence or all-cause death. These novel observations underscore the importance of smoking status in the interpretation of BMI data and, to our knowledge, represent the first data showing that obesity adversely impacts survival in patients with EAC.
A complex interaction between BMI and smoking status has been increasingly appreciated, as smoking has been shown to be associated with reduced BMI and increased mortality risk.17–19,43 Consistent with our findings in patients with EAC, stronger associations between obesity and cancer-related or all-cause mortality have been observed in never, as compared with ever, smokers in multiple prospective cohort studies.17–20 These studies indicate that the prognostic impact of BMI on mortality may be underestimated when combining data for smokers and nonsmokers, whereas mortality rates for never smokers seem to more accurately estimate the true prognostic impact of obesity. Accordingly, these findings17–20,43,44 support stratification by smoking status as performed in our study.
Studies in EAC and other cancers have shown that overweight status and/or obesity is associated with favorable survival as compared with normal weight, although results have been inconsistent.23,24,26 We found that overweight patients had favorable prognosis compared with normal weight patients that was of borderline statistical significance and limited to ever smokers. Notably, most patients with upper digestive cancers are overweight, not obese,45,46 and are ever smokers.47,48 In contrast to other studies, Cancer Prevention Study II (CPS II), a population-based US study, found a significant upward trend in cancer-related mortality risk as BMI increased above normal weight in patients with esophageal and gastric cancers.20 However, unlike our study, CPS II included squamous cell carcinomas in its upper digestive cancer subpopulation, weight was collected at various time points relative to cancer diagnosis, and patients had heterogeneous stage and therapy. By contrast, our study cohort was homogeneous in that all patients had adenocarcinomas, and subcardial gastric cancers were excluded. Furthermore, body weight was recorded at cancer diagnosis, and all patients underwent potentially curative esophagectomy. The majority of our study patients were male, which is consistent with the male predominance of EAC in the general population,6,49 and may potentially limit the applicability of our findings to women. In CPS II, the associations between obesity and cancer mortality were strongest in men, and a greater effect of excess weight on all-cause mortality in men has been observed in some,18,19 but not other,17,43 studies.
Mechanistic evidence has been reported, which may help explain the complex interaction between adiposity, smoking, nicotine, and tumor progression. Adiposity in overweight and obese patients has been linked to hyperinsulinemia and upregulation of insulin growth factor signaling and proinflammatory mediators, which may create a more favorable microenvironment for tumor cell survival, proliferation, and progression.50 In addition, insulin-resistant adipocytes can activate macrophages that then release proinflammatory molecules, including tumor necrosis factor-α and interleukin-6, which in turn promote tumor growth.51–53 In contrast to the carcinogenic effects of smoking, nicotine exposure may create a tumor microenvironment that is less favorable for progression of some tumor types. In this regard, nicotine exposure in both genetically obese and diet-induced obese mice has been shown to improve insulin sensitivity and to suppress the inflammation generated by adipose tissue.54 Specifically, nicotine was shown to reduce tumor necrosis factor-α and interleukin-6 expression by epithelial cells of the human aerodigestive tract that are directly exposed to cigarette smoke.55 Through its influence on the amount of circulating adipocyte-derived leptin and sensitivity to leptin, smoking may exert effects on energy metabolism and body weight, complicating the relationship between cancer and BMI. Smoking in humans can lower circulating leptin levels in a manner that is independent from the amount of body fat.56 Leptin is increased in obese subjects and has been identified as a growth factor for cancers arising from the gastrointestinal epithelium.57 Nicotine may also enhance the sensitivity of hypothalamic leptin receptors and increase appetite inhibition, resulting in reduced weight.58 The withdrawal of this nicotinic mechanism with smoking cessation may help explain the frequent observation of weight gain in abstinent smokers.
Strengths of our study include measurement of BMI values by trained personnel at a uniform time point relative to diagnosis and treatment. Our study population was large and homogeneous with regard to histopathology and surgical therapy, with exclusion of patients receiving neoadjuvant therapy. Clinicopathologic and survival data were verified by review of individual patient records. Smoking data were collected using standard questionnaires, and our categorization of smoking (ie, never v ever) is in common usage, including prior studies in EAC,24,59 other cancers,20 and other prognostic studies of BMI.17,18 The robustness of our findings is supported by the consistent associations across all three end points (ie, DSS, OS, DFS). Although retrospective design can weaken data collection, internal validity is preserved because ascertainment and collection of study variables (eg, pathologic stage, vital status) did not differ by BMI or smoking status. Our cohort is generalizable to other patients with EAC in the West by numerous parameters, including stage, age, and sex.60
Limitations of our study include its retrospective design and the fact that, despite our large study population, only 171 patients (22%) were obese. Recognized limitations of BMI include the lack of a direct measure of central adiposity such as the waist-to-hip ratio, which may be more informative. We also did not address weight change after surgical therapy, which may also provide important information. In addition, it is possible that our results may not extend to patients with distant metastases or cancer-involved surgical margins, or who received neoadjuvant chemoradiotherapy, because these patients were excluded. For these reasons, it is important to validate our findings in an independent cohort. Although cigarette smoking has been extensively investigated as an exposure, no single model of analyzing smoking in relation to cancer outcomes may be entirely satisfactory.21,61
In conclusion, obesity is independently associated with increased mortality among never, but not ever, smokers. Elucidation of the biologic mechanisms underlying the interaction between smoking and obesity awaits further study. Our findings are relevant to patient management in that they can provide prognostic information that can inform postoperative risk stratification.
Acknowledgment
We thank Karen J. Hanson and Candace L. Kostelec for administrative assistance, and Patrick A. Burch for assistance in review of medical records.
Footnotes
Supported by the National Cancer Institute (Grant No. K12 CA90628-10U to H.H.Y., Grant No. K05 CA142885 to F.A.S.) and National Center for Research Resources (Center for Translational Science Activities, Grant No. UL1 RR024150) at the National Institutes of Health.
The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Harry H. Yoon, Mark A. Lewis, Stephen D. Cassivi, Robert B. Diasio, Frank A. Sinicrope
Collection and assembly of data: Harry H. Yoon, Mark A. Lewis, Maliha Khan, Stephen D. Cassivi
Data analysis and interpretation: Harry H. Yoon, Mark A. Lewis, Qian Shi, Frank A. Sinicrope
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Heidl G, Langhans P, Krieg V, et al. Comparative studies of cardia carcinoma and infracardial gastric carcinoma. J Cancer Res Clin Oncol. 1993;120:91–94. doi: 10.1007/BF01200730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heidl G, Langhans P, Mellin W, et al. Adenocarcinomas of esophagus and cardia in comparison with gastric carcinoma. J Cancer Res Clin Oncol. 1993;120:95–99. doi: 10.1007/BF01200731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pohl H, Welch HG. The role of overdiagnosis and reclassification in the marked increase of esophageal adenocarcinoma incidence. J Natl Cancer Inst. 2005;97:142–146. doi: 10.1093/jnci/dji024. [DOI] [PubMed] [Google Scholar]
- 4.Pohl H, Sirovich B, Welch HG. Esophageal adenocarcinoma incidence: Are we reaching the peak? Cancer Epidemiol Biomarkers Prev. 2010;19:1468–1470. doi: 10.1158/1055-9965.EPI-10-0012. [DOI] [PubMed] [Google Scholar]
- 5.Crane SJ, Locke GR, 3rd, Harmsen WS, et al. Survival trends in patients with gastric and esophageal adenocarcinomas: A population-based study. Mayo Clin Proc. 2008;83:1087–1094. doi: 10.4065/83.10.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jemal A, Siegel R, Xu J, et al. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 7.Ayazi SHJ, Chan LS, DeMeester SR, et al. Obesity and gastroesophageal reflux: Quantifying the association between body mass index, esophageal acid exposure, and lower esophageal sphincter status in a large series of patients with reflux symptoms. J Gastrointest Surg. 2009;13:1440–1447. doi: 10.1007/s11605-009-0930-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Engeland A, Tretli S, Bjorge T. Height and body mass index in relation to esophageal cancer; 23-year follow-up of two million Norwegian men and women. Cancer Causes Control. 2004;15:837–843. doi: 10.1023/B:CACO.0000043434.21558.ea. [DOI] [PubMed] [Google Scholar]
- 9.Chow WH, Blot WJ, Vaughan TL, et al. Body mass index and risk of adenocarcinomas of the esophagus and gastric cardia. J Natl Cancer Inst. 1998;90:150–155. doi: 10.1093/jnci/90.2.150. [DOI] [PubMed] [Google Scholar]
- 10.Abnet CC, Freedman ND, Hollenbeck AR, et al. A prospective study of BMI and risk of oesophageal and gastric adenocarcinoma. Eur J Cancer. 2008;44:465–471. doi: 10.1016/j.ejca.2007.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Becker S, Dossus L, Kaaks R. Obesity related hyperinsulinaemia and hyperglycaemia and cancer development. Arch Physiol Biochem. 2009;115:86–96. doi: 10.1080/13813450902878054. [DOI] [PubMed] [Google Scholar]
- 12.Komninou D, Ayonote A, Richie JP, Jr, et al. Insulin resistance and its contribution to colon carcinogenesis. Exp Biol Med (Maywood) 2003;228:396–405. doi: 10.1177/153537020322800410. [DOI] [PubMed] [Google Scholar]
- 13.McWilliams RR, Matsumoto ME, Burch PA, et al. Obesity adversely affects survival in pancreatic cancer patients. Cancer. 2010;116:5054–5062. doi: 10.1002/cncr.25465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Parr CL, Batty GD, Lam TH, et al. Body-mass index and cancer mortality in the Asia-Pacific Cohort Studies Collaboration: Pooled analyses of 424,519 participants. Lancet Oncol. 2010;11:741–752. doi: 10.1016/S1470-2045(10)70141-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Geyer SM, Morton LM, Habermann TM, et al. Smoking, alcohol use, obesity, and overall survival from non-Hodgkin lymphoma: A population-based study. Cancer. 2010;116:2993–3000. doi: 10.1002/cncr.25114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sinicrope FA, Foster NR, Sargent DJ, et al. Obesity is an independent prognostic variable in colon cancer survivors. Clin Cancer Res. 2010;16:1884–1893. doi: 10.1158/1078-0432.CCR-09-2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Adams KF, Schatzkin A, Harris TB, et al. Overweight, obesity, and mortality in a large prospective cohort of persons 50 to 71 years old. N Engl J Med. 2006;355:763–778. doi: 10.1056/NEJMoa055643. [DOI] [PubMed] [Google Scholar]
- 18.Berrington de Gonzalez A, Hartge P, Cerhan JR, et al. Body-mass index and mortality among 1.46 million white adults. N Engl J Med. 2010;363:2211–2219. doi: 10.1056/NEJMoa1000367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pischon T, Boeing H, Hoffmann K, et al. General and abdominal adiposity and risk of death in Europe. N Engl J Med. 2008;359:2105–2120. doi: 10.1056/NEJMoa0801891. [DOI] [PubMed] [Google Scholar]
- 20.Calle EE, Rodriguez C, Walker-Thurmond K, et al. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348:1625–1638. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 21.Cook MB, Kamangar F, Whiteman DC, et al. Cigarette smoking and adenocarcinomas of the esophagus and esophagogastric junction: A pooled analysis from the international BEACON consortium. J Natl Cancer Inst. 2010;102:1344–1353. doi: 10.1093/jnci/djq289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cescon DW, Bradbury PA, Asomaning K, et al. p53 Arg72Pro and MDM2 T309G polymorphisms, histology, and esophageal cancer prognosis. Clin Cancer Res. 2009;15:3103–3109. doi: 10.1158/1078-0432.CCR-08-3120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sundelöf M, Lagergren J, Ye W. Patient demographics and lifestyle factors influencing long-term survival of oesophageal cancer and gastric cardia cancer in a nationwide study in Sweden. Eur J Cancer. 2008;44:1566–1571. doi: 10.1016/j.ejca.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 24.Trivers K. Demographic and lifestyle predictors of survival in patients with esophageal or gastric cancers. Clin Gastroenterol Hepatol. 2005;3:225–230. doi: 10.1016/s1542-3565(04)00613-5. [DOI] [PubMed] [Google Scholar]
- 25.Kleinberg L, Forastiere AA. Chemoradiation in the management of esophageal cancer. J Clin Oncol. 2007;25:4110–4117. doi: 10.1200/JCO.2007.12.0881. [DOI] [PubMed] [Google Scholar]
- 26.Morgan MA, Lewis WG, Hopper AN, et al. Prognostic significance of body mass indices for patients undergoing esophagectomy for cancer. Dis Esophagus. 2007;20:29–35. doi: 10.1111/j.1442-2050.2007.00637.x. [DOI] [PubMed] [Google Scholar]
- 27.Grotenhuis BA, Wijnhoven BP, Hotte GJ, et al. Prognostic value of body mass index on short-term and long-term outcome after resection of esophageal cancer. World J Surg. 2010;34:2621–2627. doi: 10.1007/s00268-010-0697-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bower MR, Martin RC., 2nd Nutritional management during neoadjuvant therapy for esophageal cancer. J Surg Oncol. 2009;100:82–87. doi: 10.1002/jso.21289. [DOI] [PubMed] [Google Scholar]
- 29.Suntharalingam M, Moughan J, Coia LR, et al. The national practice for patients receiving radiation therapy for carcinoma of the esophagus: Results of the 1996-1999 Patterns of Care Study. Int J Radiat Oncol Biol Phys. 2003;56:981–987. doi: 10.1016/s0360-3016(03)00256-6. [DOI] [PubMed] [Google Scholar]
- 30.Yoon HH, Khan M, Shi Q, et al. The prognostic value of clinical and pathologic factors in esophageal adenocarcinoma: A Mayo cohort of 796 patients with extended follow-up after surgical resection. Mayo Clin Proc. 2010;85:1080–1089. doi: 10.4065/mcp.2010.0421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Incidence of initiation of cigarette smoking: United States, 1965–1996. MMWR Morb Mortal Wkly Rep. 1998;47:837–840. [PubMed] [Google Scholar]
- 32.World Health Organization. WHO Global Database on Body Mass Index. http://apps.who.int/bmi/index.jsp?introPage=intro_3.html.
- 33.Plaisant N, Senesse P, Azria D, et al. Surgery for esophageal cancer after concomitant radiochemotherapy: Oncologic and functional results. World J Surg. 2005;29:32–38. doi: 10.1007/s00268-004-7455-8. [DOI] [PubMed] [Google Scholar]
- 34.Lagarde SM, ten Kate FJ, Reitsma JB, et al. Prognostic factors in adenocarcinoma of the esophagus or gastroesophageal junction. J Clin Oncol. 2006;24:4347–4355. doi: 10.1200/JCO.2005.04.9445. [DOI] [PubMed] [Google Scholar]
- 35.Stahl M, Wilke H, Stuschke M, et al. Clinical response to induction chemotherapy predicts local control and long-term survival in multimodal treatment of patients with locally advanced esophageal cancer. J Cancer Res Clin Oncol. 2005;131:67–72. doi: 10.1007/s00432-004-0604-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap): A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Corrao MA, Guindon GE, Cokkinides V, et al. Building the evidence base for global tobacco control. Bull World Health Org. 2000;78:884–890. [PMC free article] [PubMed] [Google Scholar]
- 38.Al-Delaimy W, White M, Mills A, et al. La Jolla, CA: University of California, San Diego; 2010. Final Summary Report of: Two Decades of the California Tobacco Control Program: California Tobacco Survey, 1990–2008. http://www.cdph.ca.gov/programs/tobacco/Documents/CDPH_CTS2008%20summary%20report_final.pdf. [Google Scholar]
- 39.Skipworth J, Foster J, Raptis D, et al. The effect of preoperative weight loss and body mass index on postoperative outcome in patients with esophagogastric carcinoma. Dis Esophagus. 2009;22:559–563. doi: 10.1111/j.1442-2050.2009.00939.x. [DOI] [PubMed] [Google Scholar]
- 40.Flegal KM, Graubard BI, Williamson DF, et al. Cause-specific excess deaths associated with underweight, overweight, and obesity. JAMA. 2007;298:2028–2037. doi: 10.1001/jama.298.17.2028. [DOI] [PubMed] [Google Scholar]
- 41.Orpana HM, Berthelot JM, Kaplan MS, et al. BMI and mortality: Results from a national longitudinal study of Canadian adults. Obesity (Silver Spring) 2010;18:214–218. doi: 10.1038/oby.2009.191. [DOI] [PubMed] [Google Scholar]
- 42.Prentice AM, Jebb SA. Beyond body mass index. Obes Rev. 2001;2:141–147. doi: 10.1046/j.1467-789x.2001.00031.x. [DOI] [PubMed] [Google Scholar]
- 43.Whitlock G, Lewington S, Sherliker P, et al. Body-mass index and cause-specific mortality in 900 000 adults: Collaborative analyses of 57 prospective studies. Lancet. 2009;373:1083–1096. doi: 10.1016/S0140-6736(09)60318-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Willett WC, Hu FB, Colditz GA, et al. Underweight, overweight, obesity, and excess deaths. JAMA. 2005;294:551. doi: 10.1001/jama.294.5.551-a. author reply 552–553. [DOI] [PubMed] [Google Scholar]
- 45.Figueroa JD, Terry MB, Gammon MD, et al. Cigarette smoking, body mass index, gastro-esophageal reflux disease, and non-steroidal anti-inflammatory drug use and risk of subtypes of esophageal and gastric cancers by P53 overexpression. Cancer Causes Control. 2009;20:361–368. doi: 10.1007/s10552-008-9250-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Corley DA, Kubo A, Zhao W. Abdominal obesity and the risk of esophageal and gastric cardia carcinomas. Cancer Epidemiol Biomarkers Prev. 2008;17:352–358. doi: 10.1158/1055-9965.EPI-07-0748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang ZF, Kurtz RC, Sun M, et al. Adenocarcinomas of the esophagus and gastric cardia: Medical conditions, tobacco, alcohol, and socioeconomic factors. Cancer Epidemiol Biomarkers Prev. 1996;5:761–768. [PubMed] [Google Scholar]
- 48.Zhai R, Liu G, Asomaning K, et al. Genetic polymorphisms of VEGF, interactions with cigarette smoking exposure and esophageal adenocarcinoma risk. Carcinogenesis. 2008;29:2330–2334. doi: 10.1093/carcin/bgn210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nordenstedt H, El-Serag H. The influence of age, sex, and race on the incidence of esophageal cancer in the United States (1992–2006) Scand J Gastroenterol. 2011;46:597–602. doi: 10.3109/00365521.2011.551890. [DOI] [PubMed] [Google Scholar]
- 50.Fantuzzi G. Adipose tissue, adipokines, and inflammation. J Allergy Clin Immunol. 2005;115:911–919. doi: 10.1016/j.jaci.2005.02.023. [DOI] [PubMed] [Google Scholar]
- 51.Olefsky JM, Glass CK. Macrophages, inflammation, and insulin resistance. Annu Rev Physiol. 2010;72:219–246. doi: 10.1146/annurev-physiol-021909-135846. [DOI] [PubMed] [Google Scholar]
- 52.Suganuma M, Okabe S, Marino MW, et al. Essential role of tumor necrosis factor alpha (TNF-alpha) in tumor promotion as revealed by TNF-alpha-deficient mice. Cancer Res. 1999;59:4516–4518. [PubMed] [Google Scholar]
- 53.Oka M, Yamamoto K, Takahashi M, et al. Relationship between serum levels of interleukin 6, various disease parameters and malnutrition in patients with esophageal squamous cell carcinoma. Cancer Res. 1996;56:2776–2780. [PubMed] [Google Scholar]
- 54.Wang X, Yang Z, Xue B, et al. Activation of the cholinergic antiinflammatory pathway ameliorates obesity-induced inflammation and insulin resistance. Endocrinology. 2011;152:836–846. doi: 10.1210/en.2010-0855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li Q, Zhou X, Kolosov VP, et al. Nicotine suppresses inflammatory factors in HBE16 airway epithelial cells after exposure to cigarette smoke extract and lipopolysaccharide. Transl Res. 2010;156:326–334. doi: 10.1016/j.trsl.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 56.Koc B, Bulucu F, Karadurmus N, et al. Lower leptin levels in young non-obese male smokers than non-smokers. Ups J Med Sci. 2009;114:165–169. doi: 10.1080/03009730902761631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Endo H, Hosono K, Uchiyama T, et al. Leptin acts as a growth factor for colorectal tumours at stages subsequent to tumour initiation in murine colon carcinogenesis. Gut. 2011;60:1363–1371. doi: 10.1136/gut.2010.235754. [DOI] [PubMed] [Google Scholar]
- 58.Hodge AM, Westerman RA, de Courten MP, et al. Is leptin sensitivity the link between smoking cessation and weight gain? Int J Obes Relat Metab Disord. 1997;21:50–53. doi: 10.1038/sj.ijo.0800362. [DOI] [PubMed] [Google Scholar]
- 59.Hayashi Y, Correa AM, Hofstetter WL, et al. The influence of high body mass index on the prognosis of patients with esophageal cancer after surgery as primary therapy. Cancer. 2010;116:5619–5627. doi: 10.1002/cncr.25745. [DOI] [PubMed] [Google Scholar]
- 60.Rice TW, Rusch VW, Ishwaran H, et al. Cancer of the esophagus and esophagogastric junction: Data-driven staging for the seventh edition of the American Joint Committee on Cancer/International Union Against Cancer Cancer Staging Manuals. Cancer. 2010;116:3763–3773. doi: 10.1002/cncr.25146. [DOI] [PubMed] [Google Scholar]
- 61.Samet JM, Thun MJ, de Gonzalez AB. Models of smoking and lung cancer risk: A means to an end. Epidemiology. 2007;18:649–651. doi: 10.1097/EDE.0b013e3181271afa. [DOI] [PubMed] [Google Scholar]